3.2. Effect of Water-Soluble Heat-Stable Salts on Triethylene Glycol Foaming Bulleted Lists Look Like This
According to the previous study [
18] on the experiment of continuous heating of triethylene glycol, when 48h continuous heating of triethylene glycol which will generate, di-ethylene glycol, tetraethylene glycol, pentaethylene glycol, hexaethylene glycol and glycol and other impurities account for more than 60% of the total impurities, but also generate a small number of ethers, cyclization and acid impurities. In this case, water-soluble inorganic salts and their hydrolysis products play the role of catalysts, they promote the decomposition of triethylene glycol molecules, intermolecular and intramolecular condensation reactions, thus accelerating the deterioration of triethylene glycol, making changes in the foaming properties and surface tension, which is specifically reflected in the enhancement of foaming properties,triethylene glycol is coated by the bubbles, and ultimately resulting in the increase in the amount of triethylene glycol carrying loss.
However, the results of the current cycle regeneration experiments show that the impurity content of the produced triethylene glycol samples is extremely low, with the total mass fraction of impurities at a low level of less than 0.2%. This result strongly suggests that the decomposition effect of water-soluble inorganic salts on triethylene glycol is not significant under the specific conditions of cyclic regeneration due to the short heating duration. The source of organic impurity generation is mainly the decomposition reaction of a small number of triethylene glycol molecules due to their own nature under high temperature environment. Although the amount of organic impurities generated in a single cycle is limited, if these impurities continue to accumulate over a long period of time, they will inevitably have a significant negative effect on the properties of the triethylene glycol solution, including the enhancement of foaming properties and the increase in the amount of triethylene glycol carry-over loss. The influence of inorganic salts on the foaming properties of triethylene glycol during the cycling experiments goes beyond their own decomposition reactions. In fact, the morphology presented by the inorganic salt in the triethylene glycol solution plays a key role. When the salt crystals are small, similar in size, and can be dispersed relatively homogeneously in the triethylene glycol, the solution exhibits a higher foaming height and a longer defoaming time. The rationale behind this is that the foaming of triethylene glycol is closely related to the stability of the foam and the aggregation behavior of the salt crystals at the gas-liquid interface. Those fine solid particles are easily attached to the foam bilayer film, so that the flow of liquid at the film encounters greater resistance, and the process of discharging liquid out of the bubble film is blocked, thus making it difficult for the foam to burst [
19,
20]. Ultimately, the foaming characteristics and foam stability of the whole solution are significantly enhanced.
Figure 2.
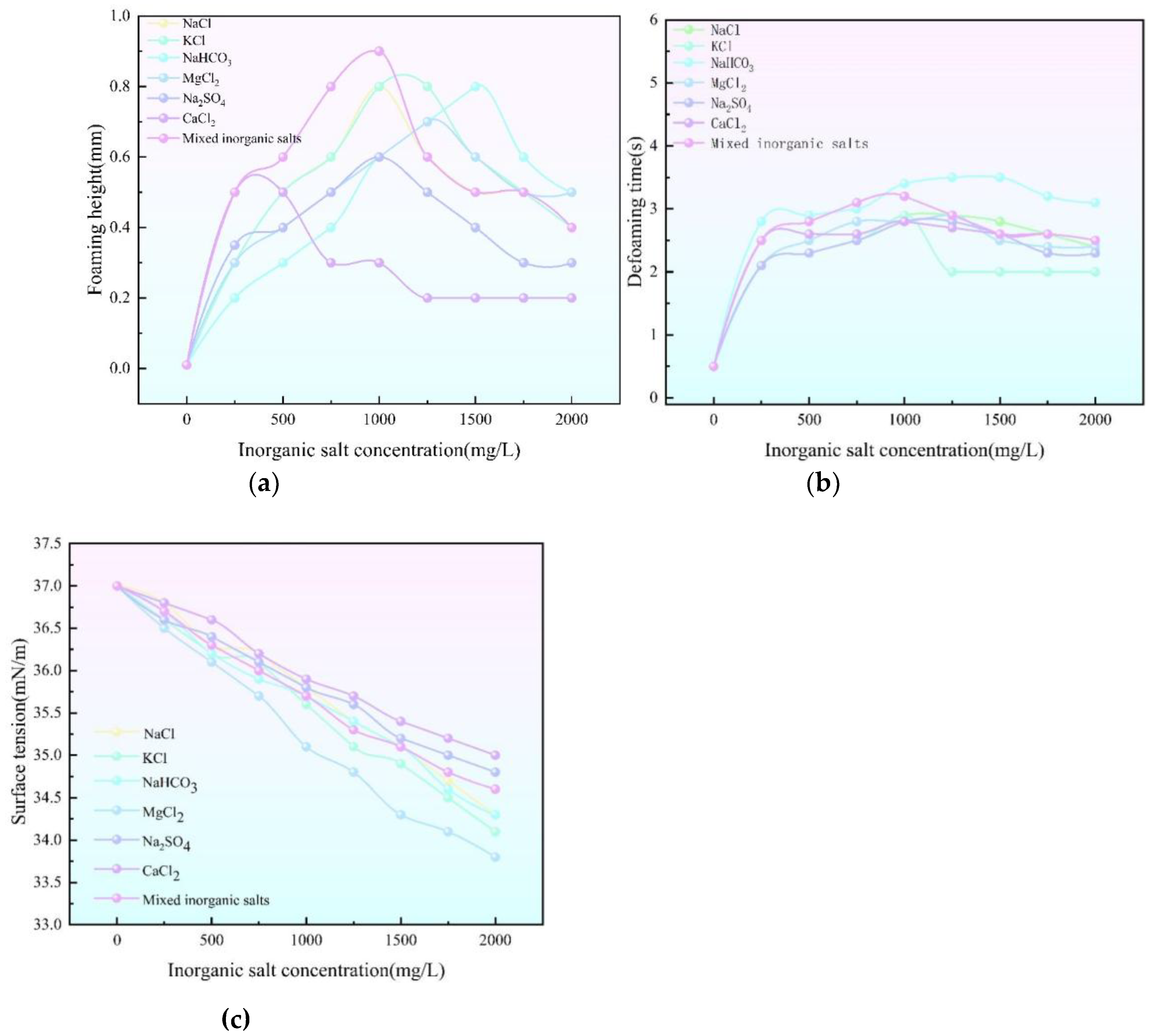
(a) Relationship between foaming height and inorganic salt concentration curve (b) Relationship between defoaming time and inorganic salt concentration curve (c) Relationship between surface tension and inorganic salt concentration.
