1. Introduction
1.1. Natura 2000 and Proteus anguinus
The European Union has developed various legislative measures, instruments, and programs to protect the environment and ensure sustainable development. These measures, which are important, are supported by multiple policies, legislation, and financial resources that guide Member States in conserving natural resources and consistently reducing environmental impacts. The Habitats Directive (92/43/EEC) and the Birds Directive (2009/147/EEC) are key to nature protection in the European Union (EU). The protected areas of these two directives were established by Natura 2000, a program of immense significance [
1]. The main objectives of Natura 2000 are to conserve habitats such as grasslands, wetlands, forests, and karst systems, to protect species such as butterflies, large carnivores, birds, and amphibians, to promote sustainable development in agriculture, and to reduce human impacts on the environment. Areas for conserving endangered bird species are called Special Protected Areas (SPA), and areas for preserving habitat types are called Sites of Community Importance (SCI).
Slovenia has 355 areas included in Natura 2000. Special attention is paid to karst habitats and Proteus anguinus, which is endemic to the Dinaric Karst. Proteus anguinus is an amphibian that lives in the groundwater of the Dinaric Karst from the Soča River basin near Trieste in Italy, through central and southern Slovenia and southwestern Croatia to the Trebišnjica River in Bosnia and Herzegovina. It is the only European representative of the family Proteidae, the only representative of the genus Proteus, and the only cave vertebrate in Europe. According to the IUCN classification, this species is critically endangered on the Red List. In ecology, it is known as a good environmental indicator.
1.2. Ecology of the Species Proteus anguinus
The body of the species Proteus anguinus is elongated, delicate, and eel-like. Adults also have external gills. It is colorless (without pigment), whitish-yellowish, or whitish-reddish, and its color comes from surface capillaries. For this reason, it was named "human fish" [
2]. A few specimens with dark color pigments have also been found, indicating that scarce pigmented specimens met the sun, most likely due to heavy rains that brought them from underground to the surface [
3]. The head is large and elongated, with a rounded snout (pear-shaped). Their eyes are visible at first, but later, they are covered by skin and are barely visible from the outside, and there is a leathery fin on the vertically flattened tail, which is shorter than the body. It has a developed inner ear, an organ of balance and hearing. Their legs are tiny, with three poorly developed toes on the front legs and two toes on the hind legs. It grows from 23 to 25 cm, although specimens of 40 cm length have also been found. Males are slightly smaller than females [
4].
The human fish is oviparous (lays eggs). It reaches sexual maturity very slowly, reaching full maturity between the ages of 14 and 18 years. Females can produce about 35 eggs at a time, and the incubation period is about 133 days, which is a rather lengthy process adapted to an environment with low temperatures and stable conditions [
5]. The eggs are laid in a protected place in cavities. Larvae hatch from the eggs, which are about 2 cm long. At this stage, the larvae are independent and adapted to water life. The larvae already have some characteristics. They have functional eyes that are visible and sensitive to light. External gills are developed and allow efficient breathing in water [
6]. Most larvae retain characteristics of their youth, such as external gills, an example of neoteny. Neoteny is a biological phenomenon in which an organism retains some characteristics of youth. This means that even if a human fish, a term used to describe a fish that resembles a human in some way, reaches sexual maturity, it retains these characteristics. However, these are usually characteristic of developmental stages [
7].
They have a slow metabolism because there are times when there is no food, and when there is food, they can eat a lot and store it for later resources in case of hunger. They feed on microscopic organisms such as plankton and small aquatic invertebrates. Adults are predators and feed on smaller organisms such as crustaceans, snails, and worms. They can survive for long periods without food because they store energy in body fat. Some sources state that they can last up to 10 years without food. Life expectancy also depends on nutrition, living conditions, and metabolism. Under appropriate conditions, their lifespan is around 100 years [
8]. The ecology of the species Proteus anguinus is very important for understanding habitat needs.
1.3. Habitat of Species Proteus anguinus
Researchers interested in ecology and environmental protection play a crucial role in preserving the habitat of the Proteus anguinus species. Karst habitats, such as the ones in Slovenia, are suitable living environments for the Proteus anguinus. Unpolluted, clean water rich in oxygen, free of organic matter, with a slightly acidic pH or neutral value, and a constant temperature between 8-12°C are essential for their survival [
9]. Ecology and habitat understanding provide us with important information about the key needs for environmental protection Proteus anguinus.
In Slovenia, the most famous habitat areas of the species Proteus anguinus are in the central part of the country, mainly in the Notranjska region, where there are numerous karst caves and underground watercourses that are ideal for its existence. Among these, the world-famous "Postojna Cave" stands out as the most famous home of the Proteus anguinus. This unique ecosystem, with its vast system of underground rivers and lakes that flow through the karst cavities, provides the perfect conditions for the survival of Proteus anguinus. The underground water is cold and stable, with low temperatures, creating a wonder of nature that we can only marvel at.
