Submitted:
13 February 2025
Posted:
13 February 2025
You are already at the latest version
Abstract
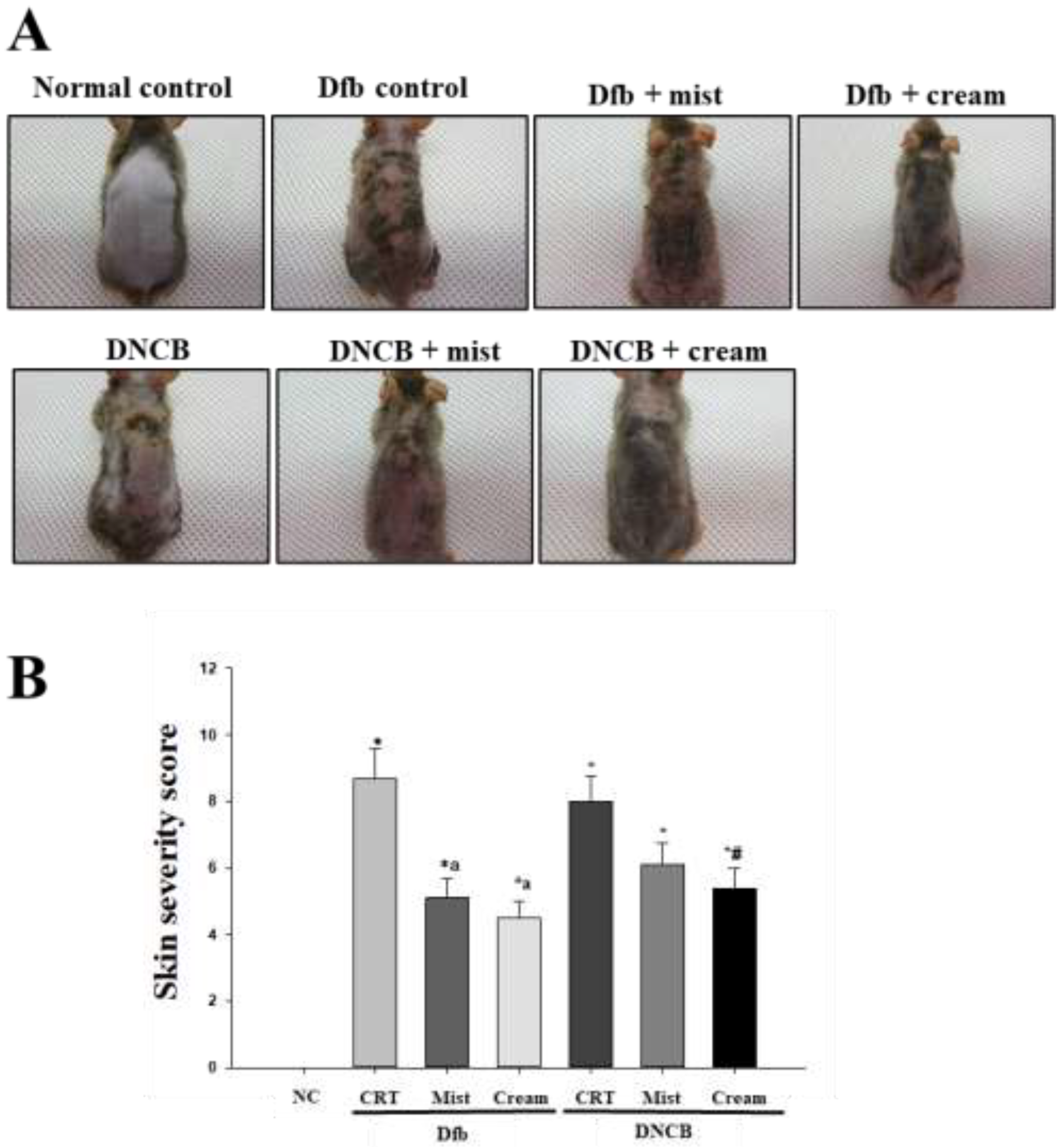
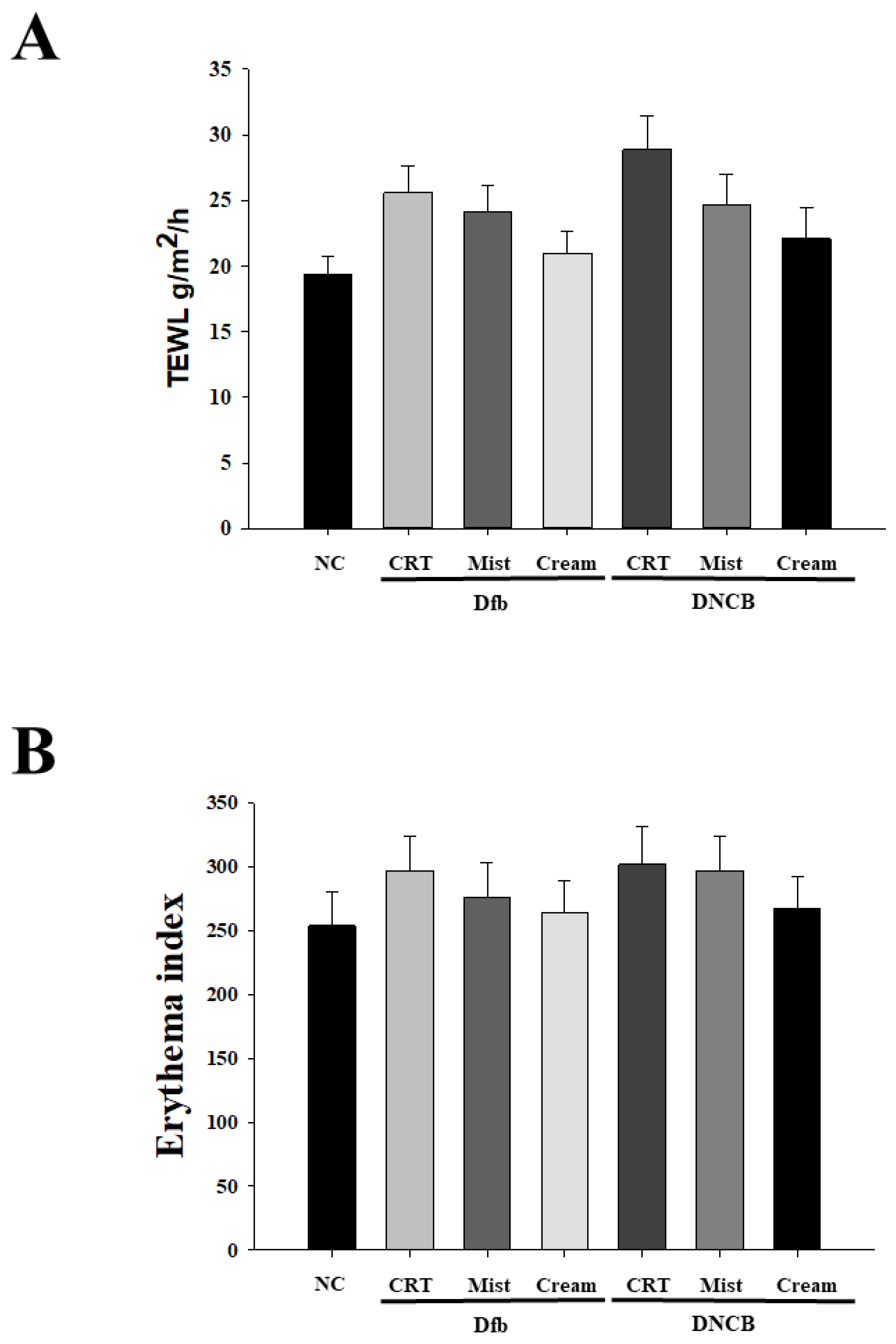
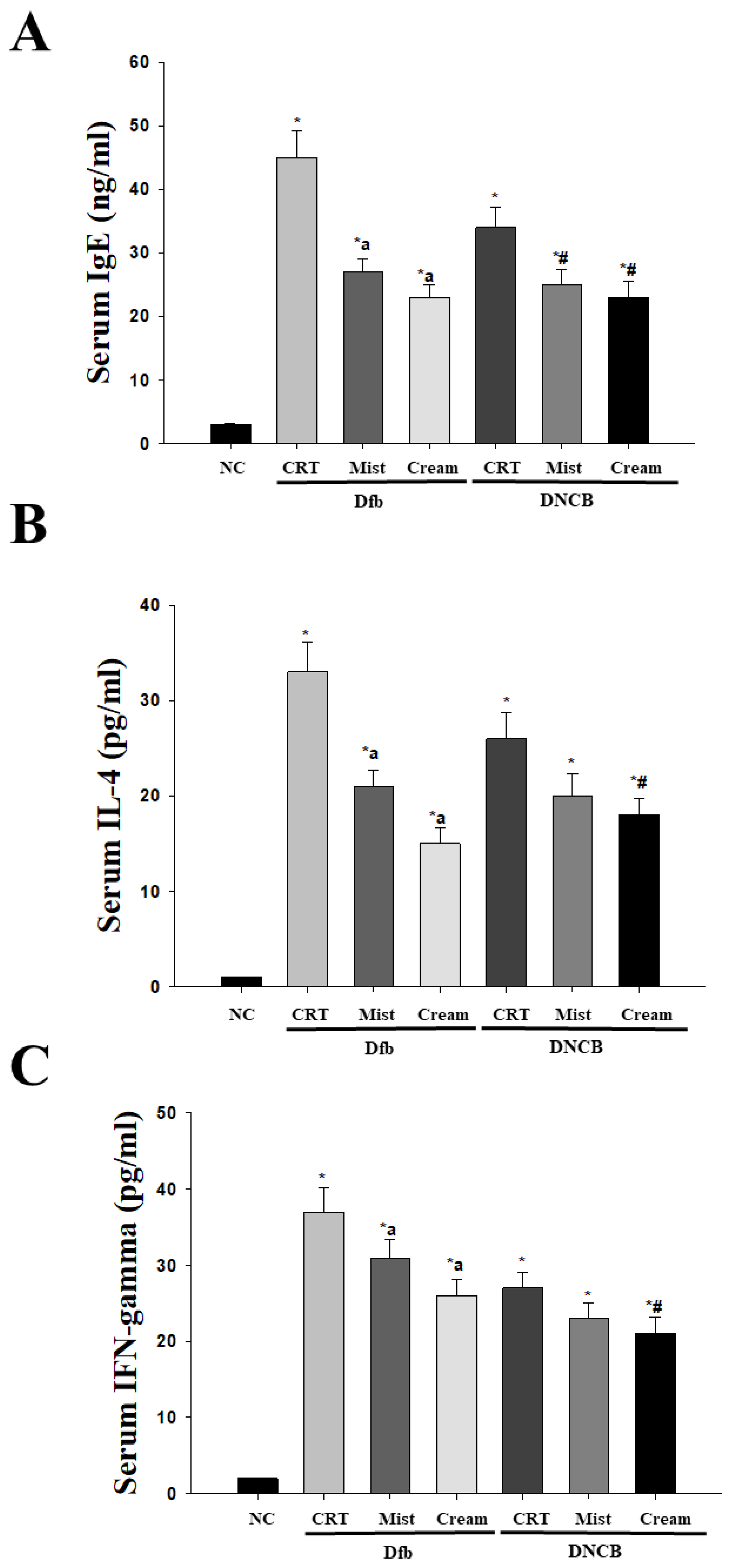
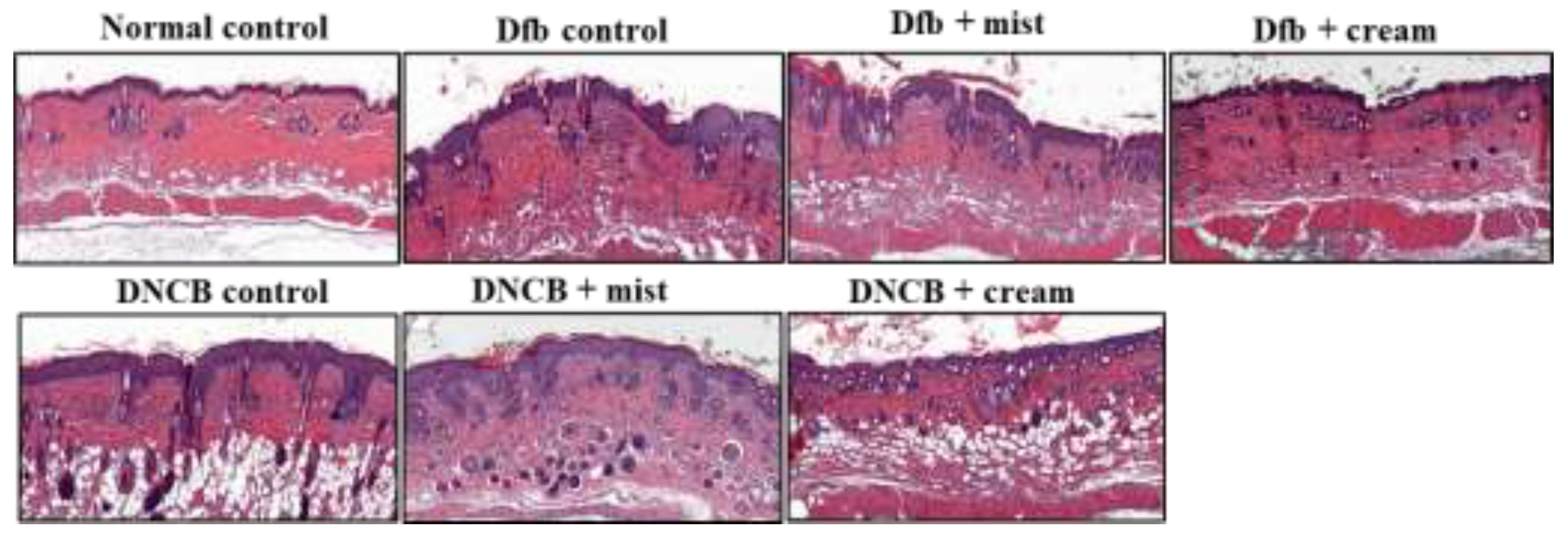
Chronic pruritus and inflammatory skin lesions, characterized by high recurrence, are hallmarks of atopic dermatitis (AD). Despite its increasing prevalence, the development of therapeutic agents for