Submitted:
25 January 2025
Posted:
27 January 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Se Dietary Intake and Health Implications
Se in the Human Body
Se Toxicity in Humans
Se Deficiency in Humans
Alleviation of Se Malnutrition in Humans Through Food Intake
Selenium in the Environment
Se in Plants
Se in Cereals
Se in Soil
Se Content in Upland and Lowland Soils
Approaches to Manipulation of Se in Plants for Better Human Health, Crop Yield and Soil Remediation
Improving Bioavailability of Se in Soil
Se Uptake by Plants
Se Toxicity in Plants
The Ability of Different Plants to Metabolize and Accumulate Se
Prevention of Se Pollution
Se Phytoremediation by Se Hyperaccumulators and Se tolerance
Se Reduction by Microbes
Utilizing Se Detoxification in Plants for Phytoremediation
Se Biofortification
Genetic Biofortification or Breeding for Biofortification
Gene Transformation for Se Biofortification
Agronomic Se Biofortification
Factors Affecting Biofortification
Assessment of Biofortification
Economic Benefits of Se Biofortification
Knowledge Gap
Conclusions
References
- Abdulah, R., K. Miyazaki, M. Nakazawa and H. Koyama (2005) Chemical forms of Se for cancer prevention. Journal of Trace Elements in Medicine and biology 19(2): 141-150.
- Acuña, J. J., M. A. Jorquera, P. J. Barra, D. E. Crowley and M. de la Luz Mora (2013) Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biology and fertility of soils 49(2): 175-185.
- Agalou, A., A. Roussis and H. P. Spaink (2005) The Arabidopsis Se-binding protein confers tolerance to toxic levels of Se. Functional plant biology 32(10): 881-890.
- Ajwa, H.A., Bañuelos, G.S. and Mayland, H.F., (1998) Se uptake by plants from soils amended with inorganic and organic materials. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. 27 (5):1218-1227.
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminene, P.; Hietaniemi, V.; Aspila, P.; et al. (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. Journal of Trace Elements in Medicine and Biology 31:142–147.
- Allander, E. (1994) Kashin-Beck disease. An analysis of research and public health activities based on a bibliography 1849-1992. Scandinavian Journal of Rheumatology 23(99): 1-36.
- Amarya, S., K. Singh and M. Sabharwal (2015) Changes during aging and their association with malnutrition. Journal of Clinical Gerontology and Geriatrics 6(3): 78-84.
- Anderson, J. W. (1993) Se interactions in sulfur metabolism. Sulfur nutrition and assimilation in higher plants: regulatory, agricultural and environmental aspects. SPB Academic Publishing, The Hague, The Netherlands: 49-60.
- Arvy, M. (1993) Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). Journal of Experimental Botany 44(6): 1083-1087.
- Avila, F. W., V. Faquin, Y. Yang, S. J. Ramos, L. R. Guilherme, T. W. Thannhauser and L. Li (2013) Assessment of the anticancer compounds Se-methylselenocysteine and glucosinolates in Se-biofortified broccoli (Brassica oleracea L. var. italica) sprouts and florets. Journal Agric Food Chem 61(26): 6216-6223.
- Azaizeh, H.A., Gowthaman, S. and Terry, N., (1997) Microbial Se volatilization in rhizosphere and bulk soils from a constructed wetland. Journal of Environmental Quality. 26(3): 666-672.
- Banuelos, G. (2006) Phyto-products may be essential for sustainability and implementation of phytoremediation. Environmental Pollution 144(1): 19-23.
- Azaizeh, H.A., Salhani, N., Sebesvari, Z. and Emons, H., (2003). The potential of rhizosphere microbes isolated from a constructed wetland to biomethylate Se. Journal of Environmental Quality, 32(1): 55-62.
- Banuelos, G. and D. Meek (1990) Accumulation of Se in plants grown on Se-treated soil. Journal of Environmental Quality 19(4): 772-777.
- Banuelos, G. and Z. Lin (2009). Development and Uses of Biofortified Agricultural Products, Boca Raton, FL: CRC Press.
- Bañuelos, G., Terry, N., LeDuc, D.L., Pilon-Smits, E.A. and Mackey, B., (2005) Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of Se-contaminated sediment. Environmental Science & Technology, 39(6), 1771-1777.
