Submitted:
25 February 2025
Posted:
26 February 2025
You are already at the latest version
Abstract
An effective deposition of cinchonine layer on platinum metal surface can be easily achieved by the cathodic reduction of cinchonine hydrochloride methanolic solution at a controlled potential of -220 mV vs. the silver standard electrode (SSE). A coated screen-printed platinum electrode has proven suitable for cinchonine determination in water, urine and serum at ug L-1 level concentrations by differential pulse voltammetry in phosphate buffer solution (pH=7.0). The limits of detection (LOD) and the limits of quantitation (LOQ) are 0.6 ug L-1 and 1.8 ug L-1 respectively.
Keywords:
1. Introduction
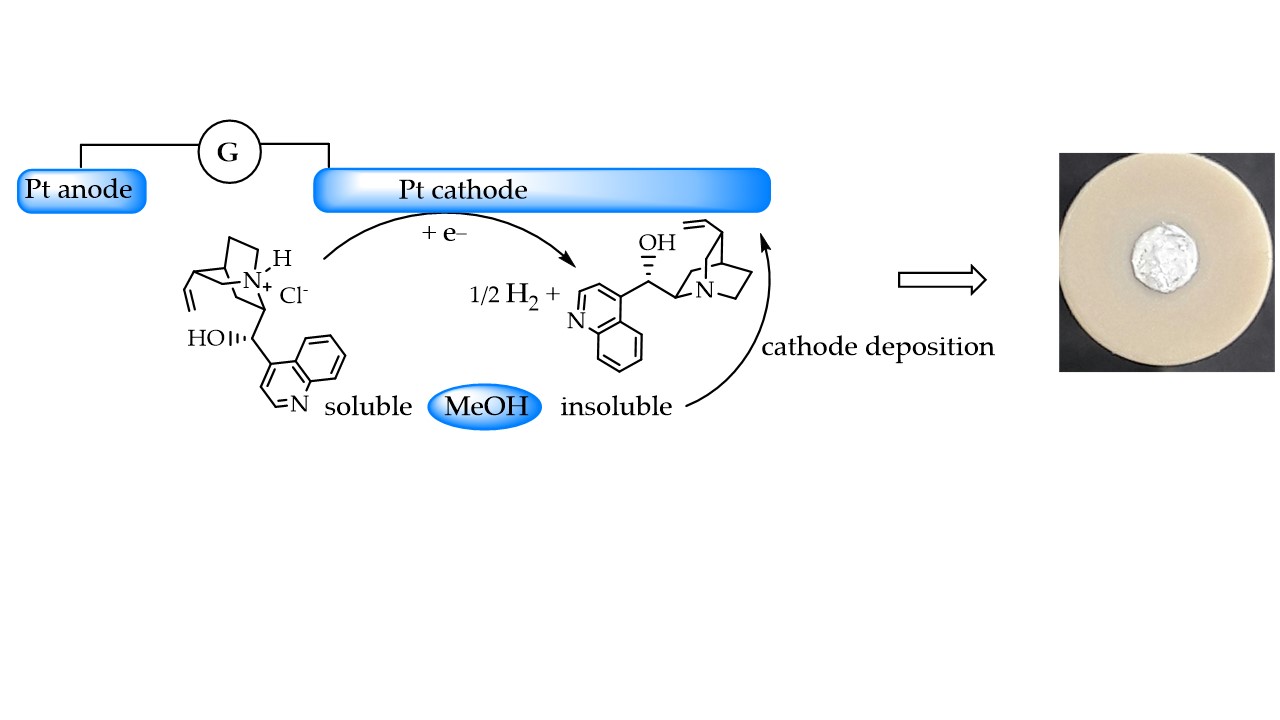
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation and Apparatus
2.3. Procedures
2.3.1. Solutions
2.3.2. Electrode Preparation
2.3.3. Differential Pulse Voltammetry Measurements
2.3.4. Data Treatment
3. Results and Discussion
3.1. Preliminary Studies
3.2. Electrode Testing
3.3. Selectivity
3.3. Application of Sensor: Determination of Cinchonine in the Urine and Serum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SPE | Screen–printed electrode |
| SP-Pt/CN | Screen–printed platinum electrode coated with cinchonine |
| DPV | Differential pulse voltammetry |
| RSD | Relative standard deviation |
References
- Beitollahi, H.; Mohammadi, S.Z.; Safaei, M.; Tajik, S. Applications of electrochemical sensors and biosensors based on modified screen-printed electrodes: a review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, G.; Xu, C.; Wu, J.; Zhanga, X.; Liu, J. Applications of electrochemical biosensors based on functional antibody-modified screen-printed electrodes: a review. Anal. Methods 2022, 14, 7–16. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical Overview: Screen-Printed Electrochemical Sensing Platforms. ChemElectroChem 2024, 11, e202400370–1. [Google Scholar] [CrossRef]
