1. Introduction
Some of the restorative substances functions as the reservoir of fluoride and releases the fluoride gradually to the oral cavity to prevent secondary caries and restoration failure[
1]. This study could be effectively used for developing the improved regimes for the delivery of topical fluoride. The influence of temperature, time, and storage conditions on fluoride release followed by the recharge of restorative materials such as silorane based composite and Methacrylate based composite[
2]. Methacrylate based composite (MBC) resins are effectively employed as an esthetic restorative material[
3]. Methacrylate composites are a blend of silinated inorganic filler, monomeric resin matrix, polymerization initiator and inhibitors to prevent premature polymerization during storage and color pigments[
4]. Methacrylate resins present with a polymerization shrinkage of 2-3% by volume. Clinically this leads to marginal intensity loss, microleakage, debonding, micro cracks and post-operative sensitivity. The associated complications might be solved by altering the polymerization chemistry[
5]. One such alternative is the development of innovative silorane based composite (SBC) resin. The silorane composite that includes the ring opening monomer with the oxirane and silorane structural moieties ensures low shrinkage in the polymerization of greater than 1 % that improves the biocompatibility, optical properties and resistance[
6]. The cationic ring opening mechanism of the cyclic epoxides is needed for the development of new composite (dental) with lower shrinkage [
7].
Secondary caries causes restoration failure which remains as an inevitable issue in the restorative dental field. The fluoride promotes oral health by prevention of dental caries in all age groups[
8]. On the restoration of a specific tooth, those sites act as fluoride reservoir and release fluoride in a sustained manner into the oral fluids and hence the prevention of dental caries. The fluoride ion release from the restoration materials could be a greater advantage due to that the fluoride forms complexes with adjacent enamel or dentine, thereby preventing demineralization. The performance of restorative material under the circumstances of differing temperature, storage and time that induces the conditions of oral cavity that influences the fluoride release. Fluoride releasing restorative materials has adequate mechanical and esthetics properties, but low fluoride release and recharge abilities. Hence the anti-cariogenic property of present fluoride-releasing restorative materials is uncertain.
[
9] compared the parameters on fluoride release (FR) and recharge of esthetic restorative materials compomer, MBC, SBC and glass ionomer cement (GIC). Fluoride re-release was found to be more in GIC and in Dyract AP when compared with the distilled water. Further it was found to be more significant at high temperature and at the duration of three weeks.
[
10] determined the impact of glass ionomer resin and adhesive systems on the FR and concurrent pH changes over 168 days. This study investigated cention, alkasite, giomer and filtek. L Fluoride release differs among the analysed materials and depended on the utilization of coatings and dental adhesives. Further the study observed that the pH of all the substances, time points and coating types differed significantly.
[
11] evaluated the impact of powder-to-liquid (PLR) ratio on the setting time, compressive strength, fluoride release on SPG. Increased PLR and decreased cumulative fluoride release has been observed in the study. These comparative results demonstrated that the increasing PLR, and decreasing setting time, compressive strength and fluoride release of the restorative materials.
[
12]investigated the impacts of radiotherapy on the chemical, mechanical and surface properties of the restorative materials like an alkasite and a glass hybrid. The study found no statistical differences and only a minor changes has been found in terms of discoloration.
[
13] compared the FR from GIC, and other four restorative materials. The study used ANOVA, post hoc test to determine the comparative analysis. Accordingly the study also found that alkasite is much better than other materials.
The following limitations are found among the existing studies, the clinical case customized implementation were not effectively performed. Likewise the longevity of the material were generally not assessed. The effect of storage were not critically investigated by the existing studies. The challenge has been associated with the low mechanical, compressive strength of the material.
A drastic reduction in fluoride release is observed in a restorative material generally after three days. This feature can be partially counteracted by recharging with topical fluorides or fluoridated dentifrice[
14]. But the recharge differs among various class of fluoride releasing materials[
15]. Hence the fluoride release and recharge becomes more important[
16]. The major objective of the study is to analyze and compare the impact of time, temperature and storage on the Fluoride release of MBC in comparison with SBC.