- Barberon, M., P. Berthomieu, M. Clairotte, N. Shibagaki, J. C. Davidian and F. Gosti (2008) Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1; 1 and SULTR1; 2. New Phytologist 180(3): 608-619.
- Barrow, N. and B. Whelan (1989) Testing a mechanistic model. VII. The effects of pH and of electrolyte on the reaction of selenite and selenate with a soil. Journal of Soil Science 40(1): 17-28.
- Bawa, S., K. Dhillon and S. Dhillon (1992) Screening of different fodders for Se absorption capacity. Indian Journal of Dairy Science 45: 457-457.
- Beilstein, M., P. Whanger and G. Yang (1991) Chemical forms of Se in corn and rice grown in a high Se area of China. Biomedical and environmental sciences: BES 4(4): 392-398.
- Bendich, A. (2001) Dietary reference intakes for vitamin C, vitamin E, Se, and carotenoids institute of medicine washington, DC: National Academy Press, 2000 ISBN: 0-309-06935-1. Nutrition 17(4): 364.
- Bhattacharya, A. (2011). Methylselenocysteine – a promising antiangiogenic agent for overcoming drug delivery barriers in solid malignancies for therapeutic synergy with anticancer drugs. Expert Opinion on Drug Delivery, 8(6), 749–763. [CrossRef]
- Blaylock, M. J., D. E. Salt, S. Dushenkov, O. Zakharova, C. Gussman, Y. Kapulnik, B. D. Ensley and I. Raskin (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environmental Science & Technology 31(3): 860-865.
- Boldrin, P. F., V. Faquin, S. J. Ramos, K. V. F. Boldrin, F. W. Ávila and L. R. G. Guilherme (2013) Soil and foliar application of Se in rice biofortification. Journal of Food Composition and Analysis 31(2): 238-244.
- Brinch-Pedersen, H., S. Borg, B. Tauris and P. B. Holm (2007) Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. Journal of Cereal Science 46(3): 308-326.
- Brown, T. A. and A. Shrift (1981) Exclusion of Se from proteins of Se-tolerant Astragalus species. Plant Physiology 67(5): 1051-1053.
- Bujdoš, M., A. Muľová, J. Kubova and J. Medveď (2005) Se fractionation and speciation in rocks, soils, waters and plants in polluted surface mine environment. Environmental geology 47(3): 353-360.
- Caballero, B. (2002) "Global patterns of child health: the role of nutrition. Annals of Nutrition and Metabolism 46(Suppl. 1): 3-7.
- Cakmak, I. (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and soil 302(1-2): 1-17.
- Cakmak, I. (2009) Agronomic approaches in biofortification of food crops with micronutrients. The Proceedings of the International Plant Nutrition Colloquium XVI. UC Davis: Department of Plant Sciences. Retrieved from https://escholarship.org/uc/item/3d46b1th.
- Carey, A. M., K. G. Scheckel, E. Lombi, M. Newville, Y. Choi, G. J. Norton, A. H. Price and A. A. Meharg (2012) Grain accumulation of Se species in rice (Oryza sativa L.). Environ Sci Technol 46(10): 5557-5564.
- Chaney, R. L., J. S. Angle, C. L. Broadhurst, C. A. Peters, R. V. Tappero and D. L. Sparks (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. Journal of Environmental Quality 36(5): 1429-1443.
- Chen, L., Yang, F., Xu, J., Hu, Y., Hu, Q., Zhang, Y. and Pan, G., 2002. Determination of Se concentration of rice in China and effect of fertilization of selenite and selenate on Se content of rice. Journal of Agricultural and Food Chemistry, 50(18): 5128-5130.
- Chen, Y.-W., H.-Y. T. Truong and N. Belzile (2009) Abiotic formation of elemental Se and role of iron oxide surfaces. Chemosphere 74(8): 1079-1084.
- Clark, L. C., G. F. Combs, B. W. Turnbull, E. H. Slate, D. K. Chalker, J. Chow, L. S. Davis, R. A. Glover, G. F. Graham and E. G. Gross (1996) Effects of Se supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. Jama 276(24): 1957-1963.
- Cohen, J. I. and R. Paarlberg (2002) Explaining restricted approval and availability of GM crops in developing countries. AgBiotechNet 4(097): 1-6.
- Combs, G. F. (2001) Se in global food systems. British Journal of Nutrition 85(05): 517-547.
