1. Introduction
Newcastle disease (ND) is a highly contagious viral disease that mainly affects birds, especially chickens. It is characterized by rapid coverage of a large number of birds, high mortality, pneumonia, encephalitis and the manifestation of hemorrhagic syndrome in the form of multiple punctate hemorrhages in internal organs, causes huge economic damage and belongs to especially dangerous infections (OIE, 2012). ND outbreaks can be devastating, and the mortality rate from cycling ND in poultry reaches 100% [1].
This disease is caused by the Newcastle disease (ND) virus, avian paramyxovirus-1, belonging to the genus Avulavirus of the family Paramyxoviridae, which has an envelope of a negative-polar single-stranded RNA genome approximately 15.2 kb in size, encoding six structural proteins: nucleocapsid protein (NP), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase (HN), and large RNA-dependent polymerase (L). Among these proteins, F is considered to be the main molecular determinant of ND pathogenicity [2,3].
Newcastle disease virus isolates vary in their pathogenicity for chickens and may include at least 3 pathotypes: lentogenic (low virulence), mesogenic (moderate virulence), and velogenic (high virulence). Velogenic ND remains a serious threat to poultry production due to high mortality and reduced egg production in laying hens [4]. This classification system plays a key role in the diagnosis and control of disease progression, as well as in the development of effective vaccine strategies [5,6].
For many years, live attenuated vaccines prepared from La Sota, B1 genotype II strains have been used in the global poultry industry to reduce the threat of ND [7,8]. A single injection of 105 EID50 of live ND vaccine, which can cause 100% protection against clinical disease, is sufficient to quickly stimulate the immune response. Despite all these advantages of live attenuated vaccines, they also have their drawbacks. First of all, it is worth noting that live viruses are able to restore virulence and cause diseases even in vaccinated birds. In addition, these vaccines can cause respiratory reactions in young birds, which, if severe, can lead to secondary bacterial infections. Also, these strains, developed in the 1940s, are 21–23% genetically different from the viruses currently circulating in different parts of the world. Significant antigenic and genetic diversity was observed between different ND genotypes, despite the fact that they are all APMV-1 serotypes. Currently, the most common ND genotype circulating in Asia [9,10], the Middle East [1,] and South Africa [12], South America [8] and Europe [13] is genotype VII, associated with the ongoing fourth disease pandemic that began in the late 1980s [14].
Given the existing problem of genotype mismatch and the associated decrease in the effectiveness of the vaccines used, it is very important to develop a safer and more effective inactivated Newcastle disease vaccine. They should be available to farmers and be able to effectively prevent the development of disease in birds. Several recent studies have shown that inactivated vaccines developed from currently circulating genotype strains provide better protection than traditional vaccines [15].
Since 2021, the Research Institute for Biological Safety Problems (RIBSP) has been conducting research on the development of inactivated Newcastle disease vaccines within the framework of PCF O.001B, resulting in the development of a safe and effective inactivated vaccine based on the recently circulating ND strain.
Inactivated vaccines used to protect poultry require the selection of strains and adjuvants in order to induce a sufficient level of immune response. The safety of the adjuvant in the emulsion of the water-oil composition is important for the development of an effective inactivated Newcastle disease vaccine [16]. The use of the ready-to-use Montanide ISA system as an adjuvant reduces the number of technological steps in the preparation of the emulsion, thereby significantly distinguishing the overall the duration of vaccine preparation compared to the use of multicomponent adjuvants that require additional time and money, the cost of preparing emulsions [17].
2. Materials and Methods
2.1. The ND Virus F Gene Sequencing
This study used a previously prepared virus suspension from developing chicken embryos free of specific pathogens. Viral RNA extraction was performed using a validated QIAamp Viral RNA Mini Kit (Qiagen, Germany), according to the manufacturer's instructions.
Reverse transcription was performed with the UltraScript cDNA Synthesis Kit (PCR Biosystems Ltd, UK) [18], Amplification of the F gene was performed using the VeriFi Mix kit (PCR Biosystems Ltd) and sequencing primers the sequence of which is presented in
Table 1:
PCR purification of products was carried out using the innuPREP DOUBLEpure Kit (Analytik Jena GmbH + Co. KG, Germany), according to the manufacturer's instructions.
The ND virus F-gene sequencing was performed using dideoxynucleotides, according to the Sanger method, using the BigDye Terminator v3.1 Cycle Sequencing Kit from Thermo Fisher Scientific (USA) and with overlapping primers (
Table 1) used during the preparation phase. The resulting products were purified using the BigDye Xterminator kit (Thermo Fisher Scientific, USA) and then sequenced using the 3130XL genetic analyzer from Applied Biosystems and Hitachi (USA). After the sequencing was completed, the nucleotide sequence data was analyzed using the Sequencher v.5.4 software developed by Gene Codes Corporation (USA).
2.2. Phylogenetic Analysis of ND Virus F Gene
Phylogenetic analysis based on the F protein gene was performed using the MEGA 11 program using the adjacent conjunction method with 1000 bootstrapping repeats [19]. The sequences used for phylogenetic analysis were derived from various genotypes of the ND strain registered with GeneBank (NCBI).
The evolutionary history was constructed using the Neighbor-Joining method [20], and the Optimal Phylogenetic Tree was presented. Below the branches, the percentages of duplicate trees in which related taxa were grouped as part of a bootstrap test (1000 iterations) are indicated [21]. The tree is depicted to scale, with the length of the branches measured in the same units as the evolutionary distances that were used to build the tree. These distances are calculated using the maximum likelihood method [22] and are expressed as the number of nucleotide substitutions per site. 59 nucleotide sequences were involved in the analysis. All undefined positions have been eliminated for each pair of sequences.
