Submitted:
03 February 2025
Posted:
04 February 2025
You are already at the latest version
Abstract
Keywords:
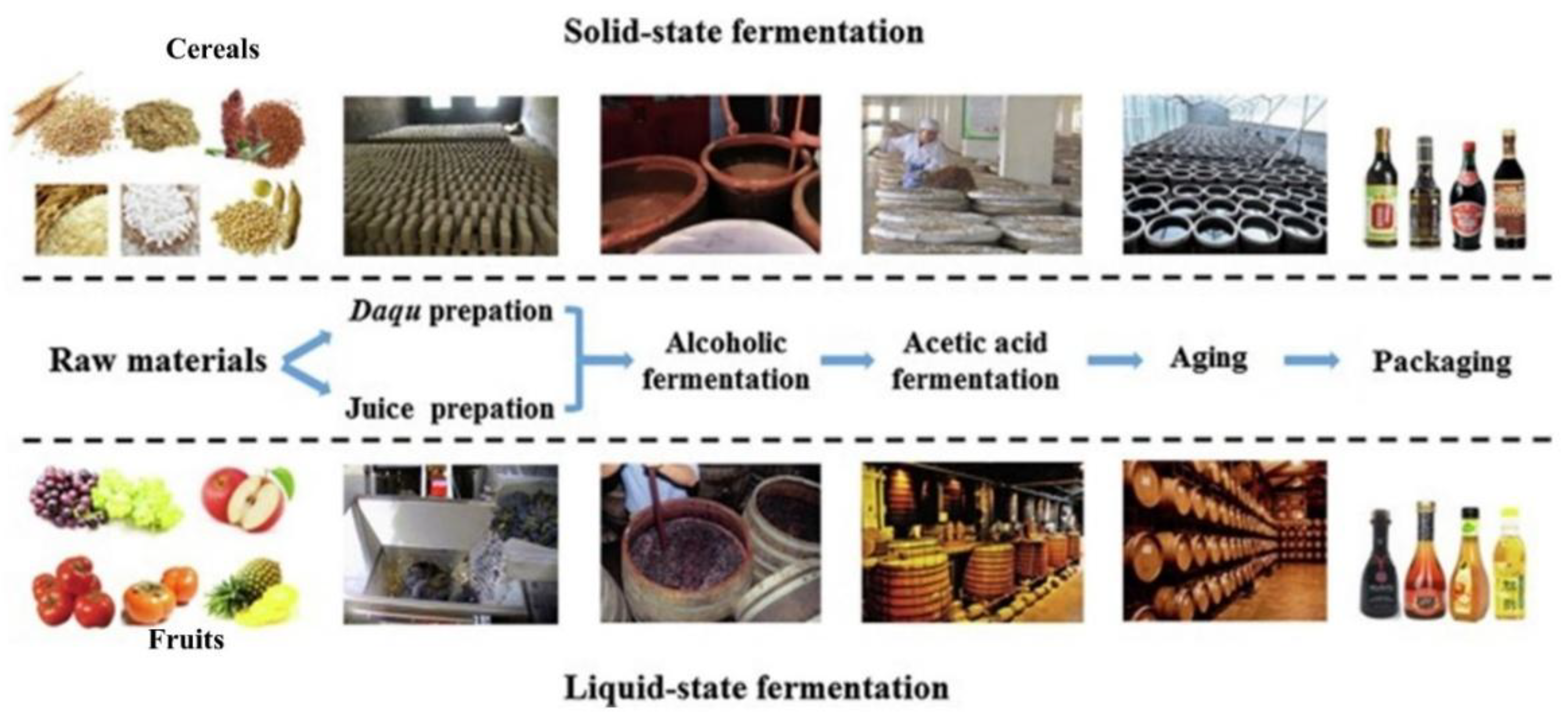
1. Introduction
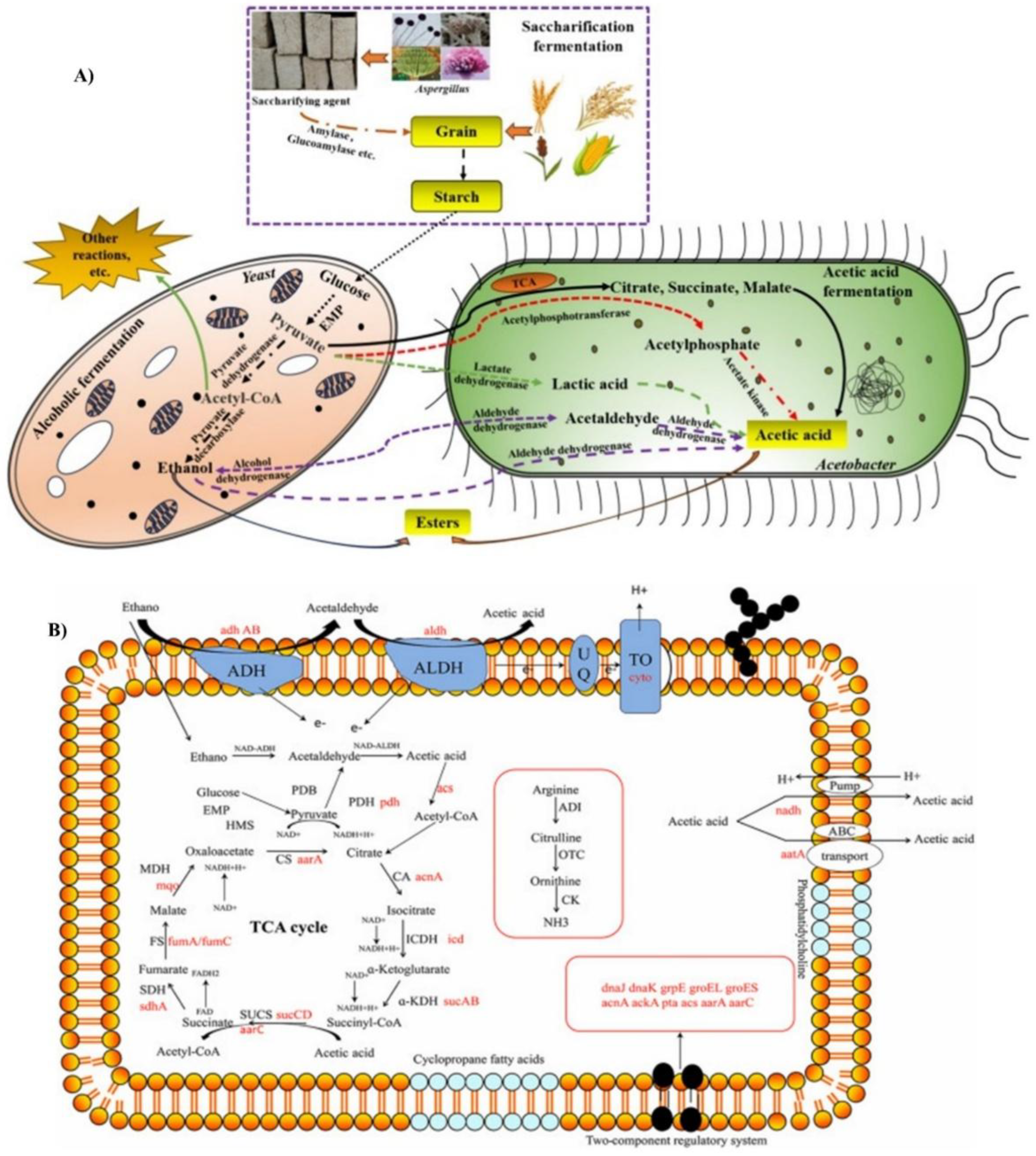
2. Diversity and Application of LAB During Vinegar Fermentation
3. Vinegar Functional Compounds
3.1. Organic Acids
3.2. Phenolic Compounds
3.3. Melanoidins and Tetramethylpyrazine
3.4. Other Bioactive Compounds
4. Functional Quality and Safety Improvements of Vinegar
4.1. Vinegar Quality Improvement
4.2. Vinegar Safety Performance Improvement
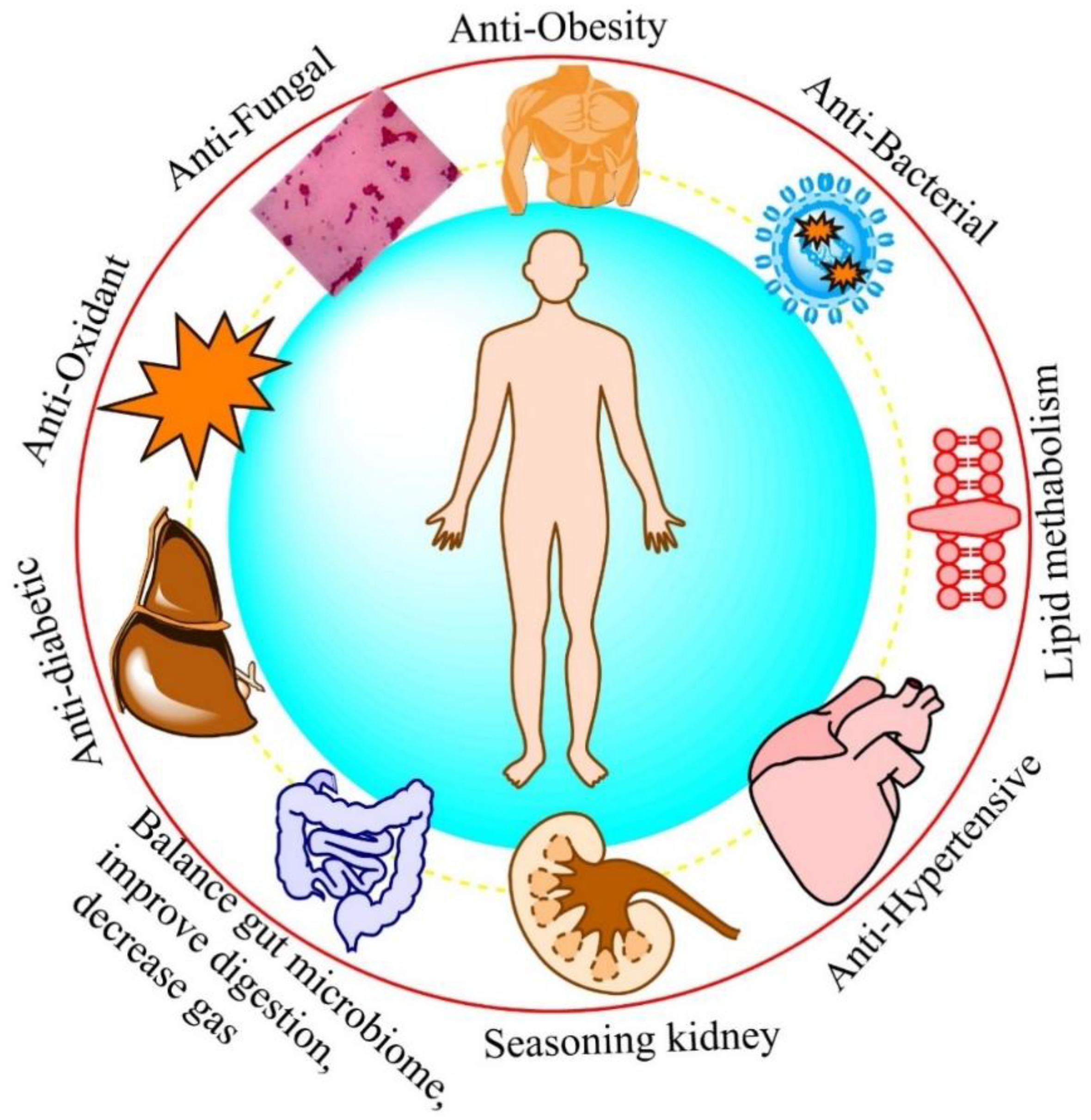
5. Vinegar Health Benefits
5.1. Anti-Microbial Activity
5.2. Antioxidant Potential
5.3. Anti-Inflammatory Activity
6. Conclusions
Funding
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Zhu, W. Liu, Y. Lan, X. Li, L. Luo, X. Duan, M. Lei, G. Liu, Z. Yang, X. Mai, Y. Sun, L. Wang, S. Lu, L. Ou, W. Wu, Z. Mai, D. Zhong, C. Cai, Z. Zhao, W. Zhong, Y. Liu, Y. Sun, and G. Zeng, Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. eBioMedicine. 2019, 45, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L. and P. Giudici, Vinegars of the World, in Vinegars of the World, L. Solieri and P. Giudici, Editors(2009), Springer Milan: Milano. p. 1-16. [CrossRef]
- Plessi, M. , VINEGAR, in Encyclopedia of Food Sciences and Nutrition (Second Edition), B. Caballero, Editor 2003), Academic Press: Oxford. p. 5996-6004. [CrossRef]
- Hemke, J., P. Rasane, S. Kaur, P. Kumbhar, and J. Singh, Vinegar: A traditional functional food. Think India Journal. 2019, 22, 581–629. [Google Scholar]
- Fang, G.-Y., L. -J. Chai, X.-Z. Zhong, and Y.-J. Jiang, Deciphering the succession patterns of bacterial community and their correlations with environmental factors and flavor compounds during the fermentation of Zhejiang rosy vinegar. International Journal of Food Microbiology. 2021, 341, 109070. [Google Scholar] [CrossRef]
- Garcia-Parrilla, M.C. J. Torija, A. Mas, A.B. Cerezo, and A.M. Troncoso, Chapter 25 - Vinegars and Other Fermented Condiments, in Fermented Foods in Health and Disease Prevention, J. Frias, C. Martinez-Villaluenga, and E. Peñas, Editors(2017), Academic Press: Boston. p. 577-591. [CrossRef]
- Wu, J.J., Y. K. Ma, F.F. Zhang, and F.S. Chen, Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiology. 2012, 30, 289–297. [Google Scholar] [CrossRef]
- Maske, B.L., G. V. de Melo Pereira, A. da Silva Vale, D.S. Marques Souza, J. De Dea Lindner, and C.R. Soccol, Viruses in fermented foods: are they good or bad? Two sides of the same coin. Food Microbiology. 2021, 98, 103794. [Google Scholar] [CrossRef]
