Submitted:
26 January 2025
Posted:
27 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
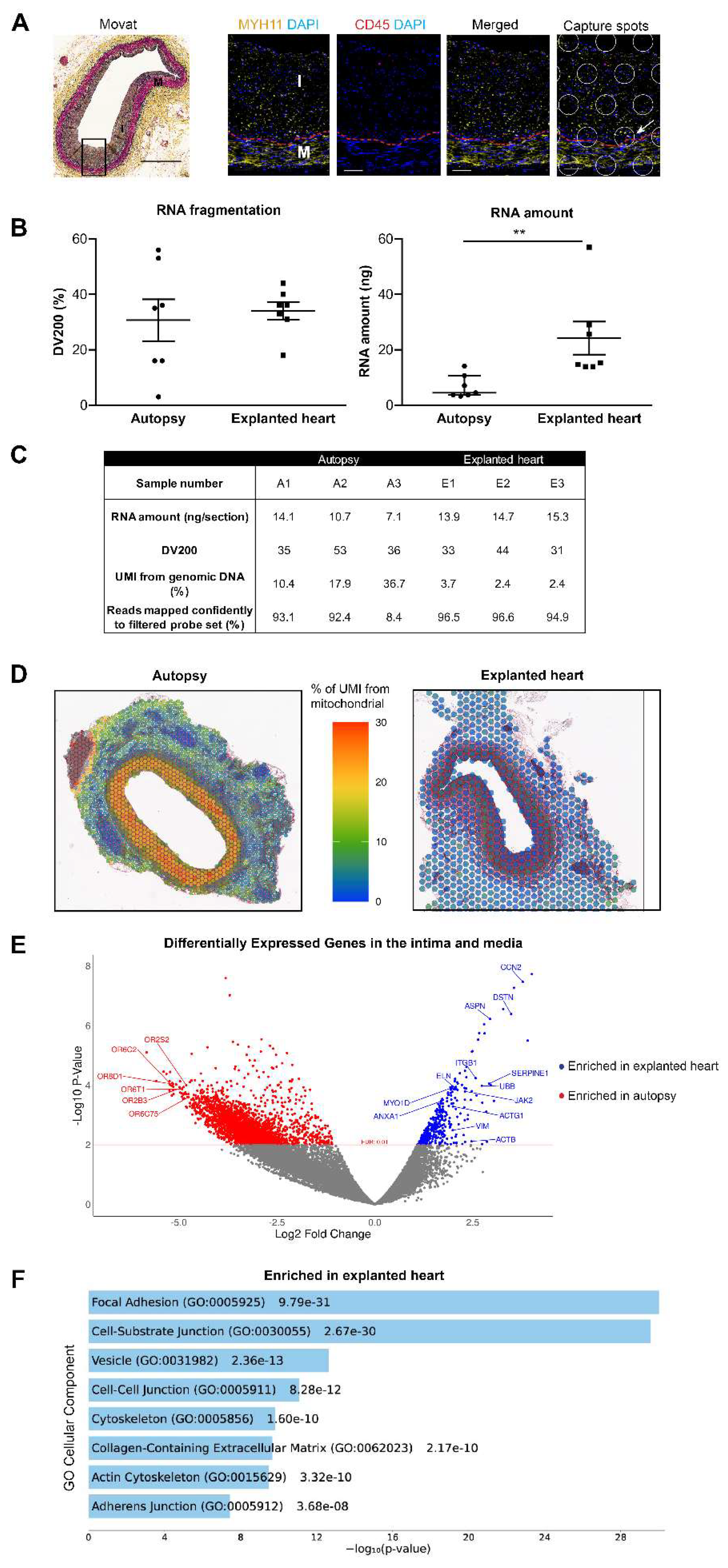
Human Samples
Immunofluorescence Staining and Confocal Microscopy
RNA Extraction and Quality Tests
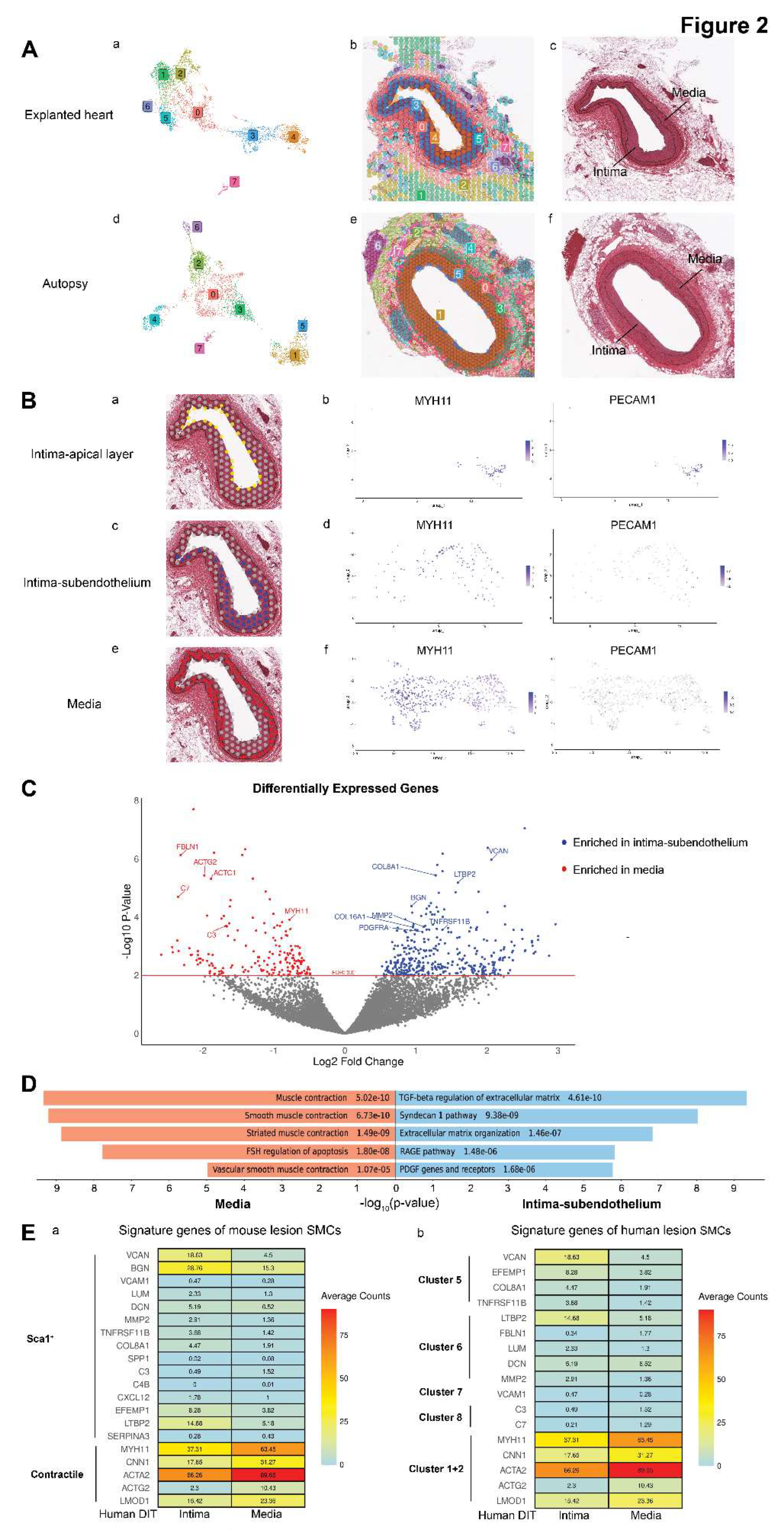
Spatial Gene Expression Analysis
Differential Gene Expression Analysis
Comparison to SMCs in Single-Cell RNA Sequencing Studies
Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIT | Diffuse intimal thickening |
| EC | Endothelial cells |
| FFPE | Formalin-fixed paraffin-embedded |
| MYH11 PIT SMC UMI |
Myosin heavy chain 11 Pathological intimal thickening Smooth muscle cell Unique molecule identifier |
References
- Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000-1012. [CrossRef]
- Restrepo C, Strong JP, Guzman MA, Tejada C. Geographic comparisons of diffuse intimal thickening of the aorta. Atherosclerosis. 1979;32:177-193. [CrossRef]
- Nakashima Y, Chen YX, Kinukawa N, Sueishi K. Distributions of diffuse intimal thickening in human arteries: preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. 2002;441:279-288. [CrossRef]
- Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr., Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992;85:391-405. [CrossRef]