2.3. Inactivation and Formulation of Vaccines
For the preparation of experimental batches of vaccines, the strain of the Newcastle disease virus "PMV-1/Astana/chicken/49/98" was used, produced by inoculating 10-11 day-old chicken embryos into the allantoic cavity. The level of hemagglutinating activity of viral strains was determined based on the hemagglutination reaction (according to the OIE Terrestrial Guide, 2018 here), conducting a series of double dilutions of the material in 96-well plates, after which 1% suspension of rooster erythrocytes was added.
The virus was inactivated using a formaldehyde solution with a concentration of 0.05%. To analyze the residual virulence of the inactivated material, three consecutive blind passages were performed in 10-day-old chicken embryos by inoculation into the allantoic cavity according to the technique described in [23].
When developing emulsion vaccines of the "water-in-oil" type using oil adjuvants of the Montanide ISA 70 VG (vaccine-1) and Montanide ISA 78 VG (vaccine-2) series (Seppic, France), we followed the manufacturer's instructions. The antigen was prepared with hemagglutinating activity at the level of 1:64, and all procedures were performed according to [16,24].
The quality of the experimental batch of the inactivated Newcastle disease vaccine was evaluated according to the following criteria: sterility, safety, pH level, kinetic viscosity, emulsion stability and immunogenic efficacy.
The stability of the emulsion was studied by testing its long-term storage at temperatures of 4 °C and 25 °C, according to the method given in [25].
2.4. Harmlessness Tests of Inactivated Newcastle Disease Vaccines
The studies were carried out in specialized premises that fully meet sanitary and hygienic requirements. The temperature in the room ranged from 20 to 24 °C, the humidity level did not exceed 50%, and the lighting followed a day-night mode. Throughout the period of monitoring immunogenic efficacy and safety, the chicks received adequate nutrition and had unlimited access to water and feed. All experiments with animals were conducted in accordance with the principles of bioethics that govern the practice of working with laboratory animals.
To assess the safety of the vaccines, 20 chickens at the age of 28 days were used. The chickens were randomly divided into two groups: G1, which received the inactivated Vaccine-1 vaccine (n=10), and G2, which received the inactivated Vaccine-2 vaccine (n=10). The vaccine was considered safe if within 10 days after the administration of the vaccine in a 5-fold dose, vaccinated chickens did not experience clinical symptoms of the disease (such as depression, loss of sensitivity, cyanosis of visible mucous membranes, etc.) [26], as well as cases of mortality.
The local tissue reaction at the site of vaccination in chickens was assessed on the 10th day after vaccination. For this purpose, the animals were taken out of the experiment by an overdose of Propapol (0.5 cm3 intravenously), after which an autopsy was performed. The condition of the muscles in the injection area was analyzed and the degree of tissue reaction was determined according to the following gradation: moderate (pallor of the muscles in the injection area and the absence of vaccine residues), moderate (the muscles were pale or red, the presence of vaccine residues), severe (inflammation of superficial and deep muscles and vaccine residues at the injection sites) [27].
2.5. Determination of Vaccine Immunogenicity
Before use, the vaccine vials were kept at a temperature of 18-20 °C for 8-10 hours. Prior to vaccination, blood serum was taken from chickens to assess the immune state and analyzed simultaneously with sera obtained 7, 14, 21 and 28 days after vaccination in the hemagglutination inhibition reaction (HAI). A group of 30 chickens that were 28 days old were used to assess the immunogenicity of the vaccines. By randomization, the chickens were divided into three groups: G3 – chickens that received Vaccine-1 (n=10), G4 – chickens that received Vaccine-2 (n=10), and a control group (K) (n=10).
2.6. Test for the Efficacy of Inactivated Newcastle Disease Vaccines After a Single Vaccination
To assess the efficacy of the vaccines studied on day 28, G3, G4 and K chickens participating in the immunogenicity experiment were infected with an epizootic virulent strain of ND virus at a dose of 100,000 EID50/cm3 (105) with a volume of 0.5 cm3 injection into the pectoral muscle. For 10 days after viral infection, the chickens were examined daily and assessed on a scoring scale. The assessment of clinical manifestations in birds was as follows: 0 points – no symptoms, 1 point – loss of appetite or refusal to drink, 2 points – mild respiratory symptoms or greenish diarrhea, 3 points – severe condition before death, 4 points – death. Mean values were evaluated as the total score for each group. Birds that had the infection and did not show clinical signs were considered protected. According to the monograph of the European Pharmacopoeia, a vaccine was considered effective if the level of actual protection reached 50% [23].
2.7. Statistical Analysis
Statistical analysis of all experimental data was performed using Graph Pad Prism software version 8.0 (Graph Pad Software Inc., La Jolla, California, USA). The titer of specific antibodies was expressed in the mean value with standard errors. The statistical difference in antibody titers between intervals was assessed using the Student test (t-test). At the same time, P <0.05 was considered significant.
2.8. Ethics Statement
The study and use of animals in experiments were carried out with the approval of the Local Ethics Commission on Bioethics of the RIBSP (Protocol No1 of 10.01.2021).