- Budak, N.H., E. Aykin, A.C. Seydim, A.K. Greene, and Z.B. Guzel-Seydim, Functional Properties of Vinegar. Journal of Food Science. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Honsho, S., A. Sugiyama, A. Takahara, Y. Satoh, Y. Nakamura, and K. Hashimoto, A red wine vinegar beverage can inhibit the renin-angiotensin system: experimental evidence in vivo. Biological and Pharmaceutical Bulletin. 2005, 28, 1208–1210. [Google Scholar] [CrossRef]
- Perumpuli, P. and D.M.N. Dilrukshi, Vinegar: A functional ingredient for human health. International Food Research Journal. 2022, 29, 959–974. [Google Scholar] [CrossRef]
- Chen, H., T. Chen, P. Giudici, and F. Chen, Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Comprehensive Reviews in Food Science and Food Safety. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Sui, Y., J. Liu, Y. Liu, Y. Wang, Y. Xiao, B. Gao, and D. Zhu, In vitro probiotic characterization of Lactobacillus strains from fermented tangerine vinegar and their cholesterol degradation activity. Food Bioscience. 2021, 39, 100843. [Google Scholar] [CrossRef]
- Safari, R., S. H. Hoseinifar, S. Nejadmoghadam, and M. Khalili, Apple cider vinegar boosted immunomodulatory and health promoting effects of Lactobacillus casei in common carp (Cyprinus carpio). Fish & Shellfish Immunology. 2017, 67, 441–448. [Google Scholar] [CrossRef]
- Leal Maske, B., A. F. Murawski de Mello, A. da Silva Vale, J.G. Prado Martin, D.L. de Oliveira Soares, J. De Dea Lindner, C.R. Soccol, and G.V. de Melo Pereira, Exploring diversity and functional traits of lactic acid bacteria in traditional vinegar fermentation: A review. International Journal of Food Microbiology. 2024, 412, 110550. [Google Scholar] [CrossRef] [PubMed]
- Trček, J., A. Mahnič, and M. Rupnik, Diversity of the microbiota involved in wine and organic apple cider submerged vinegar production as revealed by DHPLC analysis and next-generation sequencing. International Journal of Food Microbiology. 2016, 223, 57–62. [Google Scholar] [CrossRef]
- Haruta, S., S. Ueno, I. Egawa, K. Hashiguchi, A. Fujii, M. Nagano, M. Ishii, and Y. Igarashi, Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. International Journal of Food Microbiology. 2006, 109, 79–87. [Google Scholar] [CrossRef]
- Shi, J., Y. Liu, W. Feng, X. Chen, Y. Zhu, and X. Liang. Investigation of Bacterial Diversity in Traditional Meigui Rice Vinegar by PCR-DGGE Method. in Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). 2014, Berlin, Heidelberg: Springer Berlin Heidelberg.