- Combs, G. F. and W. P. Gray (1998) Chemopreventive agents: Se. Pharmacology & therapeutics 79(3): 179-192.
- Dauchy, X., M. Potin-Gautier, A. Astruc and M. Astruc (1994) Analytical methods for the speciation of Se compounds: a review. Fresenius' journal of analytical chemistry 348(12): 792-805.
- Brummell, D.A., Watson, L.M., Pathirana, R., Joyce, N.I., West, P.J., Hunter, D.A., McKenzie, M.J. (2011) Biofortification of Tomato (Solanum lycopersicum) Fruit with the Anticancer Compound Methylselenocysteine Using a Selenocysteine Methyltransferase from a Selenium Hyperaccumulator, Journal of Agricultural and Food Chemistry 2011 59 (20):10987-10994. [CrossRef]
- Dawe, D., R. Robertson and L. Unnevehr (2002) Golden rice: what role could it play in alleviation of vitamin A deficiency? Food Policy 27(5): 541-560.
- De Kok, L. J. (1993) Sulfur nutrition and assimilation in higher plants. Workshop on Sulfur Metabolism in Higher Plants 1992: Garmisch-Partenkirchen, Germany), SPB Academic.
- de Souza, M. P., E. A. Pilon-Smits, C. M. Lytle, S. Hwang, J. Tai, T. S. Honma, L. Yeh and N. Terry (1998) Rate-limiting steps in Se assimilation and volatilization by Indian mustard. Plant Physiology 117(4): 1487-1494.
- Dhillon, K.S. and Dhillon, S.K., (2001) Restoration of Se-contaminated soils. In Environmental Restoration of Metals-Contaminated Soils. Lewis Publishers Boca Raton, FL.
- Dhillon, K. S. and S. K. Dhillon (2009) Se concentrations of common weeds and agricultural crops grown in the seleniferous soils of northwestern India. Science of the total environment 407(24): 6150-6156.
- Dhillon, K.S. and Bañuelos, G.S., (2017) Overview and prospects of Se phytoremediation approaches. Se in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects, pp.277-321.
- Diyabalanage, S., Dangolla, A., Mallawa, C., Rajapakse, S. and Chandrajith, R., (2020) Bioavailability of Se (Se) in cattle population in Sri Lanka based on qualitative determination of glutathione peroxidase (GSH-Px) activities. Environmental geochemistry and health, 42: 617-624.
- Djanaguiraman, M., D. D. Devi, A. K. Shanker, J. A. Sheeba and U. Bangarusamy (2005) Se–an antioxidative protectant in soybean during senescence. Plant and Soil 272(1-2): 77-86.
- Dörr, A. J. M., N. Pacini, M. C. Abete, M. Prearo and A. C. Elia (2008) Effects of a Se-enriched diet on antioxidant response in adult crayfish (Procambarus clarkii). Chemosphere 73(7): 1090-1095.
- Ellis, D. R. and D. E. Salt (2003) Plants, Se and human health. Current Opinion in Plant Biology 6(3): 273-279.
- Ellis, D. R., T. G. Sors, D. G. Brunk, C. Albrecht, C. Orser, B. Lahner, K. V. Wood, H. H. Harris, I. J. Pickering and D. E. Salt (2004) Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biology 4(1): 1.
- El-Ramady, H.R., Abdalla, N., Alshaal, T., Elhenawy, A.S., Shams, M.S., Faizy, S.E.D.A., Belal, E.S.B., Shehata, S.A., Ragab, M.I., Amer, M.M. and Fári, M., (2015) Giant reed for Se phytoremediation under changing climate. Environmental chemistry letters, 13: 359-380.
- Elrashidi, M., D. Adriano, S. Workman and W. Lindsay (1987) Chemical equilibria of Se in soils: a theoretical development1. Soil Science 144(2): 141-152.
- Etteieb, S., Zolfaghari, M., Magdouli, S., Brar, K.K. and Brar, S.K., (2021) Performance of constructed wetland for Se, nutrient and heavy metals removal from mine effluents. Chemosphere, 281, p.130921.
- Eswayah, A.S., Smith, T.J. and Gardiner, P.H., (2016) Microbial transformations of Se species of relevance to bioremediation. Applied and environmental microbiology, 82(16): 4848-4859.
- Eurola, M. H., P. I. Ekholm, M. E. Ylinen, P. T. Varo and P. E. Koivistoinen (1991) Se in Finnish foods after beginning the use of selenate-supplemented fertilisers. Journal of the Science of Food and Agriculture 56(1): 57-70.
