1. Introduction
The fortunes of density separation as a method of concentrating Foraminifera from sandy matrixes declined since its drawbacks (that include toxicity, low recovery rates and poor or marginal recovery of agglutinated/arenaceous tests) become clear (Schönfeld et al., 2012).
In the early 1980’s, the usage of Carbon Tetrachloride (CCl4 – density 1.5867 g/cm3) for the density separation of Foraminifera from sandy matrixes (see e.g., de Vernal et al., 2010) was widespread and – with the only aid of a fume hood – open to any university student (as in the case of the author). Increased environmental awareness and improvements in workplace safety rules resulted in severe restrictions of the use of toxic organic compounds, while less toxic or harmless alternatives emerged (including Zinc Chloride and since the late 1980’s the variable density solutions of Polytungstates reported e.g., by Savage, 1988), soon becoming the golden standard to concentrate Foraminifera from loose sandy substrates. The Introduction in Parent et Al. (2018) provides an exhaustive overview on all the main recent techniques, and provide comparative tests of Trichloroethylene, Zinc Chloride, and Sodium Polytungstate. Parent et Al. (2018) cite the use of Bromoform, Zinc Bromide and Calcium Bromide (density 1.65 g/cm3) advocated also by Thomsen (Thomsen, 1989; Thomsen, 1991).
Both their cost and the difficulty of supply keep such alternatives out of the reach of the avocational micropaleontologist who, usually, is compelled to hand-pick Foraminifera from the sandy matrix, an exacting and time-consuming activity. Furthermore, hand picking Foraminifera under the microscope results necessarily in an incomplete collection, which is affected by the perceptive and cognitive biases of the operator, and gets increasingly ineffective as the microfossils size decreases. Considering that also hand picking of Foraminifera has its cons, anybody more concerned about time expenditure than about the above-mentioned drawbacks of density separation may gladly adopt a limited risk and low-cost version of the density separation technique: this short report illustrates the results obtained by the usage of Perchloroethylene (Tetrachloroethylene, Cl2C=CCl2), a chemical that can be easily obtained as stain remover and is commonly used in dry-cleaner’s, whose density of 1.622 g/cm3 compares favourably with that of Carbon Tetrachloride, and that has been widely used for zoological and paleontological studies involving Foraminifera until recently (Coulbourn & Resig, 1975; Debenay, 2012; Kotthoff et al., 2017; Murray & Alve, 1999) as an alternative to Trichloroethylene (a chemical included in the comparative analysis by Parent et Al., 2018).
2. Materials and Methods
Warning: the activities described here were performed only after reading and understanding a Safety Data Sheet of the Perchloroethylene. References include Sigma-Aldrich (2019) and Univar Solutions (2022).
A sandy clay sample of approximately 1 kg was collected from an outcrop of the Argille di Fangario formation (Langhian/Serravallian age) on the slope of the Giara di Gesturi plateau, in the territory of the Commune of Assolo (Sardinia, Italy) at latitude/longitude 39° 47’ 45.258”/ 8° 53’ 7.040”.
The sample was dried and wet sieved above a 64μ sieve to retain the sandy matrix, recovering around 205 g of sand.
The sand was granulometrically fractioned by dry sieving in the following dimensional categories: above 590μ (sterile, weight around 62 g), 590μ - 250μ, 250μ (28 g) - 125μ (65g), 125μ - 64μ (50 g). Fractioning is a practice originally intended to ease hand-picking of Foraminifera, but in this case, it allowed to check whether the recovery rate by density separation varies for each category.
Accurate measurements or precise statistical data are out of the scope of this short report, that is aimed at obtaining a first impression of the effectiveness and of the efficacy of home-made density separation. Although no weight data about the non-sinking portion was obtained for the lack of a high-precision scales, figures illustrate the relative size of the two matrix portions (sinking and non-sinking, the latter including floating and suspended fraction).
The rate of retention of Foraminifera in the sinking portion was not measured: considering the relative rarity of Foraminifera in the untreated matrix, their successful massive extraction by density separation results in their further increased rarefaction, that would impose long and exhausting sessions to ascertain the presence and the quantity of the very few Foraminifera remaining in the sinking portion. Furthermore, as many including Schönfeld et al. (2012) have demonstrated, one can take for granted that density separation by definition cannot be exhaustive: its efficacy decreases for those tests, such as the agglutinated/arenaceous ones, whose prevalently sandy composition leads to a density comparable with that of loose sand granules. The images and the short narrative will give a convincing impression of the efficacy of the method.
This is not at all a faunal study: calculation of depth and oceanicity index were not performed, although the overwhelming presence of planktonic species is evident: again, this report is entirely focused on the process of home-managed “heavy fluid” separation.
To allow the evaluation of the relative efficiency of the two techniques, the author collected by hand-picking a given volume of Foraminifera in successive session totalling around 10 hours of work (see the Conclusions).
