1. Introduction
Dental restorative procedures are based on the use of different techniques, classified as direct and indirect, and materials, depending on the specific clinical needs.
The main objective is to restore reduced masticatory function. The most used materials for direct methods are resin-based composites, which are used through adhesive techniques and performed in both incremental layering and bulk-fill modes [
1].
However, in recent years, some of the research on new dental materials has focused on bioactive materials that not only restore masticatory function but also interact with the hard tissues of teeth and play an important role in maintaining long-term dental health. This is because these materials, such as glass ionomer cements and alkasites, are designed to promote the remineralization of dental tissues and inhibit the formation of caries through active biological mechanisms. [
2]
Recent studies have shown that alkasites release significant amounts of fluoride (F-) and calcium (Ca²⁺) ions. This release contributes to restoring the integrity of the tooth enamel and balancing the pH of the oral environment. [
3] In addition, this mechanism also ensures stable preservation of the microenvironment around the restoration remains stable and protective and elevates the material's ability to resist demineralization and contributing to long-term oral health. [
4] Other studies have shown that glass ionomer cements also support the remineralization of demineralized dentin by releasing ions through the biomineralization process. Recent results have shown the formation of carbonate apatite with an increase in the Ca/P ratio. [
5] This process occurs because glass ionomer cements have a chemical composition that acts as a buffer against the pH of the surrounding fluid. Indeed, when exposed to an acidic environment, as in caries, these materials neutralize the acidity and stabilize the pH to a weak acid. This alkalization may be related to the release of various ions by these materials, which due to their chemical properties can reduce the acidity of the environment [
6].
Demineralization, which plays a fundamental role in the development of primary and secondary caries, can be prevented by increasing the pH through the release of high concentrations of ions in acidic environments. This process reduces the amount of free hydrogen ions and helps to neutralize acidity. [
7] Therefore, the scientific literature has shown that bioactive materials can induce the formation of apatite and help stabilize the pH in conditions where the oral cavity becomes acidic, such as in caries development. This property, therefore, strengthens the resistance of the enamel to demineralization processes. [
8]
For these reasons, the release of ions such as calcium (Ca²⁺), fluoride (F-), hydroxide (OH-), strontium (Sr²⁺) and phosphate (PO₄³-) by bioactive substances is crucial in the prevention of dental caries.
Fluoride is particularly advantageous because its long-term sustained release has a significant effect on secondary caries formation. Glass ionomer cements have the property of prolonged fluoride release by ion exchange mechanism [
9]. Indeed, the release of fluoride from such bioactive materials contributes to increased resistance to acids by promoting the formation of fluorapatite on the tooth surface, which is less soluble than hydroxyapatite [
10].
There is literature showing that even at very low concentrations, it is effective in inhibiting enamel demineralization and promoting remineralization, especially in cycles of pH variation, where the presence of fluoride is essential and contributes to slowing the progression of caries [
11]. In this context, it has been shown that fluoride-containing bioactive glasses can have excellent synergistic interaction with calcium and phosphate ions. This synergistic effect is important because it contributes to the formation of a protective layer under acidic conditions, thereby further increasing biological activity. [
12]
Strontium is an element that has been shown to be useful in the treatment of dentine hypersensitivity and contributes to caries control. This alkaline earth metal has similar properties to calcium. Therefore, it can be used as a substitute for enamel formation. [
13] Indeed, the conversion of hydroxyapatite to strontium apatite has demonstrated its ability to increase the resistance of teeth to acid [
14].
Furthermore, the substitution of strontium in remineralizing composites not only helps in the treatment of dentine hypersensitivity, but may also confer antibacterial properties, thus reducing the risk of caries through the prolonged release of ions. [
15] In fact, scientific studies have shown that these cations reduce acid production by
Streptococcus mutans in bio membranes by increasing the pH of the surrounding environment [
16].
The release of cations such as calcium by bioactive substances is of great importance as it helps to reduce material loss, decrease demineralization events and prolong the remineralization time [
17]. In addition, since calcium ions act as stabilizing agents within the demineralized collagen matrix, they are an essential component of maintaining mineral homeostasis in dental tissue. [
18]. Very small amounts of calcium are required to create a saturated environment conducive to mineral precipitation [
19]. In fact, calcium is also added to resin-based composite resins due to its ability to release minerals that remineralize the tooth structure [
20]. Minimal changes in calcium concentration have a greater effect than similar changes in phosphate concentration. Furthermore, in vitro experiments have shown that calcium is approximately 20 times more effective than phosphate in inhibiting enamel dissolution, suggesting that calcium ion concentration is a limiting factor in enamel remineralization [
21].
The release of hydroxyl ions is also very beneficial, contributing significantly to lowering the pH and acting as an alkalizing agent [
22]. Scientific studies have also shown that a decrease in pH increases the likelihood of fracture of dental tissue and reduces mechanical properties. [
23] The acidic environment exacerbates microleakage and can lead to an increased presence of cariogenic bacteria, compromising the structural integrity of the dental material and surrounding dental tissue. [
24]
Furthermore, these ions have antimicrobial effects through inhibition of bacterial enzyme systems [
25]. Studies have shown that hydroxyl ions can alter the integrity of bacterial cytoplasmic membranes, disrupting both lipid structure and nutrient transport. These ions break down lipopolysaccharides in the cell walls of Gram-negative bacteria, contributing to the reduction of toxins and inflammation. [
26]
Phosphate ions play an important role in dentin remineralization, binding to calcium and promoting apatite formation. To facilitate this process, it is fundamentally important that the materials used for restoration release mineral ions such as calcium and phosphate and act as a stable site for the nucleation process [
27]. The remineralization and protective effect caused by this ion release of bioactive materials is not limited to the tissue immediately after placement of the restoration, but extends to other areas of the oral cavity, such as the floor of deep cavities with marginal leakage, the margin of the restoration and non-carious Class V lesions where the cementum is exposed. Various studies have shown that. [
28] Additionally, bioactive materials that release phosphate ions can cooperate in maintaining the structural stability of the tooth and in protecting the restoration from marginal lesions, offering a long-term preventive effect against secondary caries [
29]
The aim of this study is to evaluate the short- and long-term release of fluoride (F⁻), calcium (Ca²⁺), hydroxyl (OH⁻), strontium (Sr²⁺), silicon (Si) and phosphate (PO₄³⁻) ions from different restorative materials at different pH levels and temperatures. The study aims to determine how some environmental factors, such as pH and temperature, influence the ion release properties of these materials, and how these factors influence their bioactive properties.
3. Results
3.1. pH Measurements
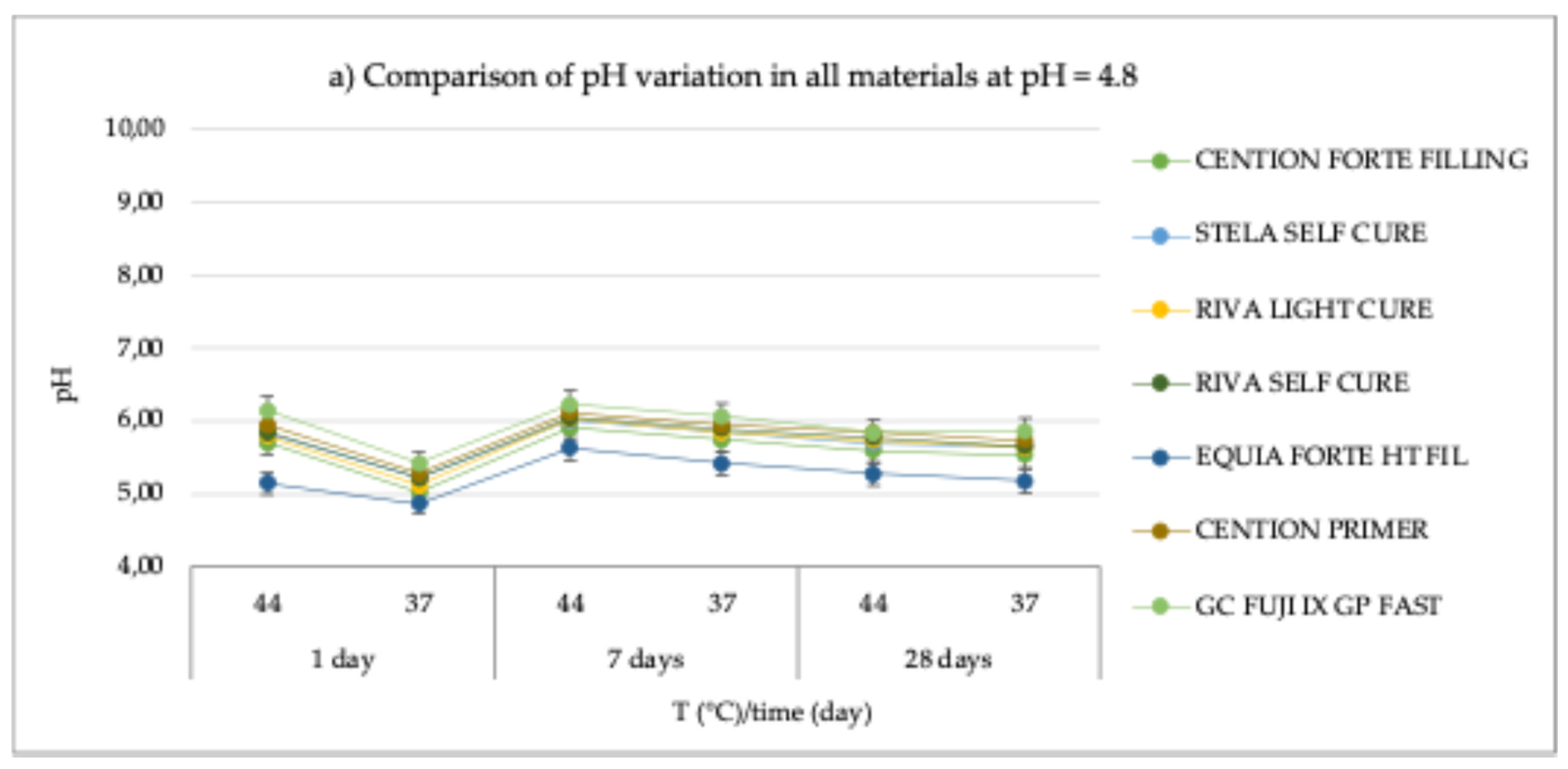
The results of the pH measurements carried out for the various materials under the established conditions (acidic, basic, and neutral pH; two temperatures: 37°C and 44°C; and three different observation times: 24 hours, 7 days, and 28 days) are detailed in
Table 2 and graphically illustrated in
Figure 1.
For all materials, a similar behavior was observed, with relatively small variations in pH values. Under acidic conditions (pH = 4.8), all materials showed an increase of ap-proximately one pH unit compared to the initial value (
Figure 1a). Specifically, for the
Cention Forte Filling Material, the pH values ranged from 5.02 ± 0.06 at 37°C after 24 hours to 5.91 ± 0.07 at 44°C after 7 days, with an average of 5.58 ± 0.31. Similarly, for
Stela Self Cure, the observed pH values varied between 5.22± 0.03 at 37°C after 24 hours and 6.01 ± 0.07 at 44°C after 7 days, with a mean value of 5.71 ± 0.27.
Riva Light Cure HV showed a range of 5.12 ± 0.04 to 6.03 ± 0.03 under the same conditions, with an average of 5.68 ± 0.31. In the case of
Riva Self Cure, the pH values were between 5.22 ± 0.07 and 6.05 ± 0.08, observed at 37°C after 24 hours and at 44°C after 7 days, respectively, yielding a mean of 5.74 ± 0.29. The
Equia Forte HT Fil material exhibited slightly lower values, ranging from 4.87 ± 0.03 at 37°C after 24 hours to 5.63 ± 0.04 at 44°C after 7 days, with an average of 5.25 ± 0.26.
Cention Primer material showed higher values, with pH measurements ranging from 5.28 ± 0.07 to 6.12 ± 0.06 under the same conditions, corresponding to an average of 5.82 ± 0.29. Finally,
GC Fuji IX GP Fast material presented the highest observed pH values, ranging between 5.42 ± 0.07 and 6.23 ± 0.04, with an average of 5.93 ± 0.29.