Figure 2.
(a) Relationship between foaming height and inorganic salt concentration curve (b) Relationship between defoaming time and inorganic salt concentration curve (c) Relationship between surface tension and inorganic salt concentration.
With the gradual increase of inorganic salt concentration, the foaming height showed the change trend of rising and then falling. Specific data showed that CaCl₂ had the weakest effect on the foaming height, which was only 0.37 mm when the concentration reached 1000 mg/L; on the contrary, KCl had a large effect, which was as high as 0.85 mm when the concentration was 1100 mg/L. Although CaCl₂ and KCl exhibited relatively large crystal sizes, the crystals of CaCl₂ were not only large, but also less homogeneous. Although CaCl₂ and KCl exhibited relatively large crystal sizes, CaCl₂ crystals were not only large in size, but also poorly homogenized, which resulted in poor uniformity of dispersion in triethylene glycol. The larger the particle size and the heavier the mass of the salt crystals, the more difficult it is to achieve effective adhesion on the surface of the bubble liquid film, coupled with the different sizes of CaCl₂ particles, even if some of the particles are attached to the liquid film, it will lead to an uneven force on the liquid film, which will undermine the structural stability of the foam, and ultimately be reflected in the weakening of the foaming performance of triglyceride. Substances with higher surface activity are able to diffuse faster into the gas-liquid interface and form a tightly arranged adsorption layer, leading to an increase in foaming height. However, it is not the strongest foaming ability due to the large size of its own crystals.
The change of defoaming time with the concentration of inorganic salts is more complicated, and the overall trend shows an increase first, followed by a small decrease, and finally leveles off. Among them, Na₂SO₄ has the smallest effect on the defoaming time, in the concentration range of 0 - 2000mg/L, in most cases, compared with other inorganic salts of the same concentration, the defoaming time is shorter, for example, when the concentration is 1250mg/L, the defoaming time is only 2.8s. This is attributable to the fact that the Na₂SO₄ crystals are incorporated into the tri-glycol solution, the particle size is smaller and the particle homogeneity is good, although the foaming time is only 2.8s. This is attributed to the fact that the Na₂SO₄ crystals were incorporated into the triglyceride solution with small particle size and good particle homogeneity, which could induce the generation of crystalline alcohols in the solution and lead to a significant increase in viscosity, although the foaming ability was weak. During the foaming stage, the generation of bubbles is inhibited, and when the foam persists, the high-viscosity foam film slows down the discharge by virtue of the strong surface viscosity and enhances the foam stability, which is conducive to rapid defoaming. On the contrary, the effect of NaHCO₃ on defoaming time was most prominent, in the concentration range of 0-2000mg/L, almost the whole defoaming time was higher than that of other inorganic salts with the same concentration, and the defoaming time reached 2.8s in the concentration of 1500mg/L. The crystal particles of NaHCO₃ were fine and homogeneous, which were highly dispersed in the tri-glycol solution, and the microscopic particles were tightly attached to the surface of the foam bilayer film, which strongly blocked the liquid membrane surface and strongly hindered the liquid membrane. The tiny particles were tightly attached to the surface of the foam bilayer, which strongly blocked the liquid flow in the liquid film and hindered the discharge of the bubble liquid film, which greatly enhanced the foam stability and greatly increased the antifoaming difficulty.
The surface tension decreased linearly with the increase of inorganic salt content. The quantitative data showed that MgCl₂ had the greatest effect on the surface tension, which was as low as 33.8 mN/m when the concentration of inorganic salts reached 2000 mg/L, and CaCl₂ had the least effect, which was 35 mN/m at this time, and qualitatively, the degree of different inorganic salts altering the interaction of the triethylene glycol molecules varied, and the surface tension was greatly reduced by the more effective disruption of the intermolecular forces on the surface of triethylene glycol with MgCl₂, while CaCl₂ had a relatively moderate effect. MgCl₂ was more effective in destroying the intermolecular forces on the surface of triethylene glycol, which led to a significant decrease in the surface tension, while the effect of CaCl₂ was relatively mild.