3. Results
3.1. Genetic and Biological Characteristics
As a result of the research, it was possible to successfully extract viral RNA, which became the basis for the subsequent polymerase chain reaction. Analysis of the results using a transilluminator MiniBIS Pro and software GelCapture confirmed the presence of amplified products, which indicates the correctness of the steps performed and the effectiveness of the primers used The F-gene of the ND virus.
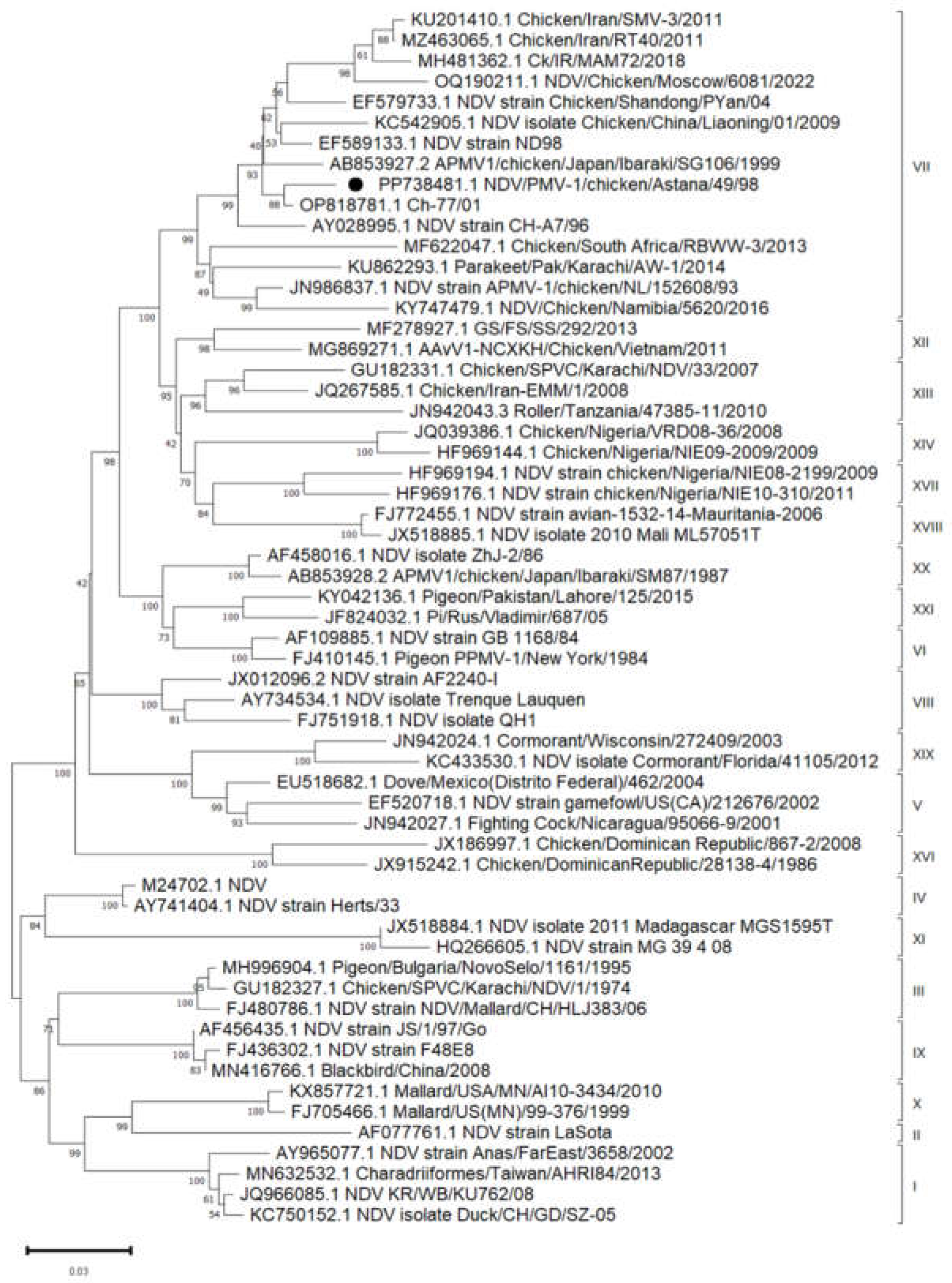
According to the data (
Figure 1), the "PMV-1/Astana/Chicken/49/98" strain is located on the same branch as strains from China (OP818781.1), Japan (AB853927.2), Iran (KU201410.1) and the Russian Federation (OQ190211.1), but was not closely related to widely used vaccine strains, such as Clone 30 and La Sota (AF077761.1). These results identify possible risks associated with the use of existing vaccines that do not provide adequate protection against new strains. This highlights the importance of monitoring virulent strains, especially in the context of the spread and evolution of viruses.
The complete F gene sequences of the virulent ND virus reviewed in this study have been uploaded to GenBank (NCBI) and are available at the following access numbers: PP738481.1.
3.2. Phylogenetic Analysis of F Protein
Phylogenetic analysis of the F gene showed that the strain "PMV-1/Astana/chicken/49/98" belongs to the genotype VII class II, which contained amino acid sequences of RRQKRF, indicating its velogenicity. It is noteworthy that the closest to the "PMV-1/Astana/chicken/49/98" strain was ch-77/01 isolate from China, with an identity percentage of 98.26%. This may indicate that virulent strains are actively circulating in regions with high poultry density.
