Submitted:
27 February 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
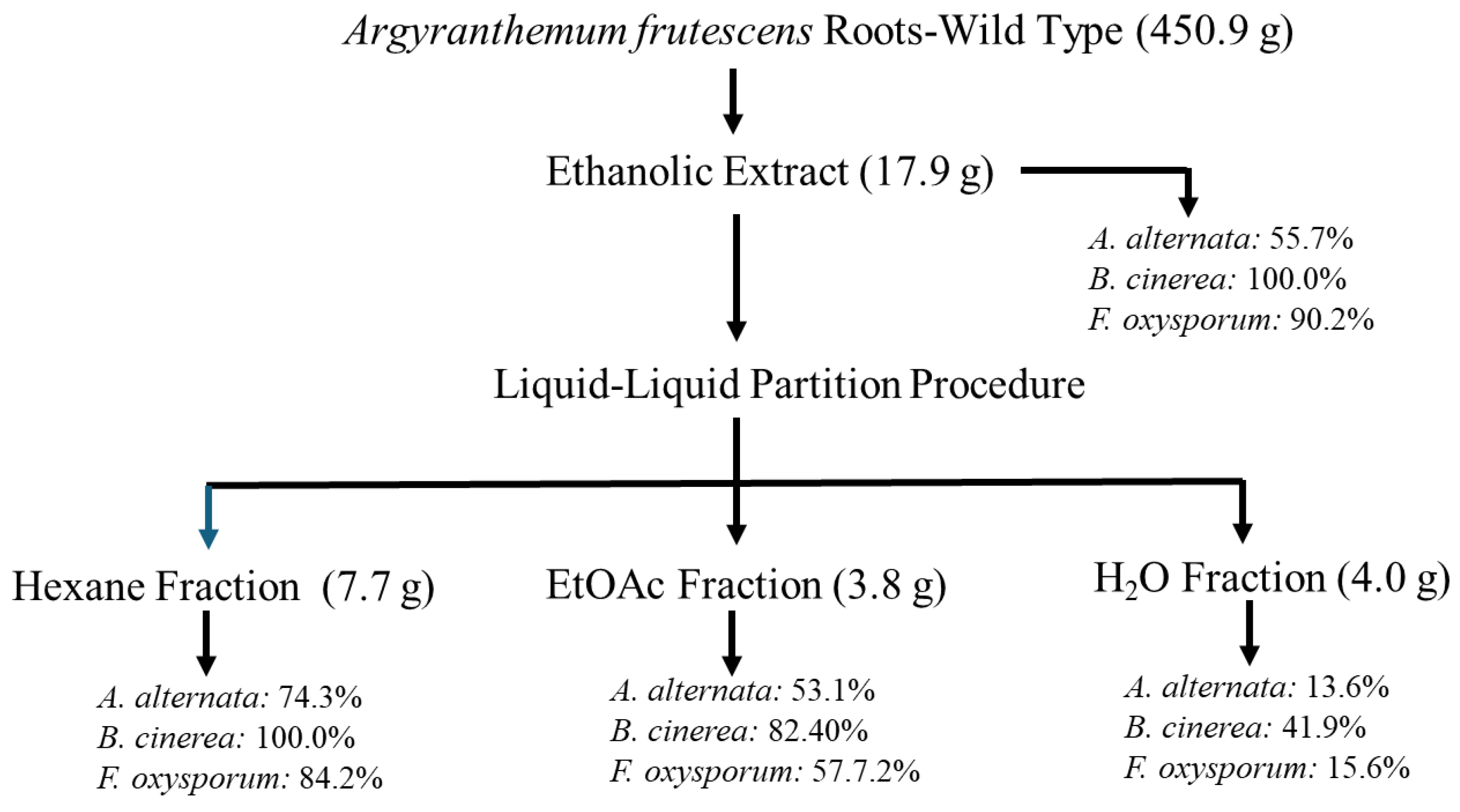
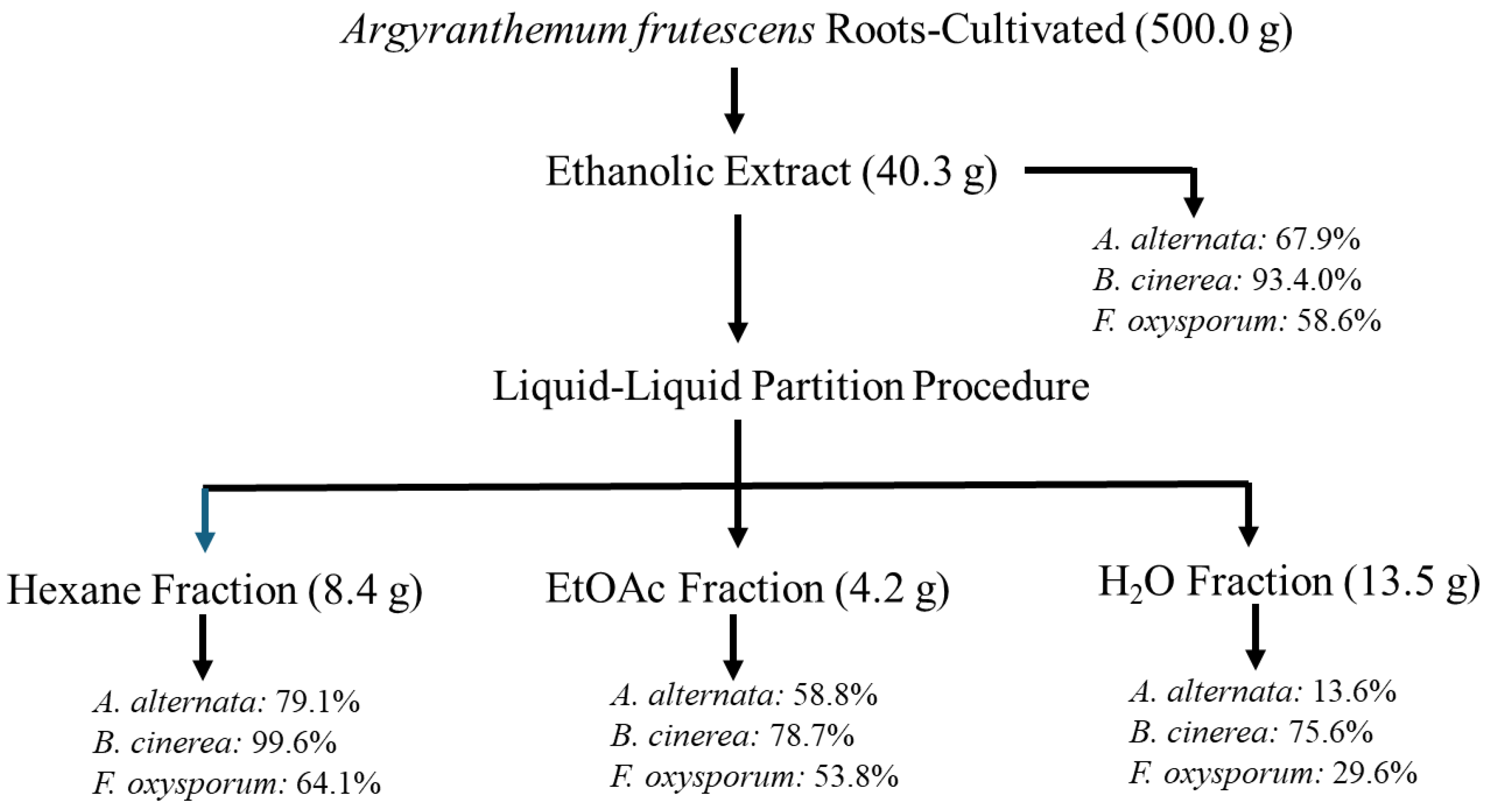