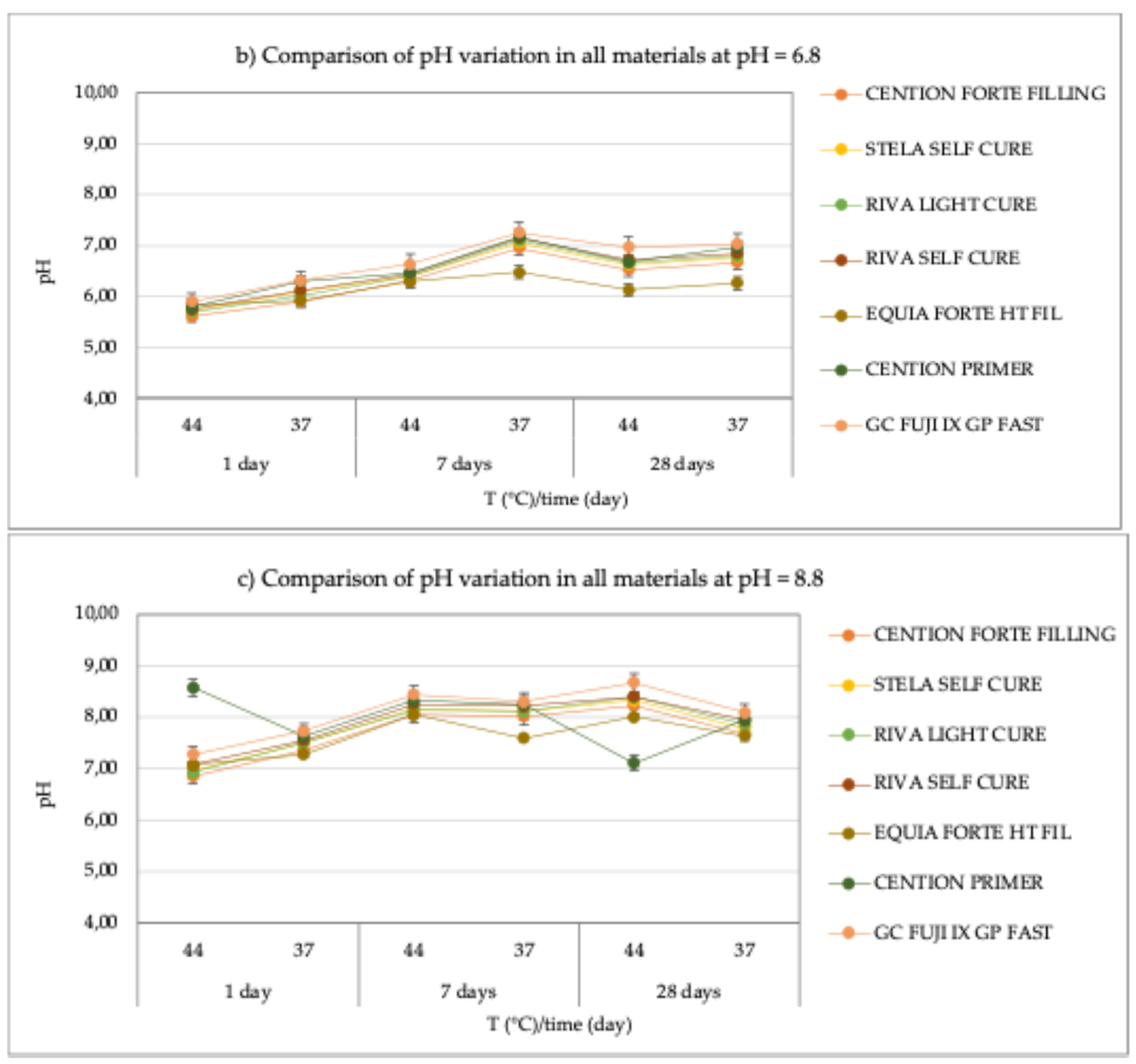
Even in a neutral environment (pH = 6.8), only small pH variations were recorded (
Figure 1b). However, unlike in the acidic environment, all materials initially showed a decrease in pH of approximately one unit during the first 24 hours, followed by a stable behavior thereafter. Specifically, for the
Cention Forte Filling Material, pH values ranged from 5.61 ± 0.08 at 44°C after 24 hours to 6.95 ± 0.08 at 37°C after 7 days, with an average value of 6.33 ± 0.50. Similarly, for
Stela Self Cure, the pH ranged from 5.71 ± 0.06 at 44°C after 24 hours to 7.05 ± 0.04 at 37°C after 7 days, resulting in an average value of 6.45 ± 0.48. The material
Riva Light Cure HV exhibited a similar trend, with pH values ranging from 5.70 ± 0.07 at 44°C after 24 hours to 7.10 ± 0.06 at 37°C after 7 days, and an average value of 6.45 ± 0.52. For
Riva Self Cure, pH values were observed between 5.75 ± 0.07 at 44°C after 24 hours and 7.15 ± 0.08 at 37°C after 7 days, giving an average value of 6.51 ± 0.51. In the case of
Equia Forte HT Fil, pH values ranged from 5.84 ± 0.04 at 44°C after 24 hours to 6.48 ± 0.04 at 37°C after 7 days, with a mean of 6.15 ± 0.24. For
Cention Primer, the pH ranged from 5.79 ± 0.03 at 44°C after 24 hours to 7.17 ± 0.08 at 37°C after 7 days, with an average value of 6.56 ± 0.49. Finally, the
GC Fuji IX GP Fast material showed pH values ranging between 5.90 ± 0.07 at 44°C after 24 hours and 7.25 ± 0.04 at 37°C after 7 days, with an average of 6.68 ± 0.51.
In a basic environment (pH = 8.8), a behavior like that observed in the acidic en-vironment was noted, with an initial decrease in pH of approximately two units during the first 24 hours, followed by a subsequent increase, returning to the starting value. Specifically, for the Cention Forte Filling Material, pH values ranged from 6.85 ± 0.04 at 44°C after 24 hours to 8.23 ± 0.04 at 44°C after 28 days, with an average value of 7.70 ± 0.52. For Stela Self Cure, pH values ranged from 6.95 ± 0.06 at 44°C after 24 hours to 8.33 ± 0.04 at 44°C after 28 days, with a mean value of 7.81 ± 0.52. Similarly, for Riva Light Cure, pH values ranged from 6.93 ± 0.08 at 44°C after 24 hours to 8.39 ± 0.07 at 44°C after 28 days, with an average value of 7.82 ± 0.53. For Riva Self Cure, pH values ranged between 7.08 ± 0.06 at 44°C after 24 hours and 8.40 ± 0.05 at 44°C after 28 days, with a mean of 7.91 ± 0.50. In the case of Equia Forte HT Fil, pH values varied from 7.08 ± 0.05 at 44°C after 24 hours to 8.05 ± 0.07 at 44°C after 7 days, with an average value of 7.61 ± 0.39. For GC Fuji IX GP Fast, pH ranged from 7.28 ± 0.04 at 44°C after 24 hours to 8.67 ± 0.05 at 44°C after 28 days, with a mean of 8.08 ± 0.51. Only for Cention Primer were decreasing pH values observed as the observation time increased. Specifically, the pH values ranged from 7.11 ± 0.07 at 44°C after 28 days to 8.58 ± 0.04 at 44°C after 24 hours, with an average value of 7.96 ± 0.54.
In conclusion, despite variations across different materials and conditions, the pH changes observed were relatively consistent, with all materials exhibiting similar trends. Under acidic conditions, there was an approximate increase of one pH unit, while in neutral and basic environments, an initial decrease in pH was followed by stabilization or a return to baseline values. These patterns suggest that the tested materials demonstrate stable pH profiles over time across various environmental conditions, with some varia-tions indicating differing susceptibilities to pH changes. These findings provide valuable insight into the long-term behavior of these materials, which is essential for under-standing their potential applications in diverse fields.
3.2. Release of Fluoride and Phosphate Ions
The releases of fluoride and phosphate ions obtained for all materials across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days) are showed in
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7. Specifically, for the
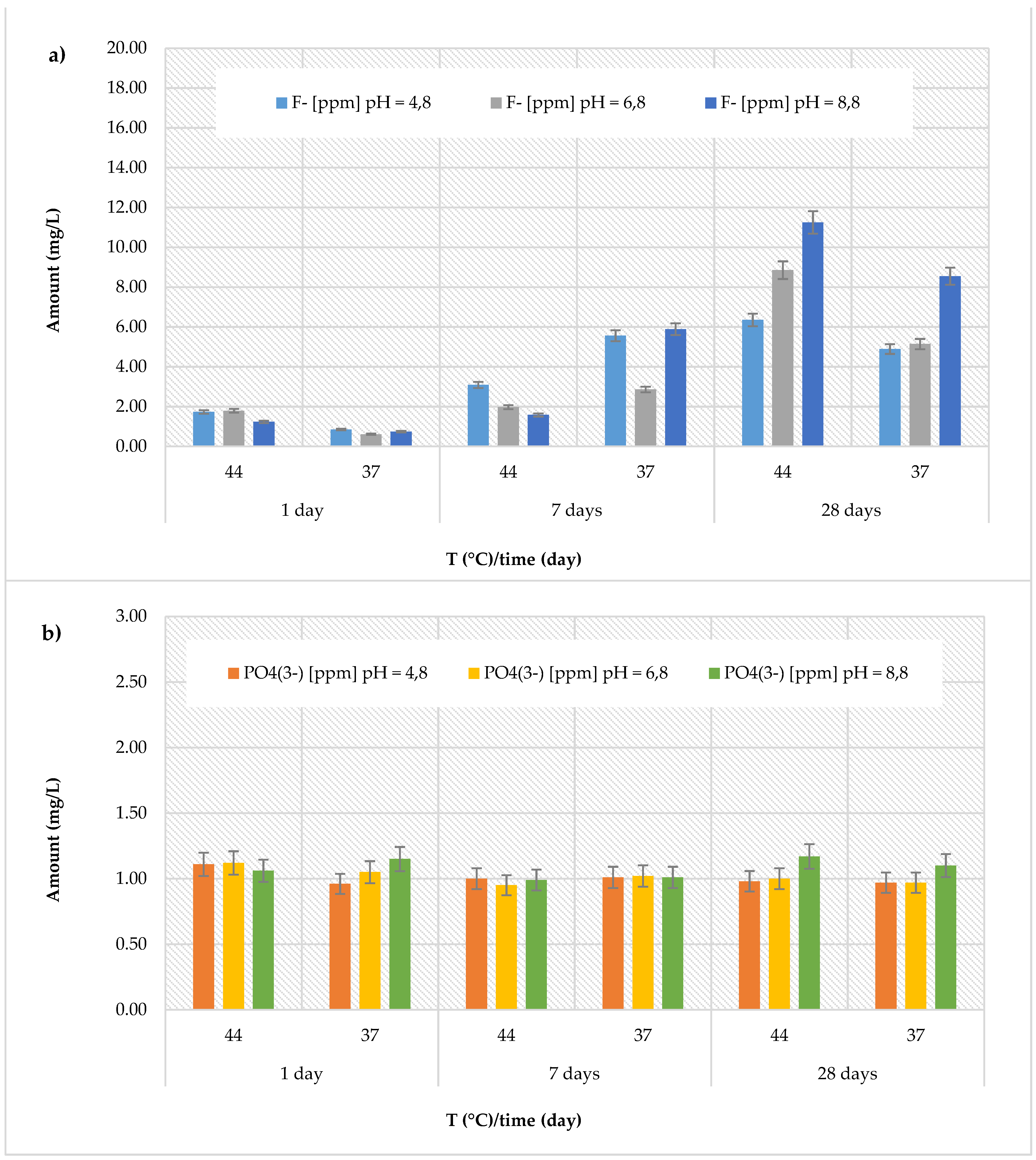
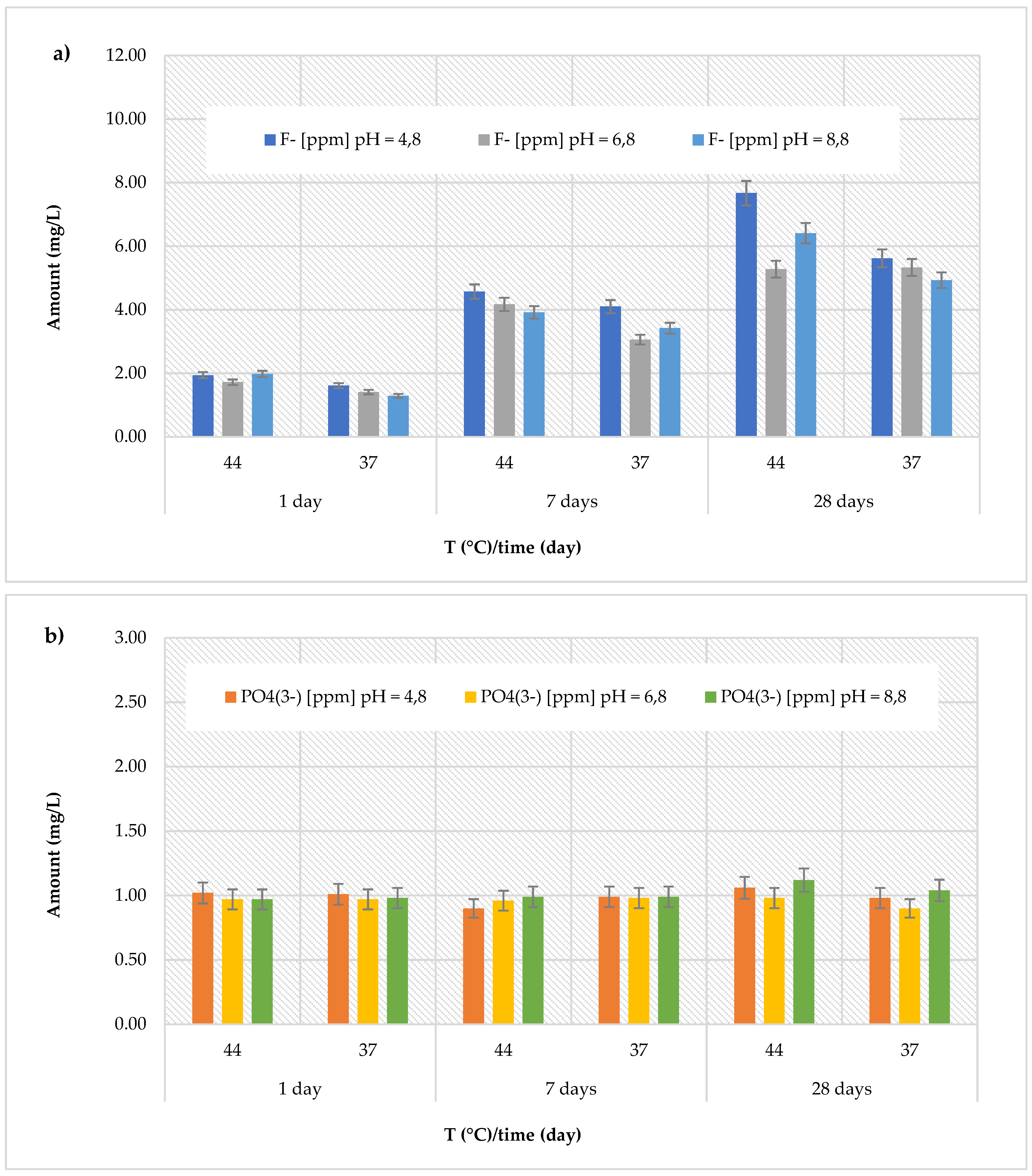
Cention Forte Filling Material, the release of fluoride ions showed an increasing trend with the observation times, temperature, and pH (
Figure 2a). In fact, the highest concentration (11.25 ± 0.71 mg/L) was found after 28 days at 44 °C in the basic environment (pH = 8.8). The observed concentrations ranged from 0.85 ± 0.13 to 6.35 ± 0.22 mg/L at pH = 4.8 (average value 3.75 ± 2.20 mg/L), from 0.61 ± 0.07 to 8.85 ± 0.25 mg/L at pH = 6.8 (average value 3.54 ± 3.01 mg/L), and from 0.74 ± 0.09 to 11.25 ± 0.71 mg/L at pH = 8.8 (average value 4.87 ± 4.39 mg/L). Regarding the release of phosphate ions (
Figure 2b), in the
Cention Forte Filling Material, a nearly constant trend was observed, with values ranging from 0.95 ± 0.10 mg/L (at pH = 6.8 and T = 44 °C, after 7 days) to 1.17 ± 0.26 mg/L (at pH = 8.8 and T = 44 °C, after 28 days).