3.3. Physical and Chemical Parameters of Vaccines
The physicochemical characteristics of the vaccine are key information about its composition and properties. These indicators play a critical role in assessing its quality and effectiveness, as well as in ensuring safety during use. As part of our study, we analyzed the following vaccine parameters: sterility, safety, pH, kinetic viscosity, and stability.
Based on the results of our analysis, the physicochemical characteristics of emulsified vaccines fully comply with the established requirements. During temperature control, the appearance of a transparent aqueous fraction at the bottom of the test tubes, which are generally known for visual inspection, was not noticed. When stored for more than three months at 25 °C or for 12 months at 4 °C, the vaccines remained stable and unaffected. The kinematic viscosity was 38.62±0.01 and 39.20±0.01, which is within the permissible range (20-150 mm²/s). Analysis of the concentration of hydrogen ions showed that the level fully meets the requirements. The measured pH values for the inactivated Vaccine-1 were 7.23±0.00, and for Vaccine-2 – 7.32±0.00, which indicates the neutral acidity of both vaccines. Culture on nutrient media did not reveal the growth of bacteria, fungi, as well as mycoplasmas.
These results confirm the high efficiency of the virus inactivation process and demonstrate the safety and efficacy of vaccines in preventing disease.
3.4. Vaccine Safety Assessment
For 10 days, the safety of vaccines against Newcastle disease based on the strain "PMV-1/Astana/chicken/49/98" with various adjuvants was checked. During the observation period, all birds involved in the study remained alive, and no deviations from physiological norms were found.
Autopsy of the tissues at the injection site showed that there were no pathological changes in the muscles in the injection area, they remained pale, and no vaccine residues were found.
3.5. Analysis of Immunogenicity Assessment of Inactivated Emulsified Vaccines
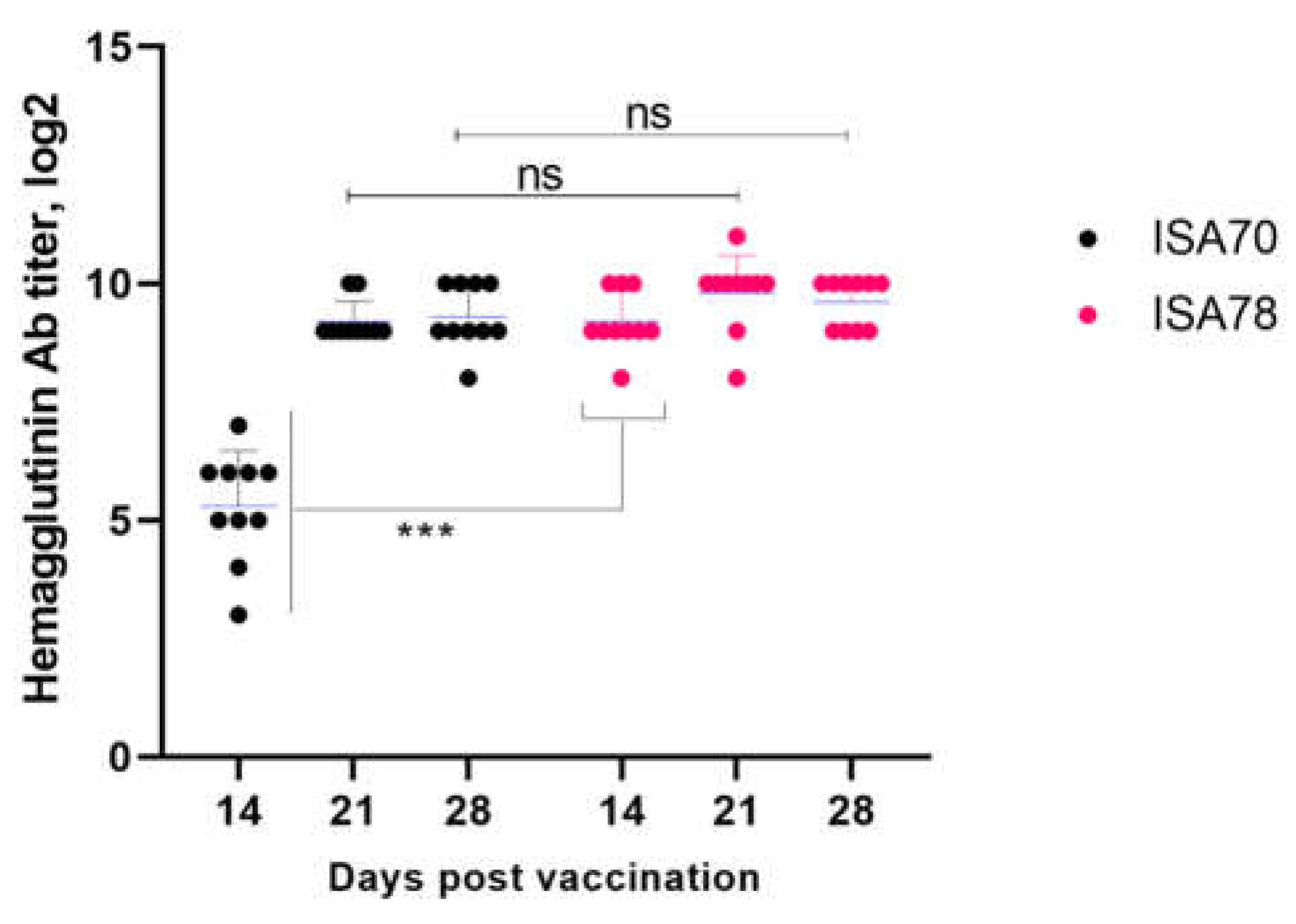
One of the goals of this study was to evaluate the vaccine's ability to induce an immune response in the body against Newcastle disease. As part of the work, experiments were carried out on birds with a subsequent assessment of their effectiveness. A graphical representation of the results of the study is given in
Figure 2.
