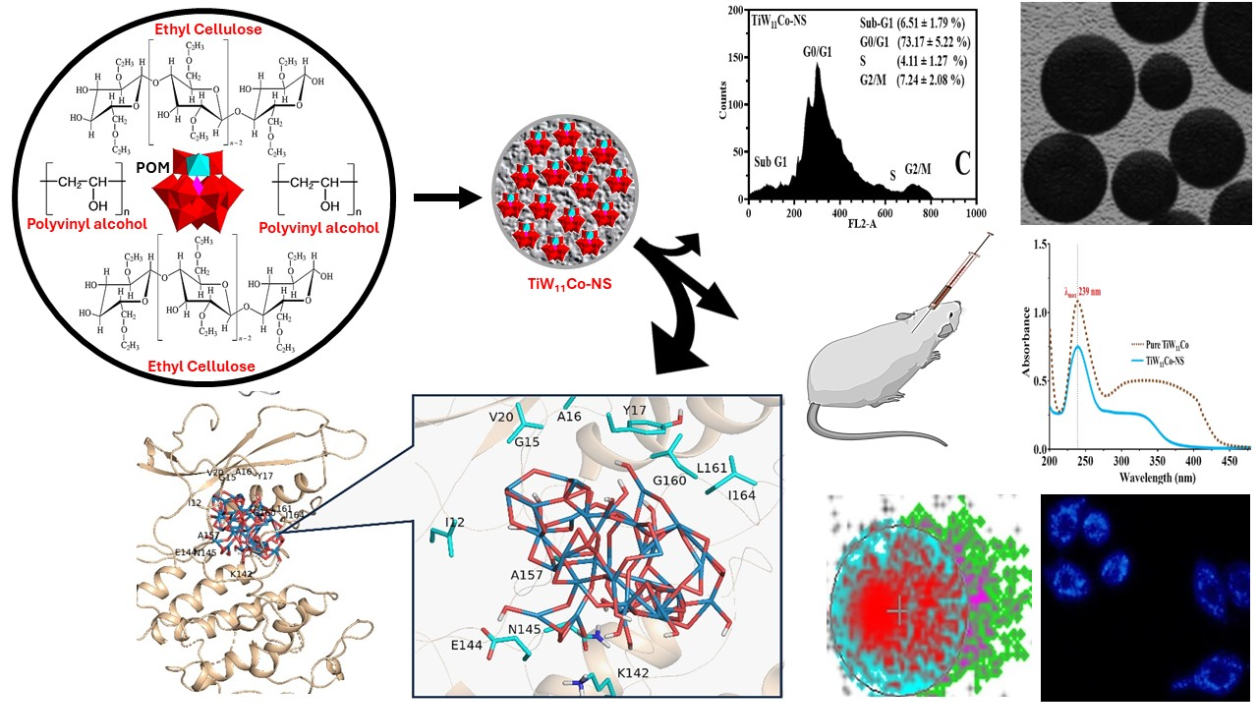
1. Introduction
Cancer is an anthology of problems classified by the proliferation of peculiar cells that undergo unregulated division, resultant in the formation of a unified neoplastic growth represented as a tumor [
1]. Normal cells may be disturbed by faults that arise during cell division, leading to genetic mutations. Moreover, exposure to dangerous environmental pollutants can also induce gene mutations [
2]. Metastasis pertains to the process by which a tumor has the capacity to distribute and subvert adjacent tissues and anatomical structures [
3]. Metastatic malignancies are distinguished by elevated mortality rate and present greater difficulties in terms of medical intervention [
4]. Cancer is considered one of the leading causes of death on a global scale. As per the World Health Organization (WHO), cancer accounted for approximately 20% of all documented fatalities in 2023 [
5]. The identification and management of cancer present a complex and demanding task given the diverse nature of cancer cells and the intricacies involved in their staging [
6].
Polyoxometalates (POMs) refer to clusters composed of inorganic transition metal oxides (namely vanadium, molybdenum, and tungsten) that possess a negative charge. The transition metals at their greatest oxidation state are connected by oxo-ligands (O
2-) inside a three-dimensional framework [
7]. The diverse nature of their molecular makeup and features allows for a wide range of applications in the treatment of metabolic illnesses, such as cancer and diabetes, as well as infectious diseases caused by bacteria, viruses, and parasites [
8,
9,
10]. POMs exhibit several limitations, such as their relatively larger dimensions in comparison to other nano-sized compounds used in cancer treatment [
11]. Additionally, their integration into cellular structures poses challenges, and their stability under physiological pH conditions is compromised [
12,
13].
There has been a tremendous increase in research dedicated to nanosponges (NS) and its potential use in personalized drug delivery, which has attracted considerable interest in the scientific community [
14,
15]. The precision of NS delivery systems in controlling the release rates of medications or guiding drugs to precise cellular sites is anticipated to have a substantial influence on the healthcare system [
16]. The nanosized delivery system e.g. NS, has distinct advantages for drug delivery purposes, owing to its remarkable stability, substantial carrier capacity, and the ability to accommodate both hydrophilic and hydrophobic compounds [
17]. The work in this specific field is spurred by the potential of NS for accurate and targeted delivery of pharmaceuticals [
15,
18]. The objective of this study was to analyze the formation, physical, pharmacological, and biological effects of NS containing TiW
11Co.
2. Results and Discussion
2.1. Characterization and Optimization of TiW11Co-NS: A Physical Perspective
A complete comparison of numerous important parameters that might influence the formulation of TiW
11Co-NS were shown in
Table 2. Variations in concentration were applied to each formulation, which were labelled as F1, F2, F3, and F4, to investigate the influence that these factors had on the features of NS.
The percentage of TiW11Co (% w/v) was consistently maintained at 1% w/v throughout all formulations, demonstrating a steady base concentration for the NS. A clear difference was observed among the formulations in the concentration of EC and PVA in terms of weight percent (w/v). Both EC and PVA were evaluated at two different concentrations: 50% and 100% w/v. All formulations were prepared at 10,000 rpm to ensure uniformity in mixing. Characterization results had provided clarity regarding the ways in which these factors affect NS characteristics.
When designing NS, there are two crucial issues that require careful study. Firstly, it is essential that NS possess sufficient size to impede renal excretion [
19]. Additionally, it is essential that these entities possess a sufficiently diminutive size to elude phagocytosis and subsequent elimination by the reticuloendothelial system (RES) [
20]. The available evidence indicated that biomolecules with molecular weights exceeding 40 kDa and NS ranging from 10 to 500 nm have ability to infiltrate the capillary network and amass inside the interstitial regions of tumors enables the use of passive targeted strategies [
21]. Furthermore, a research investigation had provided evidence indicating that the human immune system exhibits a higher level of efficacy in the removal of NS with a size exceeding 200 nm [
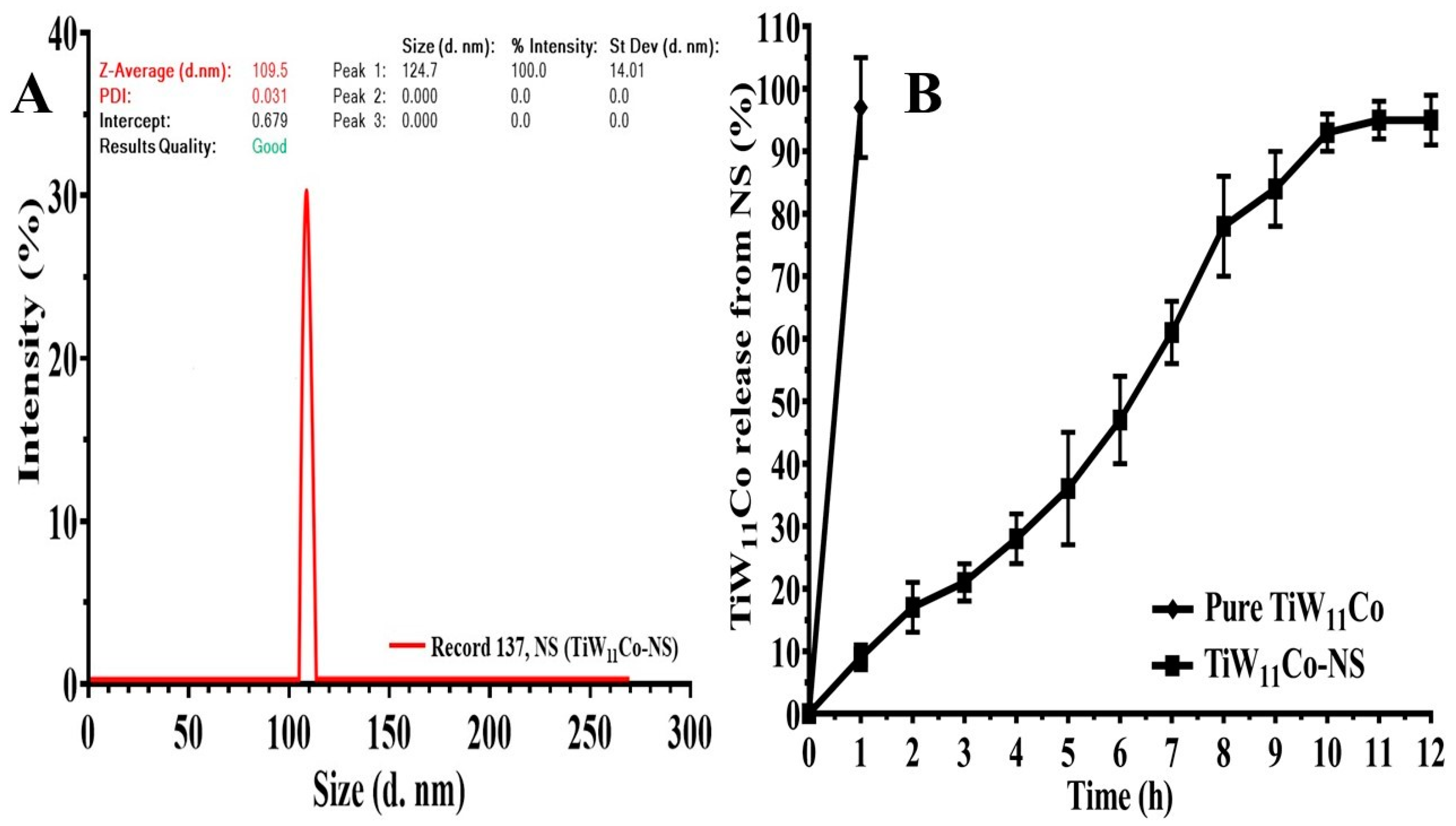
22]. The analysis of NS size diversity revealed notable disparities across the different formulations, with average NS sizes ranging from 109.5 nm to 471 nm. The results demonstrated changes in the concentrations of EC and PVA had an impact on NS size, with lower concentrations of EC resulting in smaller NS. The potential enhancement of NS stabilization in F2 via the increase in PVA concentration may lead to a reduction in NS diameters (
Figure 3 (A). This may be attributed to many processes, including greater surface covering, altered nucleation kinetics, and enhanced control of particle growth dynamics. Likewise, it was observed that formulations F3 and F4 exhibited larger NS sizes due to the greater percentage of EC (100% w/v) and the varying amount of PVA. The elevated viscosity and polymer concentration in F3 and F4 might facilitate the aggregation of NS during the formulation process, leading to an increase in NS sizes. Furthermore, the increased concentration of PVA in F4 (100%w/v) relative to F3 (50% w/v) has the potential to improve NS dispersion and reduce their aggregation propensity. Therefore, F4 revealed a somewhat reduced NS size in contrast to F3.
The adaptation in loading efficiency uncovered across formulations F1 to F4 might be accredited as disparities in the composition of the formulations and their related impacts on TiW11CoNS properties. The noticed improvement in loading efficiency from F1 to F2 and from F3 to F4 might be elucidated by the role of PVA as a stabilizing agent and its influence on the encapsulation process. The robust stabilizing impact of PVA might lead to more efficient encapsulation of TiW11Co within the NS when utilizing formulations with higher concentrations of PVA (F2 and F4). The elevated concentration of PVA facilitated the creation of additional binding cavities for the TiW11Co-NS, hence enhancing their encapsulation and reducing the likelihood of premature release during the formation process. Moreover, the elevated viscosity resulting from higher concentrations of EC and PVA (F3 and F4) might also enhance the loading efficiency by reducing the rate of diffusion of TiW11Co molecules during encapsulation. Consequently, this might facilitate enhanced entrapment within the NS matrix.
