Submitted:
22 February 2025
Posted:
24 February 2025
You are already at the latest version
Abstract
Targeted drug delivery has become a miraculous tool in the new millennium of medicine to deliver the greatest therapeutic action, minimize systemic side effects, and attain site-specific drug activity. This review offers advances in liposomal, nanoparticle, and vesicular drug delivery systems with emphasis on their capacity for maximal bioavailability and regulation of drug release. There has been controversy over the use of some of these other carriers such as tablet formulations, proniosomes, and other age-new carriers. Introduction of nanotechnology with biodegradable items and use of artificial intelligence has added new dimensions to patient-specific medicine based on the patient's need.In spite of these developments, regulatory problems, biocompatibility, and scale-up manufacture are concerns that persist as barriers to universal clinical use. Some of the recent technologies such as 3D-printed pharmaceuticals, stimuli-responsive nanocarriers, and drug design using artificial intelligence have the potential to bypass these concerns. Future direction is also set here in the focus on the application of green drug delivery concepts, precision medicine, and combination regimens in setting the future of targeted drug delivery systems. Through ongoing research and technology development, these technologies are poised to revolutionize medicine by maximizing therapeutic effect, reducing side effects, and maximizing drug delivery.
Keywords:
1. Introduction to Drug Targeting Strategies
2. Liposomal and Vesicular Drug Delivery Systems
2.1. Liposomal Drug Delivery
| Feature | Benefit |
|---|---|
| PEGylation | Extends circulation time, prevents rapid clearance |
| Ligand Conjugation | Enables site-specific targeting using antibodies/peptides |
| Reduced Toxicity | Protects healthy tissues from high drug concentrations |
2.2. Proniosomes and Vesicular Systems
| Therapeutic Area | Application | Key Benefits | References |
|---|---|---|---|
| Cancer Therapy | Liposomal doxorubicin, paclitaxel formulations | Improved efficacy, reduced systemic toxicity | [10] |
| Infectious Diseases | Liposomal antibiotics, antifungal formulations | Enhanced drug penetration to infection sites | [11] |
| Neurological Disorders | Liposomes for Alzheimer’s, Parkinson’s treatment | Better blood-brain barrier penetration | [10,11] |
| Pulmonary Drug Delivery | Inhalable liposomes for asthma, tuberculosis | Increased lung retention time, targeted therapy | [12] |
3. Nanoparticle-Based Drug Delivery Systems
| Nanoparticle Type | Composition | Key Applications |
|---|---|---|
| Polymeric Nanoparticles | Biodegradable polymers (e.g., PLGA, chitosan) | Sustained drug release, improved drug stability |
| Metallic Nanoparticles | Gold, silver, iron oxide | Antibacterial, anticancer, bioimaging |
| Lipid Nanoparticles | SLNs, NLCs, liposomes | Enhanced solubility, improved stability, drug targeting |
| Quantum Dots & Magnetic NPs | Semiconductor materials, magnetic nanoparticles | Imaging, diagnostics, stimuli-responsive drug release |