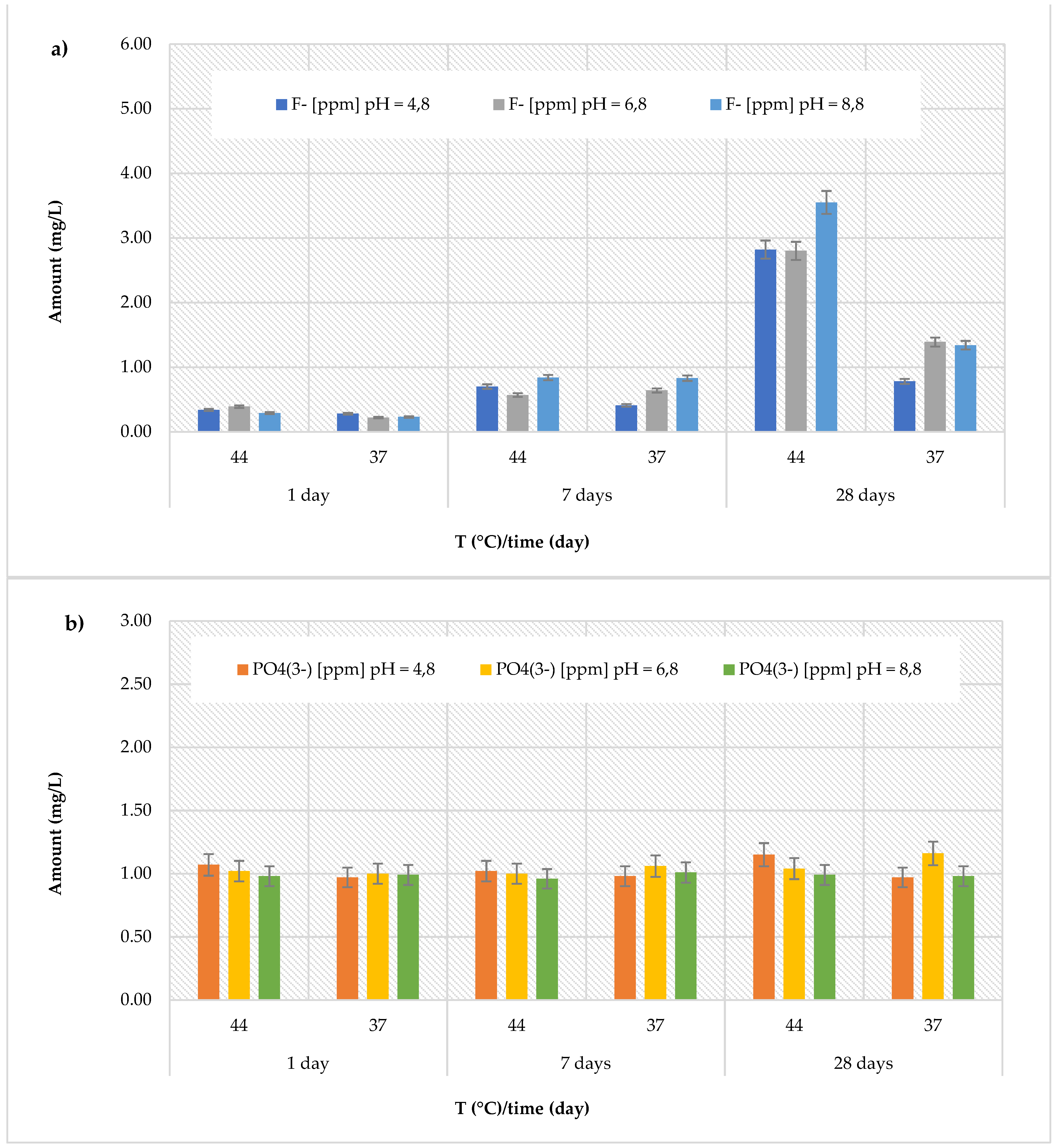
Similarly, for the
Stela Self Cure material, the highest concentration of fluoride released (although much lower than the
Cention Forte Filling Material) was found under the same conditions (
Figure 3a). In fact, the highest concentrations (3.55 ± 0.24 mg/L) were found after 28 days at 44 °C, at pH = 8.8. Additionally, the recorded amount for this material were in range 0.28 ± 0.07 to 2.82 ± 0.25 mg/L at pH = 4.8 (average value 0.89 ± 0.97 mg/L), from 0.22 ± 0.04 to 2.80 ± 0.26 mg/L at pH = 6.8 (average value 1.00 ± 0.96 mg/L), and from 0.23 ± 0.06 to 3.55 ± 0.24 mg/L at pH = 8.8 (average value 1.18 ± 1.23 mg/L). Regarding the release of phosphate ions (
Figure 3b), like the
Cention Forte Filling Material, in the
Stela Self Cure material a constant trend was observed, with values ranging from 0.96 ± 0.12 mg/L (at pH = 8.8 and T = 44 °C, after 7 days) to 1.16 ± 0.13 mg/L (at pH = 6.8 and T = 37 °C, after 28 days).
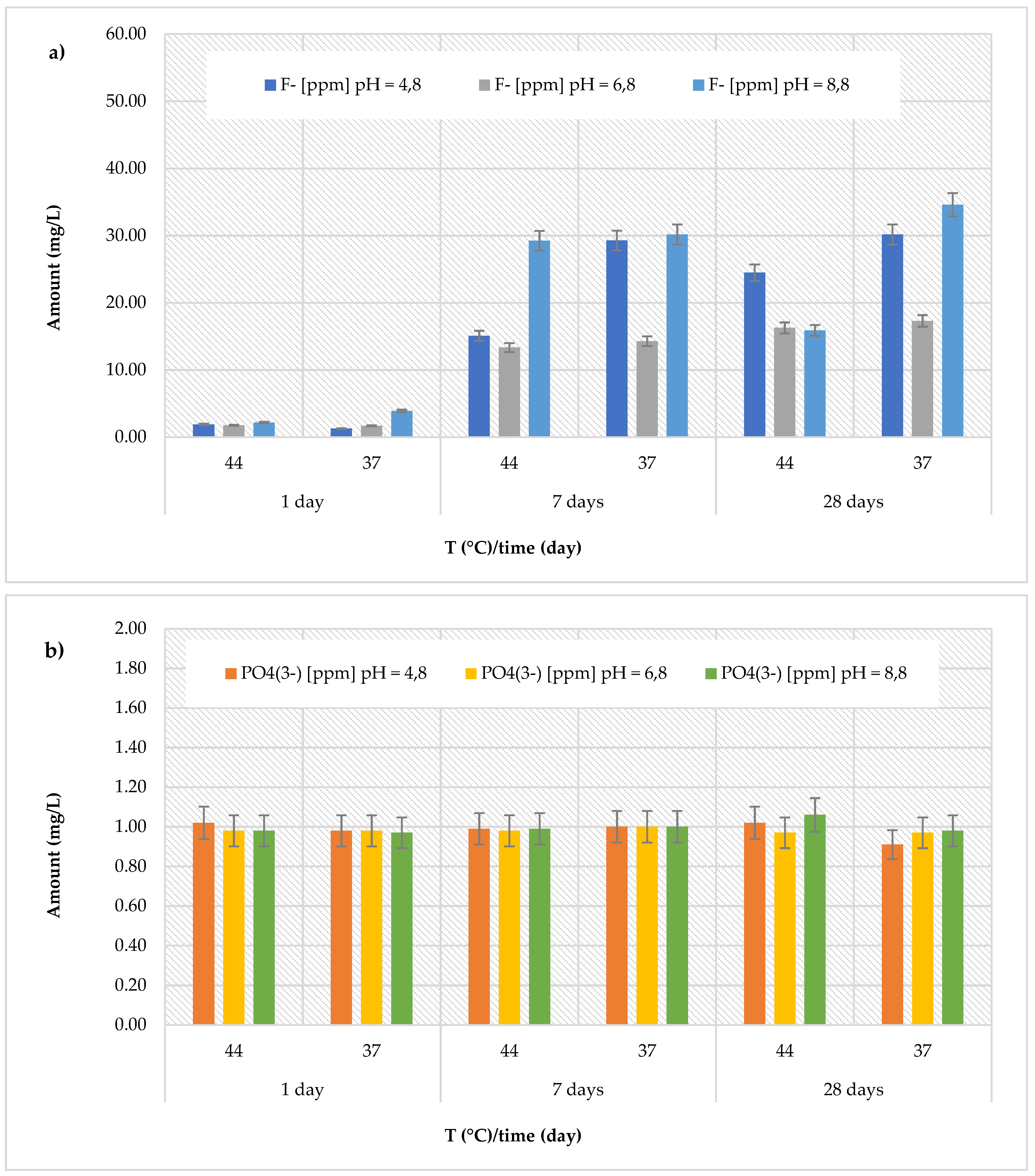
The
Riva Light Cure material showed higher amount than the Cention Forte Filling material, but conversely, with the highest fluoride release recorded (40.14 ± 0.32 mg/L) after 28 days in acidic environment (pH = 4.8) at 44°C (
Figure 4a). Furthermore, the cumulative fluoride release was in range from 1.51 ± 0.25 to 40.14 ± 0.25 mg/L at pH = 4.8 (average value 10.43 ± 14.79 mg/L), from 2.04 ± 0.29 to 8.69 ± 0.33 mg/L at pH = 6.8 (average value 5.38 ± 2.96 mg/L), and from 2.63 ± 0.16 to 12.91 ± 0.44 mg/L at pH = 8.8 (average value 5.68 ± 3.79 mg/L). Regarding the release of phosphate ions (
Figure 4b), the recorded values were higher than those observed for the previously discussed materials, ranging from 0.99 ± 0.12 mg/L (at pH = 8.8 and T = 37 °C, after 28 days) to 3.06 ± 0.19 mg/L (at pH = 8.8 and T = 44 °C, after 28 days). Similarly, the
Riva Self Cure material indicated the highest fluoride release (7.67 ± 0.49 mg/L) after 28 days at 44 °C in the acid environment (pH = 4.8). The recorded amount was in range from 1.61 ± 0.31 to 7.67 ± 0.49 mg/L at pH = 4.8 (average value 4.25 ± 2.28 mg/L), from 1.41 ± 0.34 to 5.33 ± 0.47 mg/L at pH = 6.8 (average value 3.50 ± 1.71 mg/L), and from 1.29 ± 0.33 to 6.41 ± 0.44 mg/L at pH = 8.8 (average value 3.66 ± 1.88 mg/L). Regarding the release of phosphate ions (
Figure 4b), the detected amount ranging from 0.90 ± 0.16 mg/L (at pH = 4.8 and T = 44 °C, after 7 days) to 1.12 ± 0.20 mg/L (at pH = 8.8 and T = 44 °C, after 28 days).
In contrast, for the
Equia Forte HT Fil, the highest fluoride release (34.59 ± 0.63 mg/L) was observed after 28 days in basic environment (pH = 8.8) at 37°C (
Figure 5a). Particularly, the cumulative fluoride release ranged from 1.27 ± 0.22 to 30.15 ± 0.43 mg/L at pH = 4.8 (average value 17.02 ± 13.10 mg/L), from 1.70 ± 0.34 to 17.30 ± 0.36 mg/L at pH = 6.8 (average value 10.78 ± 7.14 mg/L), and from 2.18 ± 0.23 to 34.59 ± 0.63 mg/L at pH = 8.8 (average value 19.32 ± 14.09 mg/L). Regarding the release of phosphate ions (
Figure 5b), the cumulative release was in range from 0.91 ± 0.11 mg/L (at pH = 4.8 and T = 37 °C, after 28 days) to 1.06 ± 0.16 mg/L (at pH = 8.8 and T = 44 °C, after 28 days).
