Submitted:
12 February 2025
Posted:
13 February 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
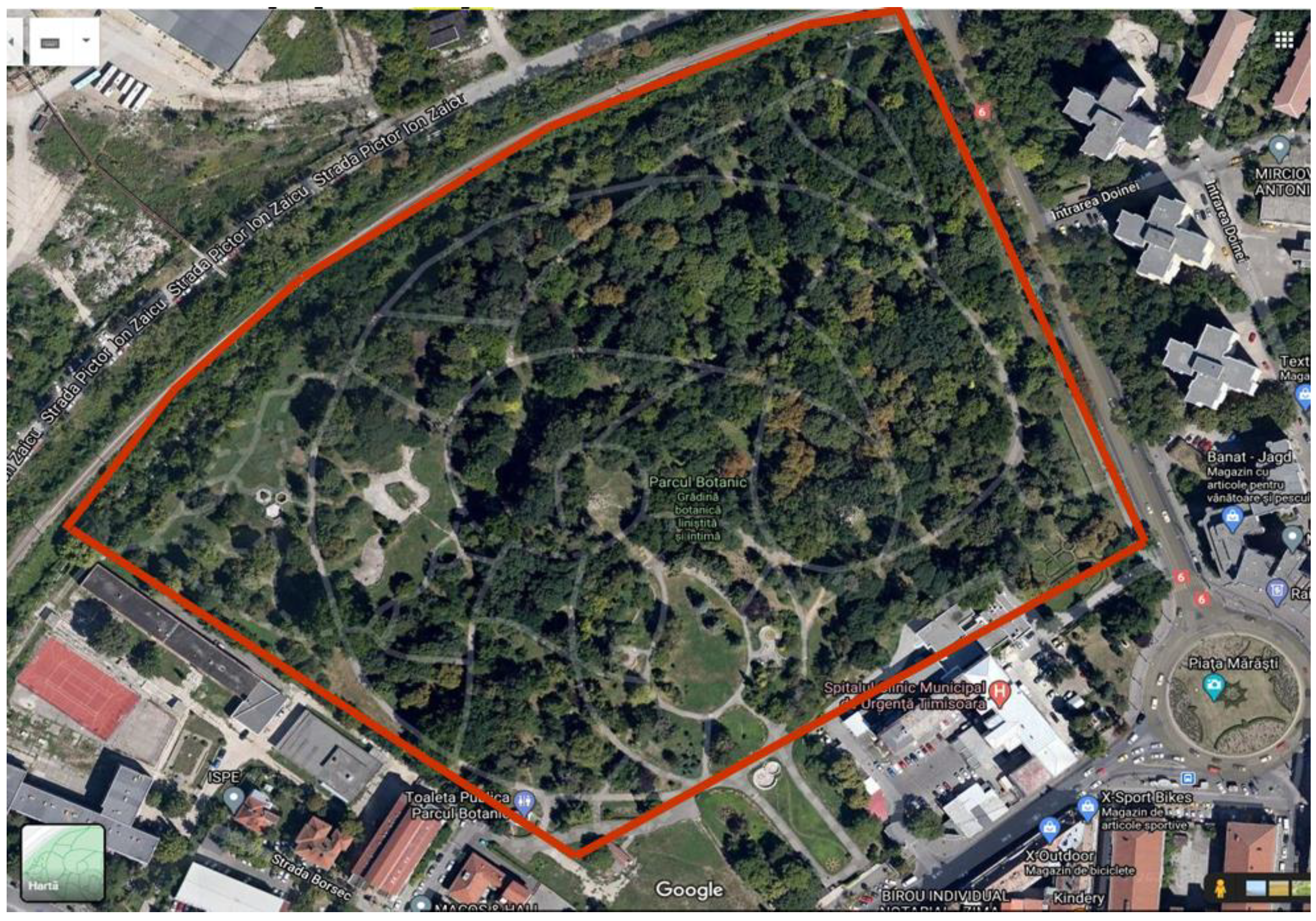
Research Site
Research Methodology
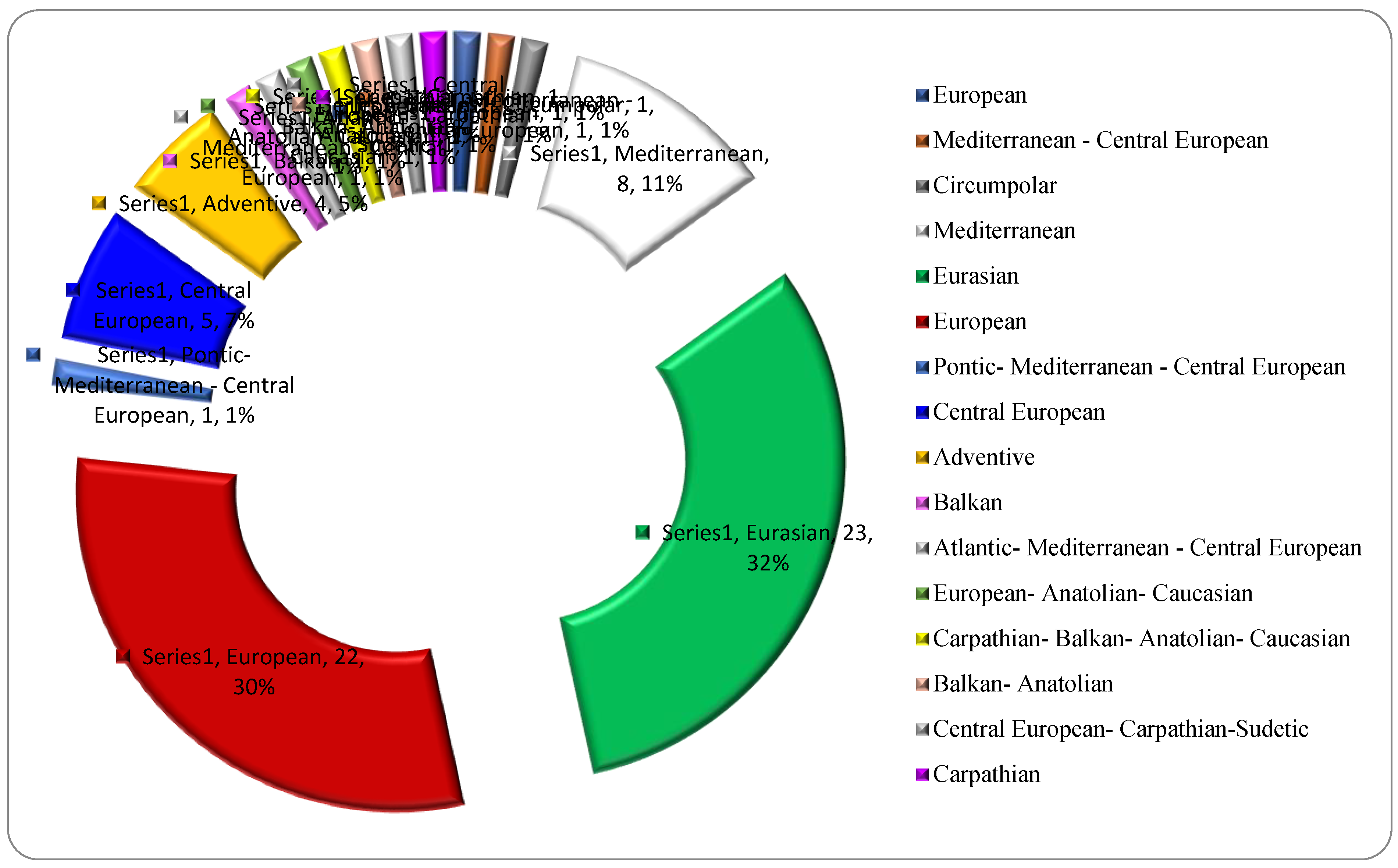
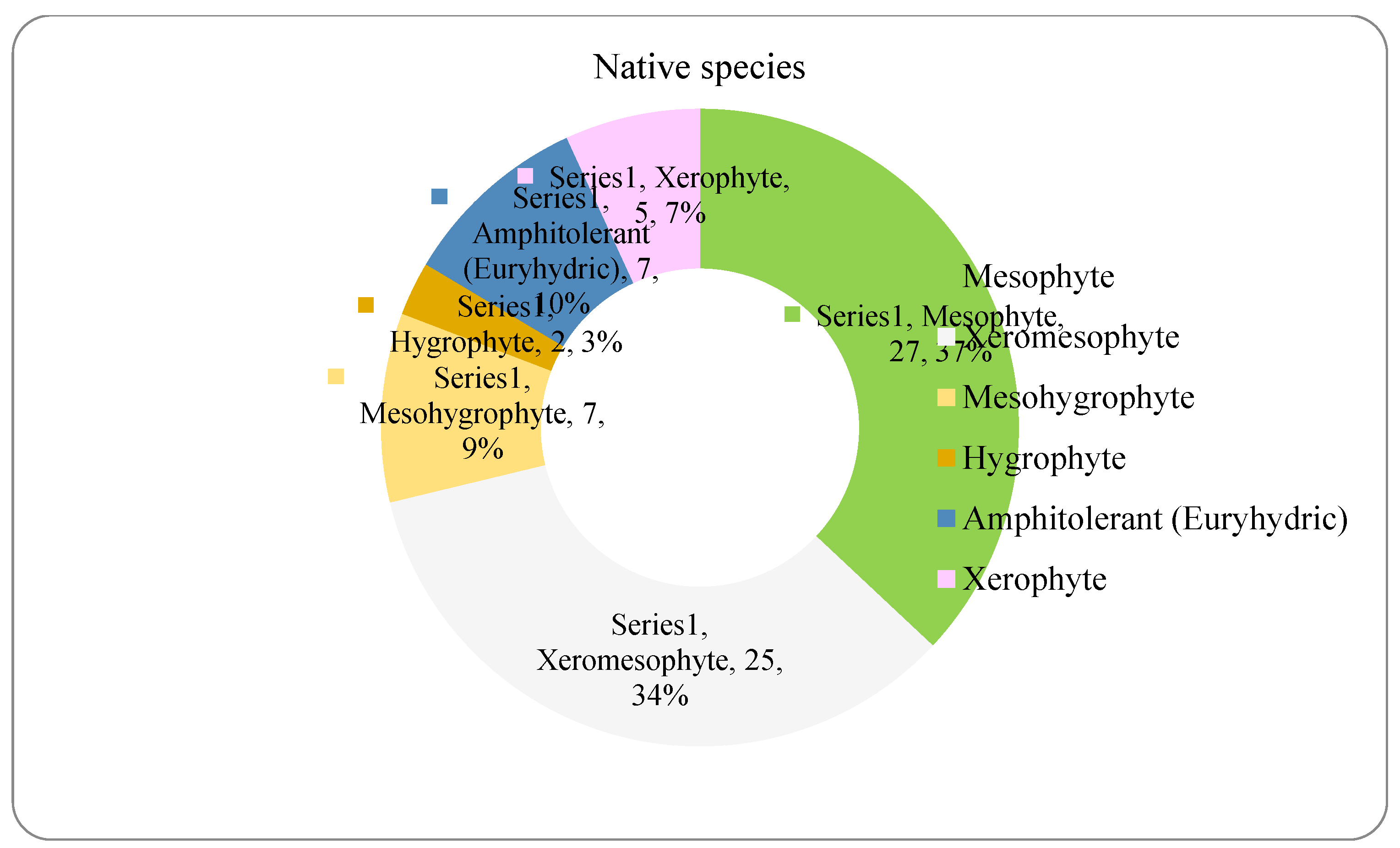
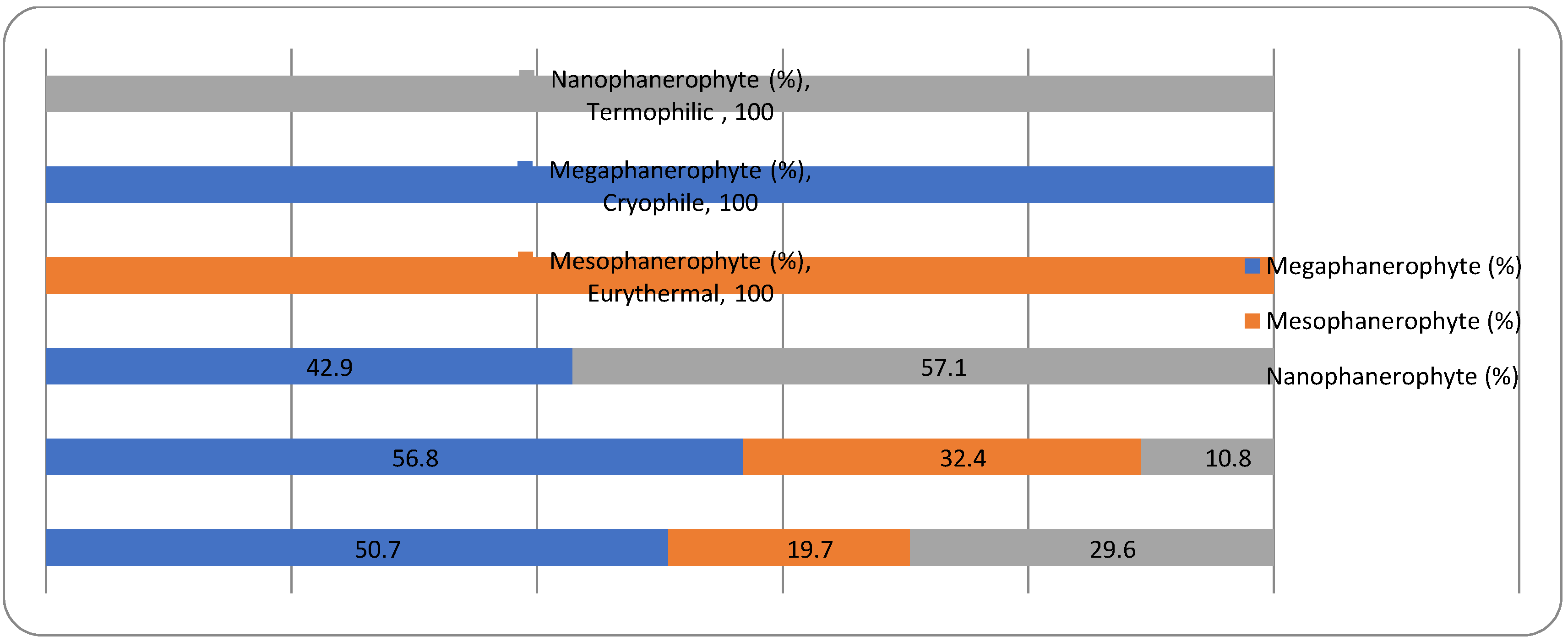
Results and Discussion
Conclusions
Acknowledgments
References
- Andronova, M., and A. Platonov. 2022. Sucrose in the tissues of annual shoots of introduced woody plants. Lesnoy Zhurnal-Forestry Journal 1: 62–76. [Google Scholar] [CrossRef]
- Bartoli, F., V. Savo, and G. Caneva. 2022. Biodiversity of urban street trees in Italian cities: a comparative analysis. Plant Biosystems 156, 3: 649–662. [Google Scholar] [CrossRef]
- Campbell-Arvai, V., R. Vergel, M. Lindquist, N. Fox, and D. Van Berkel. 2024. Tree selection for a virtual urban park: Comparing aided and unaided decision-making to support public engagement in greenspace design. Urban Forestry & Urban Greening 99: 128447. [Google Scholar] [CrossRef]
- Carpenter, W., and A. Goodenough. 2014. How robust are community-based plant bioindicators? Empirical testing of the relationshipbetween Ellenberg values and direct environmental measures in woodland communities. Community Ecology 15: 1–11. [Google Scholar] [CrossRef]
- Chew, Y. H., A. M. Wilczek, M. Williams, S. M. Welch, J. Schmitt, and K. J. Halliday. 2012. An augmented Arabidopsis phenology model reveals seasonal temperature control of flowering time. New Phytologist 194, 3: 654–665. [Google Scholar] [CrossRef]
- Chytrý, M., L. Tichý, and J. Roleček. 2002. Local and regional patterns of species richness in Central European vegetation types along the pH/calcium gradient. Folia Geobotanica 38: 429–442. [Google Scholar] [CrossRef]
- Ciocârlan, V. 2000. Flora ilustrată a României, Editura Ceres, București. (in Romanian) [Google Scholar]
