Submitted:
11 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Lambda-cyhalothrin is a synthetic pyrethroid insecticide that is widely used to control leaf-eating pests. Because of increased insecticide resistance, an under-standing of sublethal cross-generational effects of insecticides is important. We exam-ine the effects of sublethal concentrations (LC) (LC10, LC20, and LC40) of lamb-da-cyhalothrin on growth, reproduction, and detoxification enzyme activities of F0 and F1 generation Henosepilachna vigintioctomaculata. Lambda-cyhalothrin is toxic to adult H. vigintioctomaculata, with an LC40 at 48 h of 0.355 mg L−1. At sublethal concentrations, lambda-cyhalothrin significantly reduces the longevity and average fecundity of F0 and F1 adults, and durations of egg, larval, and pupal stages and adult preoviposition period. Additionally, increased lambda-cyhalothrin concentration significantly de-creases net reproductive rates, and both finite and intrinsic rates of increase of the F1 generation, and significantly increases average generation cycle. Detoxification en-zyme activity of F1 adults treated with sublethal concentrations of lambda-cyhalothrin for 48 h trends upwards. Results indicate that low concentrations of lamb-da-cyhalothrin induce glutathione S-transferase and carboxylesterase activities and inhibit multifunctional oxidase activity. We demonstrate the ability of lamb-da-cyhalothrin to control H. vigintioctomaculata, provide a theoretical basis for the ef-fective control of this pest, and identify an appropriate concentration to do so.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Insecticide
2.2. Insecticide
2.3. Determining Biological Activity
2.4. Effects of Lambda-cyhalothrin Sublethal Treatment on F0 and F1 H. vigintioctomaculata
2.5. Determining Detoxifying Enzyme Activities
2.6. Data Analysis
3. Results
3.1. Toxicity of Lambda-cyhalothrin to Henosepilachna vigintioctomaculata Adults
