Submitted:
17 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
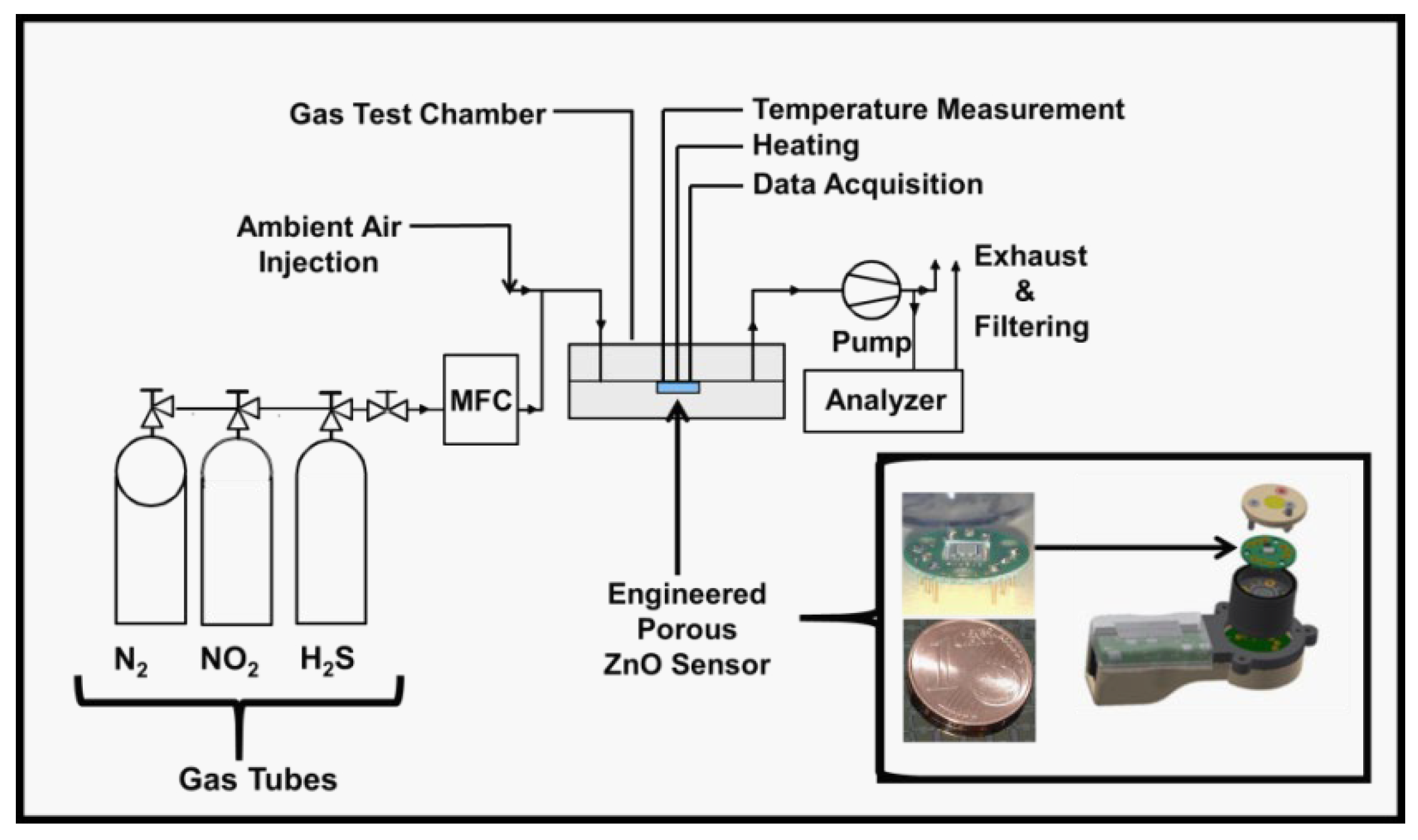
2. Experimental
3. Result
3.1. Conception and Creation of Engineered Porosity ZnO with Oxygen Vacancies ()
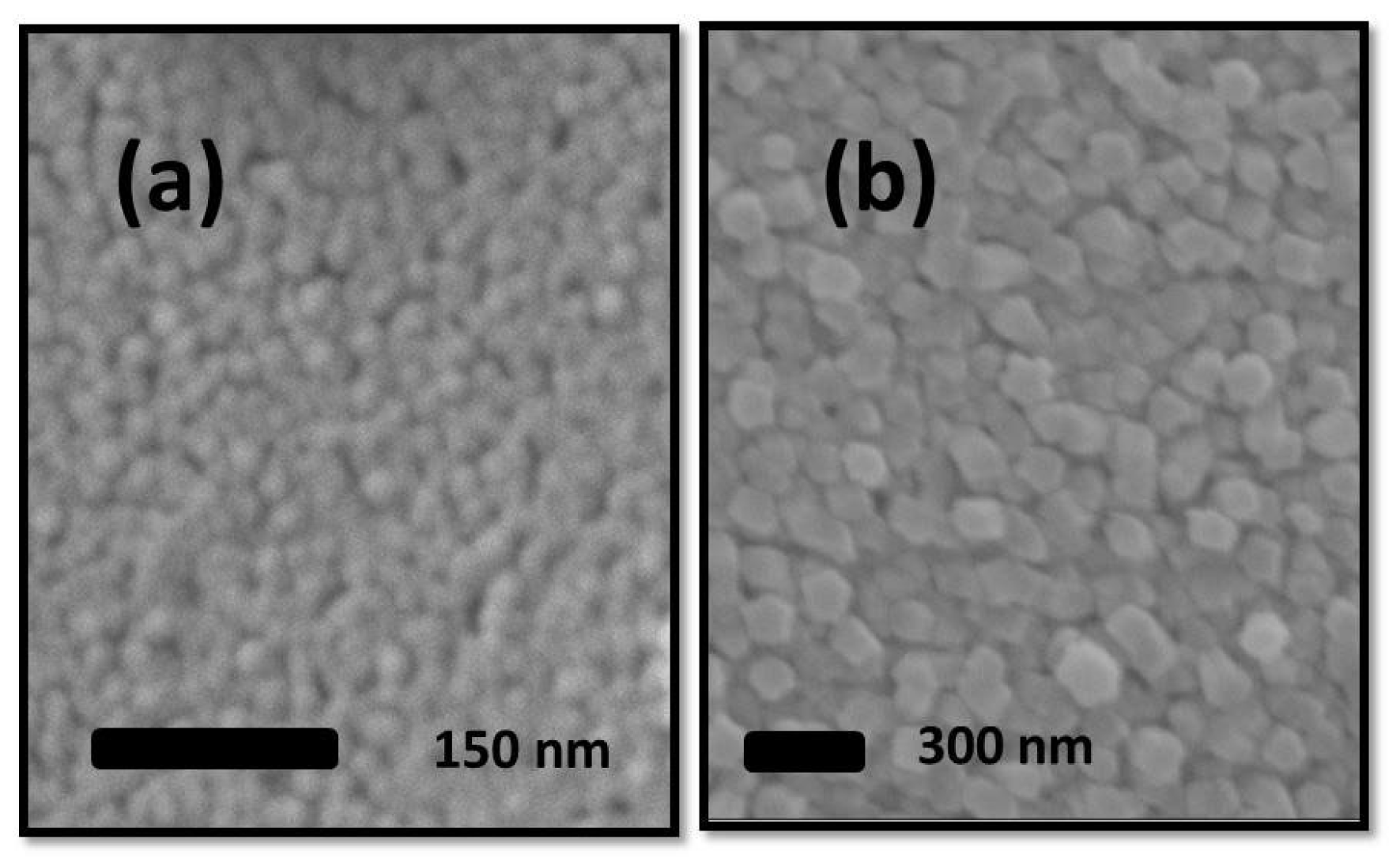
3.2. ZnO Microstructure Analysis
3.3. Zinc oxide (ZnO) Electrical Resistance Investigation

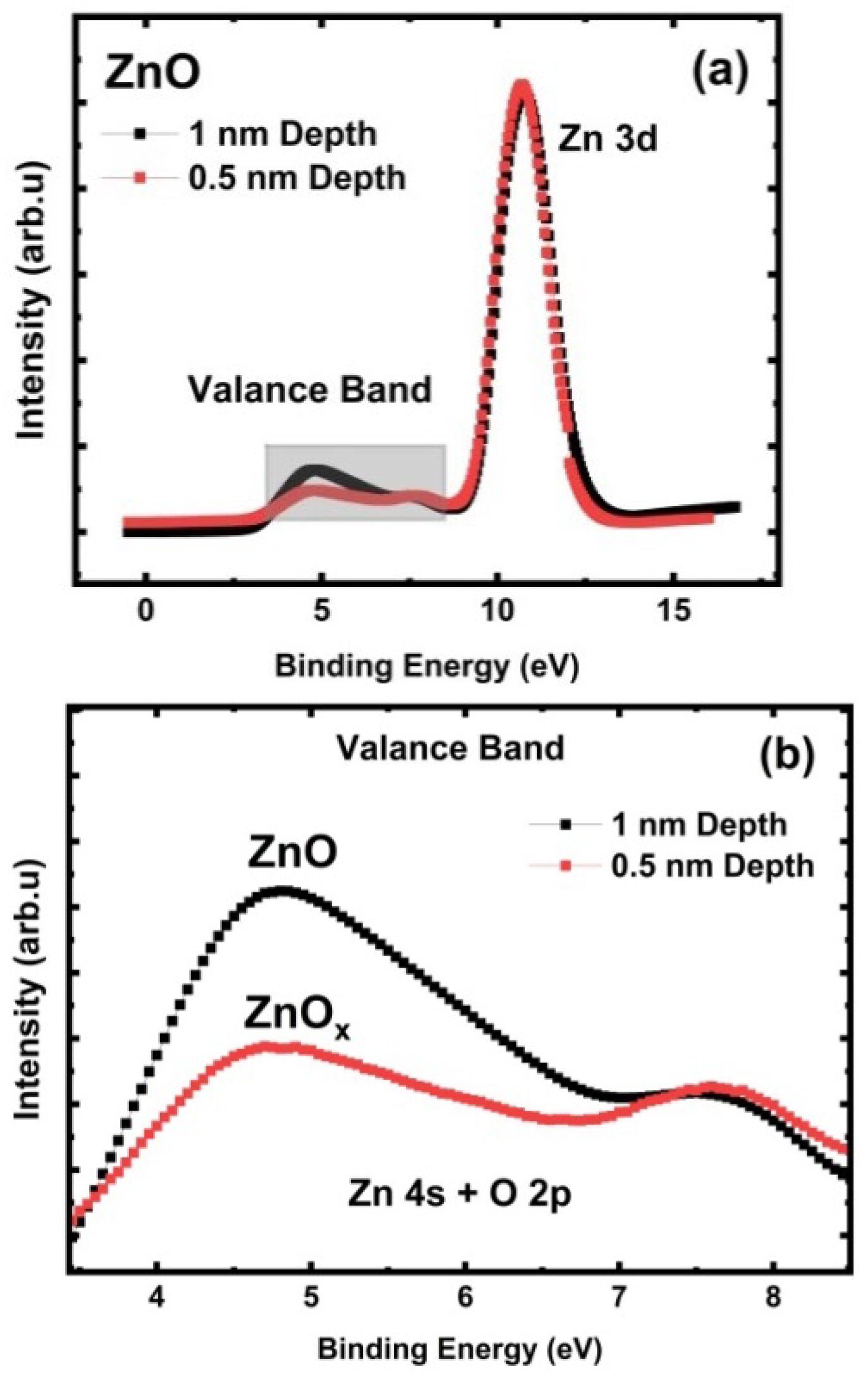
3.4. ZnO Valence Band Investigation
3.5. ZnO Auger Peak (AES) Analysis for Stoichiometric Understanding
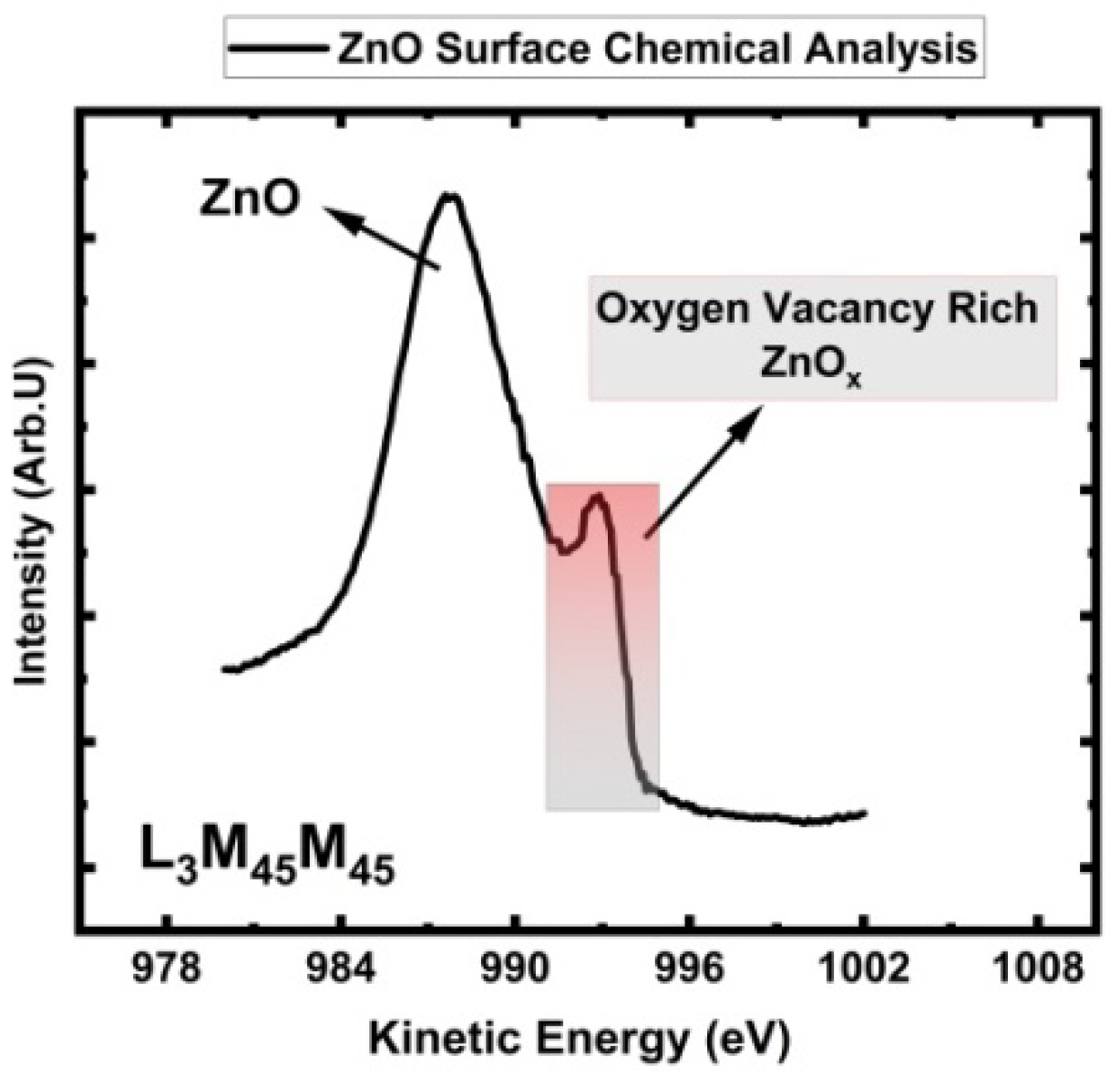
| Electron Level | Zn 3s in ZnO |
Zn 3p1/2 in ZnO | Zn 3p3/2 in ZnO | Zn 3d in ZnO | O 1s in ZnO |
L3M4,5M4,5 in ZnO |
L3M4,5M4,5 in Zn |
| Ciftyurek and Schierbaum (this work) | 140.0 | 91.6 | 88.8 | 10.7 | 529.8 | 987.9 | 992.3 |
| Vesely and Langer [66] | 139.8 | 92.0 | 89.0 | 10.5 | 530.9 | 988.9 | - |
| Gaarenstroom and Winograd [67] | - | - | - | 10.7 | - | 987.7 | - |