- Allahverdian S, Ortega C, Francis GA. Smooth Muscle Cell-Proteoglycan-Lipoprotein Interactions as Drivers of Atherosclerosis. Handb Exp Pharmacol. 2022;270:335-358. [CrossRef]
- Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14-23. [CrossRef]
- Slenders L, Tessels DE, van der Laan SW, Pasterkamp G, Mokry M. The Applications of Single-Cell RNA Sequencing in Atherosclerotic Disease. Front Cardiovasc Med. 2022;9:826103. [CrossRef]
- Wang Y, Nanda V, Direnzo D, Ye J, Xiao S, Kojima Y, Howe KL, Jarr KU, Flores AM, Tsantilas P, et al. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc Natl Acad Sci U S A. 2020;117:15818-15826. [CrossRef]
- Mocci G, Sukhavasi K, Ord T, Bankier S, Singha P, Arasu UT, Agbabiaje OO, Makinen P, Ma L, Hodonsky CJ, et al. Single-Cell Gene-Regulatory Networks of Advanced Symptomatic Atherosclerosis. Circ Res. 2024;134:1405-1423. [CrossRef]
- Wang Y, Gao H, Wang F, Ye Z, Mokry M, Turner AW, Ye J, Koplev S, Luo L, Alsaigh T, et al. Dynamic changes in chromatin accessibility are associated with the atherogenic transitioning of vascular smooth muscle cells. Cardiovasc Res. 2022;118:2792-2804. [CrossRef]
- Gastanadui MG, Margaroli C, Litovsky S, Richter RP, Wang D, Xing D, Wells JM, Gaggar A, Nanda V, Patel RP, et al. Spatial Transcriptomic Approach to Understanding Coronary Atherosclerotic Plaque Stability. Arterioscler Thromb Vasc Biol. 2024;44:e264-e276. [CrossRef]
- Cole JE, Monaco C. Spatial Transcriptomics: A New Frontier in Atherosclerosis Research? Arterioscler Thromb Vasc Biol. 2024;44:2291-2293. [CrossRef]
- 10xGenomics. How do I determine if my tissue block or archived section will be compatible with the Visium CytAssist for FFPE assay? https://kb.10xgenomics.com/hc/en-us/articles/7776416227981-How-do-I-determine-if-my-tissue-block-or-archived-section-will-be-compatible-with-the-Visium-CytAssist-for-FFPE-assay. Accessed Oct 19, 2024.
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-1275. [CrossRef]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411-420. [CrossRef]
- Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296. [CrossRef]
- Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16:1289-1296. [CrossRef]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [CrossRef]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90-97. [CrossRef]
- Zhu Y, Wang L, Yin Y, Yang E. Systematic analysis of gene expression patterns associated with postmortem interval in human tissues. Sci Rep. 2017;7:5435. [CrossRef]
- 10xGenomics. Visium CytAssist Spatial Gene Expression for FFPE: Robust data analysis with minimal impact of genomic DNA. Technical Note (CG000605). 2022.