2. Materials and Methods
The present study made a comparative analysis of the recharge capacities and the amounts of fluoride release in the distilled water and artificial saliva at 4, 37, 55°C of MBC in comparison with MBC over the time period of 1, 7, 14, and 28 days.
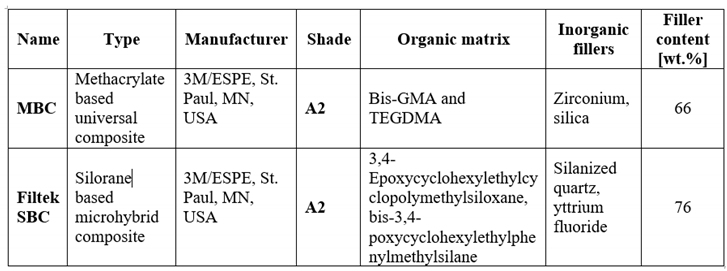
Table 1 specifies the information regarding the composites in the current study.
2.1. Test Specimen Preparation
An individual operator developed all the samples to decrease the variability among them. Teflon dimension molds of 10mm diameter x 2mm thickness has been employed for the preparation of samples. The unprocessed substances were overfilled intentionally into the mold and being sandwiched in between the glass slide and the transparent matrix strip. The excessive materials are being expelled with the light force on the mold followed by the light curing through the up and down for the suggested duration. The intensity of the light caring units had been calibrated before polymerization of all the samples by Hilux curing of light meter. The processed samples were being conditioned at 100% relative humidity for a period of 24 hours at 37°C.
2.2. Release of Flouride
Sixty samples of MBC and SBC were prepared separately. 30 samples were immersed in artificial saliva of 30 ml test tube or 20 ml of distilled water. These samples are subdivided further into three groups consisting 10 in each group and stored at 4 °C in the refrigerator, 55°C in the water bath and 37°C in the incubator respectively for a period of 24 hours. The samples are then removed after 24 hours and being washed with the distilled water and then consequently dried with blotting paper before transforming to a fresh jar comprising artificial saliva or 20 ml of the distilled water.
This procedure was found to be continued for 7, 14, and 28 days of the time interval. The solutions at the end of the first, seventh, fourteenth, and twenty-eighth days were collected. The release of fluoride release has been determined with the fluoride-sensitive electrode. Orion TM 2109 XP Fluoride Analyzer has been connected to the Orion Ion Analyser by the addition of a 1:1 quantity of TISAB solution. For every sample, the analysis has been performed three times and to obtain accurate results, the mean value has been considered.
The comparison between the immersion media has been processed with the student T test and the comparison of time, materials, and temperatures was performed with Tukey’s postoc and ANOVA. These data are derived by the mean value by considering the significant p-value.
2.3. Recharge of Fluoride
The individual specimen has been recharged at a speed of 5000 ppm of neutral NaF solution for a period of five minutes and then repeated for 3 weeks. After every episode of recharge. The re-release of fluoride has been estimated at a period of one, three and seven days. The comparison was performed with the student’s t test with immersion media. Tukey posthoc and ANOVA was done for revealing the comparative analysis between the time interval and materials. The data has been represented as mean and standard deviation with significant.
