Submitted:
24 February 2025
Posted:
24 February 2025
You are already at the latest version
Abstract
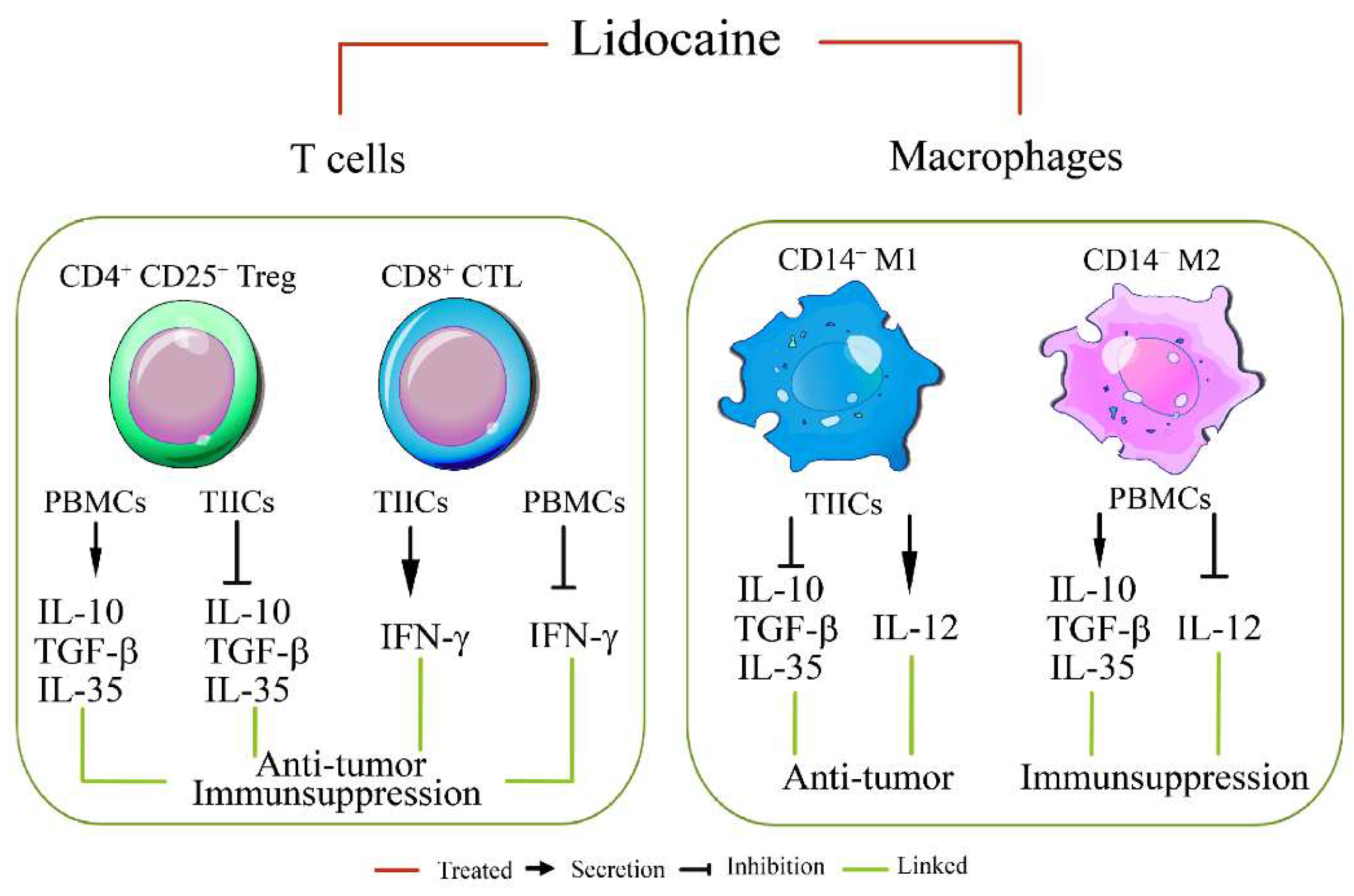
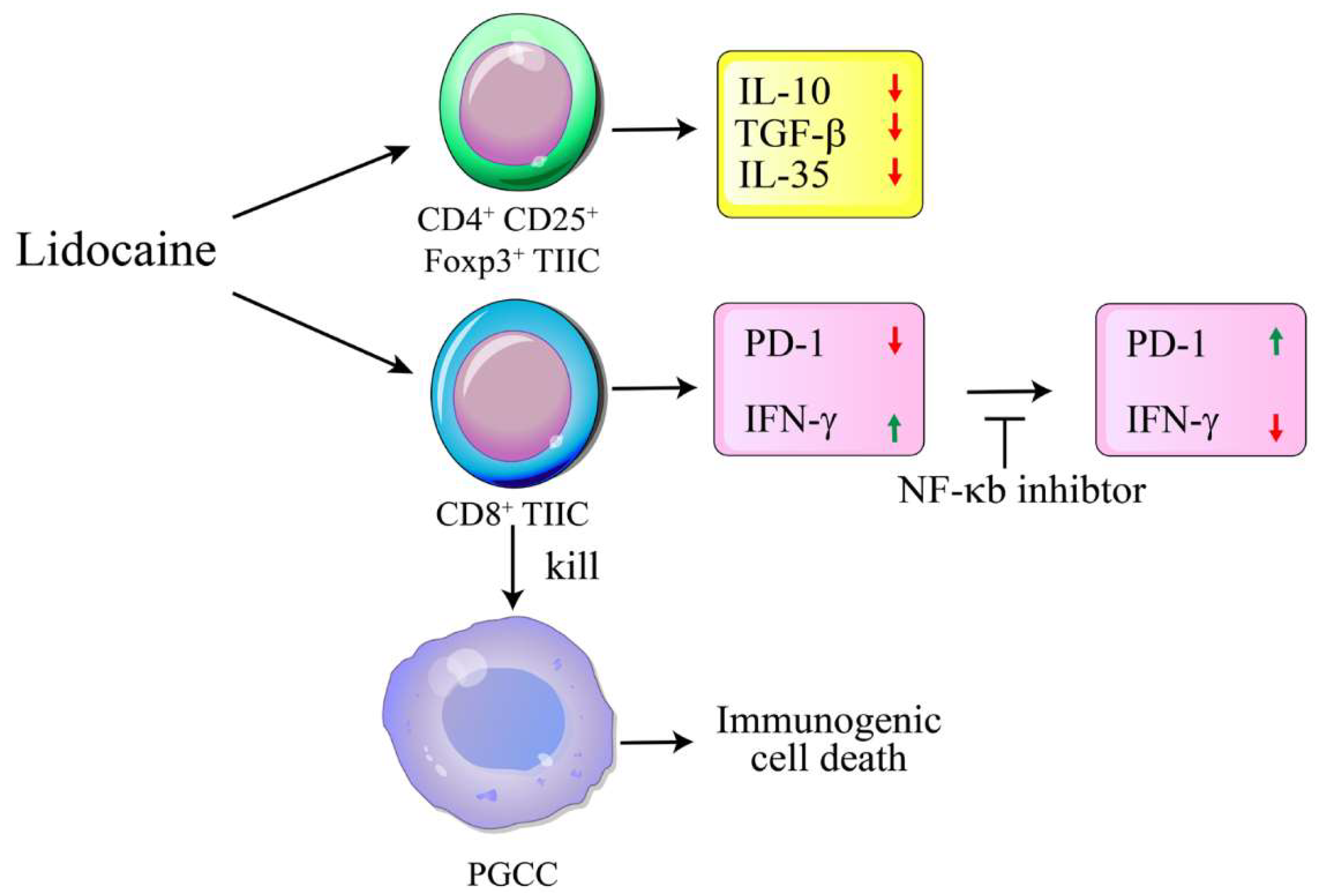
Lidocaine, a local anesthetic, has been shown to modulate immune responses. This study examines its effects on cytokine production in peripheral blood mononuclear cells (PBMCs) from healthy donors and tumor-infiltrating immune cells (TIICs) from gastric cancer patients. PBMCs from healthy donors and TIICs from gastric cancer patients were treated with lidocaine. Cytokine production was assessed using flow cytometry and cytokine assays, with a focus on IFN-γ, IL-12, IL-10, TGF-β, and IL-35 levels. Cytotoxicity against primary gastric cancer cells (PGCCs) was also evaluated. Lidocaine inhibited IFN-γ production in CD8+ PBMCs and IL-12 in CD14+ PBMCs while increasing anti-inflammatory cytokines (IL-10, TGF-β, IL-35) in CD4+CD25+ and CD14+ cells. In TIICs, lidocaine enhanced IFN-γ and IL-12 production in CD8+ and CD14+ cells, while reducing IL-10, TGF-β, and IL-35 levels, promoting an M1-like phenotype in macrophages. Lidocaine also increased IFN-γ production and cytotoxicity in CD8+ TIICs via NF-κB activation. Importantly, lidocaine did not affect the viability of PBMCs, TIICs, or PGCCs at concentrations up to 1.5 mM. Lidocaine reprograms the tumor immune microenvironment, enhancing anti-tumor immune responses, suggesting its potential to modulate immune functions in gastric cancer.