- Eurola, M., Ekholm, P.A.I.V.I., Ylinen, M.A.I.J.A., Koivistoinen, P.E.K.K.A. and Varo, P.E.R.T.T.I., (1990) Effects of Se fertilization on the Se content of cereal grains, flour, and bread produced in Finland. Cereal Chem, 67(4): 334-337.
- Fan, M.-S., F.-J. Zhao, S. J. Fairweather-Tait, P. R. Poulton, S. J. Dunham and S. P. McGrath (2008a) Evidence of decreasing mineral density in wheat grain over the last 160 years. Journal of Trace Elements in Medicine and Biology 22(4): 315-324.
- Fang, Y., L. Wang, Z. Xin, L. Zhao, X. An and Q. Hu (2008) Effect of foliar application of zinc, Se, and iron fertilizers on nutrients concentration and yield of rice grain in China. Journal of Agricultural and Food Chemistry 56(6): 2079-2084.
- FAO (2005). The State of Food Insecurity in the World (2005) Eradicating World Hunger-Key to Achieving the Millennium Development Goals, Food & Agriculture Org., Rome, Italy. Available at: https://www.fao.org/4/a0200e/a0200e00.htm.
- Feist, L. J. and D. R. Parker (2001) Ecotypic variation in Se accumulation among populations of Stanleya pinnata. New Phytologist 149(1): 61-69.
- Felton, S., M. Landolt, R. Grace and A. Palmisano (1996). Effects of Se dietary enhancement on hatchery-reared coho salmon, Oncorhynchus kisutch (Walbaum), when compared with wild coho: hepatic enzymes and seawater adaptation evaluated. Aquaculture Research 27(2): 135-142.
- Fendorf, S., M. Herbel, K. Tufano and B. Kocar (2008) Biogeochemical processes controlling the cycling of arsenic in soils and sediments, Biophysico-chemical Processes of Heavy Metals and Metalloids in Soil Environments, 313-338.Wiley: Hoboken, NJ.
- Fernandes, K., R. Berton and A. Coscione (2014) Se biofortification of rice and radish: effect of soil texture and efficiency of two extractants. Plant soil and environment 60(3): 105.
- Fiedler, J. L., D. R. Dado, H. Maglalang, N. Juban, M. Capistrano and M. V. Magpantay (2000) Cost analysis as a vitamin A program design and evaluation tool: a case study of the Philippines. Social Science & Medicine 51(2): 223-242.
- Finley, J. W. (2005). Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids, glucosinolates, polyphenols and selenocompounds. Annals of Botany 95(7): 1075-1096.
- Fogel, R. W. (2004) The escape from hunger and premature death, 1700-2100: Europe, America, and the Third World, Cambridge University Press.
- Fordyce, F. (2005) Se deficiency and toxicity in the environment. In: Essentials of medical geology–Impacts of the natural environment on public health (Eds O Selinus, B Alloway, JA Centeno, RB Finkelman, R Fuge, U Lindh, P Smedley) pp. 373-415, Elsevier-Academic Press, London.
- Fordyce, F.M., Johnson, C.C., Navaratna, U.R., Appleton, J.D. and Dissanayake, C.B., (2000) Se and iodine in soil, rice and drinking water in relation to endemic goitre in Sri Lanka. Science of the Total Environment, 263(1-3): 127-141.
- Frankenberger Jr., W.T., & Benson, S. (1994). Se in the Environment (1st ed.). CRC Press. Pp 480. Available at: . [CrossRef]
- Frankenberger Jr, W. T., & Arshad, M. (2001). Bioremediation of selenium-contaminated sediments and water. Biofactors, 14(1-4): 241-254.
- Freeman, J. L., C. F. Quinn, M. A. Marcus, S. Fakra and E. A. Pilon-Smits (2006a) Se-tolerant diamondback moth disarms hyperaccumulator plant defense. Current Biology 16(22): 2181-2192.
- Freeman, J. L., L. H. Zhang, M. A. Marcus, S. Fakra, S. P. McGrath and E. A. Pilon-Smits (2006b) Spatial imaging, speciation, and quantification of Se in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant physiology 142(1): 124-134.
