1. Introduction
Hearing impairment, a prevalent disability in contemporary society, constitutes a substantial global health challenge. The World Health Organization (WHO) anticipates that by 2050, one in ten individuals worldwide will experience disabling hearing loss [
1]. Beyond its evident impact on communication abilities, this condition exacts a considerable economic toll, estimated at approximately 750 billion dollars annually [
2]. It is crucial to investigate the etiological factors of deafness, as the WHO estimates that approximately half of all hearing loss is preventable [
3]). Global trends in hearing loss are influenced by a variety of factors, including noise and air pollution [
4], exposure to heavy metals [
5], and the increasing use of agrochemicals, particularly pesticides [
6].
Acute and chronic exposure to pesticides is associated with an array of health issues, from nausea and dizziness to chronic neurological problems and cancer [
7,
8]. However, pesticide exposition has been linked to sensory impacts, particularly on hearing. Studies reported by Petty et al. [
9] showed damage to the eighth cranial nerve, with symptoms including nausea and tinnitus after pesticide exposition. Later research, like that by Crawford et al. [
10] and Rabinowitz et al. [
11], showed that people who were exposed to organophosphates (OP) pesticides had a higher chance of losing their hearing. Also, Dundar et al. [
12] found cases of people suddenly losing hearing after breathing in OPs. Moreover, Guida et al. [
13] observed significant hearing loss in individuals exposed to a combination of pesticides and noise, emphasizing the compounded impact of these environmental factors. This study corroborates earlier research by Teixeira and Brandao [
14], documenting sensorineural hearing loss in agricultural workers exposed to pesticides. Perry and May [
15] further confirmed that these effects were independent of noise exposure from agricultural machinery. On the other hand, Carbaryl, a commonly used acetylcholinesterase inhibitor in agriculture, is another substance associated with auditory system damage. Prakash Krishnan Muthaiah et al. [
16] identified a significant correlation between exposure to carbaryl and damage to the basal region of the cochlea in rats on postnatal day three. This finding establishes a potential link between cochlear damage and various auditory disorders observed in individuals exposed to pesticides, including central auditory processing. A pivotal challenge in diagnosing these issues stems from the complexity of detecting cochlear damage through standard clinical tests. Consequently, individuals exposed to pesticides may experience undiagnosed auditory processing problems, potentially resulting in significant, albeit subclinical, hearing loss.
Animal model studies have elucidated the impact of noise exposure on auditory capacity, revealing temporary changes without immediate or chronic loss of hair cells. Significant damage to the connections between inner hair cells (IHCs) and cochlear neurons has been observed [
17,
18,
19]. Notably, this reduction in neural connections may not be apparent in conventional hearing tests, which typically measure the perception of weak sounds or utilize otoacoustic emissions testing. Conversely, noise-induced hearing loss primarily affects the outer hair cells (OHCs) before impacting other cochlear areas [
20], with animal studies revealing varying degrees of OHCs changes after exposure to OP, ranging from mild alterations to severe damage or loss [
8]. These changes are associated with reduced auditory sensitivity and frequency discrimination ability [
21]. Consequently, standard hearing assessments may lack the sensitivity to detect this type of synaptic loss. Even a small number of intact IHCs can facilitate tone detection in quiet environments [
17,
22]. This reduction in synaptic connections, termed cochlear synaptopathy, has been linked to various factors, including noise exposure, aging, and ototoxic substances [
19,
23,
24].
We presume that a similar process can occur with exposure to pesticides in rural inhabitants that do not work in agriculture, which potentially induces subtle alterations in auditory processing, which are initially undetectable by conventional audiological assessment. This study investigates the effects of pesticide exposure on auditory function in a cohort of young rural residents, evaluating audiometric thresholds, suprathreshold auditory brainstem responses (ABRs), and distortion-product otoacoustic emissions (DPOAEs). By examining these measures, this research aims to elucidate the nuanced impact of pesticide exposure on auditory health, highlighting the need for further investigation to fully comprehend the complex relationship between these exposures and auditory dysfunction and ultimately to inform preventative strategies for vulnerable populations.
2. Materials and Methods
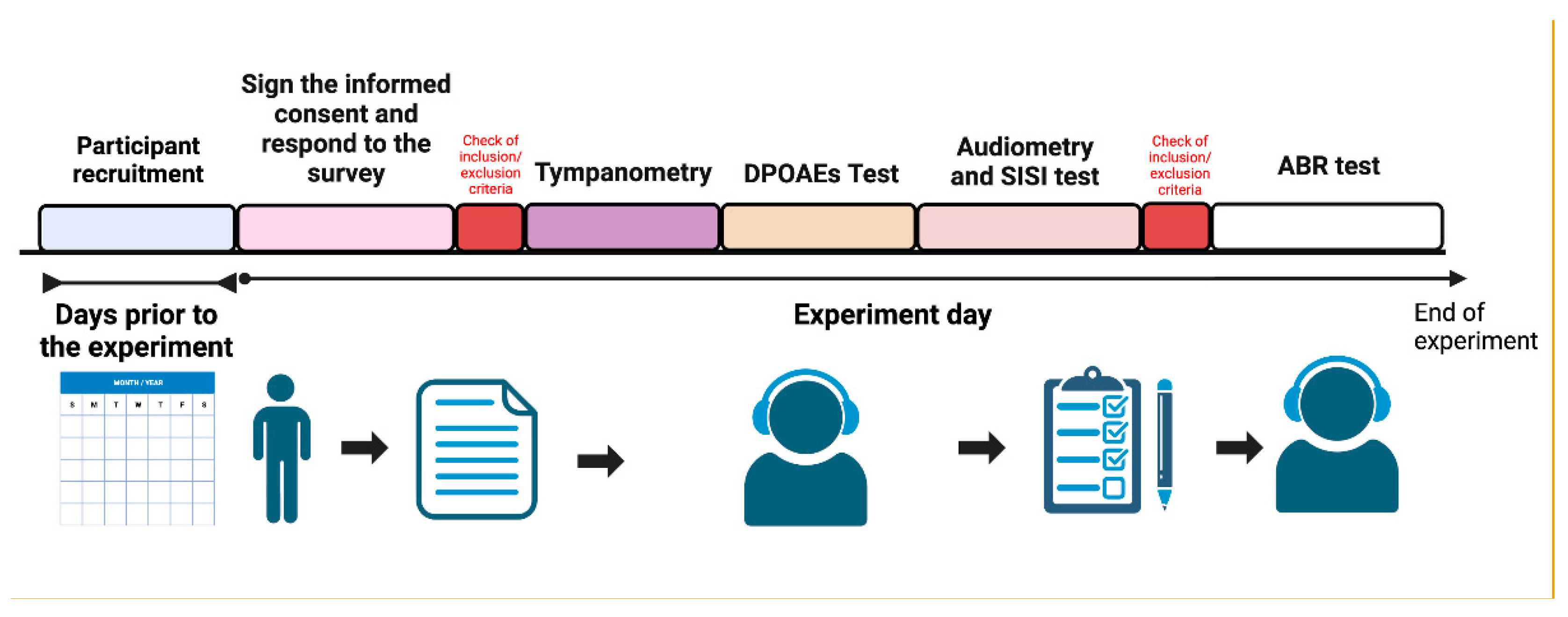
All evaluations were conducted in a sound-attenuated chamber with an average ambient noise level of 30 dB. An expert audiologist performed them. Neither the participants nor the experimenter knew to which group the participants belonged. We evaluated normality for each statistical test performed in this study using the Shapiro-Wilk test.