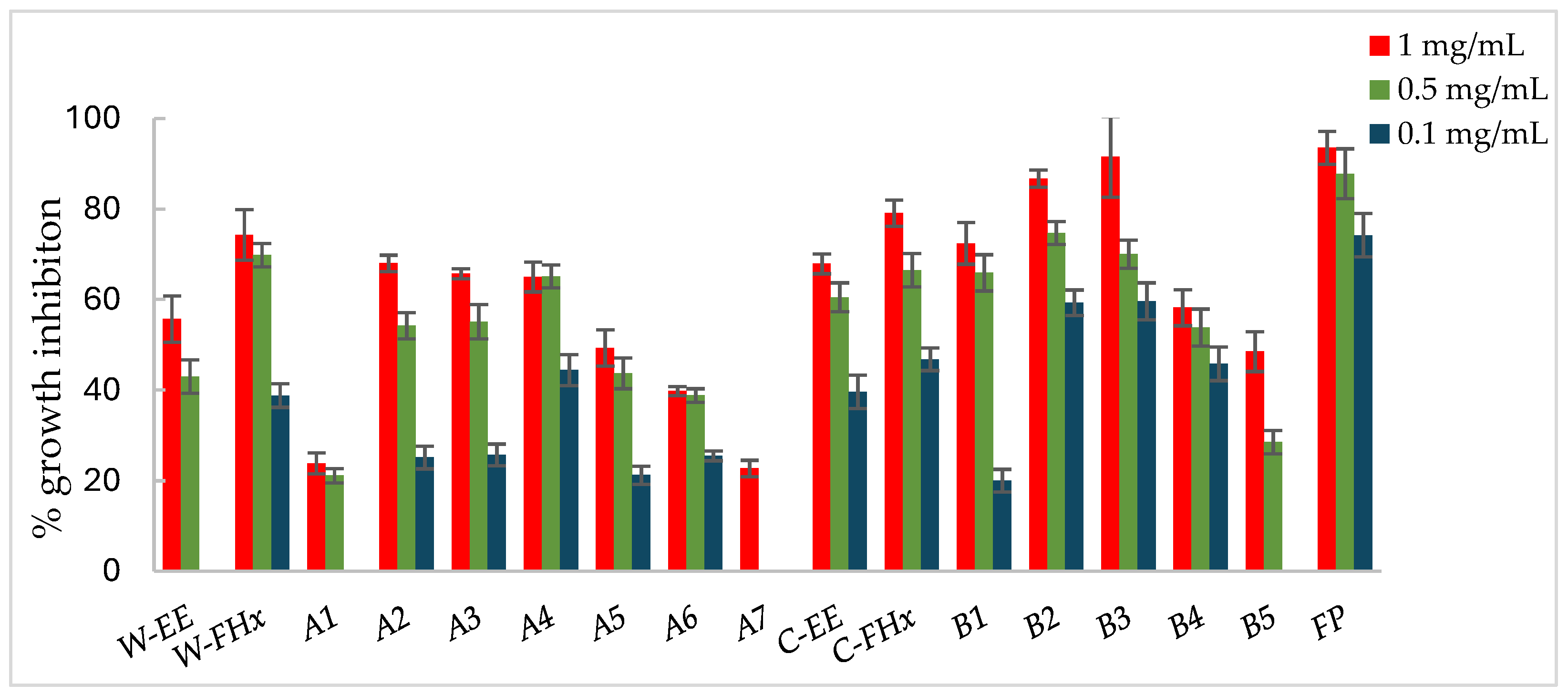
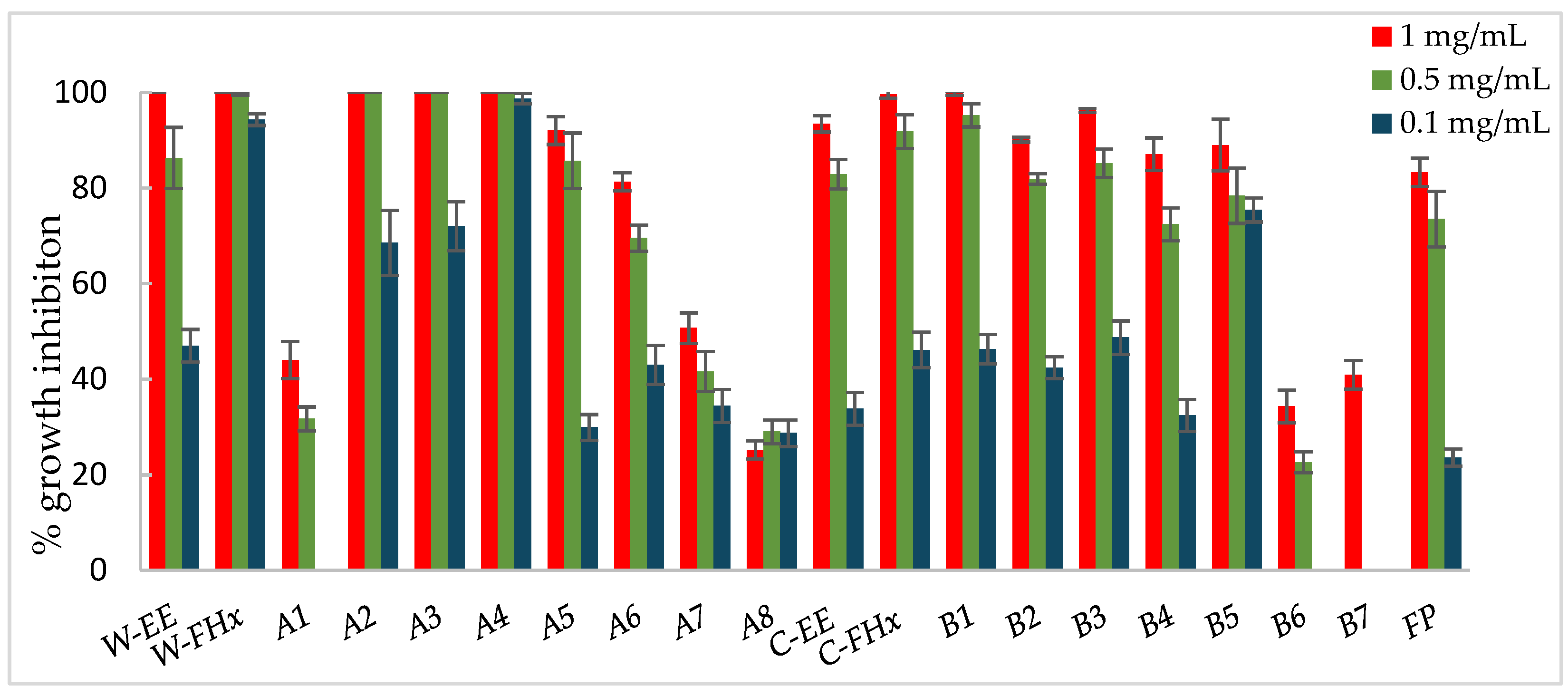
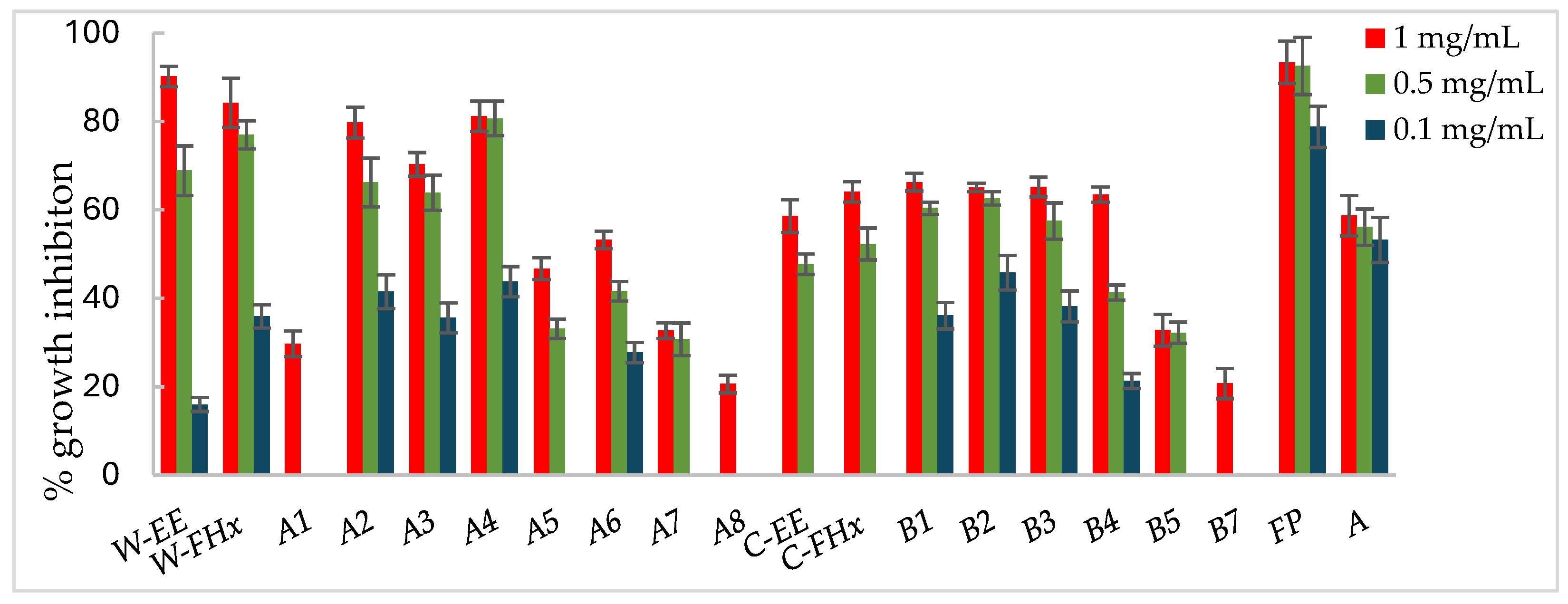
2.1. Bioassay-Guided Fractionation
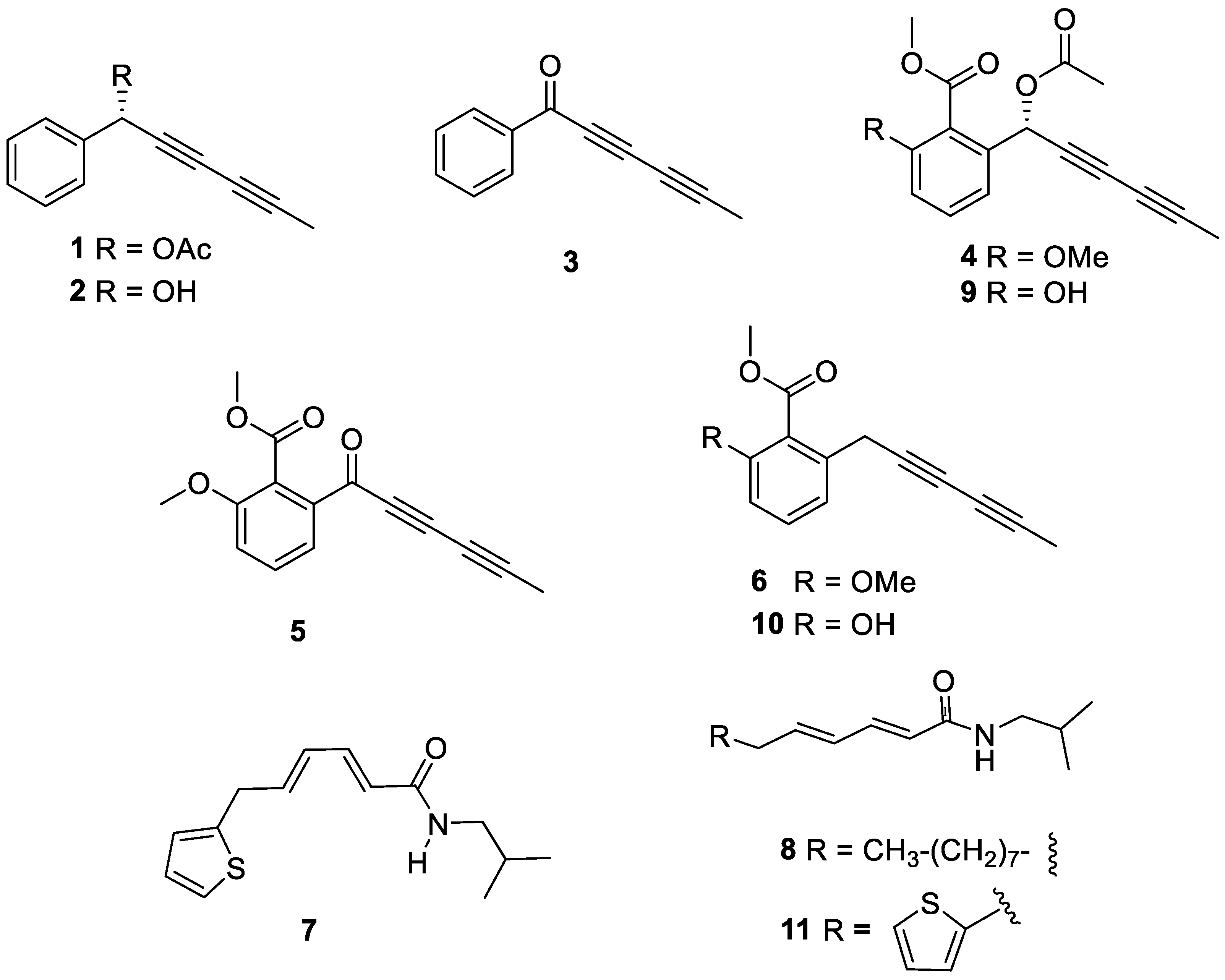
