Submitted:
10 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Preparation of Sensors
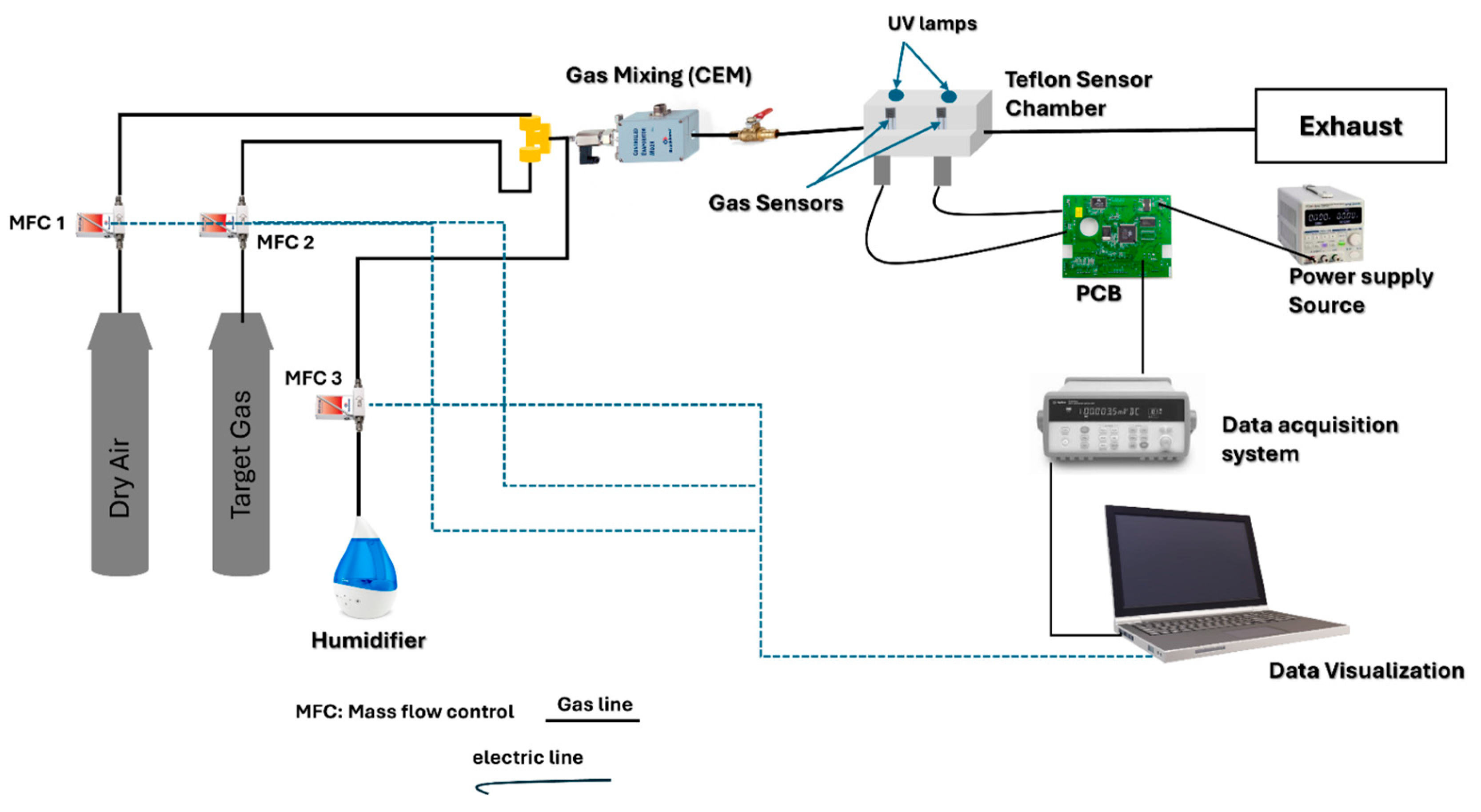
2.3. Gas sensing measurements