The sensory system of the Proteus anguinus is a marvel of evolution, uniquely adapted to life in an aquatic cave environment. The lack of light and the associated inability to use vision for orientation are compensated for by other senses better developed than amphibians living in surface environments. They perceive events in their surroundings through smell and vibrations, which they monitor with sensory organs in their skin. Their eyes are stunted and covered by skin, as they are of no use in an environment without light, but the entire surface of their body is sensitive to light. This unique adaptation is a testament to nature's wonder and the Proteus anguinus's resilience [
10].
1.4. Activities with Environmental Impacts that Pollute the Habitat of the Proteus anguinus
The Proteus anguinus, a species highly dependent on its environment, is particularly sensitive to environmental pollution. This sensitivity makes it an excellent indicator of the health of its ecosystem. The Proteus anguinus habitat, which includes rivers, lakes, and water streams that extend through limestone caves, is characterized by oxygen-rich water. Oxygen availability is crucial for the Proteus anguinus's survival, as it breathes through external gills, lungs, and skin [
11]. Any pollution or decrease in oxygen levels severely threatens the Proteus anguinus's survival, underscoring the urgent need for environmental protection and conservation. It is our responsibility to ensure that the Proteus anguinus's habitat remains clean and oxygen-rich, as this is crucial for its survival and the preservation of its unique ecological role.
The Proteus anguinus breathes with gills and lungs, so it is also crucial to have enough dissolved oxygen in the water [
12]. Water in karst systems must contain more than 7 mg/L of dissolved oxygen for the Proteus anguinus to live and reproduce undisturbed [
13].
Reproduction is rare, meaning it only happens every few years, depending on environmental conditions. If the conditions are not stable for a long time, they cannot reproduce. The ideal water temperature for the Proteus anguinus is between 8 °C and 12 °C. Due to its thermal stability, groundwater rarely experiences sudden temperature changes, which allows this species to survive successfully in its specific environment. The female lays 12–70 eggs, approximately 12 mm in size. She carefully placed the eggs in safe places, such as under stones or cracks, to protect them from predators and mechanical damage [
14]. Egg development is a process that takes several months and depends on the ambient/water temperature. The eggs need clean, oxygen-rich water for normal development [
15].
The Proteus anguinus, being extremely sensitive to pollution, requires pristine water for survival. Underground systems, where the Proteus anguinus often reside, are generally nutrient-poor, making any presence of nitrates, phosphates, or pesticides immediately noticeable. A drop in oxygen concentration due to pollution can quickly affect Proteus's nutrition and reproduction [
16]. The most significant pollutants in the habitat of the Proteus anguinus are direct discharges of polluted water into the environment from agricultural production and wastewater that reaches the habitat through the water sink or drain system. Water from agriculture, often pH acid/based with a large amount of organic matter, can significantly reduce oxygen concentration in the groundwater and directly affect the ecology of the Proteus anguinus. Climate change further exacerbates these problems, altering the groundwater's oxygen circulation dynamics and temperature [
17]. This underscores the need for proactive measures to mitigate the impact of climate change on the habitat of the Proteus anguinus and the urgency of these measures to ensure the survival of this unique species.
1.5. Dairy Wastewater and Impacts on Ecology Proteus anguinus
In the Slovenian Karst, one of the most vulnerable natural environments [19], the ecology of Proteus anguinus, one of the primary pollutants, is severely affected by the dairy industry, which discharges its wastewater into nature. The most significant amount of wastewater is generated in the production and cleaning processes. For example, cheese production produces more carbohydrates and proteins, while butter produces more lipids (19). Casein, a phosphoprotein in mammalian milk [20], can be found in wastewater and various fats, carbohydrates, lactose, and other proteins [21]. Some waters may even contain elements such as nickel, sodium, chlorine, potassium, iron, copper, manganese, and magnesium, which can be harmful in specific quantities [22] to the habitat of the species Proteus anguinus. One of the main effects of dairy wastewater pollution is pH change, which can lead to eutrophication [23] and oxygen depletion due to the high amount of organic matter and fats [24]. A sudden increase in organic matter can significantly impact the oxygen regime. Microorganisms that deplete this sudden increase in organic matter use the available oxygen in the water. A slight decrease in oxygen levels can still negatively affect Proteus anguinus's slow metabolism and prey availability. Since, over time, a sustained influx of organic matter can reduce the quality of the habitat to the point where it is no longer beneficial to the species, this can lead to habitat degradation [25].
Due to the importance of hygiene, dairy wastewater can usually contain detergents, disinfectants, salts, and bases in addition to waste generated during production [26]. One of the most common types of treatment in the dairy industry is cleaning in place - CIP [27]. It is practical and efficient and removes all dirt and bacteria from pipes, containers, and other equipment. There are five basic steps in CIP: pre-rinsing, to remove the maximum amount of milk residue; alkaline cleaning, in which a hot alkaline solution is used as a detergent solution to remove milk residue; rinsing, in which the chemical alkaline cleaning solution is removed; acid cleaning, in which a hot acid solution neutralizes any remaining alkali and removes mineral dirt, if present; and finally, post-rinsing, which eliminates the last traces of soil and chemicals [28]. Therefore, the water used in this part of the dairy industry operation may contain solutions of alkalis and acids, which can affect the overall pH of the water. Proteus anguinus, which thrives at a neutral pH of 7, can be more seriously affected by the low pH caused by acid solutions. In addition, low pH conditions can allow more dissolved substances and metals in the water, which are also harmful to the species.