Subjects
Sixty-three Chilean volunteers, aged 18 to 35 years and native Spanish speakers, participated in this study. Participants were recruited through online platforms. Following data collection, participants were categorized into exposed (E) and unexposed (UE) groups based on their residential proximity to agricultural areas. Evaluations were conducted blinded, with group assignments occurring after completing all assessments. Twelve individuals were excluded due to not meeting pre-established inclusion or exclusion criteria, resulting in a final sample size of 51. This specific age range (18-35 years) was selected to minimize the potential confounding effects of age-related hearing loss and more accurately assess pesticide exposure's impact on auditory function. Ethical approval for this study (project number 221-2020) was granted by the Ethical and Scientific Committee for Research on Human Subjects of the University of Chile, adhering to the principles outlined in the Declaration of Helsinki. All participants provided written informed consent.
We applied the following inclusion criteria to determine participant eligibility for the experimental group: people who live within a radius approximately 400 meters or less from the monoculture areas in the last three years and people who are 35 years old or younger. On the other hand, individuals with a medical history of persistent otitis during childhood, adolescence, and adulthood were excluded, as were those whose workplaces exposed them to constant or variable noise levels equal to or exceeding 80 dB; candidates who share their homes with family members who work in monoculture fields; volunteers involved in the management and cultivation of residential gardens and using agricultural chemicals. Additionally, individuals with neuropsychiatric conditions that could lead to heightened sensitivity to auditory stimuli and who chronically consumed ototoxic medications or substances linked to hearing loss were also ineligible [
25]. Finally, people who had had accidents that caused traumatic tympanic membrane ruptures or who had visible ruptures and/or a Type B tympanogram, earwax blockage, or a Pure-Tone Average (PTA) threshold of 20 dB or higher were omitted.
For the control (UE) group, we applied the same inclusion and exclusion criteria as the exposed group, with two key differences: participants in the control group lived more than 500 meters away from monoculture areas in the last three years. All other criteria, including age restrictions, medical history requirements, and audiological conditions, remained the same for both groups. This ensured that the primary difference between the groups was their pesticide exposure through proximity to agricultural areas or occupational activities.
The experimental protocol is shown in
Figure 1.
Agricultural Work and Pesticide Exposure
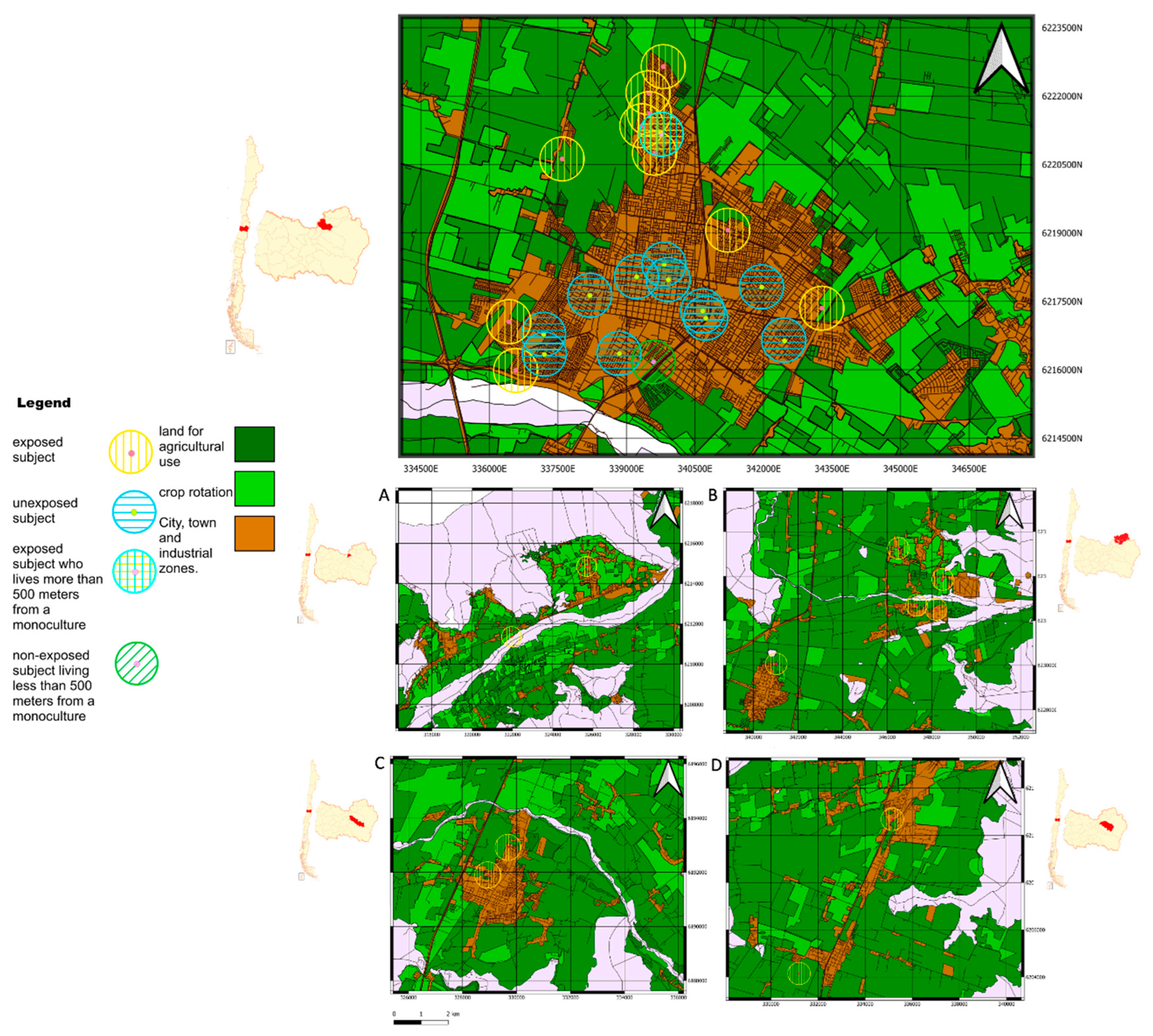
Upon arrival, participants completed a comprehensive questionnaire. This instrument gathered data on several key areas, including general health status, auditory health history, socioeconomic background, biographical information, occupational history, and detailed pesticide exposure history. The latter included inquiries about insecticide use in the home and personal gardening practices. Prior to its administration in the study, the questionnaire underwent pilot testing to ensure clarity, validity, and reliability of the collected data (Supplementary material 1 shows the questionnaire translated into English). To categorize participants based on their residential history over the past three years, we used Geographic Information System (GIS) software (QGIS version 3.30.0) to calculate the distance between their residences and nearby fruit monoculture fields. This software facilitated the analysis, resulting in a geographic map that depicted information about agricultural monocultures, crop rotation fields, and urban settlements (
Figure 1). We employed the proximity criterion established by Fenske et al., (2002) to define pesticide exposure, classifying individuals residing approximately 400-meter radius of a monoculture field as exposed. This threshold was selected due to the prevalence of organophosphate (OP) pesticide spraying in the region. For constructing the map, we utilized two layers and locations: the 2017 Census, which is the latest Census in Chile as of the publication date, and the "
Land Use Change Monitoring in the O'Higgins Metropolitan Region, 2016" layer from the IDE [
26] for establishing monoculture layers and addresses of participants studied in the research.
