Submitted:
18 February 2025
Posted:
20 February 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Study Site, Sourcing and Processing of Test Materials
Experimental Diets and Design
Experimental Animals, Design and Management
Carcass Analysis
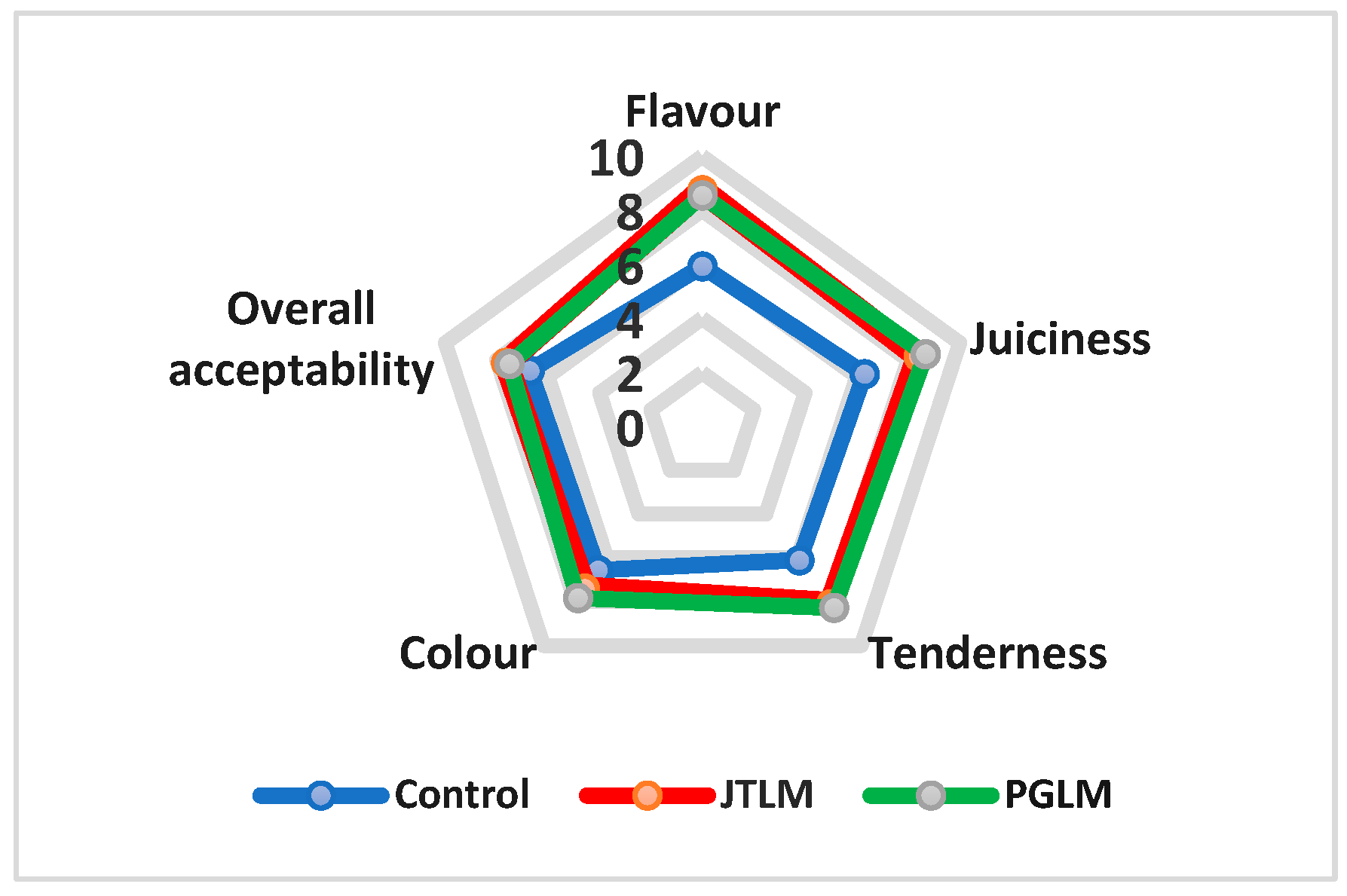
Sensory Evaluation
Cooking Loss
Thermal Shortening
Water Holding Capacity
Statistical Analysis
Results and Discussion
Carcass Traits of Pigs Fed Jathropha Tanjorensis and Psidium Guajava Leaf Meal
Physicochemical Properties of Pork from Pigs Fed Jathropha Tanjorensis and Psidium Guajava Leaf Meal
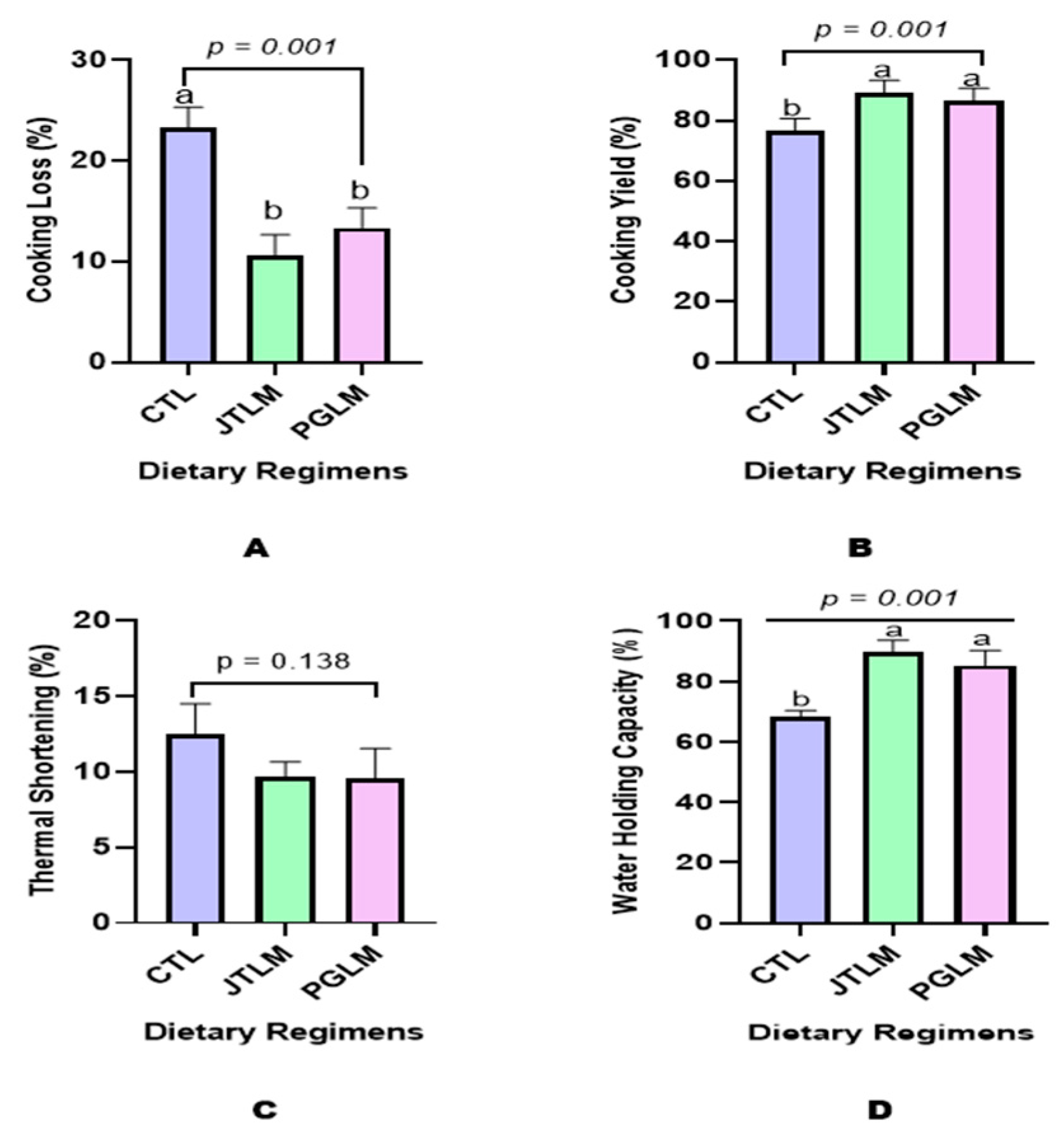
Cooking Loss
Cooking Yield
Thermal Shortening
Water-Holding Capacity
Conclusion
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Nakrachata-Amon, T.; Vorasayan, J.; Pitiruek, K.; Arunyanart, S.; Niyamosoth, T.; Pathumnakul, S. Optimizing vertically integrated pork production supply chain: A Lagrangian heuristic approach. Heliyon, 2024, 10(6), e26407. [CrossRef]
- Yang, P.; Yu, M.; Ma, X.; Deng, D. Carbon Footprint of the Pork Product Chain and Recent Advancements in Mitigation Strategies. Foods (Basel, Switzerland), 2023, 12(23). [CrossRef]
- Lin-Schilstra, L.; Backus, G.; Snoek, H.; Mörlein, D. Consumers’ view on pork: Consumption motives and production preferences in ten European Union and four non-European Union countries. Meat Science, 2022, 187, 108736. [CrossRef]
- Ekpo, J.S.; Okon, U.M. Effects of multiple spices and processing methods on the organoleptic quality of pork. Acta Periodica Technologica, 2025. [CrossRef]