- Zhang, Q., C. Fu, C. Zhao, S. Yang, Y. Zheng, M. Xia, Y. Yan, F. Lang, and M. Wang, Monitoring microbial succession and metabolic activity during manual and mechanical solid-state fermentation of Chinese cereal vinegar. LWT. 2020, 133, 109868. [Google Scholar] [CrossRef]
- Li, T., X. Wang, C. Li, Q. Fu, X. Xu, J. Sun, C. Wang, J. Du, B. Wang, and X. Shi, Investigation of microbial succession and volatile compounds dynamics during the fermentation of traditional cereal vinegar in Xinjiang. LWT. 2023, 186, 115258. [Google Scholar] [CrossRef]
- Ye, X.; Yu, Y.; Liu, J.; Zhu, Y.; Yu, Z.; Liu, P.; Wang, Y.; Wang, K. Seasonal environmental factors drive microbial community succession and flavor quality during acetic acid fermentation of Zhenjiang aromatic vinegar. Frontiers in Microbiology 2024, 15. [Google Scholar] [CrossRef]
- Wang, Z.-M., Z. -M. Lu, J.-S. Shi, and Z.-H. Xu, Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Scientific Reports. 2016, 6, 26818. [Google Scholar] [CrossRef]
- Gan, X., H. Tang, D. Ye, P. Li, L. Luo, and W. Lin, Diversity and dynamics stability of bacterial community in traditional solid-state fermentation of Qishan vinegar. Annals of Microbiology. 2017, 67, 703–713. [Google Scholar] [CrossRef]
- Nie, Z., Y. Zheng, M. Wang, Y. Han, Y. Wang, J. Luo, and D. Niu, Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresource Technology. 2013, 148, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Fu, J., J. Feng, G. Zhang, J. Liu, N. Li, H. Xu, Y. Zhang, R. Cao, and L. Li, Role of bacterial community succession in flavor formation during Sichuan sun vinegar grain (Cupei) fermentation. Journal of Bioscience and Bioengineering. 2023, 135, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, J., Q. Li, K. Hu, J. Li, E. Durán-Guerrero, S. Liu, M. Guo, and A. Liu, Microbial characterization of Sichuan Baoning vinegar: lactic acid bacteria, acetic acid bacteria and yeasts. Archives of Microbiology. 2024, 206, 59. [Google Scholar] [CrossRef] [PubMed]
- Dong, K., W. Li, Q. Xu, Z. Hong, S. Zhang, B. Zhang, Y. Wu, H. Zuo, J. Liu, Z. Yan, and X. Pei, Exploring the correlation of metabolites changes and microbial succession in solid-state fermentation of Sichuan Sun-dried vinegar. BMC Microbiology. 2023, 23, 197. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Liu, Z.; Zhang, C.; Song, Y. Preparation of Rose Vinegar Rich in Gamma-Linolenic Acid and Analysis of its Nutritional Composition and Antioxidant Activity. American Journal of Biochemistry and Biotechnology. 2023, 19. [Google Scholar] [CrossRef]
- Jang, S.-W., H. H. Oh, K.E. Moon, B.-M. Oh, D.-Y. Jeong, and G.-S. Song, Lactic acid bacteria-malted vinegar: fermentation characteristics and anti-hyperlipidemic effect. Food Science and Biotechnology. 2024, 33, 1425–1436. [Google Scholar] [CrossRef]
- Zhang, L., M. Wang, H. Song, W. Liang, X. Wang, J. Sun, and D. Wang, Changes of microbial communities and metabolites in the fermentation of persimmon vinegar by bioaugmentation fermentation. Food Microbiology. 2024, 122, 104565. [Google Scholar] [CrossRef]
- Tang, H., H. Liang, J. Song, W. Lin, and L. Luo, Comparison of microbial community and metabolites in spontaneous fermentation of two types Daqu starter for traditional Chinese vinegar production. Journal of Bioscience and Bioengineering. 2019, 128, 307–315. [Google Scholar] [CrossRef]
- Song, J., J. -H. Zhang, S.-J. Kang, H.-Y. Zhang, J. Yuan, C.-Z. Zeng, F. Zhang, and Y.-L. Huang, Analysis of microbial diversity in apple vinegar fermentation process through 16s rDNA sequencing. Food Science & Nutrition. 2019, 7, 1230–1238. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and Functional Properties of Lactic Acid Bacteria Isolated From Wild Fruits and Flowers Present in Northern Argentina. Frontiers in Microbiology 2019, 10. [Google Scholar] [CrossRef]
- Chen, Y., Y. Huang, Y. Bai, C. Fu, M. Zhou, B. Gao, C. Wang, D. Li, Y. Hu, and N. Xu, Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT. 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Zhu, Y., F. Zhang, C. Zhang, L. Yang, G. Fan, Y. Xu, B. Sun, and X. Li, Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Scientific Reports. 2018, 8, 8612. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.-J., T. Qiu, Z.-M. Lu, Y.-J. Deng, X.-J. Zhang, J.-S. Shi, and Z.-H. Xu, Modulating microbiota metabolism via bioaugmentation with Lactobacillus casei and Acetobacter pasteurianus to enhance acetoin accumulation during cereal vinegar fermentation. Food Research International. 2020, 138, 109737. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, N.; Li, G. The Effect of In Vitro Gastrointestinal Digestion on the Antioxidants, Antioxidant Activity, and Hypolipidemic Activity of Green Jujube Vinegar. Foods 2022, 11, 1647. [Google Scholar] [CrossRef]
- Al-Rousan, W.M., A. N. Olaimat, T.M. Osaili, A.A. Al-Nabulsi, R.Y. Ajo, and R.A. Holley, Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiology. 2018, 73, 61–66. [Google Scholar] [CrossRef]
- Xia, T., B. Zhang, W. Duan, J. Zhang, and M. Wang, Nutrients and bioactive components from vinegar: A fermented and functional food. Journal of Functional Foods. 2020, 64, 103681. [Google Scholar] [CrossRef]
- Shi JiYong, S.J. X. Zou XiaoBo, H.X. Huang XiaoWei, Z.J. Zhao JieWen, L.Y. Li YanXiao, H.L. Hao LiMin, and Z.J. Zhang JianChun, Rapid detecting total acid content and classifying different types of vinegar based on near infrared spectroscopy and least-squares support vector machine. 2013.
- Nie, Z., Y. Zheng, S. Xie, X. Zhang, J. Song, M. Xia, and M. Wang, Unraveling the correlation between microbiota succession and metabolite changes in traditional Shanxi aged vinegar. Scientific Reports. 2017, 7, 9240. [Google Scholar] [CrossRef]
- Xu, W., Q. Xu, J. Chen, Z. Lu, R. Xia, G. Li, Z. Xu, and Y. Ma, Ligustrazine formation in Zhenjiang aromatic vinegar: changes during fermentation and storing process. Journal of the Science of Food and Agriculture. 2011, 91, 1612–1617. [Google Scholar] [CrossRef]
- Bureau, S., I. Ścibisz, C. Le Bourvellec, and C.M.G.C. Renard, Effect of Sample Preparation on the Measurement of Sugars, Organic Acids, and Polyphenols in Apple Fruit by Mid-infrared Spectroscopy. Journal of Agricultural and Food Chemistry. 2012, 60, 3551–3563. [Google Scholar] [CrossRef]
- Gao, Q., Y. Song, Y. Liang, Y. Li, Y. Chang, R. Ma, X. Cao, and S. Wang Dynamics of Physicochemical Properties, Functional Compounds and Antioxidant Capacity during Spontaneous Fermentation of Lycium ruthenicum Murr. (Qinghai–Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Wang, Z., T. Li, F. Liu, C. Zhang, H. Ma, L. Wang, and S. Zhao, Effects of ultrasonic treatment on the maturation of Zhenjiang vinegar. Ultrasonics Sonochemistry. 2017, 39, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Sáiz-Abajo, M.J., J. M. González-Sáiz, and C. Pizarro, Prediction of organic acids and other quality parameters of wine vinegar by near-infrared spectroscopy. A feasibility study. Food Chemistry. 2006, 99, 615–621. [Google Scholar] [CrossRef]
- Ren, M., X. Wang, C. Tian, X. Li, B. Zhang, X. Song, and J. Zhang, Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. Journal of Food Processing and Preservation. 2017, 41, e12937. [Google Scholar] [CrossRef]
- Zhu, H., J. Zhu, L. Wang, and Z. Li, Development of a SPME-GC-MS method for the determination of volatile compounds in Shanxi aged vinegar and its analytical characterization by aroma wheel. Journal of Food Science and Technology. 2016, 53, 171–183. [Google Scholar] [CrossRef]
- Lalou, S., E. Hatzidimitriou, M. Papadopoulou, V.G. Kontogianni, C.G. Tsiafoulis, I.P. Gerothanassis, and M.Z. Tsimidou, Beyond traditional balsamic vinegar: Compositional and sensorial characteristics of industrial balsamic vinegars and regulatory requirements. Journal of Food Composition and Analysis. 2015, 43, 175–184. [Google Scholar] [CrossRef]
- Fushimi, T., K. Suruga, Y. Oshima, M. Fukiharu, Y. Tsukamoto, and T. Goda, Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. British Journal of Nutrition. 2006, 95, 916–924. [Google Scholar] [CrossRef]