3. Results
3.1. For Fluoride Release
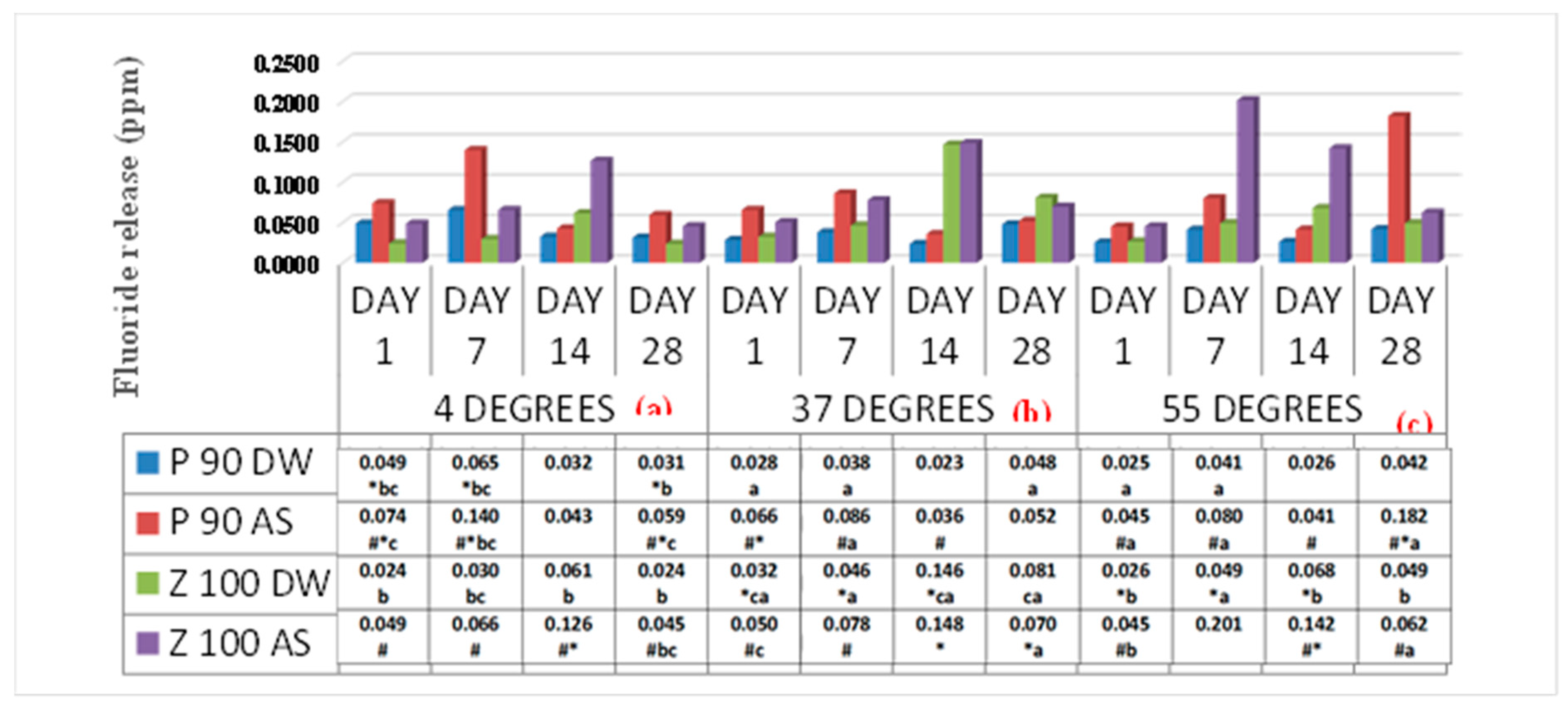
Comparison between immersion media (distilled water and artificial saliva) (
Figure 1) shows fluoride release was considerably higher (<0.001) in artificial saliva than distilled water. Further, at a lower temperature in artificial saliva, fluoride release was more significant in SBC. As the temperature increased, MBC showed comparatively higher fluoride release. Also, in comparison to the time, in artificial saliva, day 7 was high in SBC, whereas day 14 was highest in MBC.
Comparison between materials (SBC and MBC) irrespective of immersion media (
Figure 1) shows that at lower temperatures, SBC releases higher fluoride than MBC, but at higher temperature fluoride release was greater in MBC.