On the other hand, karst areas have a low filtration natural capacity for wastewater because of their geological composition; therefore, they can reach the habitat of the Proteus anguinus almost unchanged. As such, they first affect the replacement of invertebrates that are the leading food of the Proteus anguinus and then the reproduction of the population itself, for which constant living conditions are necessary. To survive, the Proteus anguinus needs water with strict conditions: pH close to neutrality (6.5 -7.5) and oxygen above 7.0 mg or 91%. The wastewater treatment process, especially the first phase of chemical treatment, where the pH is balanced and organic matter is removed, is critical in maintaining these conditions and directly affecting the water's oxygen amount.
1.6. Environmental Protection of the Habitat of Proteus anguinus
It is interesting to look at the environmental protection of habitats from the perspective of local practice regarding managing industrial wastewater, which threatens underground karst habitats. The well-known Krepko eco-dairy in Slovenia is in this area. This production produces significant amounts of treated wastewater that is then discharged directly into the environment. This production has advanced technologies for reducing wastewater pollution and protecting underground waste habitats. The company has its wastewater treatment plant installed next to the company. The wastewater treatment plant has three stages of treatment: primary, secondary, and tertiary. The primary stage includes chemical water treatment methods (neutralization, coagulation, flocculation). This research aims to test the effectiveness of various chemicals for a better process of neutralization, coagulation, and flocculation, and consequently, a better method of wastewater treatment to protect the habitat of the ecology of Proteus anguinus.
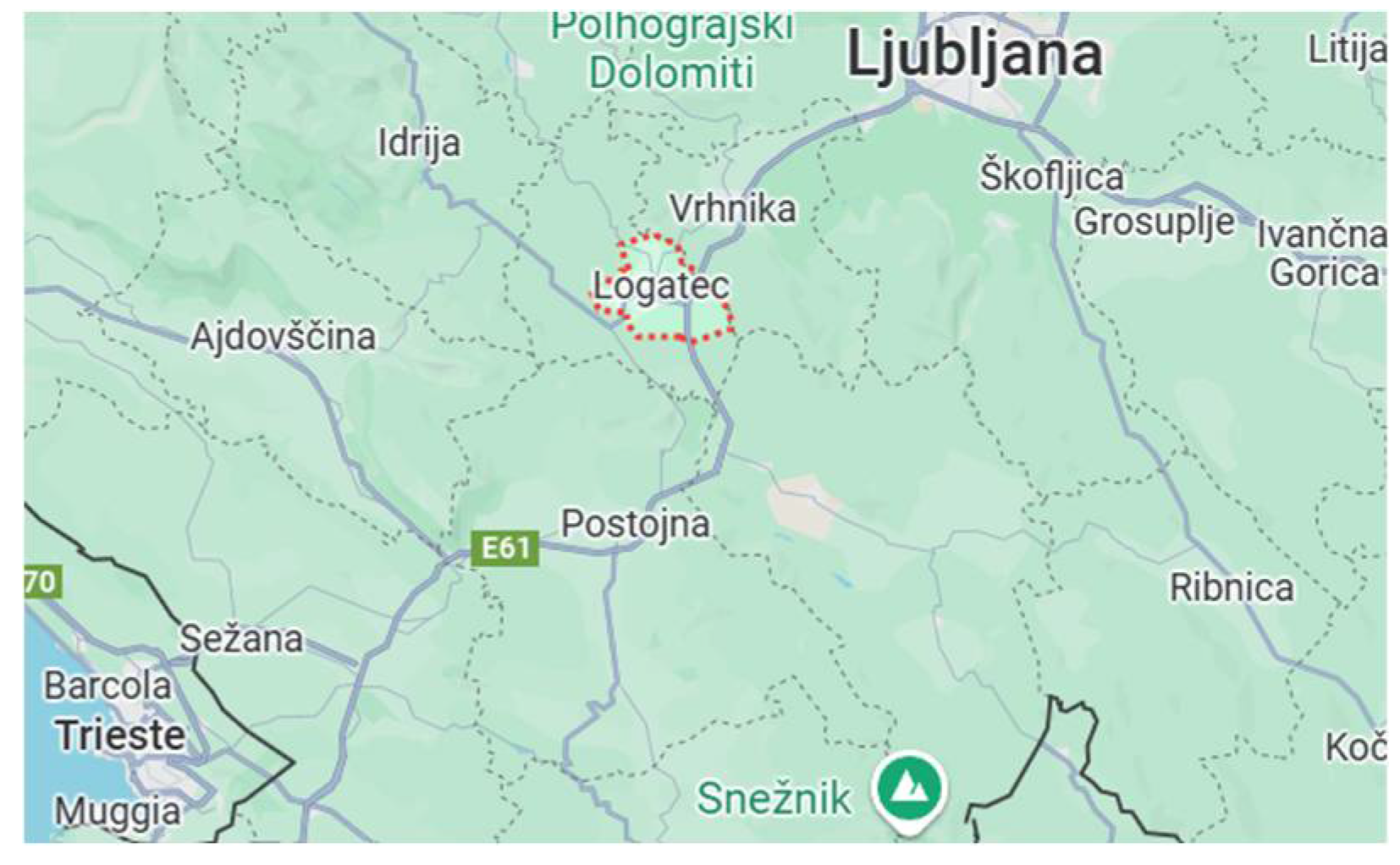
Figure 1.