The purpose of setting the mixed salt is to simulate the real situation in the field, and the mixed salt is NaCl+KCl+CaCl₂+NaHCO₃+MgCl₂+Na2SO₄ (10:2:0.8:0.5:0.4:0.3), and it can be concluded that the curves of foaming height and interfacial tension of the mixed salt are the most similar to those of NaCl, which indicates that NaCl dominates in the mixed salt in terms of foaming height and interfacial tension; in terms of defoaming time and MgCl2, MgCl2 dominates. It shows that NaCl dominates in the foaming height and interfacial tension in the mixed salt; in the defoaming time and the curve of MgCl2 is similar, and the proportion of MgCl2 in the mixed salt is only 0.4, but it dominates in the defoaming time. Through the above analysis, combined with the surface tension, foaming height, defoaming time and the proportion of the content, we can get the rankings of inorganic salts on the size of the influence on foaming performance. MgCl2> NaHCO3> KCl> NaCl> Na2SO4> CaCl2. So in the actual production process, we have to strictly control the content of inorganic salts to reduce their effect on the foaming and defoaming of triethylene glycol.
3.3. Effect of Solid Impurities on the Foaming of Triethylene Glycol
As with the effect of salt crystallization on the foaming performance of triethylene glycol, the morphology of solid impurities in triethylene glycol has a great relationship with the foaming performance of triethylene glycol, the morphology of solid impurities in triethylene glycol has a great relationship with the foaming performance of triethylene glycol, when the particles of the solid impurities are smaller and uniform in size, the higher the foaming height of the triethylene glycol, the longer the defoaming time, the smaller solid particles are adhered to the liquid film of the foaming bilayer, which increases the The smaller solid particles adhered to the foam bilayer liquid film, increasing the resistance to liquid flow at the liquid film and enhancing the foaming and foam stability of the solution [
22,
23,
24]. When the solid impurity particles are large, they are not easily adhered to the bubble liquid film due to gravity, and the foaming performance is reduced; when the solid impurity particles are of different sizes, the particles adhering to the bubble liquid film are not uniformly stressed, which reduces the stability of the foam.
Figure 3.
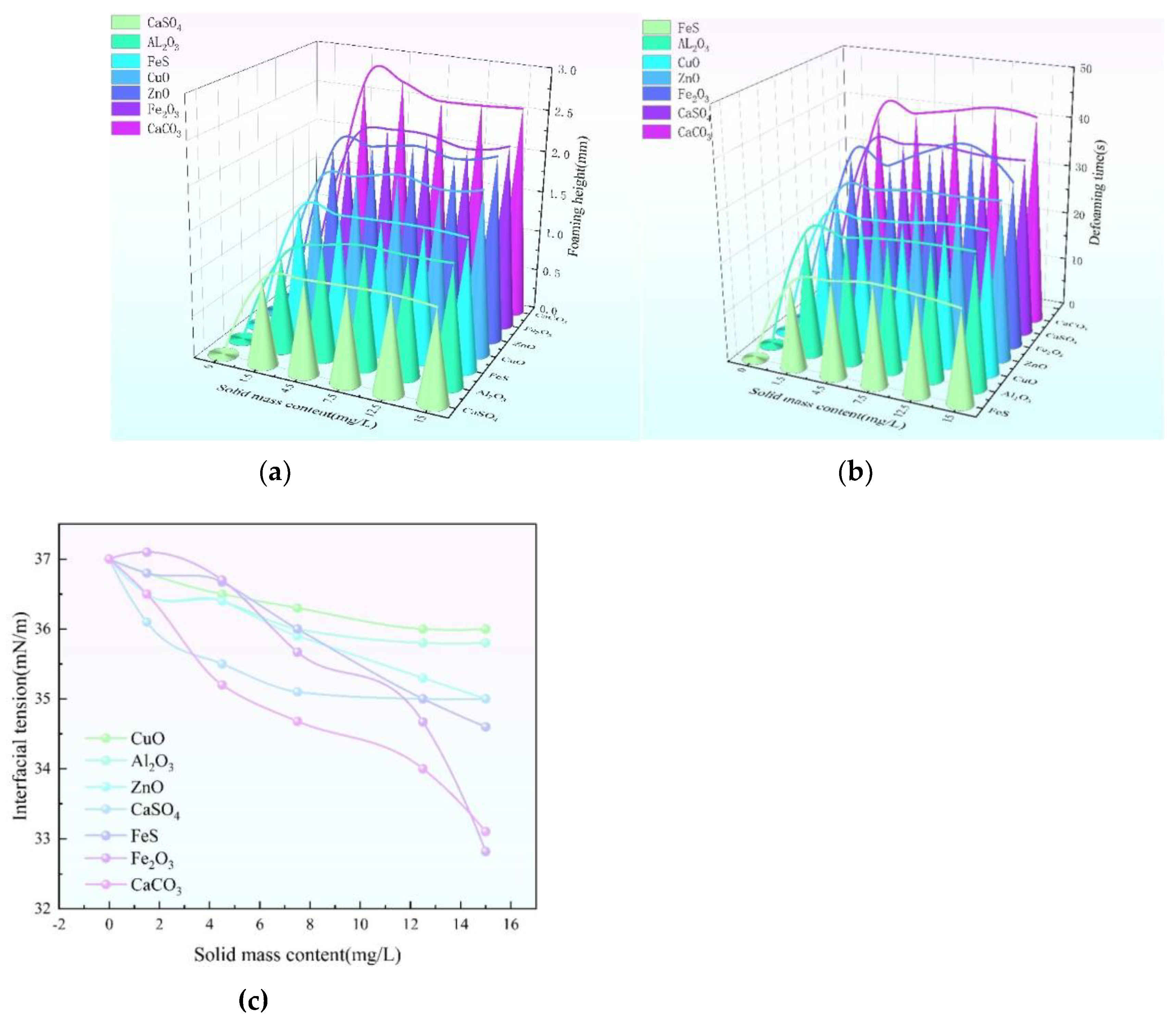
(a) Relationship between the foaming height and the concentration curve of solid impurities (b) Relationship between defoaming time and solid impurity concentration curve (c) Relationship between surface tension and solid impurity concentration.