- Shen, F., J. Feng, X. Wang, Z. Qi, X. Shi, Y. An, Q. Zhang, C. Wang, M. Liu, B. Liu, and L. Yu, Vinegar Treatment Prevents the Development of Murine Experimental Colitis via Inhibition of Inflammation and Apoptosis. Journal of Agricultural and Food Chemistry. 2016, 64, 1111–1121. [Google Scholar] [CrossRef]
- Sanarico, D., S. Motta, L. Bertolini, and A. Antonelli, HPLC Determination of Organic Acids in Traditional Balsamic Vinegar of Reggio Emilia. Journal of Liquid Chromatography & Related Technologies. 2003, 26, 2177–2187. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Graziosi, R.; Maietti, A.; Tedeschi, P.; Marchetti, A.; Plessi, M. Traditional balsamic vinegar and balsamic vinegar of Modena analyzed by nuclear magnetic resonance spectroscopy coupled with multivariate data analysis. LWT - Food Science and Technology 2015, 60, 1017–1024. [Google Scholar] [CrossRef]
- Cocchi, M., C. Durante, M. Grandi, P. Lambertini, D. Manzini, and A. Marchetti, Simultaneous determination of sugars and organic acids in aged vinegars and chemometric data analysis. Talanta. 2006, 69, 1166–1175. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J., J. M. González-Sáiz, and C. Pizarro, Multi-objective optimisation strategy based on desirability functions used for chromatographic separation and quantification of l-proline and organic acids in vinegar. Analytica Chimica Acta. 2005, 528, 63–76. [Google Scholar] [CrossRef]
- Caligiani, A., D. Acquotti, G. Palla, and V. Bocchi, Identification and quantification of the main organic components of vinegars by high resolution 1H NMR spectroscopy. Analytica Chimica Acta. 2007, 585, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M., Y. Ogasawara, K. Endou, H. Akano, T. Nakajima, T. Aoyama, and K. Nakamura, Fermentation-induced changes in the concentrations of organic acids, amino acids, sugars, and minerals and superoxide dismutase-like activity in tomato vinegar. International Journal of Food Properties. 2017, 20, 888–898. [Google Scholar] [CrossRef]
- Liu, F. and Y. He, Application of successive projections algorithm for variable selection to determine organic acids of plum vinegar. Food Chemistry. 2009, 115, 1430–1436. [Google Scholar] [CrossRef]
- Corsini, L., R. Castro, C. G. Barroso, and E. Durán-Guerrero, Characterization by gas chromatography-olfactometry of the most odour-active compounds in Italian balsamic vinegars with geographical indication. Food Chemistry. 2019, 272, 702–708. [Google Scholar] [CrossRef]
- Morales, M.L., A. G. Gonzalez, and A.M. Troncoso, Ion-exclusion chromatographic determination of organic acids in vinegars. Journal of Chromatography A. 1998, 822, 45–51. [Google Scholar] [CrossRef]
- Dykes, L. and L.W. Rooney, Sorghum and millet phenols and antioxidants. Journal of Cereal Science. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Shimoji, Y., Y. Tamura, Y. Nakamura, K. Nanda, S. Nishidai, Y. Nishikawa, N. Ishihara, K. Uenakai, and H. Ohigashi, Isolation and Identification of DPPH Radical Scavenging Compounds in Kurosu (Japanese Unpolished Rice Vinegar). Journal of Agricultural and Food Chemistry. 2002, 50, 6501–6503. [Google Scholar] [CrossRef]
- Aykın, E., N. H. Budak, and Z.B. Güzel-Seydim, Bioactive Components of Mother Vinegar. Journal of the American College of Nutrition. 2015, 34, 80–89. [Google Scholar] [CrossRef]
- Budak, H.N. and Z.B. Guzel-Seydim, Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. Journal of the Science of Food and Agriculture. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Veberic, R., M. Trobec, K. Herbinger, M. Hofer, D. Grill, and F. Stampar, Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. Journal of the Science of Food and Agriculture. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Perron, N.R. and J.L. Brumaghim, A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochemistry and Biophysics. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Cerezo, A.B., W. Tesfaye, M.J. Torija, E. Mateo, M.C. García-Parrilla, and A.M. Troncoso, The phenolic composition of red wine vinegar produced in barrels made from different woods. Food Chemistry. 2008, 109, 606–615. [Google Scholar] [CrossRef]
- Budak, N.H., D. Kumbul Doguc, C.M. Savas, A.C. Seydim, T. Kok Tas, M.I. Ciris, and Z.B. Guzel-Seydim, Effects of Apple Cider Vinegars Produced with Different Techniques on Blood Lipids in High-Cholesterol-Fed Rats. Journal of Agricultural and Food Chemistry. 2011, 59, 6638–6644. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H., P. Kadiroğlu, N.B. Demircan, and S. Selli, Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. Journal of the Institute of Brewing. 2017, 123, 407–416. [Google Scholar] [CrossRef]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit Antioxidants during Vinegar Processing: Changes in Content and in Vitro Bio-Accessibility. International Journal of Molecular Sciences 2016, 17, 1658. [Google Scholar] [CrossRef]
- Bertelli, D., A. Maietti, G. Papotti, P. Tedeschi, G. Bonetti, R. Graziosi, V. Brandolini, and M. Plessi, Antioxidant Activity, Phenolic Compounds, and NMR Characterization of Balsamic and Traditional Balsamic Vinegar of Modena. Food Analytical Methods. 2015, 8, 371–379. [Google Scholar] [CrossRef]
- Xie, X., Y. Zheng, X. Liu, C. Cheng, X. Zhang, T. Xia, S. Yu, and M. Wang, Antioxidant Activity of Chinese Shanxi Aged Vinegar and Its Correlation with Polyphenols and Flavonoids During the Brewing Process. Journal of Food Science. 2017, 82, 2479–2486. [Google Scholar] [CrossRef]
- Yusoff, N.A., M. F. Yam, H.K. Beh, K.N. Abdul Razak, T. Widyawati, R. Mahmud, M. Ahmad, and M.Z. Asmawi, Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia. Asian Pacific Journal of Tropical Medicine. 2015, 8, 595–605. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, T.; Du, P.; Duan, W.; Zhang, B.; Zhang, J.; Zhu, S.; Zheng, Y.; Wang, M.; Yu, Y. Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process. Molecules. 2018, 23, 2949. [Google Scholar] [CrossRef]
- Xu, Q.P., Z. H. Ao, and W.Y. Tao, Antioxidative activity of Heng-shun aromatic vinegar extracts. China Brewing. 2004, 7, 16–18. [Google Scholar]
- Xu, Q.P., W. Y. Tao, and Z.H. Ao, Bioactivity of ethanol supernate of vinegar. Journal of Food Science and Biotechnology. 2005, 24, 76–80. [Google Scholar]
- Verzelloni, E., D. Tagliazucchi, and A. Conte, From balsamic to healthy: Traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food and Chemical Toxicology. 2010, 48, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Kharchoufi, S., J. Gomez, C. Lasanta, R. Castro, F. Sainz, and M. Hamdi, Benchmarking laboratory-scale pomegranate vinegar against commercial wine vinegars: antioxidant activity and chemical composition. Journal of the Science of Food and Agriculture. 2018, 98, 4749–4758. [Google Scholar] [CrossRef]
- Beh, B.K., N. E. Mohamad, S.K. Yeap, K.L. Lim, W.Y. Ho, H.M. Yusof, S.A. Sharifuddin, A. Jamaluddin, K. Long, and N.B. Alitheen, Polyphenolic profiles and the in vivo antioxidant effect of nipa vinegar on paracetamol induced liver damage. RSC Advances. 2016, 6, 63304–63313. [Google Scholar] [CrossRef]
- Kim, H., H. -D. Hong, H.-J. Suh, and K.-S. Shin, Structural and immunological feature of rhamnogalacturonan I-rich polysaccharide from Korean persimmon vinegar. International Journal of Biological Macromolecules. 2016, 89, 319–327. [Google Scholar] [CrossRef]
- Zou, B., G. Xiao, Y. Xu, J. Wu, Y. Yu, and M. Fu, Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chemistry. 2018, 255, 23–30. [Google Scholar] [CrossRef]
- Fan, J., Y. Zhang, X. Chang, B. Zhang, D. Jiang, M. Saito, and Z. Li, Antithrombotic and Fibrinolytic Activities of Methanolic Extract of Aged Sorghum Vinegar. Journal of Agricultural and Food Chemistry. 2009, 57, 8683–8687. [Google Scholar] [CrossRef]
- Shimoji, Y., H. Kohno, K. Nanda, Y. Nishikawa, H. Ohigashi, K. Uenakai, and T. Tanaka, Extract of Kurosu, a Vinegar From Unpolished Rice, Inhibits Azoxymethane-Induced Colon Carcinogenesis in Male F344 Rats. Nutrition and Cancer. 2004, 49, 170–173. [Google Scholar] [CrossRef]
