1. Introduction
The prevalence of Parkinson's disease (PD) is significantly increasing, affecting about 1–2% of the world's older adult population [
1]. Although complex, the pathogenesis of this still incurable disease is directly linked to the degeneration of dopaminergic neurons in the brain's substantia nigra. This neurodegeneration appears to be triggered by states of oxidative stress, which in turn lead to mitochondrial dysfunction, mainly in Complex I of oxidative phosphorylation, oxidation of strategic molecules neurotransmission as dopamine, and conformational alteration in a large number of proteins [
2]. These alterations contribute to the production and deposition of cytosolic inclusion bodies primarily composed of the protein α-synuclein (Lewy bodies) and black pigments known as neuromelanin [
3].
The mitochondrial dysfunction and accumulation of these modified molecules have been directly associated with neural death in several ways, such as apoptosis and pyroptosis [
3]. Both damaged mitochondria and other residues are expelled from the interior of cells into the extracellular environment, probably contributing to Damage-associated Molecular Patterns (DAMPs) leading to states of neuroinflammation and systemic inflammation present in PD [
4]. From there, these circuits of oxidative stress, mitochondrial dysfunction, and molecule damage reinforce each other, leading to the onset of PD's clinical symptoms and progression.
Within this context, a strategy to mitigate the impact of oxidative-inflammatory states and mitochondrial dysfunction in PD could involve ingesting foods or supplements rich in antioxidant and anti-inflammatory bioactive molecules. Evidence from pre-clinical and clinical studies indicates that the consumption of various antioxidant molecules, especially those in the flavonoid category, can benefit PD by reducing oxidative and inflammatory states perpetuated during the disease [
5]. In this context, developing nutritional supplements rich in flavonoids and proteins could benefit these patients [
6].
A potential source of these molecules involves agro-industrial byproducts generated in the processing of fruits. These residues possess a significant volume of residues, generally constituting 65% to 70% of the total fruit mass [
7]. This is the case of some Amazonian diet fruits, such as cocoa shell bean (CBS) (
Theobroma cacao L.), which contains polyphenols, methylxanthines (especially theobromine), carbohydrates (fibers), and proteins in its chemical matrix [
8]. Thus, it is possible that a combined extract of cocoa seed husk and guarana seed powder could produce a formulate rich in flavonoids with relevant functional properties to DP, including improvement of neurofunctional states, reduction of mitochondrial damage, and immunomodulation of proinflammatory markers [
9].
Aqueous extracts combining CBS and guarana powder rich in flavonoids (GuaCa) were produced and chemically characterized to test this assumption. A Guaca extract of reference was chosen to evaluate its potential toxicity in an in vivo red Californian earthworm (Eisenia fetida) experimental model. Potential neurofunctional and immunomodulatory GuaCa´s effect was also tested in SH-SY5Y neuron cultures and peripheral blood mononuclear cells (PBMCs) cultures obtained from volunteers diagnosed with PD.
2. Material and Methods
2.1. Experimental Design and GuaCa Extracts Preparation
Initially, a total of 18 aqueous extracts were produced with variations in the proportion of CBS and roasted ground guarana seeds, as well as adjustments in temperature and pH . Details of each extract are presented in
Table 1. Hot extracts were identified with the abbreviation HE, cold extracts with the abbreviation CE, and extracts processed at both temperatures with the abbreviation HCE. The extraction time was standardized to 24 hours across all extracts. After this period, the extracts were freeze-dried, packaged, and stored at -3°C until use. Chemical characterization of total polyphenols, flavonoids, and catechins, and antioxidant and genoprotective capacity of all extracts were quantified. A representative GuaCa extract near 75th percentile of flavonoid concentration was chosen to perform the complementary analysis. Additionally, Ultra–Performance Liquid Chromatograph (UPLC) coupled with mass spectrometry (MS) was used to identify the principal peaks of molecules present in the GuaCa extract, which predominantly comprised flavonoids, xanthines, and proteins, including caffeine and catechin. According to the literature, caffeine from guarana extract can present potential beneficial antioxidant and antifatigue effects in DP [
10].
The potential toxicity of GuaCa at different concentrations was tested using the Red Californian earthworm (
Eisenia fetida) as an experimental model. This model is commonly used in ecotoxicity studies and research involving extracts, nutritional formulations, pharmaceuticals, and pollutants [
11].
The GuaCa concentrations that did not trigger higher earthworms’ mortality were used to evaluate GuaCa's in vitro neurofunctional effects assessed in the SH-SY5Y (CRL-2266 ™) human neuron-like cell line. The viability of cells supplemented with GuaCa was determined using the MTT assay. A concentration of GuaCa that caused a potential therapeutic effect, observed through a significant increase in neural viability, was utilized in the subsequent tests. This same concentration was also used in in vitro assays with PBMCs from volunteers diagnosed with PD. Neurofunctional indicators of GuaCa were investigated through the analysis of four biochemical markers.
The first involved quantifying the DNA damage rate, which is elevated in PD, determined by the levels of 8-Hydroxydeoxyguanosine [
12]. The second involved assessing the rate of mitochondrial damage by quantifying the NDUFS7 protein of Mitochondrial Complex I in the extracellular medium [
2]. The third evaluated the effect of GuaCa supplementation on the modulation of brain-derived neurotrophic factor (BDNF) and its tropomyosin receptor kinase type B (TrkB), which are directly involved in the neuropathology of PD. The BDNF promotes the survival of dopaminergic neurons in the substantia nigra and increases the functional activity of striatal neurons. Therefore, BDNF deficiency increases in PD and is associated with disease severity and long-term complications [
13]. For this reason, inducing an increase in BDNF levels could be a desirable functional property.
As growing evidence shows the involvement of cellular senescence in the pathogenesis of neurodegenerative diseases, including PD, it was also evaluated whether the GuaCa extract could differentially modulate the enzyme beta-galactosidase (β-Gal) [
14] .
An additional
in vitro protocol also evaluated whether supplementation of the culture medium with GuaCa could exhibit any immunomodulatory properties in peripheral blood mononuclear cells (PBMCs) obtained from volunteers diagnosed with PD. In this protocol, similar to [
15], the GuaCa effects on cellular proliferation and the pro-inflammatory cytokine interleukin 6 (IL-6) levels were determined. Additionally, alterations in the cytomorphological patterns of cell size and extracellular residual debris that can indicate PBMCs' inflammatory activation or degenerative states were evaluated.