The increased polymer content resulting from higher EC concentrations (F3 and F4) led to enhanced entrapment efficiency (78.32 ± 6.18 and 85.9 ± 3.21) due to the formation of a denser and more cohesive matrix. Therefore, this facilitated the effective entrapment of a larger amount of TiW11Co. Formulations containing higher concentrations of PVA, specifically F2 and F4, exhibited enhanced entrapment efficiencies for TiW11Co. This was attributed to the improved accessibility of PVA molecules, which might promote the development of a more consistent and stable NS structure. Consequently, the entrapment efficiencies for F2 and F4 were higher, with values of 82.16 ± 5.91 and 85.9 ± 3.21, respectively.
Furthermore, variations in the uniformity of NS size distribution might be seen by the examination of polydispersity index (PDI) evaluations; lower PDIs suggest a more limited range of sizes. The observed NS PDI values, between 0.031 to 0.136, suggested that various formulas provide varying degrees of NS size homogeneity. This phenomenon might perhaps be attributed to variations in the quantities of polymers used. The decrease in PDI observed between F1 and F2 indicated that the formulation F2 has a more homogeneous size distribution of nanostructures compared to F1. The observed improvement in NS uniformity may be attributed to the higher concentration of PVA and lower quantity of EC. This might be expected to promote the longevity of the NS, resulting in a narrower size range and a decreased PDI. In terms of PVA and EC content, Formulation F4 exhibited the highest levels. The elevated concentration might lead to NS aggregation or an uneven dispersion of sizes of NS. This phenomenon might impede the formation of uniform NS and therefore resulting into an insufficient stabilization, hence leading to elevated PDI values.
Assessing the zeta potential is necessary for evaluating the charge, stabilization, and dispersion properties of TiW
11Co-NS. Higher zeta potential levels are indicative of increased stability inside the entire structure, whereas lower values suggest a propensity for colloids to agglomerate [
23,
24,
25]. Zeta potential values ranging from -24.91 mV to -27.08 mV were observed, indicative of surface charge characteristics. The results indicated that changes in the concentrations of EC and PVA had an impact on the surface potential of the TiW
11Co-NS, thereby influencing their stability and their interactions with biological systems. The PVA had an influence on the surface properties of NS, namely zeta potential, by its interaction with the NS surface and subsequent alteration of the charged group configuration. It is anticipated that formulations F2 and F4, characterized by a greater concentration of PVA, would exhibit an increased quantity of PVA molecules that were readily available for adhesion onto NS surface. This phenomenon would result in a more pronounced modification of the surface charge. Furthermore, the heightened concentration of PVA in formulation F2 has the potential to influence the aggregation properties and colloidal stability of the NS. The use of PVA molecules as steric stabilizers might be attributed to their ability to form a protective barrier surrounding NS, so impeding their aggregation via the mechanism of steric repulsion. The higher concentration of PVA in formulation F2 had provided enhanced steric stabilization, resulting in a more uniformly distributed and stable colloidal system. This might perhaps account for the observed differences in zeta potential between F2 and F1. The significance of spotting a little negative charge on the cell membrane lies in its ability to enhance electrostatic interactions involving the membrane surface as well as positively charged NS [
26]. Insights into the preferential accumulation of NS with a small positive charge at tumor sites after systemic injection have been derived from animal studies [
27]. The experiment's results highlighted the significant influence of different concentrations of EC and PVA on several parameters related to TiW
11Co-NS, as shown in
Table 2.
Table 2.
Physical characteristics of NS at different concentrations of EC and PVA, with a constant TiW11Co percentage of 1% w/v (mean ± SD, n = 3).
Table 2.
Physical characteristics of NS at different concentrations of EC and PVA, with a constant TiW11Co percentage of 1% w/v (mean ± SD, n = 3).
| |
F1 |
F2 |
F3 |
F4 |
| TiW11Co (% w/v) |
1 |
1 |
1 |
1 |
| EC (% w/v) |
50 |
50 |
100 |
100 |
| PVA (% w/v) |
50 |
100 |
50 |
100 |
| rpm |
10,000 |
10,000 |
10,000 |
10,000 |
| Particle size Mean± SD (nm) |
126 ± 4 |
109.5 ± 8 |
471 ± 6 |
324 ± 7 |
| Loading efficiency |
23.48 ± 7.19 |
34.25 ± 4.37 |
40.57 ± 5.64 |
42.74 ± 6.02 |
| Entrapment efficiency |
75.11 ± 6.61 |
82.16 ± 5.91 |
78.32 ± 6.18 |
85.9 ± 3.21 |
| PDI Mean ± SD |
0.095 ± 0.0027 |
0.031± 0.0094 |
0.104±0.083 |
0.136±0.098 |
| Zeta potential Mean ± SD (mV) |
-24.91 ± 6.21 |
-27.08 ± 3.79 |
-18.57± 4.05 |
-20.34± 2.18 |
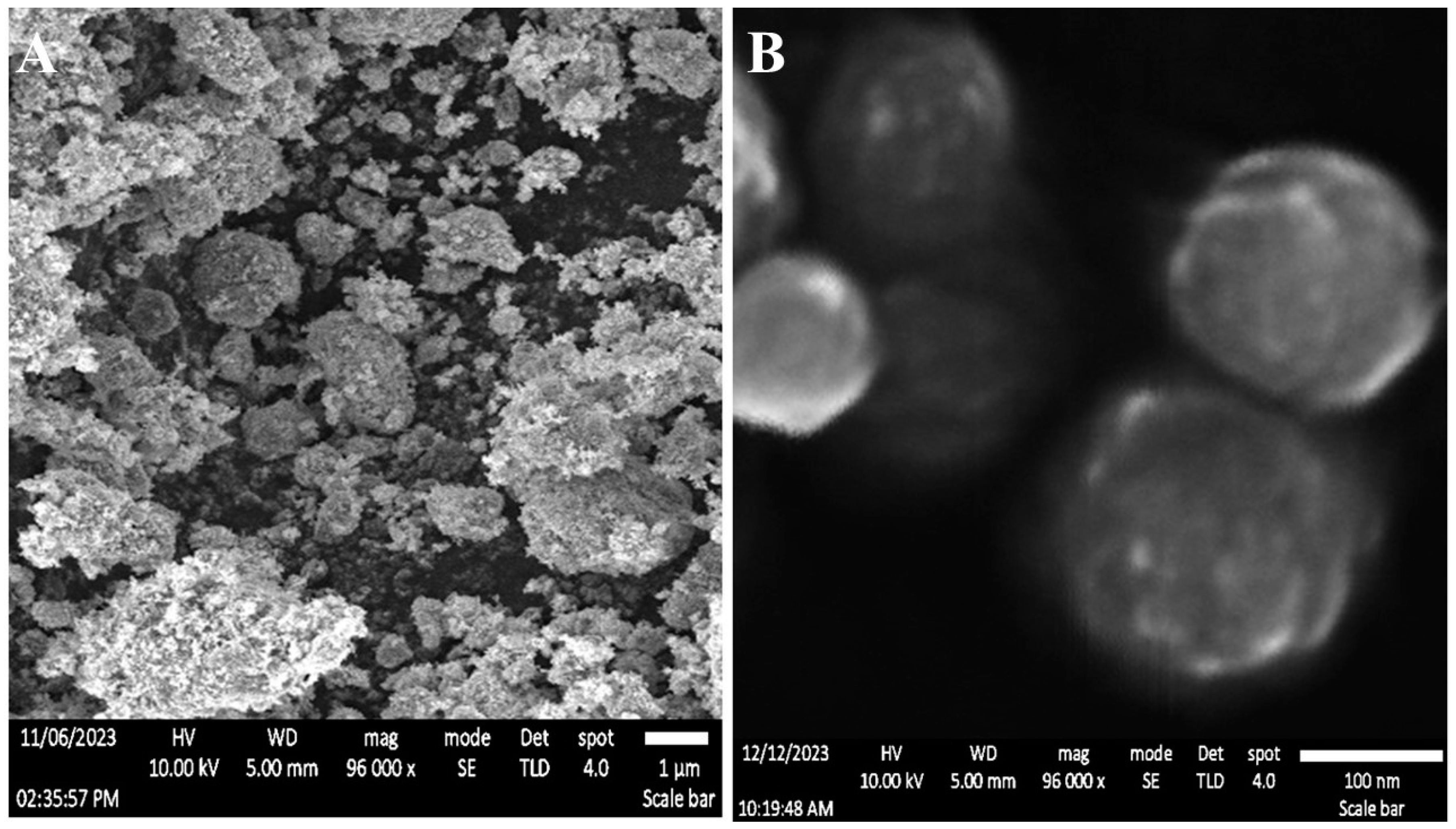
2.2. Scanning Electron Microscopy
The physical characteristics of NS were significantly enhanced with the incorporation of additives. One of the additives that has received a lot of attention for its potential to improve the porous structure of NS is PVA. This indicates that the optimal use of PVA helps create a porous network in the NS matrix (see
Figure 1 A, B), which might lead to better drug loading, release dynamics, and ultimate NS system performance.
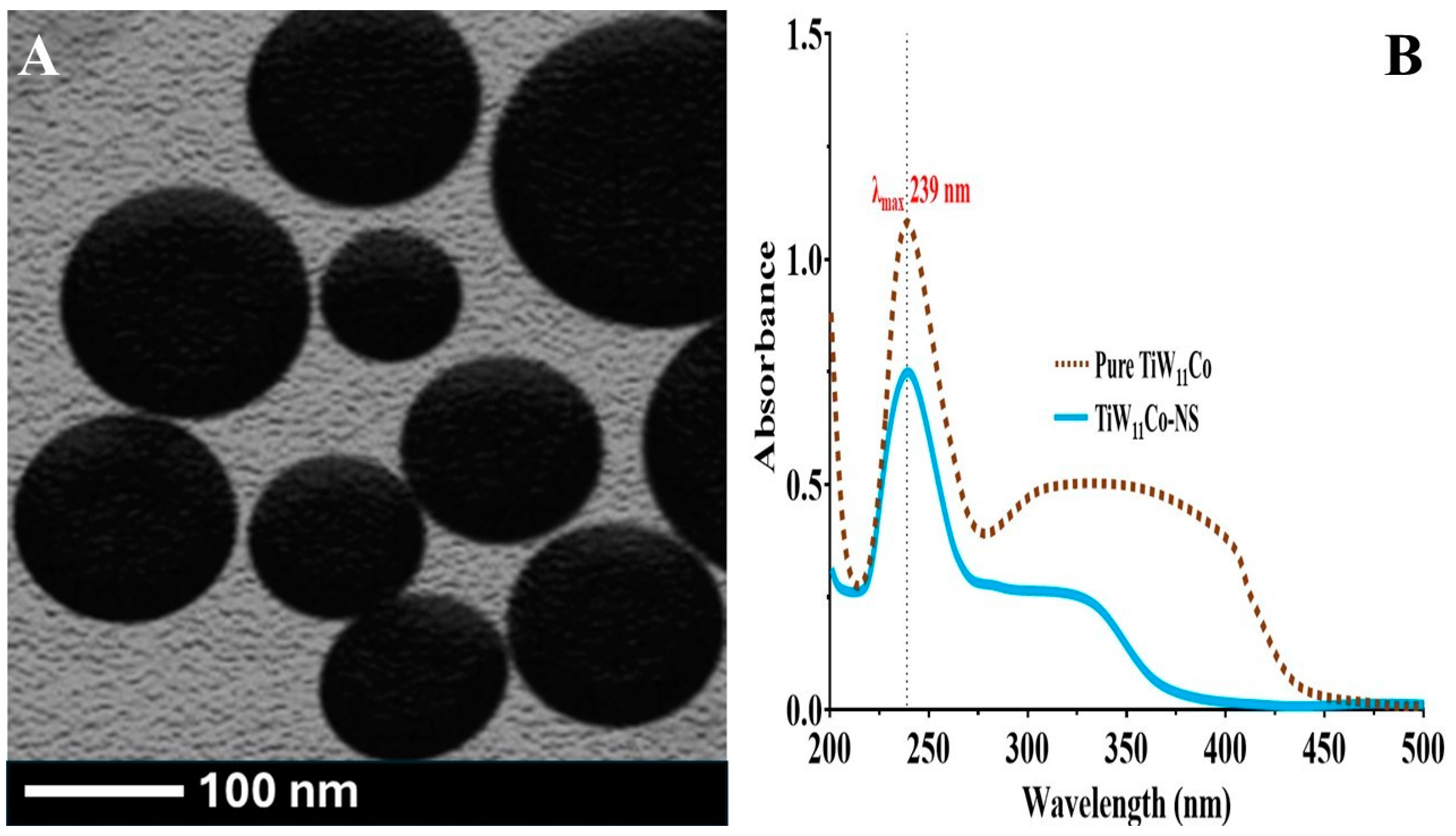
2.3. Transmission Electron Microscopy
Transmission electron microscopy (TEM) research was used to assess the surface characteristics of TiW
11Co-NS. The morphology of the TiW
11Co-NS, as seen in
Figure 1(A, B) had a spherical form and smooth, unbroken edges, as evidenced by the absence of any flaws. Previous research on the surface structure of TiW
11Co-NP at varying TiW
11Co proportions was consistent with our findings [
28]. The homogeneity in the structural features of NS underscored the repeatability and reliability of our findings. The outcomes of our study provided further support for the enhancement of our understanding of the surface characteristics of TiW
11Co-NS and their possible implications for various applications.