| Kowalczyk [68] | - | - | - | - | - | - | 991.9 |
| Wagner [69] | - | - | - | - | - | - | 992.0 |
| Schoen [65] | 139.6 | 91.8 | 88.7 | 10.3 | 530.3 | 988.5 | 992.5 |
| Barr and Hackenberg [70] | - | - | - | 10.3 | 530.3 | 987.9 | 992.0 |
| Klein and Hercules [71] | - | - | - | 10.4 | - | 988.2 | 992.3 |
| Strohmeier and Hercules [72] | 139.2 | - | 88.3 | - | 529.9 | 988.9 | 992.4 |
| Powell [73] | - | - | - | - | - | - | 992.4 |
| Ley and Kowalczyk [74] | - | - | - | 10.4 | - | - | 991.9 |
| Wehner and Mercer [75] | - | - | - | - | - | 988.1 | 992.1 |
| Dake and Baer [76] | - | - | - | - | - | 988.1 | 992.2 |
3.6. Concentration of Oxygen Vacancy ( in Engineered Porosity ZnO
3.6.1. Zn 3p Analysis
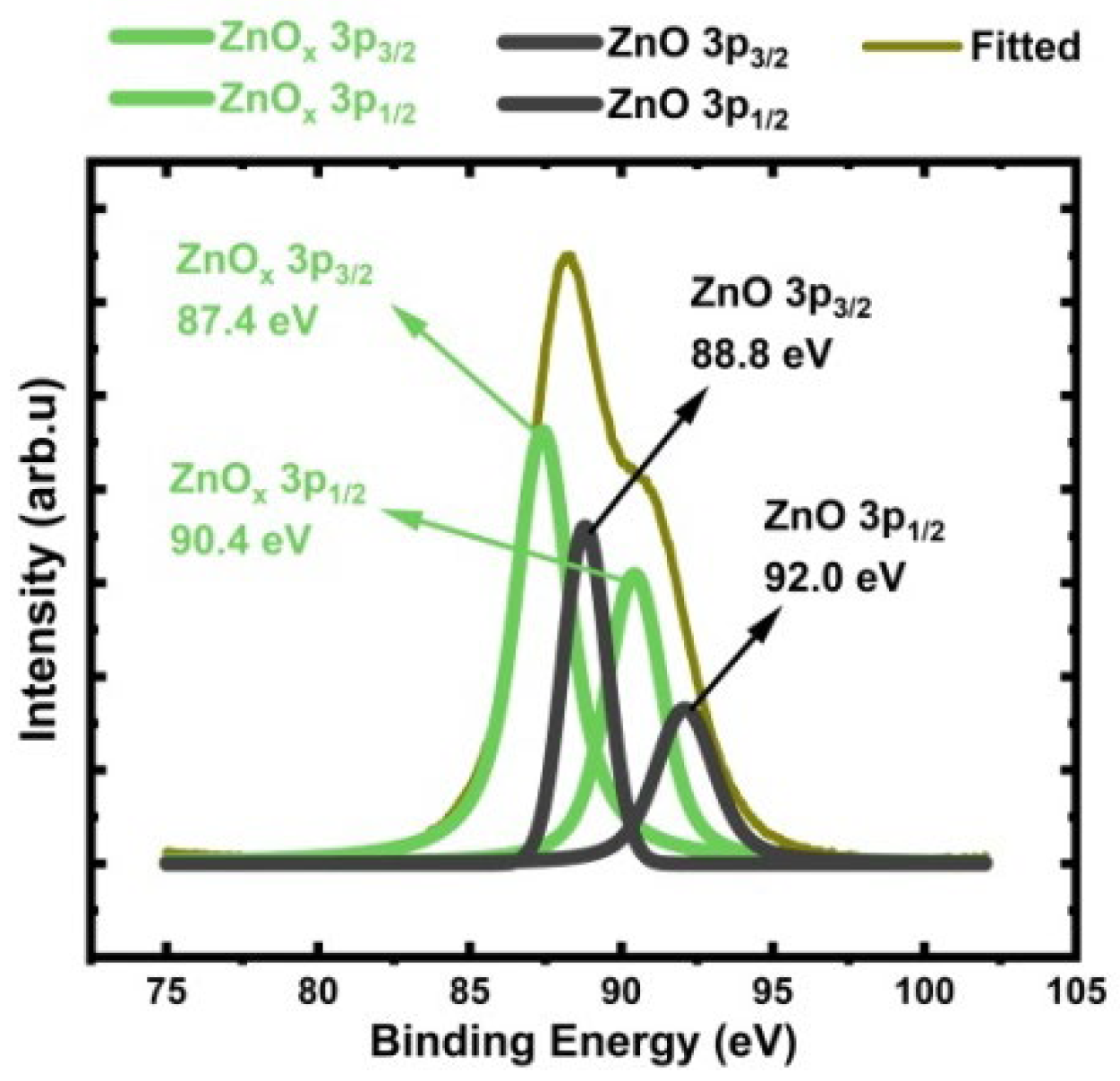
3.6.2. Oxygen 1s Analysis
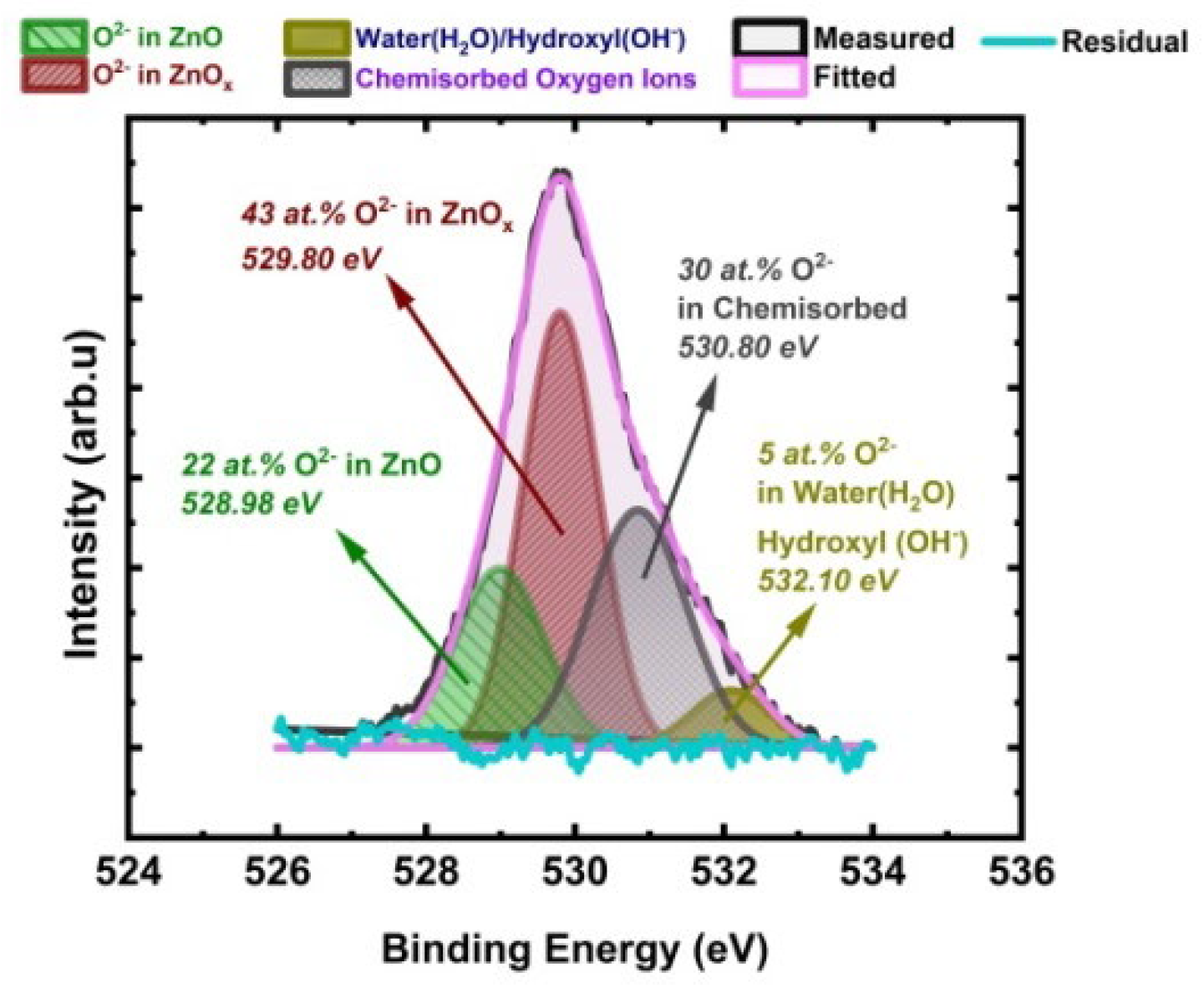
3.6.3. Zinc (Zn) to Oxygen (O) Stoichiometric Quantification (ZnO)
3.7. Gas Sensor Testing for H2S and NO2