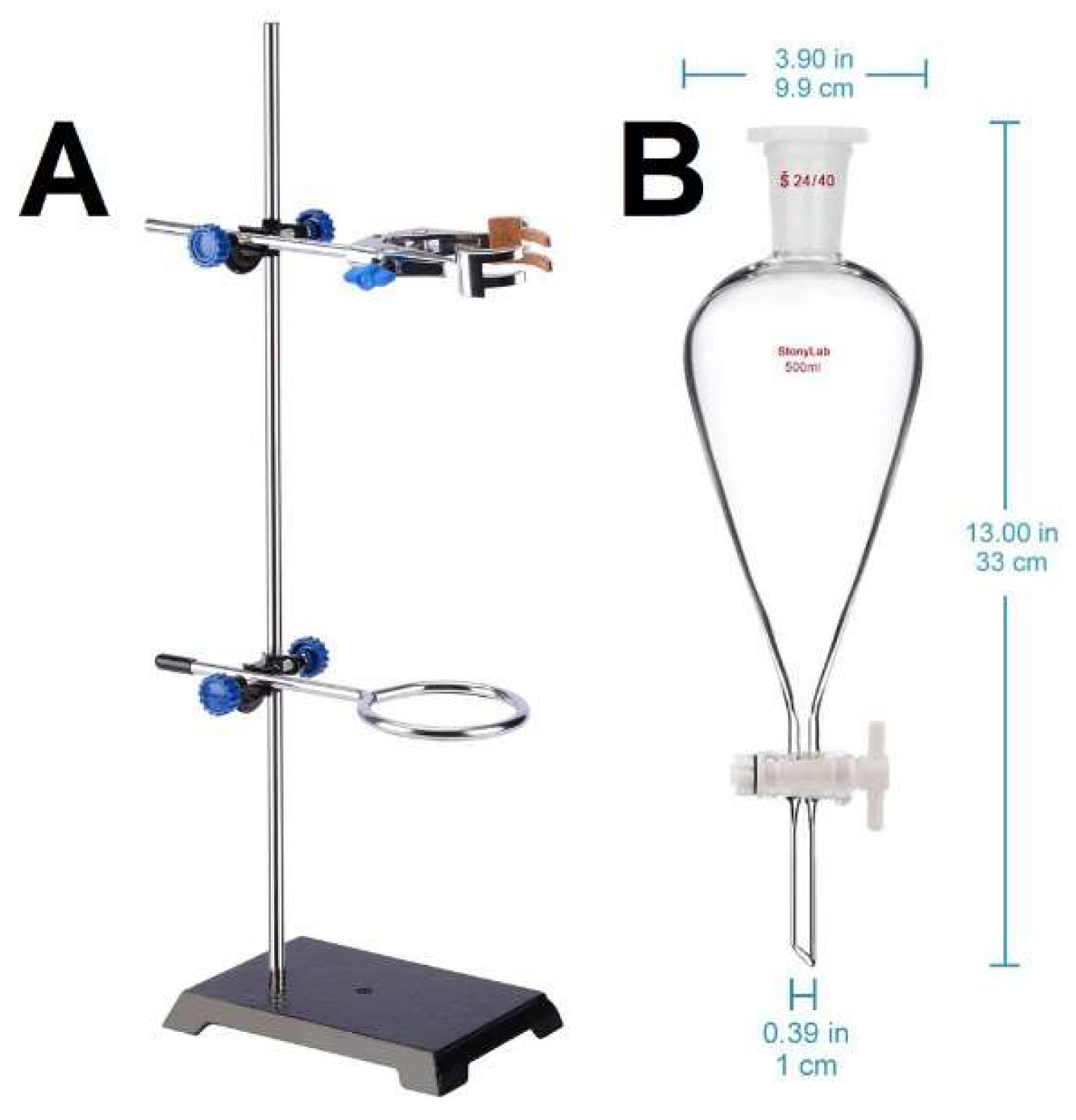
Density separation was performed by a pear-shaped separatory funnel, commercially noted as “Squibb funnel”, with a capacity of 500 ml (“Stonylab 500ml Separatory Funnel”, see the References), mounted on its special stand (“Stonylab Lab Stand Set”, see the References), as shown in
Figure 1.
The detachable Teflon tap (“stopcock”) as provided with the separatory funnel had a 3mm wide hole, a size that could have slowed down or compromised the release of the sandy phase during the separation process. For that reason, the hole diameter was progressively brought to 5mm (the same internal diameter of the funnel’s drain) by suitably using an electric drill with increasing diameter drill bits (3.5 mm, 4 mm, 4.5 mm and 5 mm) to ensure smoothness and axiality of the larger diameter hole.
Figure 3.
The PCE packaging used in this study.
Figure 3.
The PCE packaging used in this study.
The “heavy liquid” used in this study was pure Tetrachloroethylene (TCE), in this case «Multichimica Percloro Puro» (see the References). The total cost of the equipment including TCE slightly exceeded 100 Euro, shipping included.
Each granulometric fraction was separately treated. All the activities involving the usage of TCE were performed outside of the author’s residence (garage and adjacent area) to avoid the accumulation of dangerous fumes in the house. The author constantly wore eye protection and rubber gloves, as well as a disposable apron.