2.4. Material characterization
3. Results
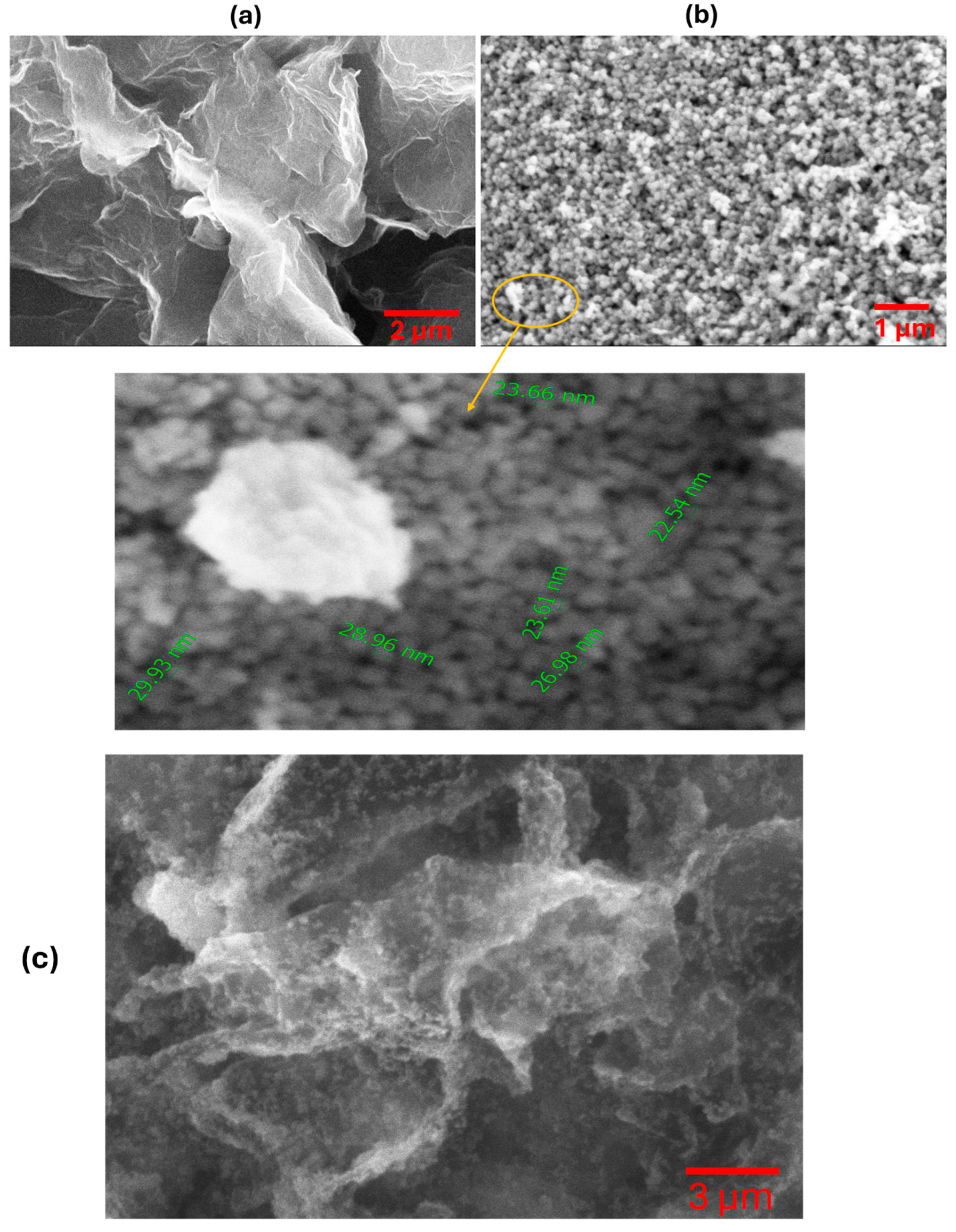
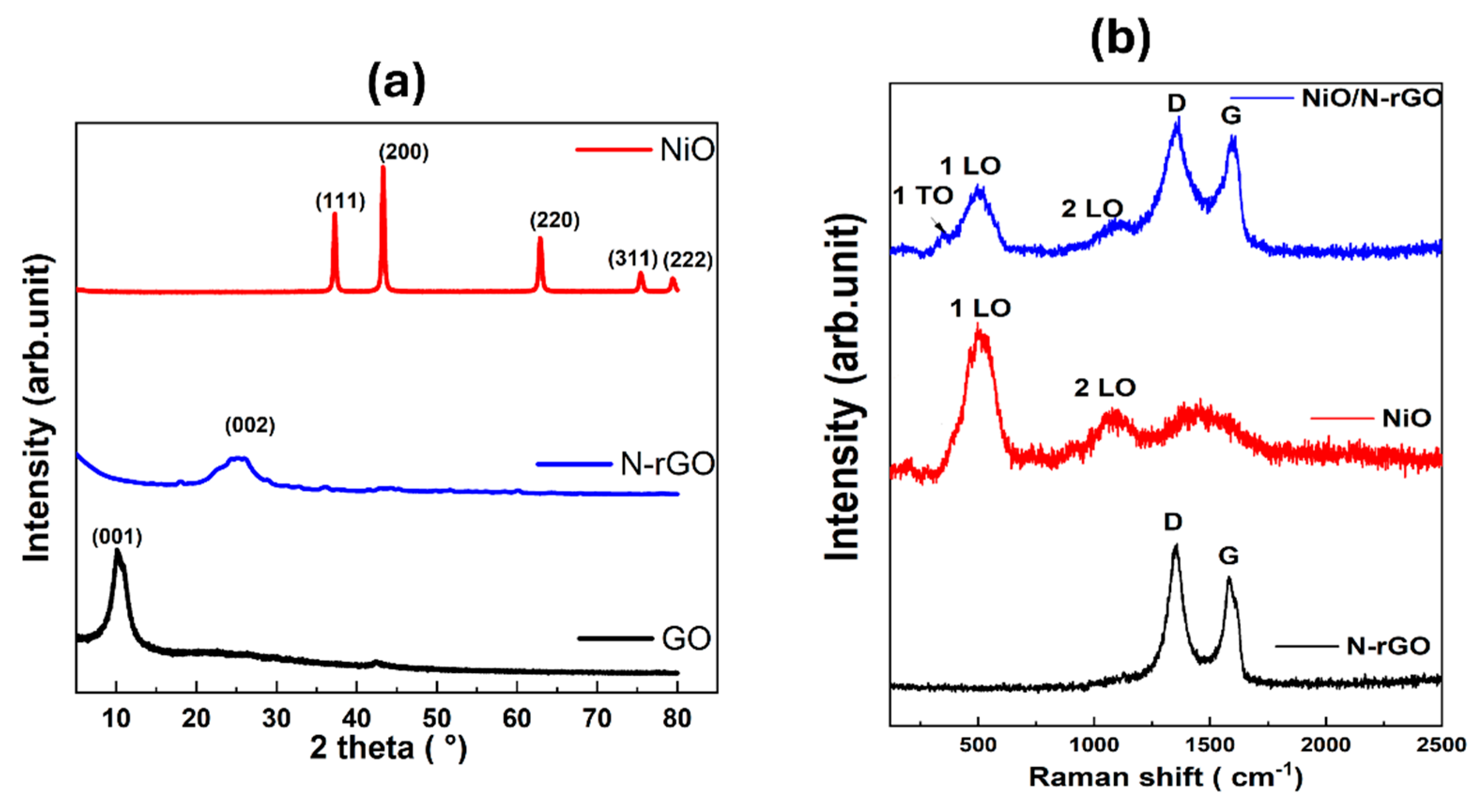
3.1. Characterization
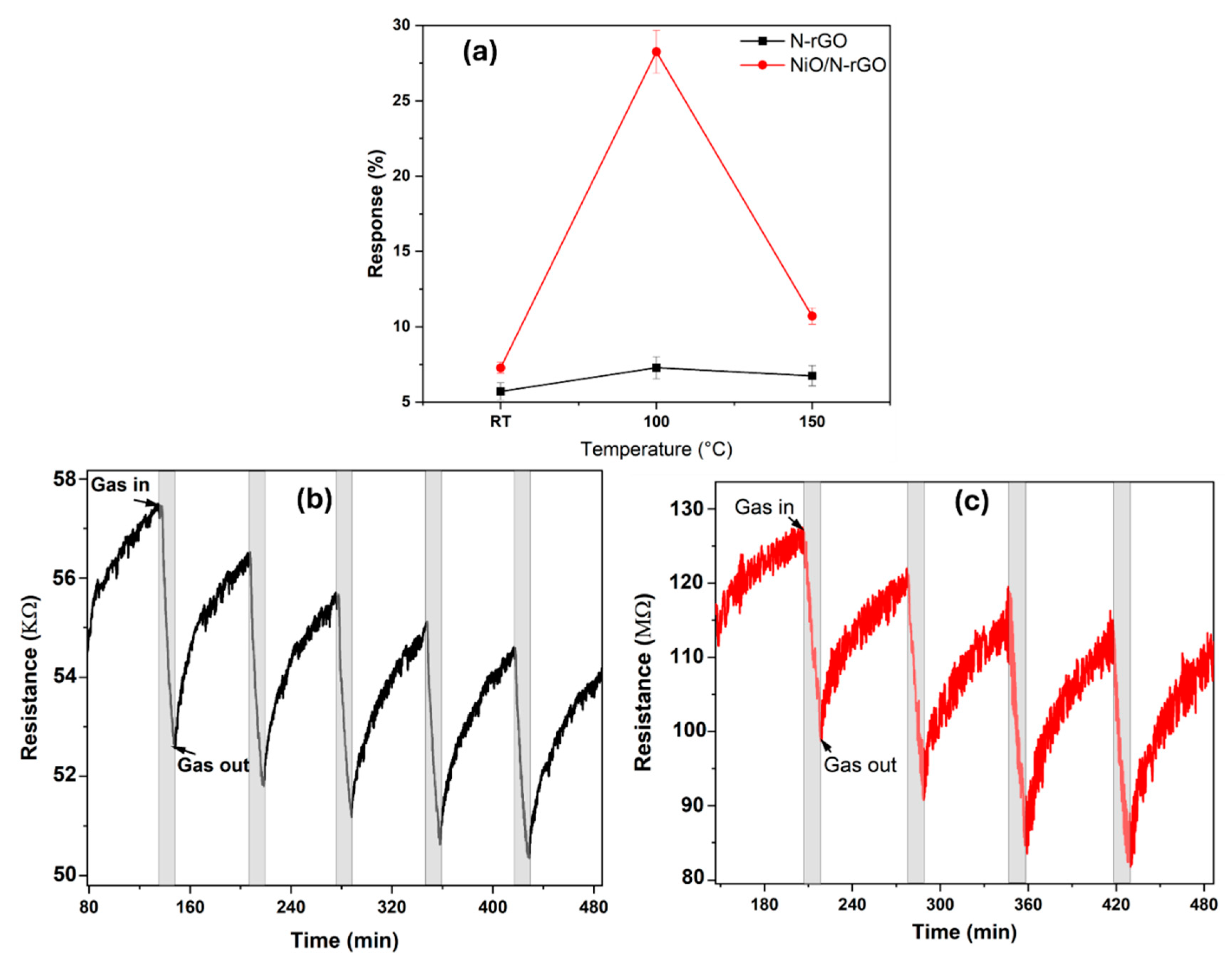
3.2. Gas sensing characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- H. Salonen, T. Salthammer, and L. Morawska, Environ Int 130, 104887 (2019).