- Fu, L.-H., X.-F. Wang, Y. Eyal, Y.-M. She, L. J. Donald, K. G. Standing and G. Ben-Hayyim (2002) A selenoprotein in the plant kingdom mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chlamydomonas reinhardtii glutathione peroxidase. Journal of Biological Chemistry 277(29): 25983-25991.
- Gailer, J., S. Madden, M. F. Burke, M. B. Denton and H. V. Aposhian (2000) Simultaneous multielement-specific detection of a novel glutathione–arsenic–Se ion [(GS) 2AsSe]− by ICP AES after micellar size-exclusion chromatography. Applied organometallic chemistry 14(7): 355-363.
- Galeas, M. L., L. H. Zhang, J. L. Freeman, M. Wegner and E. A. Pilon-Smits (2007) Seasonal fluctuations of Se and sulfur accumulation in Se hyperaccumulators and related nonaccumulators. New Phytologist 173(3): 517-525.
- Ganther, H. (1986) Pathways of Se metabolism including respiratory excretory products. International Journal of Toxicology 5(1): 1-5.
- Garvin, D. F., R. M. Welch and J. W. Finley (2006) "Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. Journal of the Science of Food and Agriculture 86(13): 2213-2220.
- Gatlin 3rd, D. and R. P. Wilson (1984) Dietary Se requirement of fingerling channel catfish. The Journal of nutrition 114(3): 627-633.
- Giray, B. and F. Hincal (2004) Se status in Turkey. Journal of radioanalytical and nuclear chemistry 259(3): 447-451.
- Goldhaber, S. B. (2003) Trace element risk assessment: essentiality vs. toxicity. Regulatory Toxicology and Pharmacology 38(2): 232-242.
- Gómez-Ariza, J.L., Pozas, J.A., Giraldez, I. and Morales, E., (1998) Speciation of volatile forms of Se and inorganic Se in sediments by gas chromatography–mass spectrometry. Journal of Chromatography A, 823(1-2): 259-277.
- Graham, R. D., R. M. Welch, D. A. Saunders, I. Ortiz-Monasterio, H. E. Bouis, M. Bonierbale, S. De Haan, G. Burgos, G. Thiele and R. Liria (2007) Nutritious subsistence food systems. Advances in Agronomy 92: 1-74.
- Graham, R., D. Senadhira, S. Beebe, C. Iglesias and I. Monasterio (1999). Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crops Research 60(1): 57-80.
- Grant, T. D., M. Montes-Bayón, D. LeDuc, M. W. Fricke, N. Terry and J. A. Caruso (2004) Identification and characterization of Se-methyl selenomethionine in Brassica juncea roots. Journal of Chromatography A 1026(1): 159-166.
- Grčman, H., D. Vodnik, Š. Velikonja-Bolta and D. Leštan (2003) Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. Journal of Environmental Quality 32(2): 500-506.
- Gregorio, G. B., D. Senadhira, H. Htut and R. D. Graham (2000) Breeding for trace mineral density in rice. Food and Nutrition Bulletin 21(4): 382-386.
- Grybos, M., M. Davranche, G. Gruau and P. Petitjean (2007) Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction? Journal of colloid and interface science 314(2): 490-501.
- Guerinot, M. L. and D. E. Salt (2001) Fortified foods and phytoremediation. Two sides of the same coin. Plant Physiology 125(1): 164-167.
- Gupta, M. and Gupta, S., (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Frontiers in plant science, 7, p.2074.
- Guzmán Mar, J. L., L. Hinojosa Reyes, G. Mizanur Rahman and H. S. Kingston (2009) Simultaneous extraction of arsenic and Se species from rice products by microwave-assisted enzymatic extraction and analysis by ion chromatography-inductively coupled plasma-mass spectrometry. Journal of agricultural and food chemistry 57(8): 3005-3013.
- Haddad, L., J. Ross, A. Oshaug, L. E. Torheim and B. Cogill (2004). 5th report on the world nutrition situation. Nutrition for improved development outcomes. (Vol. 5). United Nations System Standing Committee on Nutrition.
- Hageman, S.P., van der Weijden, R.D., Weijma, J. and Buisman, C.J., (2013) Microbiological selenate to selenite conversion for Se removal. Water research, 47(7), pp.2118-2128.
- Hansen, D., P. J. Duda, A. Zayed and N. Terry (1998) Se removal by constructed wetlands: role of biological volatilization. Environmental Science & Technology 32(5): 591-597.