3.7.1. An overview of the sensing mechanism and the involvement of oxygen ions in metal oxide semiconductor (MOS) sensors’ gas sensing reactions
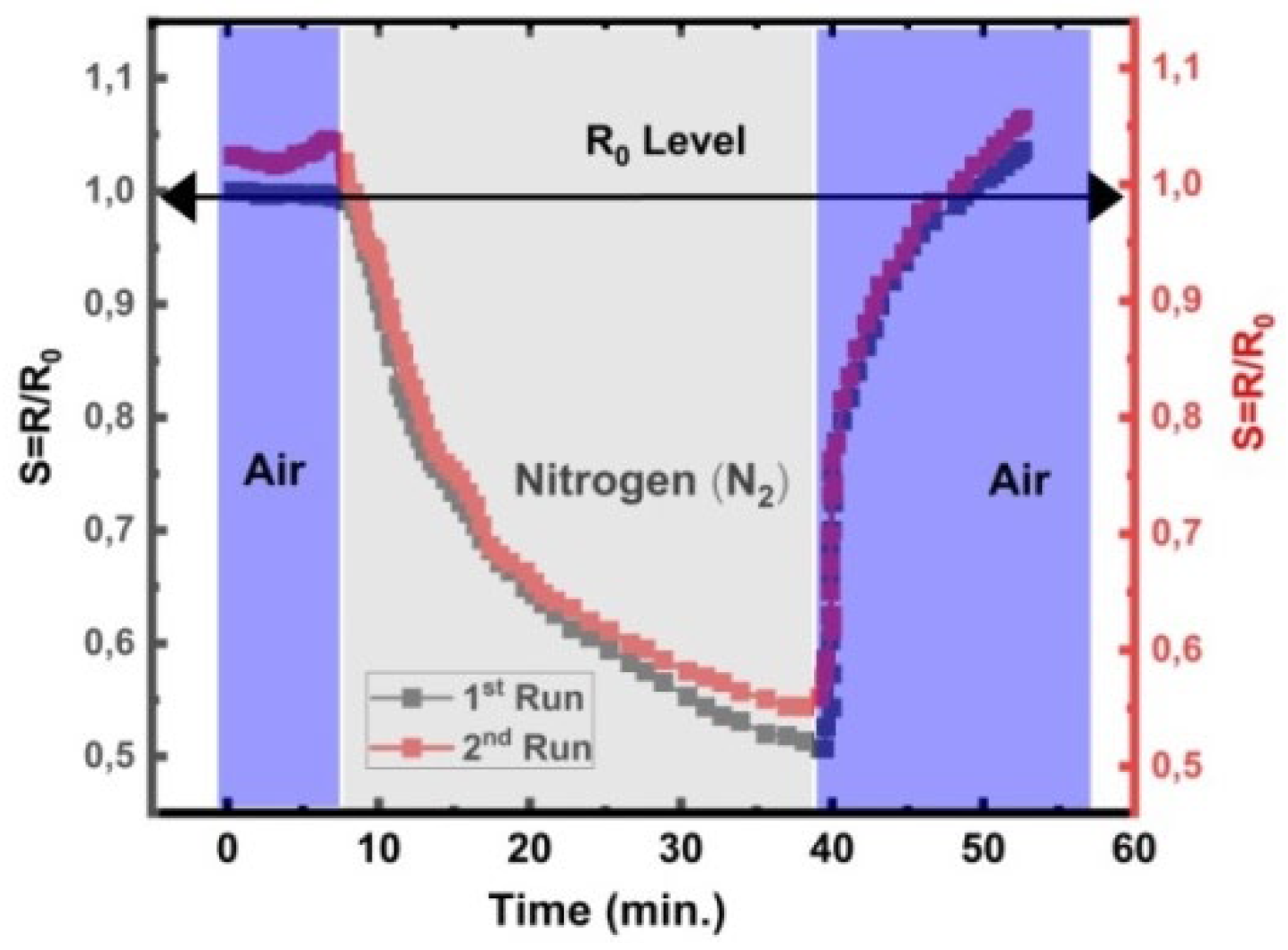
3.7.2. Initial Tests on Adsorption Kinetics with Oxygen (O2) and Nitrogen (N2)
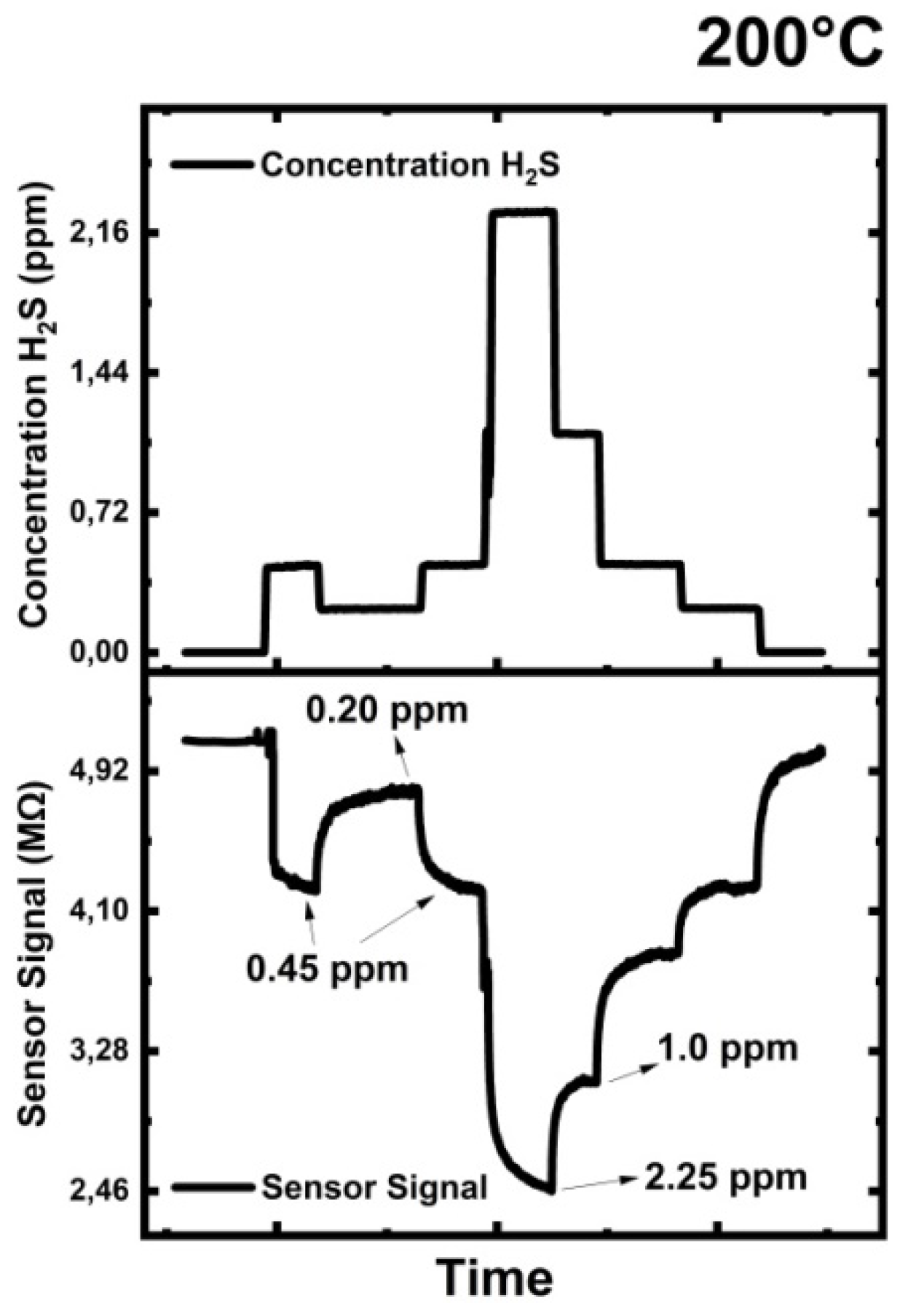
3.7.3. Hydrogen Sulfide (H2S) Testing
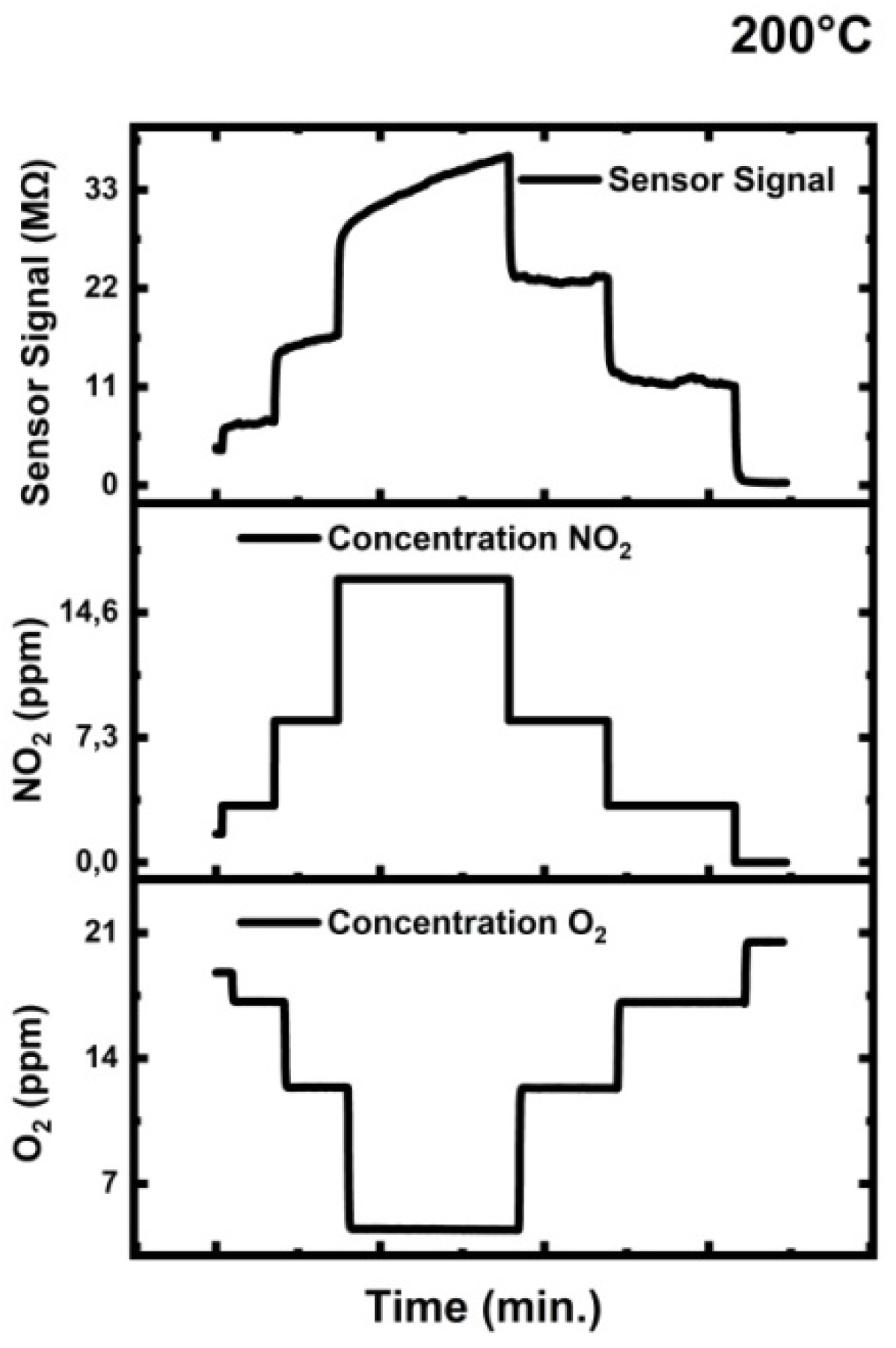
3.7.4. Nitrogen Oxide (NO2) Testing
| Sensing Material Composition and Physical State & Form |
Sensor Testing Temperature (°C) | Test Gas Concentration | Response Magnitude (Rair/Rgas) for H2S (Rgas/Rair) for NO2 |
|---|---|---|---|
| Engineered Porosity ZnO (this work) | 200 | 2.25 ppm H2S | 2.2 (This Work) |
| CuO/SnO2-ZnO core shell NWs [89] | 25 | 10 ppm H2S | 1.6 |
| ZnO/SnO2 nano-fibers [90] | 250 | 50 ppm H2S | 63.3 |
| ZnO Nano-rods [91] | 250 | 10 ppm H2S | 20 |
| Cu–ZnO nanograins [92] | 250 | 15 ppm H2S | 0.9 |