Keywords:
1. Introduction
2. Results
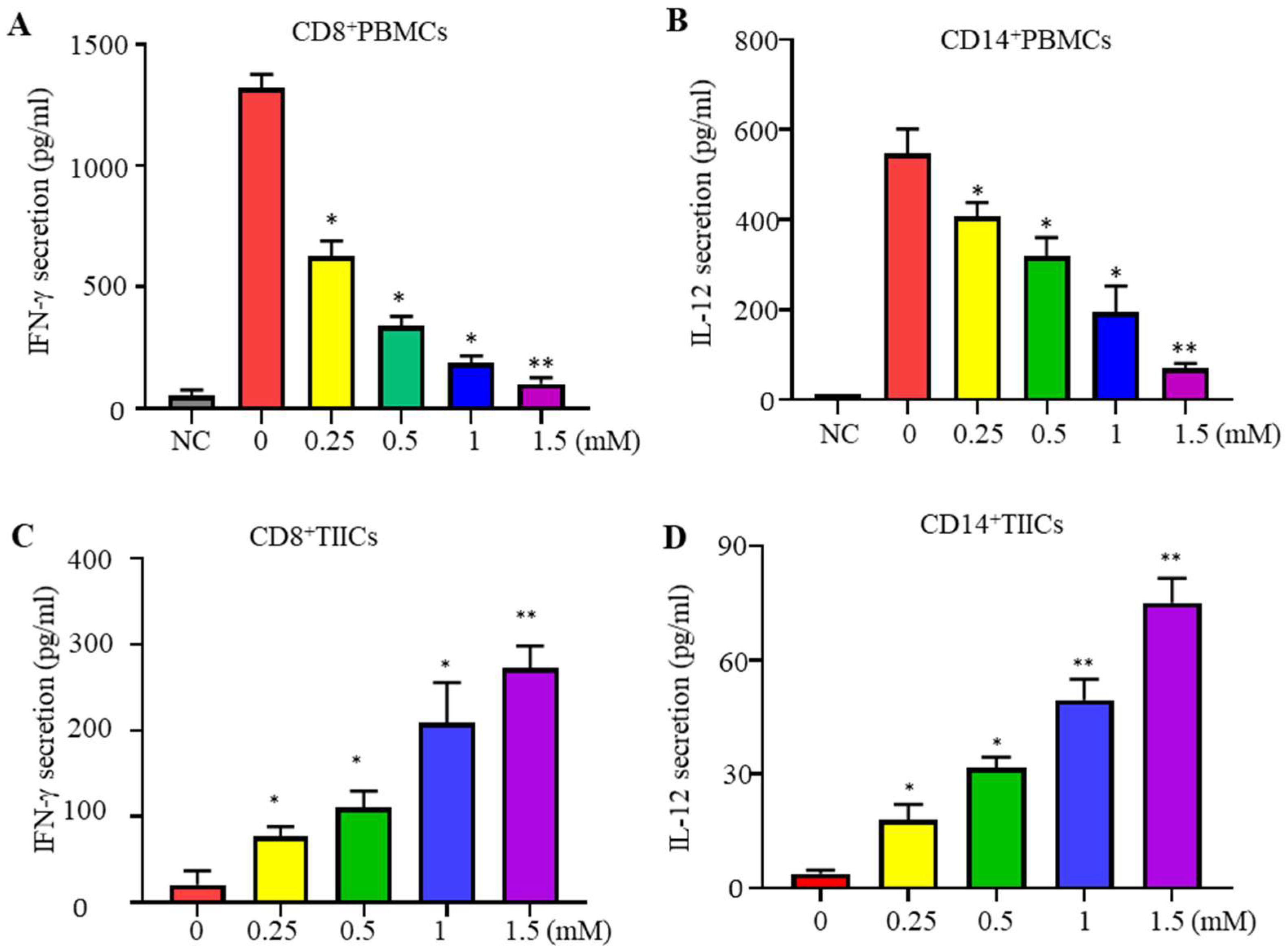
2.1. Lidocaine Inhibits IFN-γ Production by Sorted CD8+PBMCs and IL-12 Production by Sorted CD14+PBMCs
2.2. Lidocaine Increases the Anti-Cancer-Related Cytokines IFN-γ by Sorted CD8+ TIICs and IL-12 by Sorted CD14+ TIICs
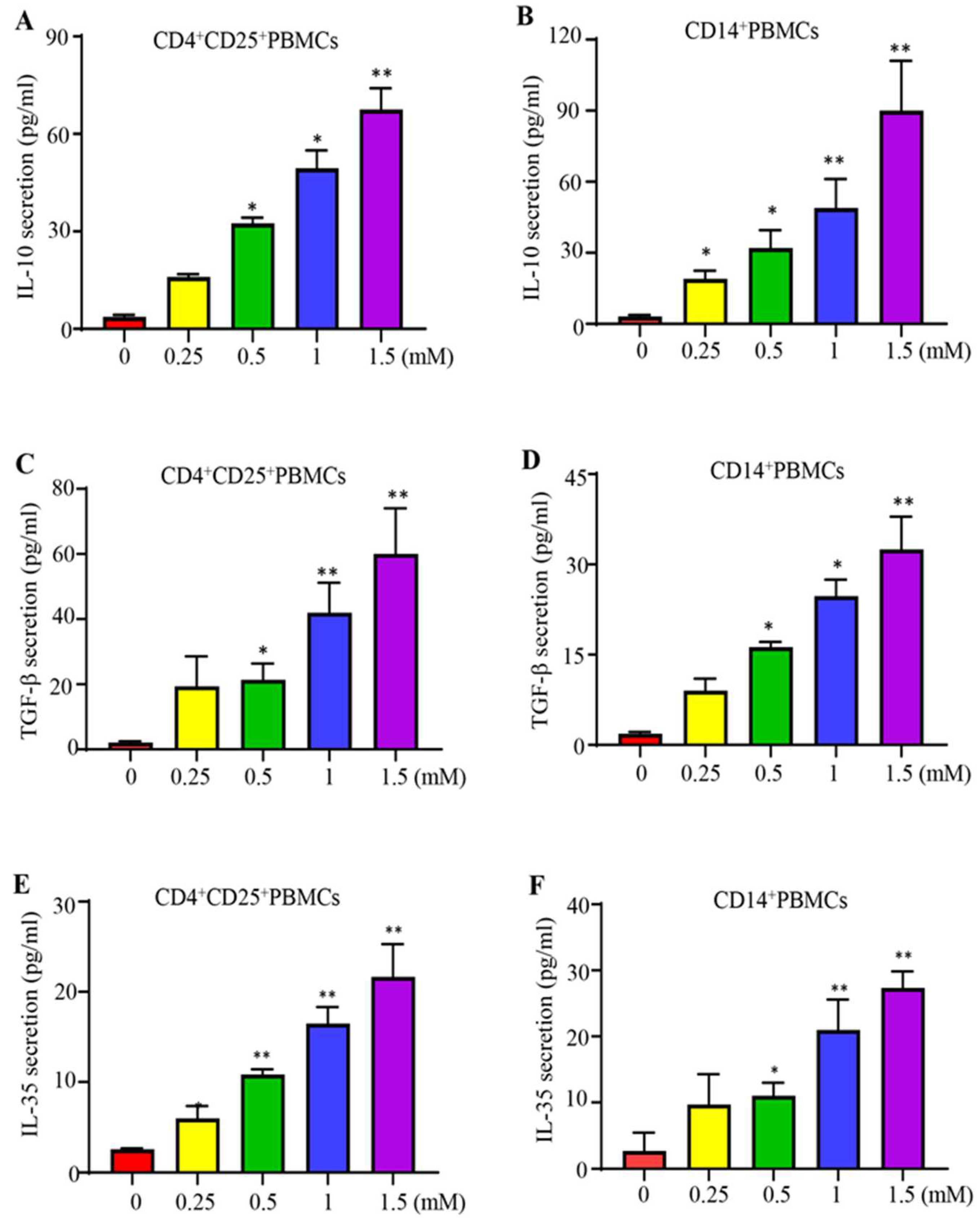
2.3. Lidocaine Increases the Production of Anti-Inflammatory Cytokine IL-10 by Sorted CD4+CD25+PBMCs and Sorted CD14+PBMCs
2.4. Lidocaine Increases the Secretion of the Treg-Related Cytokine TGF-β by CD4+CD25+ Peripheral Blood Mononuclear Cells (PBMCs) and the M2 Macrophage-Associated Cytokine TGF-β by CD14+ PBMCs
2.5. Lidocaine Increases a Novel Immunomodulator Cytokine IL-35 by Sorted CD4+CD25+PBMCs and Sorted CD14+PBMCs
2.6. Lidocaine Does Not Affect the Viability of Sorted CD4+CD25+, CD8+, and CD14+ PBMCs
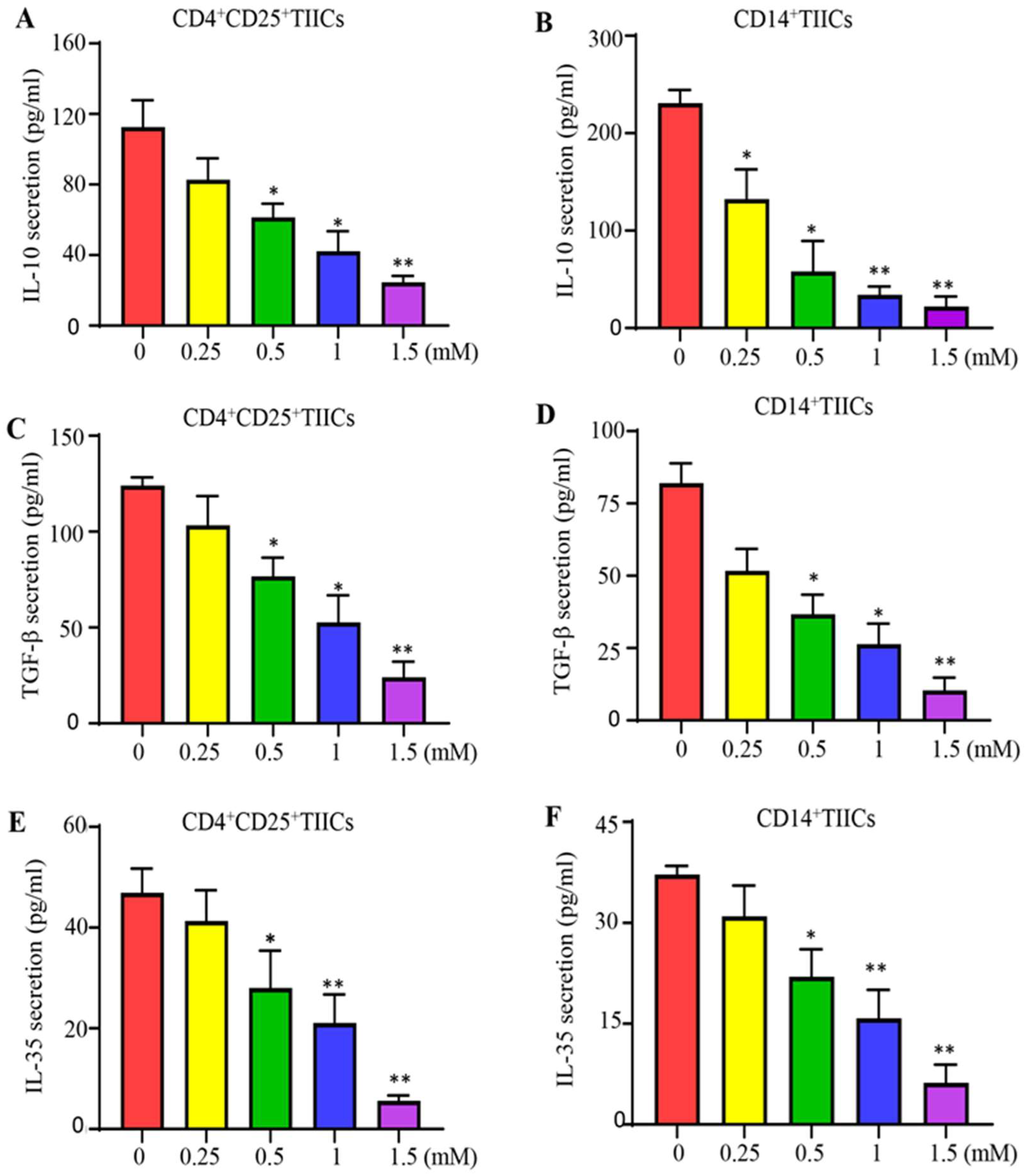
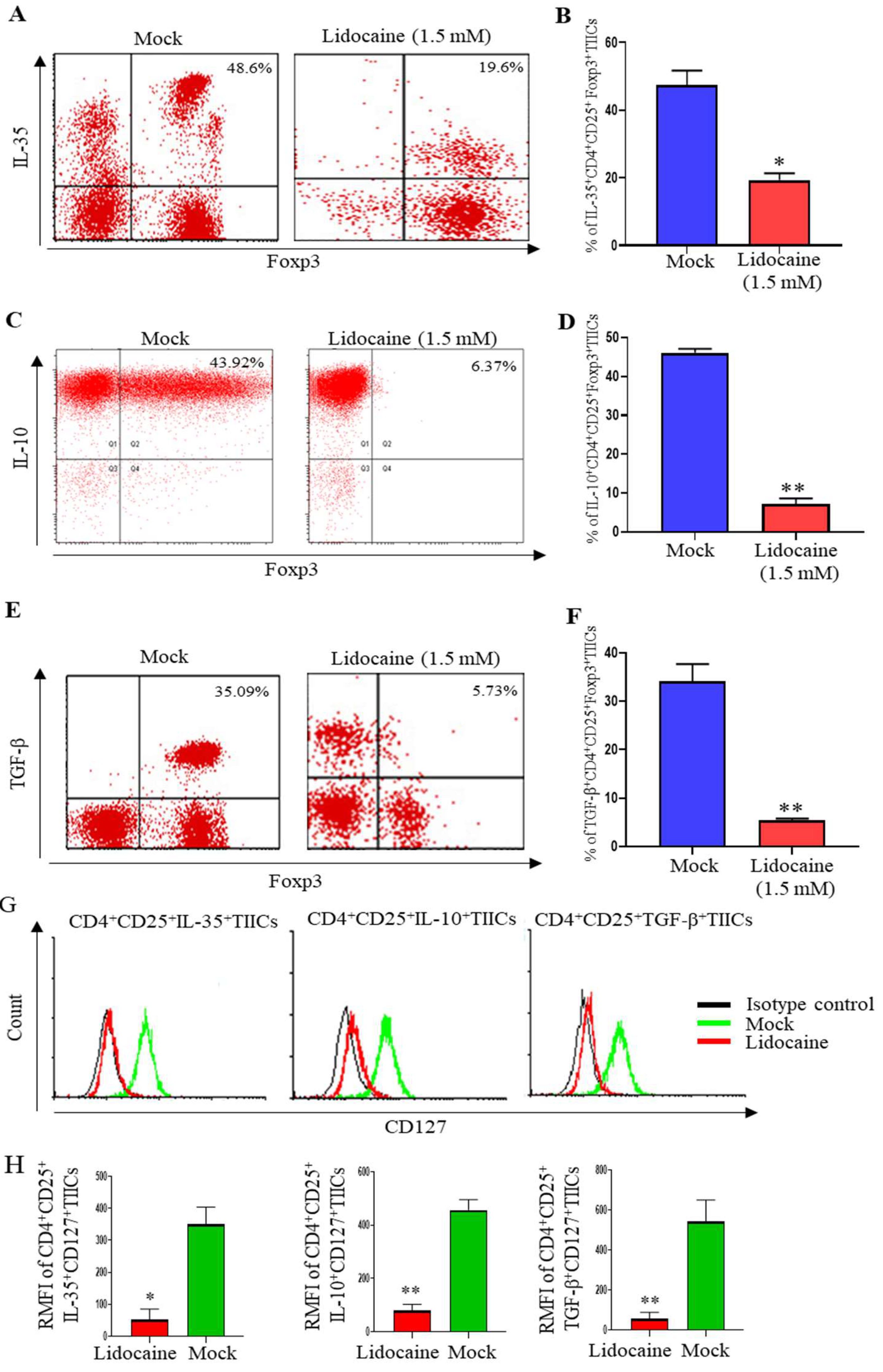
2.7. Lidocaine Inhibits IL-10, TGF-β, and IL-35 Production by Sorted CD4+CD25+ and Sorted CD14+TIICs
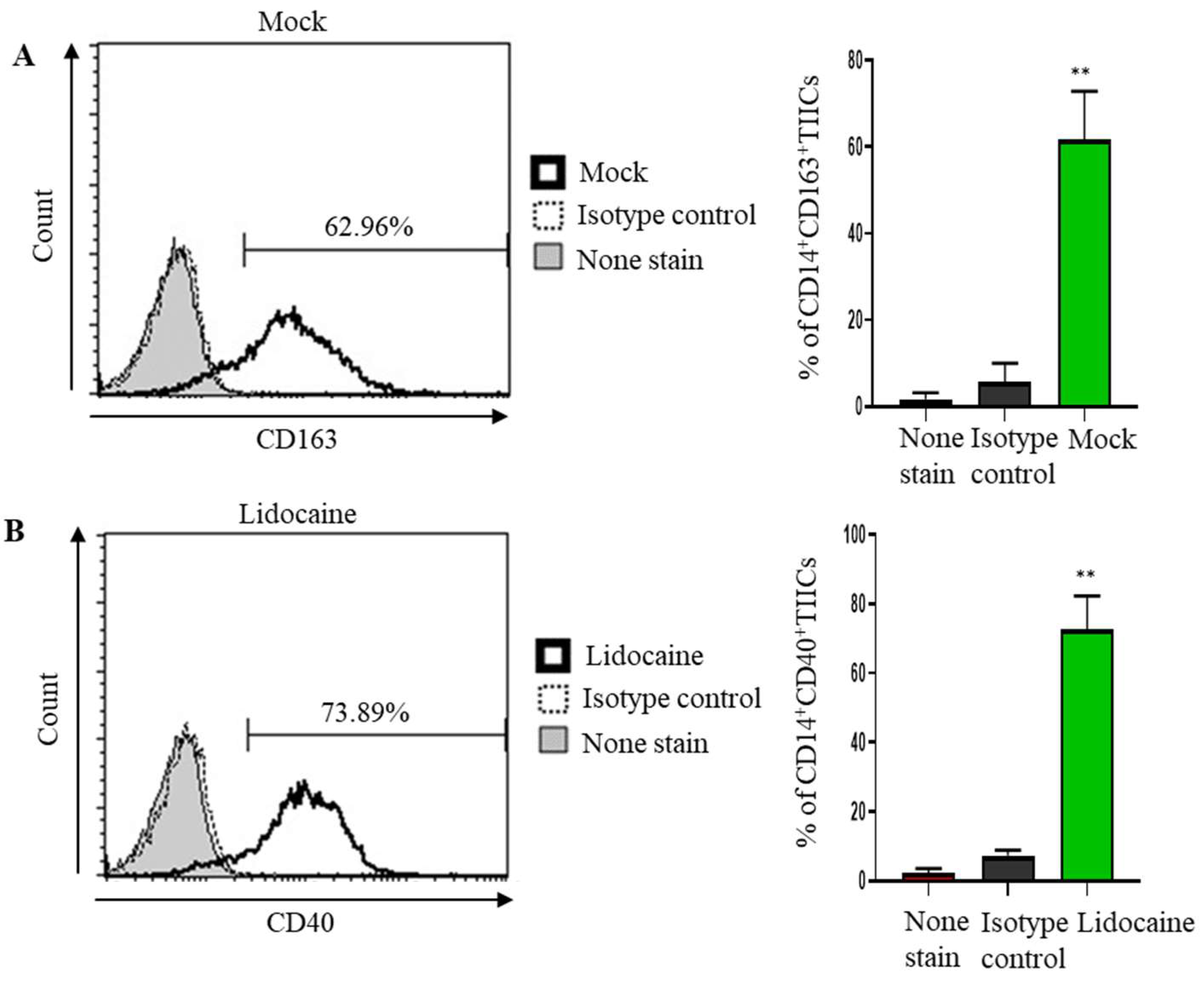
2.8. Lidocaine May Modulate Macrophage Polarization, Promoting a Pro-Inflammatory and Potentially Anti-Tumor Immune Environment
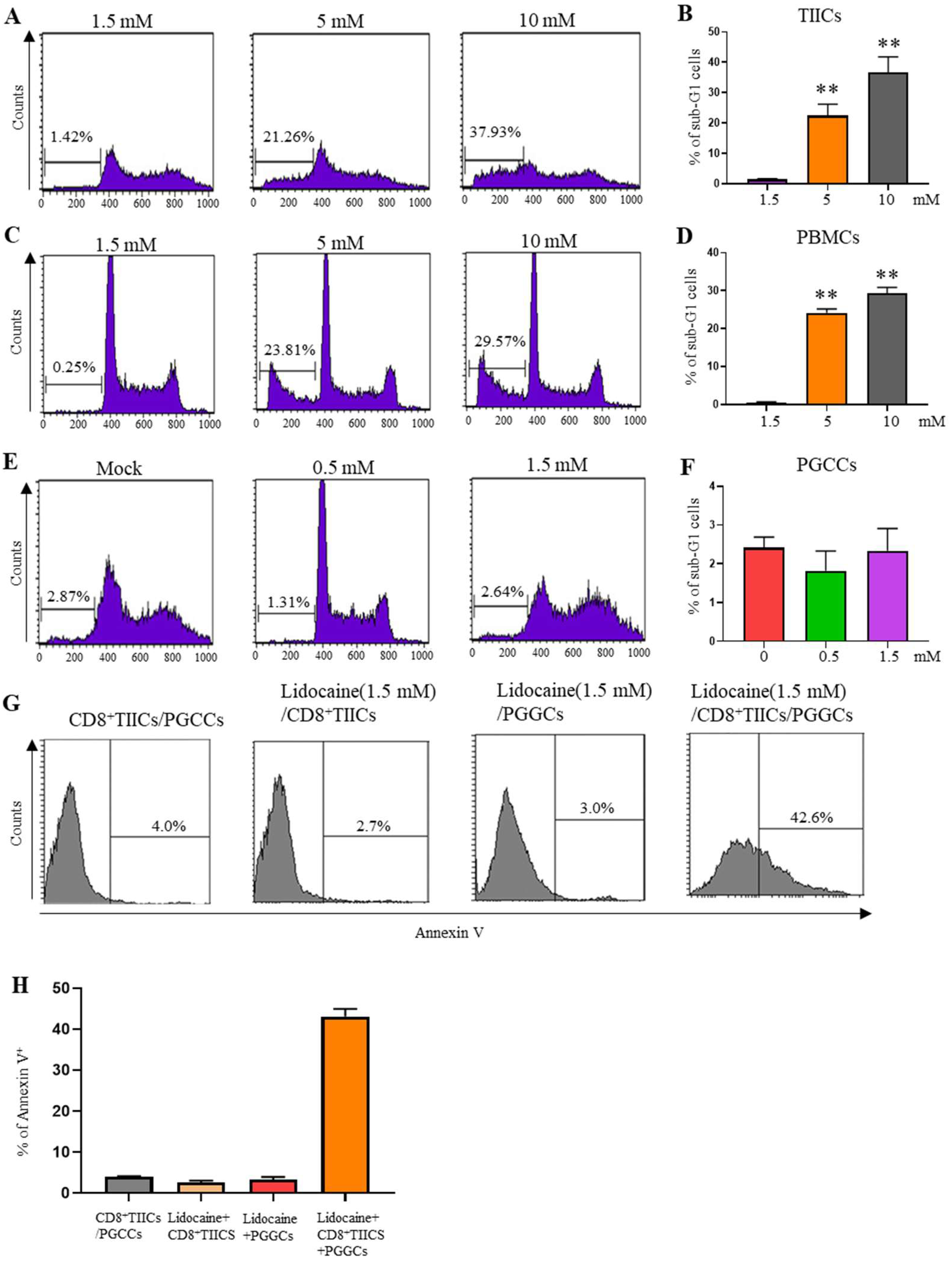
2.9. Lidocaine Does Not Affect the Viability of Sorted CD4+CD25+, CD8+, and CD14+ TIICs