Hearing Assessment
Pure-Tone Audiogram Thresholds
We captured air thresholds between 125 and 16000 Hz using an AC40 audiometer (Interacoustics™®, Middelfart, Denmark), DD45 headphones, and a B-71 bone oscillator. Our approach adhered to the clinical standards outlined by ANSI S3.6, 2010. To check the exclusion criteria, we calculated the pure-tone average hearing threshold for frequencies 0.5, 1, 2, and 4 kHz (PTA). Then, we determined the audiometric threshold until 16KHz. The SISI (Short Increment Sensitivity Index) test was used to evaluate the ability to detect intensity increments. For the SISI test, we aimed to identify cochlear injury with a specific frequency 20 dB above the audiometric threshold for two minutes. A total of 20 distinct sound modulations occurs within this two-minute testing window. The SISI assessment is iterated across frequencies of 500, 1000, 2000, 4000, and 8000 Hz. Participants are directed to signal any perceived variations in loudness through a response trigger linked to a counter.
Distortion-Product Otoacoustic Emissions (DPOAE)
To quantify DPOAE, we employed the Titan Interacoustics system. Primary tones (f1 and f2) were introduced at 65- and 55-dB SPL, respectively, with an f2/f1 ratio 1.22. We employed six distinct f2 frequencies: 1000, 1500, 2000, 3000, 4000, and 6000 Hz, measured in both right and left ears. The presence of DPOAEs was automatically determined by the equipment and visually confirmed by an expert audiologist.
Auditory Brainstem Responses
Our suprathreshold ABR waves I and V measurements utilized the Eclipse EP25 instrument alongside authorized research equipment (Interacoustics™, Middelfart, Denmark) to induce the ABR response. Stimuli were presented as CE-chirps through ER-2B insert earphones at intensities of 80 dB, each lasting for 100 μs. Responses were recorded using active electrodes positioned on both mastoids and the vertex as reference points and a ground electrode placed on the lower forehead. Waves I and V were identified using two sets of 4,000 repetitions and a 150/3000 Hz band-pass filter, and the recordings used had a reproducibility of 99%. To facilitate subsequent analysis, mean latencies and amplitudes of each participant's waves I and V were measured. The peak-to-valley amplitudes were determined by assessing the distance between the positive and negative peaks through expert visual inspection by three independent experienced audiologists. The wave V/I ratio was calculated by dividing the amplitude of wave V by that of wave I [
27]. When a wave was absent or could not be identified, it was excluded from the respective analysis.
3. Results
Study Population and Geographical Characterization
A total of 63 participants were initially included in this research, with 51 meetings all study inclusion criteria after applying our strict selection protocol. We implemented a comprehensive screening methodology to address potential confounding variables between rural and urban settings and ensure that any observed differences could be specifically attributed to indirect pesticide exposure. All participants completed a detailed survey (Appendix 1) that evaluated previous exposure to ototoxic substances, history of acoustic trauma, occupational noise exposure, family history of hearing disorders, previous ear pathologies, and current auditory symptoms. The information collected also included socioeconomic and demographics status, employment history, and a detailed description of pesticide exposure, including insecticide use in the home and personal gardening activities. This survey, combined with our exclusion criteria, allowed us to control factors that could affect auditory function independently of pesticide exposure.
The study comprised two groups: the "E" group (n=31) with a mean age of 25.94 ± 6.45 years and the "UE" group (n=20) with a mean age of 28.2 ± 5.34 years, showing no significant age difference when analyzed using the Mann-Whitney test (p=0.152). The sex distribution, assessed through Fisher's Exact Test, showed no significant differences (p=0.147), with the exposed group consisting of 10 males (32.26%) and 21 females (67.74%), while the unexposed group had 11 males (55%) and nine females (45%). A chi-square test revealed no significant differences in economic perception between groups (p=0.502) regarding socioeconomic factors. In the exposed group, 13 participants (41.94%) reported high economic status, 16 (51.61%) medium, and 2 (6.45%) low, while in the unexposed group, 11 (55%) reported high status, 7 (35%) medium, and 2 (10%) low. Lifestyle factors were also comparable between groups, with Fisher's Exact Test showing no significant differences in alcohol consumption (exposed: 54.84% yes, 45.16% no; unexposed: 60% yes, 40% no; p=0.778) or smoking habits (exposed: 22.58% yes, 77.42% no; unexposed: 30% yes, 70% no; p=0.743).
Previous agricultural work experience, analyzed using Fisher's Exact Test, showed no significant differences between groups (p=0.348), with 11 participants (35.48%) in the exposed group and 4 (20%) in the unexposed group reporting prior farming experience that did not meet the inclusion criteria threshold of four seasons (equivalent to 4-6 months) of agricultural work. This prior experience typically consisted of sporadic or short-term agricultural work that fell below the exposure threshold required for classification in the exposed group. We specifically excluded individuals with workplace noise exposure ≥80 dB, chronic exposure to ototoxic medications, history of recurrent ear infections, recent acoustic trauma, family history of early onset hearing loss, or neurological conditions affecting auditory processing. The sole significant difference between groups, determined through t-test analysis, was their proximity to agricultural areas. The exposed group resided at a mean distance of 281.23 ± 371.19 meters from agricultural activities, while the unexposed group's average distance was 2872.89 ± 3367.88 meters (p<0.0001). This careful matching of groups across multiple variables, combined with our comprehensive hearing health assessment, provides a robust methodological framework for investigating the specific effects of pesticide exposure on auditory function.
The study of pesticide use in this region is critical due to its substantial contribution to national pesticide sales. In 2019, the region accounted for over half (51.23%) of all declared pesticide sales nationwide, with a volume of 28,019,055.99 kg/L out of the country's total sales of 54,451,488.15 kg/L [
28]. Consequently, the region exhibits a notable disparity between its agricultural area and pesticide application. While occupying only 8.2% of the national land dedicated to crops, it accounts for approximately 51.23% of the total pesticide use in the country, demonstrating a remarkably high intensity of agrochemical application [
28,
29]. Using Geographic Information System (GIS) mapping provided by the Chilean government, participants were classified based on their residential proximity to agricultural areas. A geo-referenced map (
Figure 2) illustrates the participants' residences in relation to the nearest agricultural fields. Rancagua, Doñihue, San Francisco de Mostazal, Rengo and Requinoa are cities in Chile characterized by residential areas located near to agricultural zones, with many of its residential neighborhoods falling within the exposure radius of farming activities. This classification based on residential proximity and occupational exposure is fundamental to understanding the potential impact of agricultural spraying and related activities on participants, playing a crucial role in interpreting subsequent findings related to auditory health and audiological risks associated with agricultural environments. Therefore, the significant differences observed in auditory measures between groups can be more confidently attributed to pesticide exposure rather than to general rural-urban lifestyle differences or other environmental factors.
Audiological and Psychoacoustic Evaluations
All subjects were evaluated with electrophysiological and perceptual auditory tests, including tonal audiometry, high frequency audiometry, the Short Increment Sensitivity Index (SISI) test, DPOAEs and ABR. A rigorous health examination confirmed the absence of outer and middle ear pathologies, absence of diagnosed neurological diseases, absence of chronic pharmacological and/or ototoxic therapy, absence of exposure to intense and/or occupational noise, and absence of tinnitus.