- I. Manisalidis, E. Stavropoulou, A. Stavropoulos, and E. Bezirtzoglou, Front Public Health 8, (2020).
- H. Mei, F. Zhang, T. Zhou, and T. Zhang, Sensors 24, 7188 (2024).
- P. Recum and T. Hirsch, Nanoscale Adv 6, 11 (2024).
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H.-D. Lee, and K. Jayaramulu, Chem Soc Rev 53, 2530 (2024).
- J. Ma, M. Zhang, L. Dong, Y. Sun, Y. Su, Z. Xue, and Z. Di, AIP Adv 9, (2019).
- W. Yuan and G. Shi, J Mater Chem A Mater 1, 10078 (2013).
- R. Ghosh, M. Aslam, and H. Kalita, Mater Today Commun 30, 103182 (2022).
- C. Walleni, S. B. Malik, G. Missaoui, M. A. Alouani, M. F. Nsib, and E. Llobet, ACS Omega (2024).
- 1H. Wang, T. Maiyalagan, and X. Wang, ACS Catal 2, 781 (2012).
- W. Ouyang, D. Zeng, X. Yu, F. Xie, W. Zhang, J. Chen, J. Yan, F. Xie, L. Wang, H. Meng, and D. Yuan, Int J Hydrogen Energy 39, 15996 (2014).
- A. Ariharan, B. Viswanathan, and V. Nandhakumar, Graphene 06, 41 (2017).
- M. Shaik, V. K. Rao, M. Gupta, K. S. R. C. Murthy, and R. Jain, RSC Adv 6, 1527 (2016).
- Y.-S. Chang, F.-K. Chen, D.-C. Tsai, B.-H. Kuo, and F.-S. Shieu, Sci Rep 11, 20719 (2021).
- A. Mirzaei, S. P. Bharath, J.-Y. Kim, K. K. Pawar, H. W. Kim, and S. S. Kim, Chemosensors 11, 334 (2023).
- C. Wang, L. Yin, L. Zhang, D. Xiang, and R. Gao, Sensors 10, 2088 (2010).
- S. Yang, G. Lei, H. Xu, Z. Lan, Z. Wang, and H. Gu, Nanomaterials 11, 1026 (2021).
- S. Masroor, in Inorganic Anticorrosive Materials (Elsevier, 2022), pp. 85–94.
- N. Khomarloo, E. Mohsenzadeh, H. Gidik, R. Bagherzadeh, and M. Latifi, RSC Adv 14, 7806 (2024).
- G. F. Fine, L. M. Cavanagh, A. Afonja, and R. Binions, Sensors 10, 5469 (2010).
- S. Yang, G. Lei, H. Xu, Z. Lan, Z. Wang, and H. Gu, Nanomaterials 11, 1026 (2021).
- S. K. Ayyala and J. A. Covington, Chemosensors 9, 247 (2021).
- M. P. Deshpande, K. N. Patel, V. P. Gujarati, K. Patel, and S. H. Chaki, Adv Mat Res 1141, 65 (2016).
- C. Walleni, N. Hamdaoui, S. B. Malik, M. F. Nsib, and E. Llobet, SSRN (2023).
- A. A. Khaleed, A. Bello, J. K. Dangbegnon, M. J. Madito, F. U. Ugbo, A. A. Akande, B. P. Dhonge, F. Barzegar, D. Y. Momodu, B. W. Mwakikunga, and N. Manyala, J Mater Sci 52, 2035 (2017).
- T. Kamal, J Alloys Compd 729, 1058 (2017).
- S. Srirattanapibul, P. Nakarungsee, C. Issro, I.-M. Tang, and S. Thongmee, Mater Sci Semicond Process 137, 106221 (2022).