2.10. Lidocaine Inhibits IL-10, TGF-β and IL-35 Production by the High Levels of FoxP3(FoxP3+) But Low Levels of CD127(CD127-) CD4+CD25+ TIICs
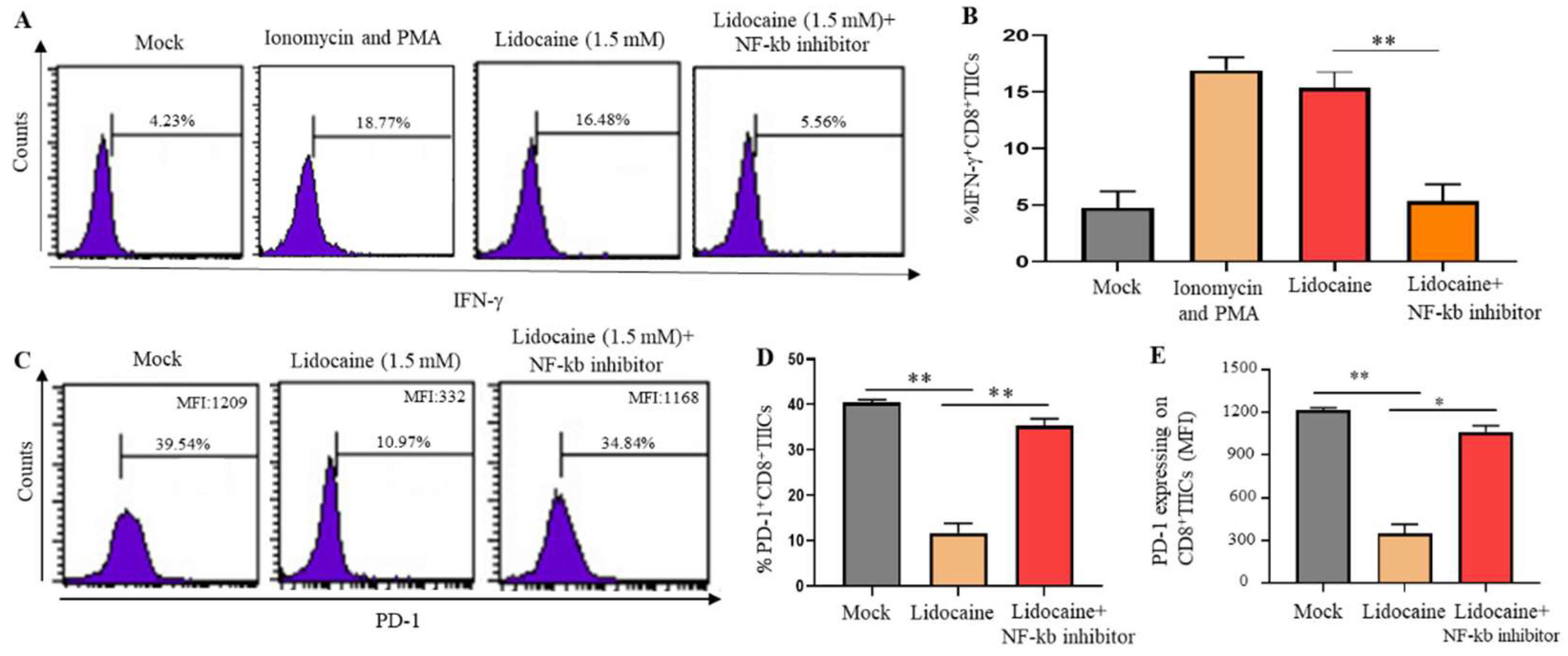
2.11. A Significant Decrease in PD-1 and Significant Increase IFN-γ Expression Was Observed in Lidocaine-Treated CD8+ TIICs Through the NF-κB Signaling Pathway
2.12. Lidocaine Does Not Affect the Viability of Sorted GRN+ Primary Gastric Cancer Cells
2.13. Lidocaine-Treated CD8+TIICs Induced Primary Gastric Cancer Cell Death
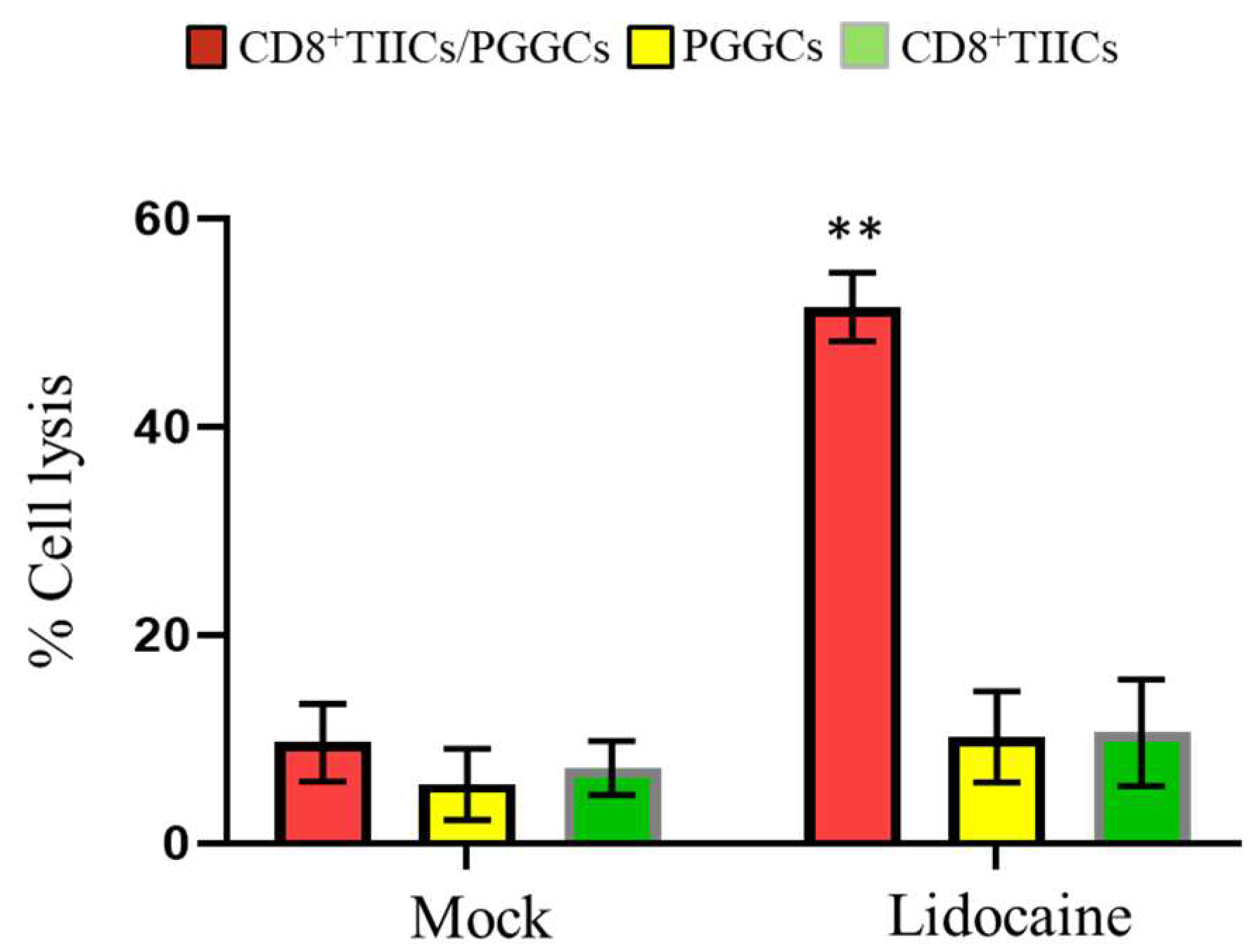
2.14. Lidocaine Enhances the Killing Activity of CD8+ TIICs Against Gastric Cancer
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of PBMCs from Healthy Adult Volunteers
4.2. Reagents and Antibodies
4.3. Isolation of TIICs from Gastric Cancer Patients
4.4. Primary Gastric Cancer Cells Culture from Fresh Surgical Malignant Gastric Tissues
4.5. Sorting of CD8+ Cells, CD4+CD25+ Cells, CD14+ Cells, and GRN+ PGCCs
4.6. Human CD8+, CD4+CD25+, CD14+PBMCs and CD8+, CD4+CD25+, CD14+ TIICs Viability
4.7. CD8+ Primary T Cells and CD14+ Primary Macrophage Activation
4.8. Analysis of Cytokines by ELISA
4.9. Foxp3 Staining and Intracellular Cytokine Staining
4.10. Detection of PD-1+ Analysis of TIICs by Flow Cytometry
4.11. Apoptosis Assays
4.12. Detection of Cytotoxicity of CD8+TIICs and PGCCs