Figure 3.
(a) Relationship between the foaming height and the concentration curve of solid impurities (b) Relationship between defoaming time and solid impurity concentration curve (c) Relationship between surface tension and solid impurity concentration.
In terms of foaming performance, compared with the foaming performance of triethylene glycol with only distilled water added, the foaming height and defoaming time of triethylene glycol increased significantly after the addition of solid impurities, and then the foaming height and defoaming time did not change significantly with the increase of this concentration [
25,
26].The effect of CaCO₃ on the height of the bubbles was the most significant, and the foaming height was as high as 2.6 mm when the concentration of the solids reached 1.5 mg/L; in contrast, CaSO₄ had the weakest effect, which was due to the fact that CaCO₃ had only 1 mm foaming height for the same concentration. The effect of CaCO₃ on the bubble height was the most significant, with a bubble height of 2.6 mm at a solid concentration of 1.5 mg/L. In contrast, the effect of CaSO₄ was the weakest, with a bubble height of 1 mm at the same concentration, which was attributed to the fact that CaCO₃ exhibits the characteristics of small and uniform particle size in triethylene glycol, which enables it to adsorb gases more efficiently and promotes the generation and stabilization of bubbles, thus leading to the outstanding performance of the bubble height. On the other hand, CaSO₄ can easily lead to the formation of crystalline alcohols, which leads to a significant increase in the viscosity of the system and seriously impedes the generation of bubbles, thus limiting the bubble height.
The defoaming time also increases with the mass content of solids, firstly it rises to 1.5mg/L and then it tends to level off, CaCO₃ has the biggest influence on the defoaming time of the bubbles, when the concentration of solids is 1.5mg/L, the defoaming time is up to 38s; AL₂O₃ has the smallest influence, and the defoaming time is 25s under this concentration, it's because the particles of CaCO₃ are uniformly fine, and they can be attached to the liquid membrane of bubbles tightly in the foam system. The reason is that CaCO₃ particles are uniform and fine, which can closely attach to the bubble film in the foam system and strongly hinder the flow of liquid in the film, greatly enhancing the stability of the foam, resulting in a drastic increase in the difficulty of defoaming; FeS particles are not uniform in size, which makes the stability of the formed foam structure poor, and the liquid is easy to be discharged, and the process of defoaming is rapid, so the time of defoaming is the shortest.
The surface tension decreased continuously with increasing solid mass content. When the solid mass content reaches 15 mg/L, CaCO₃ has the greatest effect on the surface tension, which decreases to 33.1 mN/m, and CuO has the smallest effect, with a surface tension of 36 mN/m. This is because of the differences in the interactions between different solid impurities and the molecules of triethylene glycol, and CaCO₃ is more effective in altering the molecular arrangement and interactions of the molecules on the surface of triethylene glycol, which contributes to the significant reduction of the surface tension, and CuO is relatively weak in this ability. CaCO₃ is able to change the arrangement and interaction of molecules on the surface of triglyceride more effectively, leading to a significant reduction of the surface tension, while the ability of CuO is relatively weak.
This leads to the order of strength of the solid's influence on the surface impurities with uniform size of solid impurities, CaCO3> Fe2O3> CaSO4> ZnO> CuO> Al2O3> FeS.
3.4. Effect of Corrosion Inhibitor and Foam Inhibitor on the Foaming of Triethylene Glycol
After adding chemical additives, the foaming performance is significantly enhanced, and the surface tension is greatly reduced. This is because the corrosion inhibitor and foam inhibitor contain surfactant components, which can significantly reduce the surface tension of the solution, thus facilitating the foaming of the solution. On the foam bilayer liquid film, the surfactant is adsorbed to the gas-liquid interface in a directional manner, and its lipophilic group points to the gas while the hydrophilic group interacts with the water, which makes the liquid not easy to become thinner and the elasticity and strength of the liquid film is significantly enhanced, which ultimately leads to the formation of the foam stability is greatly increased [
27]. The addition of chemical additives significantly changed the foaming properties, surface tension and other properties of triethylene glycol, indicating that chemical additives have a great influence on the degradation of triethylene glycol.
Figure 4.