2.2. Materials and Reagents
The CBS was commercially obtained from Jupará Chocolates Artesanais (Salvador, Bahia, Brazil), and guarana powder was obtained from a Brazilian government-owned company- Embrapa Amazonia Oriental (Maués, Amazonas, Brazil). All chemicals and solvents utilized in this research were sourced from Sigma-Aldrich (Saint. Louis, Missouri, United States of America), including Histopaque®-1077, which isolated PBMCs and protocols involving biochemical reagents. Materials for cell culture experiments were obtained from Vitrocell-Embriolife (Campinas, São Paulo, Brazil). The molecular biology reagents were supplied by Qiagen (Hilden, North Rhine-Westphalia, Germany), Invitrogen (Carlsbad, California, United States of America), and Bio-Rad Laboratories (Hercules, California, United States of America). DNA calf thymus (dsDNA) was purchased from Invitrogen (Eugene, Oregon, United States of America); Enzyme-linked immunosorbent assay (ELISA) kits containing inflammatory markers were purchased from Elabscience (Houston, Texas, United States of America).
2.4. Spectophotometric Quantification of Main Chemical Groups
In all protocols, quantification of polyphenols, flavonoids, and catechins was performed by spectrophotometry assays, and the average of the triplicates was calculated for statistical analysis. The total phenolic content was quantified using Folin–Ciocalteu’s method [
16], using a calibration curve prepared from standard gallic acid solutions. The mg GAE/mL calibration curve equation was y = 0.0689x – 0.0482, r² = 0.9766. The extract samples were measured at wavelengths ranging from 760 to 765 nm. The aluminum chloride colorimetric method was used to determine flavonoids [
16]. The data were expressed in µg/mL quercetin equivalents, calculated from a standard curve (y = 0.0098x + 0.0328; r² = 0.9862). The assay described by [
17] was used to determine catechins. The catechin content was expressed as in µg/mL. The calibration curve for catechin yielded the equation y = 0.0172x + 0.0051, r
2=0.985.
2.5. Assays of DPPH Antioxidant Capacity and Genomodifier Capacity Assays
The DPPH method (2,2-diphenyl-1-picrylhydrazyl stable free radical) was used to measure the antioxidant capacity of the lyophilized extracts as previously described [
18]. The reaction mixture was read in microplates of 96 wells at an absorbance of 520 nm. Three calibration curves were prepared using ascorbic acid (AA, y=6.6916x+32.15y = 6.6916x + 32.15y=6.6916x+32.15, r
2=0.9783); gallic acid (GAE, y=37.817x−31.118y, r
2=0.9813); and quercetin (QE, y=10.718x+33.928y = 10.718x + 33.928 r
2=0.9748), with concentrations ranging from 0.250 to 100 µg/mL. The results are presented as the percentage inhibition of the DPPH radical.
The genomodulatory capacity was evaluated using the fluorimetric method (GEMO assay), which is a cell-free protocol employing calf thymus double-stranded DNA (dsDNA) exposed to different concentrations of the test compounds (TC) for 30 minutes. Subsequently, PicoGreen®, a highly sensitive fluorescent dye for dsDNA, was added, and fluorescence was emitted proportional to the concentration of intact dsDNA. The assay was performed in triplicate using 10 μL dsDNA samples (1 mg/mL) diluted in 5% GuaCa extract solutions. A total of 100 μL of each sample was distributed into black 96-well plates and incubated for 30 minutes. Subsequently, PicoGreen® (diluted 1:200 in TE buffer) was added to the wells, and fluorescence was measured after five minutes at room temperature, with excitation at 480 nm and emission at 520 nm. The dsDNA degradation triggers a decrease in fluorescence. The average of the triplicates was calculated for statistical analysis [
19].
2.6. Chemical Characterization by UPLC-QToF-MS (Ultra-Performance Liquid Chromatograph Coupled to Time-of-Flight Mass Spectrometer) and Centesimal Analysis)
Considering that the primary group of bioactive molecules obtained from the extracts consists of flavonoids, particularly catechins present in the chemical matrices of guarana and cocoa seed husk, a chemical characterization of a reference extract of GuaCa was conducted using (UPLC-QToF-MS). Chromatographic analyses were performed using ultra-performance liquid chromatography (UPLC) on an ACQUITY UPLC I-Class PLUS system equipped with an ACQUITY UPLC HSS T3 column (100 Å, 1.8 µm, 2.1 mm × 150 mm) at the WTS Lab of Samsung SRBR M. The mobile phase consisted of solvent A – Water+0.1% formic acid and solvent B – Methanol/acetonitrile 25:75. Gradient elution followed a combined curve with the ratio of 0 min 95%A 5%B; 0.2 min 95%A 5%B; 10 min 5%A 95%B; 12 min 5%A 95%B; 14 min 95%A 5%B; 15 min 95%A 5%B, performed at a column oven temperature of 40°C, with a mobile phase flow rate of 0.3 mL/min and an injection volume of 10 µL. Mass spectrometric analyses were performed using a Xevo G2 Q-TOF (Quadrupole Time-of-Flight) mass spectrometer operating in ESI- and ESI+ ionization modes. The instrument settings were as follows: capillary voltage at 2.5 kV, sampling cone voltage at 40 V, source temperature at 100°C, and a mass range monitored from 50 to 1200 Da [
20].
The relative quantification of the main components provides essential information on the chemical composition and abundance of different compounds in plant extracts or other natural sources. In practice, this process helps to understand the properties and efficacy of these products and their potential therapeutic uses, including: (1) Identification of Bioactive Compounds: Relative quantification allows the identification of major and minor compounds in a plant extract. The major compounds are often primarily responsible for the plant's therapeutic effects. It also serves to understand the abundance of bioactive molecules, aiding in the selection of plant extracts rich in compounds of interest for subsequent studies, such as biological activity tests; (2) Quality Control of Products; (3) Standardization of Plant Extracts; (4) Evaluation of Pharmacological Activity; (5) Research and Development of New Drugs. This enables the identification of molecules that can be used as prototypes for the development of nutraceutical drugs.
The creation of the mass spectrum (MS) library for the compounds of interest was performed using the Studio SDF software. This process involved several key steps to ensure that the library accurately represented the spectrometric profiles of each compound, facilitating subsequent identification and comparative analysis. The chemical identification of the compounds of interest was carried out using the Progenesis QI software, an advanced platform for mass spectrometry data analysis, focusing on identifying and quantifying metabolites, lipids, and other biomolecules.
Centesimal analysis of GuaCa´s macro and micronutrient composition were conducted by a commercial laboratory (Terra CO. Goiania-GO, Brazil).