4.13. Lidocaine Treated-CD8+TIICs Mediated Cytotoxicity Assay Using Time-Resolved Fluorometry
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loftus, J.P.; Williams, J.M.; Belknap, J.K.; Black, S.J. In Vivo Priming and Ex Vivo Activation of Equine Neutrophils in Black Walnut Extract-induced Equine Laminitis is Not Attenuated by Systemic Lidocaine Administration. Vet. Immunol. Immunopathol. 2010, 138(1-2), 60-69. [CrossRef]
- Renzi, P.M.; Ginns, L.C. Effect of Lidocaine on Natural Killer Activity: Rapid Inhibition of Lysis. Immunopharmacol. Immunotoxicol. 1990, 12(3), 417-437. [CrossRef]
- Elizagaray, M.L.; Mazitelli, I.; Pontoriero, A.; Baumeister, E.; Docena, G.; Raimondi, C.; Correger, E.; Rumbo, M. Lidocaine Reinforces the Anti-inflammatory Action of Dexamethasone on Myeloid and Epithelial Cells Activated by Inflammatory Cytokines or SARS-CoV-2 Infection. Biomed. J. 2023, 46(1), 81-92. [CrossRef]
- Feng, G.; Liu, S.; Wang, G.L; Liu, G.J. Lidocaine Attenuates Lipopolysaccharide-induced Acute Lung Injury Through Inhibiting NF-kappaB Activation. Pharmacology. 2008, 81(1), 32-40. [CrossRef]
- Sun, H.; Sun, Y. Lidocaine Inhibits Proliferation and Metastasis of Lung Cancer Cell via Regulation of MiR-539/EGFR Axis. Artif. Cells Nanomed Biotechnol. 2019, 47(1), 2866-2874.