2.4. Stability Test of TiW11Co-NS
The findings demonstrated that the stability of TiW
11Co inside NS remained consistent throughout the study, as seen by unaltered wavelength (λ
max) of 239 nm. The confinement of TiW
11Co inside the NS likely provided protection from environmental factors that might facilitate degradation, such as hydrolysis or oxidation. In addition, the elements of the formulation, namely EC and PVA, did not exhibit any notable associations with TiW
11Co that might have altered its spectrum properties. This observation provided further evidence that the TiW
11Co and additives used in the formulation displayed compatibility, hence enhancing the long-term stability of TiW
11Co-NS (
Figure 2(B).
2.5. TiW11Co Release Kinetics
In
Figure 3 (B), the dissolution profiles of both pure TiW
11Co and TiW
11Co-NS were depicted. Pure TiW
11Co was completely dissolved within two hours, although the limited data points posed challenges in constructing a kinetic model. The rapid disintegration of TiW
11Co may be attributed to its exceedingly short biological half-life [
29]. Nevertheless, the release kinetics techniques provided insight into the drug delivery mechanism of TiW
11Co-NS. Various kinetic models, including 1st order, zero-order, Peppas, and Higuchi equations, were applied to analyze the release characteristics. The R
2 coefficient served as a quantitative metric for evaluating the kinetics and release patterns of TiW
11Co from NS. [
30].
The R
2 results derived from zero-order analysis (0.9713) implied that the concentration of the TiW
11Co does not exert influence on its release from NS within a specific timeframe. The kinetic model utilized in this investigation illustrated the sustained release properties of a particular chemical from TiW
11Co-NS. The Peppas model displayed a notable R
2 value (0.9783, n = 0.916), suggesting that the release mechanism of TiW
11Co from NS involves swelling, erosion, and a non-Fickian super case II type of release [
31].
Figure 3.
Determining the hydrodynamic diameter of TiW11Co-NS (A), assessing its dissolution behavior under pH 7.4 conditions (B).
Figure 3.
Determining the hydrodynamic diameter of TiW11Co-NS (A), assessing its dissolution behavior under pH 7.4 conditions (B).
3. Enzyme Inhibition Studies
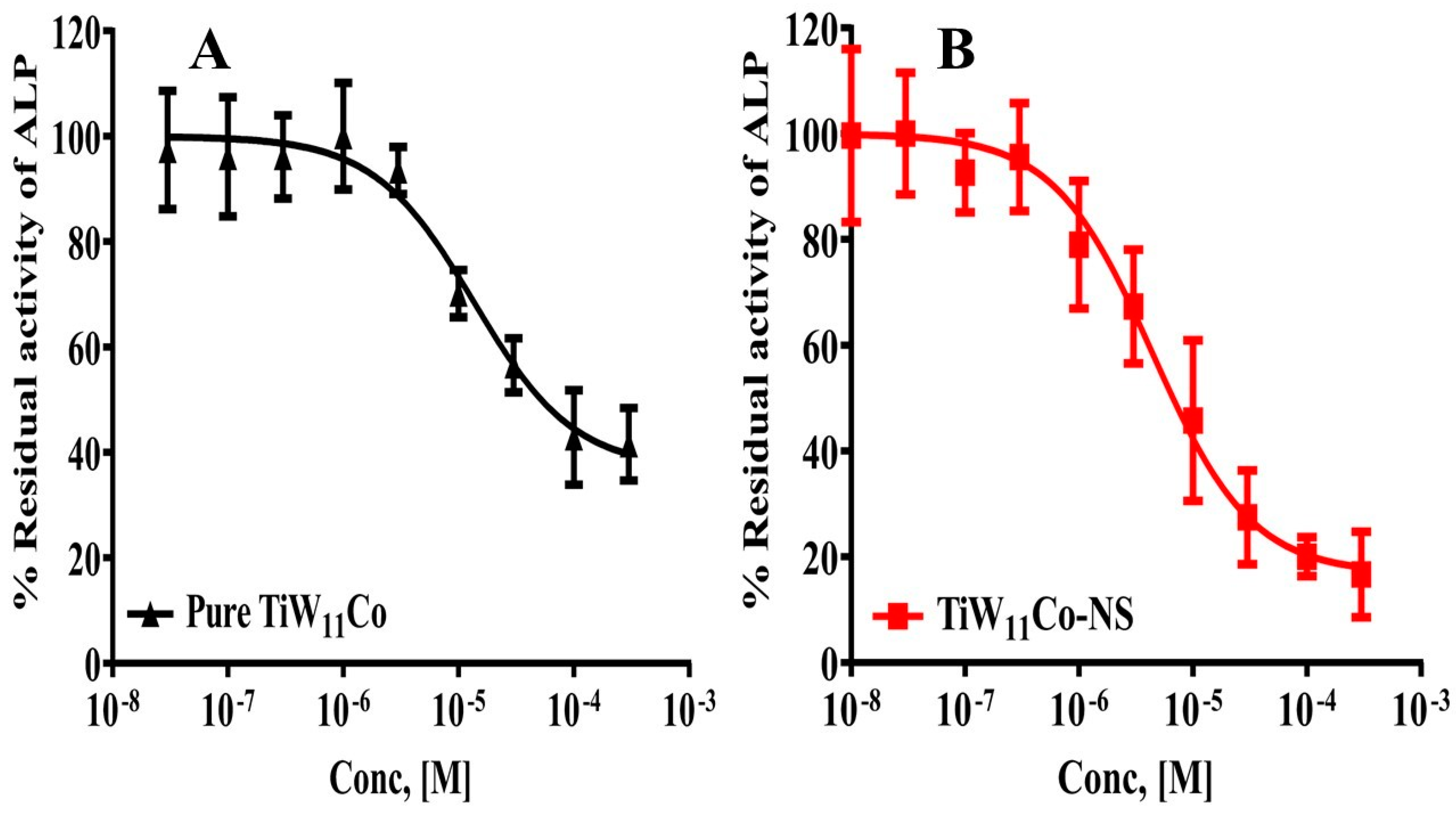
It was found that TiW
11Co-NS had a substantially stronger inhibitory activity against TNAP in comparison to pure TiW
11Co. One piece of evidence that supports this assertion is the much lower concentration that is necessary to achieve maximal inhibition. To be more specific, the IC
50 concentration of TiW
11Co-NS was found to be 1.87 ± 1.005 µM, which was the highest level of inhibition attained (
Figure 4 (A). On the other hand, pure TiW
11Co needed a higher concentration of 5.773 ± 2.823 µM to reach a comparable degree of inhibition (
Figure 4 (B).
The findings of this study indicated that the effectiveness of TiW11Co-NS was almost three times more than that of pure TiW11Co. This suggested that there might be improvements in bioavailability, solubility, or interactions with the TNAP enzyme. The use of NS could augment the surface area accessible for enzyme interactions, hence possibly resulting in improved binding and inhibitory efficacy.
The enhanced efficacy of TiW11Co-NS implied its potential as a more efficacious therapeutic agent for illnesses requiring modulation of TNAP activity. This might include circumstances such as the spread of cancer into bone, whereby TNAP might assume a pivotal function in the processes of mineralization. TiW11Co-NS might provide benefits in terms of treatment and enhanced patient compliance by necessitating lesser dosages to get the intended inhibitory effect.
4. Pharmacological Assessment
4.1. SRB Analysis
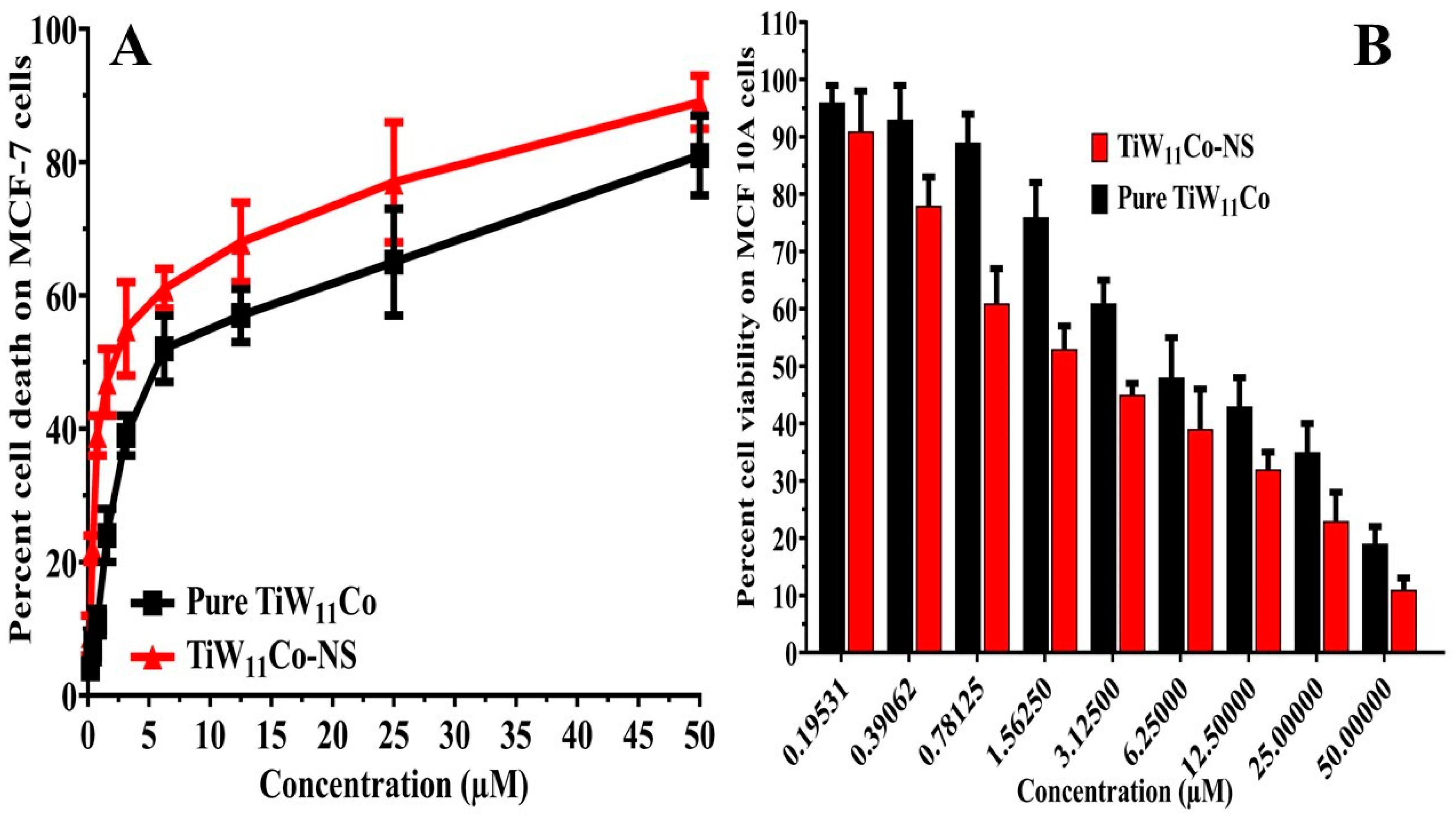
Purified TiW
11Co and TiW
11Co-NS were tested for their cytotoxic effects on MCF-7 cells using the Sulforhodamine-B (SRB) assay, the results of which were shown in
Figure 5(A). The MCF-7 cells and MCF-10A normal breast cells (
Figure 5 (B) were exposed to different doses of pure TiW
11Co and TiW
11Co-NS. The MCF-7 cell-derived IC
50 values for pure TiW
11Co and TiW
11Co-NS were calculated as 2.45 ± 1.11 and 0.976 ± 0.452 µM, respectively.