3.2. Effect of Sublethal Concentration of Lambda-cyhalothrin on Longevity and Fecundity of F0 H. vigintioctomaculata
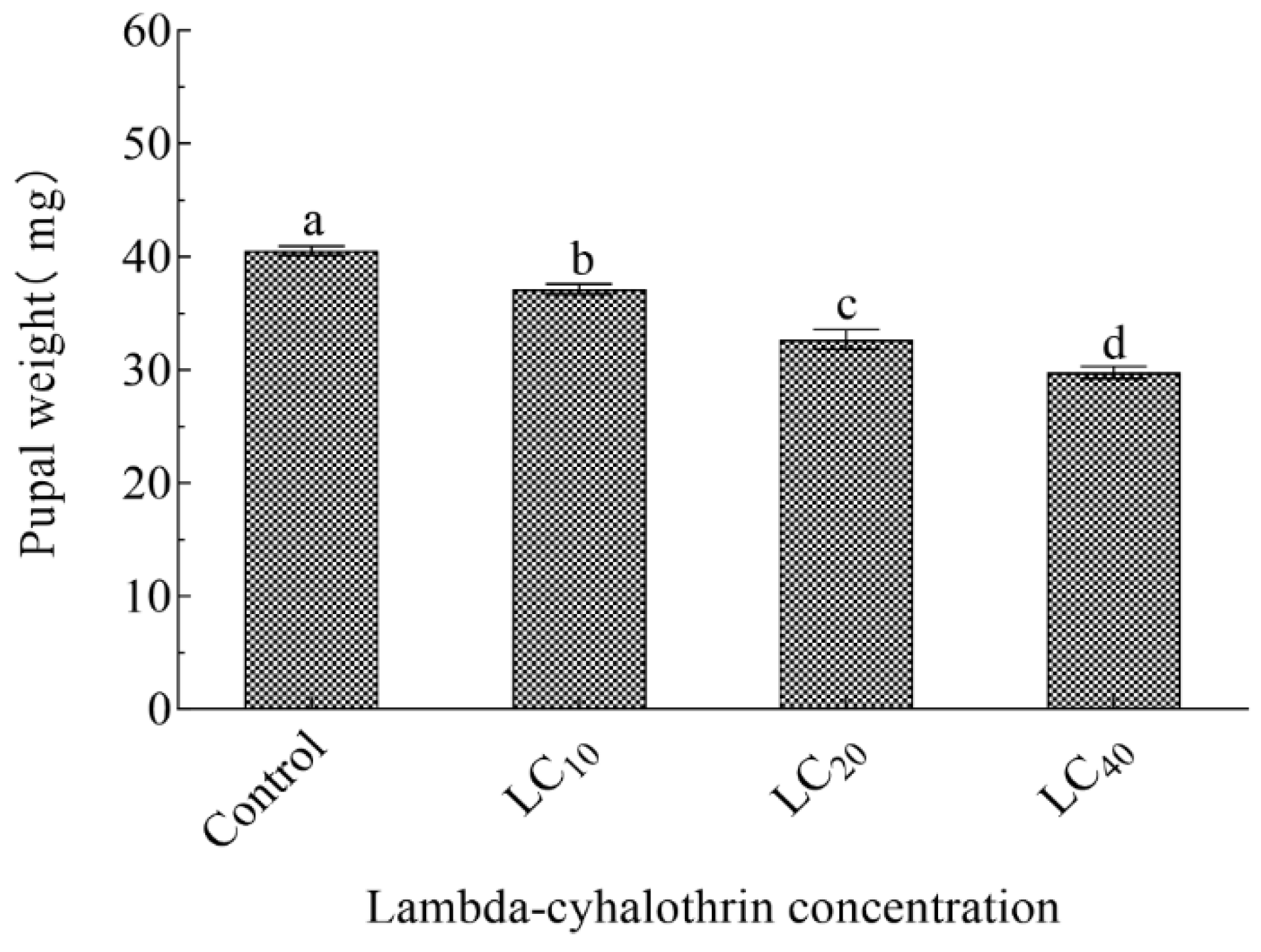
3.3. Effect of Sublethal Concentration of Lambda-cyhalothrin on Growth, Development, Fecundity, and Pupal Weight of F1 H. vigintioctomaculata
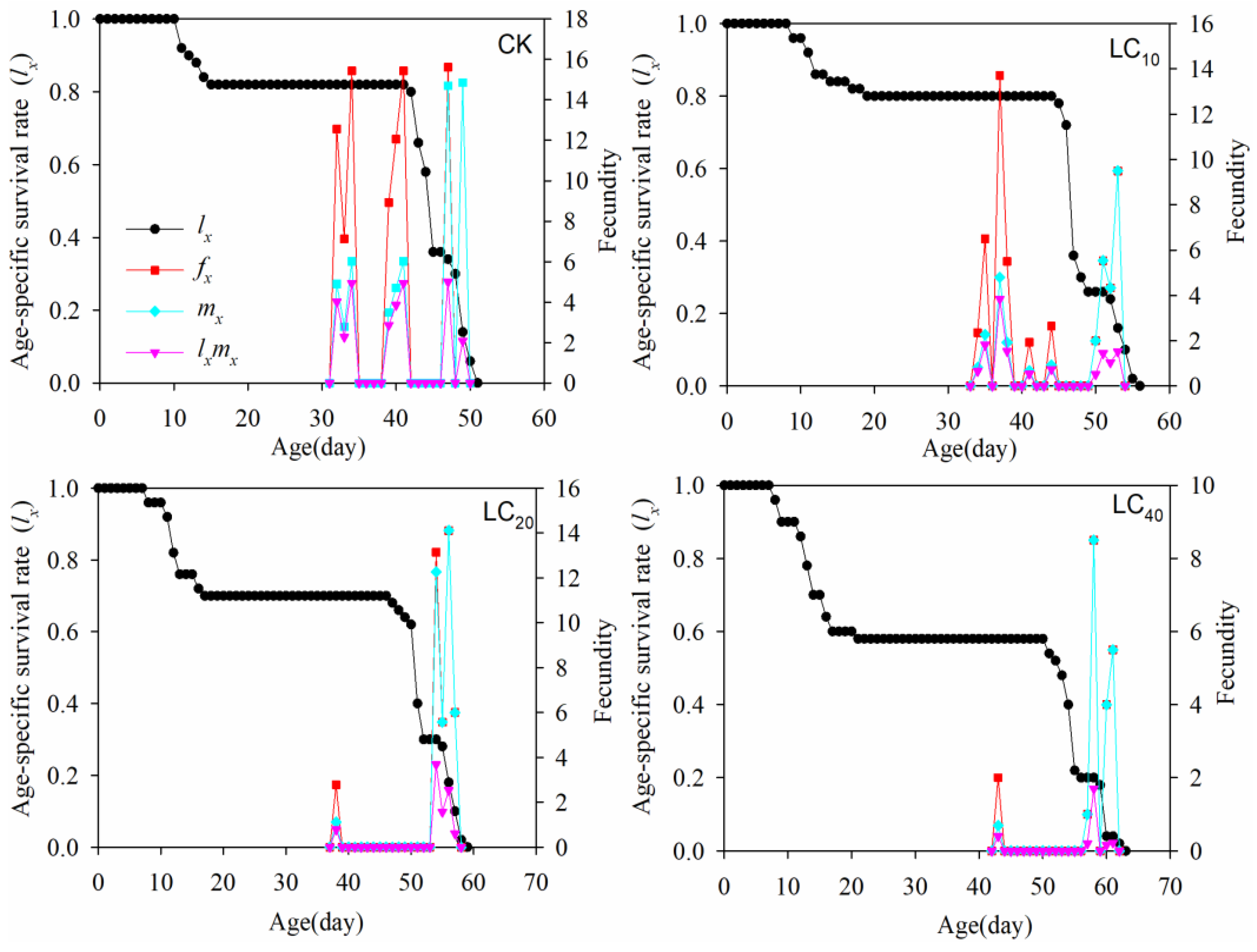
3.4. Population Parameters
3.5. Age–Stage Specific Maternity
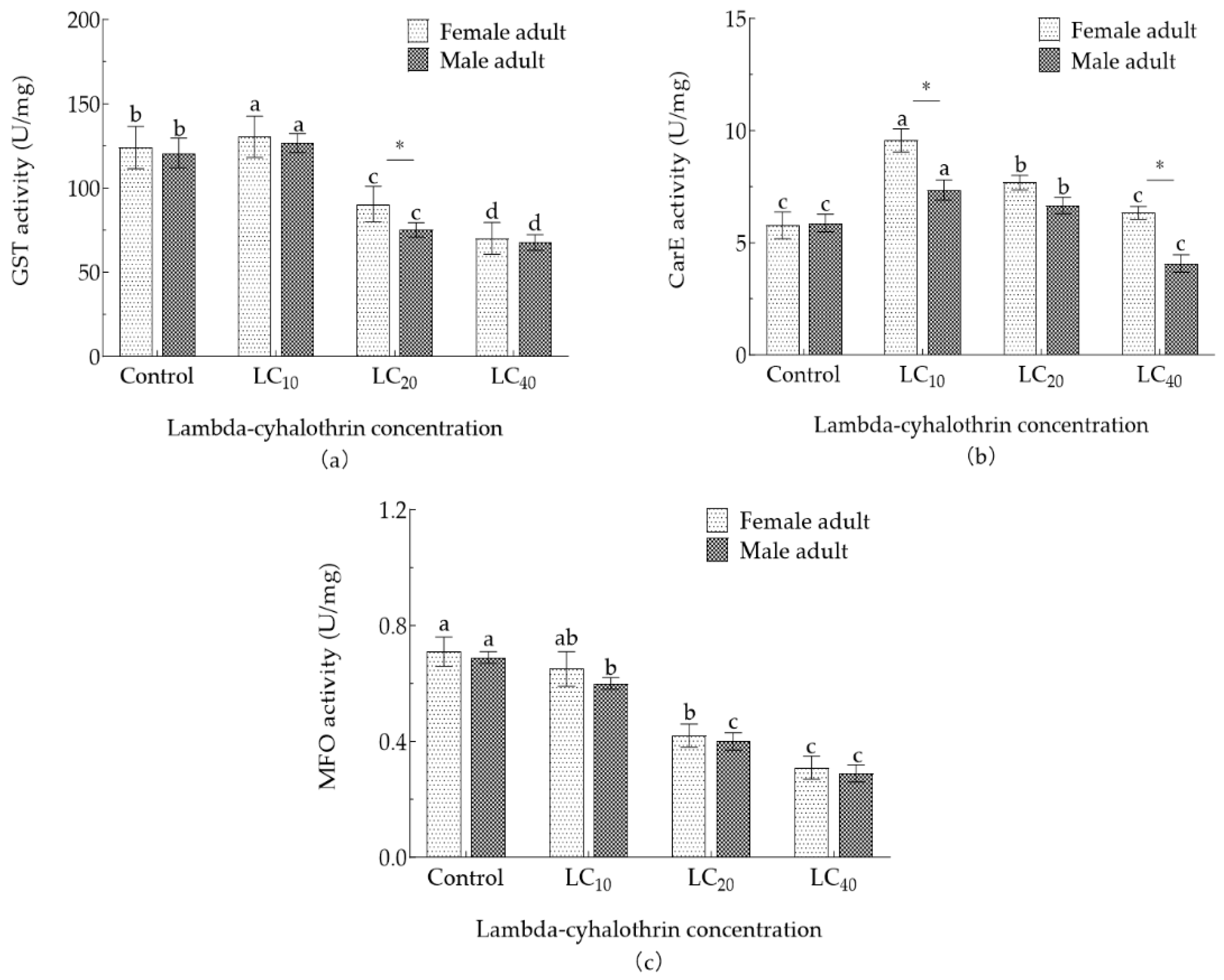
3.6. Effect of Lambda-cyhalothrin Exposure on Detoxifying Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsishina, N.V.; Ermak, M.V.; Fisenko, P.V.; Sobko, O.A. Henosepilachna vigintioctomaculata Motschulsky (Coleoptera: Coccinellidae): morphotypes in an East Asian population. J. Insect Biodivers. 2023, 38, 15–23. [Google Scholar] [CrossRef]
- Li, W.; Bashir, N.H.; Chen, H.; Li, X.; Wang, Z.; Du, L.; Tian, R. Effect of different host plants on the growth, development, and reproduction of Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae). Chin. J. Appl. Entomol. 2024, 61, 287–294. [Google Scholar]
- Ze, L.J.; Jin, L.; Li, G.Q. The compatible effects of RNA interference targeting laccase2 with biocontrol in Henosepilachna vigintioctopunctata. Entomol. Gen. 2023, 43, 117–126. [Google Scholar] [CrossRef]
- Ding, X.; Aerziguli, R.; Ji, L.; Liu, S.; Fu, K.; Tursun, A.; Guo, W. The occurrence and harm of Henosepilachna vigintioctomaculata. Xinjiang Agric. Sci. 2022, 59, 983–989. [Google Scholar]
- Tamang, S.; Alam, T.; Rana, L. Biointensive integrated pest management of potato. In Biointensive integrated pest management for horticultural crops, 1st ed.; Kumar, A., Saha, S., Choudhary, J.S., Eds.; CRC Press: London, 2021; pp. 207–240. [Google Scholar]
- Nithish, A. Study on management of damage caused by Henosepilachna vigintioctopunctata in brinjal. J. Pharmacog. Phytochem. 2020, 9, 2874–2881. [Google Scholar]
- Zhou, J.; Xu, R.; Chen, Z.; Jia, Y.; Xu, K. Phototactic behavior of Henosepilachna vigintioctomaculata motschulsky (Coleoptera: Coccinellidae). Coleopts. Bull. 2015, 69, 806–812. [Google Scholar] [CrossRef]