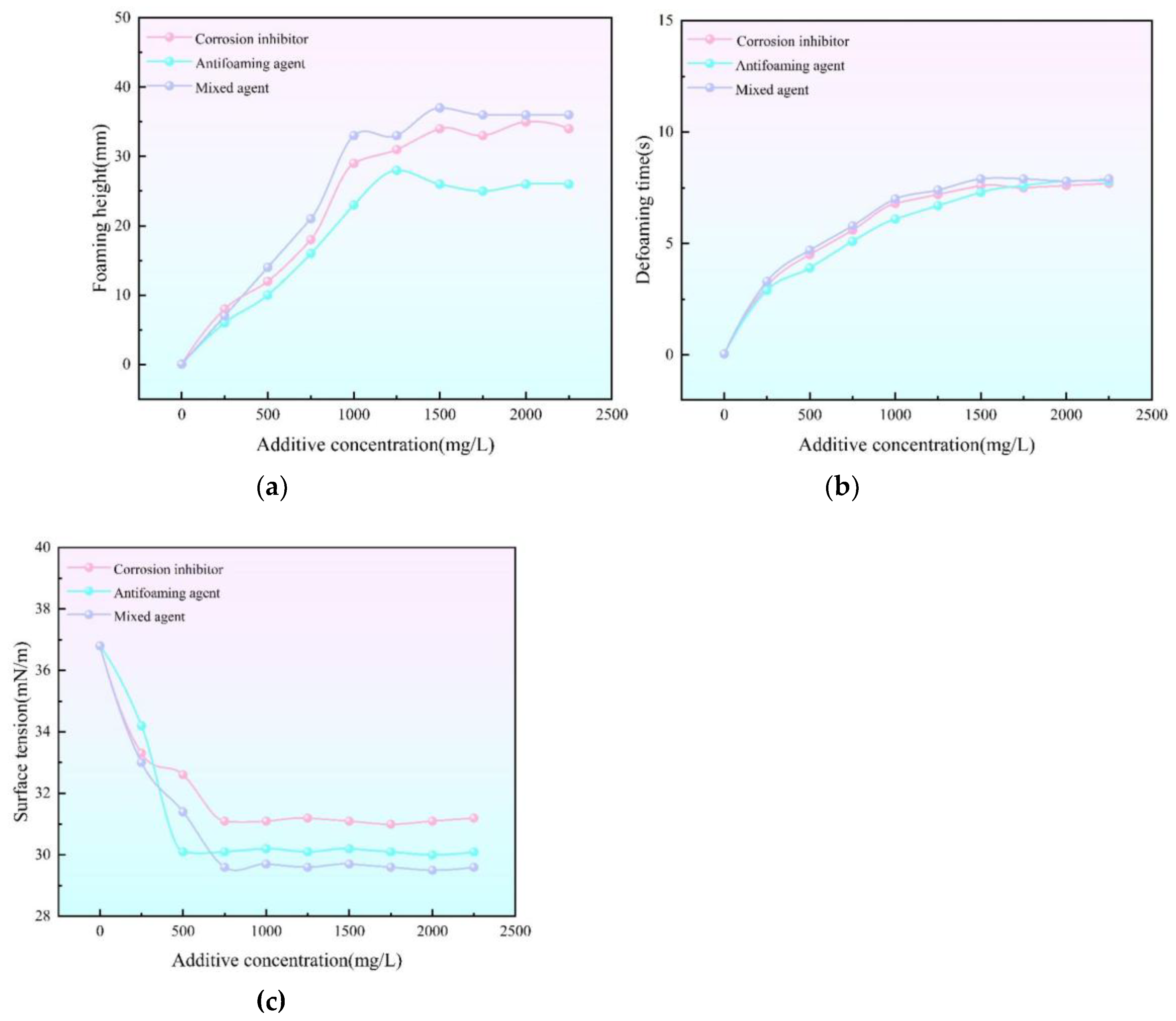
(a) Relationship curve between corrosion inhibitor, defoamer concentration and height of triethylene glycol (b) Relationship curve between corrosion inhibitor, defoamer concentration and defoaming time of triethylene glycol (c) Relationship between surface tension and solid impurity concentration.
Figure 4.
(a) Relationship curve between corrosion inhibitor, defoamer concentration and height of triethylene glycol (b) Relationship curve between corrosion inhibitor, defoamer concentration and defoaming time of triethylene glycol (c) Relationship between surface tension and solid impurity concentration.
Obtained in analyzing the role of chemical reagents on the foaming properties of triethylene glycol under a single factor in cyclic regeneration experiments. The surface tension of chemical reagents all decreased with the increase of chemical reagent content, and the interfacial tension was not changing when the chemical content reached 750 mg/L. The interfacial tension was 31.1 mN/m when the buffer content reached 750 mg/L; the interfacial tension was 30.1 mN/m when the blocking agent content reached 750 mg/L; and the interfacial tension was 29.6 mN/m when the buffer+ blocking agent (1:1) content reached 750 mg/L. The chemical reagents' foaming heights all increased with the increase of chemical reagent content, and then leveled off. Corrosion inhibitor in the concentration of about 1500mg/L reached a maximum value of 34mm, and then a small fluctuation but the overall tendency to level off; Foam inhibitor in the concentration of about 1250mg/L reached a maximum value of 28mm, and then there is a small decline but the overall tendency to level off; Corrosion inhibitor+ Foam inhibitor (1:1) in the concentration of about 1500mg/L reached a maximum value of 37mm, and then a small fluctuation but the overall tendency to level off. The overall tendency of the fluctuation is flat. The chemical reagents all increased with the increase of chemical reagent content, and then leveled off. The corrosion inhibitor reached a maximum value of 7.6s at a concentration of 1500mg/L and then leveled off; the foam inhibitor reached a maximum value of 7.8s at a concentration of 2000mg/L and then leveled off; and the corrosion inhibitor+ foam inhibitor (1:1) reached a maximum value of 7.9s at a concentration of 1500mg/L and then leveled off.