3.2. For Fluoride Recharge
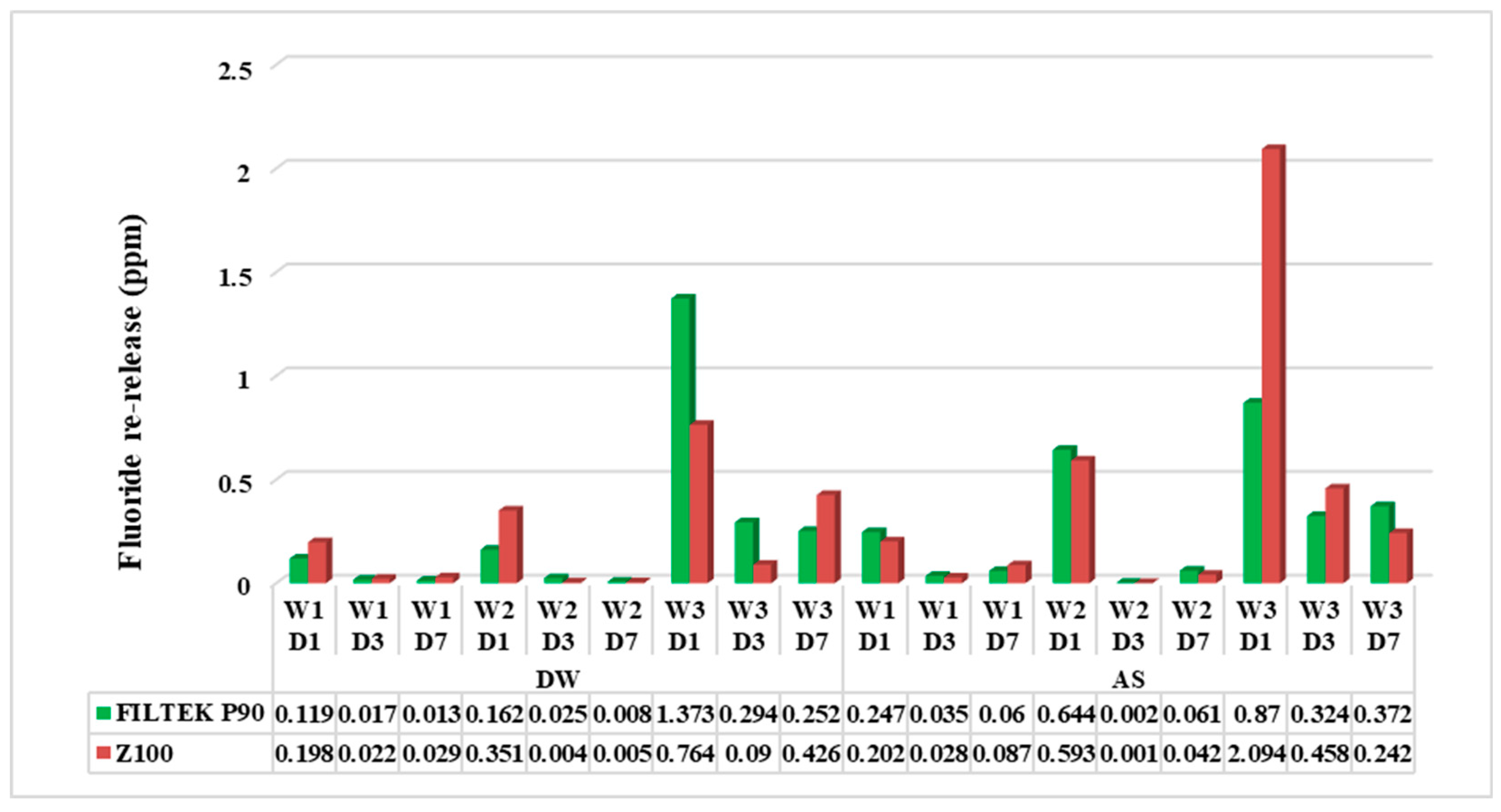
Comparison between immersion media (distilled water and artificial saliva) shows fluoride re-releasee was significantly higher (<0.001) in artificial saliva than distilled water. Further, the difference in fluoride reuptake (after recharge) in artificial saliva during the weekly recharge showed re-release progressively increased from week 1 to week 3. Also, in comparison to single days at the weekly recharge, day 1 showed higher release than day 3, and day 7 was the least.