AD remains limited. This study aimed to evaluate the therapeutic effects of deep-sea minerals (DSM) in mist and cream formulations on the development of AD-like skin lesions in NC/Nga mice exposed to either Dermatophagoides farinae body extract (Dfb) or 2,4-dinitrochlorobenzene (DNCB). To induce AD, 100 mg of Biostir AD cream containing crude Dfb or 200 µL of DNCB (1%) was topically applied to the dorsal skin of NC/Nga mice. Additionally, 200 µL of deep-sea mineral mist (DSMM) and 10 mg of deep-sea mineral cream (DSMC) were applied daily to the dorsal skin for 4 weeks. AD was assessed through visual observations, clinical scoring of skin severity, serological tests, and histological analysis. Visual and clinical evaluations revealed that DSM inhibited the formation of AD-like skin lesions. DSM also significantly affected trans-epidermal water loss and erythema. Treatment with DSM resulted in reduced serum levels of IgE, IFN-γ, and IL-4. Histological analysis indicated that DSM decreased skin thickness. Immunostaining for the CD4 antigen demonstrated reduced infiltration of CD4 T cells, which drive the Th2 response in AD, following DSM treatment. In conclusion, the cream formulation of DSM showed better results than the mist formulation. These results suggest that DSM may be an effective treatment for allergic skin inflammatory diseases, especially in cream formulation.