Analysis of the data presented in
Figure 2 suggests that vaccination of birds from both experimental groups caused a significant immune response as early as 14 days after the vaccine was administered. In birds that received inactivated Vaccine-1 with adjuvant Montanide ISA 70, active development of immunity was observed: on day 14, the level of antibodies was 5.17±0.4 log2, increasing to 9.19±0.1 log2 on day 21 and reaching 9.27±0.2 log2 on day 28. Birds treated with inactivated Vaccine-2 with adjuvant Montanide ISA 78 also showed a marked increase in antibody titers: 8.89±0.1 log2 on day 14, 9.49±0.1 log2 on day 21, and 9.68±0.2 log2 on day 28. These results indicate the formation of a strong immune response in vaccinated birds, which confirms the high effectiveness of the inactivated vaccine in protecting against Newcastle disease.
3.6. Results of Vaccine Protection After Single-Dose Vaccination
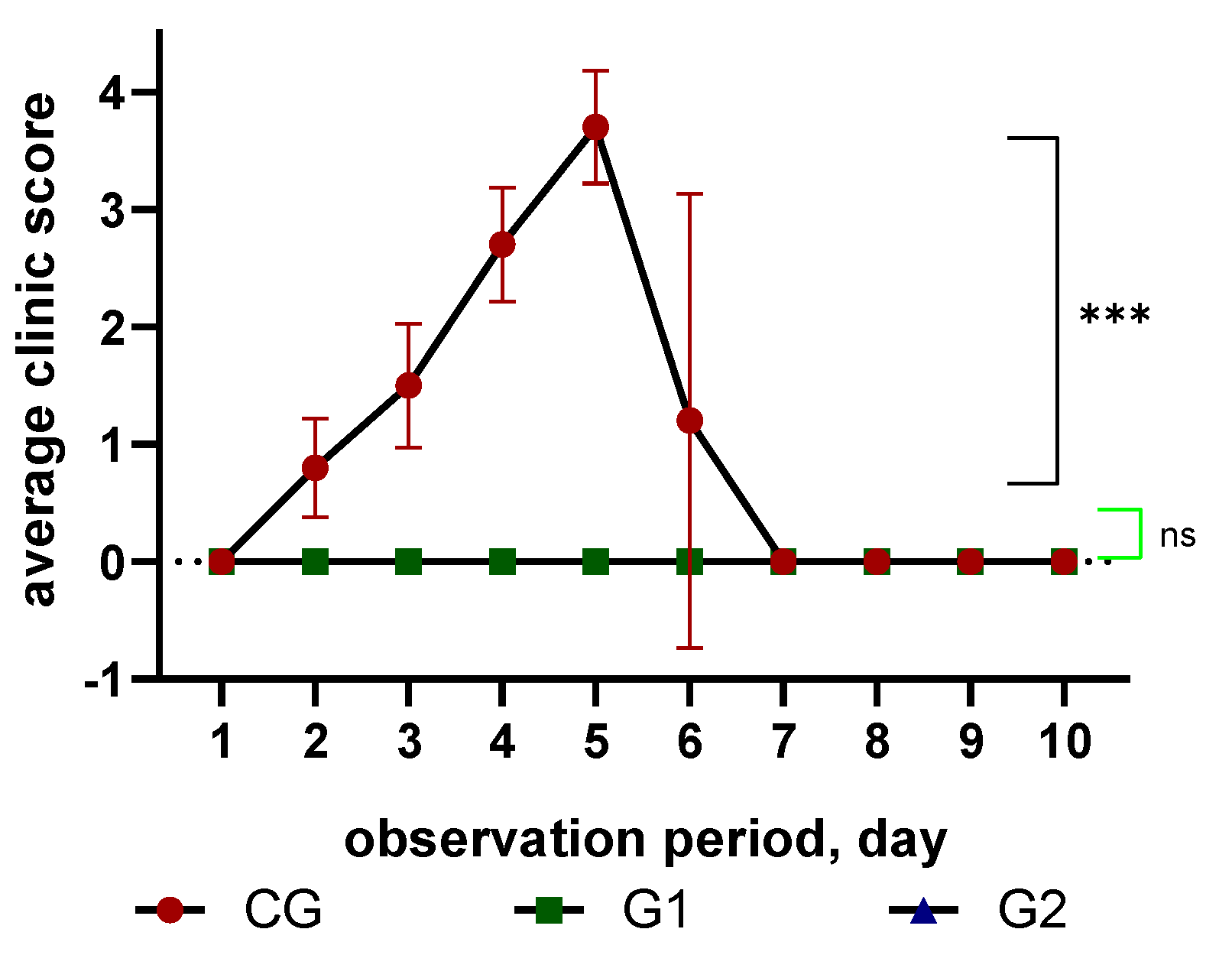
Subsequently, the protection of inactivated vaccines was assessed based on the clinical manifestations of the disease, survival and other indicators according to the point system.
Figure 3.
Comparative analysis of the severity of clinical signs in the study of the protectivity of vaccines from the strain "PMV-1/Astana/chicken/49/98" of the ND virus with different adjuvants in experimental and control birds after infection with the control strain; (R<0.0001).