Location of the Krepko Dairy (Logatec). Source: google map.
Figure 1.
Location of the Krepko Dairy (Logatec). Source: google map.
2. Materials and Methods
The chemical treatment of wastewater is not just a process; it's necessary for environmental protection technology. This treatment involves three characteristic phases: neutralization, coagulation, and flocculation. These phases primarily aim to regulate the wastewater's pH value and purify the water by removing organic matter that negatively affects the oxygen concentration in the water. The primary treatment phase is where crucial research is conducted to improve the efficiency and effectiveness of the chemical treatment process.
2.1. Materials
With its potential to significantly impact wastewater treatment, the research will analyze various chemicals for neutralization, coagulation, and flocculation, which are fundamental to the chemical phase of wastewater treatment. This study could pave the way for more efficient and sustainable wastewater management practices, addressing the urgent need for better environmental solutions. For neutralization, chemicals of different masses of sodium hydroxide (50% and 30%) from two different manufacturers will be tested. The first is Sotoplus 50% (Sotom d.o.o., Slovenia), and the second is Chemie NPK 30% (Chemie NPK d.o.o., Slovenia). Chemicals of different compositions from two manufacturers will be tested for the coagulation process. The first is Kemiklar (Sotom d.o.o., Slovenia) - Aluminum chloride 30-40%. The second one is VTA Combiflock (VTA d.o.o., Austria) - Aluminum chloride with Poly aluminum hydroxide chloride 52-65%. Chemicals with different compositions and masses of sulfamic acid and adipic acid from two different manufacturers will be tested for the flocculation process. The first one is Acefloc (Sotom d.o.o., Slovenia), which has masses of sulfamic acid and adipic acid of 2.5 %. The second one is VTA F98 (VTA d.o.o., Austria), which has masses of sulfamic acid and adipic acid of 10 % plus an addition of citric acid.
2.2. Methods
2.2.1. Instruments for Analyzing pH
Handheld instruments for on-site pH and temperature measurement are very popular. These meters are suitable for obtaining data on the neutralizer's influence on the wastewater. We used two instruments in the research: the Multi 3620 IDS and the control ProfiLine pH 3310.
2.2.2. Jar Tests
The jar test, a crucial and irreplaceable laboratory method, is pivotal in testing the effectiveness of coagulants, flocculants, and other chemicals in water treatment. This method is the cornerstone of our work, enabling us to optimize conditions and achieve the desired cleaning result. It guides us in determining the ideal concentration of chemicals, the most suitable combinations, and the time and amount of mixing needed to achieve the best results. The process is divided into several steps: preparation of wastewater samples, preparation of chemical mixtures, addition of chemical mixtures to wastewater samples, process execution, and evaluation of effectiveness.
Our process begins with the meticulous sampling and preparation of the wastewater for laboratory testing. We carefully remove solid particles from the water using appropriate laboratory methods to ensure accurate results. The next step involves the precise and meticulous preparation of the chemicals to be tested. We add the selected mixture of chemicals to each of our prepared samples and thoroughly mix them. Typically, we let the mixture of chemicals and samples stand for at least 30 minutes to allow all reactions to occur. An evaluation of effectiveness follows this. This is done through visual assessment (evaluation of floc formation, sediment quantity, and time of response) and chemical tests to check the concentration of suspended particles.
The research used a 4-Station Portable Jar Testing Apparatus (120VAC) manufactured by A&F Machine Products. Control measurements were performed with magnetic stirrers with a heater (Serie MR 3001) and an IKA RO 5-position digital magnetic stirrer that was not heated and was designed for synchronous stirring.
2.2.3. Laboratory Analysis of Chemical Oxygen Demand
The new combination of chemicals was rigorously tested in the laboratory. Over 30 days, we collected raw water and water after chemical treatment. Both glasses of water were subjected to laboratory testing for chemical oxygen demand (COD), following the predetermined procedure for Nanocolor cuvette tests. We used standard analyses with the Vario mini apparatus and the PF-12 Plus meter measurements to accurately determine the effect of these new chemicals on COD. In our analysis, we paid particular attention to the comparison with data on the impact of all substances, ensuring a thorough and valid assessment. Considering the type of raw water further strengthened the reliability of our results.