- Costa, R.G.; Ribeiro, N.L.; Nobre, P.T.; Carvalho, F.F. R.; Medeiros, A.N.; Cruz, G.R. B.; Freire, L.F. S. Biochemical and hormonal parameters of lambs using guava (Psidium guajava L.) agro-industrial waste in the diet. Tropical Animal Health and Production, 2018, 50(1), 217–221. [CrossRef]
- Ekpo, J.S.; Okon, U.M. Organoleptic Quality, Pork Characterization and Hematological Indices of Growing Pigs Fed Supplemental Diets Containing Bitter Leaf and Hospital Too Far. AKSU Journal of Agriculture and Food Science, 2023, 7(1), 22–33.
- Nwachukwu, C.U.; Aliyu, K.I.; Ewuola, E.O. Growth indices, intestinal histomorphology, and blood profile of rabbits fed probiotics- and prebiotics-supplemented diets. Translational Animal Science, 2021, 5(3). [CrossRef]
- Khalifa, I.; Barakat, H. Influencing of Guava Processing Residues Incorporation on Cupcake Characterization. Journal of Nutrition & Food Sciences, 2016, 6(4). [CrossRef]
- Batista, P.F.; Lima, M.A. C.; de Alves, R.E.; Façanha, R.V. Bioactive compounds and antioxidant activity in tropical fruits grown in the lower-middle São Francisco Valley. Revista Ciência Agronômica, 2018, 49(4). [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Research International, 2018, 106, 1095–1104. [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A. I. A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; Lorenzo, J.M. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Research International, 2018, 114, 55–63. [CrossRef]