- Chen, L.J.; Ding, Y.B.; Ma, P.L.; Jiang, S.H.; Li, K.Z.; Li, M.C.; Shi, C.X.; Du, J.; Zhou, H.D. The Protective Effect of Lidocaine on Lipopolysaccharide-induced Acute Lung Injury in Rats Through NF-κB and P38 MAPK Signaling Pathway and Excessive Inflammatory Responses. Eur. Rev. Med. Pharmacol. Sci. 2018, 22(7), 2099-2108.
- Lahat, A.; Ben-Horin, S.; Lang, A.; Fudim, E.; Picard, O.; Chowers, Y. Lidocaine Down-Regulates Nuclear Factor-kappaB Signalling and Inhibits Cytokine Production and T cell Proliferation. Clin. Exp. Immunol. 2008, 152(2), 320-327. [CrossRef]
- Johnson, B.D.; Jing, W.; Orentas, R.J. CD25+ Regulatory T cell Inhibition Enhances Vaccine-induced Immunity to Neuroblastoma. J. Immunother. 2007, 30(2), 203-214.
- Hesse, M.; Piccirillo, C.A.; Belkaid, Y.; Prufer, J.; Mentink-Kane, M.; Leusink, M.; Cheever, A.W.; Shevach, E.M.; Wynn, T.A. The Pathogenesis of Schistosomiasis is Controlled by Cooperating IL-10-producing Innate Effector and Regulatory T Cells. J. Immunol. 2004, 172(5), 3157-3166. [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) Cytotoxic T Lymphocytes in Cancer Immunotherapy: A Review. J. Cell Physiol. 2019, 234(6), 8509-8521.
- Dinarello, C.A. Anti-cytokine Therapeutics and Infections. Vaccine. 2003, 21 Suppl 2, S24-34.
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical Landscape of Oncolytic Virus Research in 2020. J. Immunother. Cancer. 2020, 8(2), e001486.