| Au modified ZnO nanowires [93] | 25 | 5 ppm H2S | 6.1 |
| Pd–SnO2–ZnO [94] | 25 | 20 ppm H2S | 0.06 |
| ZnO Nanowires [95] | 25 | 1 ppm H2S | 1.5 |
| Engineered Porosity ZnO (this work) | 200 | 15 ppm NO2 | 15 (This Work) |
| ZnO thin film [96] | 250 | 1 ppm NO2 | 2.4 |
| ZnO/SnO2-rGO nano-composite [97] | 30 | 5 ppm NO2 | 1.4 |
| UV Irradiated Au doped ZnO [40] | 25 | 1 ppm NO2 | 2 |
| Co-doped ZnO nano-capsules [98] | 280 | 100 ppm NO2 | 3.86 |
| Ni doped ZnO [99] | 200 | 100 ppm NO2 | 2 |
| Au decorated ZnO-PANI [100] | 300 | 50 ppm NO2 | 14 |
4. Conclusions
Author Contributions
Funding
Acknowledgments
References
- H. Ritchie ve P. Rosado , «Fossil fuels,» Our World in Data, 2024.
- U. E. I. Administration, " U.S. Energy Information Administration, Monthly Energy Review, Energy Overview, Table 1.2," April 2024.
- U. E. I. Administration, «Monthly Energy Review, Energy consumption by sector, Tables 2.1.a and 2.1.b,» April 2024.
- T. A. G. Institute, «What are the major sources and users of energy in the United States?,» 2017.
- S. K. Pandey, K. H. Kim and K. T. Tang, "A review of sensor-based methods for monitoring hydrogen sulfide," Trends in Analytical Chemistry, vol. 32, pp. 87-99, 2012.
- X. Zheng, P. Orellano, H. Lin, M. Jiang and W. Gaun, "Short-term exposure to ozone, nitrogen dioxide, and sulphur dioxide and emergency department visits and hospital admissions due to asthma: A systematic review and meta-analysis," Environment International, vol. 150, no. pp. 106435, 2021. [CrossRef]
- P. Orellano, N. Quaranta, J. Reynoso, B. Balbi and J. Vasquez, "Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis," PLoS ONE, vol. 12(3). 2017. [CrossRef]
- «Environmental Health Criteria 19,» IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY , Geneva, World Health Organization 1981, Hydrogen Sulfide.