2.3. Collection and Presentation of Data
We classified the collected data into tables. The first table shows the values for neutralization. Based on these data, a better neutralizer was determined. This neutralizer was used as a basis for further analysis of coagulant and flocculant. The results are shown in 2 tables. Based on these results, one coagulant and two flocculants were singled out. The third table shows the results of testing two flocculants. The results confirm (
Table 3) a better flocculant, as shown in
Table 2. After that, the new chemicals were tested for COD for 30 days. These results, when compared with previous chemicals, show significant progress, as shown in
Table 4. After that, a discussion of primary and secondary data is conducted.
3. Results
3.1. Results: Naturalization
Table 1.
Naturalization.
| Neutralization |
| Time |
Sample 1 |
Sample 2 |
Reaction |
| 0 |
3,5 |
3,5 |
No |
| 1 |
5,3 |
4,1 |
Yes |
| 2 |
7 |
5,5 |
Yes |
| 3 |
7 |
6,8 |
Yes |
| 4 |
7 |
7 |
Yes |
| 5 |
7 |
7 |
Yes |
| 6 |
7 |
7 |
Yes |
| 7 |
7 |
7 |
Yes |
| 8 |
7 |
7 |
Yes |
| 9 |
7 |
7 |
Yes |
| 10 |
7 |
7 |
Yes |
Explanation of the table: The time indicated is 0 to 10 minutes. Sample 1 is Sotoplus 50%. Sample 2 is Chemie NPK 30%. The pH value is marked from the initial raw water (3.5) state through the time reaction. The target value is pH 7, a crucial goal that guides our entire process. For both samples, the same raw water is taken, and the same value of the neutralizer is added to adjust the pH. The mixing for both samples is consistently the same.
Figure 2.
PH measurement.
Figure 2.
PH measurement.
3.2. Results: Coagulation and Flocculation
Table 2.
Coagulation and Flocculation.
Table 2.
Coagulation and Flocculation.
| Coagulation and Flocculation |
| Time |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
Reaction |
| 0 |
No |
No |
No |
No |
No |
| 1 |
No |
Initial |
No |
No |
No/Yes |
| 2 |
No |
Initial |
Initial |
No |
No/Yes |
| 3 |
No |
Good |
Initial |
No |
No/Yes |
| 4 |
No |
Good |
Initial |
Initial |
Yes |
| 5 |
Initial |
Excellent |
Initial |
Initial |
Yes |
| 6 |
Initial |
Excellent |
Initial |
Initial |
Yes |
| 7 |
Initial |
Excellent |
Good |
Initial |
Yes |
| 8 |
Initial |
Excellent |
Good |
Initial |
Yes |
| 9 |
Initial |
Excellent |
Good |
Good |
Yes |
| 10 |
Initial |
Excellent |
Good |
Good |
Yes |
Explanation of the table: The time indicated is 0-10 minutes. Four samples were tested with meticulous attention to detail. Each sample had the same value of wastewater, the same value of neutralizer, the same value of the coagulant, and the same of the flocculant. It's important to note that the pH value is 7, a key parameter in our experiment. The temperature is a constant. Sample 1 is Sotom - neutralizer, Sotom - coagulant, sotom - flocculant. Sample 2 is a Sotom-neutralizer, VTA-coagulant, and VTA-flocculant. Sample 3 is Sotom – neutralizer, VTA – coagulant, and Sotom - flocculant. Sample 4 is Sotom - neutralizer, VTA – coagulant, and Sotom - flocculant. The reaction is presented in the following way: no reaction, initial, good, and excellent.
Figure 3.
Coagulation and Flocculation.
Figure 3.
Coagulation and Flocculation.
3.3. Results: Flocculation
Table 3.
Flocculation.
| Flocculation |
| Time |
Sample 1 |
Sample 2 |
Reaction |
| 0 |
No |
No |
No |
| 1 |
Initial |
No |
No/Yes |
| 2 |
Initial |
Initial |
Yes |
| 3 |
Good |
Initial |
Yes |
| 4 |
Good |
Initial |
Yes |
| 5 |
Excellent |
Initial |
Yes |
| 6 |
Excellent |
Initial |
Yes |
| 7 |
Excellent |
Good |
Yes |
| 8 |
Excellent |
Good |
Yes |
| 9 |
Excellent |
Good |
Yes |
| 10 |
Excellent |
Good |
Yes |
Explanation of the table: The time lasts 0 to 10 minutes. Sample 1 is VTA flocculant, sample 2 is Sotom flocculant. The same wastewater was added to both samples, the same values of the VTA coagulant sample, and the same values of the Sotom flocculant sample. Temperature and electromagnetic stirring were constant.
3.4. Results COD
Table 4.
COD.
| COD raw wastewater |
COD treated wastewater - old chemicals |
COD treated wastewater - new chemicals |
| 801-900 |
501-600 |
401-500 |
| 901-1000 |
601-700 |
501-600 |
| 1001-1100 |
701-800 |
601-700 |
| 1101-1200 |
801-900 |
701-800 |
| 1201-1300 |
901-1000 |
801-900 |
| 1301-1400 |
1001-1100 |
901-1000 |
| 1401-1500 |
1101-1200 |
1001-1100 |
Explanation of the table: The table shows the input values of raw water and the output values of treated water with old (neutralizer—Chemie NPK, coagulant—Sotom, flocculant—Sotom) and new (neutralizer—Sotom, coagulant—VTA, flocculant—VTA) chemicals.