- Ciupa, V. 2010. Cadrul natural şi peisagistic al Municipiului Timişoara. Volumul I. (In Romanian). Available online: https://www.primariatm.ro/wpcontent/uploads/2020/11/Cadrul_Natural_Timisoara_vol.1.pdf (accessed on May 2024). (In Romanian).
- Ciupa, V. 2018. Timișoara-Oraș grădină, oraș al parcurilor, oraș al florilor-Monografie. In Editura ArtPress. Timișoara. (In Romanian) [Google Scholar]
- Cristea, V., D. Gafta, and F. Pedrotti. 2004. Fitosociologie. Editura Presa Universitară Clujană, Cluj-Napoca. (in Romanian) [Google Scholar]
- Di Biase, L., N. Tsafack, L. Pace, and S. Fattorini. 2023. Ellenberg indicator values disclose complex environmental filtering processes in plant communities along an elevational gradient. Biology-Basel 12, 2: 161. [Google Scholar] [CrossRef]
- Dimitrova, A., V. Stipanovic, and D. Kolevska. 2023. Collection of Experiences: 25 Years' Work on Seed Propagation of Allochthonous Woody Plants in Skopje and Their Possible Role in the Urban Landscape. Seefor-South-East European Forestry 14, 1: 53–67. [Google Scholar] [CrossRef]
- Dmuchowski, W., A. Baczewska-Dabrowska, D. Gozdowski, P. Bragoszewska, B. Gworek, I. Suwara, T. Chojnacki, A. Jóźwiak, and E. Swiezewska. 2021. Effect of salt stress in urban conditions on two Acer species with different sensitivity. PEERJ 9: e10577. [Google Scholar] [CrossRef]
- Dümpelmann, S. 2024. Tree Times: Urban Plants as Timekeepers and Seasonal Indicators. Journal of Urban History. [Google Scholar] [CrossRef]
- Ellenberg, H. 1952. Landwirtschaftliche Pflanzensoziologie II. Wiesen und Weiden und ihre standörtliche Bewertung; Ulmer: Stuttgart,Germany; pp. 1–143.17. [in German]. Available online: https://www.researchgate.net/publication/367323581_Ellenberg_Indicator_Values_Disclose_Complex_Environmental_Filtering_Processes_in_Plant_Communities_along_an_Elevational_Gradient (accessed on October 2024). (in English).