Previous studies have shown that pure TiW
11Co has anticancer effects on many cancer cells, notably KB cells [
32] and HCT-116 [
33] cell lines. when tested either alone or in combination with chitosan nanoparticles. It is worth mentioning that the composite TiW
11Co-NS were more effective than pure TiW
11Co in triggering cytotoxic responses in the cells indicated earlier. This highlights the fact that TiW
11Co could kill cancer cells on its own and that it may be much more effective when combined with NS. These results highlight the potential of TiW
11Co-NS for targeted cancer treatment, paving the way for more research into their mechanisms of action and therapeutic uses.
4.2. Method for Evaluating Cell Death Using DAPI Staining
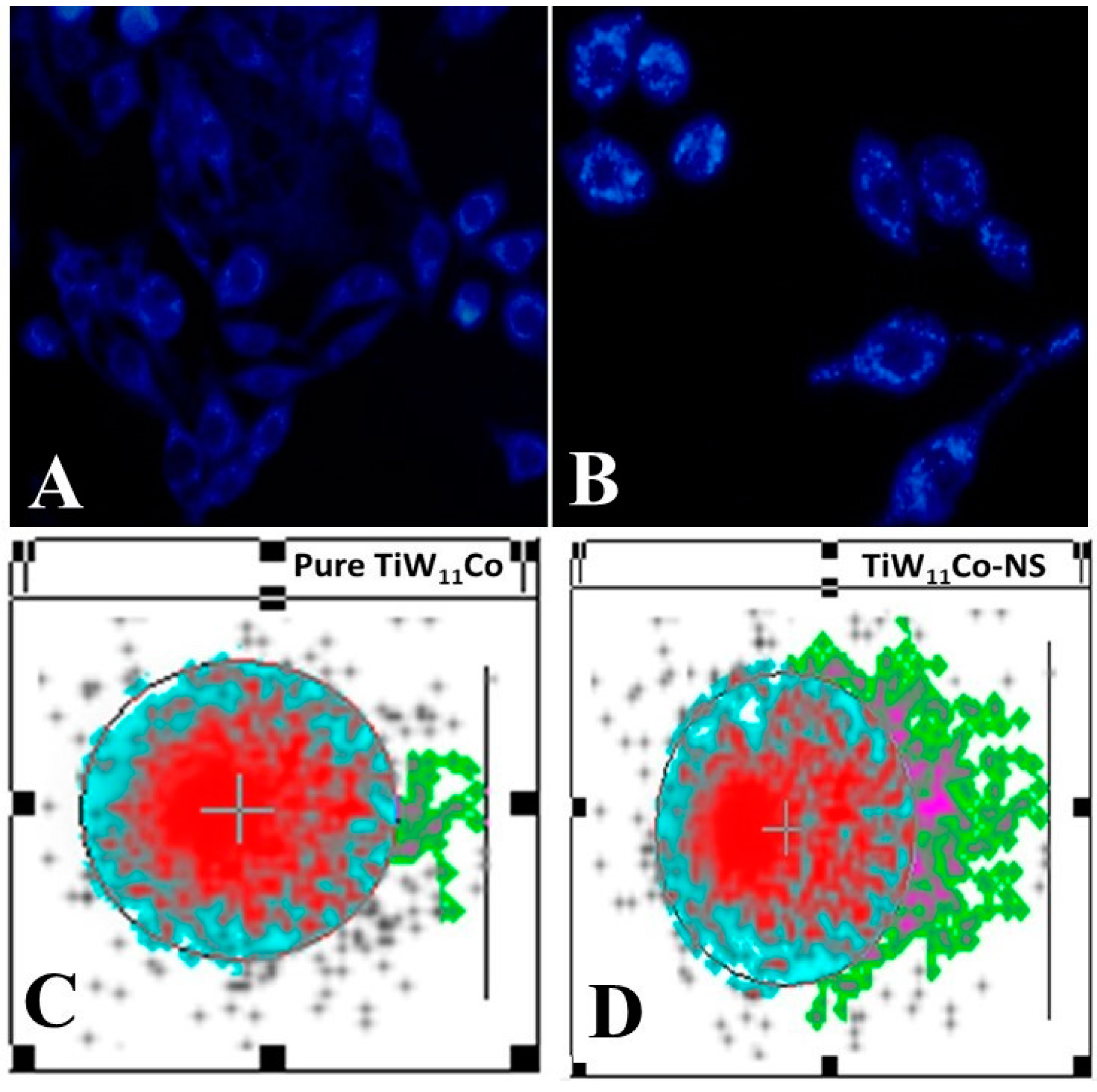
DAPI staining is an essential technique for qualitative analysis, offering valuable information on changes in nuclear structure, especially valuable for identifying apoptosis [
34]. The results obtained from the DAPI labeling of MCF-7 cells treated with pure TiW
11Co (
Figure 6 (A) and TiW
11Co-NS (
Figure 6 (B), which indicated the presence of apoptotic bodies with denatured cell membranes, were consistent with previous studies [
35,
36]. Small alterations in size, shape, and smoothness at the margins were seen in MCF-7 cells when exposed to pure TiW
11Co, suggesting a very small effect on cellular integrity. In contrast, significant characteristics suggestive of apoptosis were found in MCF-7 cells subjected to TiW
11Co-NS treatment. The presence of shattered and condensed nuclei in the studied samples indicated the occurrence of chromatin condensation, a distinctive property often associated with apoptotic cells. The observed disparity highlighted the efficacy of TiW
11Co-NS in promoting apoptosis in MCF-7 cells. The presence of apoptotic bodies, characterized by the ruptured cell membranes and condensed nucleus, indicated the coordinated cellular response to TiW
11Co-NS therapy. The observed morphological alterations align with prior research, so bolstering the dependability and replicability of our results [
37,
38].
DAPI staining was used to reveal the breakdown and condensation of nuclei, which served as an indicator of significant cellular processes linked to apoptosis, including the destruction of DNA and nuclear collapse. The observed changes might be caused by the induction of apoptotic pathways induced by TiW11Co-NS, that initiated intracellular signaling cascades leading to cellular demise.
4.3. Genotoxicity Analysis
The comet assay results, which validate DNA damage in MCF-7 cell lines that were exposed to both free TiW11Co and TiW11Co-NS, offer significant insights into the possible genotoxic consequences of these substances. Utilizing a highly sensitive method, the comet assay is employed to assess DNA damage at the cellular level. The procedure involved exposing cells to electrophoresis, which triggered the escape of fragmented DNA from the nucleus in the form of a tail resembling a comet. The quantification of DNA damage commonly employed parameters including tail moment (TM) and olive tail moment (OTM), wherein greater values signify augmented damage.
The assessment of DNA damage could be achieved through the comparison of tail DNA to total tail DNA and the evaluation of olive tail moment (OTM) and tail moment (TM). The distal portion of the DNA strand had significantly impacted by the travel of damaged fragments of DNA away from the nucleus, as indicated by these parameters.
Figure 6(C) and
Figure 4(D) were depicted the outcomes of the comet test conducted on cells that were treated with purified TiW
11Co and TiW
11Co-NS, respectively. The visual depictions provided an indication of the magnitude of DNA damage caused by each compound, with longer and more conspicuous tail denoting a more severe degree of damage.
The quantitative results of various parameters evaluated in the comet assay, such as tail DNA percentage, OTM, and TM, were estimated in
Table 3. The presented information gave a comprehensive analysis of the genotoxic impacts inflicted by pure TiW
11Co and TiW
11Co-NS on the cells of MCF-7.
The genotoxic effects of both purified TiW
11Co and TiW
11Co-NS on MCF-7 cells were evident through the detection of DNA damage, as measured by an increased proportion of TM, OTM, or tail DNA, as detailed in
Table 3. The comet assay observed enhanced mobility in the distal segment of the DNA strand, which had incurred the most significant damage, because of DNA breaks or lesions. An increased level of DNA damage induced by TiW11Co-NS in comparison to pure TiW
11Co could potentially signify improved cellular uptake or interaction with DNA due to the nanostructured configuration, thereby leading to heightened genotoxic consequences.
The findings of this study emphasized the need for additional research into the genotoxic characteristics of purified TiW
11Co and TiW
11Co-NS, particularly considering their potential use in diverse fields. Further investigation has warranted to explore the mechanisms that initiate DNA damage and evaluate the cytotoxic impacts of these compounds on a wide range of cell types [
39,
40].
Figure 6.
The evaluation of DNA damage in the MCF-7 cell line following exposure to pure TiW11Co (A) and TiW11Co-NS (B) is demonstrated through pharmacological analysis utilizing the Comet assay for both TiW11Co-NS and the pure form of TiW11Co.
Figure 6.
The evaluation of DNA damage in the MCF-7 cell line following exposure to pure TiW11Co (A) and TiW11Co-NS (B) is demonstrated through pharmacological analysis utilizing the Comet assay for both TiW11Co-NS and the pure form of TiW11Co.