- Kelíšková, P.; Matvieiev, O.; Janíková, L.; Šelešovská, R. Recent advances in the use of screen-printed electrodes in drug analysis: A review. Curr. Opin. Electrochem. 2023, 42, 101408–1. [Google Scholar] [CrossRef]
- Bard, A.J. Chemical modification of electrodes. J. Chem. Educ. 1983, 60, 4–302. [Google Scholar] [CrossRef]
- Edwards, G.A.; Bergren, A.J.; Porter, M.D. Chemically Modified Electrodes. Handb. Electrochem. 2007, 295–327. [Google Scholar] [CrossRef]
- Murray, R.W.; Ewing, A.G.; Durst, R.A. Chemically modified electrodes. Molecular design for electroanalysis. Anal. Chem. 1987, 59, 5–379A. [Google Scholar] [CrossRef]
- Durst, R.A.; Baumner, A.J.; Murray, R.W.; Buck, R.P.; Andrieux, C.P. Chemically Modified Electrodes: Recommended Terminology and Definitions, IUPAC. Pure Appl. Chem. 1997, 69, 6–1317. [Google Scholar] [CrossRef]
- White, H.S.; Leddy, J.; Bard, A.J. Polymer films on electrodes. 8. Investigation of charge-transport mechanisms in Nafion polymer modified electrodes. J. Am. Chem. Soc. 1982, 104, 18–4811. [Google Scholar] [CrossRef]
- Zen, J.-M.; Kumar, A.S.; Tsai, D.-M. Recent Updates of Chemically Modified Electrodes in Analytical Chemistry. Electroanalysis 2003, 15, 13–1073. [Google Scholar] [CrossRef]
- Perez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Arino, C.; Esteban, M. Mercury Films on Commercial Carbon Screen-Printed Devices for the Analysis of Heavy Metal Ions: a Critical Evaluation. Electroanalysis 2015, 27, 1345–1349. [Google Scholar] [CrossRef]
- Josypcuk, B.; Tvorynska, S. Screen-printed electrodes covered by mercury film or meniscus. Electrochim. Acta 2025, 513, 145565–1. [Google Scholar] [CrossRef]