- Esperon-Rodriguez, M., S. Power, M. Tjoelker, R. Marchin, and P. Rymer. 2021. Contrasting heat tolerance of urban trees to extreme temperatures during heatwaves. Urban Forestry & Urban Greening 66: 127387. [Google Scholar] [CrossRef]
- Fan, L., J. Wang, D. Han, J. Gao, and Y. Yao. 2023. Research on Promoting Carbon Sequestration of Urban Green Space Distribution Characteristics and Planting Design Models in Xi'an. Sustainability 15, 1: 572. [Google Scholar] [CrossRef]
- Fini, A., I. Vigevani, D. Corsini, P. Wężyk, K. Bajorek-Zydron, O. Failla, E. Cagnolati, L. Mielczarek, S. Comin, M. Gibin, A. Pasquinelli, F. Ferrini, and P. Viskanic. 2024. CO2 assimilation, sequestration, and storage by urban woody species growing in parks and along streets in two climatic zones. SCIENCE OF THE TOTAL ENVIRONMENT 927: 172355. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, W., A. Martini, S. Martins, M. Oliveira, L. Duenez, and W. Alves. 2024. Exploring urban forests in Minas Gerais, Brazil: floristic diversity and biome-driven insights to green infrastructure planning. Urban Ecosystems 27, 6: 2331–2347. [Google Scholar] [CrossRef]
- Galfrascoli, G., G. Bernardello, and A. Calviño. 2023. How well do trees fit the city? Lessons from an urban tree survey in Córdoba, Argentina. Boletin de la Sociedad Argentina de Botanica 58, 4: 561–572. [Google Scholar] [CrossRef]
- Géron, C., J. Lembrechts, I. Nijs, and A. Monty. 2022. Woody invaders from contrasted climatic origins distribute differently across the urban-to-rural gradient in oceanic Europe-Is it trait-related? Urban Forestry & Urban Greening 75: 127694. [Google Scholar] [CrossRef]
- Grignet, A., A. de Vaufleury, A. Papin, and V. Bert. 2020. Urban soil phytomanagement for Zn and Cd in situ removal, greening, and Zn-rich biomass production taking care of snail exposure. Environmental Science and Pollution Research 27, 3: 3187–3201. [Google Scholar] [CrossRef]
- Hamberg, L., S. Lehvävirta, D. Kotze, and J. Heikkinen. 2015. Tree species composition affects the abundance of rowan Sorbus aucuparia L.) in urban forests in Finland. Journal of Environmental Management 151: 369–377. [Google Scholar] [CrossRef]
- Heneidy, S. Z., M. W.A. Halmy, S. M. Toto, S. K. Hamouda, A. M. Fakhry, L. M. Bidak, E. M. Eid, and Y. M. Al-Sodany. 2021. Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective. Biology-Basel 10, 8: 698. [Google Scholar] [CrossRef]
- Hirons, A. D., J. H.R. Watkins, T. J. Baxter, J. W. Miesbauer, A. Male-Muñoz, K. W.E. Martin, N. L. Bassuk, and H. Sjöman. 2021. Using botanic gardens and arboreta to help identify urban trees for the future. Plants People Planet 3, 2: 182–193. [Google Scholar] [CrossRef]
- Horvat, E., M. Sipek, and N. Sajna. 2024. Urban hedges facilitate spontaneous woody plants. Urban Forestry & Urban Greening 96: 128336. [Google Scholar] [CrossRef]
- Hostyn, G., C. Schwartz, J. Côme, and S. Ouvrard. 2022. Assessment for combined phytoremediation and biomass production on a moderately contaminated soil. Environmental Science and Pollution Research 29, 39: 59736–59750. [Google Scholar] [CrossRef] [PubMed]
- Jang, J., and S. Woo. 2022. Native Trees as a Provider of Vital Urban Ecosystem Services in Urbanizing New Zealand: Status Quo, Challenges and Prospects. Land 11, 1: 92. [Google Scholar] [CrossRef]
- Jensen, J., P. Holm, J. Nejrup, M. Larsen, and O. Borggaard. 2009. The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environmental Pollution 157, 3: 931–937. [Google Scholar] [CrossRef]
- Kalberer, S. R., M. Wisniewski, and R. Arora. 2006. Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Science 171: 3–16. [Google Scholar] [CrossRef]
- Klisz, M., R. Puchalka, M. Netsvetov, Y. Prokopuk, M. Vítková, J. Sádlo, R. Matisons, M. Mionskowski, D. Chakraborty, P. Olszewski, T. Wojda, and M. Koprowski. 2021. Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate. Forest Ecology and Management 492: 119194. [Google Scholar] [CrossRef]
- Krisans, O., L. Caksa, R. Matisons, S. Rust, D. Elferts, A. Seipulis, and A. Jansons. 2022. A static pulling test is a suitable method for comparison of the loading resistance of silver birch Betula pendula Roth.) between urban and peri-urban forests. Forests 13, 1: 127. [Google Scholar] [CrossRef]
- Krisans, O., R. Matisons, M. Kitenberga, J. Donis, S. Rust, D. Elferts, and A. Jansons. 2021. Wind resistance of Eastern Baltic silver birch (Betula pendula Roth.) suggests its suitability for periodically waterlogged sites. Forests 12, 1: 21. [Google Scholar] [CrossRef]
- Lahoti, S., A. Lahoti, R. K. Joshi, and O. Saito. 2020. Vegetation structure, species composition, and carbon sink potential of urban green spaces in Nagpur City, India. Land 9, 4: 107. [Google Scholar] [CrossRef]
- Lakicevic, M., K. M. Reynolds, S. Orlovic, and R. Kolarov. 2022. Measuring dendrofloristic diversity in urban parks in Novi Sad (Serbia). Trees, Forests and People 8: 100239. [Google Scholar] [CrossRef]
- Lawesson, J. E. 2003. pH optima for Danish forest species compared with Ellenberg reaction values. Folia Geobotanica 38: 403–418. [Google Scholar] [CrossRef]
- Li, S., Y. Zhu, H. Wan, Q. Xiao, M. Teng, W. Xu, X. Qiu, X. Wu, and C. Wu. 2024. Effectiveness of potential strategies to mitigate surface urban heat island: A comprehensive investigation using high-resolution thermal observations from an unmanned aerial vehicle. Sustainable Cities and Society 113: 105716. [Google Scholar] [CrossRef]
- Lin, B. B., A. Ossola, W. Ripple, M. Alberti, E. Andersson, X. Bai, C. Dobbs, T. Elmqvist, K. Evans, N. Frantzeskaki, R. Fuller, K. Gaston, D. Haase, C. Jim, C. Konijnendijk, H. Nagendra, J. Niemela, T. McPhearson, W. Moomaw, S. Parnell, D. Pataki, and P. Tan. 2021. Integrating solutions to adapt cities for climate change. The Lancet Planetary Health 5: 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, D., V. Varachenkov, D. Manakhov, S. Mamikhin, and A. Shcheglov. 2023. 137Cs pollution in soils and plants of urban ecosystems near the Elektrostal Heavy Machinery Plant. Biology Bulletin 50, 12: 3383–3393. [Google Scholar] [CrossRef]
- Liu, Y., X. Duan, X. Li, W. Yi, G. Chen, J. Yang, D. Deng, X. Guo, Z. Yang, G. Huang, M. Hu, and C. Ye. 2024. Anti-seasonal flooding drives substantial alterations in riparian plant diversity and niche characteristics in a unique hydro-fluctuation zone. Ecology and Evolution 14, 8: e70036. [Google Scholar] [CrossRef]
- Lososová, Z., M. Chytrý, S. Cimalová, Z. Kropáč, Z. Otýpková, P. Pyšek, and L. Tichý. 2004. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. Journal of Vegetation Science 15: 415–422. [Google Scholar] [CrossRef]
- Maes, S. L., M. P. Perring, L. Depauw, M. Bernhardt-Römermann, H. Blondeel, G. Brūmelis, J. Brunet, G. Decocq, J. den Ouden, S. Govaert, W. Härdtle, R. Hédl, T. Heinken, S. Heinrichs, L. Hertzog, B. Jaroszewicz, K. Kirby, M. Kopecký, D. Landuyt, F. Máliš, T. Vanneste, M. Wulf, and K. Verheyen. 2020. Plant functional trait response to environmental drivers across European temperate forest understorey communities. Plant Biology 22, 3: 410–424. [Google Scholar] [CrossRef]
- Marcenò, C., and R. Guarino. 2015. A test on Ellenberg indicator values in the Mediterranean evergreen woods Quercetea ilicis. Rendiconti Lincei-Scienze Fisiche e Naturali 26, 3: 345–356. [Google Scholar] [CrossRef]
- Morozkin, A., S. Kalimullina, L. Salova, and T. Shpak. 2001. Status of forest ecosystems in the impact zone of the Nizhnekamsk industrial complex. Eurasian Soil Science 34, 12: 1323–1330. [Google Scholar]
- Muhammad, S., K. Wuyts, and R. Samson. 2022. Selection of plant species for particulate matter removal in urban environments by considering multiple ecosystem (dis)services and environmental suitability. Atmosphere 13, 12: 1960. [Google Scholar] [CrossRef]
- Muhlisin, J. I., B. Gunawan, and M. F. Cahyandito. 2021. Vegetation diversity and structure of urban parks in Cilegon City, Indonesia, and local residents’ perception of its function. Biodiversitas 22, 7: 2589–603. [Google Scholar]
- Muvengwi, J., H. Ndagurwa, E. Witkowski, and M. Mbiba. 2024. Woody species composition, diversity, and ecosystem services of yards along an urban socioeconomic gradient. Science of The Total Environment 912: Article 168976. [Google Scholar] [CrossRef]
- Nero, B., E. Kuusaana, A. Ahmed, and B. Campion. 2024. Carbon storage and tree species diversity of urban parks in Kumasi, Ghana. City and Environment Interactions 24: Article 100156. [Google Scholar] [CrossRef]
- Nielsen, A. B., M. van den Bosch, S. Maruthaveeran, and C. Konijnendijk van den Bosch. 2014. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosystems 17: 305–327. [Google Scholar] [CrossRef]
- Niu, C., W. Shou, L. Ma, and J. Qian. 2022. Tree height-related hydraulic strategy to cope with freeze-thaw stress in six common urban tree species in North China. Phyton-International Journal of Experimental Botany 91, 4: 811–825. [Google Scholar] [CrossRef]
- Nobre Lisboa, M. A., L. V. Alves da Silva, A. da Silva Nascimento, A. de Oliveira Silva, M. R. Alves Teixeira, M. F.R. Ferreira, S. Cardoso Fereira, A. C. Vieira da Silva, A. Viana Corales, and J. Tovares Calixto. 2024. Diversity, structure, and carbon sequestration potential of the woody flora of urban squares in the Brazilian semiarid region. Trees Forests and People 16: 100561. [Google Scholar] [CrossRef]
- Nouri, K., A. Nikbakht, M. Haghighi, N. Etemadi, M. Rahimmalek, and A. Szumny. 2023. Screening some pine species from North America and dried zones of western Asia for drought stress tolerance in terms of nutrients status, biochemical and physiological characteristics. Frontiers in Plant Science 14: 1281688. [Google Scholar] [CrossRef]
- Palaj, A., and J. Kollár. 2021. Expansion of phanerophytes above the timberline in the Western Carpathians. Biologia 76, 7: 1991–2003. [Google Scholar] [CrossRef]
- Petrushkevych, Y., and I. Korshykov. 2020. Ecological and biological characteristics of Betula pendula in the conditions of urban environment. Regulatory Mechanisms in Biosystems 11, 1: 29–36. [Google Scholar]
- Postarnak, Y., and V. Zhavoronkov. 2023. Urban dendroflora of dry subtropics of the northwestern part of the greater Caucasus on the example of the city of Gelendzhik. Russian Journal of Earth Sciences 23, 5: ES0203. [Google Scholar] [CrossRef]
- Primăria Timișoara–Registrul local al spațiilor verzi-Municipiul Timișoara-Parcul Botanic, Executant S.C. Geotop S.R.L., Beneficiar Primăria Timișoara, Faza ”Actualizarea lucrării «Cadastru verde existent (parcuri) pe baza datelor existente la beneficiar» ”(in Romanian), no document datation. (accessed on 8 May 2024).
- Rahmonov, O., A. Kowal, M. Rahmonov, and S. Pytel. 2024. Variability of concentrations of potentially toxic metals in the topsoil of urban forest parks (Southern Poland). Forests 15, 6: 1020. [Google Scholar] [CrossRef]
- Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford University Press: London. [Google Scholar]
- Ridgway, K. P., L. A. Marland, A. F. Harrison, J. Wright, J. P.W. Young, and A. H. Fitter. 2004. Molecular diversity of Frankia in root nodules of Alnus incana grown with inoculums from polluted urban soils. FEMS Microbiology Ecology 50, 3: 255–263. [Google Scholar] [CrossRef] [PubMed]
- Rogovskyi, S., L. Ishchuk, and H. Ishchuk. 2023. Chornobyl's current dendroflora: analysis of natural successions in the abandoned urban phytocoenoses. Trakya University Journal of Natural Sciences 24, 2: 5–21. [Google Scholar] [CrossRef]
- Salinitro, M., A. Alessandrini, A. Zappi, D. Melucci, and A. Tassoni. 2018. Floristic diversity in different urban ecological niches of a southern European city. Scientific Reports 8: 15110. [Google Scholar] [CrossRef]
- Sanda, V., A. Popescu, M. I. Doltu, and N. Doniţă. 1983. Caracterizarea ecologică şi fitocenologică a speciilor spontane din flora României. Studii şi comunicări, 25, Muzeul Brukenthal, Sibiu. (in Romanian) [Google Scholar]
- Sanda, V., and S. Stefanut. 2003. Atlas Florae Romaniae. I. Pinophytina. Editura Vergiliu, București (In Romanian).