Comparison between materials (SBC and MBC) irrespective of immersion media shows that MBC re-releases higher fluoride than SBC, in the first week and third week, whereas the re-release in the second week SBC was greater. The fluoride re-release significantly dropped from day 1 to day 3, but there was no significant difference from day 3 to day 7.
The fluoride release at low temperatures in artificial saliva SBC had a more significant release when compared to MBC. Fluoride release increased on day 7 but decreased over time. Fluoride re-release was greater in MBC than in SBC, and it increased with time.
4. Discussion
A rise in demand for esthetics oriented treatment from patients has revolutionized the market of esthetic materials. The release of fluoride from restoratives prevents caries, enhance remineralization, and provide an anti-cariogenic effect. The upsurge in the concentration of fluoride ions in and around restorations is beneficial for patients with a high caries index. The fluoride reservoirs and its ability to release ions are imperative for governing the success of a topical treatment. The fluoride replaced from the milieu is re-released to the neighboring tooth structure[
17]. The restorative material thus recharged by fluoride acts as a possible reserve for future release into the oral environment.
This study [
18]evaluated the in vitro fluoride release and recharge potential of SBC and MBC, as shown in
Table 1, over an extended period. The fluoride release and recharge of SBC and MBC were low. The values possibly signified the residual fluoride over the sample surface following the recharge and wash sequence. The concluding outcome could be related to cumulative changing of experimental conditions. It can be associated with manipulation technique, varying sample area exposure, the weight of the specimens, type of storage media, form, and concentration of fluoride recharging vehicle.
A material can leach ions from the mass that have been penetrated by water. During water penetration through diffusion, the surface layers will be more saturated than the inner mass[
19]. This penetration of water is different for different materials, depending on the permeability of materials. The diffusion of fluoride ions through a polymer matrix of composites is more demanding than glass ionomer cement. Glass ionomer cement is highly permeable and attains saturation quickly, resulting from the dissolution of the soluble fraction of material. The low fluoride release may be associated with the fluoride taken up by the surface[
20].
The current investigation utilization various temperature to check whether an increased surrounding temperature influences positively the recharge and release of fluoride of the restoratives. The thermal circuits formed due to extensive temperature variations in the oral cavity may result in questionable longevity of the restorative materials.
The outcomes of the present study suggested that the rise in the temperature increase the recharge capacities and fluoride release in both MBC and SBC. Further the treatment of the fluoride with the restorative materials at an increased temperature is recommended clinically to enhance the ability of recharge. Such kind of treatment could be effectively used for the development of the regimes for the delivery of topical fluoride.
The test medium also plays a major role in the release of fluoride. Certain authors used distilled water as the testing medium. It has been depicted that the release of fluoride differs in artificial saliva than the distilled water. Further the utilization of artificial saliva offers test conditions that are equivalent to oral conditions. Hence the current study test with artificial saliva. The results depicted that more amount of fluoride content was released than distilled water.
That suggested the effective utilization of the restorative materials that can be demonstrated by pH of dissolving medium. The studies represented that acidic pH testing increases the release of fluoride. The saliva investigated in the study possess pH range of 5.3 to 5.5 which explains the high release of fluoride in distilled water and artificial saliva.