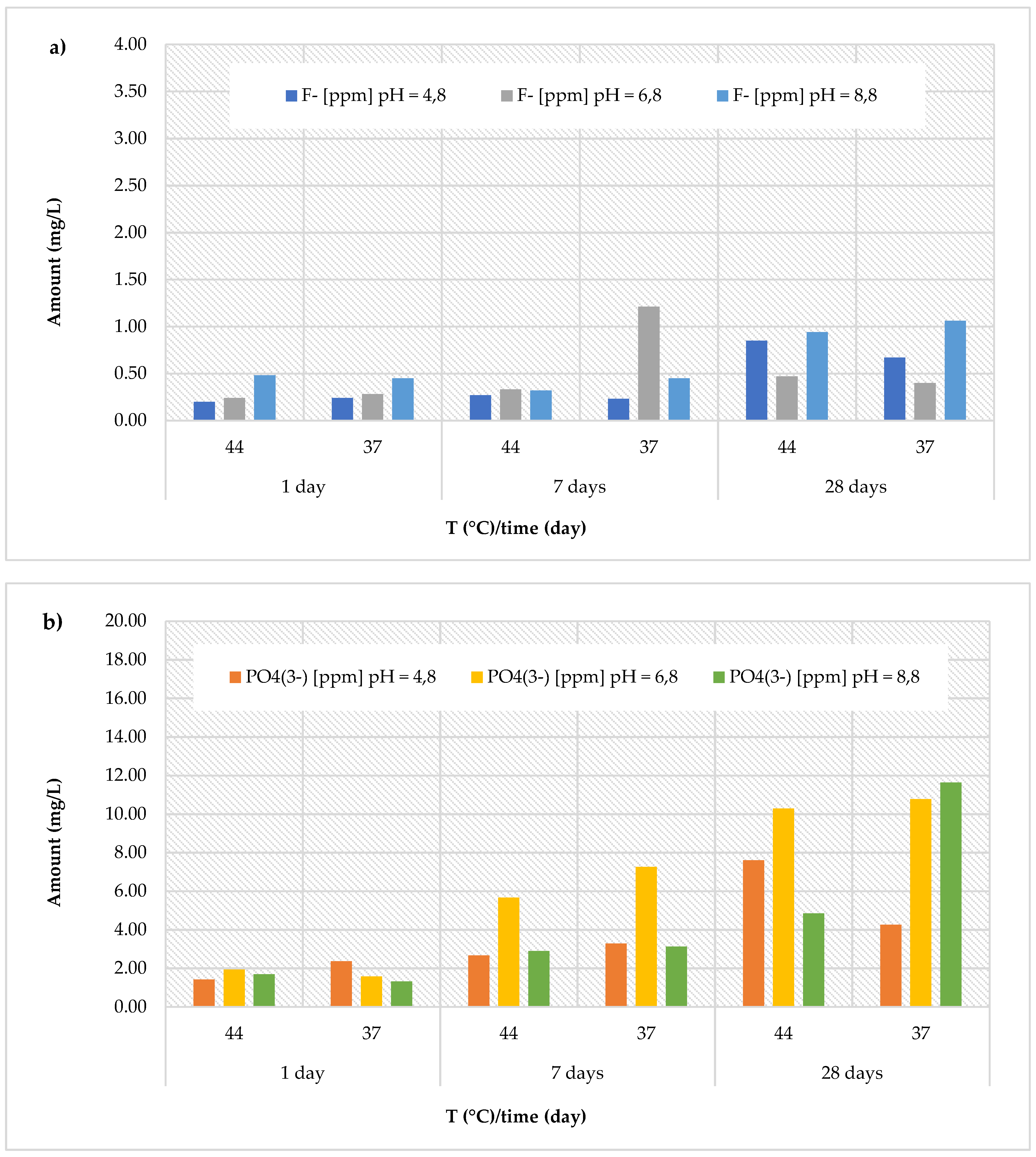
An opposite situation was observed for the
Cention Primer material, which showed a lower release of fluoride ions (
Figure 6a), but a much higher release of phosphate ions (
Figure 6b) compared to all other materials. Specifically, the cumulative concentrations of fluoride ions recorded for this material ranged between 0.20 ± 0.02 and 0.85 ± 0.10 mg/L in an acidic environment (with an average value of 0.41 ± 0.28 mg/L), between 0.24 ± 0.03 and 1.21 ± 0.15 mg/L at neutral pH (with an average value of 0.49 ± 0.36 mg/L), and between 0.32 ± 0.04 and 1.06 ± 0.13 mg/L at basic pH (with an average value of 0.62 ± 0.30 mg/L). Interestingly, the release of phosphate ions was remarkably high, with the maximum value (11.64 ± 1.40 mg/L) observed after 28 days at 37 °C in a basic environment (pH = 8.8). Specifically, the cumulative concentrations of phosphate ions ranged from 1.42 ± 0.17 to 7.60 ± 0.91 mg/L (at pH = 4.8, with an average value of 3.60 ± 2.18 mg/L), from 1.58 ± 0.19 to 10.77 ± 1.29 mg/L (at pH = 6.8, with an average value of 6.25 ± 3.97 mg/L), and from 1.32 ± 0.16 to 11.64 ± 1.40 mg/L (at pH = 8.8, with an average value of 4.26 ± 3.83 mg/L).
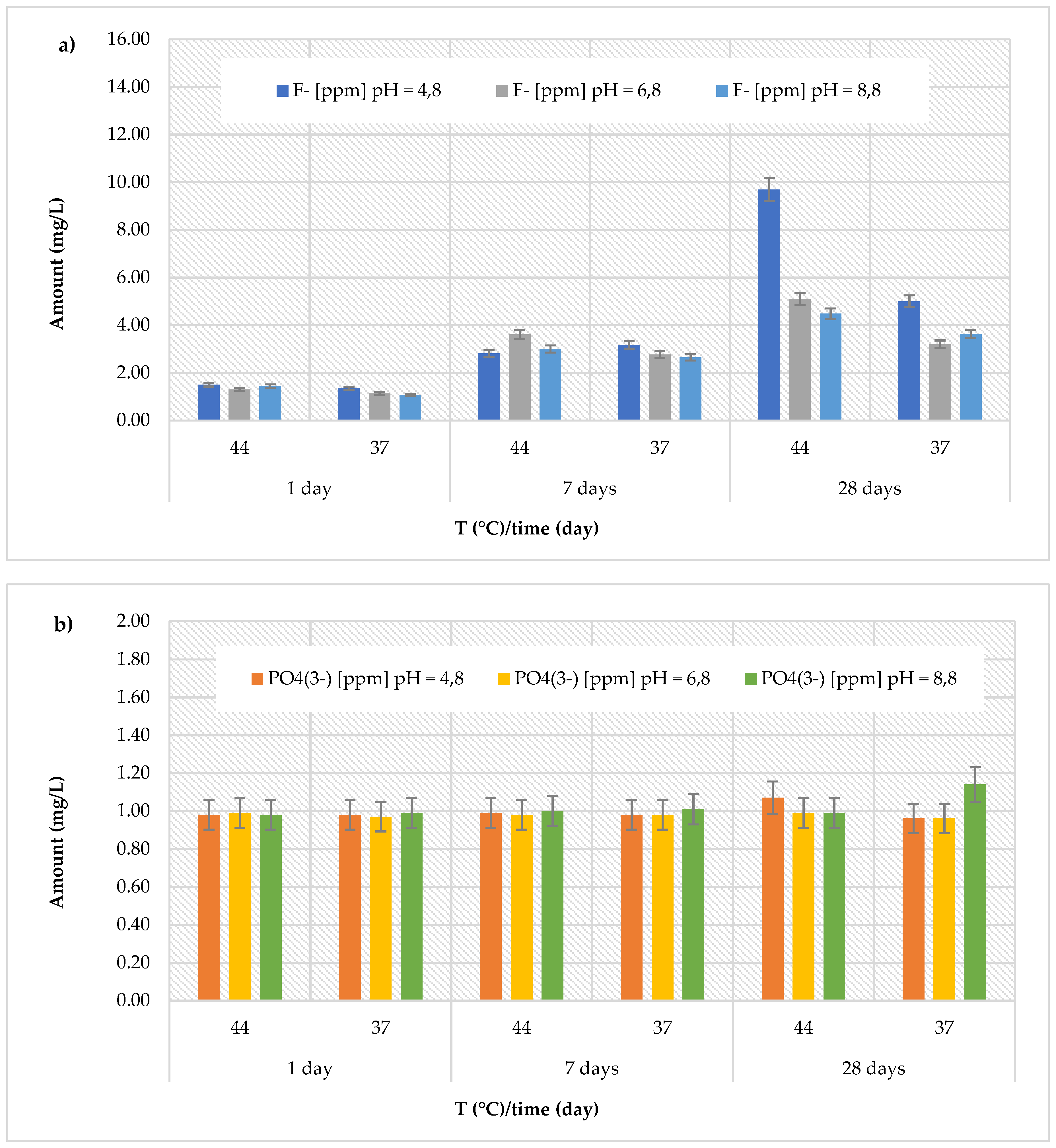
Finally, for the
GC Fuji IX GP Fast material, the highest fluoride concentrations (9.69 ± 0.57 mg/L) were detected in an acidic environment at 44 °C after 28 days (
Figure 7a). Specifically, the cumulative concentrations of fluoride ions ranged between 1.35 ± 0.26 and 9.69 ± 0.57 mg/L (with an average value of 3.92 ± 3.12 mg/L in an acidic environment), between 1.13 ± 0.19 and 5.10 ± 0.25 mg/L (with an average value of 2.85 ± 1.49 mg/L in a neutral environment), and between 1.07 ± 0.14 and 4.48 ± 0.28 mg/L (with an average value of 2.71 ± 1.29 mg/L in a basic environment). Regarding the release of phosphate ions (
Figure 7b), a consistent trend was observed, with concentrations ranging between 0.96 ± 0.05 and 1.14 ± 0.15 mg/L.
In summary, the release behaviors of fluoride and phosphate ions across the eval-uated materials revealed distinct patterns influenced by pH, temperature, and observation time. While some materials, such as the Cention Forte Filling, demonstrated a marked increase in fluoride ion release with higher temperatures and pH levels, others, like the Riva Light Cure and Equia Forte HT Fit, exhibited higher fluoride release in acidic or basic environments. Conversely, phosphate ion release generally displayed a more constant trend, with notable exceptions like the Cention Primer, which showed significantly elevated phosphate release compared to all other materials. These findings underscore the diverse ion release profiles of the tested materials, highlighting their potential variability in clinical applications depending on the surrounding environment and conditions.
3.3. Release of Calcium, Silicon, and Strontium
The results concerning the release of calcium, silicon, and strontium for all materials across three different pH levels, two temperatures (44 and 37 °C) and three different observation times (24 h, 7 days and 28 days) are presented in
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14.