Keywords:
1. Introduction
2. Results
2.1. Treatment with DSM Alleviates Dfb- or DNCB-induced AD-Like Skin Lesions in NC/Nga mice
2.2. Treatment with DSM Inhibits Skin Dehydration and Increases In Erythema Thickness in Dfb or DNCB-Induced AD-Like Skin Lesions in NC/Nga Mice
2.3. Treatment with DSM downregulates Dfb or DNCB-induced Serum IgE, IL-4, and IFN-γ levels in NC/Nga mice
2.4. Effect of DSM administration on histological features and infiltration of T cells on Dfb- or DNBC-induced AD skin lesions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Atopic Dermatitis and DSM Application
4.3. Evaluation of Skin Lesion Severity
4.4. Measurement of Serum IgE, IL-4, and Interferon Gamma (IFN-γ) Levels
4.5. Histologic Analysis and Immonohistochemistry
4.6. Statistics
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dfb | Dermatophagoides farinae body |
| DNCB | 2,4-dinitrochlorobenzene |
| DSM | Deep-sea minerals |
References
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Experimental Dermatology. 2018, 27, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Palmer, C.N.; Zhao, Y.; et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007, 127, 564–567. [Google Scholar] [CrossRef]
- Pugliarello, S.; Cozzi, A.; Gisondi, P.; Girolomoni, G. Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011, 9, 12–20. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nature Reviews Drug Discovery. 2022, 21, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Rudikoff, D. Atopic dermatitis: systemic immunosuppressive therapy. Semin Cutan Med Surg. 2008, 27, 151–155. [Google Scholar] [CrossRef]
- Vestergaard, C.; Yoneyama, H.; Matsushima, K. The NC/Nga mouse: a model for atopic dermatitis. Mol Med Today. 2000, 6, 209–210. [Google Scholar] [CrossRef]
- Vestergaard, C.; Yoneyama, H.; Murai, M.; et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J Clin Invest. 1999, 104, 1097–1105. [Google Scholar] [CrossRef]
- Arlian, L.G.; Platts-Mills, T.A. The biology of dust mites and the remediation of mite allergens in allergic disease. J Allergy Clin Immunol. 2001, 107, S406–S413. [Google Scholar] [CrossRef]
- Matsuoka, H.; Maki, N.; Yoshida, S.; et al. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003, 58, 139–145. [Google Scholar] [CrossRef]
- Sasakawa, T.; Higashi, Y.; Sakuma, S.; et al. Atopic dermatitis-like skin lesions induced by topical application of mite antigens in NC/Nga mice. Int Arch Allergy Immunol. 2001, 126, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Teplitsky, V.; Mumcuoglu, K.Y.; Babai, I.; Dalal, I.; Cohen, R.; Tanay, A. House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int J Dermatol. 2008, 47, 790–795. [Google Scholar] [CrossRef]
- Jung, B.G.; Cho, S.J.; Koh, H.B.; Han, D.U.; Lee, B.J. Fermented Maesil (Prunus mume) with probiotics inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Vet Dermatol. 2010, 21, 184–191. [Google Scholar] [CrossRef]
- Pokharel, Y.R.; Lim, S.C.; Kim, S.C.; Heo, T.H.; Choi, H.K.; Kang, K.W. Sopungyangjae-tang inhibits development of dermatitis in nc/nga mice. Evid Based Complement Alternat Med. 2008, 5, 173–180. [Google Scholar] [CrossRef]
- Ikoma, A. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: mechanisms and management of itch in atopic dermatitis. J Pharmacol Sci. 2009, 110, 265–269. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J Invest Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Hattori, K.; Nishikawa, M.; Watcharanurak, K.; et al. Sustained exogenous expression of therapeutic levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization. J Immunol. 2010, 184, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Gelfand, E.W. Mouse models of allergic diseases. Curr Opin Immunol. 2009, 1, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Shin, E.J.; Park, J.E.; Shon, Y.H. Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Mar Drugs. 2013, 11, 4193–4212. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.H; Yoo, Y.G.; et al. Inhibitory effect of deep-sea water on differentiation of 3T3-L1 adipocytes. Mar Biotechnol (NY). 2009, 11, 161–168. [Google Scholar] [CrossRef]