Figure 3.
Comparative analysis of the severity of clinical signs in the study of the protectivity of vaccines from the strain "PMV-1/Astana/chicken/49/98" of the ND virus with different adjuvants in experimental and control birds after infection with the control strain; (R<0.0001).
Control infection was carried out using the virulent strain "PMV-1/Astana/chicken/49/98". In unvaccinated chickens infected with 100,000 EID50 (105) of the control strain, characteristic clinical manifestations of the cycling form of Newcastle disease were noted, including depression, loss of response to external stimuli, cyanosis of the visible mucous membranes, comb and catkins. These symptoms began to appear on the third day after infection and gradually intensified, leading to the complete death of all chickens on the sixth day. In the G3 and G4 groups, no symptoms or deaths were reported. Statistical analysis revealed a significant difference (P<0.0001) between the control and experimental groups in terms of clinical manifestations and mortality rates (Fig. 3). The study proved the high efficacy of inactivated vaccines based on the PMV-1/Astana/Chicken/49/98 strain with different adjuvants against Newcastle disease after a single vaccination.
4. Discussion
According to the OIE, in 2024, cases of Newcastle disease (ND) were recorded in various countries, including industrialized ones. For example, Sweden, Poland, Israel, Brazil, Russia, Chinese Taipei, Botswana. In Kazakhstan, the epizootic situation with ND also remains tense, as outbreaks of the previously registered Newcastle disease continue to be observed in the countries bordering Kazakhstan [28].
To protect against Newcastle disease, poultry farms in Kazakhstan use a unified vaccination schedule, where live vaccines against this infection from the strains "La Sota", "B1" based on genotype II are actively used.
Despite the use of a live vaccine, an effective technology for the production of inactivated vaccine against Newcastle in Kazakhstan has not yet been developed. Based on the above, RIBSP has conducted research on the development of inactivated vaccines against Newcastle disease.
The current trend in the control of virulent ND virus infection is the development of genotype-appropriate vaccines based on currently circulating ND virus isolates [5]. In recent outbreaks in Taiwan and China, genotype VII virus has mainly been identified as the causative agent. present in different parts of the world [10].
According to the study, the ND virus gene was studied in detail, the phylogenetic analysis of which showed that the strain "PMV-1/Astana/chicken/49/98" belongs to the genotype VII class II and has the amino acid sequence RRQKRF. This means that the studied strain isolated in Kazakhstan is velogenic, and is not closely related to vaccine strains, such as Clone 30 and La Sota. Analysis of the genetic variability of ND strains in different regions demonstrates that new genotypes, such as VII, can cause outbreaks even in vaccinated populations where the above traditional vaccine strains are used, as evidenced by the similarity in the spread of the virus and its pathogenicity. This emphasizes the need to update vaccine strains taking into account the current viral circulation [9,10,11,12,13].
Inactivated vaccines designed to protect birds require careful selection of not only strains, but also adjuvants to ensure the necessary level of immune response. To create an effective vaccine, not only high-quality antigens are important, but also safe adjuvants that will help increase the immunogenicity of the antigen [17]. Based on our experience in developing inactivated vaccine manufacturing technologies and reviewing the available literature on different immunostimulants, we compared vaccines using the same antigen and different oil adjuvants, such as Montanide ISA 70 and Montanide ISA 78. These adjuvants are widely used in the production of vaccines for birds and have the advantage of reducing the number of technological steps in their creation compared to multicomponent adjuvants, which are time- and resource-intensive [16].
In this study, we compared two water-in-oil vaccines from the PMV-1/Astana/Chicken/49/98 strain with the above adjuvants (manufactured by Seppic, France) against Newcastle disease with a study of their safety, immunogenic properties and efficacy. The series of vaccines against Newcastle disease prepared by us were sterile and did not have foreign impurities, are safe for vaccination of chickens, did not cause manifestations of the disease after introduction into the body of chickens and met the recommendations of the OIE [29]. It was found that the kinematic viscosity of the studied vaccine batches had an acceptable level according to the criterion established as 150 mm2/s, which contributed to the fluidity of the vaccine and ensured its easy passage through the needle during injection [30]
The vaccines maintained the stability of the emulsion at a storage temperature of 4 °C for 12 months in all samples studied. At 25 °C, the duration of stability of emulsified vaccines was determined to be more than 3 months, a slight separation of the oil and water phases was observed at week 4 of follow-up, which easily recovered after intensive shaking even after 3 months of storage, turning into a homogeneous emulsion, and were permissible according to the manufacturer's recommendations (Seppic, France) [16,25].
To study the safety of these vaccines, vaccinated chickens were monitored for 10 days after vaccination. In vaccines, the adjuvant is used to enhance the humoral and cellular immune response, but prolonged exposure of mineral oil to the injection site, which causes inflammation and local tissue necrosis, can lead to a decrease in the marketable value of poultry [31] To assess macroscopic tissue injuries in the injection area, an autopsy was performed on day 10, which did not reveal damage or other inflammatory phenomena in the tissues, which is confirmed by the results of other research studies [27].