In the actual field situation, the corrosion inhibitor and antifoam agent are usually used 1:1, so the experiment set a group of comprehensive chemical additives to simulate the actual situation. Under the same conditions, the corrosion inhibitor and foam inhibitor in the defoaming time and defoaming height, with the increase in the concentration of defoaming time and height are increased, in the concentration of 1500mg / L at the turning point and after the increase in the concentration of its influence is not much, the impact of corrosion inhibitors is greater than the impact of the foam inhibitor, and in the mixture of substances in the corrosion inhibitor's influence are dominant [ 28-29]. The interfacial tension of triethylene glycol will show first decrease and then basic stabilization with the increase of corrosion inhibitor and foam inhibitor concentration. When the concentration of corrosion inhibitor and foam inhibitor exceeded 750 mg/L, the surface tension of triethylene glycol no longer changed significantly. Under the same conditions, the defoamer can make the surface tension of triethylene glycol decrease more significantly compared with the corrosion inhibitor. If the antifoam agent and corrosion inhibitor are mixed in equal amount, they will produce a positive synergistic effect, which makes the surface tension of triethylene glycol decrease further, and the antifoaming agent plays a dominant role, and its effect on the interfacial tension of triethylene glycol is most prominent.
3.5. Impact of Hydrocarbons on Foaming in Triethylene Glycol Dehydration Plants
Hydrocarbons have almost no effect on the foaming properties of triethylene glycol. An increase in interfacial tension, a key factor in foaming performance, signaled a decrease in foaming performance, but the excessive content of hydrocarbons in triethylene glycol led to an increase in foaming time and foaming height.
Figure 5.
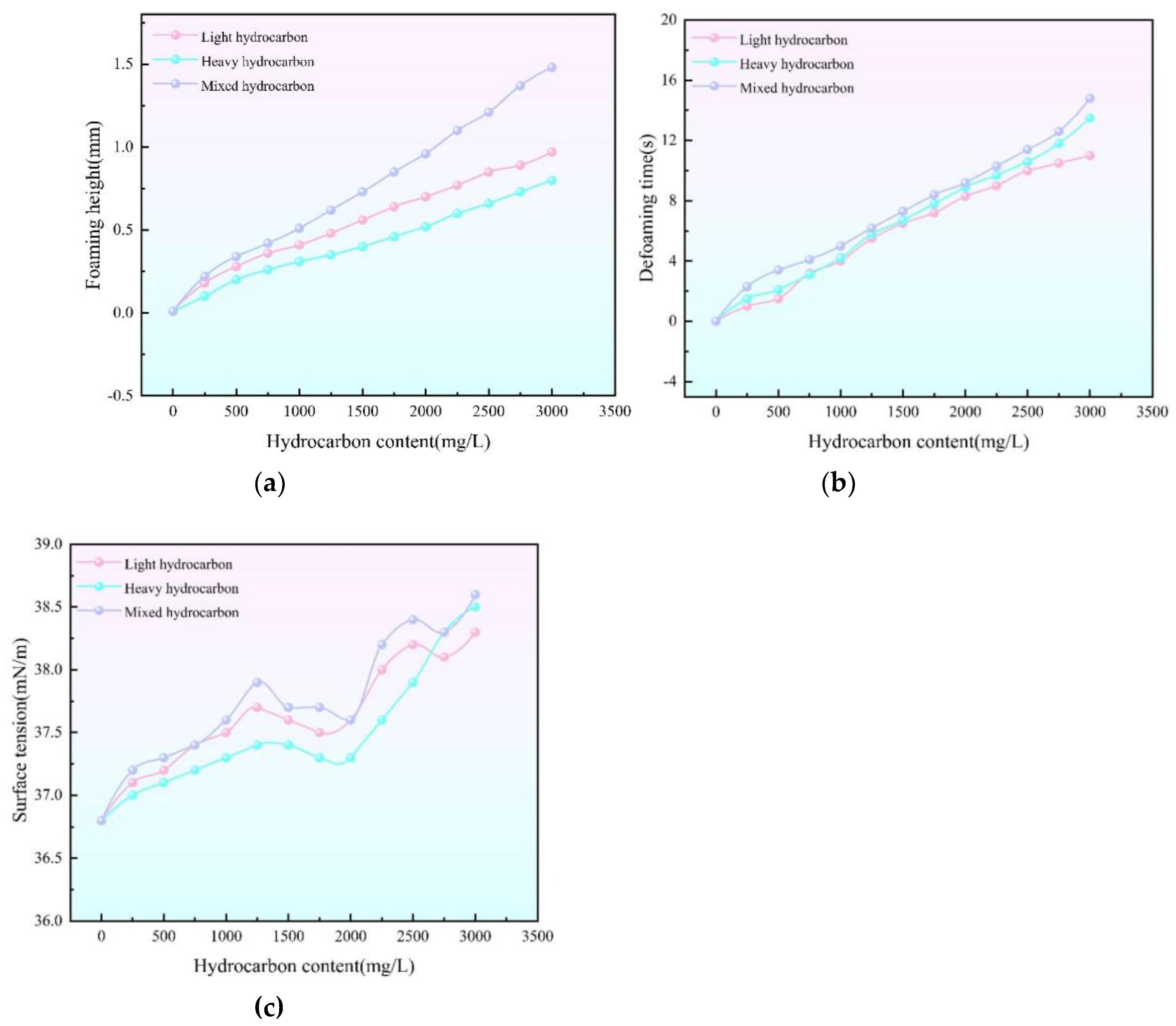
(a) Relationship curve between concentration of hydrocarbon substances and foaming height of triethylene glycol (b) Relationship curve between concentration of hydrocarbon substances and defoaming time of triethylene glycol (c) Relationship curve between hydrocarbon concentration and interfacial tension of triethylene glycol.