- Sârbu, A., D. Smarandache, and G. Pascale. 2003. Îndrumător de practică botanică: Munţii Bucegi-Baiului. Editura Universităţii din Bucureşti. (In Romanian)
- Seboko, T., C. Shackleton, and S. Ruwanza. 2024. Urban residents' knowledge of and attitudes and willingness to control woody invasive alien plants in their domestic gardens in South Africa. People and Nature 6, 5: 2077–2090. [Google Scholar] [CrossRef]
- Simovic, M., K. E. Mueller, S. M. McMahon, and J. S. Medeiros. 2024. Functional traits and size interact to influence growth and carbon sequestration among trees in urban greenspaces. Functional Ecology 38: 967–983. [Google Scholar] [CrossRef]
- Song, G., J. Wang, T. Han, Q. Wang, H. Ren, H. Zhu, X. Wen, and D. Hui. 2019. Changes in plant functional traits and their relationships with environmental factors along an urban-rural gradient in Guangzhou, China. Ecological Indicators 106: 105558. [Google Scholar] [CrossRef]
- Stagoll, K., D. B. Lindenmayer, E. Knight, J. Fischer, and A. D. Manning. 2012. Large trees are keystone structures in urban parks. Conservation Letters 5, 2: 115–22. [Google Scholar] [CrossRef]
- Stehlik, I., J. Caspersen, L. Wirth, and R. Holderegger. 2007. Floral free fall in the Swiss lowlands: environmental determinants of local plant extinction in a peri-urban landscape. JOURNAL OF ECOLOGY 95, 4: 734–744. [Google Scholar] [CrossRef]
- Teodorescu, T., W. Guidi, and M. Labrecque. 2011. The use of non-dormant rods as planting material: A new approach to establishing willow for environmental applications. Ecological Engineering 37, 9: 1430–1433. [Google Scholar] [CrossRef]
- Wright, A., and R. Francia. 2024. Plant traits, microclimate temperature and humidity: A research agenda for advancing nature-based solutions to a warming and drying climate. Journal of Ecology 112, 11: 2462–2470. [Google Scholar] [CrossRef]
- Yang, J., C. Cen, Z. Wang, and M. Jian. 2024. Impacts of spatiotemporal urban expansion on the species richness and functional traits of adults and sapling woody trees and shrubs of urban remnant forest patches. Ecological Indicators 166: 112498. [Google Scholar] [CrossRef]
- Yang, J., Z. Wang, Y. Pan, and Y. Zheng. 2023a. Woody plant functional traits and phylogenetic signals correlate with urbanization in remnant forest patches. Ecology and Evolution 13, 8: e10366. [Google Scholar] [CrossRef] [PubMed]
- Yang, S., L. Wang, T. Stathopoulos, and A. M. Marey. 2023b. Urban microclimate and its impact on built environment-A review. Building and Environment 283: 110334. [Google Scholar] [CrossRef]
- You, W., and Y. Liang. 2024. Numerical investigation of different building configurations for improving outdoor spatial ventilation conditions in strip-type residential neighbourhoods. Urban Climate 56: 102012. [Google Scholar] [CrossRef]
- Zalacáin, D., S. Martínez-Pérez, R. Bienes, A. García-Díaz, and A. Sastre-Merlín. 2019. Salt accumulation in soils and plants under reclaimed water irrigation in urban parks of Madrid (Spain). Agricultural Water Management 213: 468–476. [Google Scholar] [CrossRef]
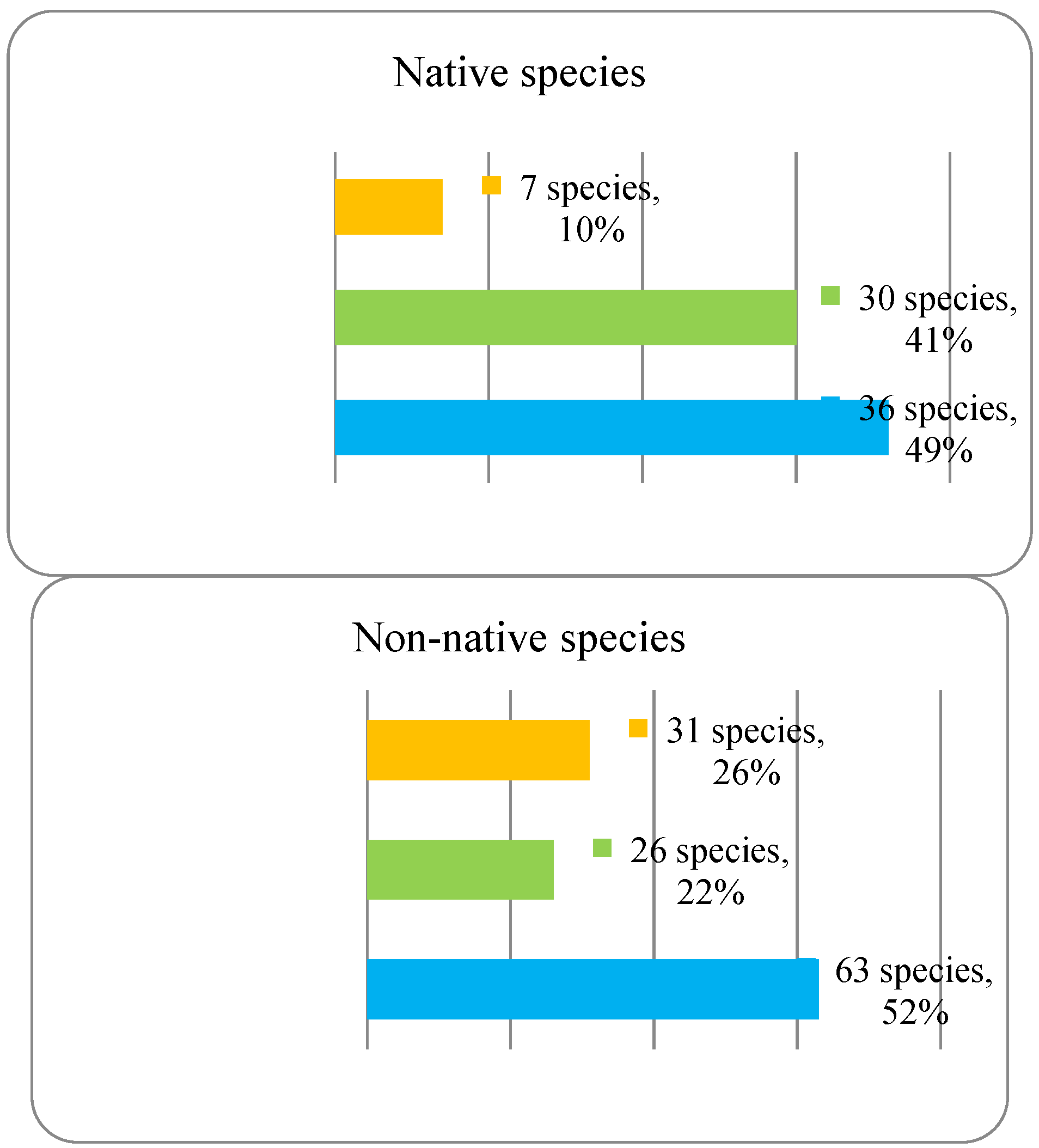
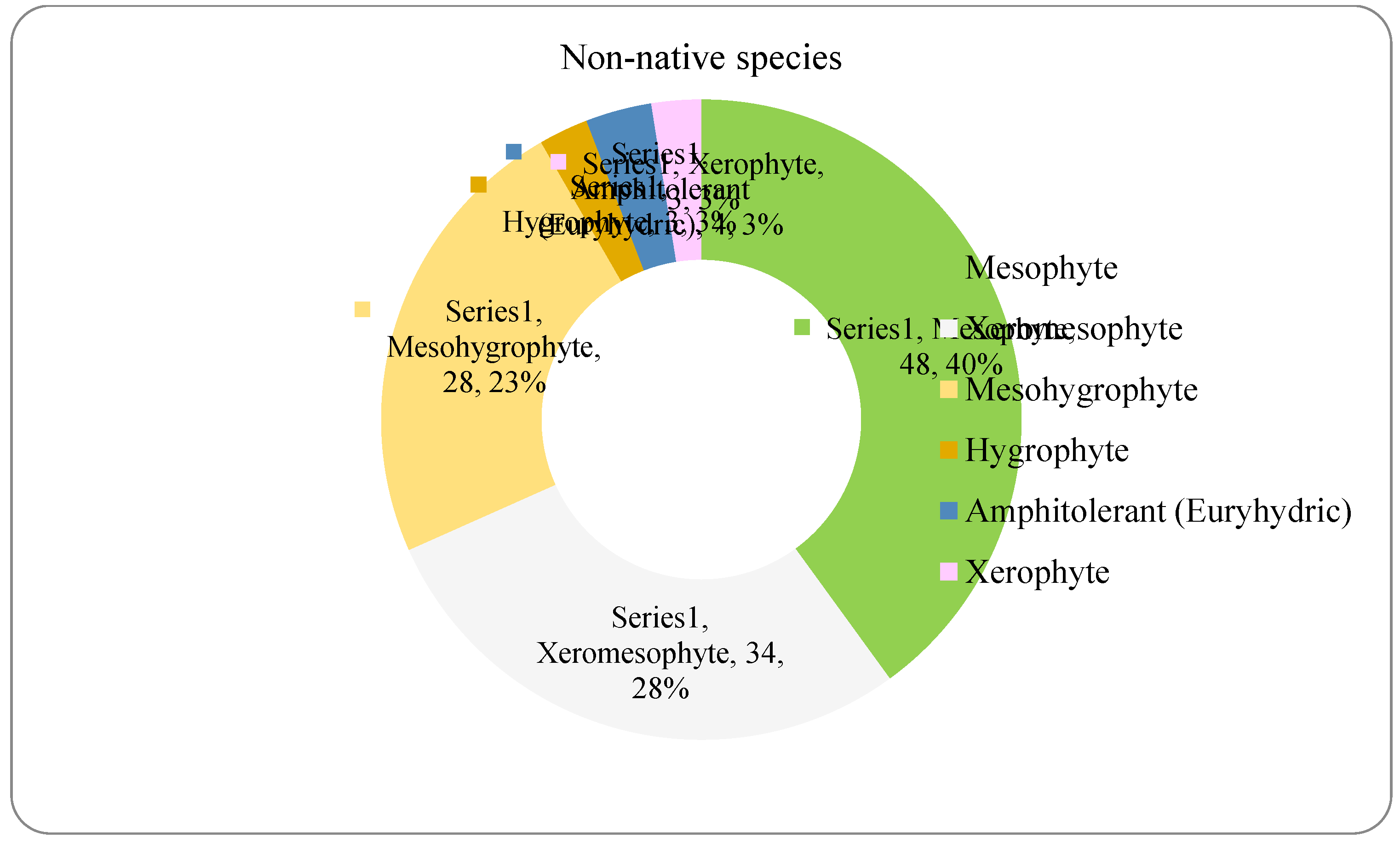

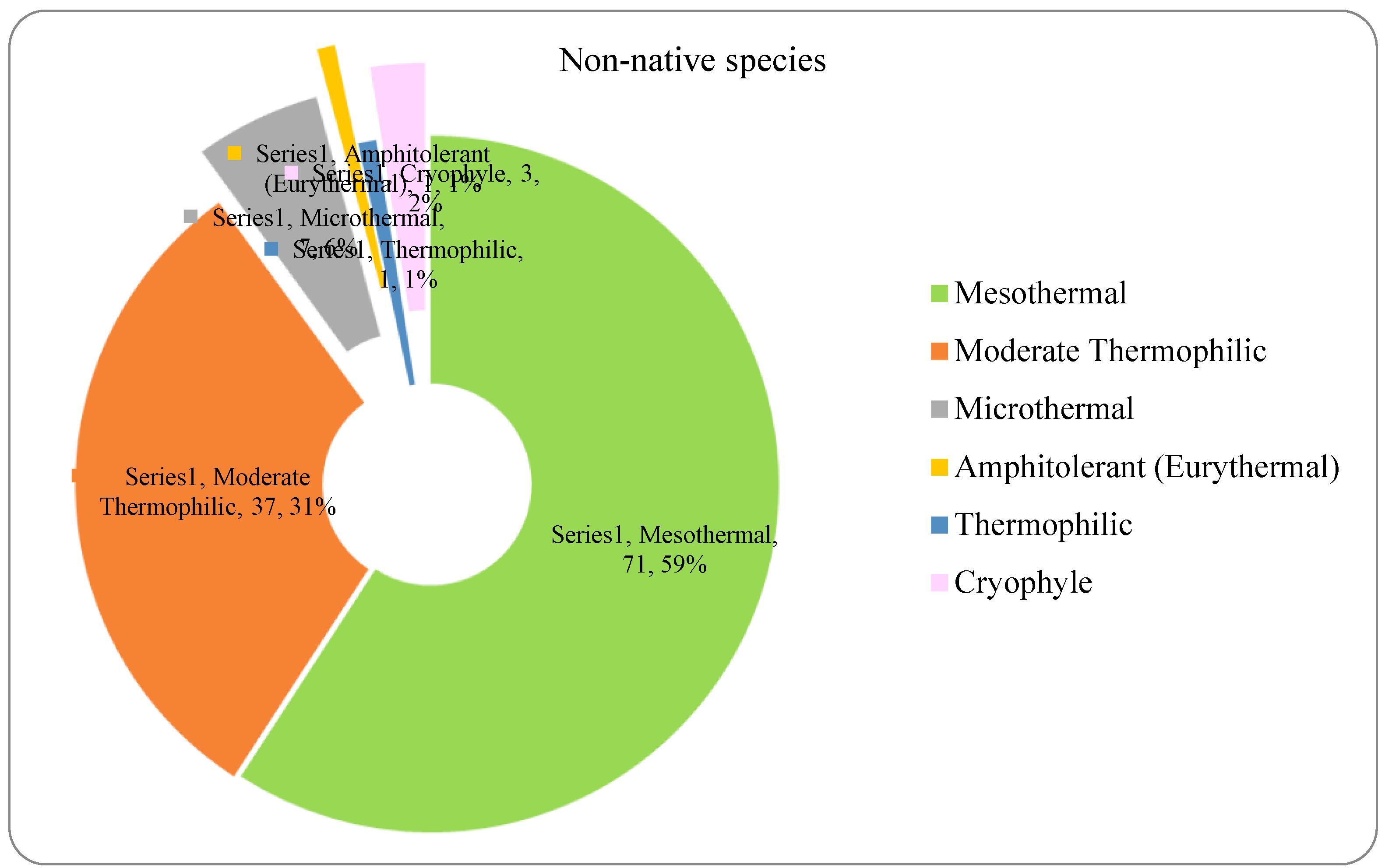
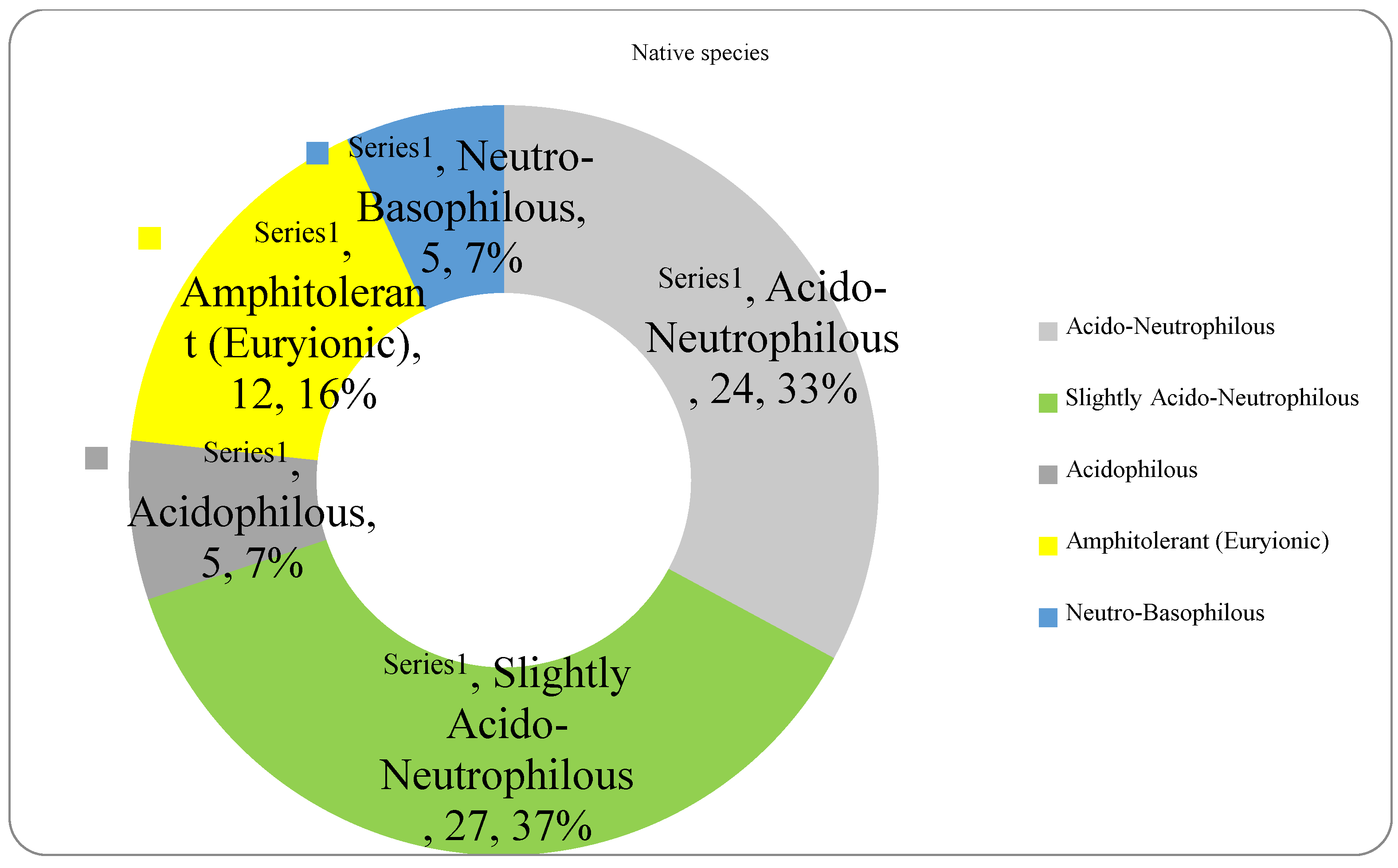
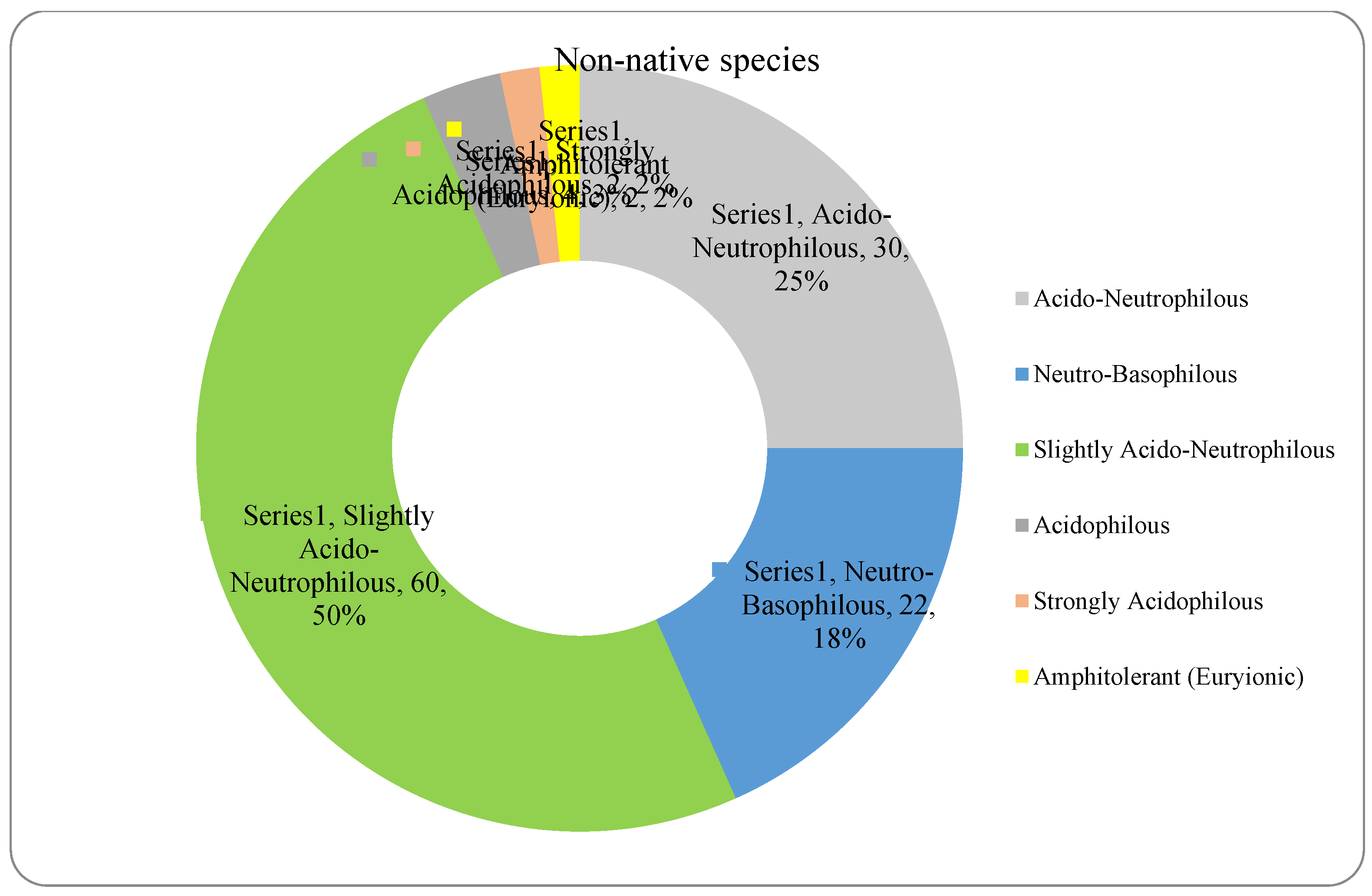
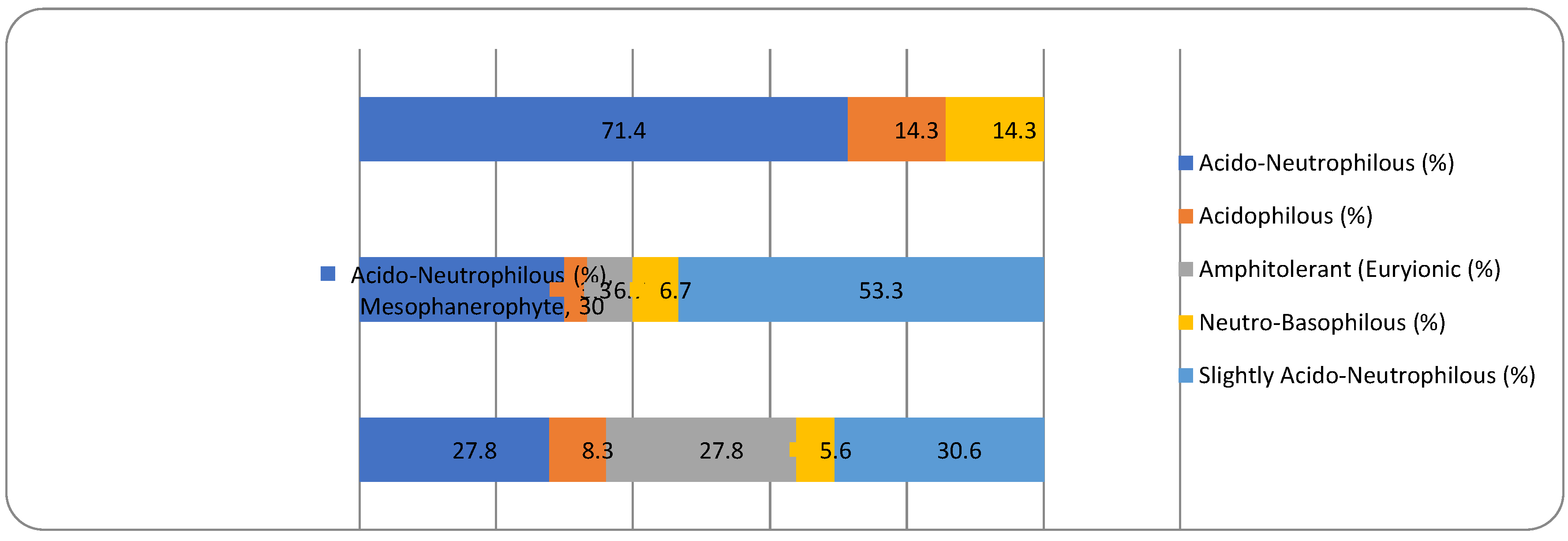
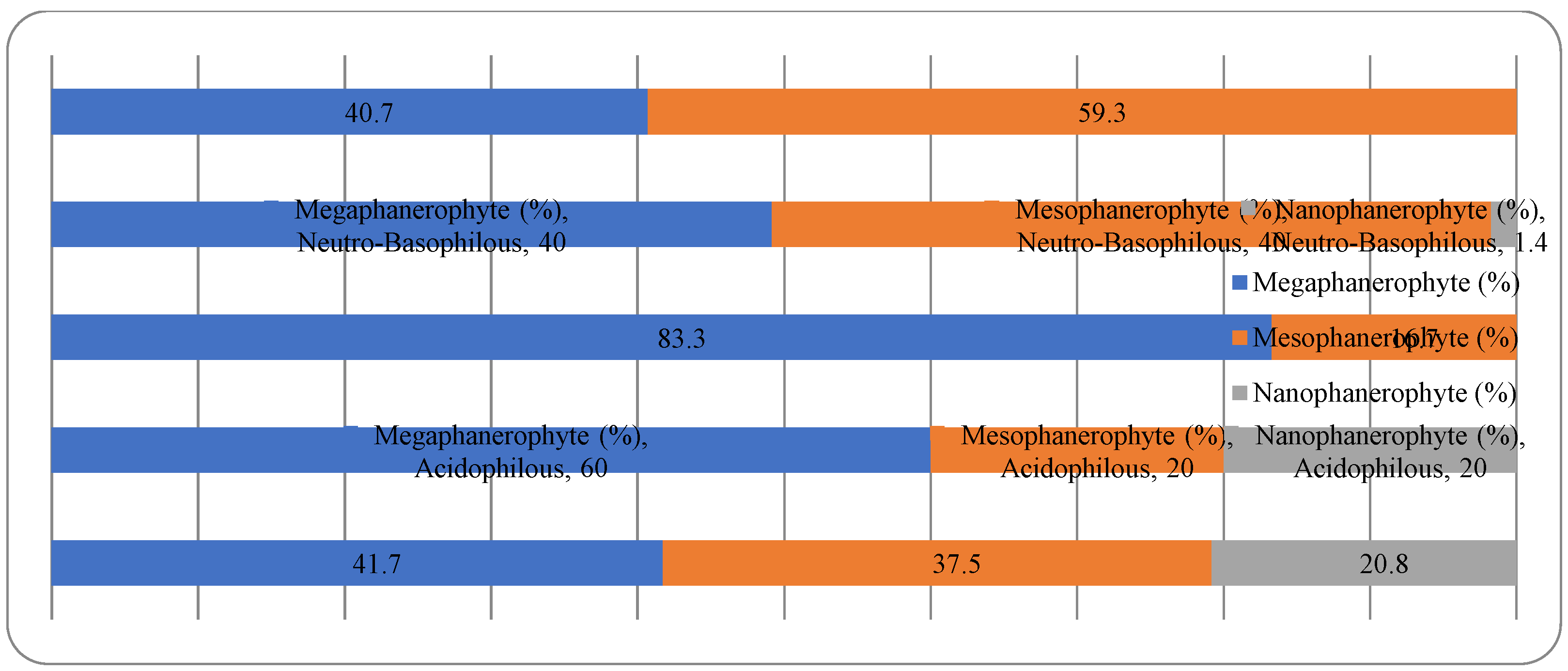
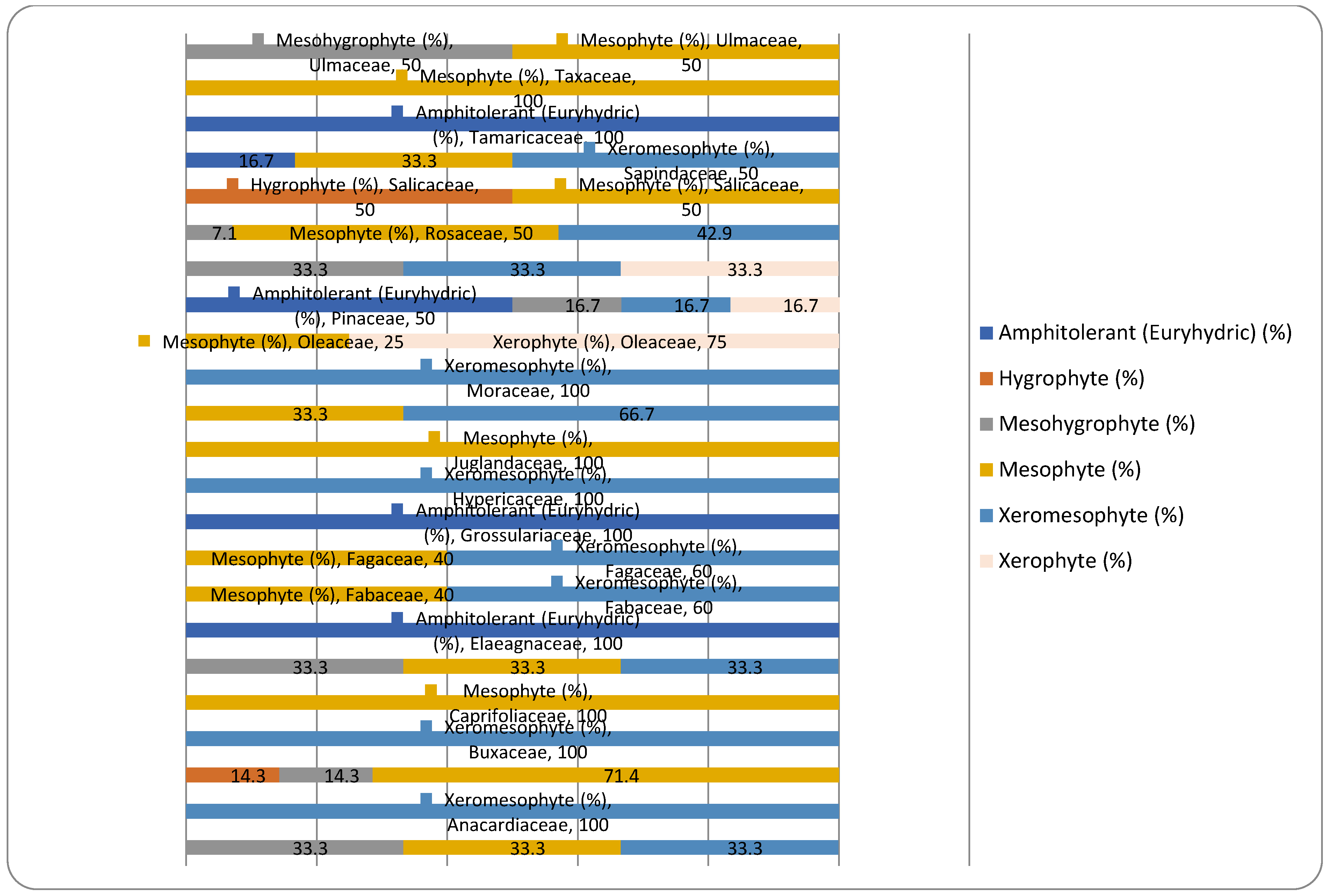
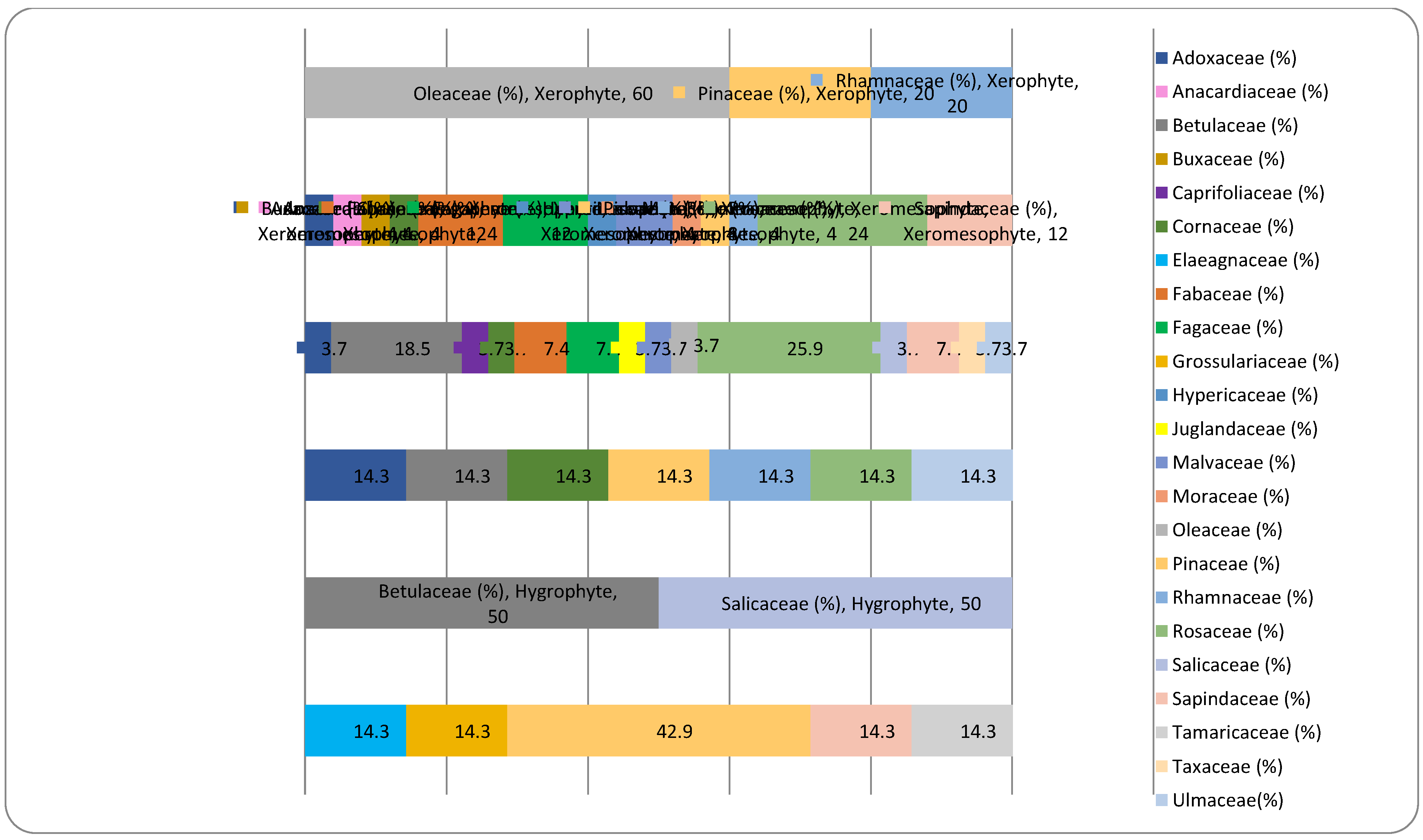

| Ecological preference index | Ecological significance description for moisture | Ecological significance description for temperature | Ecological significance description for soil reaction |
|---|---|---|---|
| 0 | Amphitolerant (Euryhydric) | Amphitolerant (Eurythermal) | Amphitolerant (Euryionic) |
| 1 - 1,5 | Xerophyte | Cryophile | Strongly Acidophilous |
| 2 - 2,5 | Xeromesophyte | Microthermal | Acidophilous |
| 3 - 3,5 | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 4 - 4,5 | Mesohygrophyte | Moderately Thermophilic | Slightly Acido-Neutrophilous |
| 5 - 5,5 | Hygrophyte | Thermophilic | Neutro-Basophilous |
| 6 | Hydrophyte | - | - |
| No. | Species | Family | Monophyletic group | Plant chorology (phytogeographic elements) | Plant life-forms | Moisture requirement | Temperature requirement | Soil reaction requirement |
|---|---|---|---|---|---|---|---|---|
| 1 | Sambucus nigra | Adoxaceae | Angiosperm | European | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 2 | Viburnum lantana | Adoxaceae | Angiosperm | Mediterranean - Central European | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 3 | Viburnum opulus | Adoxaceae | Angiosperm | Circumpolar | Mesophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 4 | Cotinus coggygria | Anacardiaceae | Angiosperm | Mediterranean | Mesophanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 5 | Alnus glutinosa | Betulaceae | Angiosperm | Eurasian | Megaphanerophyte | Hygrophyte | Mesothermal | Acido-Neutrophilous |
| 6 | Alnus incana | Betulaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesohygrophyte | Microthermal | Slightly Acido-Neutrophilous |
| 7 | Betula pendula | Betulaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Microthermal | Acidophilous |