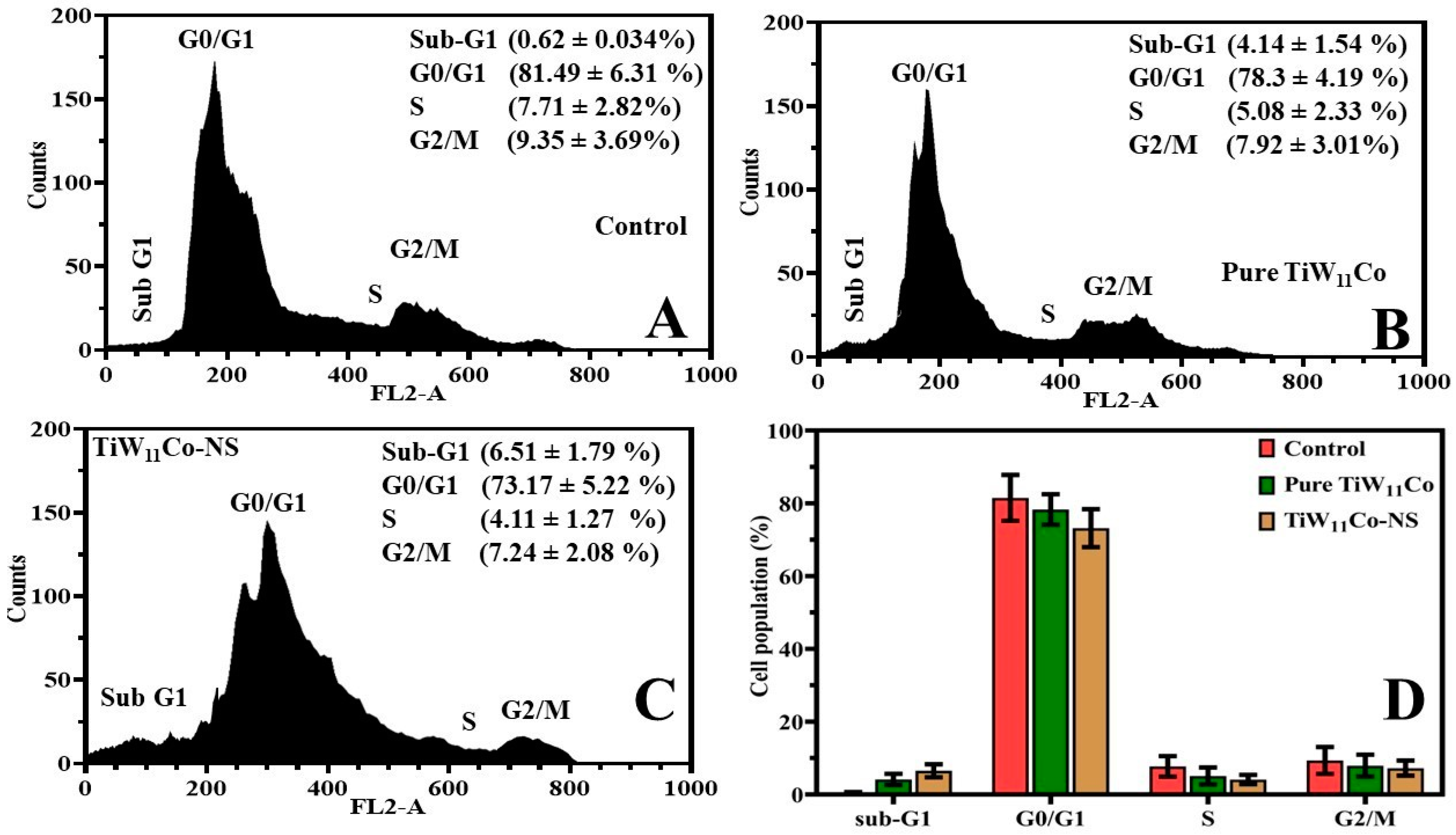
4.4. Flow Cytometry Investigation
Flow cytometry is an effective method for studying population-level cellular properties such size, complexity, and DNA concentration. Using flow cytometry, this work uncovered how the substances pure TiW
11Co and TiW
11Co-NS triggered cell cycle arrest [
41].
The administration of pure TiW
11Co led to a larger percentage of MCF-7 cells being maintained in the G0/G1 phase of the cell cycle in comparison to the control drug (
Figure 7(A, B). This observation suggested a possible halt or deceleration in the advancement of the cell cycle. Nevertheless, there was a notable rise in the proportion of cells exhibiting the sub-G1 phase, a characteristic that was often associated with apoptotic or deceased cells. This observation implies that some cells were halted in the G0/G1 phase, whereas others experienced apoptosis, resulting in cellular demise. The use of TiW
11Co-NS resulted in a subsequent augmentation in cellular demise in comparison to pure TiW
11Co (Figure (C). This is supported by a higher proportion of cells remaining in the sub-G1 phase and a smaller proportion of cells remaining in the G0/G1 phase (
Figure 7(D). The observed elevated mortality rate associated with TiW
11Co-NS implied that the nanostructured variant of the drug might augment its cytotoxic properties or cellular absorption, resulting in heightened apoptosis and cellular demise [
42].
5. In Vivo Studies
It describes an in vivo study conducted on female albino mice to corroborate findings obtained from in vitro experiments regarding the efficacy of various treatments, such as cisplatin, pure TiW
11Co, and TiW
11Co-NS [
43].
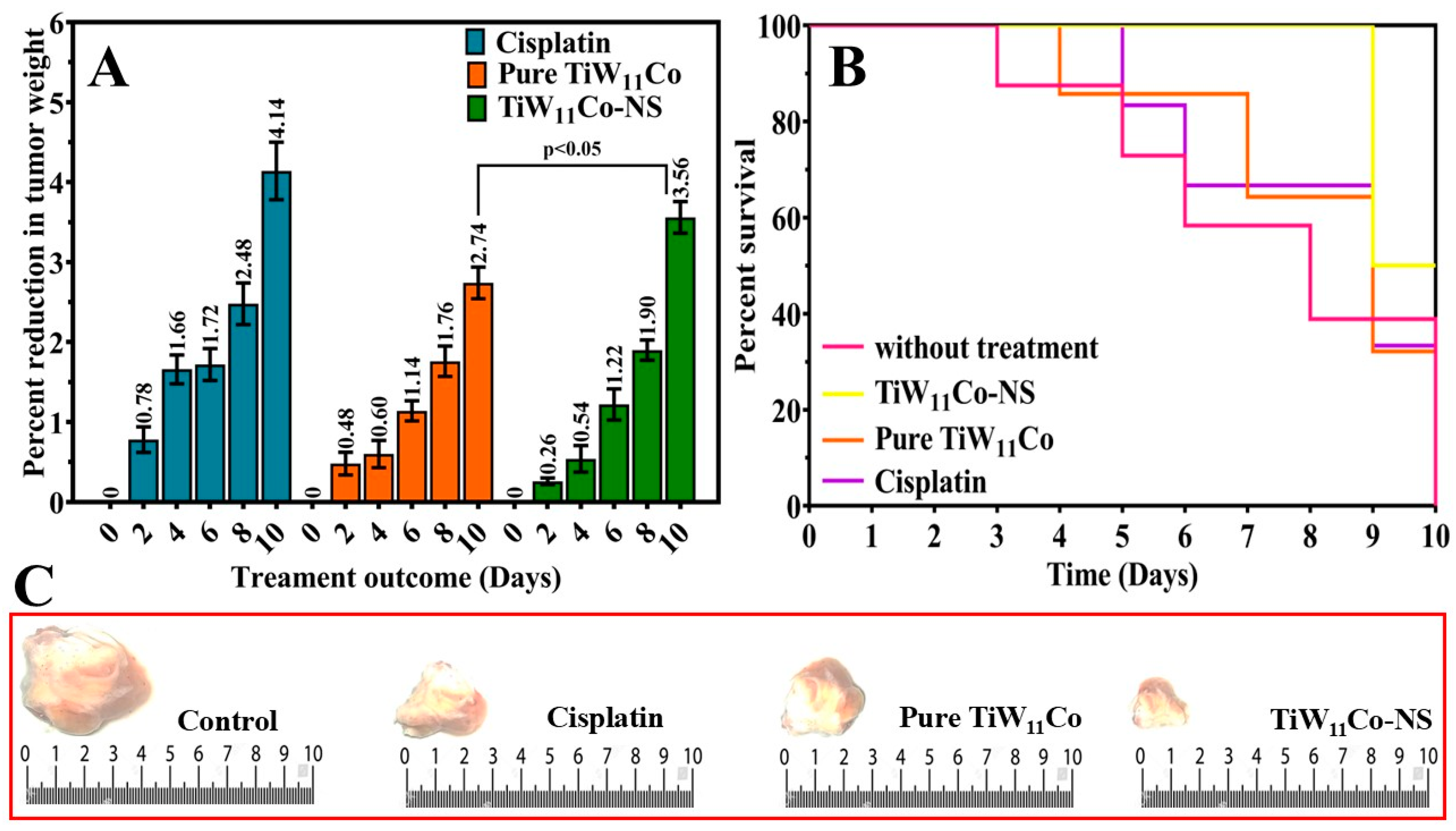
On the tenth day following administration, the comparative impact on tumor weight reduction was evaluated between mice treated with TiW
11Co-NS and those receiving pure TiW
11Co alone. When comparing pure TiW
11Co to the other group treated with TiW
11Co-NS demonstrated a more pronounced reduction in tumor weight percentage, suggesting an improved effectiveness in suppressing tumor growth (
Figure 8(A). This implies that TiW
11Co-NS might exert a more potent cytotoxic influence on tumor cells, thereby facilitating a more efficient inhibition of tumor growth [
44].
The study evaluated and compared the survival rates of mice subjected to specified dosages of pure TiW
11Co, and TiW
11Co-NS, in comparison to the reference standard of cisplatin (see
Table 1). The survival rate of mice treated with TiW
11Co-NS at a dose of 1.756 µM/kg was found to be greater in comparison to those treated with pure TiW
11Co alone, as seen in
Figure 8 (B). It has been shown in our findings that the tumor-bearing mice that were treated with TiW
11Co-NS displayed the smallest tumor size compared to all other treatment groups represented in
Figure 8 (C). This recommends that the TiW
11Co-NS were highly efficient in lowering tumor growth. These findings indicated that TiW
11Co-NS may be more effective in enhancing survival in mice than pure TiW
11Co, suggesting its potential as a better therapeutic agent.
6. Computation Methods:
6.1. Docking Studies
Molecular docking is among the most employed approaches to unveil the binding mechanisms of ligands towards various molecular targets [
45,
46,
47,
48,
49,
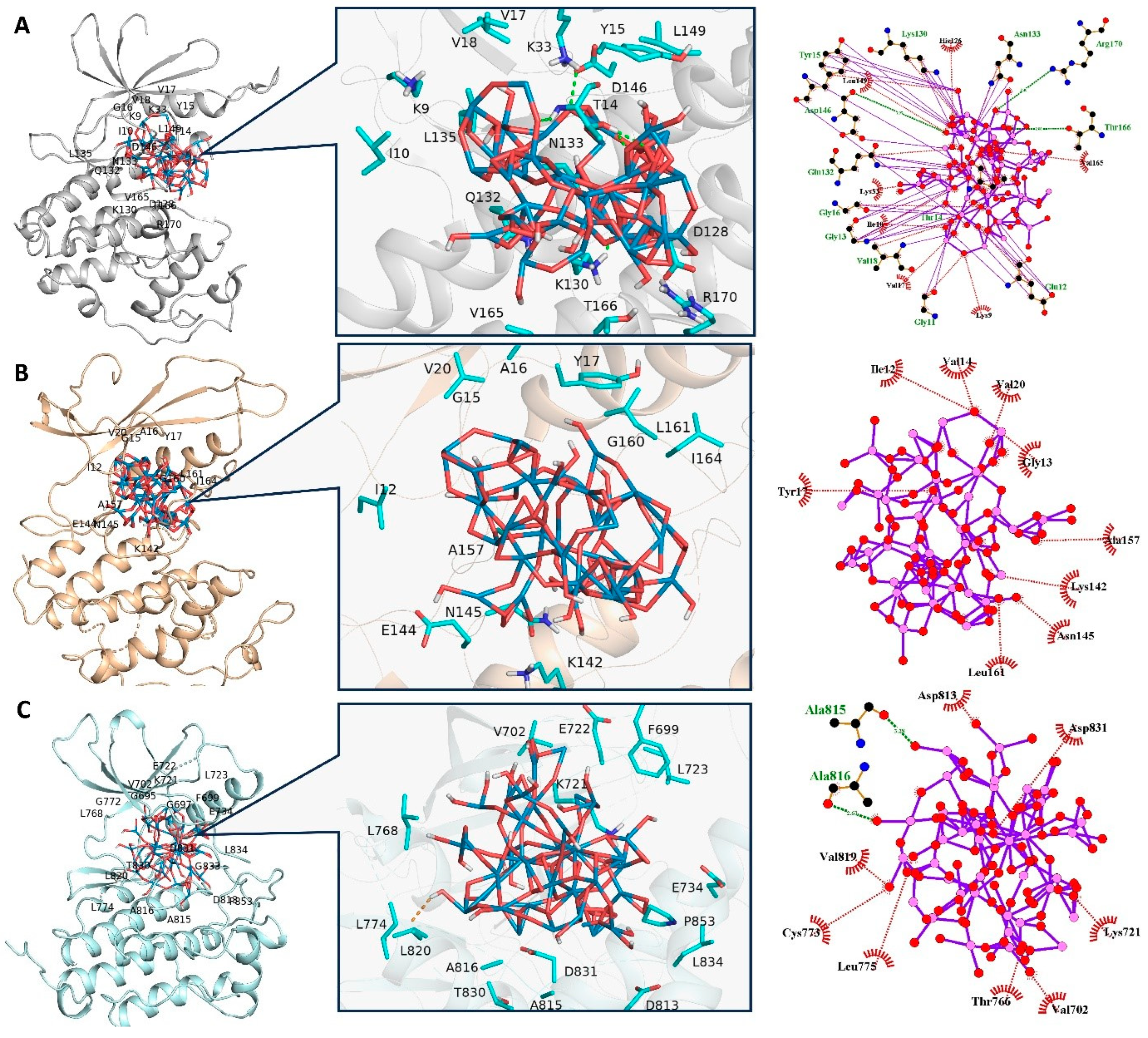
50]. To elucidate the structural and functional dynamics inherent to ligand-protein interactions, the adoption of in-silico methodologies offers substantial insights, particularly in understanding the molecular mechanisms that hinder the proliferation of cancer cells. This is exemplified through combined in-vivo and in-vitro analyses (as depicted in
Figure 9(A-C), where a marked diminution in the proliferation of MCF7 cell lines was observed after sustained exposure to POM. To deepen this understanding, a thorough review of existing literature was undertaken, pinpointing three critical proteins–Cyclin-Dependent Kinase 1 (CDK1), Cyclin-Dependent Kinase 4 (CDK4), and Epidermal Growth Factor Receptor (EGFR) – each playing a significant role in modulating distinct signaling cascades within MCF7 cells. This strategic identification of key proteins serves as a cornerstone for further exploratory research into their intricate roles and interactions within the cellular milieu [
51,
52]. Subsequently, molecular docking was employed to unveil the mechanism underpinning the anti-proliferative potential of POM.