- Shi, X.; Cao, S.; Mitsuhashi, M.; Xiang, Z.; Ma, X. Genome-wide Analysis of Molecular Changes in IL-12-induced Control of Mammary Carcinoma via IFN-gamma-independent Mechanisms. J. Immunol. 2004, 172(7), 4111-4122. [CrossRef]
- Hung, C.H.; Hsu, H.Y.; Chiou, H.C.; Tsai, M.L.; You, H.L.; Lin, Y.C.; Liao, W.T. Arsenic Induces M2 Macrophage Polarization and Shifts M1/M2 Cytokine Production via Mitophagy. Int. J. Mol. Sci. 2022, 23(22), 13879. [CrossRef]
- Kang, M.J.; Jang, A.R.; Park, J.Y.; Ahn, J.H.; Lee, T.S.; Kim, D.Y.; Lee,D.W.; Hwang, S.; Jeong, Y.J.; Park, J.H. IL-10 Protects Mice From the Lung Infection of Acinetobacter baumannii and Contributes to Bacterial Clearance by Regulating STAT3-Mediated MARCO Expression in Macrophages. Front. Immunol. 2020, 11, 270.
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A. The Inhibitory Cytokine IL-35 Contributes to Regulatory T-cell Function. Nature. 2007, 450(7169), 566-569. [CrossRef]
- Feng, J.; Wu, Y. Interleukin-35 Ameliorates Cardiovascular Disease by Suppressing Inflammatory Responses and Regulating Immune Homeostasis. Int. Immunopharmacol. 2022, 110, 108938.
- He, W.; Hao, S.; Dong, X.; Zhang, D.; Jia, Z. Circulating Cytokine Profile and Modulation of Regulatory T cells in Chronic Hepatitis B Patients with Type 2 Diabetes Mellitus. Biomol. Biomed. 2023 ,23(1), 53-62. [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020,10(3), 727-742.