- Plessi, M., D. Bertelli, and F. Miglietta, Extraction and identification by GC-MS of phenolic acids in traditional balsamic vinegar from Modena. Journal of Food Composition and Analysis. 2006, 19, 49–54. [Google Scholar] [CrossRef]
- Barnaba, C., E. Dellacassa, G. Nicolini, T. Nardin, M. Malacarne, and R. Larcher, Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction – ultrahigh-performance liquid chromatography – quadrupole-orbitrap mass spectrometry. Journal of Chromatography A. 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Á.M., R. Castro, M.C. Rodrı́guez, D.A. Guillén, and C.G. Barroso, Study of the antioxidant power of brandies and vinegars derived from Sherry wines and correlation with their content in polyphenols. Food Research International. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive Compounds in Cornelian Cherry Vinegars. Molecules. 2018, 23, 379. [Google Scholar] [CrossRef]
- Nakamura, K., Y. Ogasawara, K. Endou, S. Fujimori, M. Koyama, and H. Akano, Phenolic Compounds Responsible for the Superoxide Dismutase-like Activity in High-Brix Apple Vinegar. Journal of Agricultural and Food Chemistry. 2010, 58, 10124–10132. [Google Scholar] [CrossRef] [PubMed]
- Chen, H., Y. Zhou, Y. Shao, and F. Chen, Free Phenolic Acids in Shanxi Aged Vinegar: Changes During Aging and Synergistic Antioxidant Activities. International Journal of Food Properties. 2016, 19, 1183–1193. [Google Scholar] [CrossRef]
- Wang, H.-Y., H. Qian, and W.-R. Yao, Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chemistry. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Echavarría, A.P., J. Pagán, and A. Ibarz, Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Engineering Reviews. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Aili, W., S. Huanlu, R. Changzhong, and L. Zaigui, Key aroma compounds in Shanxi aged tartary buckwheat vinegar and changes during its thermal processing. Flavour and Fragrance Journal. 2012, 27, 47–53. [Google Scholar] [CrossRef]
- Tesfaye, W., M. L. Morales, M.C. García-Parrilla, and A.M. Troncoso, Evolution of Phenolic Compounds during an Experimental Aging in Wood of Sherry Vinegar. Journal of Agricultural and Food Chemistry. 2002, 50, 7053–7061. [Google Scholar] [CrossRef]
- Hofmann, T. , Studies on the Relationship between Molecular Weight and the Color Potency of Fractions Obtained by Thermal Treatment of Glucose/Amino Acid and Glucose/Protein Solutions by Using Ultracentrifugation and Color Dilution Techniques. Journal of Agricultural and Food Chemistry. 1998, 46, 3891–3895. [Google Scholar] [CrossRef]
- Pastoriza, S. and J.A. Rufián-Henares, Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chemistry. 2014, 164, 438–445. [Google Scholar] [CrossRef]
- Liu, J., J. Gan, S. Nirasawa, Y. Zhou, J. Xu, S. Zhu, and Y. Cheng, Cellular uptake and trans-enterocyte transport of phenolics bound to vinegar melanoidins. Journal of Functional Foods. 2017, 37, 632–640. [Google Scholar] [CrossRef]
- Tagliazucchi, D., E. Verzelloni, and A. Conte, Antioxidant properties of traditional balsamic vinegar and boiled must model systems. European Food Research and Technology. 2008, 227, 835–843. [Google Scholar] [CrossRef]
- Yang, L., X. Wang, and X. Yang, Possible Antioxidant Mechanism of Melanoidins Extract from Shanxi Aged Vinegar in Mitophagy-Dependent and Mitophagy-Independent Pathways. Journal of Agricultural and Food Chemistry. 2014, 62, 8616–8622. [Google Scholar] [CrossRef] [PubMed]
- Guo, L. and X. Yang, Separation of melanoidin from Shanxi Aged Vinegar and its antibacterial activity. Food Science. 2016, 37, 25–30. [Google Scholar]
- Li, S., P. Li, X. Liu, L. Luo, and W. Lin, Bacterial dynamics and metabolite changes in solid-state acetic acid fermentation of Shanxi aged vinegar. Applied Microbiology and Biotechnology. 2016, 100, 4395–4411. [Google Scholar] [CrossRef]
- Wang, A., J. Zhang, and Z. Li, Correlation of volatile and nonvolatile components with the total antioxidant capacity of tartary buckwheat vinegar: Influence of the thermal processing. Food Research International. 2012, 49, 65–71. [Google Scholar] [CrossRef]
- Chen, J.-C., Q. -H. Chen, Q. Guo, S. Ruan, H. Ruan, G.-Q. He, and Q. Gu, Simultaneous determination of acetoin and tetramethylpyrazine in traditional vinegars by HPLC method. Food Chemistry. 2010, 122, 1247–1252. [Google Scholar] [CrossRef]
- Chinnici, F., E. Durán Guerrero, F. Sonni, N. Natali, R. Natera Marín, and C. Riponi, Gas Chromatography−Mass Spectrometry (GC−MS) Characterization of Volatile Compounds in Quality Vinegars with Protected European Geographical Indication. Journal of Agricultural and Food Chemistry. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Chen, J., J. Tian, H. Ge, R. Liu, and J. Xiao, Effects of tetramethylpyrazine from Chinese black vinegar on antioxidant and hypolipidemia activities in HepG2 cells. Food and Chemical Toxicology. 2017, 109, 930–940. [Google Scholar] [CrossRef]
- Wang, L.S., Q. Guo, J. Han, Y.F. Zhang, and X.J. Chen, Pharmacokinetics of ligustrazine in blood, brain, and liver of mice. Chin Tradit Herb Drugs. 2009, 40, 935–8. [Google Scholar]
- Castano, G., R. Menendez, R. Mas, A. Amor, J.L. Fernandez, R.L. Gonzalez, M. Lezcay, and E. Alvarez, Effects of policosanol and lovastatin on lipid profile and lipid peroxidation in patients with dyslipidemia associated with type 2 diabetes mellitus. International journal of clinical pharmacology research. 2002, 22, 89–99. [Google Scholar] [PubMed]
- Cao, L., X. Song, Y. Song, J. Bi, S. Cong, C. Yu, and M. Tan, Fluorescent nanoparticles from mature vinegar: their properties and interaction with dopamine. Food & Function. 2017, 8, 4744–4751. [Google Scholar] [CrossRef]
- Ho, C.W., A. M. Lazim, S. Fazry, U.K.H.H. Zaki, and S.J. Lim, Varieties, production, composition and health benefits of vinegars: A review. Food Chemistry. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Bouazza, A., A. Bitam, M. Amiali, A. Bounihi, L. Yargui, and E.A. Koceir, Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharmaceutical Biology. 2016, 54, 260–265. [Google Scholar] [CrossRef]
- Shi, H., X. Zhou, Y. Yao, A. Qu, K. Ding, G. Zhao, and S.Q. Liu, Insights into the microbiota and driving forces to control the quality of vinegar. LWT. 2022, 157, 113085. [Google Scholar] [CrossRef]
- Bertan, F.A.B., E. da Silva Pereira Ronning, M.L.K. Marchioro, T.L.C. Oldoni, R.F.H. Dekker, and M.A.A. da Cunha, Valorization of pineapple processing residues through acetification to produce specialty vinegars enriched with red-Jambo extract of Syzygium malaccense leaf. Scientific Reports. 2022, 12, 19384. [Google Scholar] [CrossRef]
- Zhang, Z., Z. -h. Zhang, R. He, G. Zhao, Y. Yu, R. Zhang, and X. Gao, Research advances in technologies and mechanisms to regulate vinegar flavor. Food Chemistry. 2024, 460, 140783. [Google Scholar] [CrossRef]
- Hashimoto, M., K. Obara, M. Ozono, M. Furuyashiki, T. Ikeda, Y. Suda, K. Fukase, Y. Fujimoto, and H. Shigehisa, Separation and characterization of the immunostimulatory components in unpolished rice black vinegar (kurozu). Journal of Bioscience and Bioengineering. 2013, 116, 688–696. [Google Scholar] [CrossRef]
- Spinosa, W.A., V. d. Santos Júnior, D. Galvan, J.L. Fiorio, and R.J.H.C. Gomez, Vinegar rice (Oryza sativa L.) produced by a submerged fermentation process from alcoholic fermented rice. Food Science and Technology. 2015, 35, 196–201. [Google Scholar] [CrossRef]
- Gullo, M. and P. Giudici, Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. International Journal of Food Microbiology. 2008, 125, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M., M. J. Torija, A. Mas, M.L. Morales, and A.M. Troncoso, Changes of volatile compounds in wine vinegars during their elaboration in barrels made from different woods. Food Chemistry. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Wang, C.Y., J. Zhang, and Z.Z. Gui, Acetobacter bacteria are found in Zhenjiang vinegar grains. Genetics and Molecular Research. 2015, 14, 5054–5064. [Google Scholar] [CrossRef]
- Johnston, C.S., A. M. White, and S.M. Kent, A preliminary evaluation of the safety and tolerance of medicinally ingested vinegar in individuals with type 2 diabetes. Journal of medicinal food. 2008, 11, 179–183. [Google Scholar] [CrossRef]