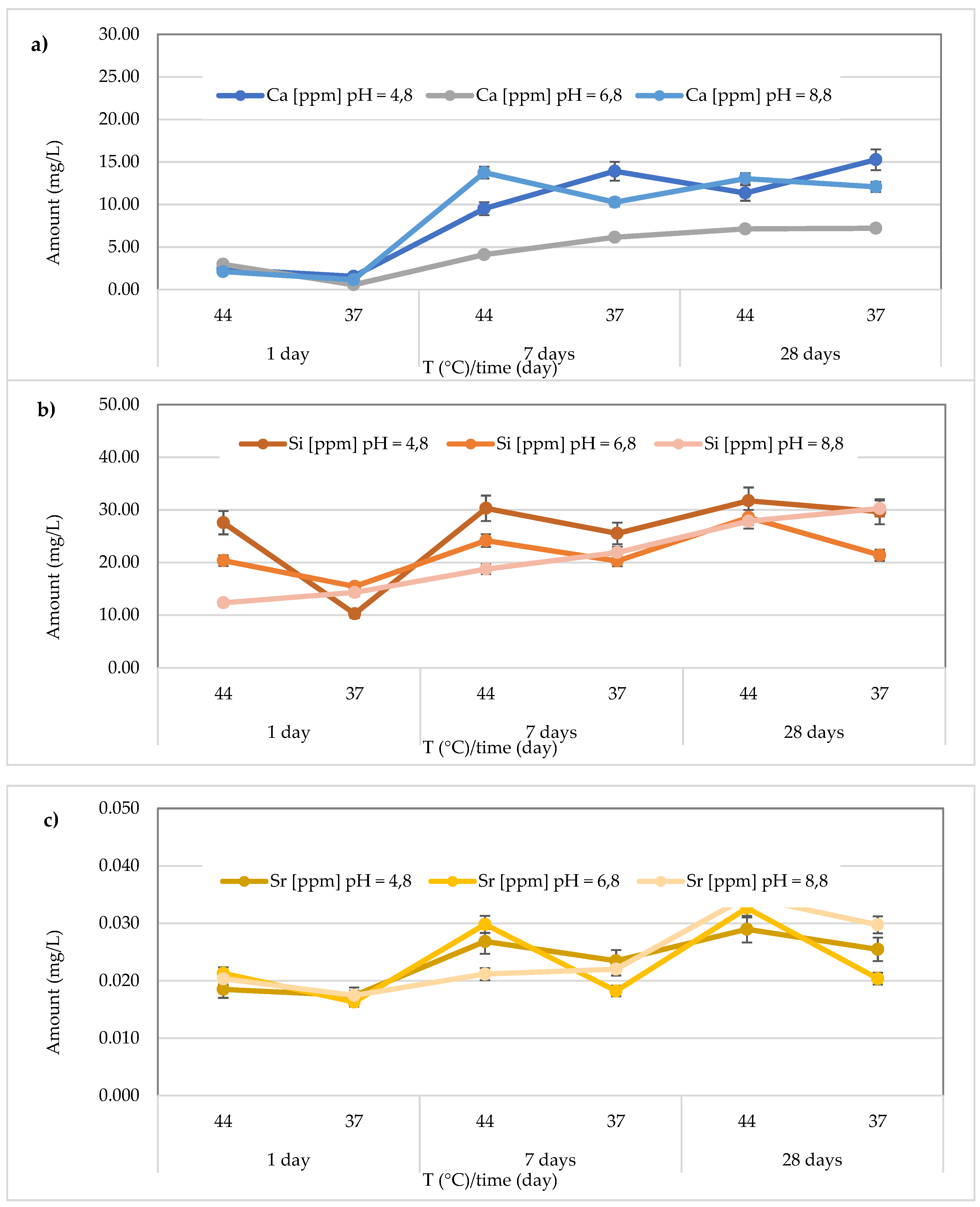
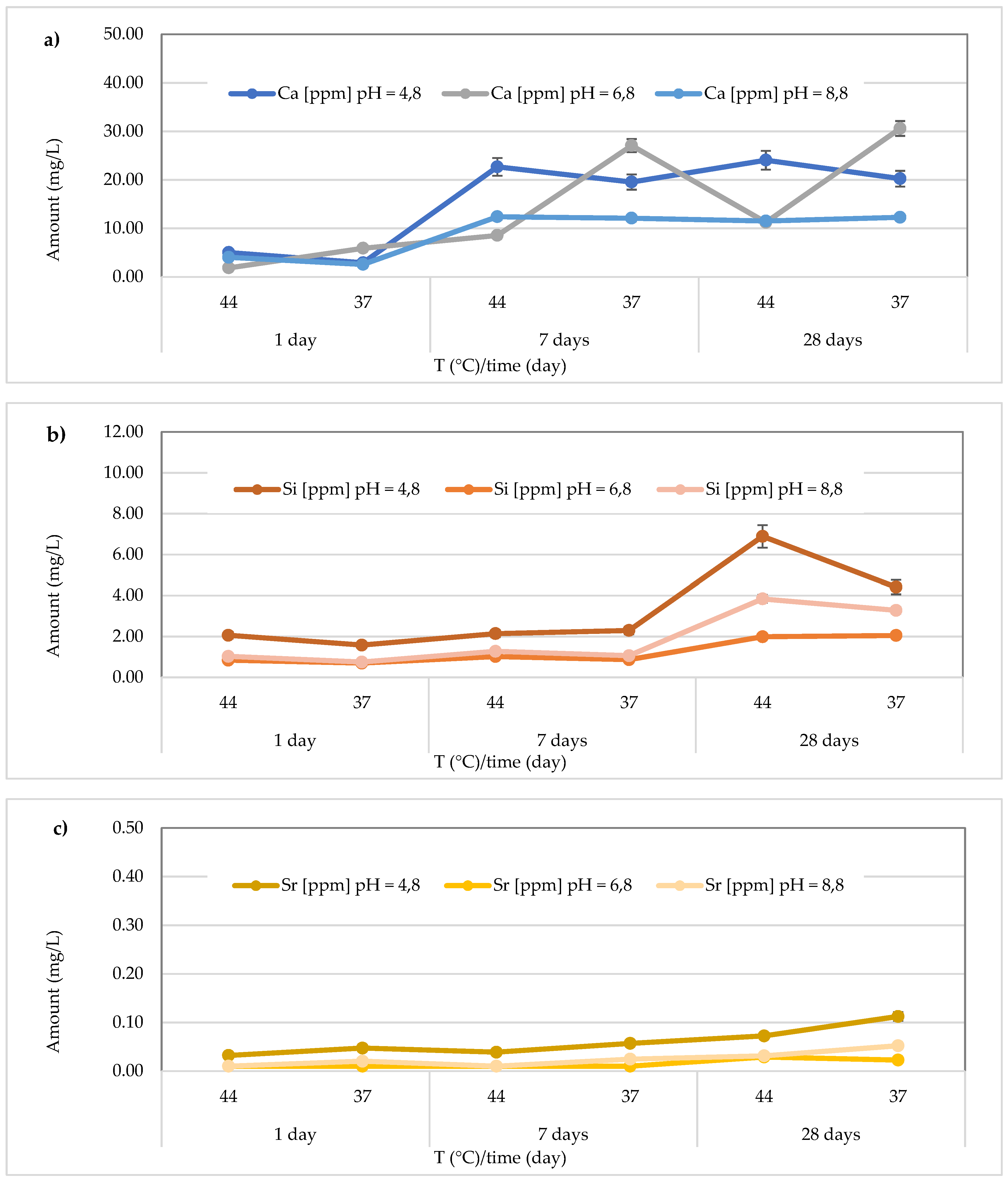
Regarding Ca2+ release, for the
Cention Forte Filling Material, the highest concentration (15.26 ± 0.45 mg/L) was found after 28 days at 37 °C in the acid environment (pH = 4.8). Specifically, the observed concentrations ranged from 1.556 ± 0.15 to 15.26 ± 0.45 mg/L at pH = 4.8 (average value 9.00 ± 5.80 mg/L), from 0.56 < 1.0 to 7.20 ± 0.23 mg/L at pH = 6.8 (average value 4.69 ± 2.64 mg/L), and from 1.15 ± 0.23 to 13.75 ± 0.28 mg/L at pH = 8.8 (average value 8.73 ± 5.63 mg/L) (
Figure 8a). In the same material, the release of Si was also observed, and the highest concentration was detected at 37 °C after 28 days in an acidic environment. Specifically, the concentrations ranged between 10.24 ± 0.42 and 31.72 ± 0.68 mg/L (mean value 25.83 ± 7.94 mg/L) in the acidic environment, 15.44 ± 0.33 to 28.56 ± 0.40 mg/L (mean value 21.69 ± 4.39 mg/L) in the neutral environment, and 12.33 ± 0.36 to 30.25 ± 0.47 mg/L (mean value 20.89 ± 7.19 mg/L) in the basic environment (
Figure 8b). Additionally, for Sr release from
Cention Forte Filling Material, shown in
Figure 8c, the highest concentration was observed in a basic environment at 44 °C after 28 days (0.035 ± 0.008 mg/L). Specifically, Sr concentrations released from the material ranged from 0.017 ± 0.003 to 0.029 ± 0.006 mg/L (mean value 0.023 ± 0.005 mg/L) in the acidic environment, 0.016 ± 0.001 to 0.033 ± 0.006 mg/L (mean value 0.023 ± 0.007 mg/L) in the neutral environment, and 0.017 ± 0.001 to 0.035 ± 0.008 mg/L in the basic environment, with a mean value of 0.024 ± 0.007 mg/L. Regarding Sb, no release was observed from
Cention Forte Filling Material, as all concentrations were below the LOQ.
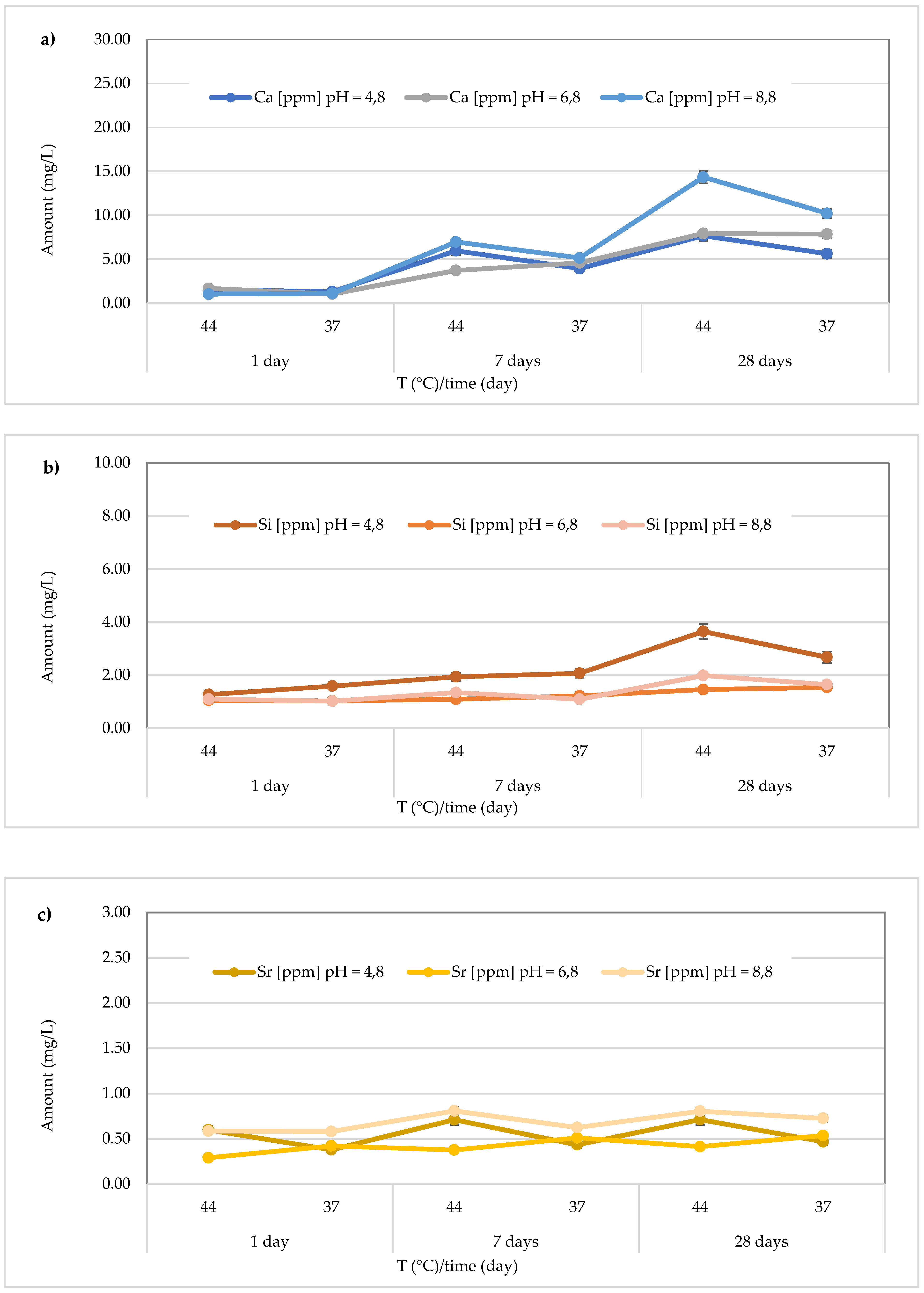
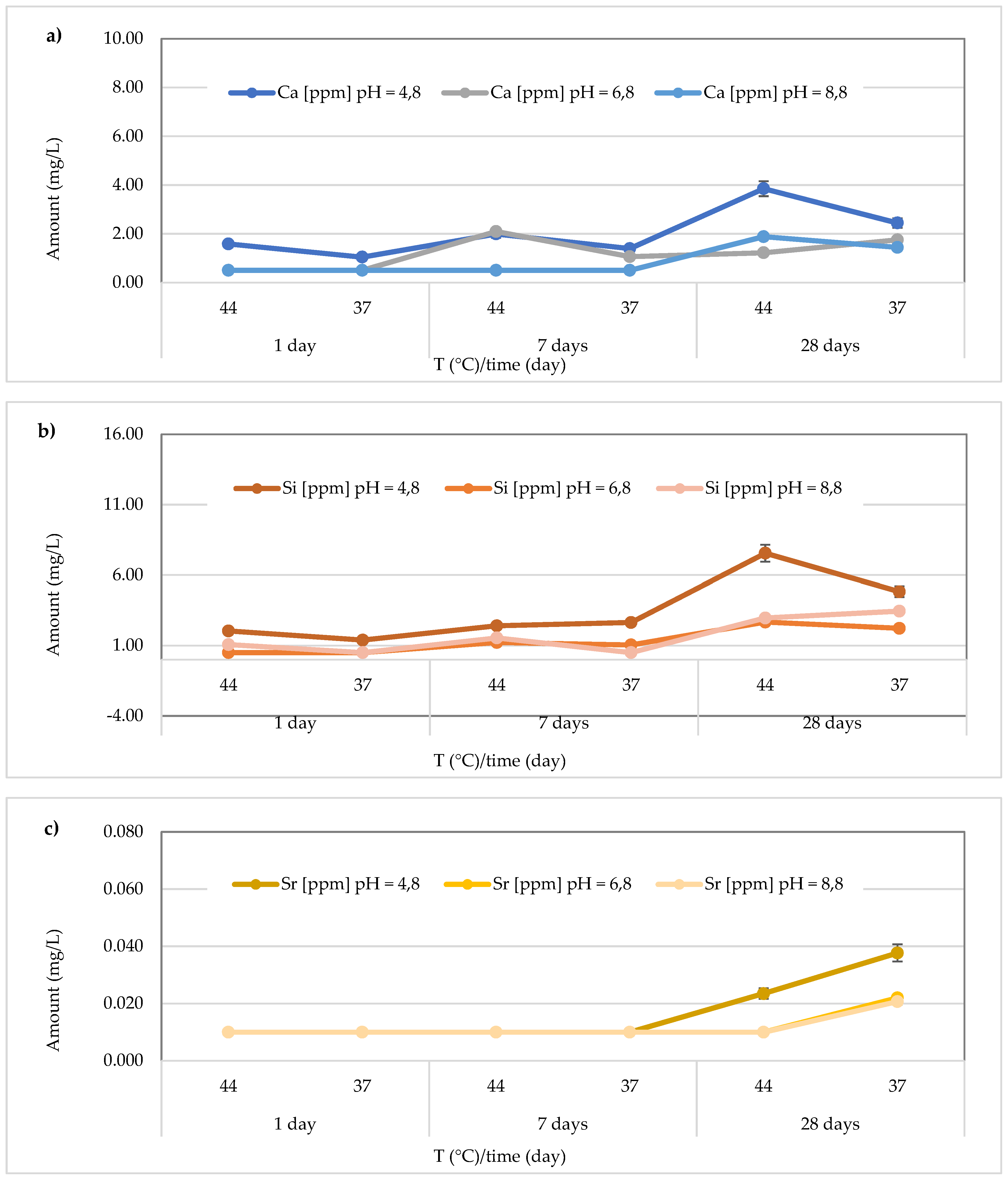
In the Stela Self Cure material, the highest Ca
2+ release (14.35 ± 0.45 mg/L), in contrast to what was observed for
Cention Forte Filling Material, was detected after 28 days at 44 °C in the basic environment (pH = 8.8). In detail, the found concentrations ranged from 1.31 ± 0.28 to 7.68 ± 0.29 mg/L at pH = 4.8 (average value 4.35 ± 2.55 mg/L), from 1.05 ± 0.05 to 7.94 ± 0.25 mg/L at pH = 6.8 (average value 4.48 ± 2.95 mg/L), and from 1.04 ± 0.11 to 14.35 ± 0.45 mg/L at pH = 8.8 (average value 6.48 ± 5.22 mg/L) (
Figure 9a). In the same material, the release of Si was also observed, and the highest concentration was detected at 44 °C after 28 days in an acidic environment. Specifically, the concentrations ranged between 1.27 ± 0.17 and 3.65 ± 0.19 mg/L (mean value 2.20 ± 0.86 mg/L) in the acidic environment, from 1.03 ± 0.11 to 1.54 ± 0.25 mg/L (mean value 1.23 ± 0.22 mg/L) in the neutral environment, and 1.02 ± 0.11 to 1.99 ± 0.24 mg/L (mean value 1.37 ± 0.38 mg/L) in the basic environment (
Figure 9b). Additionally, for Sr release from the material
Stela Self Cure, shown in
Figure 9c, the highest concentration was observed in a basic environment at 44 °C after 7 days (0.808 ± 0.017 mg/L), a value similar to that observed at the same temperature and pH after 28 days (0.805 ± 0.036 mg/L). Furthermore, Sr concentrations released from the material ranged from 0.378 ± 0.033 to 0.712 ± 0.015 mg/L (mean value 0.55 ± 0.14 mg/L) in the acidic environment, 0.290 ± 0.035 to 0.5364 ± 0.022 mg/L (mean value 0.42 ± 0.09 mg/L) in the neutral environment, and 0.580 ± 0.016 to 0.8081 ± 0.017 mg/L in the basic environment, with a mean value of 0.69 ± 0.11 mg/L.