2.2. Metabolites Identification from Active Subfractions
2.3. Antifungal Activity Assays of the Isolated Compounds
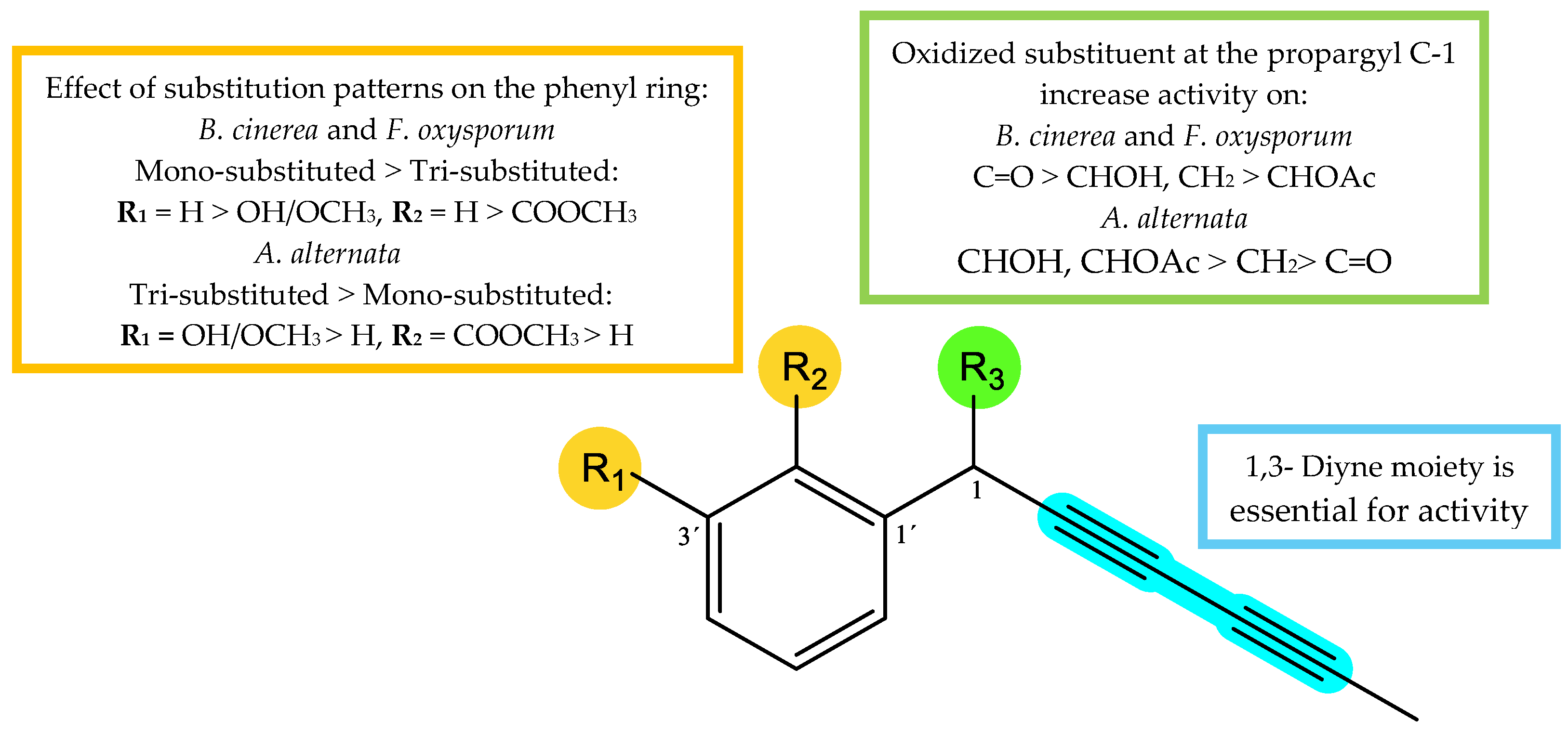
2.4. SAR Analysis
3. Materials and Methods
3.1. General
3.2. Plant Material
3.2.1. Plant Collection
3.2.2. Plant Cultivation. Seedling Production
3.3. Plant Extracts Preparation and Liquid-Liquid Partition Procedure
3.4. Bioactivity-Guided Chromatographic Fractionation and Metabolites Isolation in Wild and Cultivated A. frutescens Roots