3.1. Future Applications and Therapies
4. New Horizon for Formulation Strategy: Tablets, Proniosomes, Alternative Carriers
| Category | Description | Key Benefits | Reference |
|---|---|---|---|
| Tablet-Based Drug Delivery | Includes chewable, effervescent, and mouth-dissolving tablets | Improved patient compliance, rapid drug absorption | [19] |
| Proniosomes & Vesicular Carriers | Stable precursors to niosomes for enhanced drug encapsulation and release | Increased bioavailability, controlled drug release | [20] |
| Nanoemulsions | Oil-in-water or water-in-oil dispersions for solubility enhancement | Better drug solubility and permeability | [21] |
| Lipospheres & SLNs | Lipid-based nanoparticles for targeted drug delivery | Reduced systemic toxicity, improved drug targeting | [21] |
| Hybrid Nanocarriers | Combination of multiple delivery mechanisms | Enhanced drug loading and sustained release | [21] |
5. Personalized Medicine and Smart Drug Delivery: AI, Nanotechnology, and Biodegradable Innovations
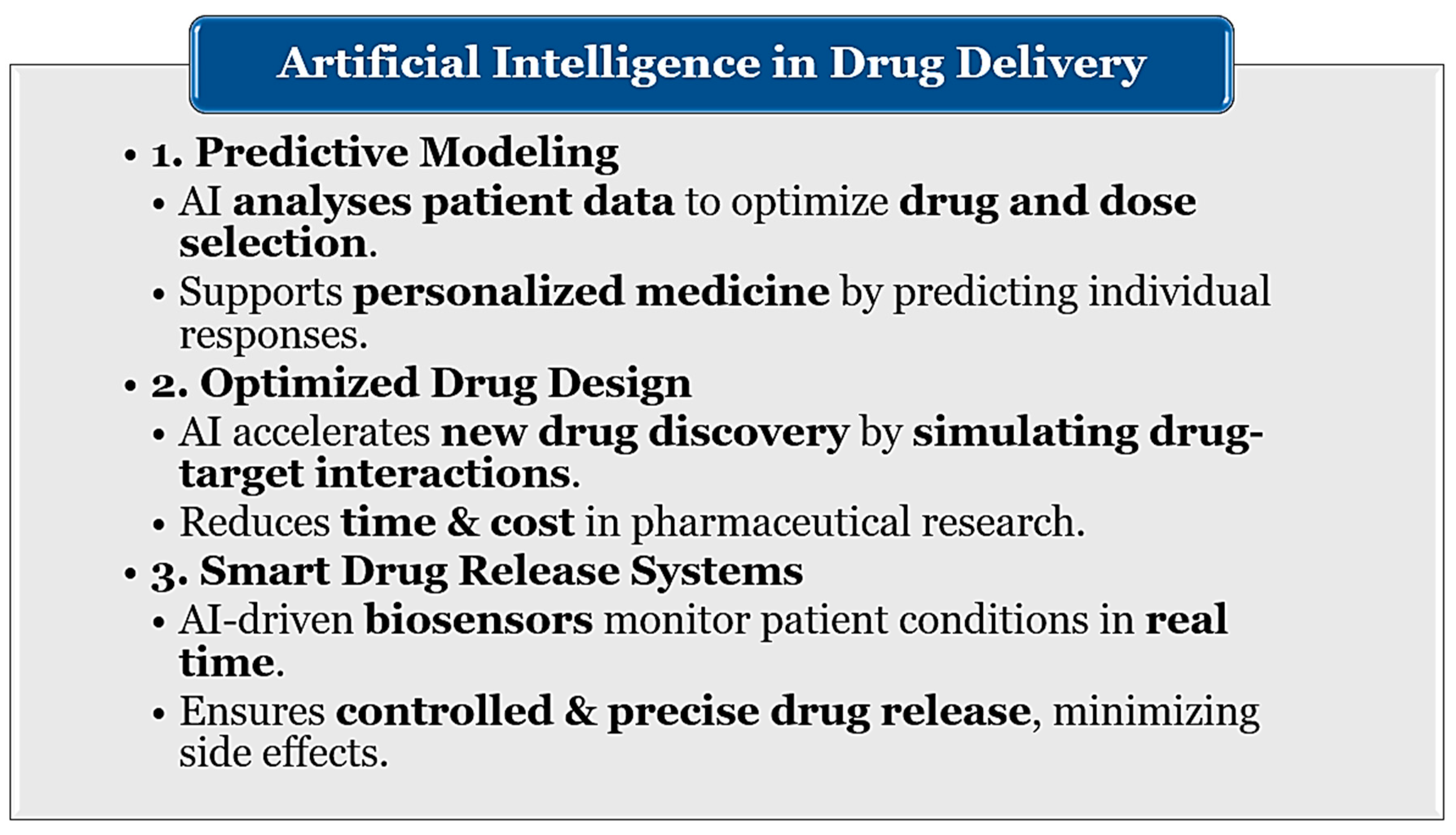
| Category | Description | Key Benefits |
|---|---|---|
| Nano-Carriers for Cancer Therapy | Targeted drug delivery using nanoparticles | Reduced toxicity, enhanced efficacy |
| Liposomes & Polymeric Nanoparticles | Controlled drug release carriers | Improved drug retention, prolonged action |
| Nanosensors in Diagnostics | Nanotechnology-based biosensors for early disease detection | Personalized treatment, early intervention |
| Biodegradable Drug Carriers | Sustainable polymer-based delivery systems | Eco-friendly, controlled release, minimal side effects |
| Hydrogel-Based Carriers | Smart hydrogels for drug encapsulation and release | Responsive drug delivery, regenerative medicine applications |
6. Future Perspectives and Challenges in Targeted Drug Delivery
| Category | Description | Key Benefits/Challenges |
|---|---|---|
| Smart Nanocarriers | Stimuli-responsive carriers release drugs based on pH, temperature, or enzymes | Precise drug targeting, controlled release, minimal side effects |
| Gene & Cell-Based Therapies | CRISPR and cell therapies for genetic disorders and cancer | Requires stable and specific delivery systems |
| 3D-Printed Drug Formulations | Personalized medicine with custom drug dosing and combinations | Improved patient adherence and treatment outcomes |
| Biocompatibility & Toxicity Concerns | Some nanocarriers pose toxicity risks | Requires extensive safety evaluations |
| Scalability & Manufacturing | High production costs and complex fabrication methods | Challenges in transitioning from lab to large-scale production |
| Regulatory Hurdles | Novel systems require strict testing and approval processes | Prolonged development timelines |
| Patient-Specific Variability | Individual differences impact drug response | AI-driven models needed for optimized treatments |
7. Conclusion
References
- Sengar, A. (2023). Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews, 4(8), 1379-1384. ISSN 2582-7421.