The brand-new separatory funnel was placed on its special stand, that in turn was placed on a small table, with the further precaution of fastening the stand to the table by two table clamps, for additional safety against spills in case of accidental impacts on the funnel or on the beaker (with a capacity of 330 cc) that was be placed directly under the funnel’s drain. For successive uses of the funnel, preliminary rinsing with a small amount of TCE (that can be filtered and recycled) may help in the removal of particles from previous use cycles.
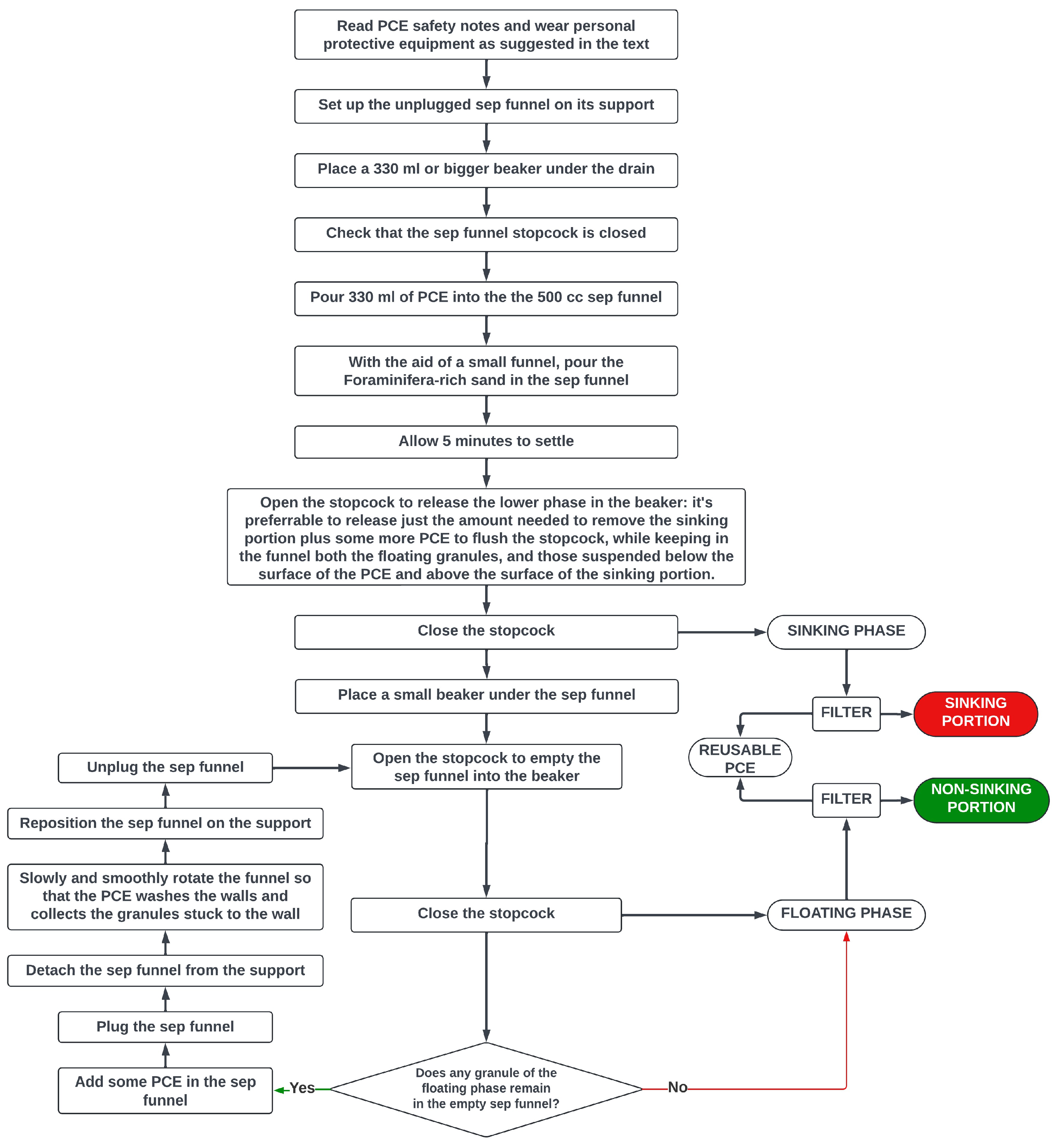
For the treatment of each separate fraction, after ensuring that the stopcock (tap) under the funnel was in the closed position, around 330 ml of TCE were poured into the funnel. The subsequent steps, illustrated in the self-explanatory
Figure 4, are repeated separately for each granulometric fraction. It’s important to remember that the open stopcock does not drain if the separatory funnel is sealed by its top plug. Unless otherwise noted, all the steps are performed keeping the funnel unplugged.
As clarified in
Figure 4, the sinking portion was removed by opening the stopcock and releasing just the sunken sand to remove plus some more PCE to flush the stopcock: that way, both the floating granules, and those suspended in the fluid above the surface of the sinking portion remained in the funnel for subsequent recovery.