- Siria, J.W.; Baldwin, R.P. Adsorption Pre-Concentration and Analysis of Dopamine at Platinum Electrode Surfaces. Anal. Lett. 1980, 13, 7–577. [Google Scholar] [CrossRef]
- Trasatti, S. Adsorption of organic substances at electrodes: recent advances. Electrochim. Acta 1992, 37, 12–2137. [Google Scholar] [CrossRef]
- Mittal, M.; Sardar, S.; Jana, A.E. Nanofabrication techniques for semiconductor chemical sensors. Handbook of Nanomaterials for Sensing Applications. Micro and Nano Technologies 2021, 119–137. [Google Scholar] [CrossRef]
- Ayala, M.C.; López, L.L.; Jaramillo-Botero, A.; Valencia, D. Electrochemical modified electrode with bismuth film for ultrasensitive determination of aluminum (III). J. Electroanal. Chem. 2022, 919, 116552–1. [Google Scholar] [CrossRef]
- Lai, J.; Ma, Z.; Mink, L.; Mueller, L.J.; Zaera, F. Influence of peripheral groups on the physical and chemical behavior of cinchona alkaloids. J. Phys. Chem. B. 2009, 113, 34–11696. [Google Scholar] [CrossRef]
- Tracy, J.W.; Webster, J.L. Drugs Used in the Chemotherapy of Protozoal Infections, 9th ed.; Hardman, J.G., Limbird, L.E., Molino, P.B., Ruddon, R.W., Gilman, A.G., Eds.; The Pharmacological Basis of Therapeutics; The McGraw Hill: New York, NY, USA, 1996; pp. 800–808. [Google Scholar]
- Sullivan, D.J. Cinchona Alkaloids: Quinine and Quinidine. In Treatment and Prevention of Malaria; Springer: Basel, Switzerland; Baltimore, MD, USA; Vol. 41, pp. 45–68. [CrossRef]
- Parveen, S.; Maurya, N.; Meena, A.; Luqman, S. Cinchonine: A Versatile Pharmacological Agent Derived from Natural Cinchona Alkaloids. Curr Top Med Chem 2024, 24(4), 343–363. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar. J. 2011, 15 (Suppl 1), 10. [Google Scholar] [CrossRef]