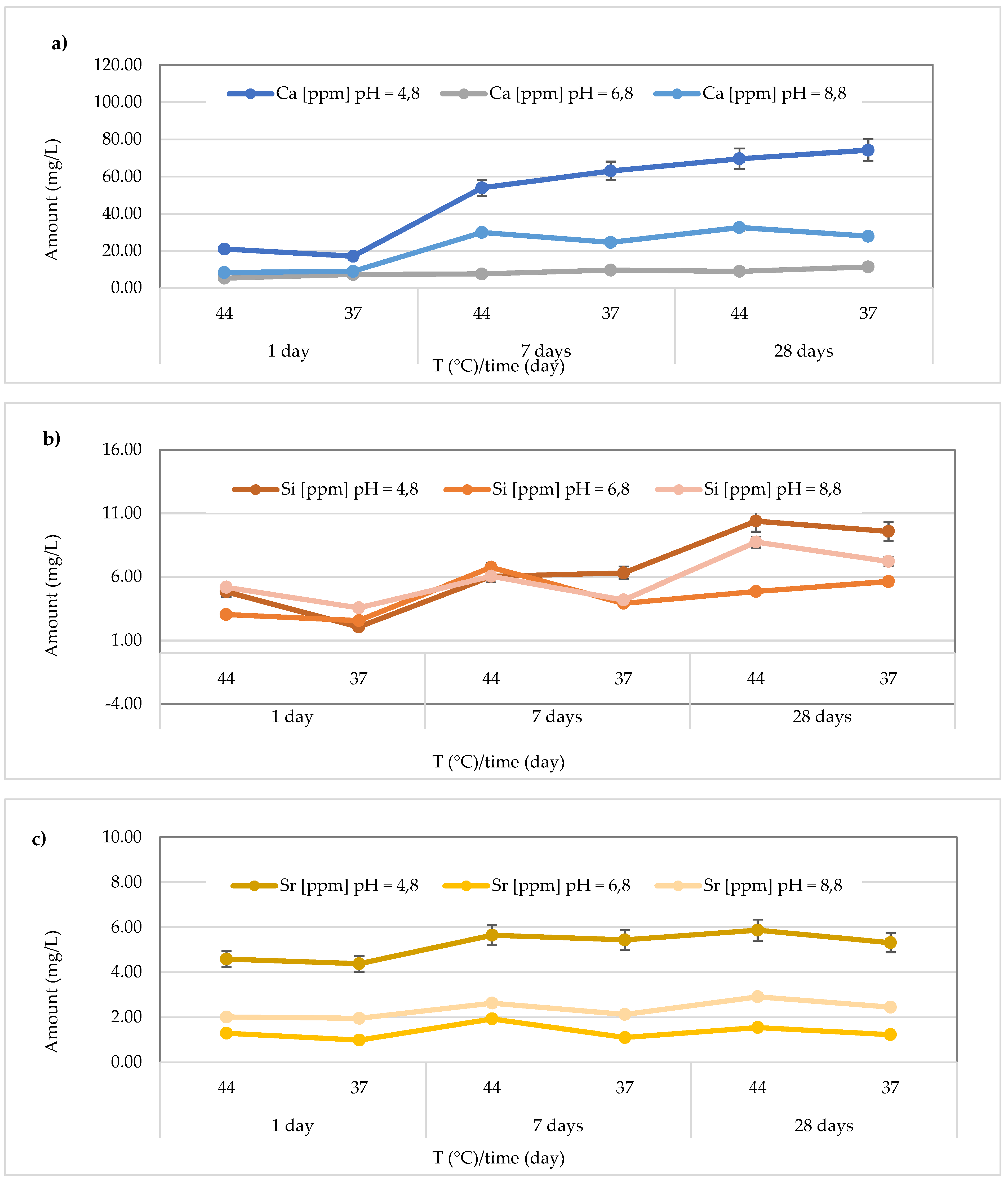
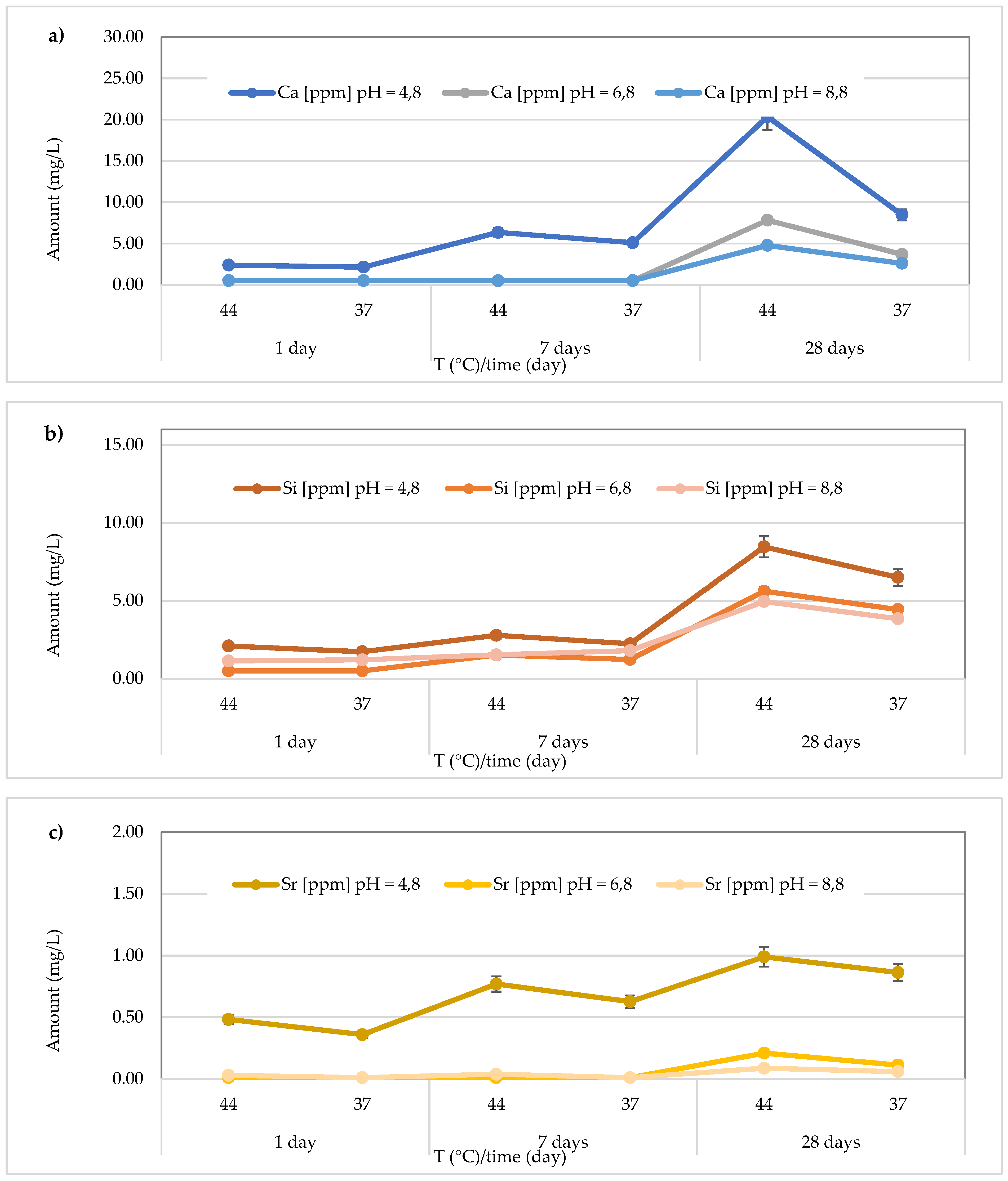
The
Figure 10 illustrates the results obtained for the
Riva Light Cure material. The highest Ca2+ release (74.23 ± 0.37 mg/L) was found after 28 days at 37 °C in the acidic media (pH = 4.8). Additionally, the detected concentrations ranged from 17.12 ± 0.18 to 74.23 ± 0.37 mg/L at pH = 4.8 (average value 49.81 ± 24.82 mg/L), from 5.31 ± 0.27 to 11.36 ± 0.50 mg/L at pH = 6.8 (average value 8.35 ± 2.10 mg/L), and from 8.32 ± to 32.58 ± 0.62 mg/L at pH = 8.8 (average value 22.04 ± 10.70 mg/L) (
Figure 10a). In the same material, the release of Si was also observed, and the highest concentration (10.39 ± 0.62 mg/L) was detected at 44 °C after 28 days in an acidic environment. Specifically, the concentrations ranged between 2.07 ± 0.20 and 10.39 ± 0.69 mg/L (mean value 6.54 ± 3.07 mg/L) in the acidic environment, 2.57 ± 0.21 to 6.75 ± 0.26 mg/L (mean value 4.47 ± 1.59 mg/L) in the neutral environment, and 3.58 ± 0.37 to 8.74 ± 0.37 mg/L (mean value 5.83 ± 1.93 mg/L) in the basic environment (
Figure 10b). Additionally, for Sr release from the material
Riva Light Cure material, shown in
Figure 10c, the highest concentration was observed in the acidic environment at 44 °C after 28 days (5.87 ± 0.06 mg/L). Furthermore, Sr concentrations released from the material ranged from 4.38 ± 0.07 to 5.87 ± 0.07 mg/L (mean value 5.21 ± 0.59 mg/L) in the acidic environment, 0.99 ± 0.08 to 1.93 ± 0.05 mg/L (mean value 1.35 ± 0.34 mg/L) in the neutral environment, and 1.96 ± 0.07 to 2.91 ± 0.14 mg/L in the basic environment, with a mean value of 2.35 ± 0.38 mg/L.