Figure 5.
(a) Relationship curve between concentration of hydrocarbon substances and foaming height of triethylene glycol (b) Relationship curve between concentration of hydrocarbon substances and defoaming time of triethylene glycol (c) Relationship curve between hydrocarbon concentration and interfacial tension of triethylene glycol.
Obtained from analyzing the role of hydrocarbons on the foaming properties of triethylene glycol under a single factor in cyclic regeneration experiments. The interfacial tension increased with the increase of hydrocarbon content, and the increase of interfacial tension was accelerated in the hydrocarbon content over 2000mg/L. The interfacial tension was 38.5mN/m at 3000mg/L of light hydrocarbon. When the content of light hydrocarbon is 3000mg/L, the interfacial tension is 38.5mN/m. When the content of heavy hydrocarbon is 3000mg/L, the interfacial tension is 38.3mN/m. When the content of light hydrocarbon + heavy hydrocarbon (4:1) is 3000mg/L, the interfacial tension is 38.6mN/m. The foaming height rises with the increase of the content of hydrocarbon. Foaming height rises the fastest when the hydrocarbon content does not exceed 500mg/L. When the content of light hydrocarbon is 3000mg/L, the foaming height is 0.97mm, when the content of heavy hydrocarbon is 3000mg/L, the foaming height is 0.8mm, when the content of light hydrocarbon + heavy hydrocarbon (4:1) is 3000mg/L, the foaming height is 1.48mm, and the defoaming time rises with the increase of the hydrocarbon content. When the hydrocarbon content exceeded 1500mg/L, the defoaming time increased faster. When the content of light hydrocarbon is 3000mg/L, the defoaming time is 11s, when the content of heavy hydrocarbon is 3000mg/L, the defoaming time is 13.5s, and when the content of light hydrocarbon + heavy hydrocarbon (4:1) is 3000 mg/L, the defoaming time is 14.8s.
Petroleum ether and gasoline were used to simulate the effects of light and heavy hydrocarbons on the properties of triethylene glycol solution, and the ratio of mixed hydrocarbons was set to 4∶ 1 to simulate the situation of hydrocarbons in the actual field. The increase of hydrocarbon concentration will increase the surface tension of triethylene glycol, though the increase is limited. Moreover, it will lead to the weakening of the foaming ability, while hydrocarbons do not have a significant effect on the foaming performance.” Under the same conditions, the influence of light hydrocarbons on the interfacial tension of triethylene glycol is greater than that of heavy hydrocarbons, and light hydrocarbons play a dominant role in the process of mixed hydrocarbons affecting the surface tension of triethylene glycol. In the influence of defoaming time, under the same conditions, heavy hydrocarbons have a greater degree of influence, and heavy hydrocarbons play a dominant role in the influence of mixed hydrocarbons. In terms of foaming height, light hydrocarbons have a greater influence under the same conditions, and light hydrocarbons dominate in the influence of mixed hydrocarbons.
3.6. Effect of Hydrogen Sulfide and MDEA on Triethylene Glycol Foaming
In the actual adsorption process of hydrogen sulfide, the stability of the MDEA solution can be disturbed by a number of external factors. Acids, hydrocarbons, oxygen and high temperatures all contribute to the formation of non-reusable degradation products and heat-stable salts in the MDEA solution. Some of the thermostable salts have a special chemical structure with both lipophilic and hydrophilic properties, which gives them excellent surface activity and foaming ability. Once these special salts are mixed in the MDEA solution, the possibility of foaming in the solution will rise sharply [
30,
31]. However, in the one-way regeneration experiments MDEA solution had little effect on the foaming property-performance of triethylene glycol, and Na
2S was used as a substitute for hydrogen sulfide for experimental safety.