2.7. In Vivo Toxicity Assessment
The potential cytotoxic effect of GuaCa at different concentrations was tested using the Red Californian earthworm (
Eisenia fetida) as an experimental model. This simple and rapid model employs an organism commonly used in ecotoxicity studies and research involving extracts, nutritional formulations, and pharmaceuticals [
15]. The protocol was similar to that described by [
11]. Briefly, the earthworms were commercially acquired, transferred, and acclimated to laboratory conditions and transferred to vials containing a tropical artificial soil sterilized at 180 °C for 30 minutes before use. The GuaCa extract solution at different concentrations was added and homogenized into the TAS at 90% humidity. The survival of the earthworms was recorded on days 1, 3, 7, 14, 21, and 28. Three replicates were performed with three earthworms in each treatment.
2.8. In Vitro Neurofunctional Assessment
The protocol was conducted using commercial SH-SY5Y lineage obtained from the American Type Cell Culture Collection (ATCC ®CRL-2266™), cultured under standardized laboratory conditions (37 °C with 5% CO
2) as previously described by [
21]. Briefly, cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM F12) with 10% foetal bovine serum supplemented with 1% penicillin/streptomycin. The cell suspension was placed in each well of a 96-well plate (1 × 10
5 cells/well) with supplementation of different GuaCa extract concentrations with a logarithmic distribution (0, 0.1, 0.3, 1, 3, 10, 30, 100, and 300 µg/mL). All experiments were independently triplicated and performed following
in vitro procedures preconized by the Organisation for Economic Co-operation and Development (OECD) Guidance Document on Good i
n vitro Method Practices [
22].
The viability analysis was performed in 24 h neuron cultures by MTT assay (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolic bromide). Briefly, cultures were centrifuged at 2500 g and resuspended in phosphate buffer (PBS, 0.01 M; pH 7.4). MTT solution (5 mg/mL dissolved in phosphate-buffer solution, PBS) was added to a 96-well plate containing cell culture with different treatments and incubated for 2 h at 37
oC. The supernatant was removed and discarded, and the cells were resuspended in 200 µL DMSO. The absorbance was read at 560 nm, and high absorbance indicated a high concentration of viable cells [
21].
One of the GuaCa concentrations that increased neuron viability was utilized to conduct additional analyses of markers for neurogenic function by quantification of 8-Hydroxydeoxyguanosine (oxDNA), BDNF, extracellular NDUFS7, mitochondrial protein, β-Gal, and 8-oxDNA. Except for β-galactosidase, which was quantified using a spectrophotometric assay as described [
23] , the other markers were determined by immunoassays using Elabscience® Biotechnology (Houston, Texas, United States of America) kits according to the manufacturer. Calibration curves: BDNF: y = 0,2569x- 0,458, r²= 0,9551; NDUFS7: y= 0,485x - 0,5177, r² = 0,9779; oxDNA: y = -0,1955x + 1,3375, r² = 0,9281.
2.9. In Vitro Immunomodulatory Effect of GuaCa on Human PD-PBMCs
The potential immunomodulatory effect was evaluated using primary human cultures from PBMCs obtained from volunteers with PD selected from patients at the Darlinda Ribeiro-FuNATI Polyclinic in Manaus-AM and Amazonas Fire Department Hospital (Amazonas Military Fire Department). Prior, these volunteers participated in an ongoing project monitoring individuals with and without a history of prior COVID-19 infection before immunization, approved by the Research Ethics Committee of the State University of Amazonas (UEA) (CAAE 47914221.1.1001.5016). All volunteers signed an informed consent form. Additionally, they were invited to participate in continued support for patients with neurological disorders (PAN Group), which offers psychological, nutritional, clinical, and nursing attention. A total of 09 volunteers from 123 study participants were able at the start of the protocol to donate blood samples selected (males = 5, females =6) 69. 7 ± 14. 11 years (45-88).
All volunteers were using levodopa as their primary medication. Regarding the stage of PD determined by the Hoehn and Yahr Scale [
24], one volunteer was at stage 0, with no visible signs of the disease; three were at stage 1, with unilateral symptoms; two were at stage 2, with bilateral symptoms but without balance deficits; and the remaining participants were at stages 4 and 5, where they already exhibit severe disability and immobility. The volunteers were instructed not to consume, within the 24 hours preceding blood collection, foods high in antioxidants, caffeinated beverages, or alcoholic beverages.
The collection and isolating of the PBMCs were performed as described by [
15]. Briefly, blood samples (20 mL) were collected by venipuncture into tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. The collected blood was transferred to tubes containing Histopaque®-1077 in a 2:1 ratio (blood:Histopaque®) for density gradient cell separation. The samples were then centrifuged at 252× g for 20 minutes. After centrifugation, the leukocyte layer was carefully collected and transferred to a new tube. The cells were washed with phosphate-buffered saline (PBS, pH 7.4) and centrifuged at 252 × g for 10 minutes to remove residues. The samples were transferred to 1 mL of RPMI 1640 culture medium containing 10% fetal bovine serum and 1% penicillin/streptomycin at a final 1 × 10
5 cells/mL concentration. The cells were distributed in 6- or 96-well plates, depending on the test, and were incubated at 37°C, 5% CO
2, and under controlled humidity conditions for 24 h before conducting the experiments. PBMCs of each volunteer were transferred for 96-plate wells containing culture medium without (Control) or with GuaCa supplementation. After 48h, some inflammatory markers were evaluated in primary cell cultures obtained from each volunteer.
Due to limitations in the volume of blood samples obtained, the evaluated markers included lymphocyte proliferation assessed by the MTT assay, cytomorphological analysis via optical microscopy indicating a higher number of cells/or large cells and extensive debris due to cellular mortality, and levels of the pro-inflammatory cytokine interleukin-6 (IL-6), was quantified by ELISA immunoassay using Elabscience® Biotechnology (Houston, Texas United States of America) kits according to the manufacturer. Calibration curve IL-6: y=68,66x-11,852, r²=0,9913.
2.10. Optical Microscopy Analysis
Considering that supplementation of the culture medium with GuaCa could trigger an antigenic response in PBMCs, a complementary analysis was conducted to evaluate the cytomorphological conditions of the cultures stained with commercially obtained Panoptic dye according to the manufacturer's instructions (Laborclin, Paraná, Brazil). The comparative analysis was performed by obtaining microphotographs evaluated using the Image J software. For this purpose, the images were converted to binary staining, allowing the determination of mean gray intensity (MGI). The average MGI was compared between treatments, considering that a higher number of cells and cellular debris would indicate a higher cell mortality rate and/or lymphocyte proliferation. For each obtained PBMC culture, 5-10 microphotographs were evaluated, and the results were expressed as mean ± standard deviation (SD).