- Luciano, G.; Vasta, V.; Monahan, F.J.; López-Andrés, P.; Biondi, L.; Lanza, M.; Priolo, A. Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chemistry, 2011, 124(3), 1036–1042. [CrossRef]
- Ekpo, J.S.; Okon, U.M. Performance and lipid profile of growing pigs fed Vernonia amygdalina and Jathropha tanjorensis leaf meal supplementation, Livestock Research for Rural Development, 2022. https://www.lrrd.net/search.
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Ruminant Research, 2011, 101(1–3), 150–159. [CrossRef]
- Adeyemi, K.D.; Audu, S.; Oloke, J.A.; Oladiji, O.E.; Salawu, K.F.; Ahmed, R.A.; Sulaimon, R.O. Influence of Crescentia cujete and Launaea taraxacifolia leaves on growth, immune indices, gut microbiota, blood chemistry, carcass, and meat quality in broiler chickens. Tropical Animal Health and Production, 2021, 53(3), 365. [CrossRef]
- Wang, Q.; Wang, L.; Li, L.; Sun, M.; Li, P.; Yu, Y.; Zhang, Y.; Xu, Z.; Gao, P.; Ma, J.; Liu, X. Effects of dietary supplementation of fermented Artemisia argyi on growth performance, slaughter performance, and meat quality in broilers. Poultry Science, 2024, 103(4), 103545. [CrossRef]
- Okon, U.M.; Ekpo, J.S.; Essien, C.A.; Thabethe, F.; Nuamah, E. Serum lipid profile and organoleptic characteristics of meat from rabbits fed diets containing selim pepper (Xylopia aethiopica) and African nutmeg (Monodora myristica). Nigerian Journal of Animal Science. 2023, 25(2), 223-235.