- Keyhanian, A.A.; Barari, H.; Mobasheri, M.T. Comparison of the efficacy of insecticides, alphacypermethrin and lambda-cyhalothrin, against canola flea beetles. Appl. Entomol. Phytopathol. 2021, 44, 113–122. [Google Scholar]
- Qiu, Y.; Chen, Z. Intergenerational effects of sublethal lambda-cyhalothrin exposure on Aphis gossypii glover (Hemiptera: Aphididae) reproduction and development. Insects 2024, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.M.; Liu, H.Y.; Xin, Z.; Xue, M. Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]
- Gul, H.; Güncan, A.; Ullah, F.; Desneux, N.; Liu, X. Intergenerational sublethal effects of flonicamid on cotton aphid, Aphis gossypii: An age-stage, two-sex life table study. Insects 2024, 15, 529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chen, Y.T.; Chen, Y.; Zhao, J.W.; Fu, J.W.; Shi, M.Z. Sublethal effects of lambda-cyhalothrin on the biological characteristics, detoxification enzymes, and genes of the papaya mealybug, Paracoccus marginatus. J. Pest Sci. 2024, 1–15. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Dermauw, W. The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Ann. Rev. Entomol. 2016, 61, 475–498. [Google Scholar] [CrossRef]
- Guo, W.; Yang, H.; Duan, M.; Xu, H.; Zhao, W.; Wang, C. Sublethal effects of tolfenpyrad and its impact on detoxifying enzymes to Spodoptera exigua. Plant Protect. 2024, 50, 168–175. [Google Scholar]
- Batool, N.; Abubakar, M.; Noureldeen, A.; Naqqash, M.N.; Alghamdi, A.; Al Dhafar, Z.M.; Baakdah, F.; Mozūratis, R. Toxicity and sublethal effect of chlorantraniliprole on multiple generations of Aedes aegypti L. (Diptera: Culicidae). Insects 2024, 15, 851. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bashir, N.H.; Naeem, M.; Tian, R.; Tian, X.; Chen, H. Age-stage, two-sex life table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at different temperatures. Insects 2024, 15, 493. [Google Scholar] [CrossRef]