2.11. Statistical Analysis
The results were analyzed using GraphPad Prism 9.5.1 software. Quantitative variables were generally compared using a one-way or two-way analysis of variance followed by the Tukey or Bonferroni post hoc test or Student T test. The effect on earthworms exposed to different concentrations of GuaCa was conducted using Kaplan-Meier survival curves. The comparison of biological markers in PBMC cultures at 6, 24, and 48 hours was performed using repeated measures analysis of variance followed by Bonferroni post hoc test. The in vitro results were presented as the mean ± standard deviation of each variable’s relative frequency (%). p-values < 0.05 were considered statistically significant.
3. Results
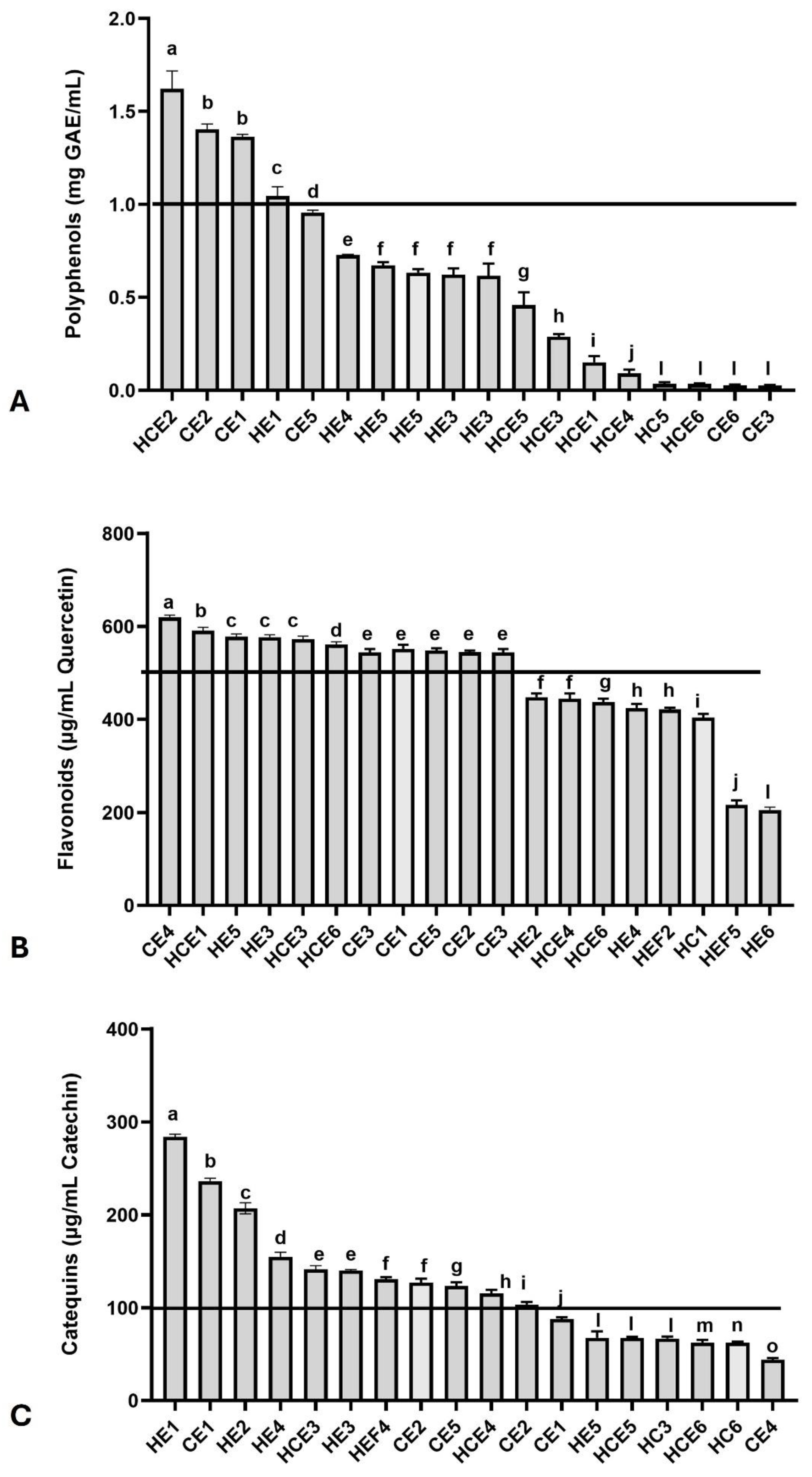
Determining total polyphenol, flavonoid, and catechin concentrations presented some variation among the 18 extracts. The four highest polyphenol concentrations (>1 mg/mL) were found in extracts HCE 2, CE2, CE1, and HE1 (
Figure 1A). On the other hand, a more significant number of extracts (n = 11) exhibited flavonoid concentrations exceeding 500 µg/mL (
Figure 1B) and catechin concentrations exceeding 100 µg/mL (
Figure 1C).