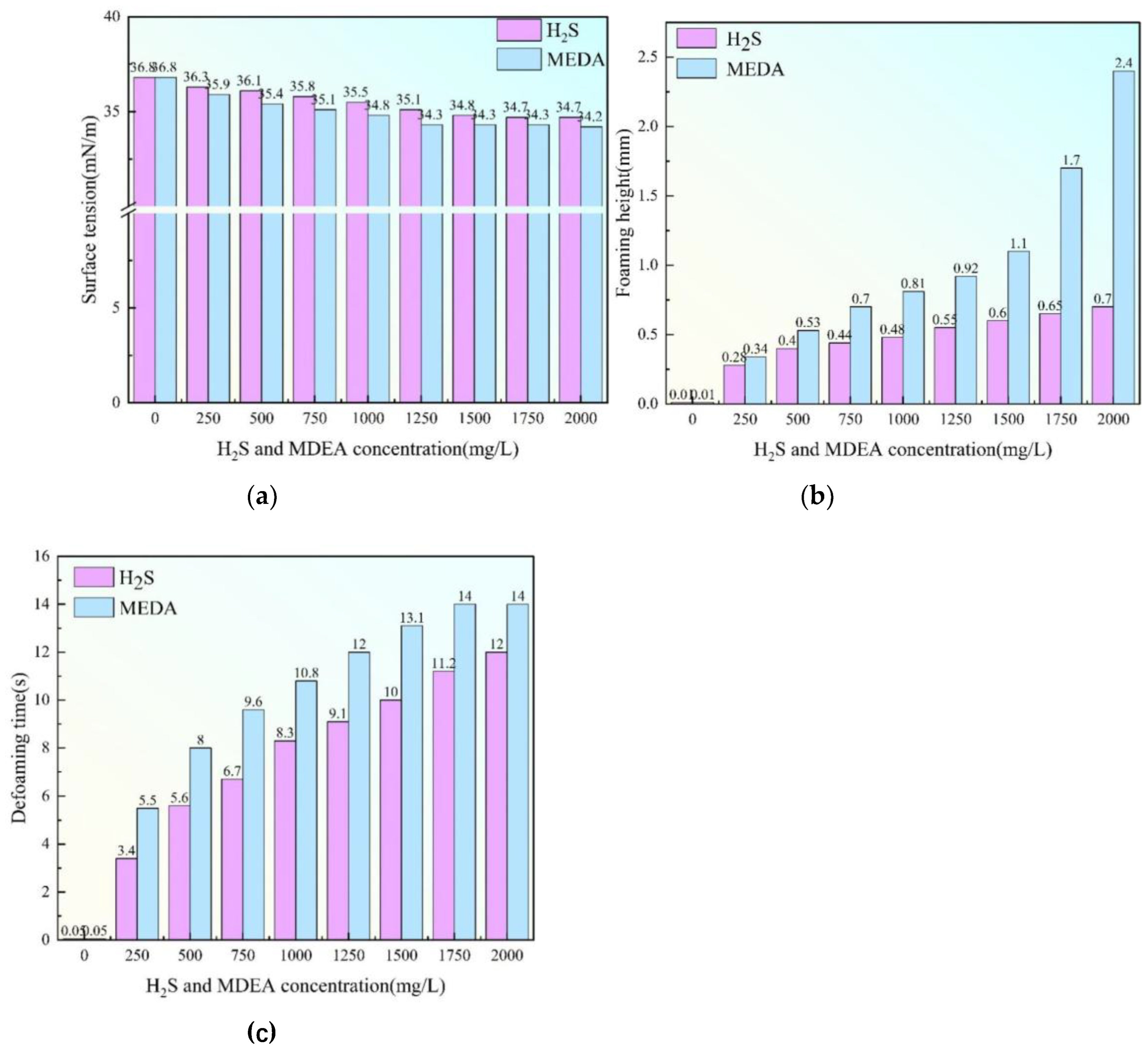
Figure 6.
(a) Relationship diagram between hydrogen sulfide, MDEA concentration and triethylene glycol foaming height(b) Relationship between hydrogen sulfide, MDEA concentration and defoaming time of triethylene glycol(c) Relationship diagram between hydrogen sulfide, MDEA concentration and surface tension of triethylene glycol.
Figure 6.
(a) Relationship diagram between hydrogen sulfide, MDEA concentration and triethylene glycol foaming height(b) Relationship between hydrogen sulfide, MDEA concentration and defoaming time of triethylene glycol(c) Relationship diagram between hydrogen sulfide, MDEA concentration and surface tension of triethylene glycol.
Obtained in the analysis of the role of hydrogen sulfide and MDEA on the foaming properties of triethylene glycol under a single factor in cyclic regeneration experiments. The surface tension of both hydrogen sulfide and MDEA decreased with the increase of chemical content, and the interfacial tension did not changing when the chemical content reached 1500 mg/L. The interfacial tension was 34.8 mN/m when the content of hydrogen sulfide reached 1500 mg/L, and the interfacial tension was 34.3 mN/m when the content of MDEA reached 1500 mg/L. The foaming height of hydrogen sulfide increased with the increase of chemical reagent content, and the increase was very slow after the concentration of 500 mg/L, and the foaming height was 0.7 mm at the concentration of 2000 mg/L. The foaming height of MDEA was 0.7 mm when the concentration of MDEA was 0.5 mm, and that of hydrogen sulfide was 0.7 mm. The foaming height of MDEA increased with the increase of chemical reagent content, and the rate of increase increased dramatically after the concentration of 1500mg/L, the concentration of 2000mg/L, the foaming height of 0.7mm to reach the maximum value of 2.4mm. The foaming time of hydrogen sulfide and MDEA increased with the increase of chemical reagent content, and the rate of increase was very fast before the concentration of 500mg/L After that, it became relatively slow. When the concentration of hydrogen sulfide was 2000mg/L, the defoaming time was 12s, and when the concentration of MDEA was 2000mg/L, the defoaming time was 14s.
The interfacial tension of triethylene glycol decreases as the concentration of hydrogen sulfide (in the form of sodium sulfide) as well as MDEA is gradually increased, although the decrease is relatively small [
32]. In contrast, the decrease in surface tension of triethylene glycol due to MDEA is slightly larger. The foaming height of triethylene glycol increases with increasing concentrations of hydrogen sulfide and MDEA, with MDEA causing a more pronounced increase in the foaming height of triethylene glycol. When the concentration of MDEA in triethylene glycol exceeded 750 mg/L, the increasing trend of foaming height was especially significant. At the same time, the defoaming time of triethylene glycol was prolonged with the rise of the concentration of hydrogen sulfide and MDEA, and MDEA would make the defoaming time of triethylene glycol become longer. In summary, it is more appropriate to control the concentration of hydrogen sulfide and MDEA in triethylene glycol below 750mg/L, so as to maintain a relatively stable and balanced performance in various aspects and reduce the adverse effects brought by too high a concentration, such as abnormal changes in interfacial tension, excessive foaming and defoaming difficulties.