| 8 | Carpinus betulus | Betulaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 9 | Corylus avellana | Betulaceae | Angiosperm | European | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 10 | Corylus colurna | Betulaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 11 | Fagus sylvatica | Betulaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Amphitolerant (Euryionic) |
| 12 | Buxus sempervirens | Buxaceae | Angiosperm | Eurasian | Nanophanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 13 | Lonicera xylosteum | Caprifoliaceae | Angiosperm | Eurasian | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 14 | Cornus alba | Cornaceae | Angiosperm | Eurasian | Nanophanerophyte | Mesohygrophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 15 | Cornus mas | Cornaceae | Angiosperm | Pontic- Mediterranean - Central European | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 16 | Cornus sanguinea | Cornaceae | Angiosperm | Central European | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 17 | Hippophae rhamnoides | Elaeagnaceae | Angiosperm | Eurasian | Mesophanerophyte | Amphitolerant (Euryhydric) | Mesothermal | Slightly Acido-Neutrophilous |
| 18 | Amorpha fruticosa | Fabaceae | Angiosperm | Adventive | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Amphitolerant (Euryionic) |
| 19 | Cercis siliquastrum | Fabaceae | Angiosperm | Eurasian | Mesophanerophyte | Xeromesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 20 | Laburnum anagyroides | Fabaceae | Angiosperm | Balkan | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 21 | Robinia pseudoacacia | Fabaceae | Angiosperm | Adventive | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Amphitolerant (Euryionic) |
| 22 | Sarothamnus scoparius | Fabaceae | Angiosperm | Atlantic- Mediterranean - Central European | Nanophanerophyte | Xeromesophyte | Mesothermal | Acidophilous |
| 23 | Castanea sativa | Fagaceae | Angiosperm | Mediterranean | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Acidophilous |
| 24 | Quercus cerris | Fagaceae | Angiosperm | Mediterranean | Megaphanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 25 | Quercus macranthera | Fagaceae | Angiosperm | European- Anatolian- Caucasian | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 26 | Quercus petraea | Fagaceae | Angiosperm | European | Megaphanerophyte | Xeromesophyte | Mesothermal | Amphitolerant (Euryionic) |
| 27 | Quercus robur | Fagaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Amphitolerant (Euryionic) |
| 28 | Ribes nigrum | Grossulariaceae | Angiosperm | Eurasian | Mesophanerophyte | Amphitolerant (Euryhydric) | Amphitolerant (Eurythermal) | Acido-Neutrophilous |
| 29 | Hypericum androsaemum | Hypericaceae | Angiosperm | Eurasian | Nanophanerophyte | Xeromesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 30 | Juglans regia | Juglandaceae | Angiosperm | Carpathian- Balkan- Anatolian- Caucasian | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 31 | Tilia cordata | Malvaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 32 | Tilia platyphyllos | Malvaceae | Angiosperm | Central European | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 33 | Tilia tomentosa | Malvaceae | Angiosperm | Balkan | Megaphanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 34 | Morus alba | Moraceae | Angiosperm | Adventive | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 35 | Fraxinus excelsior | Oleaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 36 | Fraxinus ornus | Oleaceae | Angiosperm | Mediterranean | Mesophanerophyte | Xerophyte | Mesothermal | Neutro-Basophilous |
| 37 | Jasminum fruticans | Oleaceae | Angiosperm | Mediterranean | Mesophanerophyte | Xerophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 38 | Syringa vulgaris | Oleaceae | Angiosperm | Balkan- Anatolian | Mesophanerophyte | Xerophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 39 | Abies alba | Pinaceae | Angiosperm | Central European | Megaphanerophyte | Mesohygrophyte | Mesothermal | Amphitolerant (Euryionic) |
| 40 | Larix decidua | Pinaceae | Angiosperm | Central European- Carpathian-Sudetic | Megaphanerophyte | Xeromesophyte | Amphitolerant (Eurythermal) | Amphitolerant (Euryionic) |
| 41 | Picea abies | Pinaceae | Angiosperm | European | Megaphanerophyte | Amphitolerant (Euryhydric) | Amphitolerant (Eurythermal) | Amphitolerant (Euryionic) |
| 42 | Pinus mugo | Pinaceae | Angiosperm | European | Megaphanerophyte | Amphitolerant (Euryhydric) | Microthermal | Amphitolerant (Euryionic) |
| 43 | Pinus nigra | Pinaceae | Angiosperm | Carpathian | Megaphanerophyte | Xerophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 44 | Pinus sylvestris | Pinaceae | Gymnosperm | Eurasian | Megaphanerophyte | Amphitolerant (Euryhydric) | Amphitolerant (Eurythermal) | Amphitolerant (Euryionic) |
| 45 | Rhamnus cathartica | Rhamnaceae | Angiosperm | Eurasian | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 46 | Frangula rupestris | Rhamnaceae | Angiosperm | European | Nanophanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 47 | Ziziphus jujuba | Rhamnaceae | Angiosperm | Mediterranean | Mesophanerophyte | Xerophyte | Moderate Thermophilic | Neutro-Basophilous |