- Ježek, F.; Kameník, J.; Macharáčková, B.; Bogdanovičová, K.; Bednář, J. Cooking of meat: Effect on texture, cooking loss and microbiological quality – a review. Acta Veterinaria Brno, 2019, 88(4), 487–496. [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, Y.; Yang, X.; Mao, Y.; Luo, X.; Hopkins, D.L.; Niu, L.; Liang, R. Sous vide cooking improved the physicochemical parameters of hot-boned bovine semimembranosus muscles. Meat Science, 2023, 206, 109326. [CrossRef]
- Irie, M.; Izumo, A.; Mohri, S. Rapid method for determining water-holding capacity in meat using video image analysis and simple formulae. Meat Science, 1996, 42(1), 95–102. [CrossRef]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chemistry, 2021, 357, 129727. [CrossRef]
- Ekpo, J.S.; Sam, I.M.; Okon, U.M. Performance, Carcass and Relative Organ Weights of Young Boars Fed Graded Dietary Levels of Raw Icacinia manni (Earth Ball). Journal of Agricultural Research and Development, 2021, 19(1). [CrossRef]
- Essien, C.; Sam, I.M.; Okon, U.M. Effect of Jatropha tanjeronsis leaf meal on performance, carcass and internal organ characteristics of broiler finisher chickens. 2023. https://www.researchgate.net/publication/375693443.
- Pla, M. Effect of nutrition and selection on meat quality proceeding, 8th world rabbit congress held at puebla, mexico 7th – 10th September 2004. Pp; 1337-1348.
- Nobre, P.T.; Munekata, P.E. S.; Costa, R.G.; Carvalho, F.R.; Ribeiro, N.L.; Queiroga, R.C. R. E.; Sousa, S.; da Silva, A.C. R.; Lorenzo, J.M. The impact of dietary supplementation with guava (Psidium guajava L.) agroindustrial waste on growth performance and meat quality of lambs. Meat Science, 2020, 164, 108105. [CrossRef]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between water-holding capacity and intramuscular fat content in Japanese commercial pork loin. Asian-Australasian Journal of Animal Sciences, 2018, 31(6), 914–918. [CrossRef]
- Tran, T.T. T.; Ton, N.M. N.; Nguyen, T.T.; Le, V.V. M.; Sajeev, D.; Schilling, M.W.; Dinh, T.T. N. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Science, 2020, 165, 108106. [CrossRef]
- Shen, Y.; Hong, S.; Du, Z.; Chao, M.; O’Quinn, T.; Li, Y. Effect of adding modified pea protein as functional extender on the physical and sensory properties of beef patties. LWT, 2022, 154, 112774. [CrossRef]
- Sahal, A.; Chaudhary, S.; Hussain, A.; Arora, S.; Dobhal, A.; Ahmad, W.; Kumar, V.; Kumar, S. A comprehensive review on the nutritional composition, bioactive potential, encapsulation techniques, and food system applications of guava (Psidium guajava L.) leaves. Grain & Oil Science and Technology. 2024. [CrossRef]
- Ayanwale, B.A.; Aya, V.E. Nutritional Evaluation of Cornflakes Waste in Diets for Broilers. Pakistan Journal of Nutrition, 2006, 5: 485-489.