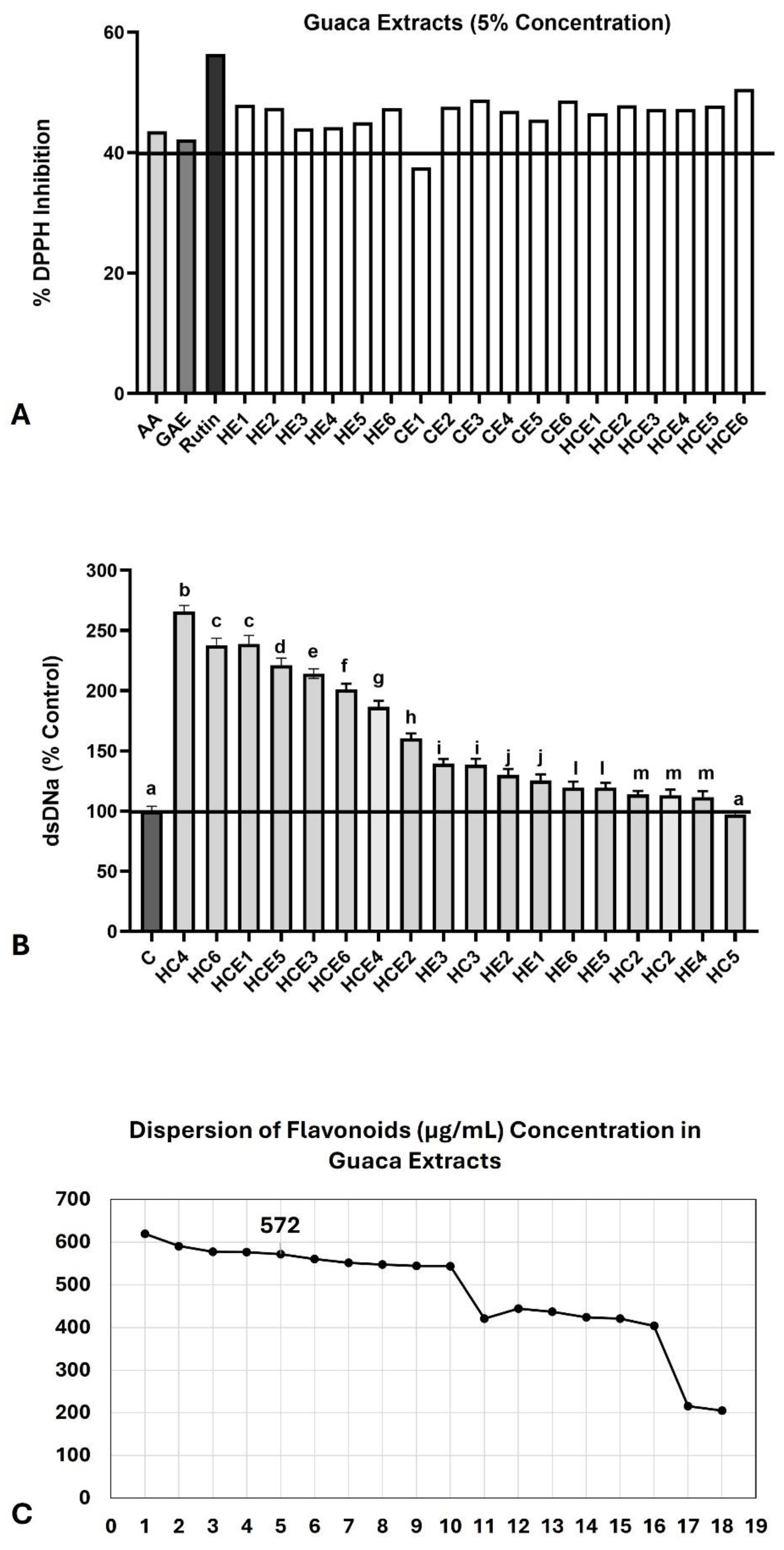
The antioxidant capacity evaluated in 5% solutions of GuaCa extracts was comparable to that observed with the standards AA (1 µg/mL) = 43.57 ± 1.1% and GAE (1 µg/mL) = 42.14 ± 2.2%. The DPPH inhibition ranged from 50.5 ± 2.1% observed in extract HCE 2 to 44.01 ± 2.4% in extract HE3 (
Figure 2A). Only extract CE1 exhibited a lower DPPH radical inhibition percentage (37.54 ± 1.8%). On the other hand, all extracts showed a significantly lower inhibition percentage than rutin (56.4 ± 2.4%). However, the highest DPPH radical inhibition observed with rutin was, on average, only 9.8% higher than that of the GuaCa extracts. Therefore, it was concluded that, in general, the antioxidant capacity of GuaCa is high.
All extracts demonstrated genoprotective capacity above that of the control solution, except for extract CE 5, whose percentage of dsDNA was similar to the control (
Figure 2B). Seven of the 18 extracts doubled the dsDNA percentage compared to the control, indicating that the extracts could protect DNA from damage caused by endogenous and exogenous factors, particularly reactive oxygen species (ROS).
Based on these evaluations, the reference GuaCa extract was selected for use in the subsequent tests. To this end, the extract closest to the 75th percentile of total flavonoid concentration was chosen, considering it relevant to allow a 25% margin of variation above and below the entire set of extracts. The percentile was 473.2; therefore, the closest identified extract was HCE 3.
A dispersion graph confirmed the position of its extract, shown in
Figure 2C. HCE 3 extract was obtained from an aqueous solution without additional acidification, with an initial temperature of 85 °C maintained for 4 h, followed by cooling and storage at 4 °C for 24 hours. The GuaCa HCE 3 extract also remained within the 75th percentile for catechin concentration and exhibited elevated antioxidant and genoprotective capacity levels.