- Lee, K.S.; Chun, S.Y.; Kwon, Y.S.; Kim, S.; Nam, K.S. Deep sea water improves hypercholesterolemia and hepatic lipid accumulation through the regulation of hepatic lipid metabolic gene expression. Mol Med Rep. 2017, 15, 2814–2822. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, D.H.; Kwon, Y.S.; Chun, S.Y.; Nam, K.S. Deep-sea water inhibits metastatic potential in HT-29 human colorectal adenocarcinomas via MAPK/NF-κB signaling pathway. Biotechnology and Bioprocess Engineering. 2014, 19, 733–739. [Google Scholar] [CrossRef]
- Bak, J.P.; Kim, Y.M.; Son, J.; Kim, C.J.; Kim, E.H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement Altern Med. 2012, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; et al. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol 120 Suppl. 1999, 1, 70–75. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T.; Leung, D.Y. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003, 112, S128–S139. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003, 12, S118–S127. [Google Scholar] [CrossRef]
- Shiohara, T.; Hayakawa, J.; Mizukawa, Y. Animal models for atopic dermatitis: Are they relevant to human disease? J Dermatol Sci. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Fukuyama, T.; Tajima, Y.; Hayashi, K.; Ueda, H.; Kosaka, T. Prior or coinstantaneous oral exposure to environmental immunosuppressive agents aggravates mite allergen-induced atopic dermatitis-like immunoreaction in NC/Nga mice. Toxicology. 2011, 289, 132–140. [Google Scholar] [CrossRef]
- Iwata, M.; Takebayashi, T.; Ohta, H.; Alcalde, R.E.; Itano, Y.; Matsumura, T. Zinc accumulation and metallothionein gene expression in the proliferating epidermis during wound healing in mouse skin. Histochem Cell Biol. 1999, 12, 283–290. [Google Scholar] [CrossRef]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch Dermatol Res. 1999, 291, 560–563. [Google Scholar] [CrossRef]
- Gambichler, T.; Kuster, W.; Kreuter, A.; Altmeyer, P.; Hoffmann, K. Balneophototherapy--combined treatment of psoriasis vulgaris and atopic dermatitis with salt water baths and artificial ultraviolet radiation. J Eur Acad Dermatol Venereol. 2000, 14, 425–428. [Google Scholar] [CrossRef]
- Halevy, S.; Sukenik, S. Different modalities of spa therapy for skin diseases at the Dead Sea area. Arch Dermatol. 1998, 134, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int J Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chun, S.Y.; Lee, M.G.; Kim, S.; Jang, T.J.; Nam, K.S. The prevention of TNF-alpha/IFN-gamma mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Suwa, E.; Yamaura, K.; Oda, M.; Namiki, T.; Ueno, K. Histamine H(4) receptor antagonist reduces dermal inflammation and pruritus in a hapten-induced experimental model. Eur J Pharmacol. 2011, 667, 383–388. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Christensen, R.; Lavrijsen, A.; Arents, B.W.M. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017, 2, CD012119. [Google Scholar]
- Giam, Y.C.; Hebert, A.A.; Dizon, M.V.; et al. A review on the role of moisturizers for atopic dermatitis. Asia Pac Allergy. 2016, 6, 120–128. [Google Scholar] [CrossRef]
- Chen, L.; Lin, S.X.; Overbergh, L.; Mathieu, C.; Chan, L.S. The disease progression in the keratin 14 IL-4-transgenic mouse model of atopic dermatitis parallels the up-regulation of B cell activation molecules, proliferation and surface and serum IgE. Clin Exp Immunol. 2005, 142, 21–30. [Google Scholar] [CrossRef]
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef]
- Singh, V.K.; Mehrotra, S.; Agarwal, S.S. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F.; Aragane, Y.; Maeda, A.; et al. Overactivation of IL-4-induced activator protein-1 in atopic dermatitis. J Dermatol Sci. 2002, 28, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Ra, C.; Kawamoto, K.; et al. IgE hyperproduction through enhanced tyrosine phosphorylation of Janus kinase 3 in NC/Nga mice, a model for human atopic dermatitis. J Immunol. 1999, 162, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Miyake, S.; Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001, 413, 531–534. Author 1, A.; Author 2, B. Title of the chapter. In Book Title, 2nd ed.; Editor 1, A., Editor 2, B., Eds.; Publisher: Publisher Location, Country, 2007; Volume 3, pp. 154–196. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