Conventional Audiometry, High-Frequency Hearing Thresholds, and SISI Test
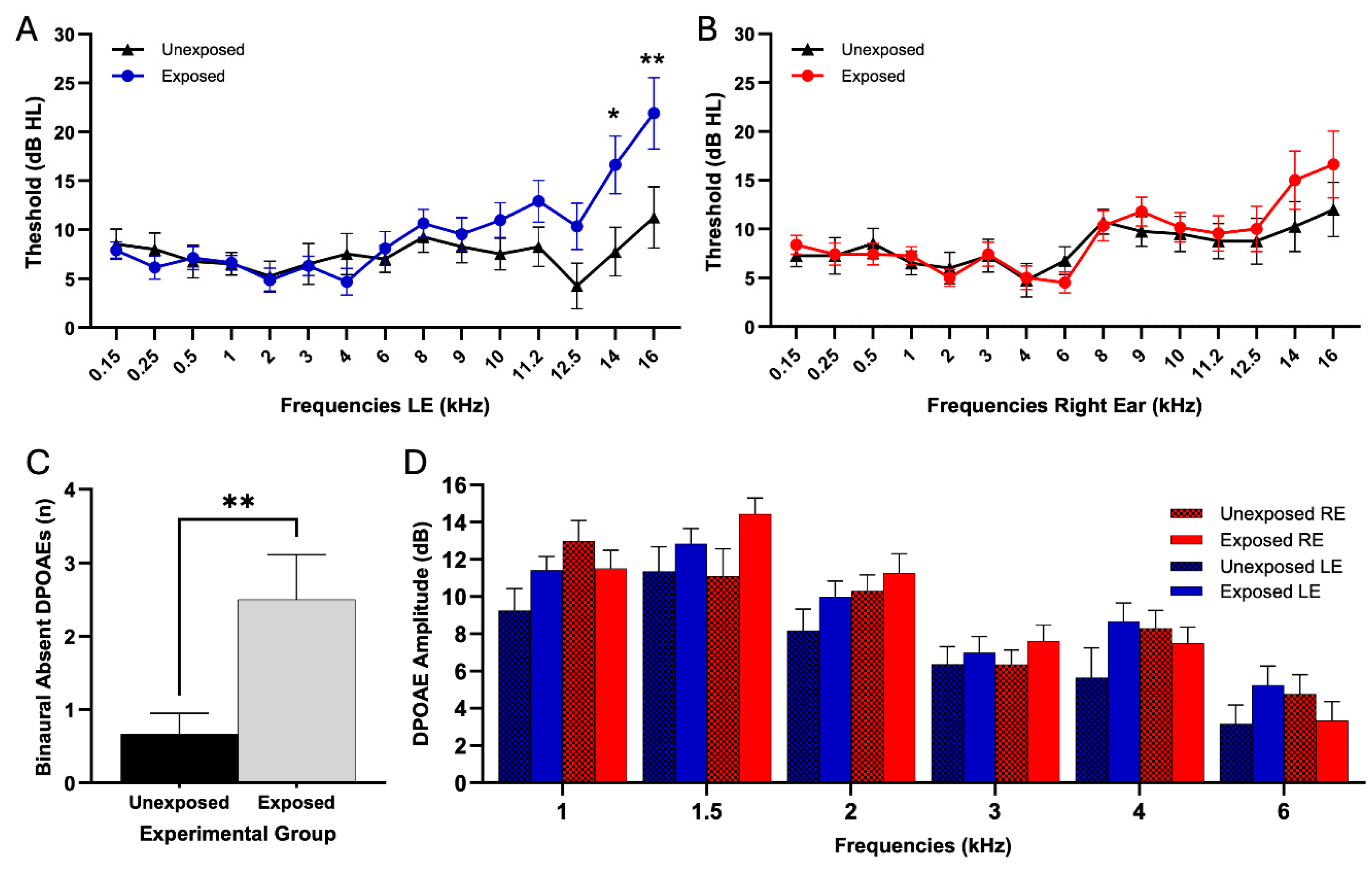
Left ear audiometric evaluation was performed examining 15 frequencies from 0.15 to 16 kHz (
Figure 3A). Two-way ANOVA revealed significant interaction effects of pesticide exposure on frequencies (F (14,733) = 1.973, p = 0.02). Bonferroni's multiple comparisons test showed significant differences at 14 kHz, where the exposed group showed significantly higher auditory thresholds than the unexposed group (E: 16.61 ± 16.45 dB; UE: 7.75 ± 11.06 dB; p = 0.02). At 16 kHz, the exposed group also showed significantly higher thresholds (E: 21.90 ± 19.66 dB; UE: 11.25 ± 13.94 dB; p = 0.002). All other frequencies evaluated in this ear showed no significant differences between groups (see
Table 1). Right ear audiometric evaluation was performed using the same protocol (
Figure 3B). Two-way analysis of variance showed no significant interaction between exposure and frequencies (F(14,732) = 0.541, p = 0.91). The results of Bonferroni's multiple comparisons test are presented in
Table 1. The SISI test ANOVA analysis revealed no significant interaction between frequencies and pesticide exposure in either ear. In the left ear, exposure showed no significant effect on frequencies responses (F (4,245) = 0, p = 0.78). Bonferroni's multiple comparisons test showed no significant differences between groups at 0.5 kHz (p = 0.46), 1 kHz (p > 0.99), 2 kHz (p > 0.99), 4 kHz (p > 0.99), and 8 kHz (p > 0.99). Similarly, in the right ear, exposure showed no significant effect on frequencies (F (4, 245) = 0, p = 0.8363), with Bonferroni's multiple comparisons test showing no significant differences between groups at 0.5 kHz (p > 0.9999), 1 kHz (p > 0.9999), 2 kHz (p > 0.9999), 4 kHz (p > 0.9999), and 8 kHz (p = 0.3455).
Otoacoustic Emissions
Analysis of DPOAEs was conducted to assess cochlear function in exposed and unexposed groups. We quantified the total number of absent DPOAEs for each frequency in both ears, comparing the total of absent DPOEAs in each subject between exposed and unexposed groups. Shapiro-Wilk test for normality revealed non-normal distributions (exposed: p=0.04, unexposed: p=0.002). Given the non-normal distribution, we used the Mann-Whitney U test for statistical comparison. Analysis of DPOAE absence revealed differences between exposed and unexposed groups. The exposed group showed a significantly higher number of absent DPOAEs than the unexposed group (E: 2.50 ± 2.11, UE: 0.667 ± 0.985, p = 0.007,
Figure 3B). These findings suggest an impact on outer hair cell function in pesticide-exposed individuals, manifesting as a higher number of DPOAE absence. This pattern resembles observations in workers exposed to industrial noise [
30] and older individuals with moderate presbycusis [
31].
