Submitted:
22 February 2025
Posted:
24 February 2025
You are already at the latest version
Abstract
The consequences of chronic sleep deprivation include memory deficits and gastrointestinal dysfunction. Studies suggest that gut microbiota plays a causal role in cognitive impairment induced by chronic sleep deprivation, but the working mechanism of the microbiota-gut-brain axis remains unclear. In this study, a chronic sleep deprivation cognitive impairment model was established by sleep deprivation instrument, and Weizmannia coagulans BC99 was given by gavage for 4 weeks. BC99 improved cognitive abnormalities in novel object recognition tests induced by chronic sleep deprivation and showed behavior related to spatial memory in the Morris water maze test. W. coagulans BC99 reduced the heart mass index of sleep-deprived mice, increased the sleep-related neurotransmitters 5-HT and DA, decreased corticosterone and norepinephrine, and increased alpha diversity and community similarity. It reduced the abundance of harmful bacteria such as Olsenella, increased the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium, and promoted the production of short-chain fatty acids (SCFAs). W. coagulansBC99 also inhibits LPS translocation and the elevation of peripheral inflammatory factors by maintaining the integrity of the intestinal barrier and inhibiting the expression of the NLRP3 signaling pathway in the jejunum, thereby inhibiting NLRP3 inflammasome in the brain of mice and reducing inflammatory factors in the brain, providing a favorable environment for the recovery of cognitive function. The present study confirmed that W. coagulans BC99 ameliorated cognitive impairment in chronic sleep-deprived mice by improving gut microbiota, especially by promoting SCFAs production and inhibiting the NLRP3 signaling pathway in the jejunum and brain. These findings may help guide the treatment of insomnia or other sleep disorders through dietary strategies.