- S. Shanavas, T. Ahamad, S. M. Alshehri, R. Acevedo, and P. M. Anbarasan, Optik (Stuttg) 226, 165970 (2021).
- A. Shanmugasundaram, N. D. Chinh, Y.-J. Jeong, T. F. Hou, D.-S. Kim, D. Kim, Y.-B. Kim, and D.-W. Lee, J Mater Chem A Mater 7, 9263 (2019).
- R. Muzyka, M. Kwoka, Ł. Smędowski, N. Díez, and G. Gryglewicz, New Carbon Materials 32, 15 (2017).
- C. Xu, Y. Li, R. A. Adams, V. G. Pol, Y. Xiao, A. Varma, and P. Chen, J Alloys Compd 884, 160927 (2021).
- X. Li, A. Zhong, S. Wei, X. Luo, Y. Liang, and Q. Zhu, Electrochim Acta 164, 203 (2015).
- M. Salavati-Niasari, F. Davar, and Z. Fereshteh, J Alloys Compd 494, 410 (2010).
- J. P. Saikia, S. Paul, B. K. Konwar, and S. K. Samdarshi, Colloids Surf B Biointerfaces 78, 146 (2010).
- D. Deep Yadav, R. Jha, S. Singh, and A. Kumar, Mater Today Proc 73, 333 (2023).
- R. A. Rochman, S. Wahyuningsih, and A. H. Ramelan, IOP Conf Ser Mater Sci Eng 509, 012119 (2019).
- X. Duan, S. Indrawirawan, H. Sun, and S. Wang, Catal Today 249, 184 (2015).
- R. Beams, L. Gustavo Cançado, and L. Novotny, Journal of Physics Condensed Matter 27, (2015).
- N. Dharmaraj, P. Prabu, S. Nagarajan, C. H. Kim, J. H. Park, and H. Y. Kim, Materials Science and Engineering: B 128, 111 (2006).
- A. Kaschner, A. Hoffmann, and C. Thomsen, Phys Rev B 64, 165314 (2001).
- N. J. Usharani and S. S. Bhattacharya, Ceram Int 46, 5671 (2020).
- S.-Y. Chu, M.-J. Wu, T.-H. Yeh, C.-T. Lee, and H.-Y. Lee, Nanomaterials 13, 1064 (2023).
- S. B. Malik, F. E. Annanouch, R. D′Souza, C. Bittencourt, M. Todorović, and E. Llobet, ACS Appl Mater Interfaces 16, 48585 (2024).
- G. Tan, D. Tang, X. Wang, L. He, T. Mu, and G. Li, Int J Electrochem Sci 17, 220551 (2022).
- J. Li, Y. Lu, Q. Ye, M. Cinke, J. Han, and M. Meyyappan, Nano Lett 3, 929 (2003).
- R. Verma, M. S. Chauhan, S. Pandey, and A. Dandia, Heliyon 9, e17162 (2023).
- W. Yan, M. A. Worsley, T. Pham, A. Zettl, C. Carraro, and R. Maboudian, Appl Surf Sci 450, 372 (2018).
- X. Song, L. Li, X. Chen, Q. Xu, B. Song, Z. Pan, Y. Liu, F. Juan, F. Xu, and B. Cao, Sens Actuators B Chem 298, 126917 (2019).
- H. Bai, H. Guo, J. Wang, Y. Dong, B. Liu, Z. Xie, F. Guo, D. Chen, R. Zhang, and Y. Zheng, Sens Actuators B Chem 337, 129783 (2021).
- A. Choudhari, B. A. Bhanvase, V. K. Saharan, P. H. Salame, and Y. Hunge, Ceram Int 46, 11290 (2020).
- W. Yan, K. Zhu, Y. Cui, Y. Li, T. Dai, S. Cui, and X. Shen, J Alloys Compd 886, 161287 (2021).
- P. Cao, Y. Cai, D. Pawar, S. T. Navale, Ch. N. Rao, S. Han, W. Xu, M. Fang, X. Liu, Y. Zeng, W. Liu, D. Zhu, and Y. Lu, Chemical Engineering Journal 401, 125491 (2020).
- M. M. Gomaa, M. H. Sayed, V. L. Patil, M. Boshta, and P. S. Patil, J Alloys Compd 885, 160908 (2021).
- Jyoti and G. D. Varma, Applied Physics A 126, 143 (2020).
- M. A. Alouani, J. Casanova-Chafer, S. de Bernardi-Martín, A. García-Gómez, F. Salehnia, J. C. Santos-Ceballos, A. Santos-Betancourt, X. Vilanova, and E. Llobet, Chemosensors 12, 256 (2024).