- Borges, I.; Dury, G.J.; Soares, A.O. Population growth parameters of Scymnus nubilus fed single-aphid diets of Aphis fabae or Myzus persicae. Insects 2024, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.J.; Choi, K.S. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under fluctuating temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Bian, Q.; Zhang, H.; Gao, X.; Liang, P. Bioassay technique for Plutella xylostella: Leaf-dip method. Chin. J. Appl. Entomol. 2013, 50, 556–560. [Google Scholar]
- Jiang, M.; Qian, X.; Zhou, Z.; Liu, Y.; Zhang, M.; Yang, Y. Impacts of sublethal doses of spinetoram on the biological traits and detoxifying enzymes of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2024, 15, 990. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H.S.I. Two new methods for study of insect population ecology. Bull. Inst. Zool Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis; National Chung Hsing University in Taiwan: Taichung City, Taiwan, China, 2023. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An introduction to the bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Dean, A.N.; Niemi, J.B.; Tyndall, J.C.; Hodgson, E.W.; O'Neal, M.E. Developing a decision-making framework for insect pest management: A case study using Aphis glycines ( H emiptera: A phididae). Pest Manag. Sci. 2021, 77, 886–894. [Google Scholar] [CrossRef]
- Havasi, M.; Zahedi Golpayegani, A.; Bandani, A. The sublethal concentration of Cyflumetofen adversely affect demographic parameters of Tetranychus urticae (Acari: Tetranychidae): Using age-stage, two-sex life tables. Int. J. Acarol. 2022, 48, 331–337. [Google Scholar] [CrossRef]
- Fan, R.; Fan, Z.; Sun, Z.; Chen, Y.; Gui, F. Insecticide susceptibility and detoxification enzyme activity of frankliniella occidentalis under three habitat conditions. Insects 2023, 14, 643. [Google Scholar] [CrossRef]