- Zhang, M.; Guo, Y.; Su, R.; Corazzin, M.; Hou, R.; Xie, J.; Zhang, Y.; Zhao, L.; Su, L.; Jin, Y. Transcriptome analysis reveals the molecular regulatory network of muscle development and meat quality in Sunit lamb supplemented with dietary probiotic. Meat Science, 2022, 194, 108996. [CrossRef]
- Latoch, A. Effect of meat marinating in kefir, yoghurt and buttermilk on the texture and color of pork steaks cooked sous-vide. Annals of Agricultural Sciences, 2020, 65(2), 129–136. [CrossRef]
- Okon, U.M.; Ekpo, J.S.; Nuamah, E. Effect of Ficus exasperata as feed on meat quality of rabbits. NSAP 2024 Proceedings, 2024, 748–752. [CrossRef]
- Ortuño, J., Mateo, L.; Rodríguez-Estrada, M.T.; Bañón, S. Effects of sous vide vs grilling methods on lamb meat colour and lipid stability during cooking and heated display. Meat Science, 2021, 171, 108287. [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why is it important to understand the nature and chemistry of tannins to exploit their potential as nutraceuticals? Food Research International, 2023, 173, 113329. [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Science, 2014, 98(3), 520–532. [CrossRef]
- Verma, A.K.; Rajkumar, V.; Banerjee, R.; Biswas, S.; Das, A.K. Guava (<italic>Psidium guajava</italic> L.) Powder as an Antioxidant Dietary Fibre in Sheep Meat Nuggets. Asian-Australasian Journal of Animal Sciences, 2013, 26(6), 886–895. [CrossRef]
- Joo, S.T.; Ha, Y.L.; Lee, J.I.; Park, G.B. Effects of dietary conjugated linoleic acid on fatty acid composition, lipid oxidation, color, and water-holding capacity of pork loin. Journal of Animal Science, 2002, 80(1), 108–112. [CrossRef]
- Abd El-Ghany, W.A. The impact of dietary guava (Psidium guajava L.) on some livestock production systems. CABI Reviews, 2024. [CrossRef]
| Parameters | Control (CTL) | JTLM | PGLM | SEM | P-values |
|---|---|---|---|---|---|
| Pre-slaughter weight (kg) | 37.56b | 42.10a | 39.00b | 0.73 | 0.004 |
| Dressed weight (kg) | 20.00b | 25.27a | 22.00b | 0.82 | 0.002 |
| Dressing (%) | 53.24 | 60.00 | 56.41 | 1.95 | 0.419 |
| Head (kg) | 3.00 | 3.10 | 3.05 | 0.17 | 0.978 |
| Shank (kg) | 0.30b | 0.70a | 0.60a | 0.07 | 0.007 |
| Belly (kg) | 1.40b | 1.74a | 1.64a | 0.05 | 0.001 |
| Breast (kg) | 1.30b | 1.90a | 1.50b | 0.09 | 0.000 |
| Thick rib chop (kg) | 1.20c | 1.94a | 1.62b | 0.11 | 0.000 |
| Rib chop (kg) | 1.10b | 1.40a | 1.20ab | 0.05 | 0.027 |
| Loin (kg) | 0.86c | 1.45a | 1.11b | 0.09 | 0.000 |
| Chump chop (kg) | 0.90b | 1.34a | 1.20a | 0.07 | 0.005 |
| Leg fillet end (kg) | 1.00 | 1.30 | 1.10 | 0.17 | 0.819 |
| Leg shank end (kg) | 1.20 | 2.00 | 1.40 | 0.21 | 0.291 |
| Kidney (% LW) | 0.35c | 0.56b | 0.86a | 0.08 | 0.000 |
| Lungs (% L.W) | 1.30b | 1.80a | 1.70a | 0.08 | 0.002 |
| Liver (% L.W) | 1.25c | 1.45b | 1.75a | 0.07 | 0.000 |
| Spleen (% L.W) | 0.37b | 0.84a | 0.54b | 0.07 | 0.003 |
| Heart (% L.W) | 0.41b | 0.61b | 0.91a | 0.08 | 0.000 |
| Abdominal fat (g) | 10.00a | 0.00b | 0.00b | 1.67 | 0.000 |
| Meat to bone ratio | 2.00 | 3.57 | 2.67 | 0.37 | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