| 48 | Cotoneaster integerrimus | Rosaceae | Angiosperm | Eurasian | Nanophanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 49 | Crataegus laevigata | Rosaceae | Angiosperm | Central European | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 50 | Crataegus monogyna | Rosaceae | Angiosperm | European | Mesophanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 51 | Crataegus pentagyn. | Rosaceae | Angiosperm | Mediterranean | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 52 | Malus sylvestris | Rosaceae | Angiosperm | European | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 53 | Prunus avium | Rosaceae | Angiosperm | European | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 54 | Prunus cerasifera | Rosaceae | Angiosperm | Eurasian | Mesophanerophyte | Xeromesophyte | Moderate Thermophilic | Amphitolerant (Euryionic) |
| 55 | Prunus padus | Rosaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 56 | Pyrus pyraster | Rosaceae | Angiosperm | European | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 57 | Rosa canina | Rosaceae | Angiosperm | European | Nanophanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 58 | Sorbus aria | Rosaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 59 | Sorbus aucuparia | Rosaceae | Angiosperm | European | Megaphanerophyte | Mesophyte | Microthermal | Acidophilous |
| 60 | Sorbus torminalis | Rosaceae | Angiosperm | European | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 61 | Spiraea salicifolia | Rosaceae | Angiosperm | Eurasian | Mesophanerophyte | Mesohygrophyte | Microthermal | Acidophilous |
| 62 | Salix viminalis | Salicaceae | Angiosperm | Eurasian | Mesophanerophyte | Hygrophyte | Microthermal | Slightly Acido-Neutrophilous |
| 63 | Populus alba | Salicaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 64 | Acer campestre | Sapindaceae | Angiosperm | European | Megaphanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 65 | Acer monspessulanum | Sapindaceae | Angiosperm | Mediterranean | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 66 | Acer platanoides | Sapindaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 67 | Acer pseudoplatanus | Sapindaceae | Angiosperm | Central European | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 68 | Acer tataricum | Sapindaceae | Angiosperm | European | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 69 | Ailanthus altissima | Sapindaceae | Angiosperm | Adventive | Megaphanerophyte | Amphitolerant (Euryhydric) | Amphitolerant (Eurythermal) | Amphitolerant (Euryionic) |
| 70 | Tamarix ramosissima | Tamaricaceae | Angiosperm | Eurasian | Mesophanerophyte | Amphitolerant (Euryhydric) | Mesothermal | Slightly Acido-Neutrophilous |
| 71 | Taxus baccata | Taxaceae | Gymnosperm | European | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 72 | Ulmus glabra | Ulmaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 73 | Ulmus minor | Ulmaceae | Angiosperm | Eurasian | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| No. | Species | Family | Monophyletic group | Plant life-forms | Moisture requirement | Temperature requirement | Soil reaction requirement |
|---|---|---|---|---|---|---|---|
| 1 | Liquidambar styraciflua | Altingiaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 2 | Rhus semialata | Anacardiaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 3 | Rhus typhina | Anacardiaceae | Angiosperm | Mesophanerophyte | Xeromesophyte | Amphitolerant (Eurythermal) | Acido-Neutrophilous |
| 4 | Kalopanax septemlobus | Araliaceae | Angiosperm | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 5 | Berberis julianae | Berberidaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Microthermal | Strongly Acidophilous |
| 6 | Berberis stenophylla | Berberidaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Microthermal | Strongly Acidophilous |
| 7 | Berberis thunbergii | Berberidaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Microthermal | Amphitolerant (Euryionic) |
| 8 | Berberishaoi | Berberidaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Microthermal | Acidophilous |
| 9 | Mahonia aquifolium | Berberidaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Amphitolerant (Euryionic) |
| 10 | Catalpa bignonioides | Bignoniaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 11 | Catalpa ovata | Bignoniaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 12 | Kolkwitzia amabilis | Caprifoliaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 13 | Lonicera fragrantissima | Caprifoliaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 14 | Lonicera tatarica | Caprifoliaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Acidophilous |