- Levy, S.; Azoulay, S. Stories About the Origin of Quinquina and Quinidine. Journal of cardiovascular Electrophysiol. 1994, 5, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Genne, P.; Duchamp, O.; Solary, E.; Pinard, D.; Belon, J.P.; Dimanche-Boitrel, M.T.; Chauffert, B. Comparative effects of quinine and cinchonine in reversing multidrug resistance on human leukemic cell line K562/ADM. Leukemia 1994, 8, 160–164. [Google Scholar] [PubMed]
- Shah, B.H.; Nawaz, Z.; Virani, S.S.; Ali, I.Q.; Saeed, S.A.; Gilani, A.H. The inhibitory effect of cinchonine on human platelet aggregation due to blockade of calcium influx. Biochem. Pharmacol. 1998, 56, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, X.; Wu, J.; Li, Y.; Li, C.; Qiao, X.; Fan, L.; Chen, Y.; Zhu, H.; Zhang, Z.; He, Y. Cinchonine and cinchonidine alleviate cisplatin-induced ototoxicity by regulating PI3K-AKT signaling. CNS Neurosci Ther. 2024, 30, 2–e14403. [Google Scholar] [CrossRef]
- Genne, P.; Ducham, O.; Solary, E.; Magnette, J.; Belon, J.P.; Chauffert, B. Cinchonine per os: Efficient circumvention of P-glycoprotein-mediated multidrug resistance. Anticancer Drug Des. 1995, 10, 103–118. [Google Scholar] [PubMed]
- Johnson, C.C.; Poe, C.F. Toxicity of Some Cinchona Alkaloids. Acta pharmacol. 1948, 4, 3–4. [Google Scholar] [CrossRef]
- Thapliyal, N.; Chiwunze, T.; Karpoormath, R.; Goyal, R.N.; Patel, H.; Cherukupalli, S. Research Progress in Electroanalytical Techniques for Determination of Antimalarial Drugs in Pharmaceutical and Biological Samples. RSC Adv. 2016, 6, 57580–57602. [Google Scholar] [CrossRef]
- Yin, F.; Xu, X. Construction and Analytical Application of a Novel Ion-Selective Capacitive Sensor for Determination of Cinchonine. Anal. Lett. 2004, 37, 15–3129. [Google Scholar] [CrossRef]
- Michele, M.; Pierre, D.; Pierre, L. Determination of cinchonine in mixtures by zero-crossing first-derivative spectrofluorimetry. Analyst 1988, 113, 929–932. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, G.; Zhang, Z. A sensitive chemiluminscence method for cinchona alkaloids. Anal. Lett. 1998, 14, 2537–2548. [Google Scholar] [CrossRef]