- C.H Foulkes, “GAS!” The Story of the Special Brigade, N&M Press, 2009.
- "EPA (2024). Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2022 U.S. Environmental Protection Agency, EPA 430R-24004.".
- "U.S. Energy Information Administration, Monthly Energy Review, Energy consumption by sector and Environment, April 2024, preliminary data for 2023.".
- "U.S. Environmental Protection Agency, Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2022, April 2024. Includes U.S., Territories.".
- "U.S. Department of Labor," Occupational Safety and Health Administration, [Online]. Available: https://www.osha.gov/annotated-pels/table-z-1. [Accessed 09 10 2024].
- "Integrated Pollution Prevention and Control (IPPC) Reference Document on Best Available Techniques for Mineral Oil and Gas Refineries," EUROPEAN COMMISSION, 2003.
- Håvard Devold, "Oil and gas production handbook An introduction to oil and gas production, transport, refining and petrochemical industry," ISBN 978-82-997886-3-2, Oslo, 2013.
- "Nitrogen Oxides (NOx), Why and How They Are Controlled," EPA 456/F-99-006R, Clean Air Technology Center (MD-12) Information Transfer and Program Integration Division, 1999.
- E. Ciftyürek, K. Sabolsky and E. M. Sabolsky, "High temperature selective sensing of hydrogen with MgO-modified SrMoO4 micro-fibers," Sensors and Actuators B: Chemical, vol. 249, no. pp. 296-310, 2017. [CrossRef]
- E. Ciftyurek, "Nano-derived sensors for high-temperature sensing of H2, SO2 and H2S," Graduate Theses, Dissertations, and Problem Reports. 7305, West Virginia University (WVU), Morgantown, West Virginia (WV), USA., 2014.
- F. . J. Corpas, "NO and H2S Contribute to Crop Resilience against Atmospheric Stressors," Int. J. Mol. Sci. , vol. 25(6), no. p. 3509, 2024. [CrossRef]
- M. Filonchyk and M. P. Peterson, "NO2 emissions from oil refineries in the Mississippi Delta," Science of The Total Environment, vol. 898, no. p. 165569, 2023. [CrossRef]
- M. Monteleone , G. Di Luca, M. Filomia , A. Fuoco, A. Figoli and J. C. Jansen, "Odours in Asphalt: Analysis of the Release of H2S from Bitumen by a Mass Spectrometric Residual Gas Analyser," Methods Protoc., vol. 7(4), no. p. 55, 2024. [CrossRef]
- B. Saruhan, A. A. Haidry, A. Yüce, E. Ciftyürek and G. C. M. Rodríguez, "A Double Layer Sensing Electrode “BaTi(1-X)RhxO3/Al-Doped TiO2” for NO2 Detection above 600 °C," Chemosensors, vol. 4 (2), no. 8, 2016/4/29.
- Selmar de Souza, Steven J Visco and Lutgard C De Jonghe, "Thin-film solid oxide fuel cell with high performance at low-temperature," Solid State Ionics, vol. 98, no. pp. 57-61, 1997. [CrossRef]
- T. Ross, J. W. Zondlo, E. Sabolsky, E. Ciftyurek, A. Koneru, T. Thomas, I. Celik, I. Liu, H. Sezer and U. Damo, "Performance and stability of large planar solid oxide fuel cells using phosphine contaminated hydrogen fuel," Journal of Power Sources, vol. 395, no. pp. 185-194, 2018. [CrossRef]
- X. Chunchuan , P. Gansor, J. Zondlo, K. Sabolsky ve E. Sabolsky, «An H2S-tolerant Ni-GDC anode with a GDC barrier layer,» Journal of The Electrochemical Society, cilt 158(11), no. p. B1405, 2011. [CrossRef]
- G. Fang, Z. Liu, C. Liu and K. Yao, "Room Temperature H2S Sensing Properties and Mechanism of CeO2-SnO2 Sol-gel Thin Films," Sensors and Actuators B: Chemical, vol. 66, no. 1-3, pp. 46-48, 2000.
- S. C. Lee, S. Y. Kim, B. W. Hwang, S. Y. Jung, D. Ragupathy, I. S. Son, D. D. Lee and J. C. Kim, "Improvement of H2S Sensing Properties of SnO2-Based Thick Film Gas Sensors Promoted with MoO3 and NiO," Sensors, vol. 13, pp. 3889-3901, 2013.
- G. Eranna, B. C. Joshi, D. P. Runthala and R. P. Gupta, "Oxide Materials for Development of Integrated Gas Sensors-A Comprehensive Review," Critical Reviews in Solid State and Materials Sciences, vol. 29, no. 3-4, pp. 111-188, 2010.
- Z. Sun, H. Yuan, Z. Liu, B. Han and X. Zhang, "A Highly Efficient Chemical Sensor Material for H2S: Fe2O3 Nanotubes Fabricated Using Carbon Nanotube Templates," Advanced Materials, vol. 17, pp. 2993-2997, 2005.
- C. Liewhiran, N. Tamaekong, A. Wisitsora and S. Phanichphant, "The Monitoring of H2S and SO2 Noxious Gases from Industrial Environment with Sensors Based on Flame-spray-made SnO2 Nanoparticles," Engineering Journal, vol. 16, no. 3, pp. 123-134, 2012.
- C. V. Gopal, S. V. Reddy, S. V. Manorama and V. J. Rao, "Preparation and Characterization of Ferrites as Gas Sensor Materials," Journal of Material Science Letter, vol. 19, no. 9, pp. 775-778, 200.
- G. N. Chaudhari, M. A. Alvi, H. G. Wankhadea, A. B. Bodade and S. V. Manorama, "Nanocrystalline chemically modified CdIn2O4 thick films for H2S gas sensor," Thin Solid Films, vol. 520, no. 11, pp. 4057–4062, 2012.
- S. V. Jagtab, V. Kadu A, V. S. Sangawar, s. V. Manorama and G. N. Chaudhari, "H2S sensing characteristics of La0.7Pb0.3Fe0.4Ni0.6O3 based nanocrystalline thick film gas sensor," Sensors and Actuators B: Chemical, vol. 131, no. 1, pp. 290-294, 2008.
- U. Kersen and L. Holappa, "Surface Characterization and H2S sensing potential of iron molybdate particles produced by supercritical solvothermal method and subsequent oxidation," Appl. Phys. A, vol. 85, pp. 431-436, 2006.
- G. N. Chaudhari, D. R. Bambole, A. B. Bodade and P. R. Padole, "Characterization of nanosized TiO2 H2S gas sensor," J. Mater. Sci., vol. 41, pp. 4860-4864, 2006.
- M. Hübner, D. Koziej, M. Bauer, N. Barsan, K. Kvashnina, M. D. Rossell, U. Weimar and J.-D. Grunwaldt, "The Structure and Behavior of Platinum in SnO2-Based Sensors under Working Conditions," Angewandte Chemie International Edition, vol. 50 (12), no. pp.2841-2844, 2011. [CrossRef]
- E. Ciftyurek, Z. Li and K. Schierbaum, "Adsorbed oxygen ions and oxygen vacancies: Their concentration and distribution in metal oxide chemical sensors and influencing role in sensitivity and sensing mechanisms," Sensors , vol. 23(1). p. 29, 2022. [CrossRef]
- E. Ciftyürek, S. Bretislav , Z. Li, V. Matolin and K. Schierbaum, "Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions," Sensors, vol. 19(21). p. 4737, 2019. [CrossRef]
- M. D. Shirsat, M. A. Bangar, M. A. Deshusses, N. V. Myung ve A. Mulchandani, «Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor,» Appl. Phys. Lett., cilt 94 (8). pp. 083502 , 2009. [CrossRef]
- Z. Cai, K.-K. Kim and S. Park, "Room temperature detection of NO2 gas under UV irradiation based on Au nanoparticle-decorated porous ZnO nanowires," Journal of Materials Research and Technology, vol. 9 (6). pp. 16289-16302, 2020. [CrossRef]
- P. Cao, Y. Cai , D. Pawar , N. S.T. , C. Rao, S. Han, W. Xu, M. Fang, X. Liu, W. Liu, D. Zhu and Y. Lu, "Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors," Chemical Engineering Journal, vol. 401, no. p. 125491, 2020. [CrossRef]
- V. Ganbavle, S. Inamdar, G. Agawane, J. Kim and K. Rajpure, "Synthesis of fast response, highly sensitive and selective Ni:ZnO based NO2 sensor," Chemical Engineering Journal, vol. 286. pp. 36-47, 2016. [CrossRef]
- M. Bonyani , S. M. Zebarjad, K. Janghorban, J.-Y. Kim, H. W. Kim ve S. S. Kim, «Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas,» Chemosensors, cilt 10(10). p. 388, 2022. [CrossRef]
- L. Mai, F. Mitschker, C. Bock, A. Niesen, E. Ciftyurek, D. Rogalla, J. Mickler, M. Erig, Z. Li, P. Awakowicz, K. Schierbaum and A. Devi, "From Precursor Chemistry to Gas Sensors: Plasma-Enhanced Atomic Layer Deposition Process Engineering for Zinc Oxide Layers from a Nonpyrophoric Zinc Precursor for Gas Barrier and Sensor Applications," Small, vol. 16, no. 2020. [CrossRef]
- D. C. Look, "Zinc Oxide Bulk, Thin Films and Nanostructures;Processing, Properties and Applications," in Chapter 2 :Doping and Defects in ZnO.