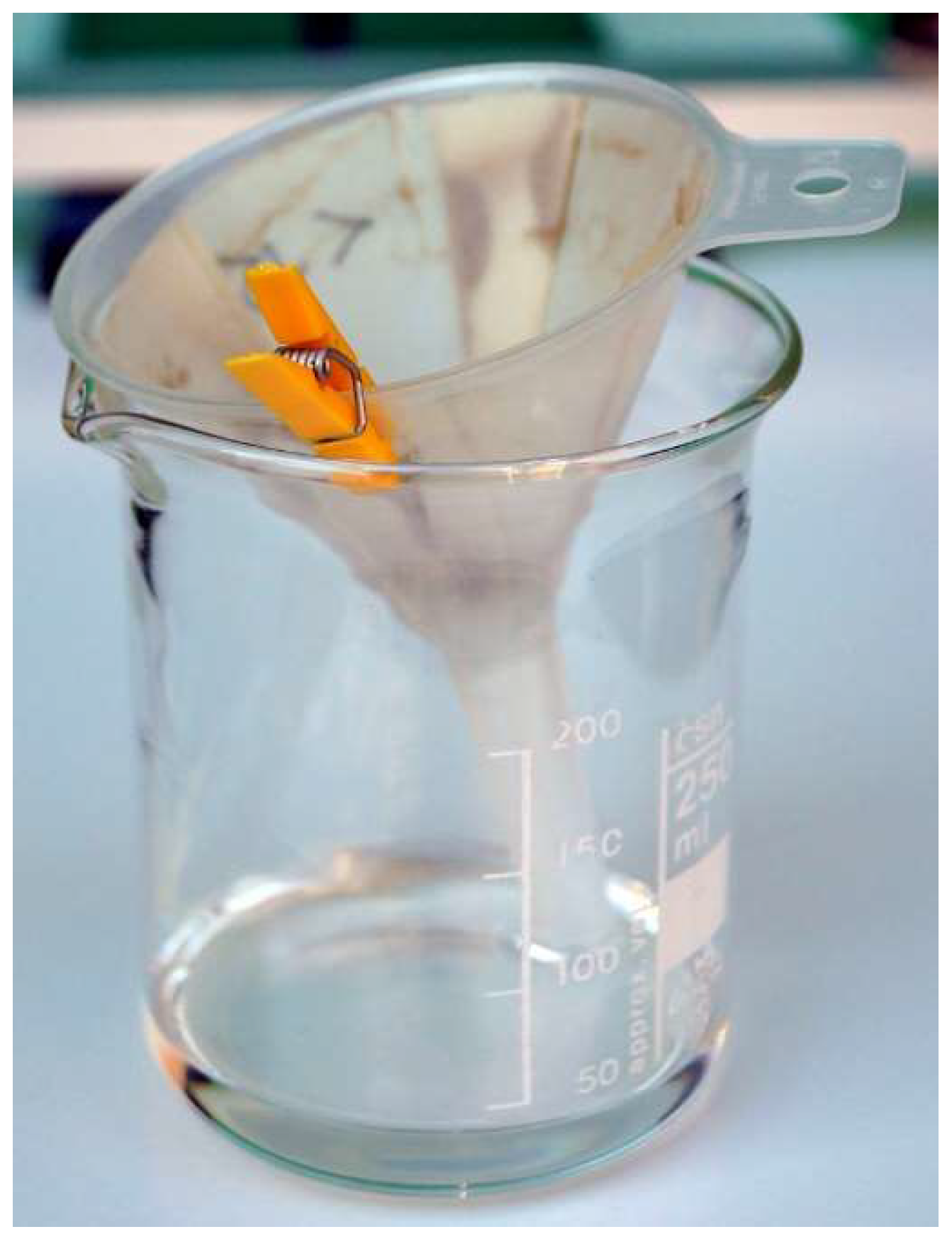
After density separation, the floating portion and the sinking portion were separated by filter paper and small funnels, in short bursts not exceeding the small funnel capacity. After complete recovery of the TCE, wet filter paper was allowed to dry until the TCE evaporated, then its contents were collected and the filter paper was disposed of to avoid inter-sample contamination in case small Foraminifera are stuck to the paper.
Figure 5 illustrates the main phases of the separation process. Particular attention was put in recovering the granules that, unavoidably, remain attached to the internal walls of the separatory funnel as the level of TCE decreases during the recovery of the sinking phase. Even though it may be almost impossible to recovery each and every Foraminifera stuck to the walls of the funnel, successive addition of small quantities of clean TCE in the funnel, (that subsequently is plugged, detached from its stand and gently rotated so that the TCE washes the walls and collects the particles in its bottom) helped greatly. Two or three passes may be necessary.
It is strongly advised to leave the fractions recovered on filter paper, in particular the relatively massive sinking fractions, to dry in an open or well-ventilated area where the fumes cannot accumulate, obviously avoiding windy contexts that may overturn the filter paper or disperse its content.