To assess the efficacy of inactivated vaccines with different adjuvants in the formation of a sustained immune response in birds against Newcastle disease, antibody titers were compared in both experimental groups, which showed that as early as 14 days after vaccination, there is a significant increase in antibody levels, which confirms the activation of the immune system, while the group that received the vaccine with Montanide ISA 78 showed higher antibody titers at all stages of follow-up, starting from day 14 compared to birds treated with Vaccine-1 with Montanide ISA 70. However, over time, antibody levels in the Vaccine-1 group increased significantly, demonstrating that the immune response to the inactivated Montanide ISA 70 adjuvanted vaccine not only begins after 14 days, but continues to evolve, peaking at day 28.
It is interesting to note that despite the high titers of antibodies, there is less growth dynamics in the Vaccine-2 group compared to the first vaccine. This may indicate differences in the mechanisms of action of adjuvants and their effect on the formation of immune memory. To compare the immunogenicity and protective efficacy of these vaccines, the responses of avian antibodies in HAI were compared with the results of control infection. From the literature [30], it is known that an antibody titer below 1:16 (on average > 4 log2) does not protect against infection with wild viruses. Evaluation of antibody titers (HAIs) in the vaccinated groups during the experiment shows that the vaccines were able to induce adequate levels of antibodies to provide protection against the virulent virus. In our experiment, vaccination provided high antibody titers in the HAI in the vaccinated groups G3 and G4, as early as 14 days after vaccination in 100% of birds, the antibody SHT reached a protective level (on average > 4 log2). On the day of infection, the mean antibody titer in the HAI in both vaccinated groups was above 7 log2, which is more than enough to protect birds from clinical signs. None of the vaccinated OG3 birds showed clinical signs after control infection (0 points). In unvaccinated birds of the CG group, clinical manifestations were observed as early as 3 days after infection with increasing severity, and eventually they all died (100% mortality). These data are confirmed by Alexander et al. (2003), where it was shown that virulent strains can cause high mortality in unvaccinated susceptible herds [32]
5. Conclusions
In conclusion, the results of the studies highlight the importance of continuous monitoring and adaptation of vaccination strategies to reduce economic losses in poultry production and control the spread of viral infections. The development of new vaccines based on topical strains, such as genotype VII, is an important task to ensure bird health and effective protection against Newcastle and ND viruses.
The developed vaccines have demonstrated their effectiveness, but differences in the dynamics of the antibody response suggest that the choice of adjuvant can have a significant impact on the vaccination outcome. More research is recommended to better understand the immunological mechanisms and optimize vaccination against Newcastle disease.
Supplementary Materials
The following supporting information can be downloaded at Preprints.org, table S1: Primer sequence for amplification of the F gene; Figure s1: Phylogenetic analysis of the F gene of the strain "PMV-1/Astana/chicken/49/98"; Figure s 2: Analysis of the immunogenicity assessment of inactivated emulsified vaccines against Newcastle disease; Figure s3: Comparative analysis of the severity of clinical signs in the study of the protectivity of vaccines from the strain "PMV-1/Astana/chicken/49/98".
Author Contributions
A.N., R.S., and A.N. contributed to the conceptualization of the study; A.N., R.S., A.M., A.S., Y.K., Y.M., B.U., Y.B., K.Y. and A.N. contributed to the study methodology; A.N., A.S., Y.M and A.M. performed the experimental analysis; A.N., Y.M., R.S.,B.U. and K.N. conducted the study’s investigations; A.N., A.M., and A.S. contributed to data curation; A.N. and A.N. wrote the original draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of PCF O.001B "Biological Safety of the Republic of Kazakhstan: Threat Assessment, Scientific and Technical Foundations of Their Prevention and Elimination".
Institutional Review Board Statement
The animal study protocol was approved by the Research Institute for Biological Safety Problems Bioethics Review Board (protocol №1, dated 10 January 2021) for studies involving animals.minutes No1 dated 01/10/2021).
Conflicts of Interest
The authors declare that there are no obvious and potential conflicts of interest associated with the publication of this article.
References
- Dahiya, S.S.; Kumar, S.; Mehta, S.C.; Narnaware, S.D.; Singh, R.; Tuteja, F.C. Camelpox: A brief review on its epidemiology, current status and challenges. Acta Trop. 2016, 158, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D. J. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections. In Diseases of Poultry. 2003, 63–99. [Google Scholar]
- De Leeuw, O. S. , Koch, G., Hartog, L., Ravenshorst, N., & Peeters, B. P. H. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein. Journal of General Virology 2005, 86, 1759–1769. [Google Scholar] [CrossRef]
- Capraro GA, Johnson JB, Kock ND, Parks GD. Virus growth and antibody responses following respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5. Virology 2008, 376, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. H. , Kwon, H. J., Kim, T. E., Kim, J. H., Yoo, H. S., & Kim, S. J. Variation of virulence of Newcastle disease viruses isolated from wild and domestic birds in Korea. Avian Diseases 2008, 52, 332–337. [Google Scholar]
- Bello, M. B. , Yusoff, K., Ideris, A., Hair-Bejo, M., Peeters, B. P. H., & Omar, A. R. Diagnostic and vaccination approaches for Newcastle disease virus in poultry: The current and emerging perspectives. BioMed Research International.
- Vijayarani, K. , Kumar, S., & Govindarajan, D. Molecular characterization of Newcastle disease virus isolates from India. Indian Journal of Virology 2010, 21, 55–59. [Google Scholar]
- Kapczynski, D. R. , Afonso, C. L., & Miller, P. J. Immune responses of poultry to Newcastle disease virus. Developmental & Comparative Immunology 2013, 41, 447–453. [Google Scholar]
- Miller, P. J. , & Koch, G. Newcastle disease. In Diseases of Poultry. 2013; pp. 89-138. Wiley-Blackwell.