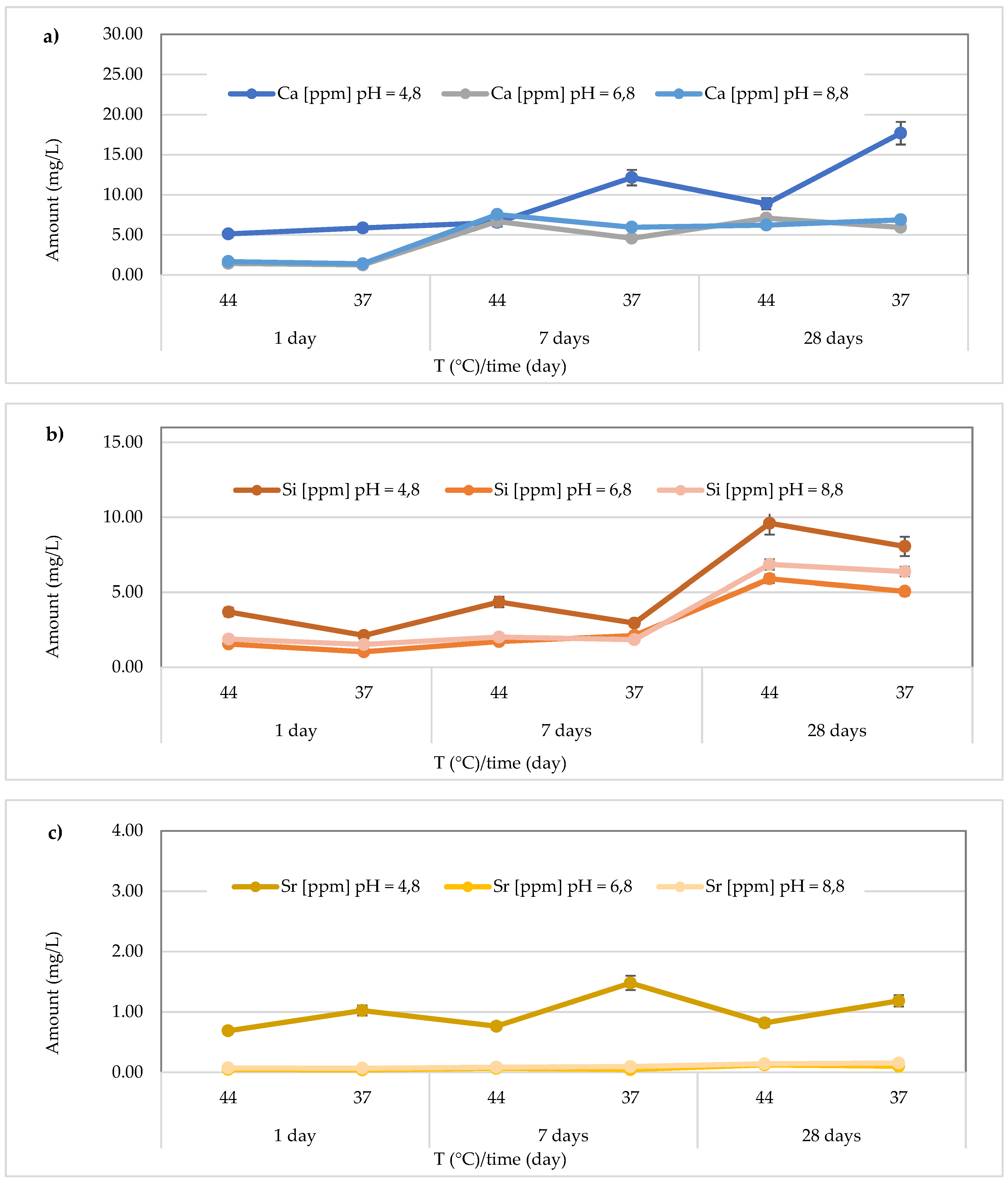
In the
Riva Self Cure material, the Ca
2+ amount released was in range 5.12 ± 0.21 – 17.69 ± 0.42 mg/L, with a mean value of 9.38 ± 4.81 mg/L at pH = 4.8, 1.25 ± 0.32 – 7.08 ± 0.23 mg/L, with a mean value of 4.49 ± 2.58 mg/L at pH = 6.8 and 1.40 ± 0.38 to 7.54 ± 0.43 mg/L, with a mean value of 4.94 ± 2.70 mg/L at pH = 8.8. Therefore, the highest release was found in the acidic environment at 37 °C after 28 days (
Figure 11a). Regarding Si release, the highest amount was found in the acidic environment after 28 days at 44 °C (9.60 ± 0.40 mg/L) (
Figure 11b). Moreover, the amount of Si ranged from 2.12 ± 0.28 to 9.60 ± 0.40 mg/L (mean value 5.13 ± 3.00 mg/L) at pH = 4.8, from 1.03 ± 0.19 to 5.89 ± 0.20 mg/L (mean value 2.89 ± 2.05 mg/L) at pH = 6.8 and from 1.52 ± 0.32 to 6.85 ± 0.19 mg/L (mean value 3.41 ± 2.49 mg/L) at pH = 8.8. Additionally, for Sr release from the material
Riva Self Cure material (
Figure 11c), the highest concentration was observed in the acidic environment at 37 °C after 7 days (1.48 ± 0.08 mg/L). However, the Sr amount ranged from 0.69 ± 0.04 to 1.48 ± 0.08 mg/L (mean value 0.99 ± 0.30 mg/L) in the acidic environment, 0.042 ± 0.012 to 0.125 ± 0.022 mg/L (mean value 0.072 ± 0.034 mg/L) in the neutral environment, and 0.070 ± 0.010 to 0.156 ± 0.017 mg/L in the basic environment, with a mean value of 0.11 ± 0.04 mg/L.
Regarding the material
Equia Forte HT Fil, the highest concentrations of Ca²⁺ were observed in the neutral medium (pH 6.8), with a maximum value of 30.60 ± 0.73 mg/L after 28 days at 37 °C (
Figure 12a). The concentrations had a mean value of 15.74 ± 9.29 mg/L (range from 2.89 ± 0.22 to 24.05 ± 0.26 mg/L) in the acidic environment, 14.20 ± 11.81 mg/L (range from 1.85 ± 0.16 to 30.60 ± 0.73 mg/L) in the neutral environment, and 9.14 ± 4.56 mg/L (range from 2.56 ± 0.25 to 12.39 ± 0.47 mg/L) in the basic environment. About the Si, the highest amount was found in the acidic environment after 28 days at 44 °C (6.89 ± 0.25 mg/L) (
Figure 12b). Moreover, the amount of Si ranged from 1.58 ± 0.27 to 6.89 ± 0.25 mg/L (mean value 3.23 ± 2.05 mg/L) at pH = 4.8, from 0.70 ± 0.09 to 2.05 ± 0.17 mg/L (mean value 1.24 ± 0.61 mg/L) at pH = 6.8 and from 0.75 ± 0.13 to 3.84 ± 0.27 mg/L (mean value 1.87 ± 1.33 mg/L) at pH = 8.8.
As observed in
Figure 12c, the
Equia Forte HT Fil material showed Sr concentrations that were almost similar under all conditions, with a maximum value of 0.112 ± 0.013 mg/L, detected after 28 days in the acidic environment at 37 °C. For the
Cention Primer material, the concentrations detected for all the studied metals were lower compared to those obtained for other materials. Specifically, for calcium, the concentrations ranged between 1.04 ± 0.12 and 3.85 ± 0.46 mg/L at acidic pH, <LOQ and 2.09 ± 0.25 mg/L at neutral pH, and <LOQ and 1.88 ± 0.23 mg/L at basic pH, with mean values of 2.05 ± 1.01 mg/L, 1.19 ± 0.65 mg/L, and 0.89 ± 0.62 mg/L, respectively (
Figure 13a). The maximum value was found after 28 days at 44 °C in an acidic environment (3.85 ± 0.46 mg/L). In the same material, the concentrations of Si obtained were in the ranges of 1.39 ± 0.17 to 7.55 ± 0.91 mg/L, <LOQ to 2.67 ± 0.32 mg/L, and <LOQ to 3.45 ± 0.41 mg/L in acidic, neutral, and basic environments, respectively. Notably, the highest release of Si was observed in an acidic environment at 44 °C after 28 days (
Figure 13b). The Sr concentrations are illustrated in
Figure 13c, showing that the highest concentrations were found in an acidic environment (0.038 ± 0.005 mg/L at 37 °C after 28 days).
Finally, for the
GC Fuji IX GP Fast material, the results are shown in
Figure 14. Specifically, regarding calcium release, the concentrations ranged between 2.14 ± 0.28 and 20.35 ± 0.47 mg/L (mean value 7.46 ± 6.75 mg/L) in an acidic environment, <LOQ to 7.82 ± 0.24 mg/L (mean value 2.25 ± 3.01 mg/L) in a neutral environment, and <LOQ to 4.77 ± 0.23 mg/L (mean value 1.56 ± 1.78 mg/L) in a basic environment. The maximum value was observed after 28 days in an acidic environment at 44 °C (
Figure 14a). For Si, the highest concentration detected was 8.45 ± 0.36 mg/L (at 44 °C, pH 4.8, after 28 days) (
Figure 14b). Similarly, for Sr, the highest concentration was found under the same conditions (0.990 ± 0.035 mg/L) (
Figure 14c).
In conclusion, the analysis of the metal ion release from the various dental materials reveals significant differences in their release profiles. Materials such as Cention Forte Filling Material and Stela Self Cure showed notable variation in metal release, with Cention Forte Filling Material exhibiting higher calcium and silicon concentrations, particularly in acidic environments, while Stela Self Cure material demonstrated the highest release of calcium in basic conditions. Conversely, materials like Cention Primer and Equia Forte HT Fil displayed much lower concentrations of metal release across all conditions, with Cention Primer showing consistently low calcium and silicon release, and Equia Forte HT Fil presenting minimal strontium release. These observations highlight the varied release behavior of different dental materials, emphasizing the importance of selecting materials based on specific clinical requirements and the potential impact on the oral environment over time.
3.4. Results of the Statistical Analysis
The results of the statistical analysis indicated that the release of the studied ions is significantly influenced by the considered factors, namely the acidity conditions of the medium, temperature, and exposure time, with different trends observed across the tested materials.
Specifically, fluoride release was significantly affected by pH (p = 0.009) and exposure time (p < 0.001), with higher fluoride concentrations generally observed under acidity stress conditions, either at acidic or basic pH levels, and over prolonged observation periods. Calcium showed a negative correlation with pH (p < 0.001), suggesting greater solubilization under acidic conditions, while time had a significant positive effect (p < 0.001), indicating sustained release over time. Silicon release exhibited statistically significant effects for all tested parameters, with pH (p < 0.001), temperature (p < 0.001), and time (p < 0.001) playing key roles, indicating that its release is influenced by multiple physicochemical factors. Strontium release was significantly affected by pH (p < 0.001), while temperature (p = 0.424) and exposure time (p = 0.432) did not show significant effects, suggesting lower susceptibility to temperature and duration changes. Lastly, phosphate release showed a significant dependence on time (p < 0.001), while pH and temperature had no statistically relevant effects (p > 0.05), suggesting greater stability compared to other ions.
Furthermore, the multivariate analysis confirmed the crucial role of exposure time and pH in regulating ion release, with significant differences observed among the tested materials (p < 0.05). These findings highlight the importance of considering environ-mental conditions when selecting restorative materials, as their behavior may vary in response to changes in pH and temperature within the oral cavity.
4. Discussion
Dental caries is a biofilm-mediated, diet-modulated, multifactorial, dynamic disease that results in a progressive decrease in dental hard tissue [
30]. Secondary caries may develop due to increased bacterial adhesion and biofilm development, especially on proximal surfaces with resin composite restorations where plaque control is difficult [
31]. It has also been amply demonstrated in the literature that polymerization shrinkage and masticatory load can lead to high levels of stress, especially in deep Class I cavities made of resin-based composite materials, favoring the development of secondary caries and dental hypersensitivity [
32,
33]. Since some resin-based composites can promote bacterial growth around them, various non-invasive or micro-invasive strategies have been proposed, including the use of bioactive restorative materials capable of releasing ions, especially fluoride and calcium, that can reduce the adhesion and proliferation of oral bacteria on their surfaces, resulting in less plaque accumulation [
34].
Bioactive restorative materials are a new development in dentistry: they maintain dental health and function through biological actions related to their antimicrobial properties, such as reducing biofilm activity, preventing demineralization of surrounding tissues, and promoting remineralization of caries-affected areas [
35]. Bioactive restorative materials also release and recharge ionic components in response to changes in pH and are moisture resistant, allowing continuous ion exchange with oral fluid [
36,
37].
The primary goal of this study was to investigate how pH and temperature affect the release of ions such as Ca²⁺, F⁻, PO₄³⁻, OH⁻, Si, and Sr²⁺ from various bioactive dental materials. This is crucial because ion release plays a central role in promoting remineralization and enhancing the biological compatibility of dental restorations. The study measured ion release over varying conditions using advanced techniques like ion chromatography and mass spectrometry, which enabled precise quantification of the ion concentrations over 28 days. The results confirmed that both pH and temperature significantly influence ion release, which, probably, in turn impacts the biological interactions and functional longevity of bioactive materials in dental applications.