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.1.1. Preparation of Probiotics Suspension
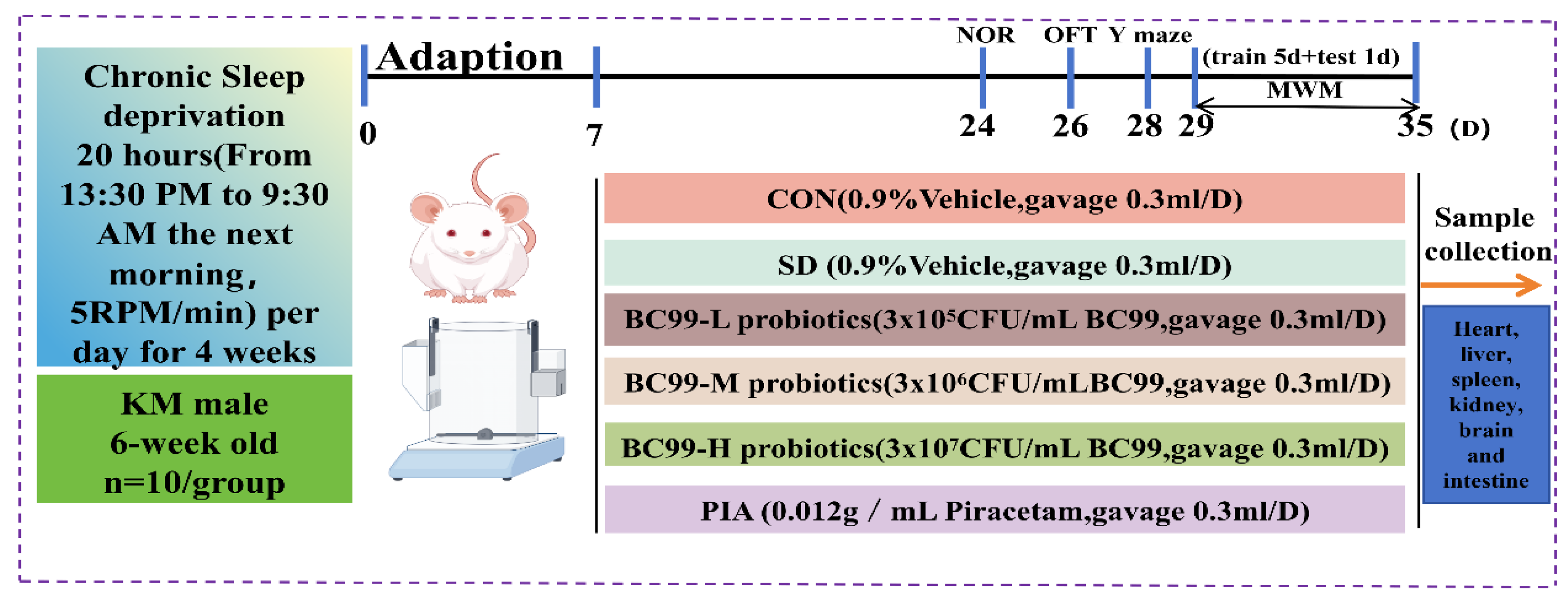
2.2. Design of Animal Experiments
2.3. Chronic Sleep Deprivation
2.4. Probiotic Therapy
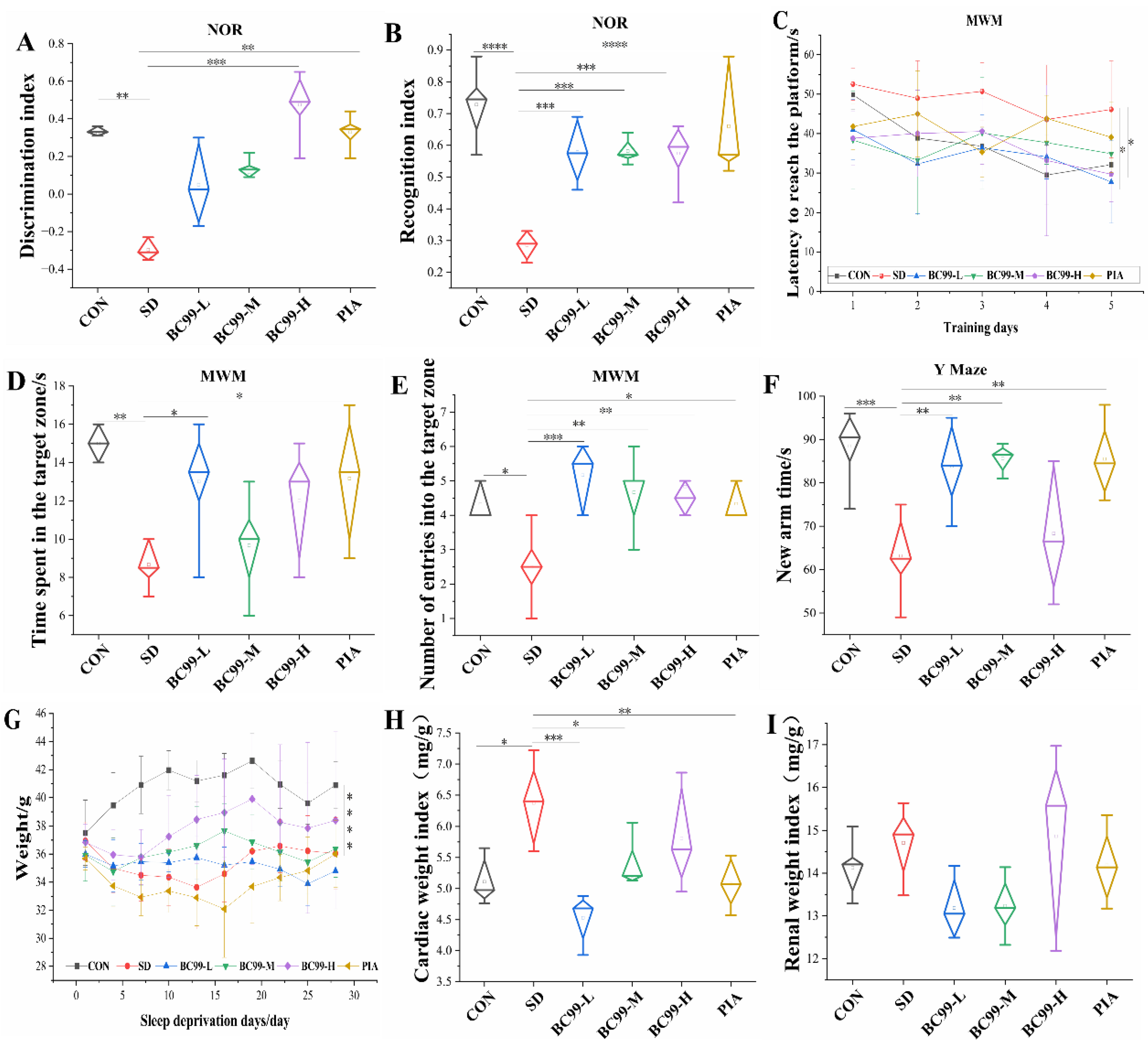
2.5. Morris Water Maze (MWM) Test
2.6. Novel Object Recognition Test
2.7. Measurement of Plasma Inflammatory Response
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Assessment of Oxidative Stress in Brain Tissue
2.10. Measurement of Fecal SCFAs
2.11. Real-Time Fluorescence Quantitative PCR Reaction
2.12. 16S rRNA Microbiota Assessment and Bioinformatics
2.13. Statistical Analysis
3. Results
3.1. Effects of W. coagulans BC99 on Behavioral and Physiological Indices of Chronic Sleep Deprivation Mice
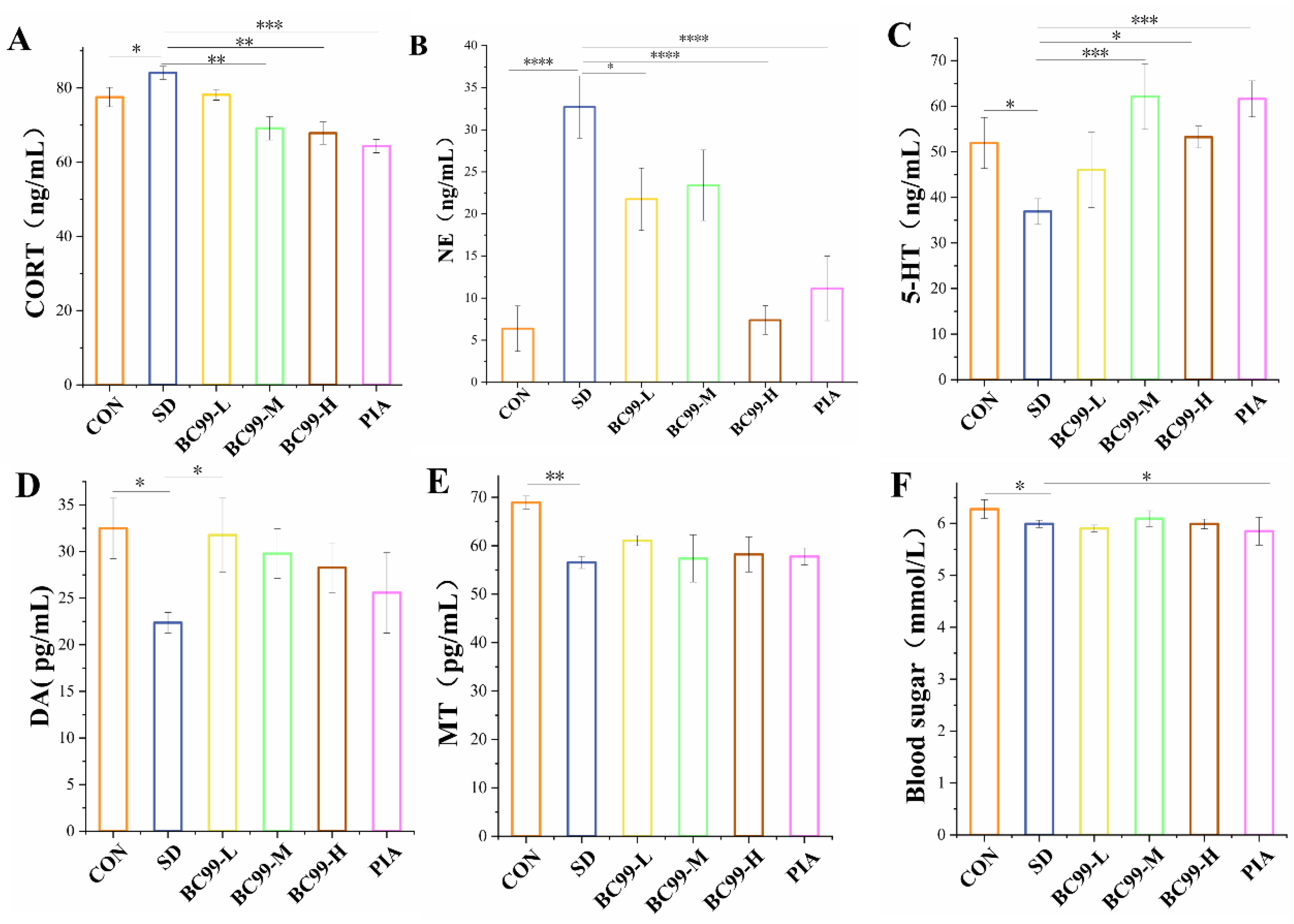
3.2. Effect of W. coagulans BC99 on Biochemical Indices in Chronic Sleep Deprivation Mice
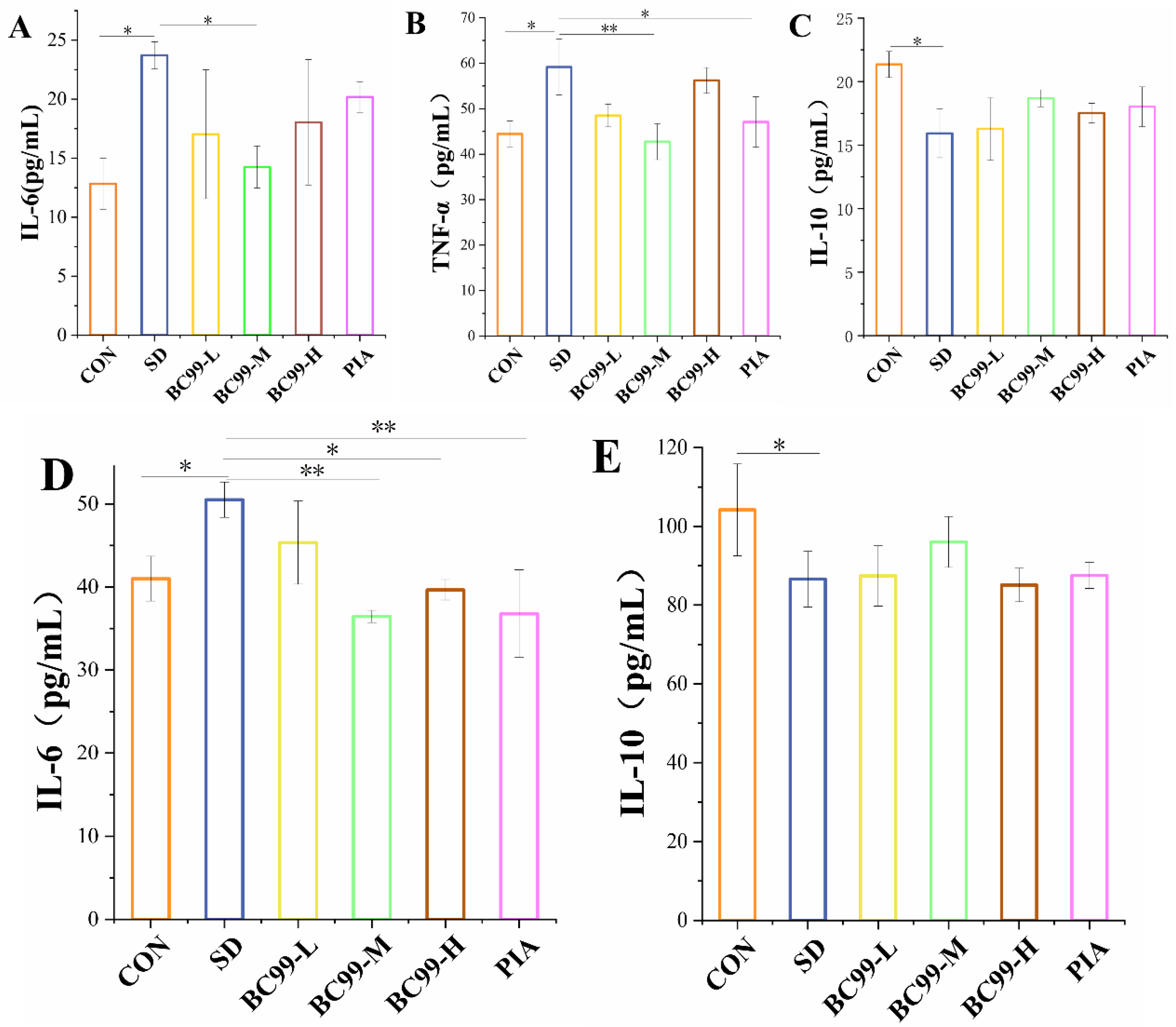
3.3. Effect of W. coagulans BC99 on Brain and Plasma Inflammation in Chronic Sleep Deprivation Mice
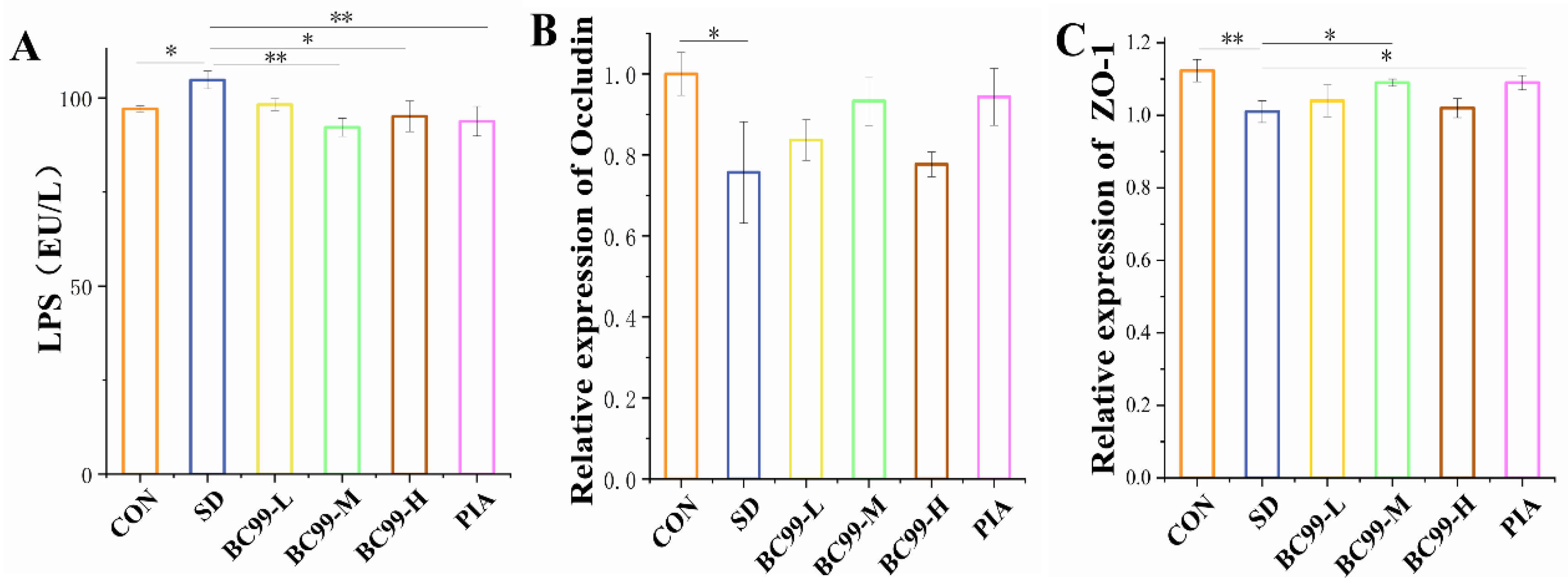
3.4. Effect of W. coagulans BC99 on Intestinal Barrier in Chronic Sleep Deprivation Mice
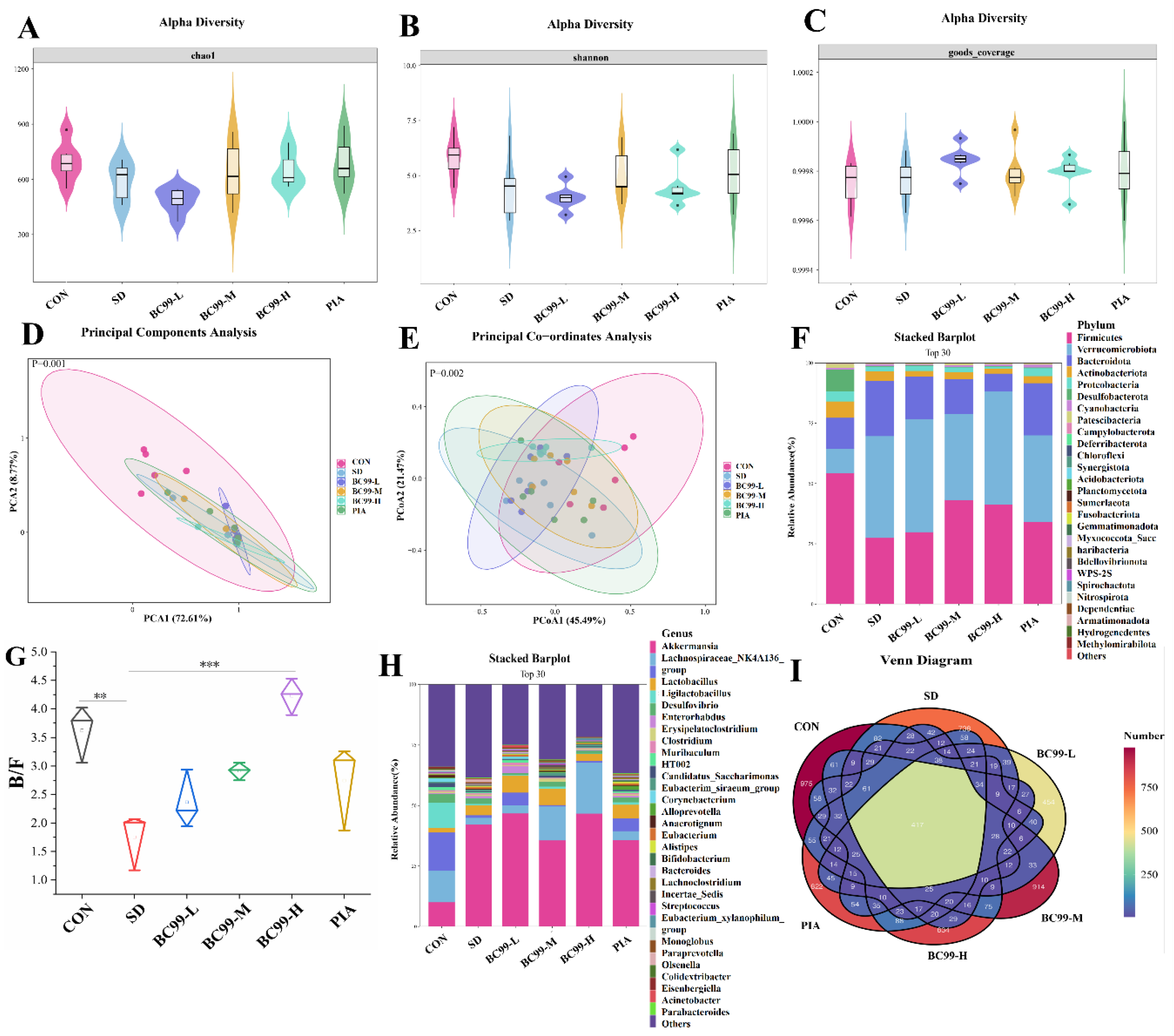
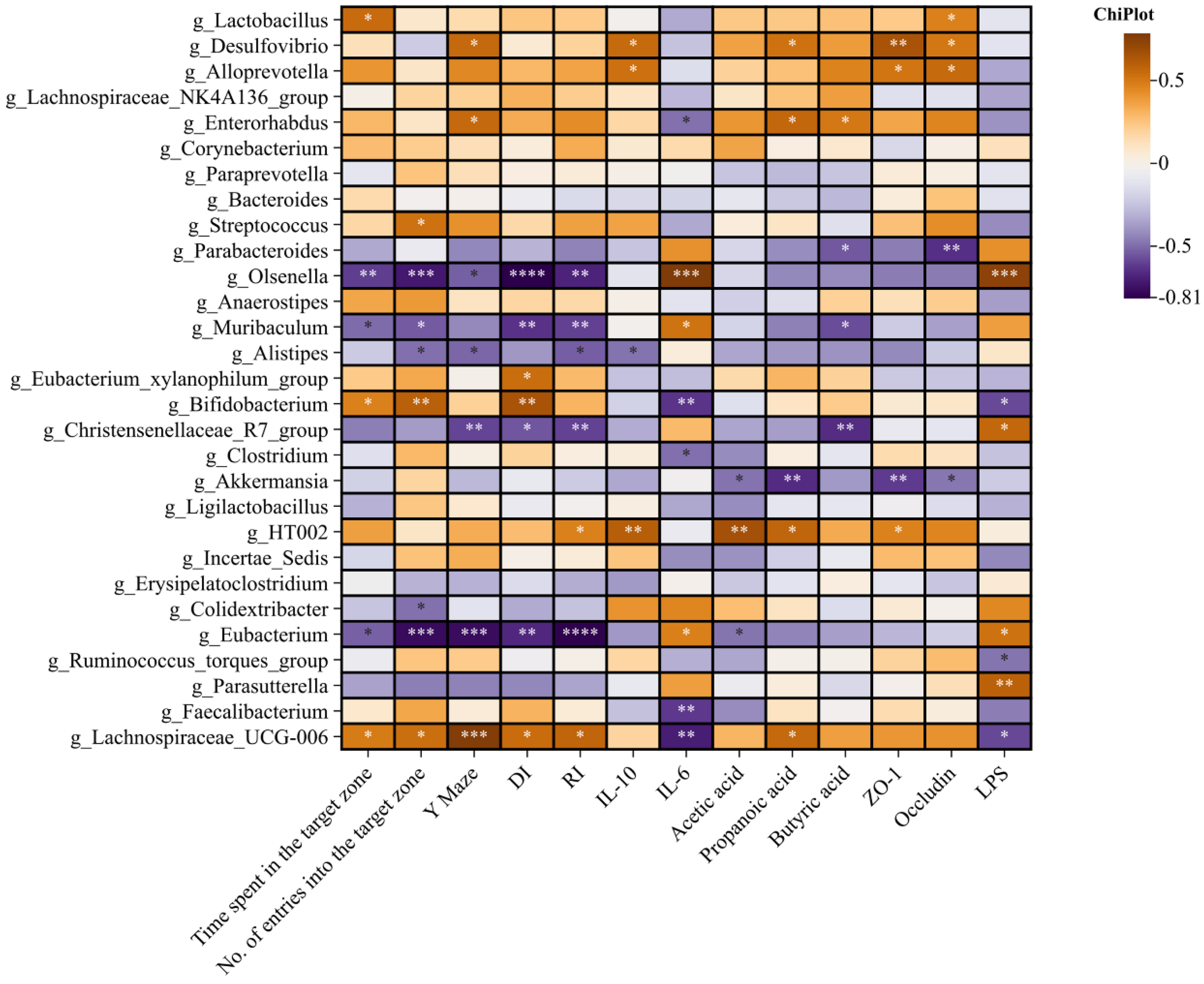
3.5. Effects of BC99 on Intestinal Flora in Chronic Sleep Deprivation Mice
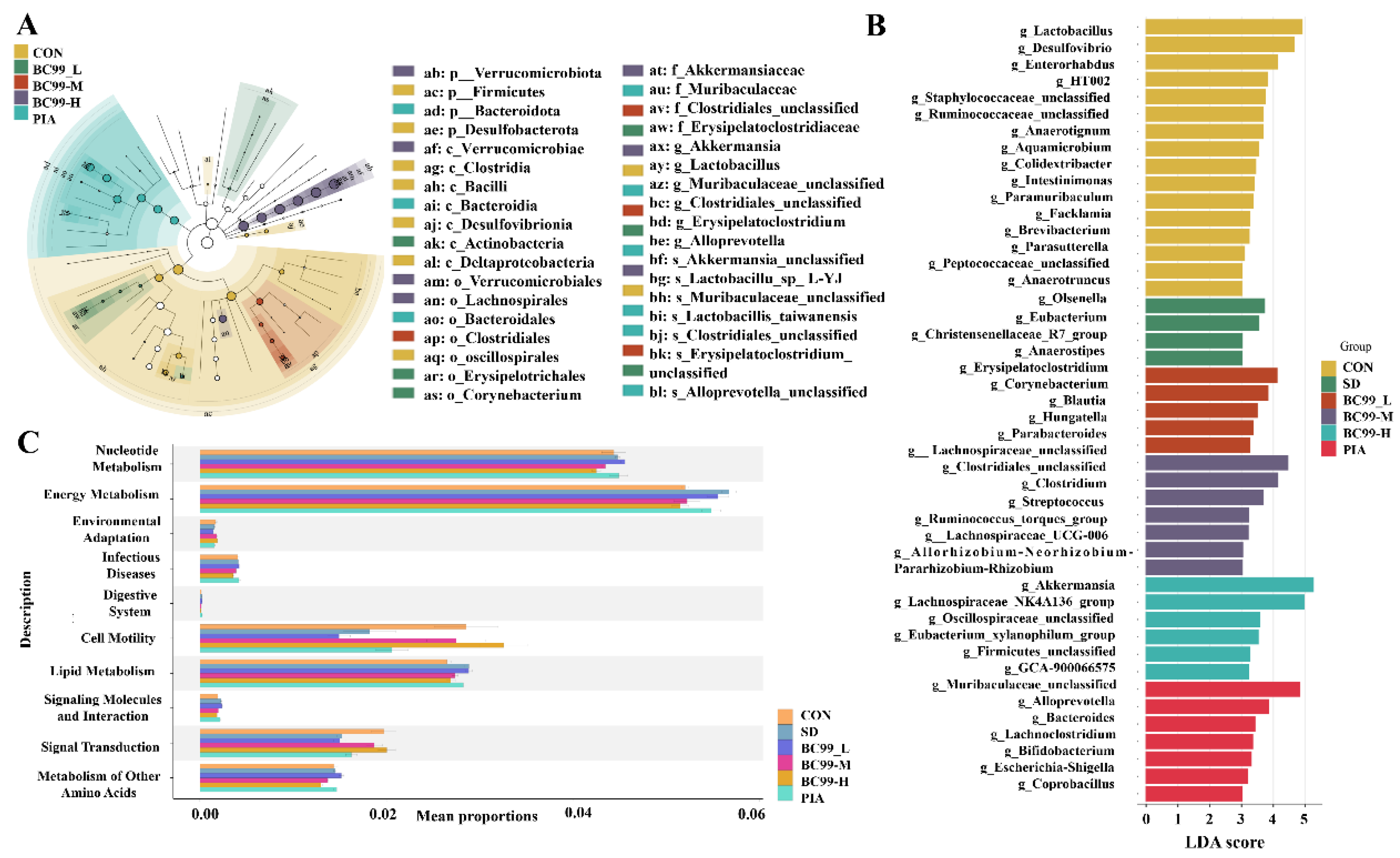
3.6. Differential Effects of W. coagulans BC99 on LEfSe and Prediction of PICRUSt2 Function in Chronic sleep Deprivation Mice