Utilizing the Consensus Score (cScore) as a metric, the most favorable ligand conformations within the active sites of the target proteins were delineated. The docking scores (cScore) for the inhibitor POM in its interactions with CDK1, CDK4, and EGFR were quantified as 48.32, 23.11, and 41.33, respectively. These values underscore the formation of a particularly stable complex between POM and CDK1, alongside an efficient binding to EGFR. Conversely, POM exhibited a markedly lower binding affinity towards CDK4. Collectively, these docking scores reflect a notable affinity of POM towards all three examined proteins, with a discernible predilection for CDK1. A comprehensive presentation of the docking results, inclusive of the scores, is provided in
Table 4. To elucidate the observed variations in docking scores, the top-ranked docking complexes were preserved for detailed visual analysis (refer to
Figure 9(A-C). The significant conformational adaptations of the inhibitor POM, which assumes an intricate configuration post energy minimization. Despite POM's complex architecture, rich in oxygen atoms, it manages to establish multiple hydrogen bond (H-bond) interactions with proximal residues in both CDK1 and EGFR complexes, corroborating its elevated docking scores. Particularly in the CDK1 complex, POM deeply infiltrates the active site, situated between the N- and C-terminal domains, engaging with residues including K9, G
11, E12, G13, T14, Y15, G16, V18, K33, H126, K130, Q132, N133, D146, T166, and R170 (as depicted in
Figure 9(A). Notably, the H-bond interaction with the catalytic residue D146 is pivotal for the inhibition of CDK1. Intriguingly, the POM-CDK1 complex demonstrates a capacity to form an H-bond with D146. As illustrated in
Figure 9(A), the inhibitor POM predominantly interacts within the solvent-exposed and catalytic regions of CDK1. In the CDK1 complex, POM can establish H-bond interactions with residues K33, Y15, D146, T166, and R170, with an average distance ranging approximately between 2.2Å and 2.9 Å. These residues have been recurrently emphasized in prior research, underscoring their integral role in the binding dynamics of potent inhibitors to CDK isoforms, particularly CDK1 [
53,
54,
55,
56]. In the context of the CDK4 complex, POM is situated within the ligand-binding cavity, yet its extensive molecular structure impedes interaction with the hinge region. This spatial constraint is likely responsible for POM's comparatively lower docking scores in the CDK4 system, as it fails to establish any hydrogen bond (H-bond) interactions within the CDK4 active site. Moving to the EGFR system, POM forms a limited number of H-bond contacts. The binding site for the EGFR-POM complex encompasses residues V702, K721, T766, C773, L775, D813, A815, D831, and A816. Mirroring the situation in the POM-CDK4 complex, POM in the EGFR-inhibitor complex predominantly localizes near the solvent-exposed region, not engaging the hinge residues. For an enhanced visual comprehension of these interactions, the 2D interaction diagrams of the inhibitor-protein complexes are elucidated in
Figure 9 (A-C). Conclusively, these computational analyses shed light on the preferential binding affinity of POM towards CDK1. There is a strong congruence between these computational findings and the experimental data, presenting a cogent rationale for the significant chemo-preventive potential of POM against MCF7 cell lines.
Utilizing a variety of evaluation methods, aCScore determines the compatibility between ligands and their respective binding sites. bCrash-score acts as a vigilant guard, identifying instances of unwarranted intrusion into the binding regions. cAttention is paid to the polar characteristics of ligands. dD-score meticulously scrutinizes the interplay of hydrogen bonds, as well as the intricate dance of energies within and between ligand-protein and ligand-ligand complexes. ePMF-score delves into the realm of Helmholtz free energies, dissecting the interactions between individual protein-ligand atom pairs. fG-score captures the essence of charge dynamics and van der Waals forces governing the interplay between proteins and their bound ligands. gChem-score, with its nuanced approach, rewards instances of hydrogen bonding, lipophilic contacts, and the subtle dance of rotational entropy, all while incorporating an intercept term for added depth.
7. Materials and Methods
The research used materials obtained from Sigma Aldrich (St. Louis, MO, USA) and were utilized in their original form without any further purification, unless specified otherwise. These materials included hexa-potassium mono-hydrogen mono-titano-undeca-tungsto-cobaltate (II) mono-hydrate (referred to as TiW11Co), Ethyl cellulose (EC), 100 kD, poly(vinyl alcohol) (PVA) 30 kD, acetic acid, dialysis membrane with a molecular weight cutoff (MWCO) of 10 kDa, dimethyl sulfoxide (DMSO), Human breast cancer cell line (MCF–7, HTB-22TM) (ATCC, CA-USA), Human breast normal cell line (MCF-10A, CRL-10317) (ATCC, CA-USA), Dulbecco's modified Eagle's medium, phosphate buffer (pH 7.4), trichloroacetic acid, tris-base and Triton X-100.
8. Synthesis of NS
The emulsion solvent diffusion method was used to synthesize NS with varying concentrations of EC and PVA (see
Table 2). Briefly, in this technique, dispersed phase (organic phase) was prepared by ultrasonic stirring of EC (50-100% w/v) in 20 mL of ethanol. Then PVA (50-100% v/v) and TiW
11Co (1% w/v) were dissolved in 150 mL of water to prepare aqueous continuous phase by stirring on hot water bath at 60°C. Afterward, dispersed phase was slowly incorporated in the continuous phase using syringe (5 mL, 22 G needle). This mixture was stirred at 10,000 rpm for 30 min with high speed homogenizer (stalwart,SMT-30K Van Nuys, L.A. USA). To remove any adsorbed PVA, the TiW
11Co-NS had washed multiple times with ultra-pure water. Finally, the TiW
11Co-NS were extracted by centrifugation (8000×
g, 20 min) (Hettich EBA 200S, Singapore). The extracted NS were subjected to lyophilization by placing them in a glass vial and subjecting them to overnight at -40°C in a single chamber LSCplus Martin Christ™ freezer Germany. The lyophilized material was kept at 4°C until the next round of experiments.
8.1. Entrapment Efficiency
The methodology that was formerly delineated endured only minor adjustments to calculate the entrapment efficiency (EE) percentage [
57]. The TiW
11Co-NS, weighing 657 mg (equivalent to 6.57 mg TiW
11Co), were solubilized in 5 mL of phosphate-buffered saline (PBS) with a pH of 7.4. Subsequently, the solution was introduced into a dialysis membrane and subjected to agitation for one hour at a temperature of 37ºC using a magnetic stirrer operating at a speed of 100 rpm. At scheduled periods (0 and 1 hr), a sample volume of 5 mL was drawn, and absorbance was recorded at a wavelength of 239 nm on a UV-visible spectrophotometer (Shimadzu UV 1900i, Tokyo, Japan) without any further dilution.
The % EE was estimated using the following equation,
8.2. Assessing Hydrodynamic Dimension and Zeta Potential: A Measurement Investigation
The TiW
11Co-NS were dispersed in distilled ultrapure water to determine their hydrodynamic size. The Malvern Zetasizer Nano ZS (Cambridge, UK) was then used to examine the particle sizes and zeta potential [
58].
8.3. Visual Examination Using Scanning Electron Microscopy (SEM)
The scanning electron microscopy (SEM) investigation was conducted using a Hitachi S-4700 (Houghton, MI, USA). The device was operated at an acceleration voltage that varied between 10 to 20 kV. The sample underwent fast dispersion in ethanol and then was deposited onto recently cleansed silicon wafers for the purpose of desiccation. In addition, a gold-sputter covering was used on the samples to enhance the conductivity of the material.
8.4. Exanimation Through Transmission Electron Microscopy (TEM)
The morphology of TiW
11Co-NS (10 µL) were analyzed using transmission electron microscopy (TEM) using a JEM-2100 (JEOL; Tokyo, Japan) operated at a voltage of 200 kV. Prior to imaging, the samples underwent negative staining using a 2% w/v uranyl acetate solution, followed by a 20-min drying period. After the drying process, the samples were affixed onto a carbon-coated copper grid with a mesh size of 300 [
59].
8.5. Release Kinetics of TiW11Co
The present work used a well-recognized methodology to examine the liberation of TiW
11Co through NS with the aim of developing kinetic models [
60]. In short, 657 mg TiW
11Co-NS was dispersed in 5 mL of pH 7.4 phosphate-buffered saline. The mixture was placed on a dialysis membrane and immersed in 100 mL of pH 7.4 phosphate-buffered saline. A 75-rpm agitator kept the system at 37°C. A UV-Vis spectrophotometer (Shimadzu UV 1900i, Tokyo, Japan) at 239 nm measured TiW
11Co release periodically. Using DDSolver software, release kinetics-based mathematical models were used to determine the release process of pure TiW
11Co from NS.
8.6. Stability Test of TiW11Co-NS
To examine the stability of TiW11Co inside NS, both the pure TiW11Co and TiW11Co-NS were kept in a controlled laboratory setting at a temperature of 25°C for a duration of 6 months. The specimens were collected at certain time intervals (0, 60, 180 days) and subjected to analysis in using the UV-vis method (Shimadzu UV 1900i, Tokyo, Japan).
9. Enzyme Inhibition Analysis
A previously described absorbance method was used for enzyme inhibition investigation [
61]. Briefly, TNAP was reconstituted in a pH 9.5 buffer solution with 50 mM Tris–HCl, 5 mM MgCl
2, 0.1 mM ZnCl
2, and 50% glycerol. The substrate, p–nitrophenyl phosphate (p–NPP), has been mixed in glycerol-free buffer. The assay commenced by combining enzyme (10 µL) with 10 µL of the test compound (both TiW
11Co and TiW
11Co-NS), followed by a 10-minute pre-incubation period at 37°C. Subsequently, the enzyme substrate (p–NPP) was added and the mixture was further incubated for 30 minutes. Formation of the yellow-colored product (p–nitrophenolate) was observed, and absorbance was measured at 405 nm using an ELISA plate reader (BioTek ELx800
TM, Instruments, Inc. USA). Each experiment was replicated three times, and the data were presented as IC
50 values calculated using Prism 5.0 software (GraphPad Software, San Diego, CA, USA).