It’s very important to avoid mixing water and TCE in the funnel. As stated above, cleaning/rinsing cycles between the treatment of different fraction of the same sample should be based on the use of TCE alone and – as long that the fractions are referred to the same sample - it’s unimportant whether isolated Foraminifera from previous use cycles are left in the funnel. The final cleaning of the glass equipment (separatory funnel, beakers…) is facilitated by the high volatility of TCE, but the shape of the funnel does not lend to an easy treatment of its inner walls, that may be facilitated by bottlebrushes, pipe cleaners and push-throughs. An effective way to grant a complete cleaning of the separatory funnel is leaving it unplugged and with the stopcock removed to facilitate the circulation of air. The residue particles, if any, will spontaneously detach from the internal walls as the TCE evaporates and fall down the drain. The process can be facilitated by sending compressed air in the superior opening of the funnel.
Once the TCE is fully evaporated, considering that there is no risk of biological contamination or organic matter transfer in successive uses, there’s no necessary of specialized labware detergents, and lukewarm soapy water may be used. It’s important to perform a final rinse with deionized water to avoid the deposit of insoluble residues (“water stains”) in the funnel.
Figure 1.
The stand and the separatory funnel used in this study.
Figure 1.
The stand and the separatory funnel used in this study.
Figure 2.
The stopcock after the increase of hole size to 5 mm.
Figure 2.
The stopcock after the increase of hole size to 5 mm.
Figure 4.
Density separation with Perchloroethylene – Workflow.
Figure 4.