The recharge capacity of the fluoride is highly significant than the release of fluoride alone. The regular contact with the restorative substances to the topical fluorides like APF and NaF or stannous fluoride in the in mouthwash or dentifrice recharges potentially, the fluoride material[
21]. In the current analysis, the NaF has been utilized for recharging the vehicle which is the common ingredient in the mouthwash and the dentrifrice. The standardized concentration of the F is generally 1000 parts/106. This study used 500 parts /106 F for the determination of fluoride recharge effectiveness at very low concentrations. The fluoride concentration replenishes at 0.2% NaF solution since the increased fluoride release after the exposure to sodium Fluoride was confined to the retained fluoride in the pore surface. These findings were in relation to the increased re-release from the first week to the third week, which suggested the topical applications of F. This will enable the prevention of caries and restoration failure as presented in
Figure 2. MBC and SBC will not differ considerably the suggested frequent application of the fluoride in the required composites. Several factors impact the material permeability, utilized concentration used, and the form of the substance[
22]. When dealing with the high permeable material, absorption and desorption of the F could occur at increased concentrations in comparison with the more permeable substances.
5. Conclusions
Results suggest that fluoride-containing dental materials had long term sustained fluoride releasing capacity. The selection of a material to be employed for a patient with high caries index must be constructed on the release values of a specific material than anticipated from the general, restorative materials. Composite needs more intermittent and more frequent application of fluoride for active release. The fluoride recharging and re-release capacity of a substance can be improved by the use of topical fluorides at higher temperatures. Topical fluoride application at low oral environment temperature should not be performed. The fluoride release is different for each material. The tooth brushing habits of the general population generally involves a fluoridated toothpaste. This habit will serve as an advantage by being a self-administered recharge regimen for secondary caries prevention. Further research can be taken to investigate the fluoride-releasing and recharging ability and the performance of composites in a clinical setting and planned in vivo studies. The future suggestion of the work deals with the investigation of more number of restorative materials. Further other than time, temperature and storage conditions, several other factors such as compressive strength has to be investigated.
Author Contributions
Conceptualization, Methodology, Validation and Writing of Original Draft, Prashanthi S Madhyastha, Validation, Data Curation and Supervision, Dilip G Naik, Software, Validation and Formal Analysis, Srikant N, Writing, Review and Editing, K. Rachel Sarah Vinodhini.
Funding
This research received no external funding
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors are thankful to the Department of Chemistry, NITK Suratkal for permitting them to utilize the instruments and other chemicals for the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SBC |
Silorane Based Composites |
| MBC |
Methacrylate Based Composites |
| GIC |
Glass Ionomer Cement |
| TISAB |
Total Ionic Strength Adjustment Buffer |
References
- Dhananjaya, K.; Chakraborty, M.; Vadavadagi, SV.; Sinha, G.; Verma, T.; Deb, S. A Scanning Electron Microscope Evaluation of the Efficacy of Different Fluoride-releasing Dental Restorative Materials to Prevent Enamel Demineralization: An In Vitro Study. J Contemp Dent Pract 2021, 22, 1292–1296. [Google Scholar] [CrossRef]
- Madhyastha, PS.; Bhat, KM.; Padma, D.; Bangera, MK.; Naik, DG.; Srikant, N. Cytotoxicity of Silorane and Methacrylate based Dental Composites on Human Pulp Cells. Journal of Orofacial Sciences 2021, 13, 13. [Google Scholar] [CrossRef]
- Ilie, N. Degradation of Dental Methacrylate-Based Composites in Simulated Clinical Immersion Media. J. Funct. Biomater 2022, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, RK.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: characteristics and future perspectives. Jpn Dent Sci Rev 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Vouzara, T.; Roussou, K.; Nikolaidis, A.K.; Tolidis, K.; Koulaouzidou, E.A. Organic Eluates Derived from Intermediate Restorative Dental Materials. Molecules 2020, 25, 1593. [Google Scholar] [CrossRef] [PubMed]
- Rohaninasab, M.; Behniafar, B.; Merrikh, F. Evaluation of the Effect of Water on the Flexural and Shear Strength of Silorane and Methacrylate-Based Composites; A Review of Long-Term Studies. Journal of Pharmaceutical Negative Results 2022, 822–828. [Google Scholar]
- Maru, VP.; Kulkarni, P.; Chauhan, R.; Bapat, SS. Evaluation and comparison of silorane resin composite to glass ionomer in occluso-proximal restorations of primary molars: A randomized controlled trial. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2022, 40, 281–287. [Google Scholar] [CrossRef]
- Hollanders, A.C.C. Closing the gap: Modifying factors in secondary caries. Radboud Institute for Health Sciences, Nijmegen Netherlands, 27th June 2022.