10. Pharmacological Assessment
10.1. Sulforhodamine B Assay
The SRB assay was employed to assess and compare the anti-proliferative effects of pure TiW
11Co and TiW
11Co-NS on both MCF-7 and MCF-10A cells [
62]. At the outset, 96-well plates were seeded with 1×10
4 cells and given 24 hr to multiply. Following this, at specified time intervals (24 hours), different doses of pure TiW
11Co and TiW
11Co-NS were added to separate wells. Prior to attachment with 40% iced trichloroacetic acid (TCA), the cells were rinsed with PBS and allowed to air-dry after the incubation time. Following that, the cells were subjected to staining using a 0.4% w/v the SRB dye for a duration of 30 min. Subsequently, a 100 µL (10 mM) solution of tris-base (pH 10.5) was administered. The microplate reader ELx808
TM (BioTek instruments, Winooski, Vermont, USA) was utilized to measure absorbance at 565 nm. IC
50 values (µg/mL) were determined using Prism 5.0 software (GraphPad Software; San Diego, California, USA).
10.2. DAPI Staining Assessment
A 2–well sterile chamber slide was used for cells culturing (1.5×104). The cells were exposed to the test chemical (dose = IC90) for a duration of 24 hours and then treated with a formaldehyde (4%) solution to fix them. The cells were subjected to a 5-minute incubation period with a solution of Triton X–100 (0.1%), followed by a subsequent washing with PBS. Afterwards, incubation of cells with DAPI (10 µg/ml) was carried out in the dark for a period of 10 min. Any unabsorbed DAPI was detached by washing with PBS for several times and cells were observed under fluorescence microscope (Nikon Eclipse–Ni Japan) at λemission 461 nm and λexcitation 358 nm.
10.3. Evaluation of Genotoxicity (Comet Test)
Quantification of DNA damage was conducted using the comet assessment [
63]. MCF-7 cells (2×10
4 cells/well) were treated with either pure TiW
11Co or TiW
11Co-NS. Prior to transfer onto comet slides, cultured cells were treated with 1% LMPA. Subsequently, the slides were submerged in an alkaline (10) lysis buffer amalgamated with Triton-X 100 and DMSO (1% and 0.1% v/v correspondingly) and 100 mM EDTA. Following this, the experiment proceeded with a time-course investigation, during which samples and buffer solution (consisting of 1 mM EDTA and 300 mM NaOH) at a pH of 13 were introduced into horizontally oriented electrophoretic-agarose chambers. An alkaline buffer was used to unwind the DNA. Following this, the glass slides were sanitized with CH
3OH and thoroughly dried. The DNA extracted from the comet was analyzed using CaspLab 1.2.3b2 software.
10.4. Analysis via Flow Cytometry
Following a 24-hour exposure to pure TiW
11Co and TiW
11Co-NS, flow cytometry investigation was performed on the MCF-7 cell line (2 × 10
4). The cells underwent trypsinization for a duration of 5 minutes at a temperature of 37°C, using a mixture of trypsin and EDTA. To mitigate the formation of cell clusters, the cell culture medium was gradually introduced. Subsequently, cells were collected in 100 µL of binding buffer over a 15-minute period after exposure to a concentration of 500 mM H
2O
2. Each cell was incubated for 15 minutes with the annexin-V FITC fluorescent marker and propidium iodide (PI) prior to being positioned in a less receptive environment. Using the proper FL2-A channel, analysis was carried out using fluorescence-triggered cell sorting (FACS) with wavelengths for emission of 600 nm for propidium iodide (PI) and 545 nm for annexin-V FITC. A comprehensive evaluation of 10,000 harvested cells was conducted in a singular iteration using the CytoFLEX equipment manufactured by Beckman-Coulter Life Sciences (Brea, C.A, USA). The results were visually shown via a graph illustrating the examination of the cell cycle [
64].
11. Studies Involving Animals
The study used mature female albino BALB/C mice with an average weight of roughly 30 g. The mice were housed at the animal facility located at Bahauddin Zakariya University in Multan, Pakistan. They were raised in controlled settings, which included a constant temperature of 25°C, a 12-hour light-dark cycle, and unrestricted access to food and water. Each steel-mesh cage housed up to five albino mice, ensuring ample space and minimizing stress due to overcrowding. In accordance with the guidelines set by the institution's ethical committee (017/PEC/2023), the animals received humane housing and care. Randomly selected groups of five mice each were assigned to four experimental groups. Afterward, female mice were inoculated with roughly 4×10
6 MCF-7 cells (100 µL). A duration of ten days was permitted for the tumors to grow to a volume ranging from 50 to 100 mm
3. Specifics concerning the various groups of mice and the treatments they received are delineated in
Table 1.
Table 1.
Animals in Control and Experimental Groups Administered Pure TiW11Co, TiW11Co-NS, and Cisplatin.
Table 1.
Animals in Control and Experimental Groups Administered Pure TiW11Co, TiW11Co-NS, and Cisplatin.
| Group |
Type of Treatment |
| 1 |
Mice that did not receive any treatment. |
| 2 |
The cancer-stricken mice were given cisplatin 9.99 µM/kg. |
| 3 |
The cancer-stricken mice were given pure TiW11Co (4.41 µM/kg) |
| 4 |
The cancer-stricken mice were given TiW11Co-NS (1.756 µM/kg) |
The tumor inhibition rate (TIR%) was utilized as a metric to evaluate the effectiveness of each formulation in combatting cancer.
Euthanasia had been done as soon as an animal showed a rapid drop in body weight of more than 20 percent. The process was advised to be completed in a few hours, ideally on the same day, in situations where the symptoms had not been extremely severe but had yet satisfied the requirements for euthanasia to reduce prolonged and needless suffering. Timely detection and reaction had been ensured by constant observation.
Daily health and behavior observations were part of routine animal well-being monitoring to identify any major signs of weight loss, tumor development, breathing problems. Throughout critical phases of the treatment, additional assessments were conducted to quickly act if the prerequisites for achieving the humane aim were met.
12. Computation Methods:
12.1. Structures Preparation and Docking Studies:
The three-dimensional conformation of TiW
11Co was constructed using SYBYL-X1.3/SKETCH module [
65]. For achieving a biologically active form, the POM structure underwent energy optimization via the Tripos force field to incorporating Gasteiger-Hückel atomic charges [
66]. Co-crystal structures for Cyclin-Dependent Kinase 1 (CDK1), Protein Kinase B-alpha (PKB-α), and Epidermal Growth Factor Receptor (EGFR) were acquired from the RCSB Protein Data Bank, corresponding to PDB entries 4YC6 [
67], 3G33 [
68], and [
69], respectively. These protein structures were processed using the integrated structure preparation tools of the SYBYL-X 1.3 Biopolymer module [
70]. The Powell algorithm facilitated energy minimization, applying a convergence gradient of 0.5 kcal (mol)^-1 across 1000 cycles. This procedure encompassed the addition of missing hydrogens, charging, and atom type assignment in accordance with the AMBER 7 FF99 force field [
71]. Following this, the Surflex-Dock module in SYBYL-X 1.3 was employed to dock the energy-optimized, bioactive POM conformation into the active sites of CDK1, CDK4, and EGFR, replicating the methods and parameters from our previous studies [
54,
55]. The top twenty docking conformations were saved and analyzed for their binding interactions within the active sites of each target. These ligand poses were evaluated using the Hammerhead scoring function [
65,
72].
13. Conclusion:
To summarize, the process of creating and analyzing nanosponges loaded with TiW11Co showed positive physical characteristics, stability, and increased capacity to kill cancer cells. Furthermore, in vitro investigations have shown enhanced inhibitory effects and apoptotic induction in comparison to TiW11Co in its pure form. The results were supported by in vivo tests, which demonstrated enhanced survival rates and tumor suppression after the injection of TiW11Co-NS. The process of molecular docking has provided more insight into the probable processes that contribute to the anticancer action of the compound, namely in its ability to target crucial proteins associated with cell cycle control and growth signaling.
The cytotoxic effects on tumor cells were boosted by the conversion of TiW11Co into hybrid TiW11Co-NS. This enhancement might be attributed to greater cellular uptake and specific delivery to the tumor location. Additional investigation might be necessary to clarify the processes that contribute to the increased effectiveness of TiW11Co-NS, as well as to optimize the dose and treatment protocols to achieve the highest possible therapeutic advantage. Furthermore, it is recommended that future research endeavors delve into the safety characteristics and possible adverse reactions of TiW11Co-NS in vivo, to ascertain its appropriateness for clinical use.
Competing Interest
There are no declared conflicting interests, according to the authors.
Disclosure from the Institutional Reviewing Board for Animal Studies
The Biosafety and Bioethics Research Committee of Bahauddin Zakariya University in Multan, Pakistan (60800). granted approval to the study protocol titled "Nanosponge-Encapsulated Polyoxometalates: Unveiling Multi-Faceted Potential Against Cancer and Metastasis through Comprehensive Preparation, Characterization, and Computational Exploration." The researchers have been instructed to adhere strictly to the protocol approved by the ethics committee (that follows guidelines written in “Chemicals 423 of the OECD”) when conducting experiments involving mice [
73].
Acknowledgments
The authors extend their appreciation to researchers supporting project number (RSP2025R357) King Saud University, Riyadh Saudi Arabia for funding this research.
Author Contributions
Conceptualization, Muhammad Sajjad and Muhammad Zubair Malik; Methodology, Muhammad Sajjad; Validation, Muhammad Sajjad, Muhammad Zubair Malik and Hamid Saeed Shah; Formal analysis, Muhammad Sajjad, Faisal Usman and Tahir Ali chohan; Investigation, Muhammad Sajjad, Faisal Usman and Tahir Ali Chohan; Resources, Ayesha Bint Umar Awan, Tanveer A. Wani, Seema zargar and Zobia Jawad; Writing—original draft preparation, Muhammad Sajjad and Hamid Saeed Shah.; Writing—review and editing, Muhammad Zubair Malik and Muhammad Sarfraz; Visualization, Muhammad Sajjad and Muhammad Sarfraz; Supervision, Muhammad Zubair Malik and Hamid Saeed Shah; Project administration, Muhammad Zubair Malik; Funding acquisition, Tanveer A. Wani and Seema zargar. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data and material generated during the current study are available upon reasonable request from the corresponding author.
References
- Pérez-Herrero, E. and A. Fernández-Medarde, Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European journal of pharmaceutics and biopharmaceutics, 2015. 93: p. 52-79. [CrossRef]
- Anusha, A., et al., Cancer immunotherapy. Journal of Pharmaceutical Sciences and Research, 2017. 9(5): p. 662.
- Welch, D.R. and D.R. Hurst, Defining the hallmarks of metastasis. Cancer research, 2019. 79(12): p. 3011-3027. [CrossRef]
- Ganesh, K. and J. Massagué, Targeting metastatic cancer. Nature medicine, 2021. 27(1): p. 34-44. [CrossRef]
- Siegel, R.L., et al., Cancer statistics, 2023. Ca Cancer J Clin, 2023. 73(1): p. 17-48.
- Alizadeh, A.M., S. Shiri, and S. Farsinejad, Metastasis review: from bench to bedside. Tumor biology, 2014. 35: p. 8483-8523. [CrossRef]
- Genovese, M. and K. Lian, Polyoxometalate modified inorganic–organic nanocomposite materials for energy storage applications: A review. Current Opinion in Solid State and Materials Science, 2015. 19(2): p. 126-137. [CrossRef]
- Aureliano, M., et al., Polyoxometalates with anticancer, antibacterial and antiviral activities, in Polyoxometalates. 2022, Jenny Stanford Publishing. p. 309-358.
- Chang, D., et al., Polyoxometalates-based nanocomposites for application in antitumor and antibacterial. Nanoscale Advances, 2022.
- Chi, G., et al., Polyoxometalates: Study of inhibitory kinetics and mechanism against α-glucosidase. Journal of Inorganic Biochemistry, 2019. 199: p. 110784. [CrossRef]
- Lu, F., et al., Polyoxometalate-Based Nanomaterials toward Efficient Cancer Diagnosis and Therapy. Chemistry–A European Journal, 2021. 27(21): p. 6422-6434. [CrossRef]
- Bijelic, A., M. Aureliano, and A. Rompel, Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angewandte Chemie International Edition, 2019. 58(10): p. 2980-2999. [CrossRef]
- Cao, Z., et al., Recent advances in synthesis and anti-tumor effect of organism-modified polyoxometalates inorganic organic hybrids. Inorganic Chemistry Communications, 2021. 134: p. 108904. [CrossRef]
- Jilsha, G. and V. Viswanad, Nanosponges: A novel approach of drug delivery system. Int J Pharm Sci Rev Res, 2013. 19(2): p. 119-123.