| 15 | Symphoricarpos albus | Caprifoliaceae | Angiosperm | Nanophanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 16 | Weigela florida | Caprifoliaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 17 | Euonymus bungeanus | Celastraceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 18 | Cercidiphyllum japonicum | Cercidiphyllaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 19 | Chamaecyparis lawsoniana | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 20 | Chamaecyparis pisifera | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 21 | Cryptomeria japonica | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 22 | Cupressus arizonica | Cupressaceae | Gymnosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 23 | Juniperus chinensis | Cupressaceae | Gymnosperm | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 24 | Juniperus horizontalis | Cupressaceae | Gymnosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 25 | Juniperus virginiana | Cupressaceae | Gymnosperm | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 26 | Thuja occidentalis | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 27 | Thuja occidentalis var. fastigiata | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 28 | Thuja orientalis | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 29 | Thuja plicata | Cupressaceae | Gymnosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 30 | Diospyros lotus | Ebenaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 31 | Albizia julibrissin | Fabaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Amphitolerant (Euryionic) |
| 32 | Caragana arborescens | Fabaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 33 | Cercis chinensis | Fabaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acidophilous |
| 34 | Gleditsia triacanthos | Fabaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 35 | Gleditsia triacanthos var. inermis | Fabaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 36 | Gymnocladus dioicus | Fabaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 37 | Robinia hispida | Fabaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 38 | Sophora japonica | Fabaceae | Angiosperm | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 39 | Quercus rubra | Fagaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 40 | Quercus macrocarpa | Fagaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Neutro-Basophilous |
| 41 | Ginkgo biloba | Ginkgoaceae | Gymnosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 42 | Deutzia scabra | Hydrangeaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 43 | Philadelphus coronarius | Hydrangeaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 44 | Philadelphus wilsonii | Hydrangeaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 45 | Hypericum patulum | Hypericaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 46 | Carya ovata | Juglandaceae | Angiosperm | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 47 | Juglans nigra | Juglandaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 48 | Pterocarya fraxinifolia | Juglandaceae | Angiosperm | Nanophanerophyte | Hygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 49 | Punica granatum | Lythraceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 50 | Liriodendron tulipifera | Magnoliaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 51 | Magnolia kobus | Magnoliaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 52 | Hibiscus syriacus | Malvaceae | Angiosperm | Nanophanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 53 | Broussonetia papyrifera | Moraceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 54 | Ficus carica | Moraceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 55 | Maclura pomifera | Moraceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 56 | Morus nigra | Moraceae | Angiosperm | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 57 | Chionanthus retusus | Oleaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 58 | Forsythia × intermedia | Oleaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 59 | Fraxinus americana | Oleaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Neutro-Basophilous |
| 60 | Ligustrum ovalifolium | Oleaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 61 | Paeonia suffruticosa | Paeoniaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 62 | Paulownia tomentosa | Paulowniaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 63 | Abies concolor | Pinaceae | Gymnosperm | Megaphanerophyte | Mesohygrophyte | Microthermal | Slightly Acido-Neutrophilous |
| 64 | Abies pinsapo | Pinaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Cryophile | Acido-Neutrophilous |
| 65 | Picea pungens | Pinaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Cryophile | Acido-Neutrophilous |
| 66 | Pinus excelsa | Pinaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Microthermal | Slightly Acido-Neutrophilous |
| 67 | Pinus strobus | Pinaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 68 | Pinus wallichiana | Pinaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Microthermal | Slightly Acido-Neutrophilous |
| 69 | Pseudotsuga menziesii | Pinaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 70 | Pseudotsuga menziesii var. glauca | Pinaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 71 | Tsuga canadensis | Pinaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Cryophile | Acido-Neutrophilous |