The pH of the oral environment is a major determinant of the solubility and ion release from bioactive materials. Acidic conditions in the oral cavity, often resulting from the consumption of acidic foods and beverages, trigger an increased release of ions such as Ca²⁺, F⁻, and PO₄³⁻. This study revealed that pH had a significant impact on ion release across all materials tested. Materials exposed to an acidic environment (pH 4.8) generally showed the highest ion release, particularly fluoride and calcium, which are key components in the remineralization of enamel. The observed peak ion releases at pH 4.8, especially in
Riva Light Cure HV, suggest that acidic conditions enhance the dissolution of material components, promoting ion release. This is consistent with previous studies, such as those by Melo et al. 2023 [
38], which found that acidic conditions increase the solubility of materials containing fluoride and calcium, resulting in higher ion concentrations in the solution. In the case of
Riva Light Cure HV, the significant fluoride release (40.14 ± 0.32 mg/L) and calcium release (74.23 ± 0.37 mg/L) observed at pH 4.8 may be beneficial for caries prevention, as these ions are integral to enamel remineralization. Similarly, the release of strontium (5.87 ± 0.06 mg/L) in these conditions aligns with the role of strontium in promoting remineralization and strengthening the structure of dental hard tissues, which is especially relevant under acidic conditions that typically lead to enamel demineralization.
Temperature is another critical factor influencing the release of ions from bioactive dental materials. According to Madi et al 2020 [
39] an increase in temperature generally results in a higher release rate of ions, including Ca²⁺, F⁻, PO
43-, Sr
2+ and Si. This is because elevated temperatures promote the dissolution of the material’s constituent compounds. Specifically, higher temperatures accelerate the solubility of silicate, phosphate, fluoride and calcium compounds, facilitating the release of these ions. This phenomenon is particularly relevant in the oral cavity, where the temperature fluctuates during the consumption of hot foods and beverages. Moreover, elevated temperatures could also contribute to a more rapid ion exchange process, potentially enhancing the therapeutic effect of bioactive materials in promoting remineralization. This was particularly noticeable in
Riva Light Cure HV, where the highest fluoride and calcium release was observed at both low pH (4.8) and higher temperature (44°C). The combined influence of acidic pH and elevated temperature (44°C) on
Riva Light Cure HV suggests that these conditions could mimic the oral environment during certain physiological conditions (e.g., the consumption of hot, acidic beverages), where ion release is maximized. This characteristic may be beneficial for patients at higher risk of caries or enamel demineralization, as the material is actively releasing fluoride and calcium to facilitate remineralization.
Strontium release, which was highest in
Riva Light Cure HV under acidic pH (4.8) and high temperature (44°C), is noteworthy. Strontium is known to enhance the remineralization process by substituting calcium in hydroxyapatite crystals, increasing their resistance to acid dissolution. The release of strontium, especially under conditions that mimic the oral cavity during acidic or high-temperature states, is likely to contribute positively to the long-term protection of dental tissues [
40,
41,
42]. These findings support the inclusion of strontium in bioactive dental materials, particularly for patients with a high caries risk or those undergoing restorative treatments.
Interestingly,
Cention Forte Filling Material showed the highest silicon release (31.72 ± 0.68 mg/L) under the conditions of pH 4.8 and 37°C. Silicon is an important component in bioactive materials due to its role in stimulating hydroxyapatite formation and promoting tissue regeneration [
43]. The release of Si from
Cention Forte Filling Material under acidic conditions could be advantageous for the long-term repair of dental tissues, as silicon promotes the formation of a mineralized layer on the material's surface, enhancing its bioactivity and biocompatibility. This finding underscores the significance of silicon in bioactive dental materials. The temperature and pH conditions at which this release occurs are relevant to the oral environment, where acidic conditions often lead to demineralization and where the release of silicon could enhance the repair of dental tissues.
Phosphate (PO₄³⁻) and hydroxide (OH⁻) ions are essential for the formation of hydroxyapatite, the primary mineral found in teeth and bones. Specifically, phosphate ions are critical in the reformation of hydroxyapatite crystals on the surface of the material, while hydroxide ions contribute to the stabilization of these crystals. As noted by Par et al 2022 the release of PO₄³⁻ and OH⁻ ions is particularly high in bioactive materials like glass ionomer cements, which are known for their ability to bond chemically to dental tissues and release fluoride, phosphate, and hydroxide ions in response to acidic conditions [
44]. Under acidic conditions, the increased availability of PO₄³⁻ and OH⁻ ions promote the deposition of remineralized hydroxyapatite, contributing to the restoration of enamel hardness and preventing further demineralization. However, excessive release of OH⁻ ions at high temperatures may lead to undesirable effects, such as material degradation and alteration of the pH balance within the oral cavity.
Finally, this in vitro study also measured ion release over different time points: 1 day, 7 days, and 28 days. The results showed a progressive release of ions over time, which suggests that bioactive materials continue to release beneficial ions in the long term. This sustained release is essential for maintaining a therapeutic effect, as continuous ion release can provide prolonged protection against demineralization and support ongoing remineralization of the tooth structure.
For instance, Riva Light Cure HV continued to release significant amounts of fluoride, calcium, and strontium over the 28-day period, reinforcing the material's potential for long-term effectiveness in preventing caries and promoting remineralization. These results align with the general properties of bioactive materials, which are designed to release ions gradually to enhance their therapeutic effect over time.
Although this study provides valuable data on ion release from bioactive materials, there are limitations. For example, the study was conducted in controlled laboratory conditions, which may not fully replicate the complexity of the oral environment, where factors like salivary flow, pH fluctuations, and dietary habits can influence the behavior of restorative materials. Future research could focus on clinical trials to validate these findings and assess the real-world performance of these materials in vivo. Additionally, it would be useful to explore how other ions, such as magnesium (Mg2+) or zinc (Zn2+), contribute to the performance of bioactive materials, particularly in their role in remineralization and tissue repair.
Figure 1.
Comparison of pH variation in all materials at a) pH = 4.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) pH = 6.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) pH = 8.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 1.
Comparison of pH variation in all materials at a) pH = 4.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) pH = 6.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) pH = 8.8, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 2.
Comparison of [F]⁻ release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 2.
Comparison of [F]⁻ release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 3.
Comparison of [F]⁻ release from Stela Self Cure material across three different pH levels two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 3.
Comparison of [F]⁻ release from Stela Self Cure material across three different pH levels two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 4.
Comparison of [F]⁻ release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 4.
Comparison of [F]⁻ release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 5.
Comparison of [F]⁻ release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 5.
Comparison of [F]⁻ release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 6.
Comparison of [F]⁻ release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 6.
Comparison of [F]⁻ release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 7.
Comparison of [F]⁻ release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 7.
Comparison of [F]⁻ release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of [PO43-] release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 8.
Comparison of Ca2+ release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 8.
Comparison of Ca2+ release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Cention Forte Filling Material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 9.
Comparison of Ca2+ release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 9.
Comparison of Ca2+ release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Stela Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 10.
Comparison of Ca2+ release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 10.
Comparison of Ca2+ release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Riva Light Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 11.
Comparison of Ca2+ release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 11.
Comparison of Ca2+ release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Riva Self Cure material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 12.
Comparison of Ca2+ release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 12.
Comparison of Ca2+ release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Equia Forte HT Fil material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 13.
Comparison of Ca2+ release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 13.
Comparison of Ca2+ release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from Cention Primer material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 14.
Comparison of Ca2+ release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Figure 14.
Comparison of Ca2+ release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); b) Comparison of Si release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days); c) Comparison of Sr release from GC Fuji IX GP Fast material across three different pH levels, two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
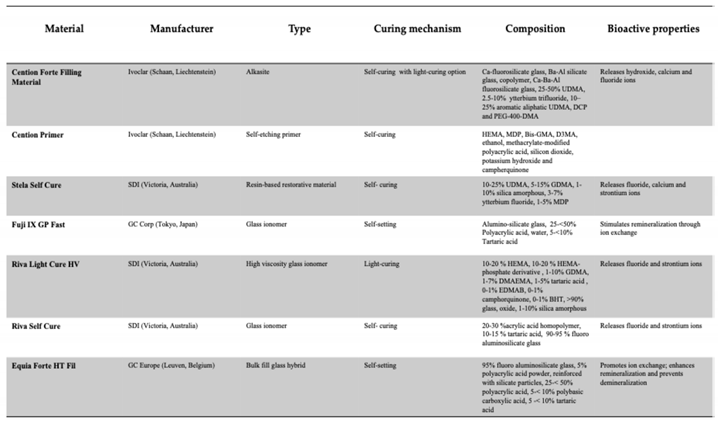
Table 1.
Type and chemical composition of the materials tested according to the available information from the manufacturer.
Table 1.
Type and chemical composition of the materials tested according to the available information from the manufacturer.
Table 2.
Average pH values with standard deviations (SD) observed for the different materials under three different acidity conditions, at two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
Table 2.
Average pH values with standard deviations (SD) observed for the different materials under three different acidity conditions, at two temperatures (44 and 37 °C) and at three different observation times (24 h, 7 days and 28 days).
| Paramenters |
Materials |
| pH |
Time |
T (°C) |
Cention Forte Filling |
Stela Self Cure |
Riva Light Cure |
Riva Self Cure |
Equia Forte Ht Fil |
CentionPrimer
|
Gc Fuji Ix Gp Fast |
| 4.8 |
1 day |
44 |
5.70 ± 0.04 |
5.83 ± 0.05 |
5.80 ± 0.08 |
5.85 ± 0.08 |
5.15 ± 0.07 |
5.95 ± 0.04 |
6.15 ± 0.03 |
| 37 |
5.02 ± 0.06 |
5.22 ± 0.03 |
5.12 ± 0.04 |
5.22 ± 0.07 |
4.87 ± 0.03 |
5.28 ± 0.07 |
5.42 ± 0.07 |
| 7 days |
44 |
5.91 ± 0.07 |
6.01 ± 0.07 |
6.03 ± 0.03 |
6.05 ± 0.08 |
5.63 ± 0.04 |
6.12 ± 0.06 |
6.23 ± 0.04 |
| 37 |
5.75 ± 0.03 |
5.85 ± 0.05 |
5.85 ± 0.06 |
5.90 ± 0.06 |
5.42 ± 0.07 |
5.95 ± 0.05 |
6.07 ± 0.06 |
| 28 days |
44 |
5.59 ± 0.04 |
5.69 ± 0.03 |
5.74 ± 0.07 |
5.78 ± 0.05 |
5.28 ± 0.03 |
5.85 ± 0.06 |
5.85 ± 0.05 |
| 37 |
5.53 ± 0.04 |
5.65 ± 0.05 |
5.64 ± 0.05 |
5.65 ± 0.03 |
5.18 ± 0.05 |
5.74 ± 0.07 |
5.86 ± 0.03 |
| 6.8 |
1 day |
44 |
5.61 ± 0.08 |
5.71 ± 0.06 |
5.70 ± 0.07 |
5.75 ± 0.07 |
5.84 ± 0.04 |
5.79 ± 0.03 |
5.90 ± 0.07 |
| 37 |
5.90 ± 0.07 |
6.10 ± 0.07 |
6.02 ± 0.08 |
6.12 ± 0.04 |
5.92 ± 0.03 |
6.29 ± 0.04 |
6.31 ± 0.03 |
| 7 days |
44 |
6.31 ± 0.07 |
6.41 ± 0.04 |
6.41 ± 0.05 |
6.45 ± 0.05 |
6.29 ± 0.04 |
6.47 ± 0.06 |
6.64 ± 0.06 |
| 37 |
6.95 ± 0.08 |
7.05 ± 0.04 |
7.10 ± 0.06 |
7.15 ± 0.08 |
6.48 ± 0.04 |
7.17 ± 0.08 |
7.25 ± 0.04 |
| 28 days |
44 |
6.53 ± 0.03 |
6.63 ± 0.03 |
6.66 ± 0.07 |
6.73 ± 0.05 |
6.13 ± 0.07 |
6.68 ± 0.06 |
6.97 ± 0.03 |
| 37 |
6.67 ± 0.05 |
6.77 ± 0.07 |
6.80 ± 0.06 |
6.85 ± 0.03 |
6.26 ± 0.08 |
6.96 ± 0.08 |
7.03 ± 0.05 |
| 8.8 |
1 day |
44 |
6.85 ± 0.04 |
6.95 ± 0.06 |
6.93 ± 0.08 |
7.08 ± 0.06 |
7.08 ± 0.05 |
8.58 ± 0.04 |
7.28 ± 0.04 |
| 37 |
7.36 ± 0.04 |
7.50 ± 0.03 |
7.5 ± 0.03 |
7.55 ± 0.08 |
7.28 ± 0.08 |
7.63 ± 0.07 |
7.73 ± 0.04 |
| 7 days |
44 |
8.06 ± 0.07 |
8.16 ± 0.05 |
8.15 ± 0.05 |
8.25 ± 0.07 |
8.05 ± 0.07 |
8.33 ± 0.04 |
8.44 ± 0.06 |
| 37 |
8.02 ± 0.05 |
8.12 ± 0.05 |
8.11 ± 0.04 |
8.22 ± 0.04 |
7.59 ± 0.08 |
8.25 ± 0.03 |
8.30 ± 0.07 |
| 28 days |
44 |
8.23 ± 0.04 |
8.33 ± 0.04 |
8.39 ± 0.07 |
8.40 ± 0.05 |
8.01 ± 0.03 |
7.11 ± 0.07 |
8.67 ± 0.05 |
| 37 |
7.68 ± 0.06 |
7.78 ± 0.07 |
7.86 ± 0.03 |
7.94 ± 0.06 |
7.64 ± 0.05 |
7.95 ± 0.06 |
8.09 ± 0.03 |