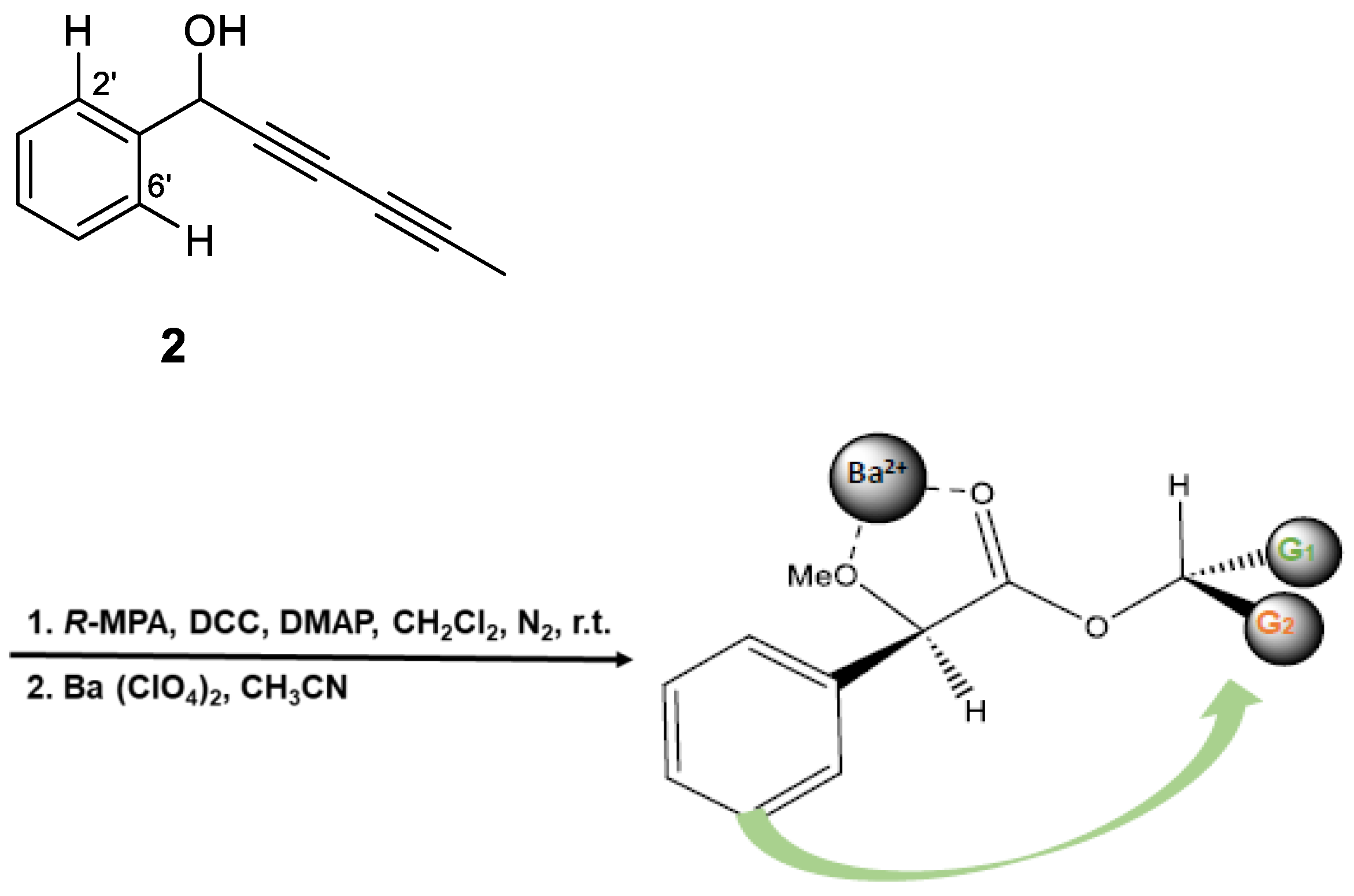
3.5. Determination of the Absolute Configuration of Capillinol 2.
3.6. Biological Assays
3.6.1. Fungal Culture
3.6.2. In Vitro Test-Assay on Mycelium
3.6.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, V.K.; Singh, R.; Kumar, A.; Bhadouria, R. Current status of plant diseases and food security. In Food Security and Plant Disease Management. Kumar, A., Droby S., Eds.; Wiley: New York, NY, USA, 2021, 19-35. https://doi.org/10.1016/B978-0-12-821843-3.00019-2. [CrossRef]
- Li, P.; Tedersoo, L.; Crowther, T.W.; Wang, B.; Shi, Y.; Kuang, L.; Li, T.; Wu, M.; Liu, M.; Luan, L.; Liu, J.; Li, D.; Li, Y.; Wang, S.; Saleem, M.; Dumbrell, A.J.; Li, Z.; Jiang, J. Global diversity andb of potential phytopathogenic fungi in a changing world. Nat. Commun. 2023, 14, 6482. https://doi.org/10.1038/s41467-023-42142-4. [CrossRef]
- John, E.; Singh, K.B.; Oliver, R.P.; Tan, K. Transcription factor control of virulence in phytopathogenic fungi. Mol. Plant Pathol. 2021, 22, 858–881. https://doi.org/10.1111/mpp.13056. [CrossRef]
- Fernandes, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria pathogenesis in animals and plants. FEMS Microbiol. Rev. 2023, 47, 1-25. https://doi.org/10.1093/femsre/fuad061. [CrossRef]
- Wang H.; Guo Y.; Luo Z.; Gao L.; Li R.; Zhang Y.; Kalaji H.M.; Qiang S.; Chen S. Recent advances in Alternaria phytotoxins: a review of their occurrence, structure, bioactivity, and biosynthesis. J. Fungi 2022, 8, 168. https://doi.org/10.3390/jof8020168. [CrossRef]
- Singh R.; Caseys C.; Kliebenstein D.J. Genetic and molecular landscapes of the generalist phytopathogen Botrytis cinerea. Mol. Plant Pathol. 2024, 25, e13404. https://doi.org/10.1111/mpp.13404. [CrossRef]
- Bi K.; Liang Y.; Mengiste T.; Sharon A. Killing Softly: A Roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211-222. https://doi.org/10.1016/j.tplants.2022.08.024. [CrossRef]
- Seepe H.A.; Nxumalo W.; Amoo S.O. Natural products from medicinal plants against phytopathogenic Fusarium species: Current research endeavours, challenges and prospects. Molecules 2021, 26, 6539. https://doi.org/10.3390/molecules26216539. [CrossRef]
- Zhou, W.; Li, M.; Achal, V.A Comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2025, 11, 100410. https://doi.org/10.1016/j.emcon.2024.100410. [CrossRef]
- Pandian, S.; Ramesh, M. Development of pesticide resistance in pests: A key challenge to the crop protection and environmental safety. In Pesticides in Crop Production: Physiological and Biochemical Action; Srivastava, P.K., Singh, V.P., Singh, A., Singh, S., Prasad, S.M., Tripathi, D.K., Chauhan, D.K., Eds.; Wiley: New York, NY, USA, 2020; pp. 1–13.