DPOAE amplitude analysis in the left ear showed no significant interaction between frequency and exposure (F (5,274) = 0.30, p = 0.91). Bonferroni's multiple comparisons test showed no significant differences between exposed and unexposed groups at any tested frequency: at 1 kHz (exposed: 11.42±5.306 dB, n=28; unexposed: 9.245±4.912 dB, n=20; p = 0.83), 1.5 kHz (exposed: 12.84±4.747 dB, n=30; unexposed: 11.36±6.600 dB, n=20; p > 0.99), 2 kHz (exposed: 9.984±5.722 dB, n=31; unexposed: 8.184±3.893 dB, n=19; p > 0.99), 3 kHz (exposed: 6.982±4.513 dB, n=28; unexposed: 6.370±3.497 dB, n=20; p > 0.99), 4 kHz (exposed: 8.862±4.690 dB, n=29; unexposed: 5.642±4.158 dB, n=19; p = 0.25), and 6 kHz (exposed: 5.246±5.099 dB, n=24; unexposed: 3.161±4.315 dB, n=18; p > 0.99). In the right ear, no significant interaction between frequency and exposure was found (F (5,276) = 1.74, p = 0.1260). Bonferroni's multiple comparisons test revealed no significant differences between exposed and unexposed groups at any frequency: at 1 kHz (exposed: 11.517±5.306 dB, n=30; unexposed: 12.985±4.912 dB, n=20; p > 0.9999), 1.5 kHz (exposed: 14.437±4.747 dB, n=30; unexposed: 11.095±6.600 dB, n=20; p = 0.1130), 2 kHz (exposed: 11.253±5.722 dB, n=30; unexposed: 10.295±3.893 dB, n=20; p > 0.9999), 3 kHz (exposed: 7.618±4.513 dB, n=28; unexposed: 6.350±3.497 dB, n=20; p > 0.9999), 4 kHz (exposed: 7.493±4.690 dB, n=29; unexposed: 8.305±4.158 dB, n=19; p > 0.9999), and 6 kHz (exposed: 3.344±5.099 dB, n=25; unexposed: 4.765±4.315 dB, n=17; p > 0.9999).
Impact of Pesticide Exposure on Auditory Brainstem Evoked Potentials (ABR)
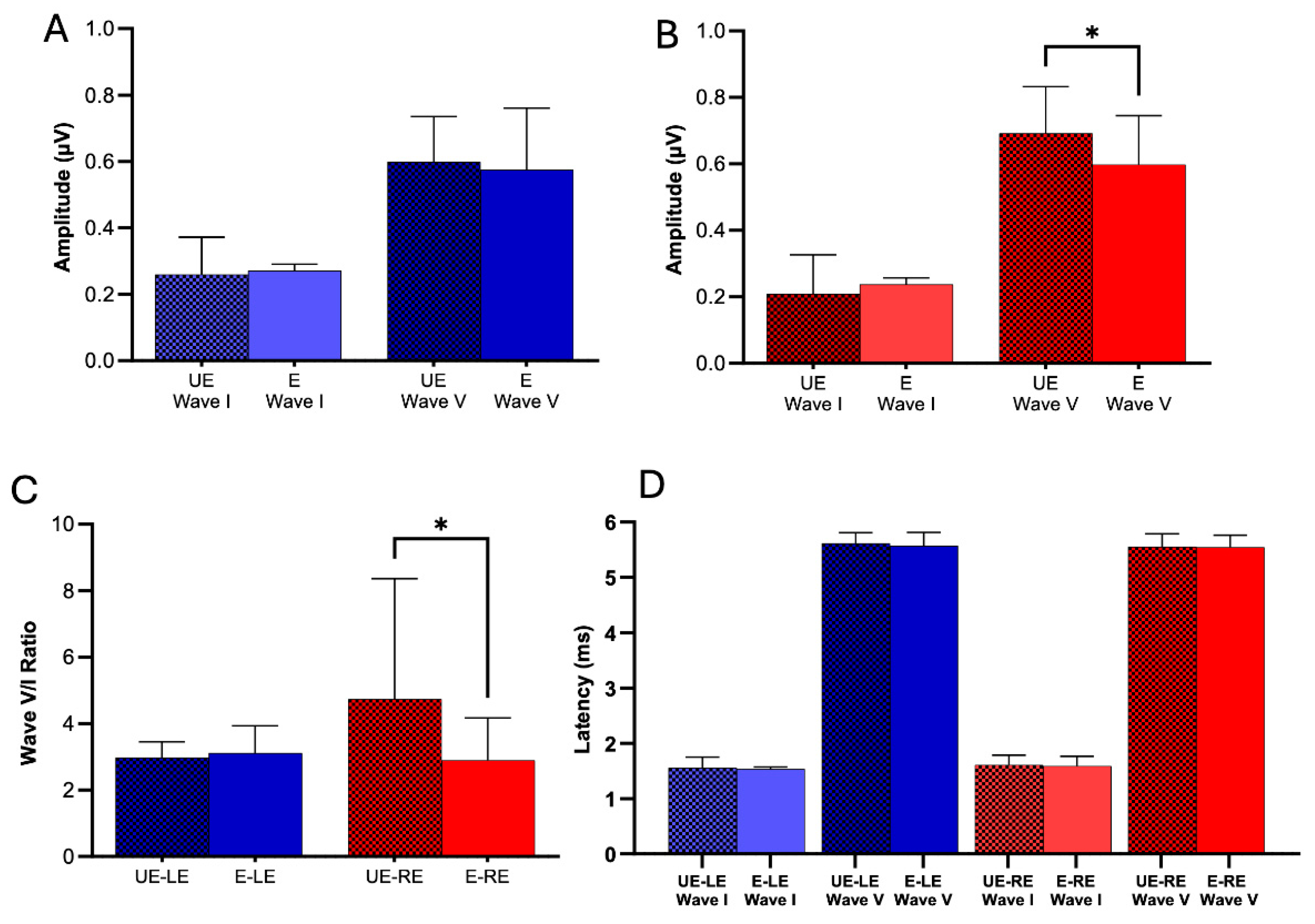
The ABR technique allows us to assess the integrity of the auditory system at both the neural and subcortical levels. In this study, wave I (auditory nerve) and wave V (inferior colliculus) were analyzed using the Shapiro-Wilk test and either the t-test or Mann-Whitney test, as appropriate, to compare the values of latencies and peak amplitudes in both groups. We studied the peak-to-valley amplitude of Wave I and Wave V. Our results reveal that pesticide exposure did not exert a significant influence on Wave I and V peak-to-peak amplitude in the left ear (
Figure 4A. ABR Wave I left ear: E: 0.272 ± 0.101, n = 28; UE: 0.259 ± 0.113, n = 17; p = 0.70. ABR Wave V left ear: E: 0.576 ± 0.185, n = 29; UE: 0.599 ± 0.136, n = 18; p = 0.64.). In the right ear, we found no significant differences in wave I; however, for wave V, unexposed subjects had a significantly greater amplitude than exposed subjects (
Figure 4B. ABR Wave I right ear: E: 0.238 ± 0.103, n = 28; UE: 0.209 ± 0.117, n = 18; p = 0.38. ABR Wave V right ear: E: 0.597 ± 0.148, n = 28; UE: 0.692 ± 0.140, n = 19; p = 0.01). Additionally, when analyzing the wave V/I ratio in the right ear, unexposed individuals showed a significantly higher ratio than those exposed to pesticides. In contrast, no significant changes were observed in the left ear (
Figure 4C. Ratio Wave V/I right ear: E: 2.89 ± 1.27, n = 28; UE: 4.74 ± 3.62, n = 18; p = 0.03. Ratio Wave V/I left ear: E: 3.11 ± 4.36, n = 28; UE: 2.98 ± 1.96, n = 17; p = 0.42).