- Jagrati, K. M., & Sengar, A. (2024). Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research, 13(13), 1155-1169. [CrossRef]
- Prajapati, R. N., Jagrati, K., Sengar, A., & Prajapati, S. K. (2024). Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research, 13(13), 1243-1262.
- Sengar, A., Jagrati, K., & Khatri, S. (2024). Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research, 13(13), 1112-1140.
- Sengar, A., Vashisth, H., Chatekar, V. K., Gupta, B., Thange, A. R., & Jillella, M. S. R. S. N. (2024). From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research, 13(16), 176-189.
- Sengar, A., Yadav, S., & Niranjan, S. K. (2024). Formulation and evaluation of mouth-dissolving films of propranolol hydrochloride. World Journal of Pharmaceutical Research, 13(16), 850-861.
- Sengar, A., Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13(18), 1424-1435.
- Sengar, A., Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063-1071.
- Sengar, A. (2024). Liposomes and Beyond: Pioneering Vesicular Systems for Drug Delivery. Preprints. [CrossRef]
- Sengar, A. (2024). Precision in Practice: Nanotechnology and Targeted Therapies for Personalized Care. International Journal of Advanced Nano Computing and Analytics, 3(2), 56-67.
- Sengar, A. (2025). Liposomal Drug Delivery Systems: An Intro as a Primer for Advanced Therapeutics. Preprints. [CrossRef]
- Sengar, A. (2025). Innovations and Mechanisms in Liposomal Drug Delivery: A Comprehensive Introduction. Preprints. [CrossRef]
- Sengar, A., Chatekar, V. K., Andhare, S. B., & Sharma, L. (2025). Clinical pharmacology of antibiotics: Mechanisms, therapeutic uses, and resistance patterns. World Journal of Pharmaceutical Research, 14(1), 1531–1545. [CrossRef]
- Sengar, A. (2025). Targeting Strategies in Liposomal Drug Delivery. Preprints. [CrossRef]
- Sengar, A. et al. (2025). Advancing urolithiasis treatment through herbal medicine: A focus on Tribulus terrestris fruits. World Journal of Pharmaceutical Research, 14(2), 91–105.
- Sengar, A. (2025). Next-Generation Liposomal Drug Delivery Systems: Core Principles, Innovations, and Targeting Strategies. Preprints. [CrossRef]
- Sengar, A. (2025). Advanced Targeting Strategies and Applications of Liposomal Drug Delivery Systems. Preprints. [CrossRef]
- Sengar, A. (2025). Innovations and Mechanisms in Liposomal Drug Delivery: A Comprehensive Introduction. J Emerg Med OA, 3(1), 01-05.
- Sengar, A. (2025). Historical Evolution and Modern Advances in Vesicular Nanocarriers. Preprints. [CrossRef]
- Sengar, A. (2025). The Interplay of Drug Delivery Systems: A Comparative Study of Nanocarriers and Vesicular Formulations. Preprints. [CrossRef]
- Sengar, A. (2025). Innovations in Drug Delivery and Advanced Therapeutics. Preprints. [CrossRef]
- Sengar, A. (2025). Personalized Medicine and Nanotechnology: Transforming Modern Therapeutics. Preprints. [CrossRef]
- Sengar, A. (2025). Advancements in Drug Delivery Systems: Targeting Strategies, Nanotechnology, and Vesicular Innovations. Preprints. [CrossRef]
- Sengar, A. (2025). Precision in Practice: Nanotechnology and Targeted Therapies for Personalized Care. Preprints.
- Sengar, A. (2025). Next-Gen Drug Delivery: Redefining Precision, Bioavailability, and Therapeutic Outcomes. Preprints. [CrossRef]
- SENGAR, A. (2025). Smart Drug Delivery: AI, Nanotech & Future Innovations. Preprints. [CrossRef]
- Ashutosh, S. (2025). Sustainable Drug Delivery Systems with Biodegradable Innovations for Targeted and Efficient Therapy. Preprints. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