- Harte, S.J.; Bray, D.P.; Nash-Woolley, V.; Stevenson, P.C.; Fernández-Grandon, G.M. Antagonistic and additive effect when combining biopesticides against the fall armyworm, Spodoptera frugiperda. Sci. Rep. 2024, 14, 6029. https://doi.org/10.1038/s41598-024-56599-w. [CrossRef]
- Aioub, A.A.A.; Ghosh, S.; AL-Farga, A.; Khan, A.N.; Bibi, R.; Elwakeel, A.M.; Nawaz, A.; Sherif, N.T.; Elmasry, S.A.; Ammar, E.E. Back to the origins: Biopesticides as promising alternatives to conventional agrochemicals. Eur. J. Plant. Pathol. 2024, 1, 697-713. https://doi.org/10.1007/s10658-024-02865-6. [CrossRef]
- Fragkouli, R.; Antonopoulou, M.; Asimakis, E.; Spyrou, A.; Kosma, C.; Zotos, A.; Tsiamis, G.; Patakas, A.; Triantafyllidis, V. Mediterranean plants as potential source of biopesticides: An overview of current research and future trends. Metabolites 2023, 13, 967. https://doi.org/10.3390/metabo13090967. [CrossRef]
- Mateo-Martín, J.; Benítez, G.; Gras, A.; Molina, M.; Reyes-García, V.; Tardío, J.; Verde, A.; Pardo-de-Santayana, M. Cultural importance, availability and conservation status of spanish wild medicinal plants: Implications for sustainability. People Nat. 2023, 5, 1512-1525. https://doi.org/10.1002/pan3.10511. [CrossRef]
- Dempewolf, H.; Rieseberg, L.H.; Cronk, Q.C. Crop domestication in the Compositae: A family-wide trait assessment. Genet. Resour. Crop Evol. 2008, 55, 1141-1157. https://doi.org/10.1007/s10722-008-9315-0. [CrossRef]
- Mandel, J.R.; Barker, M.S.; Bayer, R.J.; Dikow, R.B.; Gao, T.G.; Jones, K.E.; Keeley, S.; Kilian, N.; Ma, H.; Siniscalchi, C.M.; Susanna, A.; Thapa, R.; Watson, L.; Funk, V.A. The Compositae tree of life in the age of phylogenomics. J. Syst. Evol. 2017, 55, 405–410. https://doi.org/10.1111/jse.12265. [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. https://doi.org/10.3390/ijms22063009. [CrossRef]
- Matsuda K. Understanding pyrethrin biosynthesis: toward and beyond natural pesticide overproduction. Biochem. Soc. Trans. 2024, 52, 1927-1937. https://doi.org/10.1042/BST20240213. [CrossRef]
- Jaison, J.P.; Balasubramanian, B.; Gangwar, J.; James, N.; Pappuswamy, M.; Anand, A.V.; Al-Dhabi, N.A.; Arasu, M.V.; Liu, W.-C.; Sebastian, J.K. Green synthesis of bioinspired nanoparticles mediated from plant extracts of Asteraceae family for potential biological applications. Antibiotics 2023, 12, 543. https://doi.org/10.3390/antibiotics12030543. [CrossRef]
- Petrova, M.; Miladinova-Georgieva, K.; Geneva, M. Influence of abiotic and biotic elicitors on organogenesis, biomass accumulation, and production of key secondary metabolites in Asteraceae Plants. Int. J. Mol. Sci. 2024, 25, 4197. https://doi.org/10.3390/ijms25084197. [CrossRef]
- Bramwell D. 2011. Introduction: islands and plants. In: Bramwell D, Caujapé-Castells J, eds. The biology of island floras. Cambridge, UK: Cambridge University Press, pp. 297–304.
- White, O.W.; Reyes-Betancort, J.A.; Chapman, M.A.; Carine, M.A. Geographical isolation, habitat shifts and hybridisation in the diversification of the Macaronesian endemic genus Argyranthemum (Asteraceae). New Phytol. 2020, 228, 1953-1971. https://doi.org/10.1111/nph.16980. [CrossRef]
- Cruz Suárez, S.J. Más de 100 Plantas Medicinales, 1st ed.; Obra Social de la Caja de Canaria: Las Palmas de Gran Canarias, Spain, 2007.
- Sabotič, J.; Bayram, E.; Ezra, D.; Gaudêncio, S.P.; Haznedaroğlu, B.Z.; Janež, N.; Ktari, L.; Luganini, A.; Mandalakis, M.; Safarik, I.; Simes, D.; Strode, E.; Toruńska-Sitarz, A.; Varamogianni-Mamatsi, D.; Varese, G.C.; Vasquez, M.I. A guide to the use of bioassays in exploration of natural resources. Biotechnol. Adv. 2024, 71, 108307. https://doi.org/10.1016/j.biotechadv.2024.108307. [CrossRef]
- González A.G.; Barrera J.B.; Díaz J.G.; García T.Z.; de Paz P.P. Distribution of acetylenes and sesquiterpene lactones in Argyranthemum from Tenerife. Biochem. Syst. Ecol. 1988, 16, 17-21. https://doi.org/10.1016/0305-1978(88)90111-1. [CrossRef]
- González, A.G.; Estévez-Reyes, R.; Estévez-Braun, A.; Ravelo, A.G.; Jiménez, I.A.; Bazzocchi, I.L.; Aguilar, M.A.; Moujir, L. Biological activities of some Argyranthemum species. Phytochemistry 1997, 45, 963-967. https://doi.org/10.1016/S0031-9422(97)00063-0. [CrossRef]
- Cosoveanu, A.; Hernández, M.; Iacomi-Vasilescu, B.; Zhang, X.; Shu, S.; Wang, M.; Cabrera, R. Fungi as endophytes in chinese Artemisia spp.: juxtaposed elements of phylogeny, diversity and bioactivity. Mycosphere 2016, 7, 102-117. https://doi.org/10.5943/mycosphere/7/2/2. [CrossRef]
- Zhang, Z.; Guo, S.; Zhang, W.; Geng, Z.; Liang, J.; Du, S.; Wang, C.; Deng, Z. Essential oil and polyacetylenes from Artemisia ordosica and their bioactivities against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Ind. Crops Prod. 2017, 100, 132-137. https://doi.org/10.1016/j.indcrop.2017.02.020. [CrossRef]
- Esmaeili, G.; Fatemi, H.; Avval, M.B.; Azizi, M.; Arouiee, H.; Vaezi, J.; Fujii, Y. Diversity of chemical composition and morphological traits of eight iranian wild Salvia species during the first step of domestication. Agronomy 2022, 12, 2455. https://doi.org/10.3390/agronomy12102455. [CrossRef]
- Ee, G.C.L.; Lim, S.K.; Dzulkefly, K. Alkaloids and carboxylic acids from Piper nigrum. Asian J. Chem. 2008, 20, 5931-5940.