Centesimals analysis of the GuaCa extract revealed that it contains a significant concentration of proteins in its composition and several micronutrients that serve as cofactors in various metabolic pathways (
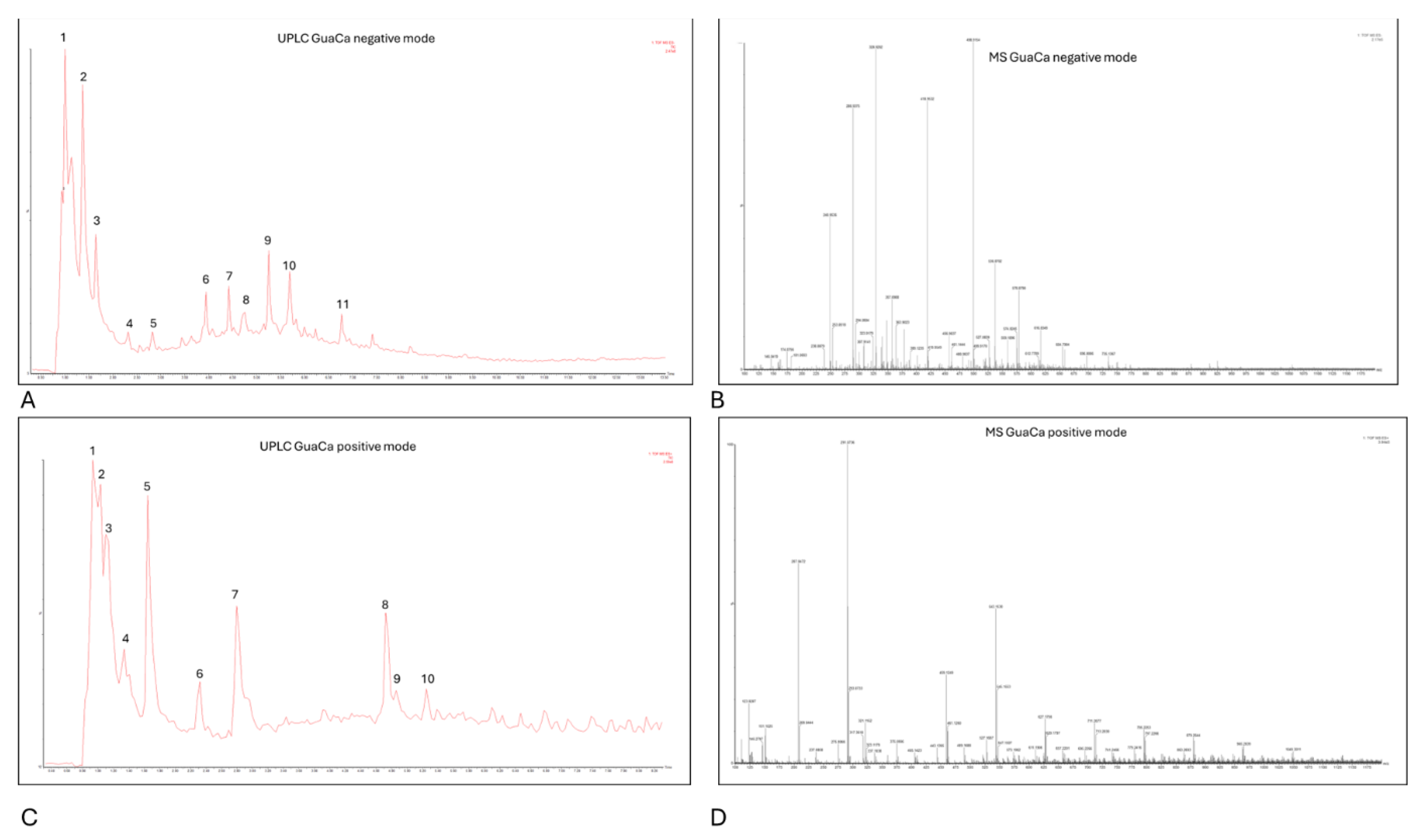
Table 2). Chemical Characterization by (UPLC-QToF-MS) identified 10 relevant peaks in both negative and positive modes in the composition of the HCE 3 extract (
Table 3,
Figure 3). Among the identified molecules, four flavonoids stand out in the negative mode (catechin, quercetin, epicatechin, and EGCG), and one flavonoid in the positive mode (kaempferol). In calculating the bioactive components' relative quantity (RQ) in the negative mode, catechin was the most abundant molecule. Using this molecule as a reference (100%), the proportion of flavonoids in the extract was estimated at 50.44%.
LC-MS also detected polypeptides, tyrosine, and alanine amino acids, consistent with the high protein content identified through centesimal analysis. In addition to these molecules, caffeine and benzoic acid were among the largest peaks detected by UPLC. In this context, as expected, the HCE 3 extract obtained from cocoa seed husk and guarana presents a composition rich in flavonoids and proteins.
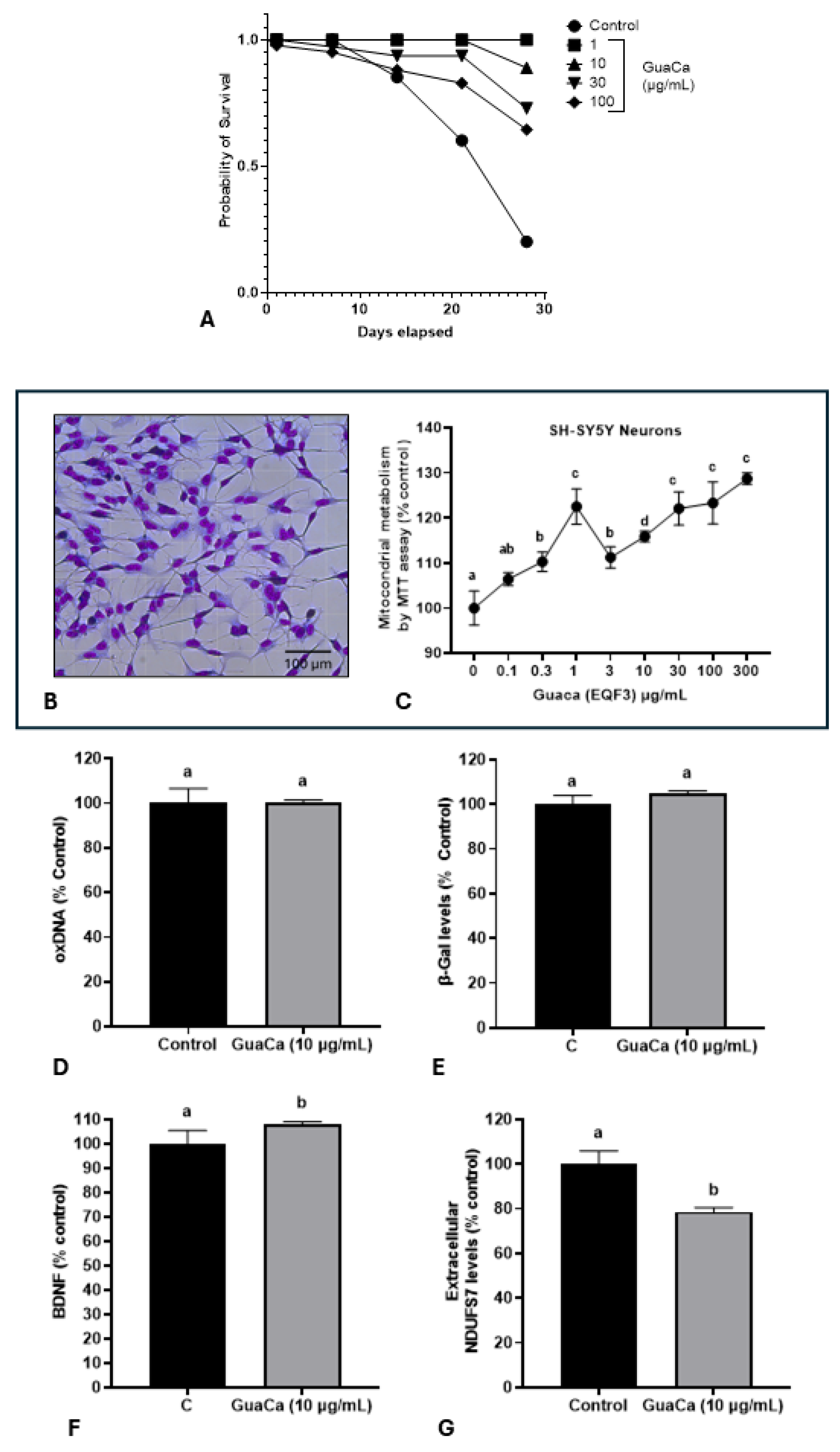
Subsequently, it was evaluated whether the HCE 3 extract at four different concentrations could have a toxic effect on
Eisenia fetida earthworms. The GuaCa concentrations of 1, 10, and 30 µg/mL significantly increased the lifespan compared to control worms or those raised in a medium supplemented with 100 µg/mL of the extract (
Figure 4A).