- Mroczek, T.; Glowniak, K.J. TLC and HPTLC assay of quinoline and quinuclidine alkaloids in cinchonae cortex and pharmaceutical preparations. J. Planar Chromatogr. 2000, 13, 6–457. [Google Scholar]
- Hu, W.; Wu, N.; Li, D.; Yang, Y.; Qie, S.; Su, S.; Xu, R.; Li, W.; Hu, M. The fluorescence distinction of chiral enantiomers: a Zn coordination polymer sensor for the detection of cinchonine and cinchonidine. J. Mater. Chem. C 2025, 13, 592–599. [Google Scholar] [CrossRef]
- Dushna, O.; Dubenska, L.; Marton, M.; Hatala, M.; Vojs, M. Sensitive and selective voltammetric method for determination of quinoline alkaloid, quinine in soft drinks and urine by applying a boron-doped diamond electrode. Microchem. J. 2023, 191, 108839–1. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, H.-F.; Liu, Z.-M.; Liu, Y.-L.; Shen, G.-L.; Yu, R.-Q. Electrochemical sensor for cinchonine based on a competitive host–guest complexation. Anal. Chim. Acta 2005, 528, 135–142. [Google Scholar] [CrossRef]
- Prideaux, E.B.R.; Winfield, F.T. The determination of quinine, cinchonine and cinchonidine with the quinhydrone electrode, and the choice of end-points in alkaloidal titrations. Analyst 1930, 55, 561–565. [Google Scholar] [CrossRef]
- Yuan, J.-B.; Tan, Y.-G.; Nie, L.-H.; Yao, S.-Z. Piezoelectric quartz crystal sensors based on ion-pair complexes for the determination of cinchonine in human serum and urine. Anal. Chim. Acta 2002, 454, 65–74. [Google Scholar] [CrossRef]
- Blaser, H.U.; Jalett, H.P.; Monti, D.M.; Reber, J.F.; Wehrli, J.T. Modified Heterogeneous Platinum Catalysts for the Enantioselective Hydrogenation of α-Ketoesters. Stud. Surf. Sci. Catal. 1998, 41, 153–163. [Google Scholar] [CrossRef]