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017, 170(6), 1120-1133.e17.
- Huang, Q.; Wu, X.; Wang, Z.; Xiangyu, C.; Lisha W.; Yijun, L.; Dan, X.; Qiao, L.; Yuhan, T.; Huayu, L.; et al. The Primordial Differentiation of Tumor-specific Memory CD8(+) T cells as Bona Fide Responders to PD-1/PD-L1 Blockade in Draining Lymph Nodes. Cell 2022, 185(22), 4049-4066.e4025.
- Jimenez-Duran, G.; Luque-Martin, R.; Patel, M.; Bernard, S.; Sharp, C.; Buchan, N.; Rea, C.; de Winther, M.P.J.; Turan, N.; Angell, D.; et al. Pharmacological Validation of Targets Regulating CD14 During Macrophage Differentiation. EBioMedicine 2020, 61,103039. [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084.
- Yu, Q.; Xu, M.; Yu, F.; Jin, Y. CD4(+)CD25(+) Regulatory T cells as a Therapeutic Target in Rheumatoid Arthritis. Cent. Eur. J. Immunol. 2014, 39(1), 100-103. [CrossRef]
- Liu, Q.; Yang, C.; Wang, S.; Shi, D.; Wei, C.; Song, J.; Lin, X.; Dou, R.; Bai, J.; Xiang, Z.; et al. Wnt5a-induced M2 Polarization of Tumor-associated Macrophages via IL-10 Promotes Colorectal Cancer Progression. Cell Commun. Signal. 2020,18(1), 51.
- Ma, X.; Gao, Y.; Chen, Y.; Liu, J.; Yang, C.; Bao, C.; Wang, Y.; Feng,Y.; Song, X.; Qiao, S. M2-Type Macrophages Induce Tregs Generation by Activating the TGF-β/Smad Signalling Pathway to Promote Colorectal Cancer Development. Onco. Targets Ther. 2021, 14, 5391-5402.
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human Macrophage Polarization in Vitro: Maturation and Activation Methods Compared. Immunobiology 2014, 219(9), 695-703. [CrossRef]
- Shao, Y.; Yang, W.Y.; Saaoud, F.; Drummer 4th, C.; Sun, Y.; Xu, K.; Lu, Y.; Shan, H.; Shevach, E.M.; Jiang,X.; et al. IL-35 Promotes CD4+Foxp3+ Tregs and Inhibits Atherosclerosis via Maintaining CCR5-amplified Treg-suppressive Mechanisms. JCI. Insight 2021,6(19), e152511.
- Lou, W.; Wang, C.; Wang, Y.; Han, D.; Zhang, L. Responses of CD4(+) CD25(+) Foxp3(+) and IL-10-secreting type I T Regulatory Cells to Cluster-specific Immunotherapy for Allergic Rhinitis in Children. Pediatr. Allergy Immunol. 2012, 23(2), 140-149.
- Peng, Y.; Laouar, Y.; Li, M.O.; Green, E.A.; Flavell, R.A. TGF-beta Regulates in vivo Expansion of Foxp3-expressing CD4+CD25+ Regulatory T cells Responsible for Protection Against Diabetes. Proc. Natl. Acad. Sci. U S A. 2004, 101(13), 4572-4577.
- Dons, E.M.; Raimondi, G.; Cooper, D.K.; Thomson, A.W. Induced Regulatory T cells: Mechanisms of Conversion and Suppressive Potential. Hum. Immunol. 2012, 73(4), 328-334. [CrossRef]
- Pinchuk, I.V.; Beswick, E.J.; Saada, J.I.; Boya, G.; Schmitt, D., Raju, G.S.; Brenmoehl, J.; Rogler, G.; Reyes, V.E.; Powell, D.W. Human Colonic Myofibroblasts Promote Expansion of CD4+ CD25high Foxp3+ Regulatory T Cells. Gastroenterology 2011, 140(7), 2019-2030.
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or Opponent in the Tumour Microenvironment? Nat. Rev. Immunol. 2022, 22(3),158-172. [CrossRef]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Amelia, A.Y.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L. Mechanistic Convergence of the TIGIT and PD-1 Inhibitory Pathways Necessitates Co-blockade to Optimize Anti-tumor CD8(+) T Cell Responses. Immunity 2022, 55(3), 512-526.e519. [CrossRef]
- Yang, R.; Pei, T.; Huang, R.; Xiao, Y.; Yan, Jiangna.; Zhu, Jinglin.; Zheng, C.; Xiao, Wei.; Huang, C. Platycodon grandiflorum Triggers Antitumor Immunity by Restricting PD-1 Expression of CD8(+) T Cells in Local Tumor Microenvironment. Front. Pharmacol. 2022, 13, 774440. [CrossRef]
- Garg, S.K.; Welsh, E.A.; Fang, B.; Hernandez, Y.I.; Rose, T.; Gray, J.; Koomen, J.M.; Berglund, A.; Mulé, J.J.; Markowitz, J. Multi-Omics and Informatics Analysis of FFPE Tissues Derived from Melanoma Patients with Long/Short Responses to Anti-PD1 Therapy Reveals Pathways of Response. Cancers (Basel) 2020,12(12), 3515.
- Bally, A.P.; Lu, P.; Tang, Y.; Austin, J.W.; Scharer, C.D.; Ahmed, R.; Boss, J.M. NF-κB Regulates PD-1 Expression in Macrophages. J. Immunol. 2015, 194(9), 4545-4554. [CrossRef]
- Xu, P.; Sun, Z.; Wang, Y.; Miao, C. Long-term Use of Indomethacin Leads to Poor Prognoses Through Promoting The Expression of PD-1 and PD-L2 via TRIF/NF-κB Pathway and JAK/STAT3 Pathway to Inhibit TNF-α and IFN-γ in Hepatocellular Carcinoma. Exp. Cell Res. 2015, 337(1), 53-60.
- Ye, L.; Zhang, Y.; Chen, Y.J.; Liu, Q. Anti-tumor Effects of Lidocaine on Human Gastric Cancer Cells in Vitro. Bratisl. Lek. Listy. 2019, 120(3), 212-217. [CrossRef]
- Hayakawa, Y.; Fox, J.G.; Gonda, T.; Worthley, D.L.; Muthupalani, S.; Wang, T.C. Mouse Models of Gastric Cancer. Cancers (Basel) 2013, 5(1), 92-130.
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical Implications of T cell Exhaustion for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19(12), 775-790.
- Wen, Z.; Sun, H.; Zhang, Z.; Zheng, Y.; Zheng, S.; Bin, J.; Liao, Y.; Shi, M.; Zhou, Rui.; Liao, W. High Baseline Tumor Burden-Associated Macrophages Promote an Immunosuppressive Microenvironment and Reduce the Efficacy of Immune Checkpoint Inhibitors Through the IGFBP2-STAT3-PD-L1 Pathway. Cancer Commun (Lond). 2023, 43(5), 562-581. [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care (New Rochelle) 2020, 9(4), 184-198. [CrossRef]
- Degboé, Y.; Rauwel, B.; Baron, M.; Boyer, J.F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3.
- Yi, M.; Li, T.; Niu M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct.Target Ther. 2024, 9(1), 176.
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How Regulatory T cells work. Nat. Rev. Immunol. 2008, 8(7), 523-532.
- Zhang, H.; Li, R.; Cao, Y.; Gu, Y.; Lin, C.; Liu, Xin.; Lv, K.; He, X.; Fang, H.; Jin, K. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann. Surg. 2022, 275(4), e626-e635. [CrossRef]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L. Cheung, J.; Sharpe, A.H. Freeman, G. J. Irving, B. A. Ahmed, R. Role of PD-1 During Effector CD8 T cell Differentiation. Proc. Natl. Acad. Sci. U S A. 2018, 115(18), 4749-4754.