- Liu, Y. , Sun C., Chi M., Wen H., Zhao L., Song Y., et al. Genetic characterization and phylogenetic analysis of Newcastle disease virus from China. Infect. Genet Evol. 2019, 75, 103958. [Google Scholar] [CrossRef]
- Orynbayev MB, Fereidouni S, Sansyzbai AR, Seidakhmetova BA,Strochkov VM, Nametov AM, et al. Genetic diversity of avian avulavirus1 (Newcastle disease virus genotypes VIg and VIIb) circulating in wildbirds in Kazakhstan. Arch Virol. 2018, 163, 1949–1954. [Google Scholar] [CrossRef]
- Habib, M. , Yaqub T., Nazir J., Shehzad W., Aziz-ul-Rahman, So hail T., et al. Genomic and biological characterization of Newcastle disease viruses isolated from migratory mallards (Anas platyrhynchos). Arch. Virol. 2018, 163, 2179–2188. [Google Scholar] [CrossRef]
- Abolnik, C. History of Newcastle disease in South Africa. Onderste poort. J. Vet. Res. 2017, 84, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, J. , Wehmann, E., Bragg, R. R., Travassos Dias, P. M., Hadjiev, G., & Werner, O. Two novel genetic groups (VIIb and VIII) responsible for recent Newcastle disease outbreaks in Southern Africa, one (VIIb) of which reached Southern Europe. Archives of Virology 1999, 144, 2087–2099. [Google Scholar]
- Miller, P. J. , Decanini, E. L., & Afonso, C. L. Newcastle disease: Evolution of genotypes and the related diagnostic challenges. Infection, Genetics and Evolution 2010, 29, 1–10. [Google Scholar]
- Miller PJ, King DJ, Afonso CL, Suarez DL. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 2007, 25, 7238–7246. [Google Scholar] [CrossRef] [PubMed]
- Montanide™ ISA 78 VG, a new powerful adjuvant for poultry vaccines. 29/04/2020. Available online: https://www.seppic.
- Cahyani JI, Widyarini S, Wibowo MH. Comparative safety and efficacy of two bivalent vaccines containing Newcastle disease LaSota and avian influenza H9N2 Sidrap isolate formulated with different oil adjuvants. Vet World. 2020, 13, 2493–2501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strochkov V, Burashev Y, Sandybayev N, Xie G, Erkkila TH, Cui H, et al. (2020) Whole Genomes of Avian orthoavulavirus 1 (NewcastleDisease Virus Genotypes VIg or new genotype XXI.) in Wild Birds in Kazakhstan. Virol Mycol. 9:183. De Leeuw, O. S., Koch, G., Hartog, L., Ravenshorst, N., & Peeters, B. P. H. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein. Journal of General Virology 2005, 86, 1759–1769.
- Kim BY, Lee DH, Kim MS, Jang JH, Lee YN, Park JK, Yuk SS, Lee JB, Park SY, Choi IS, Song CS. Exchange of Newcastle disease viruses in Korea: The relatedness of isolates between wild birds, live bird markets, poultry farms and neighboring countries. Infect. Genet. Evol. 2012, 12, 478–482. [Google Scholar] [CrossRef]
- Saitou, N. and Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K. , Nei M., and Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Newcastle disease vaccine (inactivated) // European Pharmacopoeia. Strasbourg, 2008. P. 937–939.
- Hongzhuan, Z. , Ying T., Xia S., Jinsong G., Zhenhua Z., Beiyu J., Yanyan C., Lulu L., Jue Z., Bing Y., Jing F. Preparation of the inactivated Newcastle disease vaccine by plasma activated water and evaluation of its protection effi cacy. Appl. Microbiol. Biotechnol. 2020, 104, 107–117. [Google Scholar] [CrossRef]
- Cessi, D. , Nardelli L. Vaccination against Newcastle disease: efficacy of an oil emulsion vaccine. Avian Pathol. 1974, 3, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hines, N. L. , & Miller, C. L. (2012). Avian paramyxovirus serotype-1: A review of disease distribution, clinical symptoms, and laboratory diagnostics. Veterinary Medicine International, 2012.
- Wanasawaeng, W. , Tawatsin A., Sasipreeyajan J., Poomvises P., Chansiripornchai N. Development of Inactivated Newcastle Disease Vaccine using Palm Oil as an Adjuvant. Thai J. Vet. Med. 2009, 39, 9–16. [Google Scholar] [CrossRef]
- Events management. Disease: Newcastle disease virus (Inf. with). Available online: https://wahis.woah.
- CHAPTER 3.3.14. OIE Terrestrial Manual, CHAPTER 3.3.14. NEWCASTLE DISEASE (INFECTION WITH NEWCASTLE DISEASE VIRUS). 2018. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf.
- A. I. Chegrynets, O. O. Saliy, I. A. Sobko, V. O. Krasinko. Immunological evaluation of inactivated Newcastle disease vaccine depending on adjuvant composition. Cilt 2021, 12, 490–497. [Google Scholar]
- Liu CG, Liu M, Liu F, Liu DF, Zhang Y, Pan WQ, Chen H, Wan CH, Sun EC, Li HT, Xiang WH. Evaluation of several adjuvants in avian influenza vaccine to chickens and ducks. Virol J. 2011, 8, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexander, D. J. Newcastle Disease. British Poultry Science 2003, 44, 201–217. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).