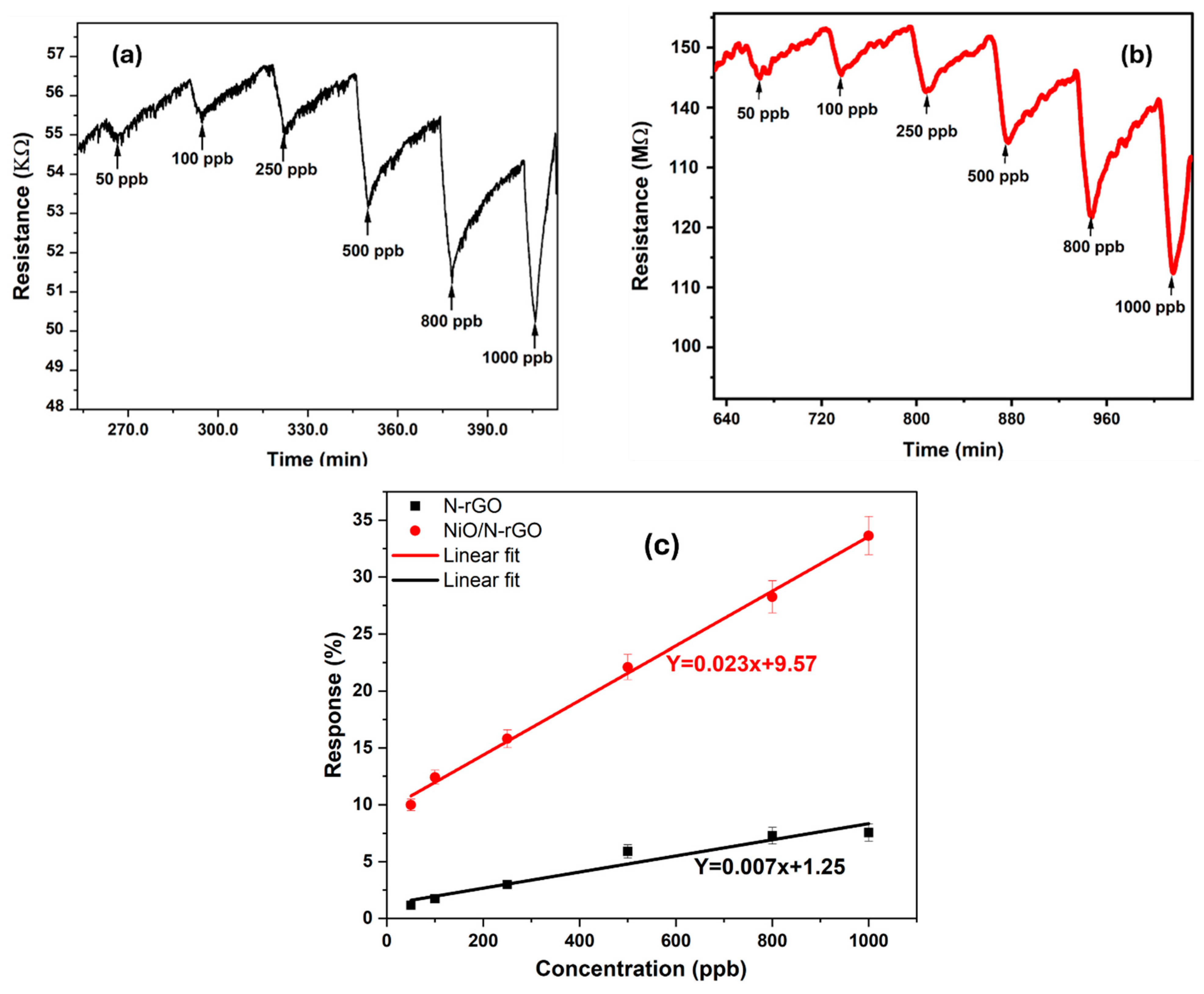
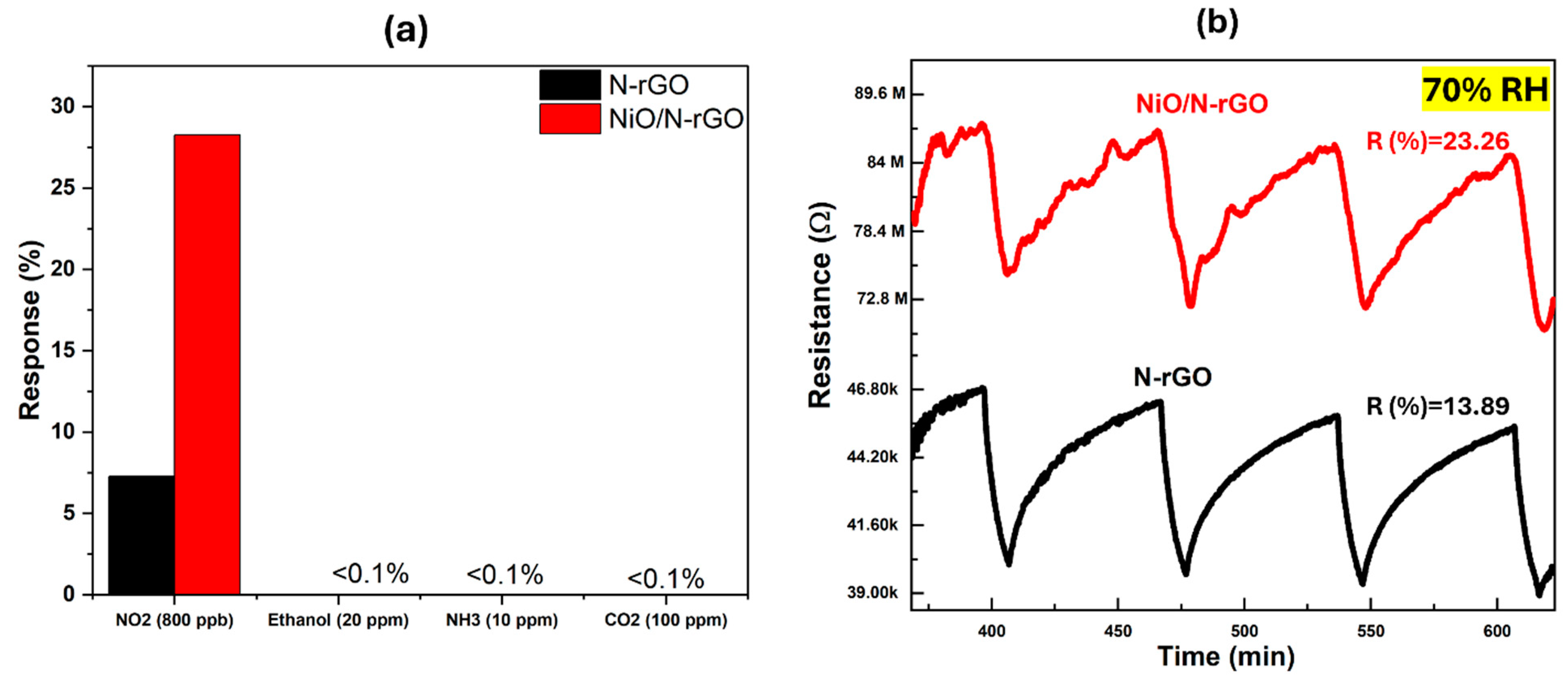
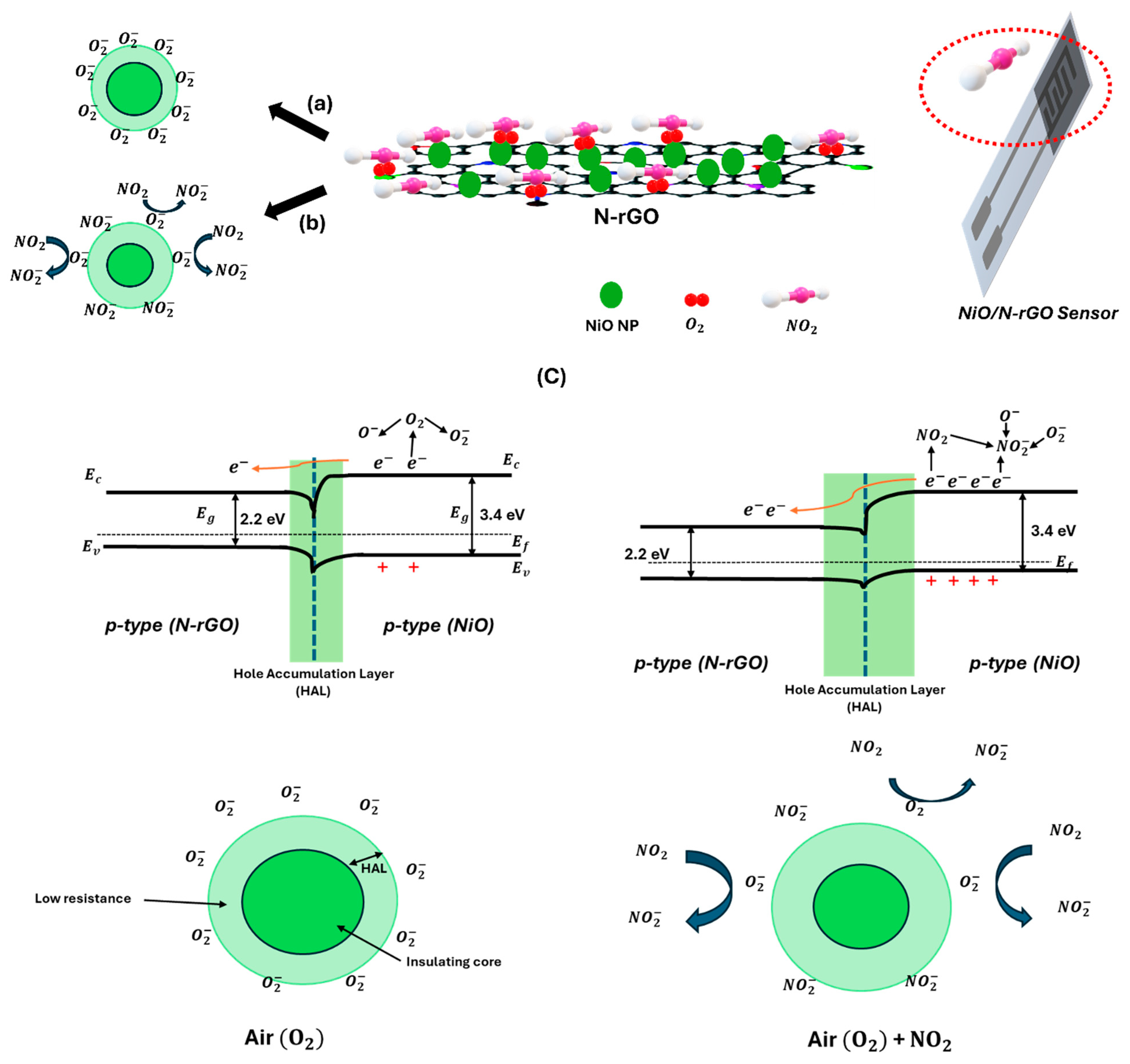