In the latency analysis, our results reveal that pesticide exposure did not significantly influence Wave I and V latency in the left ear (ABR Wave I left ear: E: 1.54 ± 0.182, n = 28; UE: 1.56 ± 0.192, n = 17; p = 0.73. ABR Wave V left ear: E: 5.57 ± 0.246, n = 29; UE: 5.61 ± 0.196, n = 18; p = 0.36. Table 2E). Similarly, in the right ear, we obtained comparable results (
Figure 4D. ABR Wave I right ear: E: 1.59 ± 0.182, n = 28; UE: 1.61 ± 0.179, n = 18; p = 0.68. ABR Wave V right ear: E: 5.55 ± 0.215, n = 28; UE: 5.55 ± 0.236, n = 19; p = 0.92).
4. Discussion
Pesticide exposure constitutes a significant public health concern in Chile due to the intensive application of these agrochemicals and a relative lack of consistent regulatory oversight. In general, pesticide use can have detrimental acute and chronic effects on human health, impacting various organ systems [
32]. Prior research has also documented associations between pesticide exposure and auditory system dysfunction [
6,
33,
34,
35,
36,
37]. In the present study, we report several auditory dysfunctions associated with pesticide exposure, including (i) elevated high-frequency auditory thresholds (
Figure 3a) and (ii) reduced wave V amplitudes of auditory brainstem evoked potentials (
Figure 4b). The O'Higgins Region stands out for its significant importance in the Chilean agricultural sector. Its contribution to the national agricultural and forestry sector represents 18.6% of this sector's Gross Domestic Product (GDP), while at the regional level, agricultural and forestry activities constitute 12.3% of the regional GDP [
29]. The region is particularly notable in fruit production, concentrating 24.8% of the national area dedicated to these crops. The region has 8.2% of the country's land area dedicated to cultivation, and this intense agricultural activity and high concentration of fruit crops characterize the region as an area with significant exposure to intensive agricultural practices [
29]. The city of Rancagua, the regional capital, shares environmental characteristics like those of the surrounding rural areas. Rancagua's urban core, though featuring an extensive network of bicycle paths, remains a relatively compact area. Its primary economic activities, centered on service provision and commerce, do not typically generate high environmental noise levels. Consequently, noise pollution in Rancagua, much like in the adjacent rural areas, is not considered a significant contributing factor to residents' auditory health. Notably, most UE participants in this study reside in Rancagua. This similarity in environmental noise exposure between Rancagua and the rural agricultural areas strengthens the study's focus on the potential impact of pesticide exposure on auditory function, as it minimizes the confounding influence of differential noise levels.
High-Frequency Thresholds, Speech Disturbance in Noise and Pesticide Exposure
Our findings revealed significant differences in auditory thresholds at 14 and 16 kHz between pesticide-exposed and unexposed individuals in the left ear (
Figure 3a). These results are consistent with animal model studies (guinea pigs), indicating that these agents primarily target the OHCs of the cochlear base, potentially impairing hearing sensitivity [
38]. Supporting this, Reischl et al. [
39], using squirrel monkeys reported significant decreases in hearing thresholds between 0.5 and 6 kHz following 40 days of parathion exposure. The mechanisms by which pesticides damage hair cells are thought to involve inducing edema of the stria vascularis and promoting the secretion of pro-inflammatory cytokines and reactive oxygen species in the spiral ligament, leading to an inflammatory response and reduced cochlear blood flow. These reactive oxygen species and pro-inflammatory cytokines may then be translocated to cochlear hair cells, activating both extrinsic and intrinsic apoptotic cascades, which in turn can induce mitochondrial dysfunction and further drive apoptosis [
40]. In humans, studies have suggested that pesticide exposure may increase the risk of hearing loss, particularly at higher frequencies, and may also reduce otoacoustic emission amplitudes and efferent suppression by contralateral noise [
33,
36]. A prospective US study of 366 adults aged 20-69, using multivariate linear and logistic regression analyses, investigated the relationship between hearing loss and decreasing pure tone averages [
35]. We hypothesize that the observed changes in high-frequency auditory thresholds associated with pesticide exposure may contribute to speech processing difficulties in noise. These subtle effects, which have not yet been studied and are not readily apparent in our paradigm, deserve special attention, especially considering the young age of the population studied. More research is needed to explore the possible long-term impact of these high-frequency disturbances on speech understanding in noisy environments.
Does Exposure to Pesticides Impair the Outer Hair Cells Functions?
DPOAEs provide a noninvasive method to assess cochlear function, particularly the integrity of PHCs [
41]. Our analysis revealed a significantly higher number of absent DPOAEs in the exposed group than the unexposed group. These results suggest a potential impact on OHC function in pesticide-exposed individuals, resembling patterns seen in workers exposed to industrial noise [
30] and in individuals with moderate presbycusis [
31]. Interestingly, we did not find a significant difference in DPOAE amplitude. The nature of pesticide-induced cochlear damage might explain the absence of significant differences in DPOAE amplitudes at most frequencies between exposed and unexposed subjects. As our high-frequency audiometry results indicate (14 and 16 kHz thresholds), pesticide damage may primarily affect the basal turn of the cochlea, which corresponds to higher frequencies than those typically assessed by standard DPOAE protocols. This unexpected result warrants further investigation and may indicate the complex effects of pesticide exposure on cochlear function, suggesting that pesticide exposure may affect the OHCs, potentially altering the cochlear amplifier.
Electrophysiological Auditory Response and Exposure to Pesticides
Our results also showed a decrease in the amplitude of ABR wave V in the right ear of exposed subjects (
Figure 4b). This finding is consistent with previous studies that report differences in some ABR parameters between exposed and non-exposed adult subjects. Alcarás et al. [
33] found significant differences in the amplitude of ABR waves in adults exposed to pesticides, which aligns with our study, where we observed a significant reduction in wave V amplitude in the right ear. Similarly, studies on newborns exposed to pesticides, such as that by Sturza et al. [
42], also report alterations in ABR parameters, highlighting the sensitivity of the developing auditory system to these toxic agents.
Moreover, Dassanayake et al. [
43] demonstrated that pesticide exposure can affect electrophysiological responses, such as event-related potentials, suggesting a broader impact on neuronal function beyond ABR. Mora et al. [
44] also found that cortical potentials are affected in exposed individuals, indicating that pesticides can alter auditory function at multiple levels of the nervous system.
When comparing and analyzing the V/I ratio, we found that non-exposed subjects had a significantly higher ratio in the right ear than exposed subjects (
Figure 4c). This contrasts with the central gain model proposed by Schaette and McAlpine [
45], where an increase in wave V amplitude is produced by auditory input deprivation. In our study, the alteration would not be caused by decreased sensory input but by functional damage in both the cochlea and the auditory nerve induced by pesticides. In the latency analysis, our results revealed that pesticide exposure did not significantly influence the latencies of waves I and V in the left ear (
Figure 4D). Similarly, we obtained comparable results in the right ear, suggesting that although the amplitude of neural responses is affected in the right ear, the speed of neural transmission remains unchanged.
These findings highlight the asymmetric effects of pesticide exposure on the auditory system. The significant reduction in wave V amplitude and the V/I ratio in the right ear contrasts with the tonal audiometry results, which showed damage in the left ear. This may reflect different vulnerabilities within the auditory system, where pesticides affect both peripheral structures in the left ear and central auditory pathways in the right ear. Previous studies have suggested that pesticides can induce oxidative stress and inflammatory responses in the cochlea, contributing to the observed neuronal damage [
36,
46] .