Density separation with Perchloroethylene – Workflow.
Figure 5.
Density separation with Perchloroethylene – In A, after some minutes since the sand was poured in the funnel loaded with TCE, the sinking phase has already accumulated to the bottom of the funnel. In B, the stopcock has just been released and the sinking phase is collected in the beaker. In C, the stopcock has been closed and the main part of the sinking portion is being filtered separately. This image allows to observe how many Foraminifera are suspended between the sinking phase and the surface of the liquid. A little more sinking sand has just been released and will be added to the portion separately filtered. In D, a small funnel lined with filter paper is placed under the drain and the remaining TCE is released in short bursts not exceeding the small funnel capacity. The TCE recovered after filtering may be recycled indefinitely. After phase D, the washing procedure described in the text was applied to recovery the Foraminifera that adhered to the internal wall of the funnel.
Figure 5.
Density separation with Perchloroethylene – In A, after some minutes since the sand was poured in the funnel loaded with TCE, the sinking phase has already accumulated to the bottom of the funnel. In B, the stopcock has just been released and the sinking phase is collected in the beaker. In C, the stopcock has been closed and the main part of the sinking portion is being filtered separately. This image allows to observe how many Foraminifera are suspended between the sinking phase and the surface of the liquid. A little more sinking sand has just been released and will be added to the portion separately filtered. In D, a small funnel lined with filter paper is placed under