- Ma, Z.; Zaera, F. Competitive Chemisorption between Pairs of Cinchona Alkaloids and Related Compounds from Solution onto Platinum Surfaces. J. Am. Chem. Soc. 2006, 128, 16414–16415. [Google Scholar] [CrossRef]
- Fietkau, N.; Bussar, R.; Baltruschat, H. The stability of adsorbed quinoline and cinchonine on poly- and monocrystalline platinum surfaces. Electrochim. Acta 2006, 51, 5626–5635. [Google Scholar] [CrossRef]
- Bakos, I.; Szabo, S.; Bartok, M.; Kalman, E. Adsorption of cinchonidine on platinum: an electrochemical study. J. Electroanal. Chem. 2002, 532, 1–2. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, I.; Zaera, F. Factors Controlling Adsorption Equilibria from Solution onto Solid Surfaces: The Uptake of Cinchona Alkaloids on Platinum Surfaces. J. Am. Chem. Soc. 2007, 129, 51–16083. [Google Scholar] [CrossRef]
- Hariyanti, H.; Kurniati, N.F.; Sumirtapura, Y.C.; Mauludin, R. Development and validation of an analytical method for the determination of nanostructured lipid carrier’s cinchonine used direct method modified by liquid-liquid extraction using high-performance liquid chromatography. J. Res Pharm. 2023, 27, 2–913. [Google Scholar] [CrossRef]
- Brankovic, S.R. Electrochemical Deposition as Surface Controlled Phenomenon: Fundamentals and Applications. J. Electrochem. Soc. 2016, Y21–Y21. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection: a closer look at the IUPAC detection. Anal. Chem. 1983, 55, 7–712A. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 5–835. [Google Scholar] [CrossRef]
- Exner, C.; Pfaltz, A.; Studer, M.; Blaser, H.-U. Heterogeneous Enantioselective Hydrogenation of Activated Ketones Catalyzed by Modified Pt-Catalysts: A Systematic Structure-Selectivity Study. Adv. Synth. Catal. 2003, 345, 1253–1260. [Google Scholar] [CrossRef]