- Madhura, S.; Madhyastha, PS.; Bangera, MK.; Natarajan, S.; Kotian, R. Effect of Time, Temperature, and Storage on Fluoride Release and Recharge of Esthetic Restorative Materials. Journal of Orofacial Sciences 2021, 13, 90–95. [Google Scholar]
- Kelić, K.; Par, M.; Peroš, K.; Šutej, I.; Tarle, Z. Fluoride-Releasing Restorative Materials: The Effect of a Resinous Coat on Ion Release. Acta Stomatol Croat. 2020, 54, 371–381. [Google Scholar] [CrossRef]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Angkananuwat, C.; Krajangta, N.; Panthumvanit, P. The effect of powder to liquid ratio on physical properties and fluoride release of glass ionomer cements containing pre-reacted spherical glass fillers. Dent Mater J. 2020, 39, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Turjanski, S.; Par, M.; Bergman, L.; Soče, M.; Grego, T.; Klarić Sever, E. Influence of Ionizing Radiation on Fluoride-Releasing Dental Restorative Materials. Polymers 2023, 15, 632. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Rashmi, S.; Pai, S.; Kini, S. Comparative Evaluation of Fluoride Release From Two Different Glass Ionomer Cement and a Novel Alkasite Restorative Material - An in Vitro Study. Pesquisa Brasileira em Odontopediatria e Clínica Integrada 2020, 20, 1590. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, KL. Secondary caries: prevalence, characteristics, and approach. Clinical oral investigations 2020, 24, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Eskandarizadeh, A. ; Mohammadzadeh, I, ; Shahravan, A, ; Bavafa, M, ; Kakooei, S, ; Torabi, M. Prevention of secondary caries by a new antibacterial compound. Dental Research Journal 2020, 17, 40–47. [Google Scholar] [PubMed]
- Al Rabiah, A.; Zahrah, A.; Malath, T.; Ebtihal, A. D.; Daniyah, A. S.; Abdullah, A. Q. Dental Composite Restorations Repair: A Systematic Review and Meta-Analysis. J. Pharm. Res. Int. 2021, 33, 707–738. [Google Scholar] [CrossRef]
- Kumari, PD.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, CR. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. Journal of oral biology and craniofacial research 2019, 9, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Makkai; Lorand, Z.; Szekely, M.; Fazakas, Z.; Nagy, LM.; Barta, KM.; Mathe, BK. Fluoride Release and Uptake Capability of Glass-ionomer Cements and Compomers Used as Dental Restorative Materials. Materiale Plastice 2019, 56, 548–53. [Google Scholar] [CrossRef]
- Hadi, MR. Effect of Increased Fluoride Contents on Fluoride Release from Glass Ionomer Cements. Systematic Reviews in Pharmacy 2020, 11, 440–443. [Google Scholar]
- Braden, M.; Clarke, RL. Water absorption characteristics of dental microfine composite filling materials: I. Proprietary materials. Biomaterials 1984, 5, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Jabin, Z.; Nasim, I.; Vishnu Priya, V. Quantitative Analysis and Effect of SDF, APF, NaF on Demineralized Human Primary Enamel Using SEM, XRD, and FTIR. Int J Clin Pediatr Dent 2021, 14, 537–541. [Google Scholar] [CrossRef]
- Yan, IG.; Zheng, FM.; Gao, SS.; Duangthip, D.; Lo, ECM.; Chu, CH. Fluoride Delivered via a Topical Application of 38% SDF and 5% NaF. International Dental Journal 2022, 72, 773–778. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).