- Launholt, T.L., C. B. Kristiansen, and P. Hjorth, Safety and side effects of apple vinegar intake and its effect on metabolic parameters and body weight: a systematic review. European Journal of Nutrition. 2020, 59, 2273–2289. [Google Scholar] [CrossRef]
- Bounihi, A., A. Bitam, A. Bouazza, L. Yargui, and E.A. Koceir, Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharmaceutical Biology. 2017, 55, 43–52. [Google Scholar] [CrossRef]
- Hlebowicz, J., G. Darwiche, O. Björgell, and L.-O. Almér, Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: a pilot study. BMC Gastroenterology. 2007, 7, 46. [Google Scholar] [CrossRef]
- Wang, X., K. Hu, F. Liu, J. Mou, J. Lai, M. Zhang, S. Wang, Q. Li, J. Li, A. Liu, X. Ao, L. He, S. Chen, Y. Yang, and S. Liu, Isolation and characterization of a gas-producing and acid-resistant bacterium from spoiled vinegar. International Journal of Food Microbiology. 2023, 394, 110167. [Google Scholar] [CrossRef]
- Lalou, S., S. A. Ordoudi, and F.T. Mantzouridou, Evaluation of safety and quality parameters of persimmon balsamic-type vinegar during multistarter culture fermentation and accelerated aging with oak chips. Food Bioscience. 2024, 57, 103526. [Google Scholar] [CrossRef]
- Yıkmış, S., F. Aksu, S.S. Altunatmaz, and B.G. Çöl Ultrasound Processing of Vinegar: Modelling the Impact on Bioactives and Other Quality Factors. Foods. 2021, 10, 1703. [Google Scholar] [CrossRef]
- Yıkmış, S., E. Bozgeyik, and M.A. Şimşek, Ultrasound processing of verjuice (unripe grape juice) vinegar: effect on bioactive compounds, sensory properties, microbiological quality and anticarcinogenic activity. Journal of Food Science and Technology. 2020, 57, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, D., M. Ward, and A.J. Shah, Antibacterial apple cider vinegar eradicates methicillin resistant Staphylococcus aureus and resistant Escherichia coli. Scientific Reports. 2021, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-F., W. -N. Yu, and B.-L. Zhang, Manufacture and antibacterial characteristics of Eucommia ulmoides leaves vinegar. Food Science and Biotechnology. 2020, 29, 657–665. [Google Scholar] [CrossRef]
- Reygaert, W.; Jusufi, I. Green tea as an effective antimicrobial for urinary tract infections caused by Escherichia coli. Frontiers in Microbiology. 2013, 4. [Google Scholar] [CrossRef]
- Cui, Y., Y. J. Oh, J. Lim, M. Youn, I. Lee, H.K. Pak, W. Park, W. Jo, and S. Park, AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiology. 2012, 29, 80–87. [Google Scholar] [CrossRef]
- Yagnik, D., V. Serafin, and A. J. Shah, Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Scientific Reports. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Desvita, H., M. Faisal, Mahidin, and Suhendrayatna, Antimicrobial potential of wood vinegar from cocoa pod shells (Theobroma cacao L.) against Candida albicans and Aspergillus niger. Materials Today: Proceedings. 2022, 63, S210–S213. [Google Scholar] [CrossRef]
- Oramahi, H.A. and T. Yoshimura, Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl. Journal of Wood Science. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Chien, L.H., M. Yasuhide, and S. Tsang-Chyi, Application of Moso Bamboo Vinegar with Different Collection Temperature to Evaluate Fungi Resistance of Moso Bamboo Materials. Journal of the Faculty of Agriculture, Kyushu University. 2008, 53, 107–113. [Google Scholar]
- Shiah, T.-C., S. -K. Wu, J.-C. Huang, and H.C. Lin, The fungi resistance of bamboo materials treated with bamboo vinegar using soaking treatment. Journal of Agriculture and Forestry, NCYU. 2006, 3, 1–22. [Google Scholar]
- Xia, T., J. Yao, J. Zhang, Y. Zheng, J. Song, and M. Wang, Protective effects of Shanxi aged vinegar against hydrogen peroxide-induced oxidative damage in LO2 cells through Nrf2-mediated antioxidant responses. RSC Advances. 2017, 7, 17377–17386. [Google Scholar] [CrossRef]
- Beh, B.K., N. E. Mohamad, S.K. Yeap, H. Ky, S.Y. Boo, J.Y.H. Chua, S.W. Tan, W.Y. Ho, S.A. Sharifuddin, K. Long, and N.B. Alitheen, Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Scientific Reports. 2017, 7, 6664. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H., Y. -J. Kim, and K.-H. Koh, Investigation into factors influencing antioxidant capacity of vinegars. Applied Biological Chemistry. 2016, 59, 495–509. [Google Scholar] [CrossRef]
- Ali, Z., H. Ma, A. Wali, I. Ayim, and M.N. Sharif, Daily date vinegar consumption improves hyperlipidemia, β-carotenoid and inflammatory biomarkers in mildly hypercholesterolemic adults. Journal of Herbal Medicine. 2019, 17–18, 100265. [Google Scholar] [CrossRef]
- Hwa, H.S., M. Kwon, H.Y. Lee, Y.M. Park, D.Y. Shin, and J.S. Choi, Immunomodulatory effect of fermented vinegar on cyclophosphamide-induced immunosuppression model. J Food Nutr Res. 2021, 9, 469–476. [Google Scholar]
- Meng, H., J. Song, Y. Li, X. Li, X. Li, J. Gou, Z. Nie, J. Wang, Y. Zheng, and M. Wang, Monascus vinegar protects against liver inflammation in high-fat-diet rat by alleviating intestinal microbiota dysbiosis and enteritis. Journal of Functional Foods. 2022, 93, 105078. [Google Scholar] [CrossRef]
- Choi, J.-H., S. -E. Park, S.-H. Yeo, and S. Kim Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes. Foods 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Xia, T., J. Zhang, J. Yao, B. Zhang, W. Duan, C. Zhao, P. Du, J. Song, Y. Zheng, and M. Wang Shanxi Aged Vinegar Protects against Alcohol-Induced Liver Injury via Activating Nrf2-Mediated Antioxidant and Inhibiting TLR4-Induced Inflammatory Response. Nutrients. 2018, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Frontiers in Microbiology. 2016, 7. [Google Scholar] [CrossRef]
- Dibner, J.J. and P. Buttin, Use of Organic Acids as a Model to Study the Impact of Gut Microflora on Nutrition and Metabolism1. Journal of Applied Poultry Research. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Zare, R., A. Abedian Kenari, and M. Yazdani Sadati, Influence of dietary acetic acid, protexin (probiotic), and their combination on growth performance, intestinal microbiota, digestive enzymes, immunological parameters, and fatty acids composition in Siberian sturgeon (Acipenser baerii, Brandt, 1869). Aquaculture International. 2021, 29, 891–910. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, K.; Zhang, H.-J.; Wu, S.-G.; Han, Y.-M.; Wu, Y.-Y.; Qi, G.-H.; Wang, J. Organic Acids as Alternatives for Antibiotic Growth Promoters Alter the Intestinal Structure and Microbiota and Improve the Growth Performance in Broilers. Frontiers in Microbiology. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D., A. Kang, W. Yao, J. Lou, Q. Zhang, B. Bao, Y. Cao, S. Yu, S. Guo, Y. Zhang, Y. Tang, and L. Zhang, Euphorbia kansui fry-baked with vinegar modulates gut microbiota and reduces intestinal toxicity in rats. Journal of Ethnopharmacology. 2018, 226, 26–35. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Frontiers in Microbiology. 2016, 7. [Google Scholar] [CrossRef]
- Luu, L.A., R. H. Flowers, Y. Gao, M. Wu, S. Gasperino, A.L. Kellams, D.C. Preston, B.J. Zlotoff, J.A. Wisniewski, and S.L. Zeichner, Apple cider vinegar soaks do not alter the skin bacterial microbiome in atopic dermatitis. PloS one. 2021, 16, e0252272. [Google Scholar] [CrossRef]
- Dębińska, A. and B. Sozańska Fermented Food in Asthma and Respiratory Allergies—Chance or Failure? Nutrients. 2022, 14, 1420. [Google Scholar] [CrossRef]
- Lee, C.S., E. H. Yi, H.-R. Kim, S.-R. Huh, S.-H. Sung, M.-H. Chung, and S.-K. Ye, Anti-dermatitis effects of oak wood vinegar on the DNCB-induced contact hypersensitivity via STAT3 suppression. Journal of Ethnopharmacology. 2011, 135, 747–753. [Google Scholar] [CrossRef]
- Prado, C.M., M. A. Martins, and I.F.L.C. Tibério, Nitric Oxide in Asthma Physiopathology. International Scholarly Research Notices. 2011, 2011, 832560. [Google Scholar] [CrossRef]
- Lv, H., Z. Li, Z. Xie, X. Hu, H. Li, J. Sun, X. Chen, and C. Wen, Innovated formulation of TCM pangolin scales to develop a nova therapy of rheumatoid arthritis. Biomedicine & Pharmacotherapy. 2020, 126, 109872. [Google Scholar] [CrossRef]