Figure 6.
COD measuring devices.
Figure 6.
COD measuring devices.
4. Discussion
4.1. Neutralization
Before wastewater can be released into the environment, it must be within a neutral pH range with a slight variation between 6.5 and 7.5. Wastewater must be approximately neutral to prevent harmful effects on the environment. Choosing a fast, effective neutralizer that reacts well with coagulant and flocculant is critical [29]. Our research, which utilized a NaOH-based neutralizer, unequivocally demonstrated the efficiency of this choice. A higher molarity of NaOH was superior, accelerating the neutralization of acidic wastewater and enabling a more effective reaction with the coagulant and flocculant. This finding should instill confidence in the effectiveness of the NaOH-based neutralizer (Picture 6).
4.2. Coagulation
Coagulation, a chemical process, is instrumental in removing solid particles from wastewater [30]. It significantly improves the color, turbidity, and presence of bacteria in wastewater. Coagulation accelerates the natural process of sedimentation, which operates on the principle of gravity. However, since this process is usually very slow, adding a coagulant can expedite it. The precision of the coagulation process is evident in its aim to increase the settling particles' diameter or form flocs through adsorption and aggregation. This is achieved by adding a coagulant, which neutralizes the surface of the particles in the wastewater, allowing them to form aggregates. These clusters are much easier to remove from wastewater using various methods (filtration, skimming, dewatering, etc.) [31]. Aluminum salt is a commonly used coagulant [32]. The results showed a better poly aluminum hydroxide and aluminum chloride (Combiflock) reaction than aluminum chloride (Kemiklar). This reaction has been demonstrated in better coagulation, lower water turbidity (less aluminum remains in the water, more transparent water), and the contribution of better flotation in the wastewater treatment process (Picture 6).
4.3. Flocculation
Flocculation is a subsequent process that follows coagulation. It involves the formation of larger particles called flocs. Flocculation occurs after adding a flocculant, usually a polymer [33]. Flocculation is based on physical and chemical processes, such as electrostatic interactions, bridging between particles (a process where the polymer molecules physically link the particles together), and particle agglomeration into larger flocs. The effectiveness of flocculation depends on the type and concentration of the chosen flocculant, mixing speed, reaction time (with coagulant), temperature, and pH value (with neutralizer). Mixing is very important in flocculation to ensure the flocculant is evenly distributed throughout the water [34]. Flocculants are categorized based on their charge: anionic polymers, cationic polymers, and neutral polymers. Anionic polymers are compounds that have negatively charged groups. Their action is based on attaching to the particle surface and consequently creating electrostatic charges between particles, stabilizing the flocs. Cationic polymers have positively charged groups, reducing the electrostatic charge between particles and helping to aggregate particles into flocs. Neutral polymers, while uncharged, play a significant role in attaching to the particle surfaces and strengthening the forces between them, making the particles more stable and easier to remove from the water [35]. Cationic polymers were used in the research. The results showed that F 98 (VTA) is a better flocculant than Acefloc (Sotom). The advantage of F 98 is the higher proportion of acids and the presence of citric acid, contributing to better connectivity of more flocs and a better process of removing organic matter from wastewater. This results in a lower amount of COD in the water.
4.4. COD
COD is of primary importance for the wastewater treatment process and ultimately for reaching enough oxygen when released into the environment, which is necessary for the survival of the Proteus Anguinus species [36]. The optimized neutralization, coagulation, and flocculation contributed to a better process of removing organic matter from wastewater and ultimately lower COD values. These results showed a smaller COD than before, a promising sign of the effectiveness of the new chemicals in improving the chemical process of wastewater treatment.
5. Conclusions
Applied research has achieved a lower concentration of COD in chemically treated water, thereby improving the chemical process of cleaning dairy waste. This improvement was achieved by optimizing neutralization, coagulation, and flocculation steps. The research showed that a neutralizer with a higher molar value of sodium hydroxide, a poly aluminum hydroxide coagulant, and a citrus acid flocculant are more effective for dairy wastewater cleaning. The study was conducted under constant temperature and mixtures of wastewater. However, there is a clear need for further research to investigate the process under different temperatures and mixtures of wastewater at the entrance (production of cheese, butter, etc.) to determine the exact burden of production on COD and the environment. Also, it is very important for future research on the impact of the chemical residue's toxicity on the tiny organisms that feed on human fish should be investigated.
In addition, it is crucial to study the consumption of chemicals and their final impact on the economic aspect of maintaining the environmental protection process. This will provide valuable insights into the research's practical implications and raise awareness about the financial implications of our environmental protection efforts.