The potential
in vitro effect of GuaCa on 72-hour cultures of differentiated SH-SY5Y neurons (
Figure 4B) was evaluated. As shown in (
Figure 4C), GuaCa supplementation at different concentrations (3 to 300 µg/mL) induced increase in the energetic metabolism indicating higher neuron viability than controls; However, GuaCa did not affect oxDNA levels, a marker of DNA damage, nor β-Galactosidase levels, an indicator of cellular aging (
Figure 4D,E).
On the other hand, the levels of the neurogenic protein BDNF significantly increased, and the extracellular levels of the NDUSF7 protein, a marker of mitochondrial damage, decreased. Thus, the collective results indicate that GuaCa possesses some potentially beneficial functional properties in neurons (
Figure 4F,G).
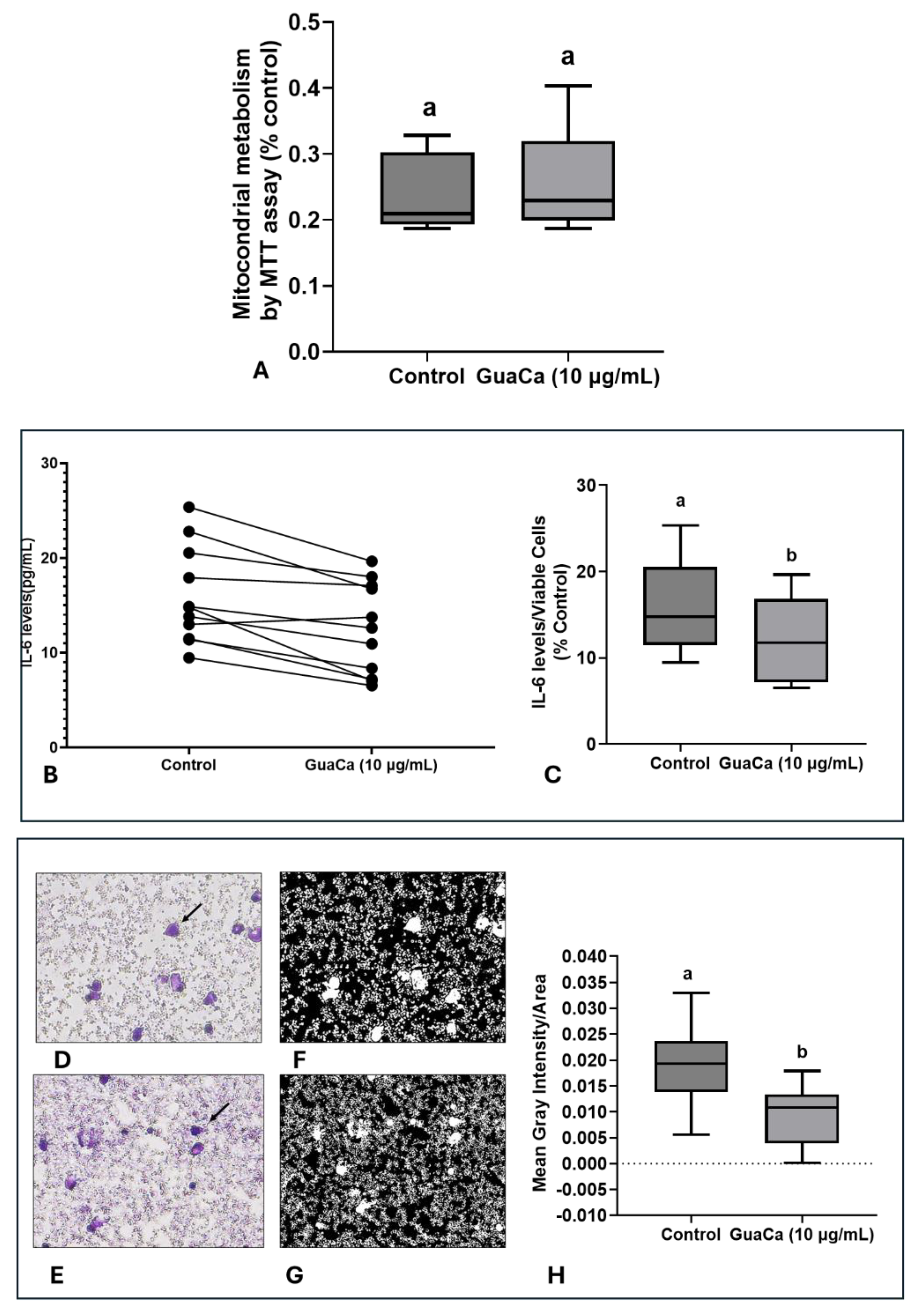
In PBMC cultures, no significant differences were detected in MTT levels, indicating that GuaCa did not induce an acute antigenic response (
Figure 5A). However, IL-6 levels were lower in PBMC cultures supplemented with GuaCa compared to controls.
Figure 5B presents the results for each volunteer's PBMC culture, comparing those with and without GuaCa supplementation, and
Figure 5C shows the mean, maximum, and minimum IL-6 levels in cultures with and without GuaCa supplementation. The analysis of PBMC cultures via optical microscopy also showed a reduced number of debris and larger cells when supplemented with GuaCa (Figures D–G).
4. Discussion
This study presents a combined extract rich in flavonoids derived from cocoa seed husk and guarana powder, exhibiting neurofunctional and immunomodulatory properties that could serve as an ingredient in producing relevant nutraceuticals for patients diagnosed with PD. There is substantial evidence that bioactive components present in functional foods and dietary supplements could have beneficial effects for PD, considering the main aspects of the pathogenesis of this neurodegenerative disorder. However, most studies focus on evaluating specific molecules or phytotherapeutics plants, highlighting the need to develop new specific functional products specifically targeted at PD. Within this perspective and considering the significant role of flavonoids in antioxidant function, genoprotection, mitochondrial protection, and immune modulation [
25], GuaCa was delineated and formulated.
The CBS was the primary source of flavonoids, as it is a residue rich in flavonoids and other chemical and nutritional components and is produced in large quantities by the cocoa production chain [
26]. However, guarana was added due to the limited number of studies specifically related to the functional properties of CBS. Guarana possesses neuroprotective properties against pollutants such as methylmercury, drugs like the chemotherapeutic vincristine, and neuropathogenic molecules such as the amyloid-beta peptide [
9].