the drain and the remaining TCE is released in short bursts not exceeding the small funnel capacity. The TCE recovered after filtering may be recycled indefinitely. After phase D, the washing procedure described in the text was applied to recovery the Foraminifera that adhered to the internal wall of the funnel.
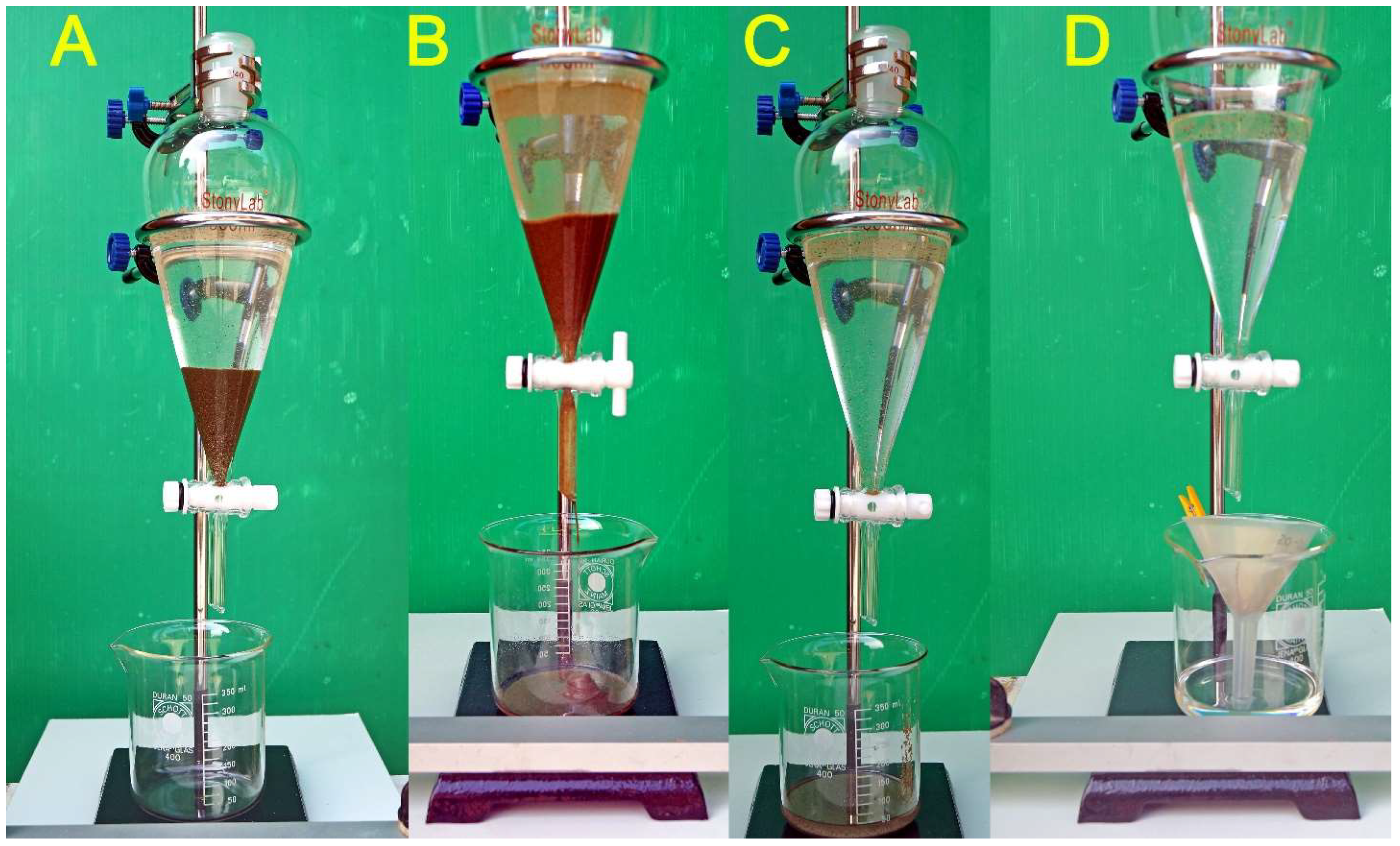
Figure 6.
Density separation with Perchloroethylene – recovering the non-sinking (floating + suspended) portion by filtering.
Figure 6.
Density separation with Perchloroethylene – recovering the non-sinking (floating + suspended) portion by filtering.
Figure 7.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 590μ - 250μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
Figure 7.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 590μ - 250μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
Figure 8.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 250μ - 125μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
Figure 8.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 250μ - 125μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
Figure 9.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 125μ - 64μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
Figure 9.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 125μ - 64μ interval, visual proportion of sinking and non-sinking (on filter paper) residue.
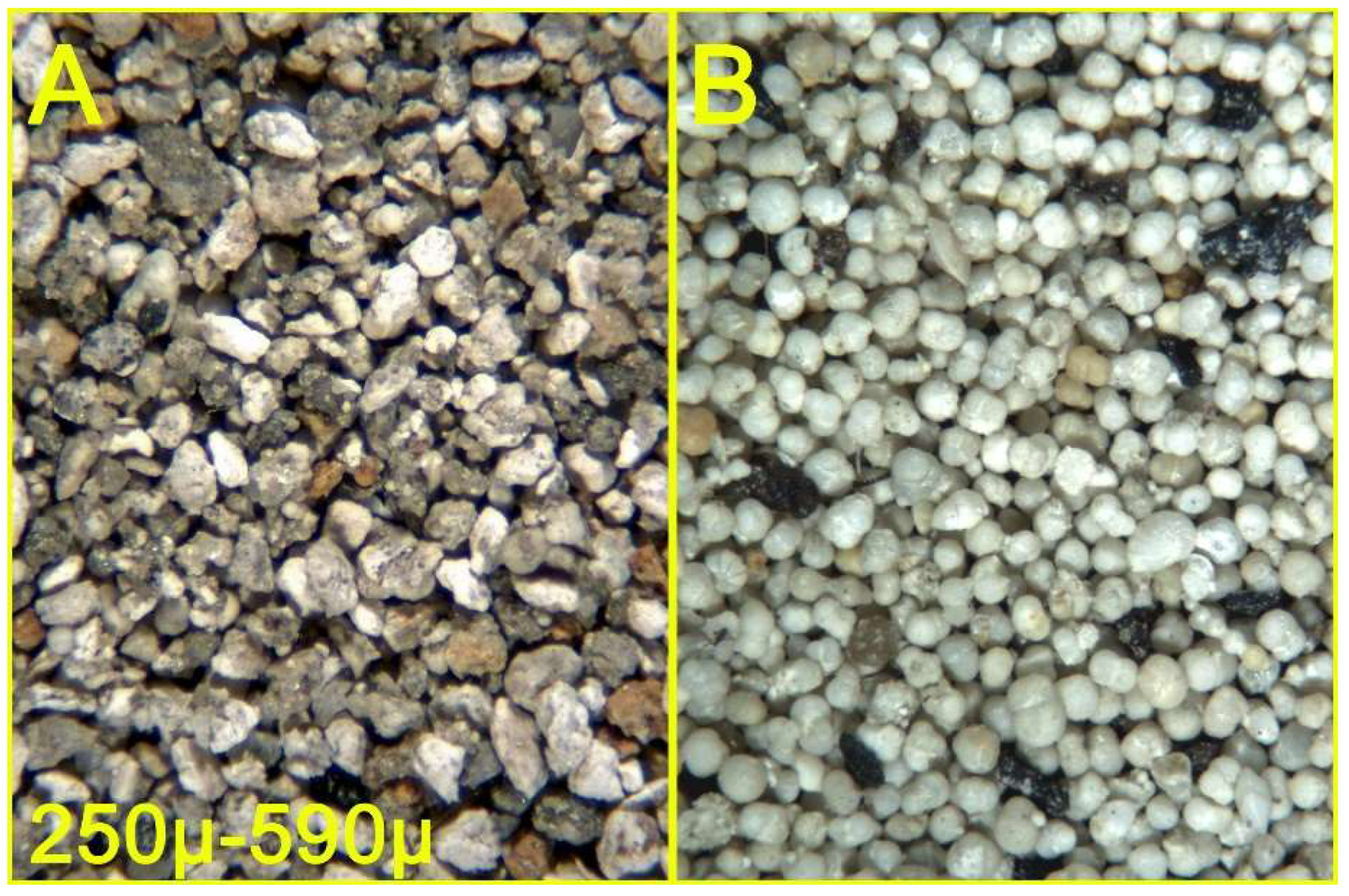
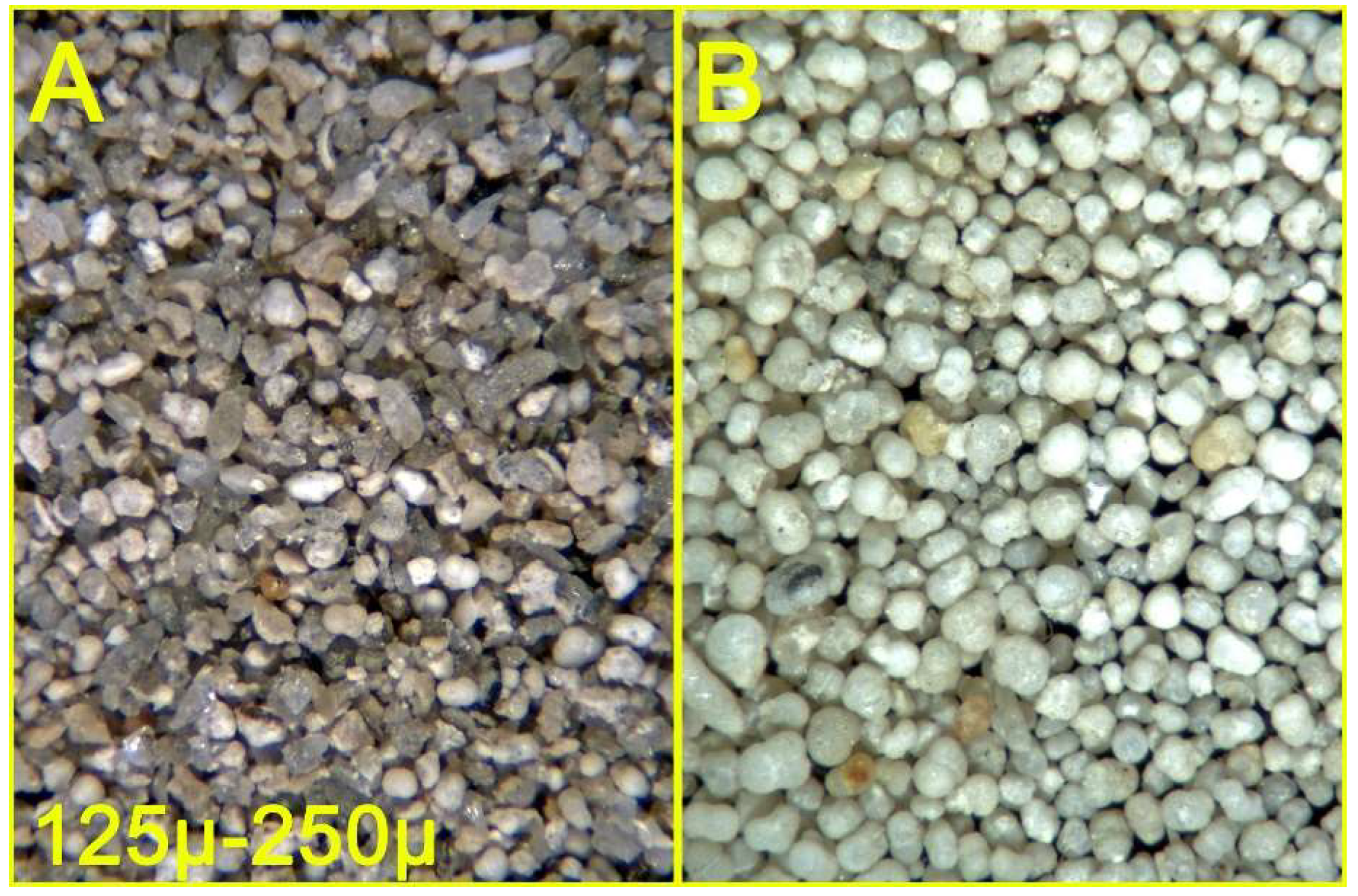
Figure 10.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 590μ - 250μ interval, before (A) and after (B) density separation.
Figure 10.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 590μ - 250μ interval, before (A) and after (B) density separation.