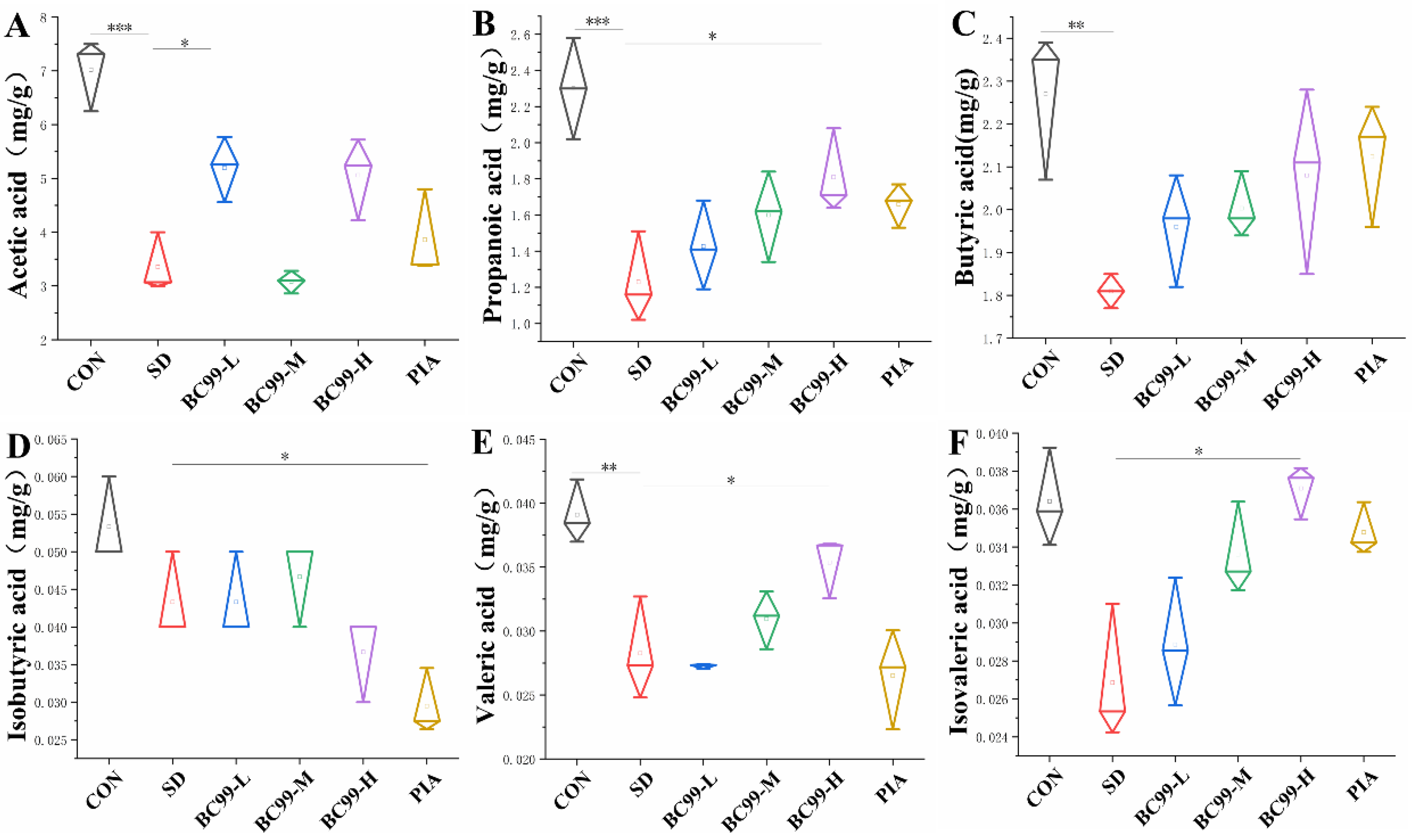
3.7. Effects of W. coagulans BC99 on Short-Chain Fatty Acids in Chronic Sleep Deprivation Mice
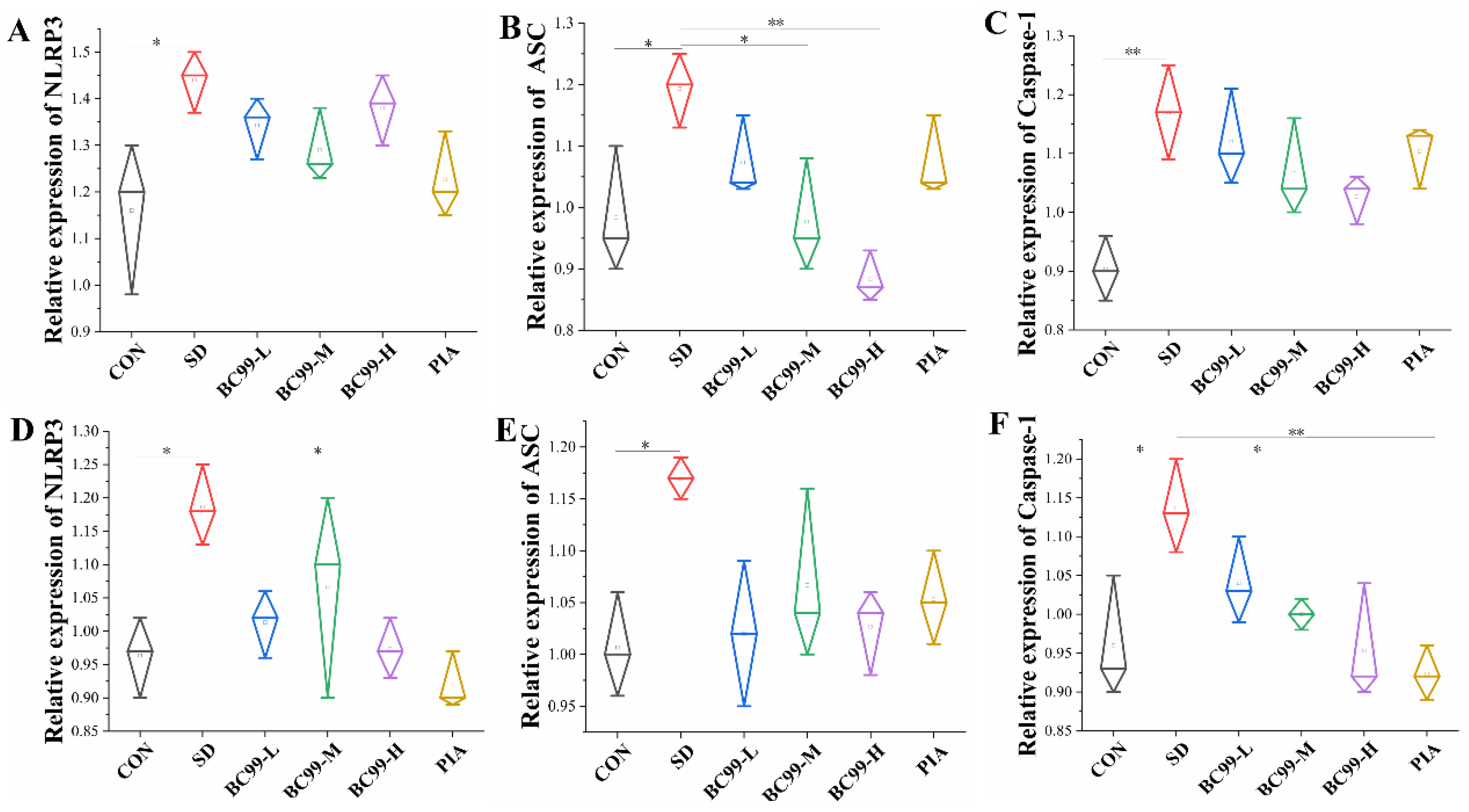
3.8. Effect of W. coagulans BC99 on NLRP3 Inflammasome Signaling Pathway in the Brain and Jejunum of Chronic Sleep Deprivation Mice
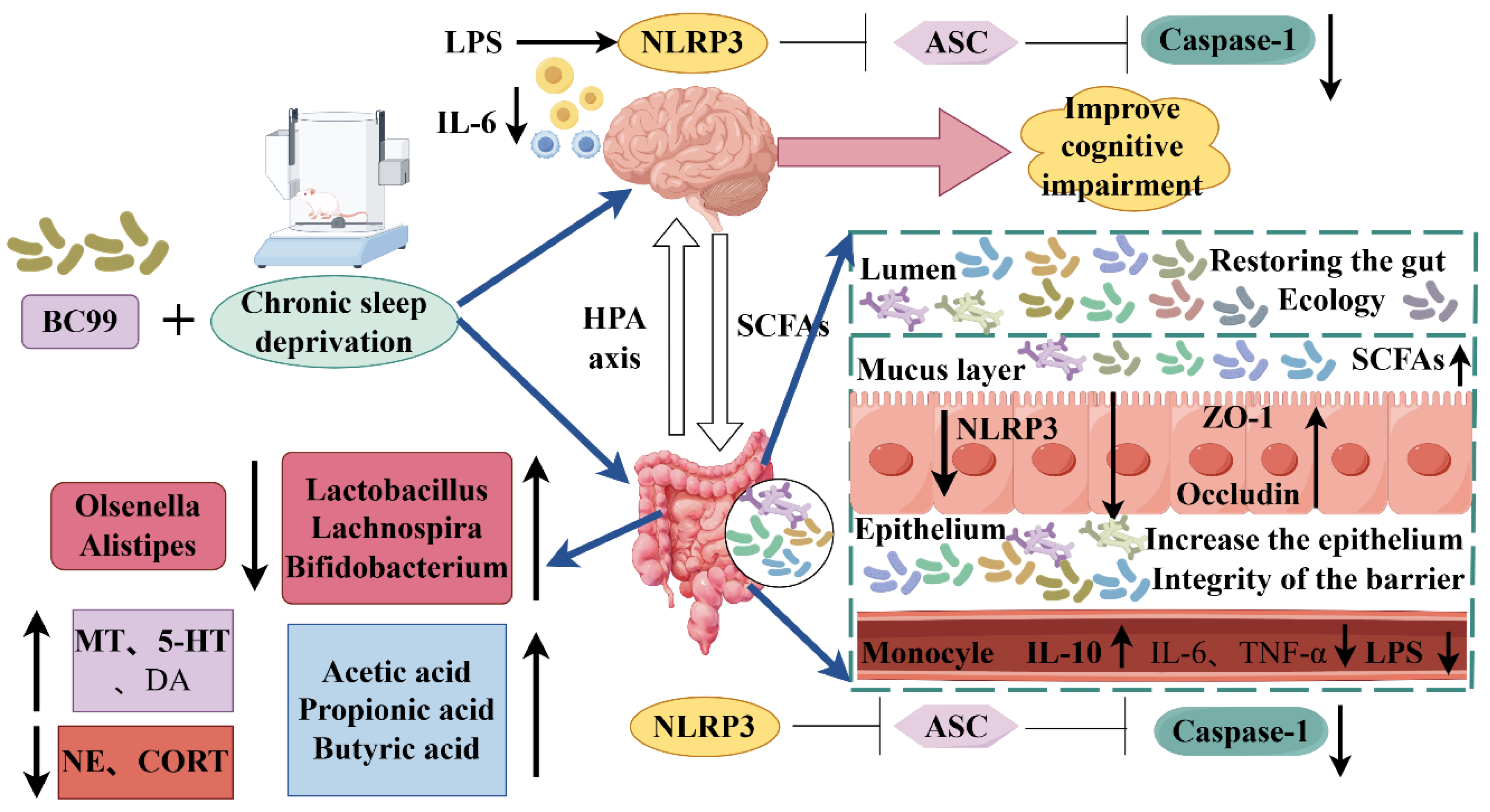
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; Baglioni, C. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; McIntyre, R.S. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affect Disord. 2020, 277, 55–64. [Google Scholar] [PubMed]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. 2016, 24, 1175–1186. [Google Scholar]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019, 16, 246–259. [Google Scholar]
- Wong, L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; Rogers, G.B.; Licinio, J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016, 21, 797–805. [Google Scholar]
- An, Q.; Li, C.; Chen, Y.; Yang, Y.; Song, R.; Zhou, L.; Li, J.; Tong, A.; Luo, Y. Scaffold hopping of agomelatine leads to enhanced antidepressant effects by modulation of gut microbiota and host immune responses. Pharmacol Biochem Behav. 2020, 192, 172910. [Google Scholar]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; Jie, Z.; Zhao, B.; Xiao, L.; Yang, L.; Zhang, T.; Liu, B.; Guo, L.; He, X.; Chen, Y.; Chen, C.; Gao, C.; Xu, X.; Yang, H.; Wang, J.; Dang, Y.; Madsen, L.; Brix, S.; Kristiansen, K.; Jia, H.; Ma, X. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020, 25, 2905–2918. [Google Scholar]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.Y.; Wang, F.; Qu, H.; Zhang, M.; Luo, T.; Wang, H.Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice. Sci Rep. 2019, 20, 19575. [Google Scholar]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016, 31, 283–93. [Google Scholar]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011, 3, 858–76. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019, 16, 461–78. [Google Scholar] [CrossRef]
- Raparelli, V.; Basili, S.; Carnevale, R.; Napoleone, L.; Del Ben, M.; Nocella, C.; Bartimoccia, S.; Lucidi, C.; Talerico, G.; Riggio, O.; Violi, F. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017, 65, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; Farré, R.; Chang, E.B.; Gozal, D. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci Rep. 2016, 14, 35405. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods. 2024, 28, 3851. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Zhao, Y.; Jin, Q.; Li, S. Protective Effects of Bacillus coagulans JA845 against D-Galatose/AlCl3Iduced Cognitive Decline, Oxidative Stress and Neuroinflammation. J Microbiol Biotechnol, 2022, 28, 32. [Google Scholar]
- Liu, Y.; Lang, H.; Zhou, M.; Huang, L.; Hui, S.; Wang, X.; Chen, K.; Mi, M. Pterostil bene improves exercise intolerance induced by sleep restriction in mice by activating AMPK/SIRT1 signaling and promoting mitochondrial biosynthesis. Journal of Third Military Medical University, 2020, 42, 259–266. [Google Scholar]
- Qi, L.; Cheng, Y.; Sun, S.; Wan, H. The administration of rhBmal1 reduces sleep deprivation-induced anxiety and cognitive impairment in mice. World J Biol Psychiatry. 2024, 25, 43–53. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; Ritz, N.L.; Jaggar, M.; Cowan, C.S.M.; Crispie, F.; Donoso, F.; Halitzki, E.; Neto, M.C.; Sichetti, M.; Golubeva, A.V.; Fitzgerald, R.S.; Claesson, M.J.; Cotter, P.D.; O'Leary, O.F.; Dinan, T.G.; Cryan, J.F. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021, 1, 666–676. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Arnsten, A.F. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000, 7, 133–46. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Steere, J.C.; Hunt, R.D. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996, 53, 448–55. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.P.; Arnsten, A.F. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007, 113, 523–36. [Google Scholar] [CrossRef] [PubMed]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C. Hippocampal 5-HT1A Receptor and Spatial Learning and Memory. Front Pharmacol. 2015, 10, 289. [Google Scholar] [CrossRef]
- Meunie, C.N.; Chameau, P.; Fossier, P.M. Modulation of Synaptic Plasticity in the Cortex Needs to Understand All the Players. Front Synaptic Neurosci. 2017, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Barajas, C.; Coronel, I.; Florán, B. . Dopamine Receptors and Neurodegeneration. Aging and Disease 2015, 6, 349–368. [Google Scholar]
- Seamans, J.K.; Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004, 74, 1–58. [Google Scholar] [CrossRef]
- Li, Y.C.; Gao, W.J. GSK-3β activity and hyperdopamine-dependent behaviors. Neurosci Biobehav Rev. 2011, 35, 645–54. [Google Scholar] [CrossRef]
- Masoud, S.T.; Vecchio, L.M.; Bergeron, Y.; Hossain, M.M.; Nguyen, L.T.; Bermejo, M.K.; Kile, B.; Sotnikova, T.D.; Siesser, W.B.; Gainetdinov, R.R.; Wightman, R.M.; Caron, M.G.; Richardson, J.R.; Miller, G.W.; Ramsey, A.J.; Cyr, M.; Salahpour, A. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol Dis. 2015, 74, 66–75. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; Liu, G.P. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer's disease. J Pineal Res. 2021, 71, 12774. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.S.; Zortea, M.; Souza, A.; Santos, V.; Biazús, J.V.; Torres, I.L.S.; Fregni, F.; Caumo, W. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: A randomized, double-blind, placebo-controlled trial. PLoS One. 2020, 17, e0231379. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Bakker, A.C.; Vanhaesebroeck, B.; Beyaert, R.; Jacob, W.A.; Fiers, W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992, 267, 5317–23. [Google Scholar] [CrossRef]
- Ingiosi, A.M. Opp, M.R.; Krueger, J.M. Sleep and immune function: glial contributions and consequences of aging. Curr Opin Neurobiol. 2013, 23, 806–11. [Google Scholar] [CrossRef]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Kumari, P.; Chauhan, G.; Roy, K.; Alam, S.; Kishore, K.; Ray, K.; Panjwani, U. Sleep deprivation induces spatial memory impairment by altered hippocampus neuroinflammatory responses and glial cells activation in rats. J Neuroimmunol. 2017, 15, 38–48. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A. ; Mc. Carley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci Lett. 2014, 19, 27–31. [Google Scholar]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 05–817. [Google Scholar] [CrossRef]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011, 26, 187–97. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010 35, 870–80. [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019 16, 461–478. [CrossRef]