- E. Ciftyürek, K. Sabolsky and E. M. Sabolsky, "Molybdenum and tungsten oxide based gas sensors for high temperature detection of environmentally hazardous sulfur species," Sensors and Actuators B: Chemical, vol. 237, no. pp. 262-274, 2016. [CrossRef]
- E. M. Sabolsky, E. Çiftyürek, C. Wildfire, K. Sabolsky, J. Taub, K. Sierros and T. H. Evans, "Nano-Derived Microsensors for Monitoring Gas Species in Harsh-Environments," ECS Transactions , vol. 61 (2), no. p. 375, 2014. [CrossRef]
- E. M. Sabolsky, C. Wildfire, E. Ciftyurek and K. Sabolsky, "Nano-Derived, Micro-Chemical Sensors for High-Temperature Applications," ECS Transactions, vol. 45 (3), no. pp. 495-506, 2012. [CrossRef]
- H. Kim, N. D. Theodore and T. L. Alford, "Comparison of texture evolution in Ag and Ag(Al) alloy thin films on amorphous," Journal of Applied Physics, vol. 95, no. p. 5180–5188, 2004. [CrossRef]
- G. L. Selman, J. G. Day and A. A. Bourne, "Dispersion Strengthened Platinum," Platinum Metals Review, vol. 18 (2), pp. 46-57, 1974.
- D.J. Frankel, G.P. Bernhardt, B.T. Sturtevant, T. Moonlight, M. Pereira da Cunha and R.J. Lad, "Stable electrodes and ultrathin passivation coatings for high temperature sensors in harsh environments," SENSORS, 2008 IEEE, no. 2008. [CrossRef]
- K. Sieradzki, K. Bailey and T. L. Alford, "Agglomeration and percolation conductivity," Appl. Phys. Lett., vol. 79, no. p. 3401–3403, 2001. [CrossRef]
- K. F McCarty, J. C Hamilton, Y. Sato, A. Saá, R. Stumpf, J. de la Figuera, K. Thürmer, F. Jones, K. A. Schmid, A. A. Talin and C. N. Bartelt, "How metal films de-wet substrates-identifying the kinetic pathways and energetic driving forces," New Journal of Physics, vol. 11(4), no. pp. 043001, 2009. [CrossRef]
- K. Sreenivas, I. Reaney, T. Maeder, N. Setter, C. Jagadish and R. G. Elliman, "Investigation of Pt/Ti bilayer metallization on silicon for ferroelectric thin film integration," J. Appl. Phys., vol. 75, no. pp. 232–239, 1994. [CrossRef]
- D. Srolovitz and M. Goldiner, "The thermodynamics and kinetics of film agglomeration," JOM, vol. 47 (3). pp. pp.31-36, 1995. [CrossRef]
- E. Çiftyürek, K. Sabolsky and E. M. Sabolsky, "Platinum thin film electrodes for high-temperature chemical sensor applications," Sensors & Actuators: B. Chemical, vol. 181. pp. 702-714, 2013. [CrossRef]
- E. Ciftyurek, C. D. McMillen, K. Sabolsky and E. M. Sabolsky, "Platinum–zirconium composite thin film electrodes for high-temperature micro-chemical sensor applications," Sensors and Actuators B: Chemical, vol. 207, no. pp. 206-215, 2015. [CrossRef]
- C. Jagadish and S. Pearton, Zinc Oxide Bulk, Thin Films and Nanostructures Processing, Properties and Applications, Elsevier Science, 2006.
- R. C. Brundle, C. A. Evans and S. Wilson, Encyclopedia of Materials Characterization Surfaces, Interfaces, Thin Films, pp-291: Butterworth-Heinemann, 1992.
- D. L. Raimondi and E. Kay, "High Resistivity Transparent ZnO Thin Films," J. Vac. Sci. Technol., vol. 7, no. pp. 96-99, 1970. [CrossRef]
- T. J. Coutts, X. Li, T. M. Barnes, B. M. Keyes, C. L. Perkins, S. E. Asher, S. B. Zhang and S.-H. Wei, "Chapter 3 - Synthesis and Characterization of Nitrogen-Doped ZnO Films Grown by MOCVD," in Zinc Oxide Bulk, Thin Films and Nanostructures Processing, Properties and Applications, 2006, pp. 43-83.
- F. Moulder, W. Stickle, P. Sobol and K. Bomben, Handbook of X-ray Photoelectron Spectroscopy, A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data: Physical Electronics Division, Perkin-Elmer Corporation, 1992.
- Y.K. Gao, F. Traeger, O. Shekhah, H. Idriss and C. Wöll, "Probing the interaction of the amino acid alanine with the surface of ZnO (1010)," Journal of Colloid and Interface Science, vol. 338, no. pp. 16-21, 2009. [CrossRef]
- C. Wöll, "The chemistry and physics of zinc oxide surfaces," Progress in Surface Science, vol. 82, no. pp. 55-120, 2007. [CrossRef]
- S. Gunnar, "Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide," Journal of Electron Spectroscopy and Related Phenomena, vol. 2, no. pp. 75-86, 1973. [CrossRef]
- D. W. Langer and C. J. Vesely, "Electronic Core Levels of Zinc Chalcogenides," Phys. Rev. B, vol. 2, no. p. 4885, 1970. [CrossRef]
- S. W. Gaarenstroom and N. Winograd, "Initial and final state effects in the ESCA spectra of cadmium and silver oxides," J. Chem. Phys., vol. 67, p. 3500–3506, 1977.
- S. P. Kowalczyk, R. A. Pollak, F. R. McFeely, L. Ley and D. A. Shirley, "L2,3M45M45 Auger Spectra of Metallic Copper and Zinc: Theory and Experiment," Phys. Rev. B, vol. 8, no. p. 2387, 1973. [CrossRef]
- C. D. Wagner, "Chemical shifts of Auger lines, and the Auger parameter," Faraday Discussions of the Chemical Society, vol. 60, 1975.
- J. J. H. Tery L. Barr, "Studies of the low temperature oxidation of alloys by X-ray photoelectron spectroscopy: CuZn," Applications of Surface Science, vol. 10, pp. 523-545, 1982.
- D. M. H. Joseph C. Klein, "Surface characterization of model Urushibara catalysts," Journal of Catalysis, vol. 82, pp. 424-441, 1983.