- Lin Y, Dong ZH, Ye TY, Yang JM, Xie M, Luo JC, Gao J, Guo AY. Optimization of FFPE preparation and identification of gene attributes associated with RNA degradation. NAR Genom Bioinform. 2024;6:lqae008. [CrossRef]
- Sidova M, Tomankova S, Abaffy P, Kubista M, Sindelka R. Effects of post-mortem and physical degradation on RNA integrity and quality. Biomol Detect Quantif. 2015;5:3-9. [CrossRef]
- Ashraf S, Frazier OH, Carranza S, McPherson DD, Taegtmeyer H, Harmancey R. A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors. Int J Mol Sci. 2023;24. [CrossRef]
- Lee LL, Chintalgattu V. Pericytes in the Heart. Adv Exp Med Biol. 2019;1122:187-210. [CrossRef]
- Tallquist MD. Cardiac Fibroblast Diversity. Annu Rev Physiol. 2020;82:63-78. [CrossRef]
- Goumans MJ, Ten Dijke P. TGF-beta Signaling in Control of Cardiovascular Function. Cold Spring Harb Perspect Biol. 2018;10. [CrossRef]
- Low EL, Baker AH, Bradshaw AC. TGFbeta, smooth muscle cells and coronary artery disease: a review. Cell Signal. 2019;53:90-101. [CrossRef]
- Merrilees MJ, Beaumont B, Scott LJ. Comparison of deposits of versican, biglycan and decorin in saphenous vein and internal thoracic, radial and coronary arteries: correlation to patency. Coron Artery Dis. 2001;12:7-16. [CrossRef]
- Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363-1369. [CrossRef]
- Panteleev MA, Korin N, Reesink KD, Bark DL, Cosemans J, Gardiner EE, Mangin PH. Wall shear rates in human and mouse arteries: Standardization of hemodynamics for in vitro blood flow assays: Communication from the ISTH SSC subcommittee on biorheology. J Thromb Haemost. 2021;19:588-595. [CrossRef]
- Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308-1316. [CrossRef]
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503-1509. [CrossRef]
- Francis GA. The Greatly Under-Represented Role of Smooth Muscle Cells in Atherosclerosis. Curr Atheroscler Rep. 2023;25:741-749. [CrossRef]
- Gomez D, Owens GK. Reconciling Smooth Muscle Cell Oligoclonality and Proliferative Capacity in Experimental Atherosclerosis. Circ Res. 2016;119:1262-1264. [CrossRef]
- Elishaev M, Hodonsky CJ, Ghosh SKB, Finn AV, von Scheidt M, Wang Y. Opportunities and Challenges in Understanding Atherosclerosis by Human Biospecimen Studies. Front Cardiovasc Med. 2022;9:948492. [CrossRef]
- Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370-377. [CrossRef]
- Ji T, Yan D, Huang Y, Luo M, Zhang Y, Xu T, Gao S, Zhang L, Ruan L, Zhang C. Fibulin 1, targeted by microRNA-24-3p, promotes cell proliferation and migration in vascular smooth muscle cells, contributing to the development of atherosclerosis in APOE(-/-) mice. Gene. 2024;898:148129. [CrossRef]
- Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M, Jorgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567. [CrossRef]
- Fischer JW, Steitz SA, Johnson PY, Burke A, Kolodgie F, Virmani R, Giachelli C, Wight TN. Decorin promotes aortic smooth muscle cell calcification and colocalizes to calcified regions in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2004;24:2391-2396. [CrossRef]
- Onda M, Ishiwata T, Kawahara K, Wang R, Naito Z, Sugisaki Y. Expression of lumican in thickened intima and smooth muscle cells in human coronary atherosclerosis. Exp Mol Pathol. 2002;72:142-149. [CrossRef]
- Hultgardh-Nilsson A, Boren J, Chakravarti S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J Intern Med. 2015;278:447-461. [CrossRef]
- Elishaev M, Li B, Zhou A, Salim K, Leeper NJ, Francis GA, Lai C, Wang Y. Multiplex Imaging for Cell Phenotyping of Early Human Atherosclerosis. J Am Heart Assoc. 2024;13:e034990. [CrossRef]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2-10. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