- Chen, L., Y. Chen, H. Yun, and Z. Jianli, Tetramethylpyrazine (TMP) protects rats against acute pancreatitis through NF-κB pathway. Bioengineered. 2019, 10, 172–181. [Google Scholar] [CrossRef]
- Chen, J., J. Chen, X. Wang, C. Wang, W. Cao, Y. Zhao, B. Zhang, M. Cui, Q. Shi, and G. Zhang, Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways. Biomedicine & Pharmacotherapy. 2016, 82, 1–7. [Google Scholar] [CrossRef]
- Ozturk, I., O. Caliskan, F. Tornuk, N. Ozcan, H. Yalcin, M. Baslar, and O. Sagdic, Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT - Food Science and Technology. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Chou, C.-H., C. -W. Liu, D.-J. Yang, Y.-H.S. Wu, and Y.-C. Chen, Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chemistry. 2015, 168, 63–69. [Google Scholar] [CrossRef]
- Seo, K.-I., J. Lee, R.-Y. Choi, H.-I. Lee, J.-H. Lee, Y.-K. Jeong, M.-J. Kim, and M.-K. Lee, Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced obese mice. Food & Function. 2014, 5, 1579–1586. [Google Scholar] [CrossRef]
- Lee, J.-H., H. -D. Cho, J.-H. Jeong, M.-K. Lee, Y.-K. Jeong, K.-H. Shim, and K.-I. Seo, New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chemistry. 2013, 141, 3241–3249. [Google Scholar] [CrossRef]
- Guan, Q.; Gong, T.; Lu, Z.-M.; Geng, Y.; Duan, W.; Ren, Y.-L.; Zhang, X.-J.; Chai, L.-J.; Shi, J.-S.; Xu, Z.-H. Hepatoprotective Effect of Cereal Vinegar Sediment in Acute Liver Injury Mice and Its Influence on Gut Microbiota. Frontiers in Nutrition. 2021, 8. [Google Scholar] [CrossRef]
- Geng, Y., Y. Yue, Q. Guan, Y. Ren, L. Guo, Y. Fan, Z.-M. Lu, J.-S. Shi, and Z.-H. Xu, Cereal Vinegar Sediment Alleviates Spontaneous Ulcerative Colitis in Il-10 Deficient Mice. Molecular Nutrition & Food Research. 2021, 65, 2001227. [Google Scholar] [CrossRef]
- Du, P., J. Song, H. Qiu, H. Liu, L. Zhang, J. Zhou, S. Jiang, J. Liu, Y. Zheng, and M. Wang Polyphenols Extracted from Shanxi-Aged Vinegar Inhibit Inflammation in LPS-Induced RAW264.7 Macrophages and ICR Mice via the Suppression of MAPK/NF-κB Pathway Activation. Molecules. 2021, 26, 2745. [Google Scholar] [CrossRef]
- Du, P., J. Zhou, L. Zhang, J. Zhang, N. Li, C. Zhao, L. Tu, Y. Zheng, T. Xia, J. Luo, J. Song, and M. Wang, GC × GC-MS analysis and hypolipidemic effects of polyphenol extracts from Shanxi-aged vinegar in rats under a high fat diet. Food & Function. 2020, 11, 7468–7480. [Google Scholar] [CrossRef]
- Coelho, E., Z. Genisheva, J.M. Oliveira, J.A. Teixeira, and L. Domingues, Vinegar production from fruit concentrates: effect on volatile composition and antioxidant activity. Journal of Food Science and Technology. 2017, 54, 4112–4122. [Google Scholar] [CrossRef]
- Davies, C.V., L. M. Gerard, M.M. Ferreyra, M.d.C. Schvab, and C.A. Solda, Bioactive compounds and antioxidant activity analysis during orange vinegar production. Food Science and Technology. 2017, 37, 449–455. [Google Scholar] [CrossRef]
- Özdemir, N., H. Pashazadeh, O. Zannou, and I. Koca, Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT. 2022, 154, 112716. [Google Scholar] [CrossRef]
- Chen, C., S. Wu, Y. Li, Y. Huang, and X. Yang, Effects of different acetic acid bacteria strains on the bioactive compounds, volatile compounds and antioxidant activity of black tea vinegar. LWT. 2022, 171, 114131. [Google Scholar] [CrossRef]
- Budak, H.N. , Alteration of antioxidant activity and total phenolic content during the eight-week fermentation of apple cider vinegar. Horticultural Studies. 2021, 38, 39–45. [Google Scholar] [CrossRef]
- Chatatikun, M., A. Tedasen, N.C. Pattaranggoon, W. Palachum, S. Chuaijit, A. Mudpan, S. Pruksaphanrat, S. Sohbenalee, K. Yamasaki, and W.K. Klangbud, Antioxidant activity, anti-tyrosinase activity, molecular docking studies, and molecular dynamic simulation of active compounds found in nipa palm vinegar. PeerJ. 2023, 11, e16494. [Google Scholar]
- Yıldızlı, G., G. Coral, and F. Ayaz, Anti-bacterial, anti-fungal, and anti-inflammatory activities of wood vinegar: a potential remedy for major plant diseases and inflammatory reactions. Biomass Conversion and Biorefinery. 2024, 14, 3633–3642. [Google Scholar] [CrossRef]
- Wei, N., X. Wang, Y. Wu, L. Liu, Y. Zhao, and R. Zhao, Comparative Study on Anti-Inflammatory Effect of Polysaccharides from Vinegar-Baked Radix Bupleuri Using Different Methods. ACS Omega. 2023, 8, 29253–29261. [Google Scholar] [CrossRef]
- El Abdali, Y., H. Saghrouchni, M. Kara, I. Mssillou, A. Allali, Y.A.B. Jardan, N.E. Kafkas, E.-M. El-Assri, H.-A. Nafidi, M. Bourhia, K.S. Almaary, N. Eloutassi, and A. Bouia Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities. Plants. 2023, 12, 3850. [Google Scholar] [CrossRef]
- Liao, W., Y. Chen, Z. Zhu, J. Chen, T. Gao, B. Limsila, Y. Techadamrongsin, L. Wang, J. Yu, C. Fu, and R. Li, Vinegar-processed Curcuma phaeocaulis promotes anti-angiogenic activity and reduces toxicity in zebrafish and rat models. Pharmaceutical Biology. 2021, 59, 408–415. [Google Scholar] [CrossRef]
| Vinegars | Organic acids | References |
|---|---|---|
| Traditional balsamic vinegar | Citric, tartaric, gluconic, malic, succinic , lactic and acetic, formic , and tartaric acids | [53,54,55] |
| Alcohol vinegar | Acetic acid | [56] |
| Cider vinegar | Acetic, citric, formic, lactic, malic, and succinic acids | [57] |
| Wine vinegar | Tartaric, malic, lactic, acetic, citric, succinic and formic acids | [47,57] |
| Tomato vinegar | Acetic, citric, formic, lactic, malic, and succinic acids | [57,58] |
| Plum vinegar | Acetic, tartaric, and lactic acids | [59] |
| Balsamic vinegar | Acetic, formic, citric, lactic, malic and succinic, tartaric, propanoic acid, 2 methylpropanoic acid, butanoic acid, 3-methylbutanoic acid, (E)- but-2-enoic acid, hexanoic acid, octanoic acid, 4-oxopentanoic acid, furan-2-carboxylic acid and 2-phenylacetic acids | [50,54,60] |
| Persimmon vinegar | Acetic, lactic, quinic, tartaric, propanedioic , malic, and succinic acids | [48] |
| Malt vinegar | Acetic, citric, lactic, and succinic acids | [56] |
| Apple vinegar | Acetic, lactic, quinic, tartaric, propanedioic, malic, succinic and citric acids | [48] |
| Kiwifruit vinegar | Acetic, lactic, quinic, tartaric, propanedioic, malic, succinic and citric acids | [48] |
| Sherry vinegar | Acetic, tartaric, lactic, malic, and citric acids | [61] |
| Zhenjiang vinegar | Acetic, 2-methyl propionate, 3-methylbutanoic, caproic, octanoic and propionic acids | [46] |
| Shanxi aged vinegar | Acetic, propionic, butyric acid, 3-methyl butyric, pentanoic, hexanoic, lactic, succinic, tartaric and citric acids | [49] |
| Black wolfberry vinegar | Lactic, acetic, sorbic, ascorbic, succinic, oxalic, malic, citric, tartaric and γ-aminobutyric (GABA) acids | [45] |
| Vinegar types | Phenolic compounds | References |
|---|---|---|
| Traditional balsamic vinegar | Furan-2-carboxylic, 5 hydroxyfuran-2-carboxylic,4-hydroxybenzoic, vanillic, protocatecuic, syringic, isoferulic, pcoumaric, gallic, ferulic and caffeic acids |
[85] |
| Balsamic vinegar | Protocatechuic, gallic, p-coumaric, syringic, caffeic, ferulic, vanillic, salicylic, homovanillic, hydroxytyrosol, gentisic, p-carboxyphenol, protocatechuic, sinapinic and p-hydroxybenzoic acids and phenol, catechin, aesculetin, epicatechin, vanillin, coniferyl alcohol, 4-methylcatechol, syringaldehyde, isopropiovanillone, scopoletin, aceto-/isoacetovanillone, isopropiosiringone, acetosyringone, isoacetosiringone, syringol, coniferylaldehyde, sinapinaldehyde, tryptophol, o-vanillina, methyl vanillate, (m + p)-cresol, 4-ethylcatechol, ocresol, vanillyl ethyl ether, guaiacol, 4-methylsyringol, 4-vinylphenol, ethyl vanillate, 3,4- xylenol, 4-vinylguaiacol, ellagic acid, 4-ethylphenol, 4-methylguaiacol, 4-ethylguaiacol, 4-allylsyringol, eugenol and isoeugenol |
[85,86] |
| Grape vinegar | Gallic, chlorogenic, caffeic, syringic, and ferulic acids and catechin and epicatechin | [65] |