Author Contributions
Conceptualization, A.Š.; methodology, A.Š.; software, A.Š.; validation, A.Š.; formal analysis, A.Š.; investigation, A.Š.; resources, A.Š.; data curation, A.Š.; writing—original draft preparation, A.Š.; writing—review and editing, A.Š., and D.B.Š.; visualization, A.Š.; supervision, A.Š.; project administration, A.Š.; funding acquisition, A.Š. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
MDPI Research Data Policies
Acknowledgments
I would like to thank you for the management of the Krepko dairy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Šobot, A.; Lukšič, A. Natura 2000 experiences in southeast Europe: comparisons from Slovenia, Croatia and Bosnia and Herzegovina. Journal of Comparative Politics, 2020, 13, 46–57. [Google Scholar]
- Bulog, B.; Mali, L. B.; Kos, M.; Mihajl, K.; Prelovsek, P.-M.; Aljančič, G. Biology and functional morphology of Proteus anguinus (Amphibia, Caudata). Acta Biologica Slovenica, 2000, 43, 85–102. [Google Scholar]
- Bizjak Mali, L.; Sket, B. History and biology of the »Black Proteus« (Proteus anguinus Parkelj Sket & Arntzen 1994; Amphibia: Proteidae): A Review. Folia biologica et geologica 2019, 60, 5–37. [Google Scholar] [CrossRef]
- Arntzen, J. W.; Sket, B. Morphometric analysis of black and white European cave salamanders, Proteus anguinus. J. Zool. Lond., 1997, 241, 699–707. [Google Scholar] [CrossRef]
- Holtze, S.; Lukač, M.; Cizelj, I.; Mutschmann, F.; Szentiks, C. A.; Jelić, D.; Hermes, R.; Göritz, F.; Braude, S.; Hildebrandt, T. B. Monitoring health and reproductive status of olms (Proteus anguinus) by ultrasound. PLoS One, 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Balázs, G.; Lewarne, B.; Herczeg, G. Extreme site fidelity of the olm (Proteus anguinus) revealed by a long-term capture–mark–recapture study. Journal of Zoology, 2020, 311, 99–105. [Google Scholar] [CrossRef]
- Mali, L. B.; Bulog, B. Ultrastructure of previtellogene oocytes in the neotenic cave salamander Proteus anguinus anguinus (Amphibia, Urodela, Proteidae). Protoplasma 2010, 246, 33–39. [Google Scholar] [CrossRef]
- Bizjak Mali, L.; Sepčić, K.; Bulog, B. Long-Term Starvation in Cave Salamander Effects on Liver Ultrastructure and Energy Reserve Mobilization. Journal of Morphology, 2013, 274, 887–900. [Google Scholar] [CrossRef]
- Durand, J. P.; Delay, B. Influence of temperature on the development of Proteus anguinus (Caudata: Proteidae) and relation with its habitat in the subterranean world. Journal of Thermal Biology, 1981, 6, 53–57. [Google Scholar] [CrossRef]
- Tesařová, M.; Mancini, L.; Mauri, E.; Aljančič, G.; Nǎpǎruş-Aljančič, M.; Kostanjšek, R.; Bizjak Mali, L.; Zikmund, T.; Kaucká, M.; Papi, F.; Goyens, J.; Bouchnita, A.; Hellander, A.; Adameyko, I.; Kaiser, J. Living in darkness: exploring adaptation of Proteus anguinus in 3 dimensions by X-ray imaging. GigaScience, 2022, 11, 1–8. [Google Scholar] [CrossRef]
- Lewarne, B.; Balázs, G. “Observed Air-Breathing Behaviour of Proteus anguinus Individuals under an Intermittent Hypoxic Scenario in their Natural Habitat, with Details of the Prevailing Environmental Conditions. ” Observations in Speleology, 2020, 6, 2–7. [Google Scholar]
- Mali, L.B.; Bulog, B. Functional morphology and environmental studies on Proteus. 2016, Natura Sloveniae, 18, 45-46.