The study conducted here initially produced different extracts by combining cocoa seed husk and roasted, ground guarana seed powder using water as the solvent. This choice was based on the premise that if the extracts exhibit relevant functional properties, they can later be enhanced using more efficient and non-polluting extraction techniques. Furthermore, the conception of the extract assumed that it can be used as an ingredient for the production of traditional nutraceuticals and nanoformulations that allow for oral or transdermal absorption, which is especially relevant for patients with mild to moderate dysphagia, which is common in DP and other neurodegenerative conditions.
Results from extracts showed that, despite variations in polyphenols, flavonoids, and catechin content, almost all extracts exhibited satisfactory concentrations of these natural chemicals. The bioactive components in the extract are consistent with those described in the literature [
8,
9]. The concentrations of total phenolics, flavonoids, and catechins observed in GuaCa were not higher than those found in extracts utilizing solvents such as alcohol and ethanol, which enhance extraction efficiency [
8]. However, the formulation obtained herein focused on evaluating a solvent-free extraction process, avoiding using solvents that may be environmental pollutants or could increase processing costs if produced industrially.
In this context, the complementary evaluation of the main chemical components of the GuaCa extract via (UPLC-QToF-MS) is relevant to understanding their potential contribution to the observed neurofunctional and immunomodulatory effects. Although it is a measure of proportionality, determining the relative quantity of the molecules with the highest peaks in the GuaCa extract suggested that a substantial portion of them are polyphenols, particularly flavonoids. In the negative mode, flavonoids accounted for 50.44% of all component molecules in the top ten UPLC peaks. Among these molecules, EGCG stands out. There is evidence that this bioactive molecule, mainly present in green tea, could have beneficial effects in delaying the neurodegeneration of the substantia nigra, regardless of the origin of PD [
27]. Quercetin is another flavonoid in the GuaCa extract that experimental studies have described as protective against the death of dopaminergic neurons in PD models [
28].
In the positive mode, kaempferol was another flavonoid detected among the top 10 UPLC peaks. Previous studies have reported the neuroprotective effects of the flavonol kaempferol against various apoptosis- and necrosis-inducing insults associated with PD [
5] . In summary, all these flavonoid molecules observed in the GuaCa extract exhibit significant antioxidant and immunomodulatory effects, as they can mitigate neuroinflammatory states present in neurodegenerative diseases such as PD.
In addition to flavonoids, LC-MS detected phenolic acids among the highest molecular peaks and benzoic acid, an aromatic carboxylic acid naturally used as an antibacterial and antifungal preservative in foods and feeds. Some studies suggest that appropriate benzoic acid levels might improve gut functions by regulating enzyme activity, redox status, immunity, and microbiota. However, since benzoic acid naturally occurs in plants and can also be added to prevent microbial contamination, we are uncertain whether the origin of this molecule in GuaCa is from the raw materials used in its production or if it was added to these commercially obtained products [
29].
The proximate analysis showed that the GuaCa extract exhibits protein concentrations close to those reported in other studies reviewed by [
8], where only cocoa bean shell was evaluated. Additionally, LC-MS analysis in the negative mode identified polypeptides with 4–5 amino acids among the ten major detected peaks, potentially including the amino acid tryptophan. Two peaks associated with key amino acids, tyrosine, and alanine, were also detected in the positive mode. Protein consumption is of great interest due to the direct and indirect effects of specific amino acids (AAs) on disease progression and their interaction with levodopa medication. Furthermore, evidence suggests that certain types of polar amino acids, such as tyrosine and alanine, which were among the significant peaks in GuaCa, may play crucial roles.
The chemical and nutritional composition of GuaCa and its potential functional effects align with the findings from the experimental protocols that were conducted. Firstly, the extract exhibited low toxicity in worms exposed to various concentrations of GuaCa over 28 days. Earthworms, which are highly sensitive to toxic agents, have also been used as experimental models for PD through exposure to rotenone, which inhibits mitochondrial complex I [
11]. Based on these findings, further testing in earthworms could explore GuaCa's potential to attenuate rotenone's neuromotor and neuroinflammatory effects. In SH-SY5Y neuron cultures, GuaCa also showed no visible cytotoxic effects, with cell viability increasing, suggesting a positive effect on cell cultures.
Even at a relatively low concentration of 10 µg/mL, GuaCa demonstrated relevant effects, as detailed below. Although GuaCa showed some capacity to reduce DNA degradation rates in GEMO assay, no significant reduction in oxDNA levels was observed in neuron cultures, indicating no apparent preventive effect. However, given that PD is associated with DNA damage, it is important to assess whether GuaCa might exhibit a more effective genoprotective effect under pathological conditions.
Conversely, the significant reduction in extracellular NDUSF7 protein levels supported the observed increase in neuron survival rates, indicating that GuaCa may help reduce mitochondrial dysfunction associated with PD development and progression [
2,
3].
One of the most notable neurofunctional actions observed was increased BDNF levels in neurons supplemented with GuaCa. BDNF, a member of the neurotrophin protein family, plays a direct role in neuronal survival, regulation, and memory function, mainly through interaction with tyrosine kinase enzyme, which catalyzes ATP phosphate group transfer to tyrosine residues in target proteins. The interaction between BDNF and TRkB enzyme enhances neurogenesis, synaptic plasticity, and neuroprotection, making it a promising therapeutic target for mental and neurodegenerative diseases like PD [
13].
We also investigated whether GuaCa could directly modulate the β-Gal enzyme, a known marker of cellular senescence that increases with cell aging [
23]. No action of GuaCa on β-Gal modulation was observed in SH-SY5Y dopaminergic neurons not exposed to agents commonly used in experimental PD models, like rotenone.
Finally, the immunomodulatory potential of GuaCa was assessed in PBMCs from PD patients. PD is generally associated with neuroinflammatory states, leading to increased levels of pro-inflammatory cytokines such as IL-6. Most individual PBMC cultures showed reduced IL-6 levels, with cytomorphological patterns indicating decreased cell size and debris concentration.
Further studies are needed to (1) determine the absolute quantity of the main GuaCa molecules; (2) evaluate the bioavailability potential of GuaCa's bioactive molecules using alternative delivery systems, such as sublingual solutions, gels, and transdermal creams; (3) assess the stability of the formulated product; and (4) conduct preclinical studies on GuaCa's potential functional effects in attenuating oxidative stress, DNA damage, and neurodegenerative and neuroinflammatory states associated with PD.
Overall, results from the protocols conducted here suggest that GuaCa may offer some preventive action against PD-related changes, such as mitochondrial damage and altered BDNF levels.