| Sherry vinegar | Gallic acid, Ellagic acid, protocatechuic, caffeoylquinic acid, protocatechualdehyde, tyrosol, p-OH-benzoic acid, catechin, p-OH-benzaldehyde, siringic, vanillin, caftaric, cis-p-coutaric, trans-p-coutaric, fertaric, caffeic, cis-p-coumaric, trans-p-coumaric, ferulic acids and quercetin 3-o-galactoside, quercetin 3-oglucuronide, kaempferol 3-o-galactoside, pelargonidine 3-o-galactoside, pelargonidine 3- o-robinobioside and aromadendrin 7-o-glucoside | [87,88] |
| Apple vinegar | Gallic, vanillic, chlorogenic, caffeic, p-coumaric, trans-ferulic, 4-pcoumaroylquinic, pcoumaroylquinic, p hydroxybenzoic, and protocatechuic acids, and (−)-epicatechin gallate and phloridzin |
[48,89] |
| Apple cider vinegar | Gallic acid, catechin, epicatechin, chlorogenic acid, caffeic acid, and p-coumaric acid | [69] |
| Persimmon vinegar | Gallic, chlorogenic, caffeic, p-coumaric, trans-ferulic, Hydroxycinnamic, acids, (−)-epicatechin gallate, gallocatechin gallate, procyanidin A2, rutin epigallocatechin phloridzin, catechin hydrate, flavanols | [48,82] |
| Red wine vinegar | Gallic acid, protocatechuic acid, caffeic acid, vanillic acid, catechin, epicatechin, caftaric acid, syringic acid, ellagic acid, p-coumaric acid, ferulic acid and chlorogenic acid | [65,68] |
| Shanxi aged vinegar | Protocatechuic, p-hydroxybenzoic, salicylic, dihydrosinapic, p-coumaric, sinapic, dihydroferulic and ferulic acids | [90] |
| Pomegranate vinegar | Gallic acid, punicalagin, catechin, vanillic acid, syringic acid, galloylglucoside, protocatechuic acid, ethyl gallate, ellagic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, ferulic acid hexoside, tyrosol and trans-p-Coumaric derivates | [79] |
| Zhenjiang aromaticvinegar | Gallic, vanillic, chlorogenic, p-coumaric and trans-ferulic acids, epicatechin and catechin hydrate | [48] |
| Types of vinegars | Bacterial strain used for fermentation | Methods employed | Quality improvement applied | References |
|---|---|---|---|---|
| Black vinegar | Acetobacter pasteurianus | Saccharification of rice by Aspergillus oryzae | A year of aging | [114] |
| Cereal vinegar | Acetobacter sp. | Submerged fermentation of rice wine (Oryza sativa L. | [115] | |
| Balsamic vinegar | Gluconacetobacter europaeus and/or Acetobacter malorum | Spontaneous acetification cooked of grape must using seed-vinager | Aged in wood barrels | [116] |
| White and red wine vinegar | Gluconacetobacter europaeus and/or Acetobacter malorum | Surface culture or submerged culture acetification | Aged in wood barrels (oak, chestnut, acacia and cherry) | [117] |
| Mature vinegar (Zhenjiang vinegar maturation) |
Acetobacter, Lactobacillus, Gluconoacetate, Bacillus | Surface culture or submerged culture acetification | Aging by ultrasonic treatment | [46,118] |
| Aromatic vinegar (Zhenjiang aromatic vinegar) | Lactobacillus acetotolerans, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus fermentum, Acetobacter pasteurianus | Solid-state fermentation | Addition of ground herbs | [22] |
| Fruit vinegar | Wild acetic bacteria strains | Acetification of pineapple pulp and peel wines using wild acetic bacteria strains | Leaf extract of Red-Jambo, Syzygium malaccense | [112] |
| Vegetable vinegars | Target activity | Objective of the study | Main findings | References |
|---|---|---|---|---|
| Leaves of Eucommia ulmoides | Antibacterial potential | Study the mechanism of action against B. subtilis | -↗antibacterial effect and yeast - Cell wall and cell membrane were damaged - ↗ increasing the cell permeability |
[128] |
| Grape, apple, Artichoke, pomegranate, Apple-Lemon, Hawthorne and sour cherry | Antimicrobial potential and antiradical activity | Distinguish between the traditional and industrailised Turkish vinegars. | -↗ antimicrobial activity in traditional vinegars compared to industrial ones -↗ antiradical potential of pomegranate vinegars compared to industrial ones |
[157] |
| Black vinegar | Antiox. Act and in vivo lipid-lowering | Investigate these activities via a hamster model | - ↘ weight gain -↗ lipid contents and hepatic Antiox. Act |
[158] |
| Tomato | Lipid and Glu metabolic enzyme | -Mechanism investigation -Arbitrated the anti-insulin and anti-obesity effect |
-↘ fat accumulation -Variations arbitrated by PPARa and AMPK increased expression. |
[159] |
| Tomato | Anti-obesity impact | Assess the efficiency in lipid accumulation | -Inhibition of lipid formation. | [160] |
| Cereal | Hepatoprotective impact | Explore the hepatoprotective impact | Changes in gut microbiota | [161] |
| Cereal | Impact on the spontaneous colitis impact | Explore the process of spontaneous colitis and investigate the variations in the in the epithelial wall function, inflammation and gut microbiota. | -Improvement of epithelium damage, -Inhibition of myeloperoxidase activity and malondialdehyde (MDA) |
[162] |
| Shanxi-aged vinegar | Anti-inflammatory activities | Investigate the anflammatory mechanism | -Enhancement of the lipid, inflammatory stress and oxidative stress. | [163] |
| Shanxi-aged vinegar | Impact on gut microbiome and metabolome | Investigate the immune/inflammation factors and explore the in vivo impact of vinegar on gut microbiome and metabolome. | - ↘Inflammatory factors - Vinegar consumption changed gut microbiota structure |
[164] |
| Orange, mango, cherry and banana | Antioxidant potential | Investigate the kinetics, chemical profile and Antiox. Act | -Total antioxidant activity was assessed at 8 and 40 times greater than a commercial vinegar. | [165] |
| Orange | Antioxidant potential | Investigate the fluctuations of chemical profile and Antiox. Act during fermentation. | Antiox. Act was linked to ascorbic acid and phenolic compounds levels. | [166] |
| Rosehip fruit (Rosa canina L.) | Antioxidant potential | Expose the chemical profile and Antiox. Act | -↗ of antioxidant activity linked to the ↘ of flavonoids | [167] |
| black tea | Antioxidant potential | -Evaluation the chemical profile and Antiox. Act | - ↗of organic acids contents, volatile compounds and the antioxidant activity | [168] |
| Apple Cider | Antioxidant potential | Investigate the change of antioxidant properties and bioactive compounds | →Antioxidant activity and phenolic substances during the acetic acid fermentation. | [169] |
| green jujube | Antioxidant potential | Study the impact of the in vitro gastrointestinal digestion on the Antiox. Act and hypolipidemic potential | -↗ correlation between Antiox. Act, TPC, TFC, and total acid contents -weak correlation with cholesterol adsorption capacity/antioxidant capacity |
[38] |
| nipa palm | Antioxidant and anti-tyrosinase activities |
-Characterize chemical profile, Antiox. Act, and anti-tyrosinase potential. -Perform molecular docking study and molecular dynamic simulation |
-Concentration-dependent anti-tyrosinase activity and antioxidant potential | [170] |
| Wood | Antimicrobial and anti-inflammatory potential | -Asses the in vivo inflammatory activity of the mammalian macrophages and antimicrobial activity against pathogenic bacteria and fungi | -Stimulation of mammalian macrophages by lipopolysaccharide -High antimicrobial activity but no anti-bacteriophage activity |
[171] |
| Cudrania tricuspidata Fruits (CTFV) | Anti-Inflammatory potential | In vitro anti-inflammatory impacts | CTFV reduced inflammatory reaction by improving inflammatory factors | [142] |
| vinegar-baked Radix Bupleuri (VBCP) | Anti-Inflammatory potential | -study the impact of extraction techniques on the physicochemical properties and biological activities of VBCP | ammonia-assisted extraction is an effective tool to achieve high anti-inflammatory | [172] |
| Apple | antioxidant, antimicrobial, antidepressant and anti-inflammatory activities |
Study the biological activity of our different apple cultivars, as well as physicochemical attributes and chemical composition |
-↗ antidepressant impact -effective against bacteria, -↗ antioxidant activity |
[173] |
| Curcuma phaeocaulis | anti-angiogenic effect | Evaluation of anti-angiogenic impact and toxicity of C. phaeocaulis via zebrafish and rat models. | -↘ toxicity and ↗anti-angiogenic activity | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