- Halli, P.; Heikkinen, J.J.; Elomaa, H.; Wilson, B.J.; Jokinen, V.; Yliniemi, K.; Franssila, S.; Lundström, M. Platinum Recovery from Industrial Process Solutions by Electrodeposition–Redox Replacement. ACS Sustainable Chem. Eng. 2018, 6, 14631–14640. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Hall, P.J. Controlling hydrogen evolution on electrodes. Int. J. Hydrog. Energy 2016, 41, 45–20807. [Google Scholar] [CrossRef]
- Ma, F.; Lennox, R.B. Potential-Assisted Deposition of Alkanethiols on Au: Controlled Preparation of Single- and Mixed-Component SAMs. Langmuir 2000, 15, 6188–6190. [Google Scholar] [CrossRef]
- Bockris, J. O’M.; Koch, D.F.A. Comparative rates of the electrolytic evolution of hydrogen on iron, tungsten, and platinum. J. Phys. Chem. 1961, 65, 11–1941. [Google Scholar] [CrossRef]
- Chunga, C.-K.; Chang, W.-T. Handbook of Manufacturing Engineering and Technology; Springer: London, UK, 2013. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. In Electrochemical Methods: Fundamentals and Applications, 2nd ed.Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Kissinger, P.T.; Heineman, W.R. In Laboratory Techniques in Electroanalytical Chemistry, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; ISBN 0-8247-9445-1. [Google Scholar]
- Yin, F.; Xu, X. Construction and Analytical Application of a Novel Ion-Selective Capacitive Sensor for Determination of Cinchonine. Anal. Lett. 2004, 37, 15–3129. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods - A Sampling of Current Approaches; Ed. Mark, T., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Ma, Z.; Zaera, F. Role of the Solvent in the Adsorption-Desorption Equilibrium of Cinchona Alkaloids between Solution and a Platinum Surface: Correlations among Solvent Polarity, Cinchona Solubility, and Catalytic Performance. J. Phys. Chem. B 2005, 109, 1–406. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B. B.; Baer, J.E.; Craig, L.C. Metabolic products of the cinchona alkaloids in human urine. J. Biol. Chem. 1951, 188, 2–567. [Google Scholar] [CrossRef]