- Hanson, B., G. F. Garifullina, S. D. Lindblom, A. Wangeline, A. Ackley, K. Kramer, A. P. Norton, C. B. Lawrence and E. A. Pilon-Smits (2003) Se accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytologist 159(2): 461-469.
- Hanson, B., S. D. Lindblom, M. L. Loeffler and E. A. Pilon-Smits (2004) Se protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytologist 162(3): 655-662.
- Hartikainen, H. (2005) Biogeochemistry of Se and its impact on food chain quality and human health. Journal of Trace Elements in Medicine and Biology 18(4): 309-318.
- Hawkesford, M.J. and F.J. Zhao, (2007) Strategies for increasing the Se content of wheat. Journal of Cereal Science, 46(3): 282-292.
- He, Z. L., J. Shentu and X. E. Yang (2010) Manganese and Se. Trace elements in soils. 1st ed. John Wiley & Sons, United Kingdom: Pp. 481-497.
- Helzlsouer, K., Jacobs, R. and Morris, S., (1985) Acute Se intoxication in the United States. In Fed Proc 44, No. 1670, p. 27.
- Hilton, J., P. Hodson and S. Slinger (1982) Absorption, distribution, half-life and possible routes of elimination of dietary Se in juvenile rainbow trout (Salmo gairdneri). Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 71(1): 49-55.
- Hira, C. K., K. Partal and K. Dhillon (2004) Dietary Se intake by men and women in high and low Se areas of Punjab. Public health nutrition 7(01): 39-43.
- Hopper, J. L. and D. R. Parker (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant and Soil 210(2): 199-207.
- Horton, S. and J. Ross (2003) The economics of iron deficiency. Food policy 28(1): 51-75.
- Hotz, C. and K. H. Brown (2004) Assessment of the risk of zinc deficiency in populations and options for its control, International nutrition foundation: for UNU.
- Hu, Y., G. L. Duan, Y. Z. Huang, Y. X. Liu and G. X. Sun (2014) Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res Int 21(5): 3955-3962.
- Huang, J. W., J. Chen, W. R. Berti and S. D. Cunningham (1997) Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environmental Science & Technology 31(3): 800-805.
- Huang, J.C., Passeport, E. and Terry, N., (2012) Development of a constructed wetland water treatment system for Se removal: use of mesocosms to evaluate design parameters. Environmental science & technology, 46(21): 12021-12029.
- Huang, Y., Wang, Q., Gao, J., Lin, Z., Banuelos, G.S., Yuan, L. and Yin, X., (2013) Daily dietary Se intake in a high Se area of Enshi, China. Nutrients, 5(3): 700-710.
- Jayaweera, G. R. and J. W. Biggar (1996) Role of redox potential in chemical transformations of Se in soils. Soil Science Society of America Journal 60(4): 1056-1063.
- Kabata-Pendias, A. (2010) Trace elements in soils and plants, CRC press.
- Kahakachchi, C., H. T. Boakye, P. C. Uden and J. F. Tyson (2004) Chromatographic speciation of anionic and neutral Se compounds in Se-accumulating Brassica juncea (Indian mustard) and in selenized yeast. Journal of Chromatography A 1054(1): 303-312.
- Kamei-Ishikawa, N., K. Tagami and S. Uchida (2007) Sorption kinetics of Se on humic acid. Journal of Radioanalytical and Nuclear Chemistry 274(3): 555-561.
- Kápolna, E. and P. Fodor (2006) Speciation analysis of Se enriched green onions (Allium fistulosum) by HPLC-ICP-MS. Microchemical journal 84(1): 56-62.
- Kelly, S., M. Baxter, S. Chapman, C. Rhodes, J. Dennis and P. Brereton (2002) The application of isotopic and elemental analysis to determine the geographical origin of premium long grain rice. European Food Research and Technology 214(1): 72-78.
- Kibriya, M. G., F. Jasmine, M. Argos, W. J. Verret, M. Rakibuz-Zaman, A. Ahmed, F. Parvez and H. Ahsan (2007) Changes in gene expression profiles in response to Se supplementation among individuals with arsenic-induced pre-malignant skin lesions. Toxicology letters 169(2): 162-176.
- Kieliszek, M. and S. Błażejak (2013) Se: significance, and outlook for supplementation. Nutrition 29(5): 713-718.