Figure 11.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 250μ - 125μ interval, before (A) and after (B) density separation.
Figure 11.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 250μ - 125μ interval, before (A) and after (B) density separation.
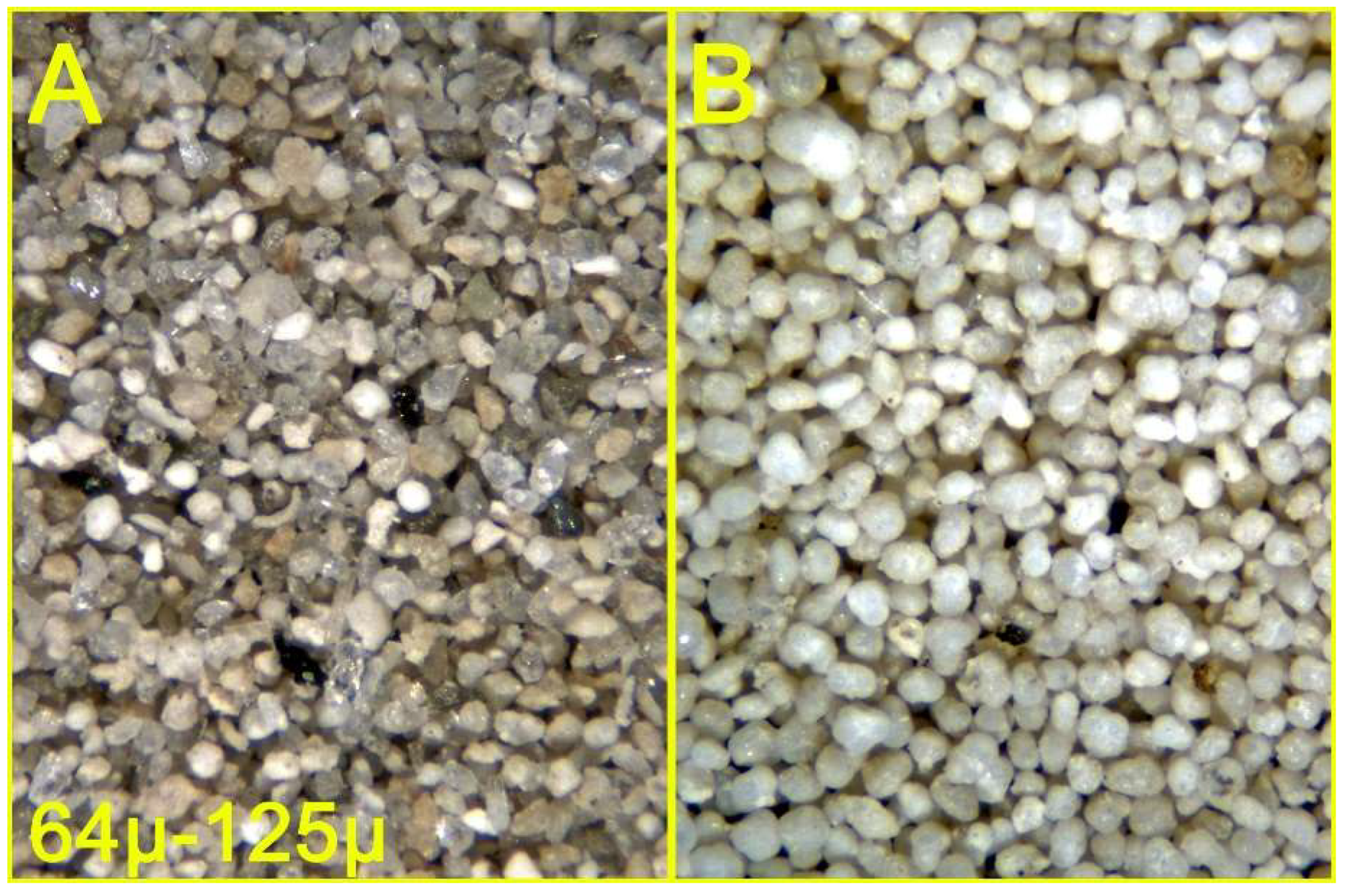
Figure 12.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 125μ - 64μ interval, before (A) and after (B) density separation.
Figure 12.
Sandy clay from the Argille di Fangario formation (see text) - Sandy fraction, 125μ - 64μ interval, before (A) and after (B) density separation.
Figure 13.
Circular area: results of around 10 hours of hand-piking of Foraminifera by wet brush from the untreated sand in
Figure 10A. Rectangular area: the small mound, whose height amply exceeds the depth of the slide, is the fruit of a 30 minutes session of density separation (
Figure 10B), and is subjectively evaluated to represent 15 times the quantity of Foraminifera in the circular area.
Figure 13.
Circular area: results of around 10 hours of hand-piking of Foraminifera by wet brush from the untreated sand in
Figure 10A. Rectangular area: the small mound, whose height amply exceeds the depth of the slide, is the fruit of a 30 minutes session of density separation (
Figure 10B), and is subjectively evaluated to represent 15 times the quantity of Foraminifera in the circular area.