- Monti, B.; Gatta, V.; Piretti, F.; Raffaelli, S.S.; Virgili, M.; Contestabile, A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson's disease: involvement of alpha-synuclein. Neurotox Res. 2010, 17, 130–41. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res. 2020, 1, 111772. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. ; The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019, 16, 461–78. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.G.; Takeo Sato, F.; Curi, R.; Vinolo, M.A.R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur J Pharmacol, 2016, 785, 50–8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Van Esch, B.C.; Henricks, P.A.J.; Garssen, J.; Folkerts, G. Time and Concentration Dependent Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α- Induced Endothelial Activation. Front Pharmacol. 2018, 9, 233. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016, 5, e73. [Google Scholar] [CrossRef]
- Baldwin, A.G.; Brough, D.; Freeman, S. Inhibiting the inflammasome: a chemical perspective. J Med Chem, 2016, 59, 1691–1710. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Inflammasome involvement in Alzheimer's disease. J Alzheimers Dis, 2016, 54, 45–53. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol, 2014, 14, 463–77. [Google Scholar] [CrossRef]
- Marcellino, D.; Suárez-Boomgaard, D.; Sánchez-Reina, M.D.; Aguirre, J.A.; Yoshitake, T.; Yoshitake, S.; Hagman, B.; Kehr, J.; Agnati, L.F.; Fuxe, K.; Rivera, A. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson's disease: studies with the P2X(7) receptor antagonist A-438079. J Neural Transm (Vienna). 2010, 117, 681–7. [Google Scholar] [CrossRef]