- Kirk, G. (2004) The biogeochemistry of submerged soils, John Wiley & Sons. Chichester.
- Kirkby, E. A. and A. E. J. Johnston (2008) Soil and fertilizer phosphorus in relation to crop nutrition. In: White, P.J., Hammond, J.P. (eds) The ecophysiology of plant-phosphorus interactions, vol 7. Springer, Dordrecht.
- Krishnan, S. and P. Dayanandan (2003). "Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.). Journal of biosciences 28(4): 455-469.
- Kryukov, G. V., S. Castellano, S. V. Novoselov, A. V. Lobanov, O. Zehtab, R. Guigó and V. N. Gladyshev (2003) Characterization of mammalian selenoproteomes. Science 300(5624): 1439-1443.
- Kulp, T. R. and L. M. Pratt (2004) Speciation and weathering of Se in Upper Cretaceous chalk and shale from South Dakota and Wyoming, USA. Geochimica et Cosmochimica Acta 68(18): 3687-3701.
- Kunli, L., X. Lirong, T. Jian'an, W. Douhu and X. Lianhua (2004) Se source in the selenosis area of the Daba region, South Qinling Mountain, China. Environmental Geology 45(3): 426-432.
- LeDuc, D. L. and N. Terry (2005) Phytoremediation of toxic trace elements in soil and water. Journal of Industrial Microbiology and Biotechnology 32(11-12): 514-520.
- LeDuc, D. L., A. S. Tarun, M. Montes-Bayon, J. Meija, M. F. Malit, C. P. Wu, M. AbdelSamie, C.-Y. Chiang, A. Tagmount and B. Neuhierl (2004) Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases Se tolerance and accumulation. Plant Physiology 135(1): 377-383.
- LeDuc, D. L., M. AbdelSamie, M. Móntes-Bayon, C. P. Wu, S. J. Reisinger and N. Terry (2006). Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances Se phytoremediation traits in Indian mustard. Environmental Pollution 144(1): 70-76.
- Lemly, A.D., (2004) Aquatic Se pollution is a global environmental safety issue. Ecotoxicology and environmental safety, 59(1): 44-56.
- Lenz, M. and P. N. Lens (2009) The essential toxin: the changing perception of Se in environmental sciences. Science of the Total Environment 407(12): 3620-3633.
- Leustek, T., M. N. Martin, J.-A. Bick and J. P. Davies (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annual review of plant biology 51(1): 141-165.
- Levander, O. A., G. Alfthan, H. Arvilommi, C. Gref, J. Huttunen, M. Kataja, P. Koivistoinen and J. Pikkarainen (1983) Bioavailability of Se to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. The American journal of clinical nutrition 37(6): 887-897.
- Li, H. F., E. Lombi, J. L. Stroud, S. P. McGrath and F. J. Zhao (2010). Se speciation in soil and rice: influence of water management and Se fertilization. J Agric Food Chem 58(22): 11837-11843.
- Li, T., Xu, H., Zhang, Y., Zhang, H., Hu, X., Sun, Y., Gu, X., Luo, J., Zhou, D. and Gao, B., (2022) Treatment technologies for Se contaminated water: A critical review. Environmental Pollution, 299, p.118858.
- Liu, Y., F. Li, X. Yin and Z. Lin (2010) Plant-based biofortification: from phytoremediation to Se-enriched agriculture products. Green chemistry for environmental sustainability. Pp. 341-356. CRC press, Boca Raton.
- Lobanov, A. V., D. E. Fomenko, Y. Zhang, A. Sengupta, D. L. Hatfield and V. N. Gladyshev (2007) Evolutionary dynamics of eukaryotic selenoproteomes: large selenoproteomes may associate with aquatic life and small with terrestrial life. Genome biology 8(9): 1.
- Low, J. W., M. Arimond, N. Osman, B. Cunguara, F. Zano and D. Tschirley (2007) A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. The Journal of nutrition 137(5): 1320-1327.
- Lu, J. and A. Holmgren (2009) Selenoproteins. Journal of Biological Chemistry 284(2): 723-727.
- Lyi, S. M., L. I. Heller, M. Rutzke, R. M. Welch, L. V. Kochian and L. Li (2005) Molecular and biochemical characterization of the selenocysteine Se-methyltransferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiology 138(1): 409-420.
- Lynch, J. P. (2007) Turner review no. 14. Roots of the second green revolution. Australian Journal of Botany 55(5): 493-512.