- Bulog, B.; Mihajl, K.; Jeran, Z.; Toman, M. J. Trace element concentrations in the tissues of Proteus anguinus (Amphibia, Caudata) and the surrounding environment. Water, Air, and Soil Pollution, 2002, 136 (1–4), 147–163. [CrossRef]
- Recknagel, H.; Premate, E.; Zakšek, V.; Aljančič, G.; Kostanjšek, R.; Trontelj, P. Oviparity, viviparity or plasticity in reproductive mode of the olm Proteus anguinus: an epic misunderstanding caused by prey regurgitation? Contributions to Zoology, 2022, 91, 153–165. [Google Scholar] [CrossRef]
- Koren, K.; Brajkovič, R.; Bajuk, M.; Vraničar, Š.; Fabjan, V. Hydrogeological characterization of karst springs of the white (Proteus anguinus anguinus) and black olm (Proteus anguinus parkelj) habitat in Bela krajina (SE Slovenia). Geologija, 2023, 66, 151–166. [Google Scholar] [CrossRef]
- Kolar, B. The threshold concentration for nitrate in groundwater as a habitat of Proteus anguinus. Natura Sloveniae, 2018, 20, 39–40. [Google Scholar] [CrossRef]
- Glavan, M.; Cvejić, R. Evaluation of Agricultural Measures to Safeguard the Vulnerable Karst Groundwater Habitat of the Black Olm (Proteus Anguinus Parkelj) from Nitrate Pollution. Sustainability, 2024, 16. [CrossRef]
- Balestra, V.; Galbiati, M.; Lapadula, S.; Zampieri, V.; Cassarino, F.; Gajdošová, M.; Barzaghi, B.; Manenti, R.; Ficetola, G. F.; Bellopede, R. Microplastic pollution calls for urgent investigations in stygobiont habitats: a case study from Classical karst. Journal of Environmental Management, 2024, 356. [CrossRef]
- Slavov, A.K. General Characteristics and Treatment Possibilities of Dairy Wastewater – A Review. Food technology and biotechnology, 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Kaur, N. Different treatment techniques of dairy wastewater. Groundwater for Sustainable Development, 2021, 14. [CrossRef]
- Al-Wasify, R. S.; Ali, M. N.; Hamed, S. R. Biodegradation of dairy wastewater using bacterial and fungal local isolates. Water Science and Technology, 2017, 76, 3094–3100. [Google Scholar] [CrossRef]
- Khalaf, A. H.; Ibrahim, W. A.; Fayed, M.; Eloffy, M. G. Comparison between the performance of activated sludge and sequence batch reactor systems for dairy wastewater treatment under different operating conditions. Alexandria Engineering Journal, 2021, 60, 1433–1445. [Google Scholar] [CrossRef]
- Tabelini, D. B.; Lima, J. P. P.; Borges, A. C.; Aguiar, A. A review on the characteristics and methods of dairy industry wastewater treatment in the state of Minas Gerais, Brazil. Journal of Water Process Engineering, 2023, 53. [CrossRef]
- Sinha, S.; Srivastava, A.; Mehrotra, T.; Singh, R. A review on the dairy industry waste water characteristics, its impact on environment and treatment possibilities. In: Emerging Issues in Ecology and Environmental Science. Editor: Jindal, T. Springer, India, 2018, 73-84. [CrossRef]
- Shete, B. S.; Shinkar, N. P. Dairy Industry Wastewater Sources, Characteristics & Its Effects on Environment. International Journal of Current Engineering and Technology, 2013, 1611-1615.
- Shete, B. S.; Shinkar, N.P. Comparative Study of Various Treatments For Dairy Industry Wastewater. IOSR Journal of Engineering, 2013, 03, 42–47. [Google Scholar] [CrossRef]
- Niamsuwan, S.; Kittisupakorn, P.; Mujtaba, I. M. Minimization of water and chemical usage in the cleaning in place process of a milk pasteurization plant. Songklanakarin Journal of Science Technology, 2011, 33, 431–440. [Google Scholar]
- Feil, A. A.; Schreiber, D.; Haetinger, C.; Haberkamp, Â. M.; Kist, J. I.; Rempel, C.; Maehler, A. E.; Gomes, M. C.; da Silva, G. R. Sustainability in the dairy industry: a systematic literature review. Environmental Science and Pollution Research, 2020. [CrossRef]
- Kumar, R.; Kumari, R. Effect of neutralization of milk on sensory, color, yield and textural characteristics of cow milk paneer. International Journal of Chemical Studies, 2019, 7, 5012–5015. [Google Scholar]
- Kuzin, E. N.; Tyaglova, Ya. V.; Gromovykh, P. S. Coagulants in the Processes of Waste Water Treatment in Dairy Complex Industry. Chemistry for Sustainable Development 2020, 28, 388–393. [Google Scholar] [CrossRef]
- Miranda, R.; Latour, I.; Blanco, A. Understanding the Efficiency of Aluminum Coagulants Used in Dissolved Air Flotation (DAF). Frontiers in Chemistry, 2020, 8. [CrossRef]
- Loloei, M.; Alidadi, H.; Nekonam, G.; Kor, Y. Study of the coagulation process in wastewater treatment of dairy industries. International Journal of Environmental Health Engineering, 2013, 2, 12. [Google Scholar] [CrossRef]
- Saritha, V.; Srinivas, N.; Srikanth Vuppala, N. V. Analysis and optimization of coagulation and flocculation process. Appl Water Sci, 2017, 7, 451–460. [Google Scholar] [CrossRef]
- Wolf, G.; Schneider, R. M.; Bongiovani, M. C.; Morgan Uliana, E.; Garcia Do Amaral, A. Application of Coagulation/Flocculation Process of Dairy Wastewater from Conventional Treatment Using Natural Coagulant for Reuse. Chem Eng Trans, 2015, 43, 2041–2046. [Google Scholar] [CrossRef]
- Girish, C. R.; Singh, S. K.; Shenoy, S. Removal of suspended solids from dairy wastewater using flocculation. International Journal of Mechanical Engineering and Technology, 2017, 8, 99–105. [Google Scholar]
- Issartel, J.; Hervant, F.; De Fraipont, M.; Clobert, J.; Voituron, Y. High anoxia tolerance in the subterranean salamander Proteus anguinus without oxidative stress nor activation of antioxidant defenses during reoxygenation. J Comp Physiol B, 2009, 179, 543–551. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).