- Brian R. Strohmeier and David M. Hercules, "Surface spectroscopic characterization of the interaction between zinc ions and γ-alumina," Journal of Catalysis, vol. 86, no. pp. 266-279, 1984. [CrossRef]
- C. Powell, "Recommended Auger-electron kinetic energies for 42 elemental solids," Journal of Electron Spectroscopy and Related Phenomena, Vols. 182 (1-2), no. pp. 11-18, 2010. [CrossRef]
- S. P. Kowalczyk, L. Ley, F. R. McFeely, R. A. Pollak and D. A. Shirley, "Relative effect of extra-atomic relaxation on Auger and binding-energy shifts in transition metals and salts," Phys. Rev. B, vol. 381, no. 9, p. 1974. [CrossRef]
- P.S. Wehner, P.N. Mercer and G. Apai, "Interaction of H2 and CO with Rh4(CO)12 supported on ZnO," Journal of Catalysis, vol. 84/1, no. pp. 244-247, 1983. [CrossRef]
- L. S. Dake, D. R. Baer and J. M. Zachara, "Auger parameter measurements of zinc compounds relevant to zinc transport in the environment," Surface and Interface Analysis, Vols. 14/1-2, no. pp. 71-75, 1989. [CrossRef]
- M.W. Wang, J.F. Swenberg, R.J. Miles, M.C. Phillips, E.T. Yua, J.O. McCaldin, R.W. Grant and T.C. McGill, "Measurement of the MgSe/Cd0.54Zn0.46Se valence band offset by X-ray photoelectron spectroscopy," Journal of Crystal Growth, vol. 138, no. pp. 508-512, 1994. [CrossRef]
- Hsin-Yen Lee, Bin-Kun Wu and Ming-Yau Chern, "Study on the Formation of Zinc Peroxide on Zinc Oxide with Hydrogen Peroxide Treatment Using X-ray Photoelectron Spectroscopy (XPS)," Electron. Mater. Lett., vol. 10, no. 1, pp. 51-55, 2014.
- O. Hirsch, K. O. Kvashnina, L. Luo, M. Sueess, P. Glatzel and D. Koziej, "High-energy resolution X-ray absorption and emission spectroscopy reveals insight into unique selectivity of La-based nanoparticles for CO2.," Proceedings of the National Academy of Sciences of the United States of America, vol. 112, no. pp. 15803-15808 , 2015. [CrossRef]
- M. J. Madou and S. R. Morrison, Chemical sensing with Solid State Devices, San Diego: Academic Press, INC, 1989.
- A. M. Azad, S. A. Akbar, S. G. Mhaisalkar, L. D. Birkefeld and K. S. Goto, "Solid State Gas Sensors A review," J. Electrochem. Soc., vol. 139, no. 12, pp. 3690-3701, 1992.
- H. Windischmann and P. Mark, "A model for the Operation of a Thin film SnOx Conductance Modulation Carbon Monoxide Sensor," J. Electrochem Soc., vol. 126, pp. 627-633, 1979.
- E. B. Varhegyi, J. Gerblinger, F. Reti, I. V. Perczel and H. Meixner, "Study of the behaviour of CeO2 in SO2 containing atmosphere," Sensors and Actuators B, Vols. 24-25 (1-3), no. pp. 631-635, 1995. [CrossRef]
- Christina Wildfire, Engin Çiftyürek, Katarzyna Sabolsky and Edward M Sabolsky, "Investigation of doped-gadolinium zirconate nanomaterials for high-temperature hydrogen sensor applications," Journal of Materials Science, vol. 49, no. pp. 4735-4750, 2014. [CrossRef]
- C. Wildfire, E. Çiftyürek, K. Sabolsky and E. Sabolsky, "Fabrication and testing of high-temperature nano-derived resistive-type microsensors for hydrogen sensing," Journal of The Electrochemical Society, vol. 161 (2), no. pp. B3094-B3102, 2013. [CrossRef]
- L. D. Birkefeld, A. M. Azad and S. A. Akbar, "Carbon Monoxide and Hydrogen Detection by Anatase Modification of Titanium Dioxide," J. Am. Ceram. Soc., vol. 75 (11), no. p. 2964, 1992. [CrossRef]
- P. Mark, "Photo-induced chemisorption on insulating CdS crystals," J. Chem. Phys. Solids., vol. 25, no. pp. 911-920, 1964. [CrossRef]
- A. M. Azad, S. G. Mhaisalkar, L. D. Birkefeld, S. A. Akbar and K. S. Goto, "Behaviour of a New ZrO2-MoO3 Sensor for Carbon Monoxide Detection," J. Electrochern. Soc, vol. 139, no. 10, pp. 2913-2920, 1992.
- Jae-Hun Kim, Ali Mirzaei , Jae Hoon Bang, Hyoun Woo Kim and Hyoun Woo Kim, "Selective H2S sensing without external heat by a synergy effect in self-heated CuO-functionalized SnO2-ZnO core-shell nanowires," Sensors and Actuators B: Chemical, vol. 300, no. p. 126981, 2019. [CrossRef]
- Zhaorui Lu, Qu Zhou, Caisheng Wang, Zhijie Wei, Lingna Xu and Yingang Gui, "Electrospun ZnO-SnO2 Composite Nanofibers and Enhanced Sensing Properties to SF6 Decomposition Byproduct H2S," Front Chem., vol. 6, no. p. 540, 2018. [CrossRef]
- Umar, Ahmad, Ibrahim, Ahmed A., Alhamami, Mohsen A., Algadi, Hassan, Ahmed, Faheem, Hussain, S., Fouad, Hassan and Akbar, Sheikh , "ZnO nanorods assembled microflower-based gas sensor for detecting formaldehyde," Materials Express, vol. 12, no. pp. 1481-1487(7), 2022. [CrossRef]
- K. Girija, K. Somasundaram, A. Topkar and Vatsa, "Highly selective H2S gas sensor based on Cu-doped ZnO nanocrystalline films deposited by RF magnetron sputtering of powder target.," J. Alloys Compd., vol. 684, no. pp. 15-20, 2016. [CrossRef]
- Niranjan S. Ramgir, Preetam K. Sharma, N. Datta, M. Kaur, A.K. Debnath, D.K. Aswal and S.K. Gupta, "Room temperature H2S sensor based on Au modified ZnO nanowires," Sensors and Actuators B: Chemical, vol. 186, no. pp. 718-726, 2013. [CrossRef]
- Hyunsu Kim, Changhyun Jin, Sunghoon Park and Chongmu Lee, "Enhanced H2S gas sensing properties of multiple-networked Pd-doped SnO2-core/ZnO-shell nanorod sensors," Materials Research Bulletin, vol. 47, no. pp. 2708-2712, 2012. [CrossRef]
- Florian Huber, Sören Riegert, Manfred Madel and Klaus Thonke, "H2S sensing in the ppb regime with zinc oxide nanowires," Sensors and Actuators B: Chemical, vol. 239, no. pp. 358-363, 2017. [CrossRef]
- A. Catto, L. da Silva, M. Bernardi, S. Bernardini, K. Aguir, E. Longo and V. Masterlaro, "Local Structure and Surface Properties of CoxZn1–xO Thin Films for Ozone Gas Sensing," ACS Applied Materials & Interfaces, vol. 8 (39), no. p. 26066–26072, 2016. [CrossRef]
- Ziying Wang, Shang Gao, Teng Fei, Sen Liu and Tong Zhang, "Construction of ZnO/SnO2 Heterostructure on Reduced Graphene Oxide for Enhanced Nitrogen Dioxide Sensitive Performances at Room Temperature," ACS Sensors, vol. 4 (8), no. p. 2048–2057, 2019. [CrossRef]
- V. S. Kamble, Y. Navale, V. Patil, N. K. Desai, S. N. Vajekar and S. Salunkhe, "Studies on structural, spectral and morphological properties of co-precipitation derived Co-doped ZnO nanocapsules for NO2 sensing applications," Journal of Materials Science: Materials in Electronics, vol. 32(8), no. 2021. [CrossRef]
- V.V. Ganbavle, S.I. Inamdar, G.L. Agawane, J.H. Kim and K.Y. Rajpure, "Synthesis of fast response, highly sensitive and selective Ni:ZnO based NO2 sensor," Chemical Engineering Journal, vol. 286, no. pp. 36-47, 2016. [CrossRef]
- M. Bonyani, S. M. Zebarjad , K. Janghorban, J.-Y. Kim , H. W. Kim ve S. S. Kim, «Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas,» Chemosensors, cilt 10 (10), no. p. 388, 2022. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