- Jiang, J.; Pan, H.; Shen, F.; Tan, Y.; Chen, S. Ketogenic Diet Alleviates Cognitive Dysfunction and Neuroinflammation in APP/PS1 Mice via the Nrf2/HO-1 and NF-κB Signaling Pathways. Neural Regeneration Research. 2023, 18, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; Shi, L.; Meng, S.Q.; Xue, Y.X.; Xie, W.; Shi, J.; Yan, W.; Wei, H.; Lu, L.; Han, Y. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Cordeira, J.; Kolluru, S.S.; Rosenblatt, H.; Kry, J.; Strecker, R.E.; McCarley, R.W. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res. 2018, 26, 124–129. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology. 2022, 137, 105641. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Diling, C.; Jian, Y.; Ting, L.; Guoyan, H.; Hualun, L.; et al. Effects of Oligosaccharides From Morinda Officinalis on Gut Microbiota and Metabolome of APP/PS1 Transgenic Mice. Front Neurol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.Y.; Wang, F.; Qu, Y.H.; Zhang, M.; Luo, T.; Wang, H.Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice. Sci Rep. 2019, 20, 19575. [Google Scholar] [CrossRef]
- Milner, M.T.; Maddugoda, M.; Götz, J.; Burgener, S.S. ; Schroder, K, The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer's disease. Curr Opin Immunol. 2021, 68, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H.; 2019. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome, 2019, 7, 116. [Google Scholar] [CrossRef]
| Gene | Primer sequence |
|---|---|
| Occludin | F: CTCGGTACAGCAGCAATGGT |
| R: TCATAGTGGTCAGGGTCCGT | |
| ZO-1 | F: ATTCAGGTCGCTCGCATGAC |
| R: ACTGCGTGGAATGATCGGAG | |
| NLRP3 | F: CCAGGAGTTCTTTGCGGCTA |
| R: GCCTTTTTCGAACTTGCCGT | |
| ASC | F: AGACCACCAGCCAAGACAAG |
| R: CTCCAGGTCCATCACCAAGT | |
| Caspase-1 | F: AACCACTCGTACACGTCTTGCC |
| R: CCAGATCCTCCAGCAGCAACTT | |
| β-Actin | F: CTGTGTTTTGGTCTTACGGTAC |
| R: AAAAAGCCTGTCTGTGATTCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