| 72 | Platanus × acerifolia | Platanaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 73 | Phyllostachys aurea | Poaceae | Angiosperm | Mesophanerophyte | Hygrophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 74 | Frangula betulifolia | Rhamnaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 75 | Securinega suffruticosa | Rhamnaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 76 | Chaenomeles japonica | Rosaceae | Angiosperm | Nanophanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 77 | Cotoneaster bullatus | Rosaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 78 | Cotoneaster melanocarpus | Rosaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 79 | Crataegus multiflora | Rosaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Moderate Thermophilic | Neutro-Basophilous |
| 80 | Crataegus phaenopyrum | Rosaceae | Angiosperm | Mesophanerophyte | Mesohygrophyte | Moderate Thermophilic | Neutro-Basophilous |
| 81 | Cydonia oblonga | Rosaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 82 | Kerria japonica | Rosaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 83 | Malus domestica | Rosaceae | Angiosperm | Megaphanerophyte | Amphitolerant (Euryhydric) | Mesothermal | Slightly Acido-Neutrophilous |
| 84 | Malus floribunda | Rosaceae | Angiosperm | Megaphanerophyte | Amphitolerant (Euryhydric) | Mesothermal | Slightly Acido-Neutrophilous |
| 85 | Prunus domestica | Rosaceae | Angiosperm | Megaphanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 86 | Prunus dulcis | Rosaceae | Angiosperm | Megaphanerophyte | Xerophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 87 | Prunus laurocerasus | Rosaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 88 | Prunus serrulata | Rosaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 89 | Prunus tomentosa | Rosaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 90 | Pyracantha coccinea | Rosaceae | Angiosperm | Mesophanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 91 | Rhodotypos kerrioides | Rosaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Acido-Neutrophilous |
| 92 | Rosa rugosa | Rosaceae | Angiosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 93 | Sorbaria sorbifolia | Rosaceae | Angiosperm | Nanophanerophyte | Mesohygrophyte | Mesothermal | Acido-Neutrophilous |
| 94 | Spiraea bumalda | Rosaceae | Angiosperm | Nanophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 95 | Spiraea × vanhouttei | Rosaceae | Angiosperm | Nanophanerophyte | Mesohygrophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 96 | Phellodendron amurense | Rutaceae | Angiosperm | Megaphanerophyte | Amphitolerant (Euryhydric) | Mesothermal | Acido-Neutrophilous |
| 97 | Ptelea trifoliata | Rutaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 98 | Tetradium daniellii | Rutaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 99 | Tetradium ruticarpum | Rutaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
| 100 | Zanthoxylum piperitum | Rutaceae | Angiosperm | Nanophanerophyte | Xerophyte | Thermophilic | Slightly Acido-Neutrophilous |
| 101 | Salix babylonica | Salicaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 102 | Salix matsudana | Salicaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 103 | Acer ginnala | Sapindaceae | Angiosperm | Mesophanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 104 | Acer laetum | Sapindaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 105 | Acer negundo | Sapindaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 106 | Acer palmatum | Sapindaceae | Angiosperm | Mesophanerophyte | Xeromesophyte | Moderate Thermophilic | Slightly Acido-Neutrophilous |
| 107 | Acer saccharinum | Sapindaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Mesothermal | Neutro-Basophilous |
| 108 | Aesculus hippocastanum | Sapindaceae | Angiosperm | Megaphanerophyte | Mesohygrophyte | Moderate Thermophilic | Neutro-Basophilous |
| 109 | Koelreuteria paniculata | Sapindaceae | Angiosperm | Megaphanerophyte | Xeromesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 110 | Xanthoceras sorbifolium | Sapindaceae | Angiosperm | Mesophanerophyte | Mesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 111 | Buddleja davidii | Scrophulariaceae | Angiosperm | Nanophanerophyte | Mesophyte | Moderate Thermophilic | Neutro-Basophilous |
| 112 | Lycium halimifolium | Solanaceae | Angiosperm | Nanophanerophyte | Xerophyte | Mesothermal | Neutro-Basophilous |
| 113 | Taxodium distichum | Taxodiaceae | Gymnosperm | Megaphanerophyte | Hygrophyte | Mesothermal | Acido-Neutrophilous |
| 114 | Taxus baccata var. globosa | Taxaceae | Gymnosperm | Nanophanerophyte | Xeromesophyte | Mesothermal | Acido-Neutrophilous |
| 115 | Celtis occidentalis | Ulmaceae | Angiosperm | Megaphanerophyte | Amphitolerant (Euryhydric) | Moderate Thermophilic | Neutro-Basophilous |
| 116 | Viburnum burejaeticum | Viburnaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 117 | Viburnum carlesii | Viburnaceae | Angiosperm | Nanophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 118 | Viburnum orientale | Viburnaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 119 | Viburnum rhytidophyllum | Viburnaceae | Angiosperm | Mesophanerophyte | Mesophyte | Mesothermal | Slightly Acido-Neutrophilous |
| 120 | Wisteria sinensis | Wisteriaceae | Angiosperm | Megaphanerophyte | Mesophyte | Moderate Thermophilic | Acido-Neutrophilous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