A key strength of this study lies in its focus on a young population residing nearby but not directly working in agricultural fields. While previous research has often examined the effects of pesticide exposure on farmworkers, where the confounding influence of occupational noise exposure is a significant factor, our findings suggest that mere proximity to monoculture fields using pesticides may be sufficient to induce measurable auditory changes. This distinction is crucial. By isolating the impact of environmental pesticide exposure independent of occupational noise, our study provides compelling evidence for the potential vulnerability of individuals living in proximity to agricultural areas. This study underscores the urgent need for developing and implementing public health policies to protect the auditory health of populations residing near monoculture agricultural operations or forestry activities where pesticides are employed. These policies should prioritize preventative measures and regular monitoring to safeguard the hearing of these at-risk communities and mitigate the long-term consequences of environmental pesticide exposure. In the same sense, it is crucial to conduct further research to explore the underlying mechanisms and determine whether these auditory changes are reversible or indicative of long-term neuronal damage.
5. Conclusions
In this work, we have reported the existence of auditory dysfunction caused by exposure to pesticides. These dysfunctions were mainly observed in high-frequency audiometry, ABR wave amplitude, and the Wave V/I ratio. These results provide evidence of the risk of exposure to these agents in the auditory system. In this sense, it is highly desirable to generate better control mechanisms and new public policies to regulate the exposure of agricultural workers. Moreover, these results suggest the need for continuous audiological monitoring of people living near to farms, mainly involving the assessment of high-frequency audiometric thresholds and ABR test.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Hearing health survey.
Author Contributions
Conceptualization, G.T. and F.M.; Methodology, G.T. and F.M.; Validation, F.M. and G.T.; Formal Analysis, F.M., G.T., C.A-S and P.J-V. and F.P-A.; Investigation, F.M.; Resources, G.T.; Data Curation, F.M., F.P-A., C.A-S.; Writing—Original Draft Preparation, F.M., G.T., C.A-S. and E.A-V.; Writing—Review and Editing, F.M., G.T., C.A-S. and E.A-V.; Visualization, F.M., F.P-A. and P.J-V.; Supervision, G.T.; Project Administration, G.T.; Funding Acquisition, G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funding by FONDECYT Iniciación código 11200881.
Institutional Review Board Statement
The institutional affiliation of the Institutional Review Board that provided consent for the research: Ethical and Scientific Committee for Research on Human Subjects of the University of Chile. Project number: 221-2020 Principal Investigator: Gonzalo Terreros. This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained to mitigate potential confounding variables associated with age-related hearing loss and provide a more precise assessment of the possible effects of pesticides on hearing.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study (audiometric data, DPOAE measurements, ABR recordings, and demographic information) can be requested by contacting the corresponding author. All shared data will be appropriately de-identified to protect participant privacy.
Acknowledgments
G.T. acknowledges funding from FONDECYT Iniciación project No. 11200881.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WHO |
World Health Organization |
| OP |
Organophosphates |
| IHCs |
Inner Hair Cells |
| OHCs |
Outer Hair Cells |
| ABRs |
Auditory Brainstem Responses |
| DPOAEs |
Distortion-Product Otoacoustic Emissions |
| GIS |
Geographic Information System |
| PTA |
Pure-Tone Average |
| SISI |
Short Increment Sensitivity Index |
| SPL |
Sound Pressure Level |
| E |
Exposed (group) |
| UE |
Unexposed (group) |
| RE |
Right Ear |
| LE |
Left Ear |
| GDP |
Gross Domestic Product |
| SEM |
Standard Error of the Mean |
| dB |
Decibel |
| kHz |
Kilohertz |
| Hz |
Hertz |
| μV |
Microvolts |
References
- Al-Mana, D.; Ceranic, B.; Djahanbakhch, O.; Luxon, L.M. Alteration in Auditory Function during the Ovarian Cycle. Hear Res 2010, 268, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hesse, G. Innenohrschwerhörigkeit: Teil I: Prävalenz, Diagnostik Und Ätiologie. Laryngorhinootologie 2016, 95, 383–391. [Google Scholar] [CrossRef]
- Chadha, S.; Kamenov, K.; Cieza, A. The World Report on Hearing, 2021. Bull World Health Organ 2021, 99, 242–242A. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Jung, G.; Jang, M.; Suh, M.-W.; Lee, J. ho; Oh, S.-H.; Park, M.K. Association of Chocolate Consumption with Hearing Loss and Tinnitus in Middle-Aged People Based on the Korean National Health and Nutrition Examination Survey 2012–2013. Nutrients 2019, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.J.; Fuente, A. The Adverse Effects of Heavy Metals with and without Noise Exposure on the Human Peripheral and Central Auditory System: A Literature Review. Int J Environ Res Public Health 2016, 13. [Google Scholar] [CrossRef]
- Terreros, G.; Cifuentes-Cabello, C.; D’Espessailles, A.; Munoz, F. Impact of Pesticide Exposure on Auditory Health: Mechanisms, Efferent System Disruption, and Public Health Implications. Toxicology 2025, 154071. [Google Scholar] [CrossRef]
- Ames, R.G.; Steenland, K.; Jenkins, B.; Chrislip, D.; Russo, J. Chronic Neurologic Sequelae to Cholinesterase Inhibition among Agricultural Pesticide Applicators. Archives of Environmental Health: An International Journal 1995, 50, 440–444. [Google Scholar] [CrossRef]
- Körbes, D.; Silveira, A.F. da; Hyppolito, M.Â.; Munaro, G. Organophosphate-Related Ototoxicity: Description of the Vestibulocochlear System Ultrastructural Aspects of Guinea Pigs. Braz J Otorhinolaryngol 2010, 76, 238–244. [Google Scholar] [CrossRef]
- Petty, C.S. Organic Phosphate Insecticide Poisoning. Am J Med 1958, 24, 467–470. [Google Scholar] [CrossRef]
- Crawford, J. Mac; Hoppin, J.A.; Alavanja, M.C.R.; Blair, A.; Sandler, D.P.; Kamel, F. Hearing Loss among Licensed Pesticide Applicators in the Agricultural Health Study. J Occup Environ Med 2008, 50, 817–826. [Google Scholar] [CrossRef]
- Rabinowitz, P.M.; Sircar, K.D.; Tarabar, S.; Galusha, D.; Slade, M.D. Hearing Loss in Migrant Agricultural Workers. J Agromedicine 2005, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dundar, M.A.; Derin, S.; Aricigil, M.; Eryilmaz, M.A. Sudden Bilateral Hearing Loss after Organophosphate Inhalation. Turk J Emerg Med 2016, 16, 171–172. [Google Scholar] [CrossRef]
- Guida, H.L.; Morini, R.G.; Cardoso, A.C.V. Avaliação Audiológica Em Trabalhadores Expostos a Ruído e Praguicida. Braz J Otorhinolaryngol 2010, 76, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.; Brandão, M.F.A. Efeitos Dos Agrotóxicos No Sistema Auditivo Dos Trabalhadores Rurais. 1998, 19, 218.