- Bohlmann, F.; Tsankova, E.; Jakupovic, J. Sesquiterpenes and acetylenes from Argyranthemum adauctum ssp. jacobaeifolium. Phytochemistry 1984, 23, 1103-1104. https://doi.org/10.1016/S0031-9422(00)82618-7. [CrossRef]
- Shahat, A.A.; Apers, S.; Pieters, L.; Vlietinck, A. J. Isolation and Complete NMR assignment of the numbing principle from Chrysanthemum morifolium. Fitoterapia 2001, 72, 89-91. https://doi.org/10.1016/S0367-326X(00)00247-1. [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. Assignment of the absolute configuration of polyfunctional compounds by NMR using chiral derivatizing agents. Chem. Rev. 2012, 112, 4603-4641. https://doi.org/10.1021/cr2003344. [CrossRef]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014-2021). Phytochemistry 2022, 201, 113288. https://doi.org/10.1016/j.phytochem.2022.113288. [CrossRef]
- D.A. Konovalov. Medicinal plants polyacetylene compounds of plants of the Asteraceae family (review). Pharm. Chem. J. 2014, 48, 613-631. https://doi.org/10.1007/s11094-014-1159-7. [CrossRef]
- Mullins, A.J.; Webster, G.; Kim, H.J.; Zhao, J.; Petrova, Y.D.; Ramming, C.E.; Jenner, M.; Murray, J.A.H.; Connor, T.R.; Hertweck, C.; Challis, G.L.; Mahenthiralingam, E. Discovery of the Pseudomonas polyyne protegencin by a phylogeny-guided study of polyyne biosynthetic gene cluster diversity. mBio 2021, 12, e00715-21. https://doi.org/10.1128/mBio.00715-21. [CrossRef]
- Chen, H.P.; Zheng, L.S.; Yang, K.; Lei, N.; Geng, Z.F.; Cai, Q.; Du, S.S.; Deng, Z.W. Insecticidal and repellant activities of polyacetylenes and lactones derived from Atractylodes lancea rhizomes. Chem. Biodivers. 2015, 12, 593-598. https://doi.org/10.1002/cbdv.201400161. [CrossRef]
- Masuda, Y.; Asada, K.; Satoh, R.; Takada, K.; Kitajima, J. Capillin, a major constituent of Artemisia capillaris Thunb. flower essential oil, induces apoptosis through the mitochondrial pathway in human leukemia HL-60 cells. Phytomedicine 2015, 22, 545-552. https://doi.org/10.1016/j.phymed.2015.03.008. [CrossRef]
- Dembitsky V.M.; Levitsky D.O. Acetylenic terrestrial anticancer agents. Nat. Prod. Commun. 2006, 1, 405-429. https://doi.org/10.1177/1934578 × 0600100512. [CrossRef]
- Islam, M.N.; Choi, R.J.; Jung, H.A.; Oh, S.H.; Choi, J.S. Promising anti-diabetic potential of capillin and capillinol isolated from Artemisia capillaris. Arch Pharm. Res. 2016, 39, 340–349. https://doi.org/10.1007/s12272-016-0715-y. [CrossRef]
- One-Way ANOVA Calculator, Including Tukey HSD. Available online: https://www.socscistatistics.com/tests/chisquare2/default2.aspx (accessed on 3 May 2024).
| Sample | A. alternata | B. cinerea | F. oxysporum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.05 | 0.01 | 0.1 | 0.0.05 | 0.01 | 0.1 | 0.05 | 0.01 | |
| 1 | 34.6±3.3 | 31.7±2.2 | 22.6±2.5 | 76.3±5.4 | 58.4±6.54 | 23.6±7.6 | 36.2±4.2 | 34.0±3.2 | 14.4±3.0 |
| 2 | 66.3±2.3 | 35.3±3.4 | 24.7±4.6 | 95.8±3.2 | 60.0±5.4 | NA | 83.6±3.8 | 44.5±4.9 | 18.2±3.8 |
| 3 | 25.5±3.8 | NA | ND | 100.0±0.0 | 92.7±7.1 | 39.9±6.8 | 94.9±1.4 | 84.4±11.7 | NA |
| 4 | 56.6±4.1 | 55.4±2.4 | 37.9±3.2 | 61.8±8.9 | 60.9±7.4 | 21.1±5.9 | 23.9±5.2 | 17.4±2.5 | NA |
| 5 | 48.1±4.2 | 40.4±4.9 | 13.3±5.2 | 90.8±4.1 | 90.8±2.3 | 70.6±4.5 | 45.6±6.3 | 31.8±4.7 | NA |
| 6 | 42.6±6.8 | 28.2±4.2 | NA | 85.1±3.2 | 73.2±2.6 | 32.2±3.1 | 23.9±2.4 | NA | ND |
| 7 | 39.3±2.3 | 38.4±1.9 | 13.8±2.9 | 39.7±4.6 | 37.4±5.6 | 20.2±5.8 | 19.5±1.5 | ND | ND |
| 8 | NA | ND | ND | NA | ND | ND | 18.9±2.5 | ND | ND |
| 9 | 71.3±2.7 | 45.3±2.9 | 33.4±6.6 | 90.2±5.5 | 80.6±2.2 | 57.4±3.0 | 41.7±1.9 | 12.7±5.9 | ND |
| 10 | 29.3±2.1 | 10.7±3.3 | NA | 70.1±6.6 | 70.0±6.1 | 23.7±4.1 | 20.6±2.0 | 18.7±8.1 | ND |
| 11 | 40.8±2.9 | NA | ND | 42.5±2.5 | 38.5±4.5 | 17.2±5.2 | 43.4±0.5 | 13.9±4.7 | ND |
| FP | 74.2 ± 4.8 | 65.5 ± 4.0 | 38.1 ± 3.8 | 23.6 ± 4.8 | 21.9 ± 3.8 | 13.5 ± 3.6 | 78.8 ± 4.7 | 44.4 ± 4.8 | 28.7 ± 2.5 |
| A | 30.4 ± 2.1 | 31.2 ± 4.0 | 33.4 ± 1.9 | 67.8 ± 4.1 | 50.0 ± 7.3 | 32.6 ± 6.1 | 53.2 ± 5.1 | 51.6 ± 5.1 | 32.2 ± 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