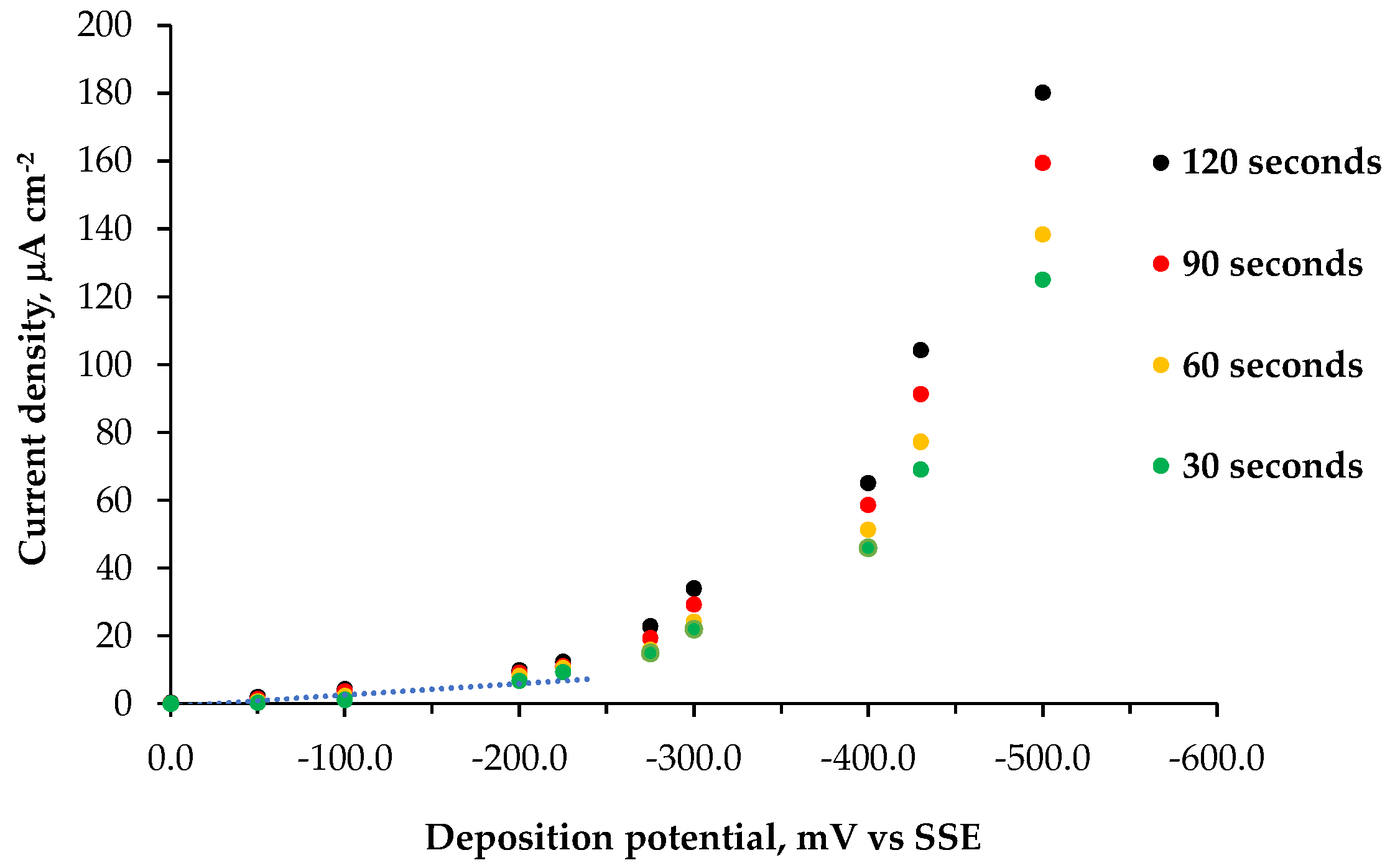
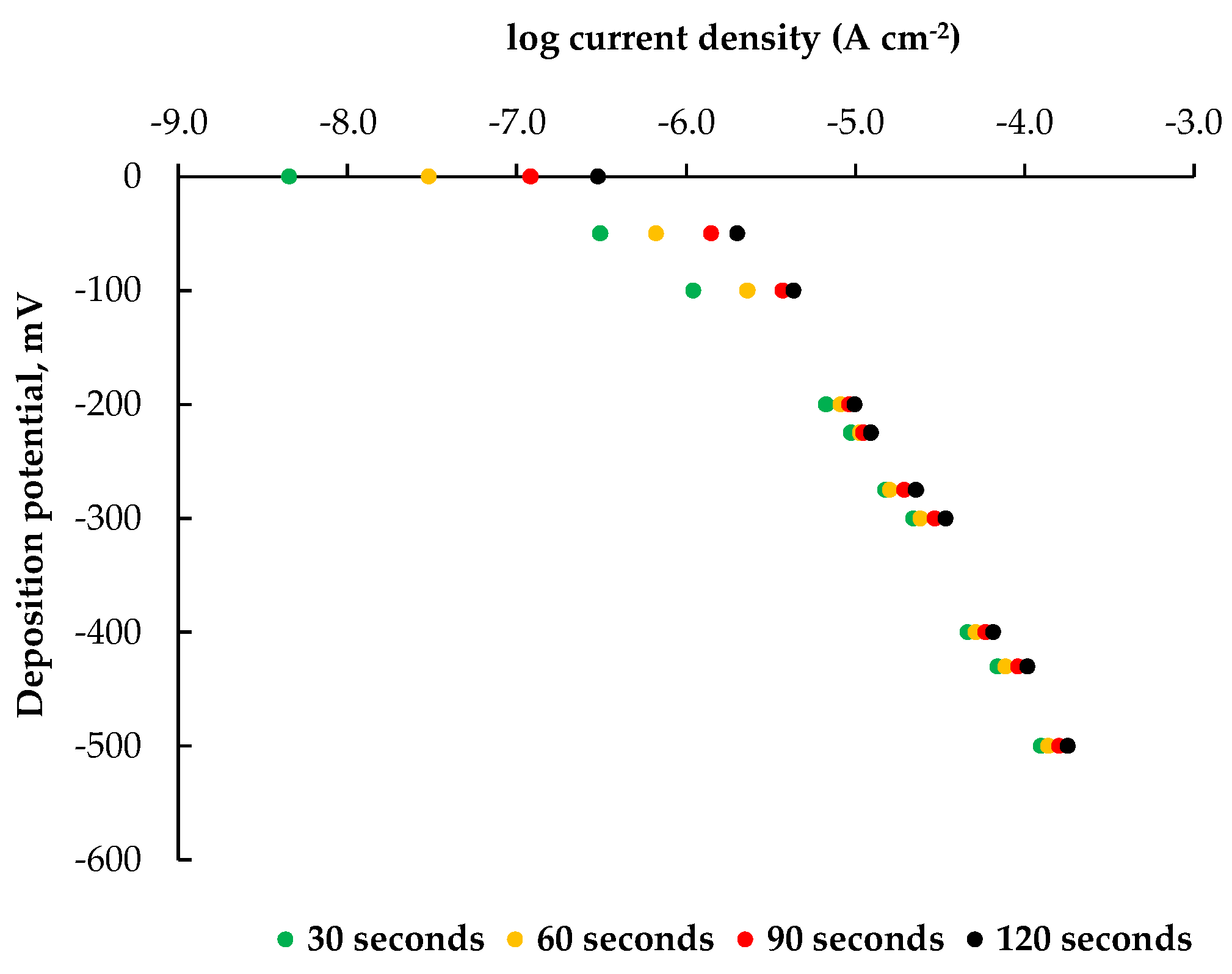
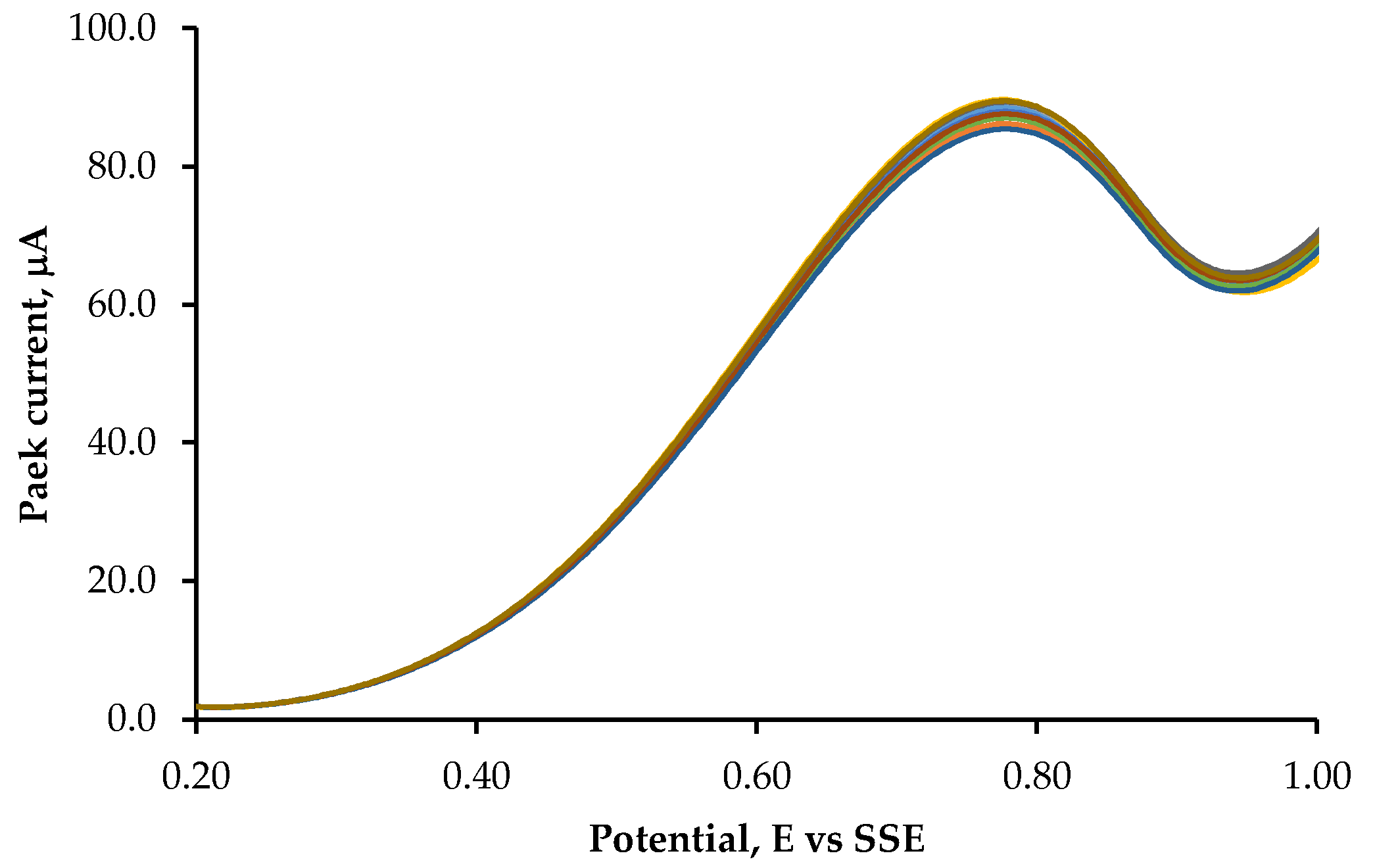
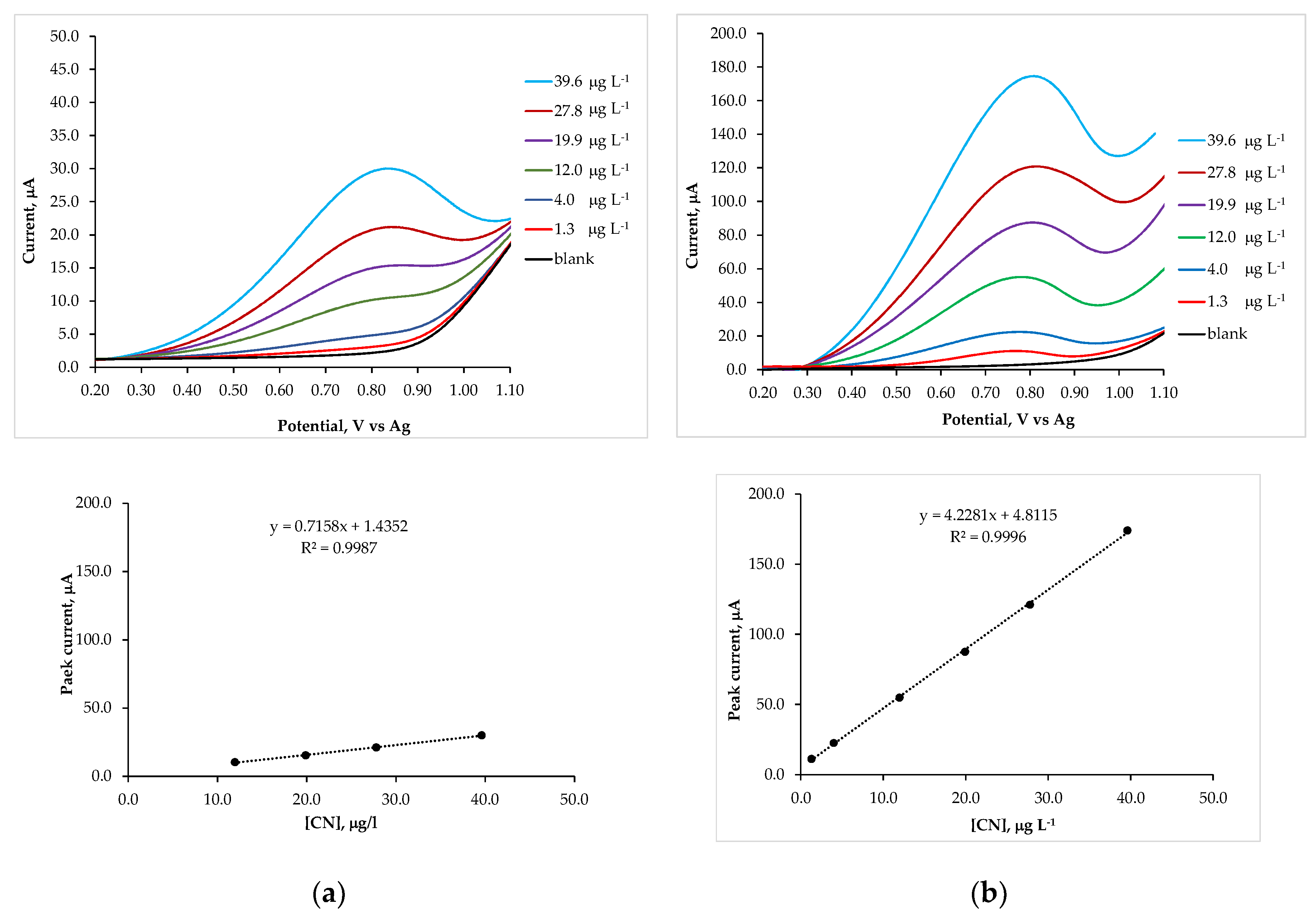
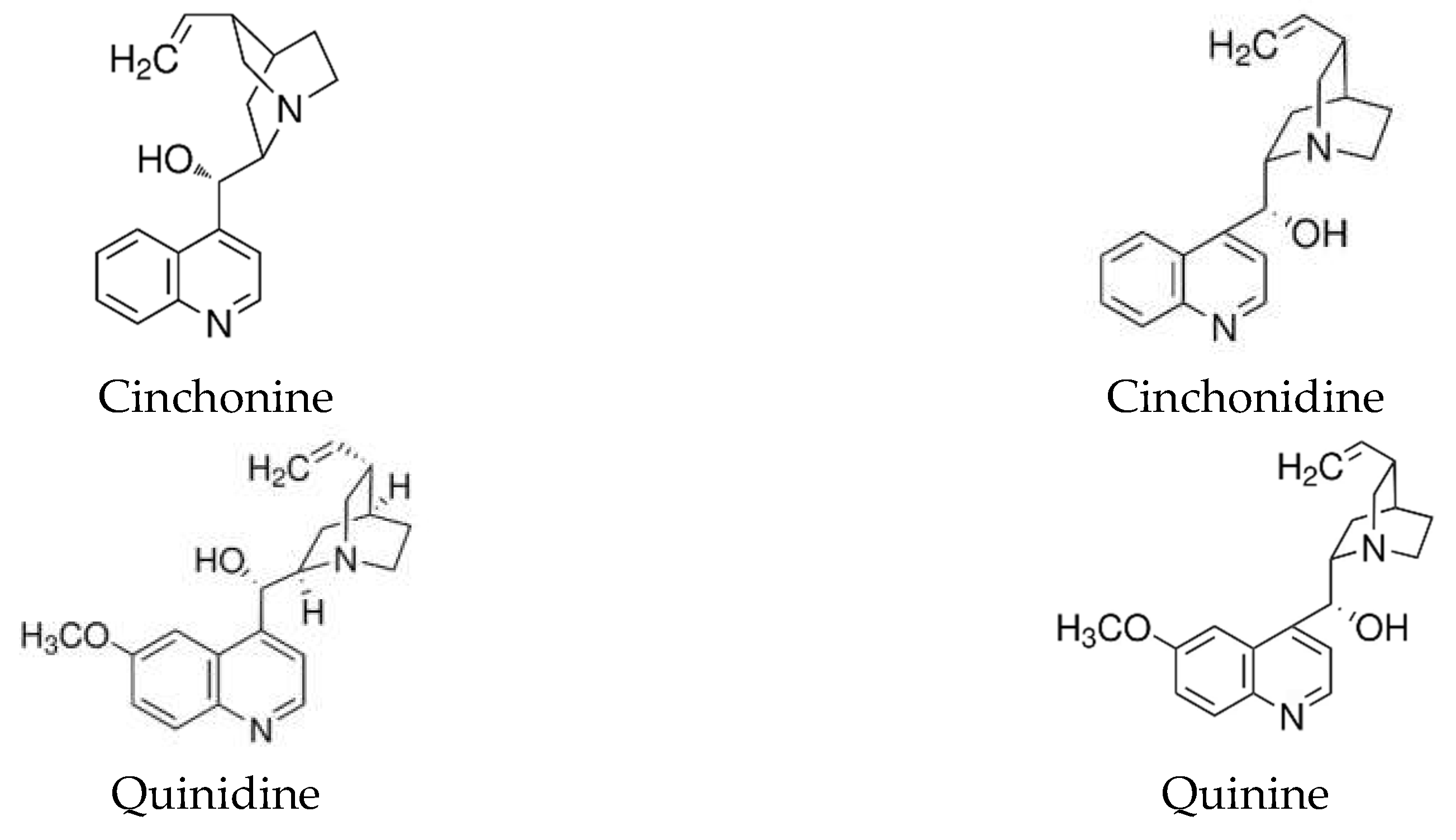
| Deposition time, s | Deposition temperature, °C | |||
| 5 | 10 | 15 | 20 | |
| 30 | 2.7 | 1.6 | 1.9 | 2.7 |
| 60 | 2.3 | 1.1 | 2.7 | 3.5 |
| 90 | 3.5 | 2.6 | 3.5 | 5.2 |
| 120 | 4.5 | 3.3 | 5.1 | 6.4 |
| Sensor Used | Limit of Detection (LOD) | Reference |
|---|---|---|
| Quinydrone electrode | 588 mg L-1 | [37] |
| GCE modified with β-CD immobilized | 0.6 mg L-1 | [36] |
| Fluorescence sensor based on a Zn coordination polymer | 129 μg L-1 | [34] |
| Gold electrode modified with ion-pair | 29.4 μg L-1 | [30] |
| complex of cinchonine–picrolonate | ||
| Sensor modified with cinchonine-monotetraphenylborate-PVC | 11.8 μg L-1 | [38] |
| SPE-Pt | 5.2 μg L-1 | This work |
| SPE-Pt/CN | 0.6 μg L-1 | This work |
| Interfering Substances | Concentration Ratio, [CN]: [Interferent] | ||
|---|---|---|---|
| 1:0.1 | 1:1 | 1:10 | |
| Recovery, % | |||
| Urea | 99.8 | 105.2 | 106.3 |
| Creatinine | 102.1 | 98.9 | 100.0 |
| Caffeine | 100.2 | 100.5 | 99.8 |
| Glucose | 98.7 | 100.2 | 101.3 |
| Cinchonidine | 100.6 | 98.6 | 92.7 |
| Quinine | 102.1 | 95.6 | 91.8 |
| Quinidine | 109.6 | High interference | High interference |
| [CN]Added, μg L-1 | Urine | Serum | ||
| Found, μg L-1 | Recovery, % | Found, μg L-1 | Recovery, % | |
| 1.99 | 2.15 | 108.0 | 1.93 | 97.0 |
| 3.96 | 3.93 | 99.2 | 4.18 | 105.5 |
| 9.76 | 10.12 | 103.7 | 9.93 | 101.8 |
| 19.05 | 19.77 | 103.8 | 19.00 | 99.8 |
| 36.36 | 36.23 | 99.6 | 36.70 | 100.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