- Cai, Z.; Zhang, S.; Wu, P.; Ren, Q.; Wei, P.; Hong, M.; Feng, Y.; Wong, C.K.; Tang, H.; Zeng, H. A Novel Potential Target of IL-35-regulated JAK/STAT Signaling Pathway in Lupus Nephritis. Clin. Transl. Med. 2021,11(2), e309. [CrossRef]
- Moeini, S.; Saeidi, M.; Fotouhi, F.; Mondanizadeh, M.; Shirian, S.; Mohebi, A.; Gorji, A.; Ghaemi, A. Synergistic Effect of Programmed Cell Death Protein 1 Blockade and Secondary Lymphoid Tissue Chemokine in the Induction of Anti-tumor Immunity by a Therapeutic Cancer Vaccine. Arch. Virol. 2017, 162(2), 333-346.
- Wu, W.; Jiang, H.; Li, Y.; Yan MX. IL-35 Expression is Increased in Laryngeal Squamous Cell Carcinoma and in the Peripheral Blood of Patients. Oncol. Lett. 2017, 13(5), 3303-3308.
- Jiang, H.; Zhang, T.; Yan, MX.; Wu, W. IL-35 Inhibits CD8(+) T cells Activity by Suppressing Expression of Costimulatory Molecule CD28 and Th1 Cytokine Production. Transl. Cancer Res. 2019, 8(4), 1319-1325. [CrossRef]
- Zeng, J.C.; Zhang, Z.; Li, T.Y.; Liang, Y. F.; Wang, H.M.; Bao, J. J.; Zhang, J.A.; Wang, W.D.; Xiang, W.Y.; Kong, B.; et al. Assessing the Role of IL-35 in Colorectal Cancer Progression and Prognosis. Int. J.Clin. Exp. Pathol. 2013, 6(9), 1806-1816.
- Turnis, ME.; Sawant, DV.; Szymczak-Workman, A.L.; Andrews, L.P.; Delgoffe, G.M.; Yano, H.; Beres, A.J.; Vogel, P.; Workman, C.J.; Vignali, D.A. Interleukin-35 Limits Anti-Tumor Immunity. Immunity. 2016, 44(2), 316-329. [CrossRef]
- Huang, Y.; Hu, H.; Liu, L.; Ye, J.; Wang, Z.; Que, B.; Liu, W.; Shi, Y.; Zeng, T.; Shi, L. et al. Interleukin-12p35 Deficiency Reverses the Th1/Th2 Imbalance, Aggravates the Th17/Treg Imbalance, and Ameliorates Atherosclerosis in ApoE-/- Mice. Mediators Inflamm. 2019, 2019, 3152040. [CrossRef]
- Olson, B.M.; Jankowska-Gan, E.; Becker, J.T.; Vignali, D.A.; Burlingham, W.J.; McNeel, D.G. Human Prostate Tumor Antigen-Specific CD8+ Regulatory T cells are Inhibited by CTLA-4 or IL-35 Blockade. J. Immunol. 2012, 189(12), 5590-5601. [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35 Suppl, S185-s198. [CrossRef]
- Pfeffer, L.M. The Role of Nuclear Factor κB in the Interferon Response. J. Interferon Cytokine Res. 2011, 31(7), 553-559.
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark Res. 2020, 8, 49. [CrossRef]
- Hayakawa, T.; Sugiyama, J.; Yaguchi, T .; Imaizumi ,A.; Kawakami, Y. Enhanced Anti-tumor Effects of The PD-1/PD-L1 Blockade by Combining a Highly Absorptive Form of NF-kB/STAT3 Inhibitor Curcumin. J. Immunother. Cancer. 2014, 2(3), P210.
- Zhou, D.; Wang, L.; Cui, Q.; Iftikhar, R.; Xia, Y.; Xu, P. Repositioning Lidocaine as an Anticancer Drug: The Role Beyond Anesthesia. Front. Cell Dev. Biol.. 2020, 17(8), 565.
- Chen, Y.Y.; Yang, W.C.; Chang, Y.K.; Wang, C.Y.; Huang, W.R.; Li, J.Y.; Chuang, K.P.; Wu, H.Y.; Chang, C.D.; Nielsen, B.L.;et al. Construction of Polycistronic Baculovirus Surface Display Vectors to Express the PCV2 Cap(d41) Protein and Analysis of Its Immunogenicity in Mice and Swine. Vet. Res. 2020, 51(1), 112.
- Dudley, M.E.; Wunderlich, J.R.; Shelton, T.E.; Even, J.; Rosenberg, S.A. Generation of Tumor-infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. J. Immunother. 2003, 26(4), 332-342. [CrossRef]
- Wu, Y.Y.; Wu. F.H.; Chen, I.C.; Liao, T.L.; Munir, M.; Liu, H.J. Oncolytic Avian Reovirus-sensitized Tumor infiltrating CD8(+) T cells Triggering Immunogenic Apoptosis in Gastric Cancer. Cell Commun. Signal. 2024, 22(1), 514. [CrossRef]
- Aziz, F.; Yang, X.; Wen, Q.; Yan, Q. A Method for Establishing Human Primary Gastric Epithelial Cell Culture from Fresh Surgical Gastric Tissues. Mol. Med. Rep. 2015, 12(2), 2939-2944. [CrossRef]
- Smoot, D.T.; Sewchand, J.; Young, K.; Desbordes, B.C.; Allen, C.R.; Naab, T. A Method for Establishing Primary Cultures of Human Gastric Epithelial Cells. Methods Cell Sci. 2000, 22(2-3), 133-136.
- Sultan, M.; Alghetaa, H.; Mohammed, A.; Osama, A.A.; Paul, J.W.; Narendra, S.; Prakash, N.; Mitzi, N. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front. Pharmacol. 2021, 12, 644281.
- Vergis, J.; Malik, S.V.S.; Pathak, R.; Kumar, M.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Exploring Galleria Mellonella Larval Model to Evaluate Antibacterial Efficacy of Cecropin A (1-7)-Melittin Against Multi-drug Resistant Enteroaggregative Escherichia Coli. Pathog. Dis. 2021, 79(3), ftab010.
- Ilkow, C.S.; Marguerie, M.; Batenchuk, C.; Mayer, J.; Neriah, D.B.; Cousineau, S.; Falls, T.; Jennings, V. A.; Boileau, M.; Bellamy, D.; et al. Reciprocal Cellular Cross-talk Within the Tumor Microenvironment Promotes Oncolytic Virus Activity. Nat. Med. 2015, 21(5), 530-536.
- Zhou, Y.; Chen, C.L.; Jiang, S.W.; Feng, Y.; Yuan, L.; Chen, P.; Zhang,L.; Huang, S.; Li, J.; Xia,J.C.; et al. Retrospective Analysis of the Efficacy of Adjuvant CIK Cell Therapy in Epithelial Ovarian Cancer Patients Who Received Postoperative Chemotherapy. Oncoimmunology 2019, 8(2), e1528411. [CrossRef]
- Snyder, K.M,; Dixon, K.J.; Davis, Z.; Hosking, M.; Hart, G.; Khaw, M.; Matson, A.; Bjordahl, R.; Hancock, B.; Shirinbak, S.; et al. IPSC-derived Natural Killer Cells Expressing the FcγR Fusion CD64/16A Can be Armed with Antibodies for Multitumor Antigen Targeting. J. Immunother. Cancer 2023, 11(12), e007280.
- Yao, L.; Hou, J.; Wu, X.; Lu, Y.; Jin, Z.; Yu, Z.; Yu, B.; Li, J.; Yang, Z.; Li, C.; et al. Cancer-associated Fibroblasts Impair the Cytotoxic Function of NK cells in Gastric Cancer by Inducing Ferroptosis via Iron Regulation. Redox Biol. 2023, 67, 102923. [CrossRef]
- Sun, L.; Jiang, G.; Ng, Y.Y.; Xiao, L.; Du, Z.; Wang, S.; Zhu, J. T cells with Split CARs Specific for NKG2D Ligands and PD-L1 Exhibit Improved Selectivity Towards Monocyte-derived Cells While Effective in Eliminating Acute Myeloid Leukaemia in Vivo. J. Cancer Res. Clin. Oncol. 2023, 149(12),10189-10201. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