- Thakre, A., Y. Gholse, and R. Kasliwal, Nanosponges: a novel approach of drug delivery system. J Med Pharm Allied Sci, 2016. 78(92): p. 78.
- Jagtap, S.R., et al., Nanosponges: a novel trend for targeted drug delivery. Journal of drug delivery and therapeutics, 2019. 9(3-s): p. 931-938. [CrossRef]
- Bhowmik, H., et al., Nanosponges: A review. International journal of applied pharmaceutics, 2018: p. 1-5.
- Iravani, S. and R.S. Varma, Nanosponges for Drug Delivery and Cancer Therapy: Recent Advances. Nanomaterials, 2022. 12(14): p. 2440. [CrossRef]
- Raj, S., et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. in Seminars in cancer biology. 2021. Elsevier. [CrossRef]
- Betzer, O., et al., The effect of nanoparticle size on the ability to cross the blood–brain barrier: an in vivo study. Nanomedicine, 2017. 12(13): p. 1533-1546. [CrossRef]
- Torchilin, V., Tumor delivery of macromolecular drugs based on the EPR effect. Advanced drug delivery reviews, 2011. 63(3): p. 131-135. [CrossRef]
- Parmar, K., J. Patel, and Y. Pathak, Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles, in Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems. 2022, Springer. p. 261-272.
- Kurpiers, M., et al., Zeta potential changing nanoemulsions based on phosphate moiety cleavage of a PEGylated surfactant. Journal of Molecular Liquids, 2020. 316: p. 113868. [CrossRef]
- Kamble, S., et al., Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. ChemistrySelect, 2022. 7(1): p. e202103084.
- Larsson, M., A. Hill, and J. Duffy, Suspension stability; why particle size, zeta potential and rheology are important. Annual transactions of the Nordic rheology society, 2012. 20(2012): p. 6.
- Albanese, A., P.S. Tang, and W.C. Chan, The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual review of biomedical engineering, 2012. 14: p. 1-16. [CrossRef]
- Zein, R., W. Sharrouf, and K. Selting, Physical properties of nanoparticles that result in improved cancer targeting. Journal of Oncology, 2020. 2020. [CrossRef]
- Wang, W., et al., Coassembled Chitosan–Hyaluronic Acid Nanoparticles as a Theranostic Agent Targeting Alzheimer’s β-Amyloid. ACS Applied Materials & Interfaces, 2021. 13(47): p. 55879-55889.
- Gumerova, N.I. and A. Rompel, Polyoxometalates in solution: speciation under spotlight. Chemical Society Reviews, 2020. 49(21): p. 7568-7601. [CrossRef]
- Morgulchik, N. and N. Kamaly, Meta-analysis of In Vitro Drug-Release Parameters Reveals Predictable and Robust Kinetics for Redox-Responsive Drug-Conjugated Therapeutic Nanogels. ACS Applied Nano Materials, 2021. 4(5): p. 4256-4268. [CrossRef]
- Qin, H., et al., Preparation and properties of lambda-cyhalothrin/polyurethane drug-loaded nanoemulsions. RSC advances, 2017. 7(83): p. 52684-52693. [CrossRef]
- Arulmozhi, V., K. Pandian, and S. Mirunalini, Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids and Surfaces B: Biointerfaces, 2013. 110: p. 313-320. [CrossRef]
- Mady, F.M. and M.A. Shaker, Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. International Journal of Nanomedicine, 2017: p. 7405-7417. [CrossRef]
- Kumar, A., et al., Synthesis of β-boswellic acid derivatives as cytotoxic and apoptotic agents. Bioorganic & medicinal chemistry letters, 2016. 26(1): p. 76-81. [CrossRef]
- Baharara, J., et al., Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna journal of medical biotechnology, 2016. 8(2): p. 75.
- Mane, S.D. and A.N. Kamatham, Ascorbyl stearate stimulates cell death by oxidative stress-mediated apoptosis and autophagy in HeLa cervical cancer cell line in vitro. 3 Biotech, 2019. 9(3): p. 115. [CrossRef]
- Skehan, P., et al., New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst., 1990. 82(13): p. 1107-1112.
- Al-Otaibi, W.A., M.H. Alkhatib, and A.N. Wali, Cytotoxicity and apoptosis enhancement in breast and cervical cancer cells upon coadministration of mitomycin C and essential oils in nanoemulsion formulations. Biomedicine & Pharmacotherapy, 2018. 106: p. 946-955. [CrossRef]
- Shah, H.S., et al., Preparation, characterization, and pharmacological investigation of withaferin-A loaded nanosponges for cancer therapy; in vitro, in vivo and molecular docking studies. Molecules, 2021. 26(22): p. 6990. [CrossRef]
- Shah, H.S., et al., Fabrication and Evaluation of Anticancer Potential of Eugenol Incorporated Chitosan-Silver Nanocomposites: In Vitro, In Vivo, and In Silico Studies. AAPS PharmSciTech, 2023. 24(6): p. 168. [CrossRef]
- Vijayan, S., K. Divya, and M. Jisha, In vitro anticancer evaluation of chitosan/biogenic silver nanoparticle conjugate on Si Ha and MDA MB cell lines. Applied Nanoscience, 2020. 10: p. 715-728. [CrossRef]
- Allaoui, A., et al., Protein hydrolysates from fenugreek (Trigonella foenum graecum) as nutraceutical molecules in colon cancer treatment. Nutrients, 2019. 11(4): p. 724. [CrossRef]
- Almalki, D.A. and D.M. Naguib, Anticancer activity of aqueous fenugreek seed extract against pancreatic cancer, histological evidence. Journal of Gastrointestinal Cancer, 2022. 53(3): p. 683-686. [CrossRef]
- Kemp, J.A., et al., “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Advanced drug delivery reviews, 2016. 98: p. 3-18.
- Habib, I., et al., Integrated Computational Approaches for Designing Potent Pyrimidine-Based CDK9 Inhibitors: 3D-QSAR, Docking, and Molecular Dynamics Simulations. Computational Biology and Chemistry, 2023: p. 108003. [CrossRef]
- Hamza, S., et al., 3D-QSAR, docking and molecular dynamics simulations of novel Pyrazolo-pyridazinone derivatives as covalent inhibitors of FGFR1: a scientific approach for possible anticancer agents. Journal of Biomolecular Structure and Dynamics, 2023: p. 1-15. [CrossRef]
- Khalid, A., et al., Phytochemical, biological, and in-silico analysis of Colutea armata Hemsl. & Lace.: A possible source of bioactive natural compounds. South African Journal of Botany, 2023. 158: p. 133-141. [CrossRef]
- Raza, A., et al., Molecular modeling of pyrrolo-pyrimidine based analogs as potential FGFR1 inhibitors: A scientific approach for therapeutic drugs. Journal of Biomolecular Structure and Dynamics, 2023: p. 1-14. [CrossRef]
- Rehman, K., et al., Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. Journal of cellular biochemistry, 2019. 120(1): p. 425-438. [CrossRef]
- Saleem, H., et al., Biochemical, Toxicological, and in-silico Aspects of Trillium govanianum Wall. ex D. Don (Trilliaceae): A Rich Source of Natural Bioactive Compounds. Chemistry & Biodiversity, 2023: p. e202301375. [CrossRef]
- Çankaya, N., M. İzdal, and S.Y. Azarkan, Synthesis, Characterization, Biological Evaluation and Molecular Docking Studies of New Oxoacrylate and Acetamide on HeLa Cancer Cell Lines. Current Computer-Aided Drug Design, 2021. 17(6): p. 838-848. [CrossRef]
- Mahnashi, M.H., et al., Cytotoxicity, anti-angiogenic, anti-tumor and molecular docking studies on phytochemicals isolated from Polygonum hydropiper L. BMC complementary medicine and therapies, 2021. 21: p. 1-14. [CrossRef]
- Chohan, T.A., et al., Molecular modeling studies to characterize N-phenylpyrimidin-2-amine selectivity for CDK2 and CDK4 through 3D-QSAR and molecular dynamics simulations. Molecular BioSystems, 2016. 12(4): p. 1250-1268. [CrossRef]
- Chohan, T.A., et al., Molecular simulation studies on the binding selectivity of 2-anilino-4-(thiazol-5-yl)-pyrimidines in complexes with CDK2 and CDK7. Molecular BioSystems, 2016. 12(1): p. 145-161. [CrossRef]
- Chohan, T.A., et al., Phytochemical profiling, antioxidant and antiproliferation potential of Euphorbia milii var.: Experimental analysis and in-silico validation. Saudi Journal of Biological Sciences, 2020. 27(11): p. 3025-3034. [CrossRef]
- He, F., et al., Identification of potential ATP-competitive cyclin-dependent kinase 1 inhibitors: De novo drug generation, molecular docking, and molecular dynamics simulation. Computers in Biology and Medicine, 2023. 155: p. 106645. [CrossRef]
- Pushpalatha, R., S. Selvamuthukumar, and D. Kilimozhi, Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-Physicochemical characterization, drug release, stability and cytotoxicity. Journal of drug delivery science and technology, 2018. 45: p. 45-53.
- Varan, C., et al., Preparation and characterization of cyclodextrin nanosponges for organic toxic molecule removal. International Journal of Pharmaceutics, 2020. 585: p. 119485. [CrossRef]
- Mohamed, N., Synthesis of hybrid chitosan silver nanoparticles loaded with doxorubicin with promising anti-cancer activity. BioNanoScience, 2020. 10(3): p. 758-765. [CrossRef]
- Shah, H.S., et al., Preparation and characterization of anticancer niosomal withaferin–A formulation for improved delivery to cancer cells: In vitro, in vivo, and in silico evaluation. Journal of Drug Delivery Science and Technology, 2020. 59: p. 101863.
- Shah, H.S., et al., Synthesis of chitosan-coated polyoxometalate nanoparticles against cancer and its metastasis. RSC advances, 2015. 5(113): p. 93234-93242. [CrossRef]
- Vichai, V. and K. Kirtikara, Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature protocols, 2006. 1(3): p. 1112-1116. [CrossRef]
- Priyadarsini, R.V., et al., The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. European journal of pharmacology, 2010. 649(1-3): p. 84-91.
- Lin, G.-J., et al., Cytotoxicity, apoptosis, cell cycle arrest, reactive oxygen species, mitochondrial membrane potential, and Western blotting analysis of ruthenium (II) complexes. JBIC Journal of Biological Inorganic Chemistry, 2013. 18: p. 873-882.
- Jain, A.N., Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. Journal of medicinal chemistry, 2003. 46(4): p. 499-511. [CrossRef]
- Powell, M.J., A fast algorithm for nonlinearly constrained optimization calculations, in Numerical analysis. 1978, Springer. p. 144-157.
- Brown, N.R., et al., CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nature communications, 2015. 6(1): p. 6769. [CrossRef]
- Takaki, T., et al., The structure of CDK4/cyclin D3 has implications for models of CDK activation. Proceedings of the National Academy of Sciences, 2009. 106(11): p. 4171-4176.
- Wood, E.R., et al., A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib) relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer research, 2004. 64(18): p. 6652-6659.
- Ghersi, D. and R. Sanchez, Beyond structural genomics: computational approaches for the identification of ligand binding sites in protein structures. J Struct Funct Genomics, 2011. 12(2): p. 109-117. [CrossRef]
- Onufriev, A., D. Bashford, and D.A. Case, Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins: Structure, Function, and Bioinformatics, 2004. 55(2): p. 383-394. [CrossRef]
- Jain, A.N., Scoring noncovalent protein-ligand interactions: a continuous differentiable function tuned to compute binding affinities. Journal of computer-aided molecular design, 1996. 10(5): p. 427-440. [CrossRef]
- Co-operation, O.f.E. and Development, Guideline for Testing of Chemicals n. 423: Acute Oral Toxicity. 2001, OECD Paris, France.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).