- Wang, D.; Gong, P.; Li, M.; Qiu, X.; Wang, K. Sublethal effects of spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Jin, D. Sublethal effects of chlorantraniliprole on the experimental populations of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Acta Entomol. Sin. 2012, 55, 1161–1167. [Google Scholar] [CrossRef]
- Ju, D.; Liu, Y.X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, Y.T.; Wang, Q.Y.; Zheng, L.Z.; Fu, J.W.; Shi, M.Z. Sublethal and transgenerational toxicities of chlorfenapyr on biological traits and enzyme activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Insects 2022, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, C.; Ma, R.; Chen, Y.; Gui, F.; Sun, Z. Effects of sublethal concentrations chlorfenapyr on growth, development and enzyme activity of Tuta absoluta. J. Environ. Entomol. 2024, 46, 1349–1357. [Google Scholar] [CrossRef]
- Bian, D.; Ren, Y.; Ye, W.; Dai, M.; Li, F.; Wei, J.; Sun, H.; Li, B. Evaluation of tolerance to λ-cyhalothrin and response of detoxification enzymes in silkworms reared on artificial diet. Ecotoxicol. Environ. Saf. 2022, 232, 113232. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Brown, P.H.; Calabrese, E.J. A gift from parent to offspring: Transgenerational hormesis. Trends Plant Sci. 2021, 26, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Ayyanath, M.-M.; Cutler, G.C.; Scott-Dupree, C.D.; Prithiviraj, B.; Kandasamy, S.; Prithiviraj, K. Gene expression during imidacloprid-induced hormesis in green peach aphid. Dose Response 2014, 12, 480–497. [Google Scholar] [CrossRef]
- Alimirzaee, S.; Khajehali, J.; Van Leeuwen, T. Hormetic effects of neonicotinoid insecticides on Rhizoglyphus robini (Acari: Acaridae). Pestic. Biochem. Physiol. 2023, 192, 105396. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Wang, Q.; Ji, X.; Wang, W.; Huang, W.; Rui, C.; Cui, L. Hormesis effects of sulfoxaflor on Aphis gossypii feeding, growth, reproduction behaviour and the related mechanisms. Sci. Total Environ. 2023, 872, 162240. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Yang, H.; Gong, M.; Long, G.; Jin, D. Role of SfJHAMT and SfFAMeT in the reproductive regulation of Sogatella furcifera and its expression under insecticide stress. Pestic. Biochem. Physiol. 2021, 173, 104779. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Yang, H.; Gong, M.; Long, G.; Jin, D. Role of insecticide-mediated transcription of the TOR and JH signaling pathway-related genesin the regulation ofreproduction in Sogatella furcifera. Entomol. Gen. 2022, 42, 771–779. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Dermauw, W. The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu. Rev. Entomol. 2016, 61, 475–498. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, T.; van Pottelberge, S.; Tirry, L. Biochemical analysis of a chlorfenapyr-selected resistant strain of Tetranychus urticae Koch. Pest Manag. Sci. 2006, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Ding, J.; Wang, Y.; Zhang, Z.; Liu, F.; Mu, W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2018, 148, 93–102. [Google Scholar] [CrossRef]
- Li, P.R.; Shi, Y.; Ju, D.; Liu, Y.X.; Wang, W.; He, Y.S.; Zhang, Y.Y.; Yang, X.Q. Metabolic functional redundancy of the CYP9A subfamily members leads to P450 -mediated lambda -cyhalothrin resistance in Cydia pomonella. Pest Manag. Sci. 2023, 79, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Kinareikina, A.; Silivanova, E. Impact of insecticides at sublethal concentrations on the enzyme activities in adult Musca domestica L. Toxics, 2023, 11, 47. [Google Scholar] [CrossRef] [PubMed]
| Insecticide | Number of samples |
LC10 (95% CL) (mg L−1) |
LC20 (95% CL) (mg L−1) |
LC40 (95% CL) (mg L−1) |
χ2 (df) |
Slope± SE | p-value |
| Lambda-cyhalothrin | 450 | 0.193 (0.112–0.265) | 0.251 (0.184–0.352) | 0.355 (0.258–0.432) | 9.53 | 3.91 ± 0.59 | 0.023 |
| Parameter | Control | Lambda-cyhalothrin (LC10) | Lambda-cyhalothrin (LC20) | Lambda-cyhalothrin (LC40) |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | |
| Male longevity (d) | 24.39 ± 0.30 a | 24.00 ± 0.23 a | 21.45 ± 0.29 b | 20.09 ± 0.33 c |
| Female longevity (d) | 26.27 ± 0.34 a | 26.00 ± 0.21 a | 24.29 ± 0.39 b | 21.79 ± 0.29 c |
| Fecundity (eggs laid/female) | 65.66 ± 9.21 a | 59.11 ± 7.09 b | 42.12 ± 5.56 c | 31.09 ± 4.76 d |
| F1 egg Hatching rate | 84.29 ± 11.55 a | 68.73 ± 9.79 b | 58.26 ± 9.60 c | 49.46 ± 7.88 d |
| Parameter (duration, days) | Treatment | |||
| Control |
Lambda-cyhalothrin (LC10) |
Lambda-cyhalothrin (LC20) | Lambda-cyhalothrin (LC40) | |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | |
| Egg (d) | 5.66 ± 0.07 c | 6.48 ± 0.07 b | 6.70 ± 0.07 b | 7.12 ± 0.05 a |
| Larva (d) | 15.05 ± 0.17 c | 16.32 ± 0.23 b | 16.60 ± 0.21 b | 18.69 ± 0.30 a |
| Pupa (d) | 4.59 ± 0.08 c | 5.40 ± 0.13 b | 5.71 ±0.15 b | 6.17 ± 0.19 a |
| Male longevity (d) | 23.53 ± 0.25 a | 22.52 ± 0.31 b | 20.12 ± 0.17 c | 18.88 ± 0.21 d |
| Female longevity (d) | 25.40 ± 0.27 a | 24.86 ± 0.18 b | 23.21 ± 0.46 c | 21.19 ± 0.26 d |
| APOP (d) | 6.20 ± 0.47 b | 7.00 ± 1.36 a | 7.00 ± 0.59 a | 7.10 ± 0.82 a |
| TPOP (d) | 23.53 ± 0.52 d | 26.75 ± 1.44 c | 29.79 ± 1.24 b | 30.45 ± 1.70 a |
| Eclosion rate % | 85.98 ± 0.13 a | 80.54 ± 0.76 b | 71.02 ± 0.54 c | 60.66 ± 0.73 d |
| Fecundity (eggs laid/female) | 55.59 ± 8.72 a | 48.79 ± 7.08 b | 32.71 ± 3.23 c | 13.40 ± 3.06 d |
| Parameters | Concentration treatments | |||
| Control | Lambda-cyhalothrin (LC10) | Lambda-cyhalothrin (LC20) | Lambda-cyhalothrin (LC40) | |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | |
| Intrinsic rate of increase, r d−1 | 0.087 ± 0.006 a | 0.062 ± 0.007 b | 0.041 ± 0.005 c | 0.017 ± 0.007 d |
| Finite rate of increase, λ d−1 | 1.081 ± 0.006 a | 1.064 ± 0.007 b | 1.041 ± 0.005 c | 1.017 ± 0.009 d |
| Net reproductive rate, R0 (offspring/individual) | 25.98 ± 6.75 a | 13.66 ± 3.64 b | 9.16 ± 1.25 c | 3.68 ± 0.96 d |
| Mean generation time, T (d) | 38.97 ± 0.46 c | 41.69 ± 0.74 b | 53.98 ± 1.80 a | 54.52 ± 1.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