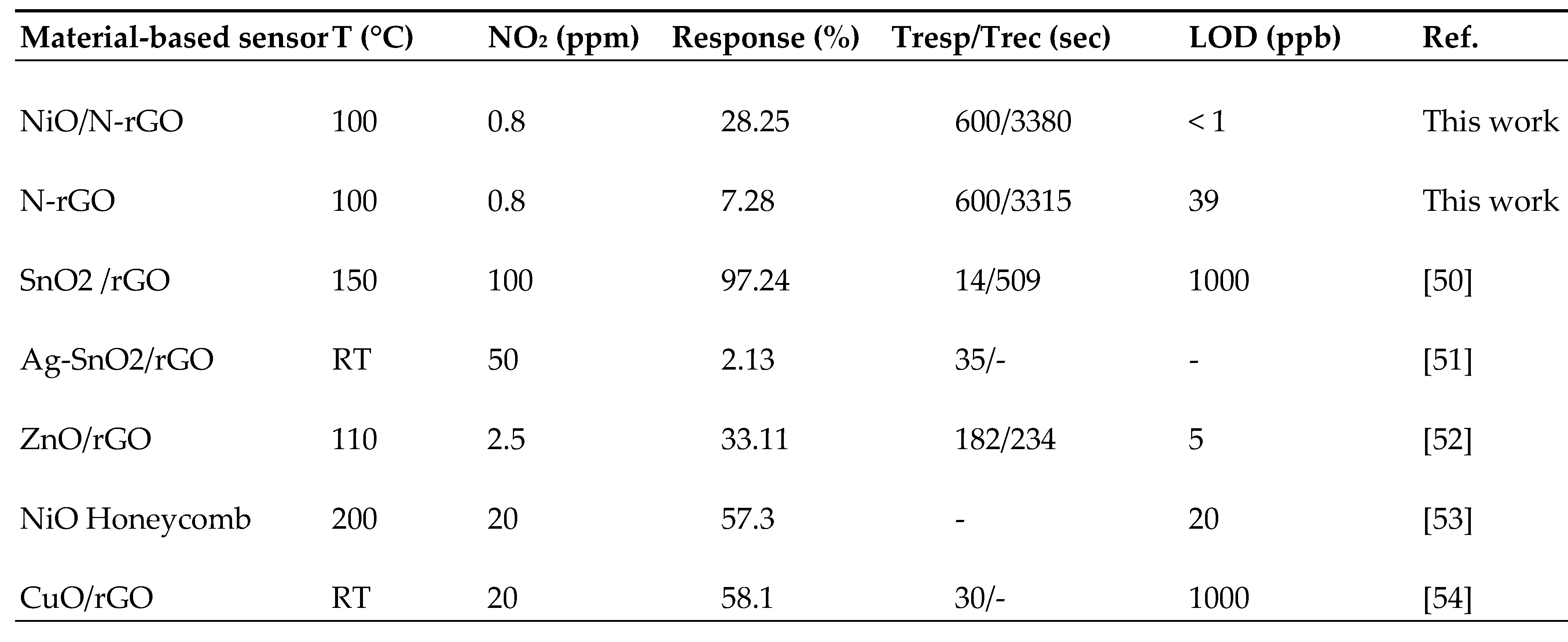
| Sensors | N-rGO | NiO/N-rGO |
|---|---|---|
| Sensitivity (10-2 ppm-1) | 709 | 2398 |
| LoD (ppb) | 39 | < 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