- Perry, M.J.; May, J.J. Noise and Chemical Induced Hearing Loss. J Agromedicine 2005, 10, 49–55. [Google Scholar] [CrossRef]
- Prakash Krishnan Muthaiah, V.; Ding, D.; Salvi, R.; Roth, J.A. Carbaryl-Induced Ototoxicity in Rat Postnatal Cochlear Organotypic Cultures. Environ Toxicol 2017, 32, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Lobarinas, E.; Salvi, R.; Ding, D. Selective Inner Hair Cell Dysfunction in Chinchillas Impairs Hearing-in-Noise in the Absence of Outer Hair Cell Loss. JARO - Journal of the Association for Research in Otolaryngology 2016, 17, 89–101. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. Journal of Neuroscience 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Salvi, R.J.; Ding, D.; Wang, J.; Jiang, H.-Y. A Review of the Effects of Selective Inner Hair Cell Lesions on Distortion Product Otoacoustic Emissions, Cochlear Function and Auditory Evoked Potentials. Noise Health 2000, 2, 9–26. [Google Scholar]
- Helleman, H.W.; Dreschler, W.A. Overall versus Individual Changes for Otoacoustic Emissions and Audiometry in a Noise-Exposed Cohort. Int J Audiol 2012, 51, 362–372. [Google Scholar] [CrossRef]
- Plack, C.J.; Barker, D.; Prendergast, G. Perceptual Consequences of “Hidden” Hearing Loss. Trends Hear 2014, 18. [Google Scholar] [CrossRef]
- Lobarinas, E.; Salvi, R.; Ding, D. Insensitivity of the Audiogram to Carboplatin Induced Inner Hair Cell Loss in Chinchillas. Hear Res 2013, 302, 113–120. [Google Scholar] [CrossRef]
- Maison, S.F.; Usubuchi, H.; Liberman, M.C. Efferent Feedback Minimizes Cochlear Neuropathy from Moderate Noise Exposure. J Neurosci 2013, 33, 5542–5552. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Fritz, J.B.; Shamma, S.A. Rapid Spectrotemporal Plasticity in Primary Auditory Cortex during Behavior. The Journal of Neuroscience 2014, 34, 4396–4408. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.G.; Lee, J.A.; Liu, Y.F.; Endriukaitis, L.; Isaac, J.L.; Bullington, W.M. Drug-Induced Ototoxicity: A Comprehensive Review and Reference Guide. Pharmacotherapy 2020, 40, 1265–1275. [Google Scholar] [CrossRef]
- Ministerio de Agricultura (MINAGRI) Descargas de capas IDE MINAGRI. Available online: https://ide.minagri.gob.cl/geoweb/descargas/ (accessed on 7 February 2024).
- Shim, H.J.; An, Y.-H.; Kim, D.H.; Yoon, J.E.; Yoon, J.H. Comparisons of Auditory Brainstem Response and Sound Level Tolerance in Tinnitus Ears and Non-Tinnitus Ears in Unilateral Tinnitus Patients with Normal Audiograms. PLoS One 2017, 12, e0189157. [Google Scholar] [CrossRef] [PubMed]
- Servicio Agrícola Ganadero (SAG), C. Declaración de Ventas de Plaguicidas de Uso Agrícola Año 2019; 2020;
- Liliana Yáñez Barrios Región Del Libertador Bernardo O´Higgins; 2021;
- Korres, G.S.; Balatsouras, D.G.; Tzagaroulakis, A.; Kandiloros, D.; Ferekidou, E.; Korres, S. Distortion Product Otoacoustic Emissions in an Industrial Setting. Noise Health 2009, 11, 103–110. [Google Scholar] [CrossRef]
- Belkhiria, C.; Vergara, R.C.; Martín, S.S.; Leiva, A.; Marcenaro, B.; Martinez, M.; Delgado, C.; Delano, P.H. Cingulate Cortex Atrophy Is Associated with Hearing Loss in Presbycusis with Cochlear Amplifier Dysfunction. Front Aging Neurosci 2019, 11. [Google Scholar] [CrossRef]
- Zúñiga-Venegas, L.; Saracini, C.; Pancetti, F.; Muñoz-Quezada, M.T.; Lucero, B.; Foerster, C.; Cortés, S. Exposición a Plaguicidas En Chile y Salud Poblacional: Urgencia Para La Toma de Decisiones. Gac Sanit 2021, 35, 480–487. [Google Scholar] [CrossRef]
- Alcarás, P.A. de S.; Zeigelboim, B.S.; Corazza, M.C.A.; Lüders, D.; Marques, J.M.; Lacerda, A.B.M. de Findings on the Central Auditory Functions of Endemic Disease Control Agents. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- França, D.M.V.R.; Lacerda, A.B.M.; Lobato, D.; Ribas, A.; Dias, K.Z.; Leroux, T.; Fuente, A. Adverse Effects of Pesticides on Central Auditory Functions in Tobacco Growers. Int J Audiol 2017, 56, 233–241. [Google Scholar] [CrossRef]
- Long, L.; Tang, X. Exploring the Association of Organochlorine Pesticides Exposure and Hearing Impairment in United States Adults. Sci Rep 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Sena, T.R.R.; Dourado, S.S.F.; Lima, L. V; Antoniolli, Â.R. The Hearing of Rural Workers Exposed to Noise and Pesticides. Noise Health 2018, 20, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Vaz, W.F.; D’Oliveira, G.D.C.; Perez, C.N.; Neves, B.J.; Napolitano, H.B. Machine Learning Prediction of the Potential Pesticide Applicability of Three Dihydroquinoline Derivatives: Syntheses, Crystal Structures and Physical Properties. J Mol Struct 2020, 1206, 127732. [Google Scholar] [CrossRef]
- Finkler, A.D.; Silveira, A.F. da; Munaro, G.; Zanrosso, C.D. Otoproteção Em Cobaias Expostas a Agrotóxico e Ginkgo Biloba. Braz J Otorhinolaryngol 2012, 78, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Reischl, P.; Gelder, G.A. Van; Karas, G.G. Auditory Detection Behavior in Parathion-Treated Squirrel Monkeys (Saimiri Sciureus). Toxicol Appl Pharmacol 1975, 34, 88–101. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, H. Research Progress on Mitochondrial Dysfunction in Diabetic Retinopathy. Antioxidants 2022, 11, 2250. [Google Scholar] [CrossRef]
- Veuillet, E.; Collet, L.; Duclaux, R. Effect of Contralateral Acoustic Stimulation on Active Cochlear Micromechanical Properties in Human Subjects: Dependence on Stimulus Variables. J Neurophysiol 1991, 65, 724–735. [Google Scholar] [CrossRef]
- Sturza, J.; Silver, M.K.; Xu, L.; Li, M.; Mai, X.; Xia, Y.; Shao, J.; Lozoff, B.; Meeker, J. Prenatal Exposure to Multiple Pesticides Is Associated with Auditory Brainstem Response at 9months in a Cohort Study of Chinese Infants. Environ Int 2016, 92–93, 478–485. [Google Scholar] [CrossRef]
- Dassanayake, T.; Gawarammana, I.B.; Weerasinghe, V.; Dissanayake, P.S.; Pragaash, S.; Dawson, A.; Senanayake, N. Auditory Event-Related Potential Changes in Chronic Occupational Exposure to Organophosphate Pesticides. Clinical Neurophysiology 2009, 120, 1693–1698. [Google Scholar] [CrossRef]
- Mora, A.M.; Baker, J.M.; Hyland, C.; Rodríguez-Zamora, M.G.; Rojas-Valverde, D.; Winkler, M.S.; Staudacher, P.; Palzes, V.A.; Gutiérrez-Vargas, R.; Lindh, C.; et al. Pesticide Exposure and Cortical Brain Activation among Farmworkers in Costa Rica. Neurotoxicology 2022, 93, 200–210. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. Journal of Neuroscience 2011, 31, 13452–13457. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. A Review on the Ethical Issues in Neurotechnology. Theoretical and Natural Science 2023, 4, 708–712. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).