- Lyons, G. H., G. J. Judson, I. Ortiz-Monasterio, Y. Genc, J. C. Stangoulis and R. D. Graham (2005a) Se in Australia: Se status and biofortification of wheat for better health. Journal of Trace Elements in Medicine and Biology 19(1): 75-82.
- Lyons, G., I. Ortiz-Monasterio, J. Stangoulis and R. Graham (2005b) Se concentration in wheat grain: Is there sufficient genotypic variation to use in breeding? Plant and Soil 269 (1-2): 369-380.
- Lyons, G., J. Stangoulis and R. Graham (2003) High-Se wheat: biofortification for better health. Nutrition Research Reviews 16(01): 45-60.
- Mao, H., J. Wang, Z. Wang, Y. Zan, G. Lyons and C. Zou (2014) Using agronomic biofortification to boost zinc, Se, and iodine concentrations of food crops grown on the loess plateau in China. Journal of soil science and plant nutrition 14(2): 459-470.
- McKenzie, R.C., Rafferty, T.S., Beckett, G.J., Arthur, J.R. (2001). Effects of selenium on immunity and aging. In: Hatfield, D.L. (eds) Selenium. Springer, Boston, MA. Pp: 21257-272. [CrossRef]
- McKevith, B., (2004) Nutritional aspects of cereals. Nutrition Bulletin, 29(2): 111-142.
- McKenzie, M.J., Hunter, D.A., Pathirana, R. et al. (2009) Accumulation of an organic anticancer selenium compound in a transgenic Solanaceous species shows wider applicability of the selenocysteine methyltransferase transgene from selenium hyperaccumulators. Transgenic Res 18, 407-424 . [CrossRef]
- McLaughlin, M. J., D. Parker and J. Clarke (1999). Metals and micronutrients–food safety issues. Field crops research 60(1): 143-163.
- Meenakshi, J., N. L. Johnson, V. M. Manyong, H. DeGroote, J. Javelosa, D. R. Yanggen, F. Naher, C. Gonzalez, J. Garcia and E. Meng (2010) How cost-effective is biofortification in combating micronutrient malnutrition? An ex ante assessment. World Development 38(1): 64-75.
- Mikkelsen, R. L., D. S. Mikkelsen and A. Abshahi (1989a) Effects of soil flooding on Se transformations and accumulation by rice. Soil Science Society of America Journal 53(1): 122-127.
- Mikkelsen, R., A. L. Page and F. T. Bingham (1989b) Factors affecting Se accumulation by agricultural crops. Se in Agriculture and the Environment (Seinagric): 65-94.
- Milovac, M., Djermanović, V. and Djujić, I., (1998) Effects of cereal supplementation with Se. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer, 17(3-4): 313-320.
- Mishra, R. R., S. Prajapati, J. Das, T. K. Dangar, N. Das and H. Thatoi (2011) Reduction of selenite to red elemental Se by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere 84(9): 1231-1237.
- Mombo, S., Schreck, E., Dumat, C., Laplanche, C., Pierart, A., Longchamp, M., Besson, P. and Castrec-Rouelle, M., 2016. Bioaccessibility of selenium after human ingestion in relation to its chemical species and compartmentalization in maize. Environmental geochemistry and health, 38: 869-883.
- Montes-Bayón, M., D. L. LeDuc, N. Terry and J. A. Caruso (2002) Se speciation in wild-type and genetically modified Se accumulating plants with HPLC separation and ICP-MS/ES-MS detection. Journal of Analytical Atomic Spectrometry 17(8): 872-879.
- Morgan, J., G. Bending and P. White (2005) Biological costs and benefits to plant–microbe interactions in the rhizosphere. Journal of Experimental Botany 56 (417): 1729-1739.
- Mouta, E. R., W. J. d. Melo, M. R. Soares, L. R. F. Alleoni and J. C. Casagrande (2008) Adsorção de selênio em latossolos. Revista Brasileira de Ciência do Solo: 1033-1041.
- Munier-Lamy, C., S. Deneux-Mustin, C. Mustin, D. Merlet, J. Berthelin and C. Leyval (2007) Se bioavailability and uptake as affected by four different plants in a loamy clay soil with particular attention to mycorrhizae inoculated ryegrass. Journal of environmental radioactivity 97(2): 148-158.
- Navarro-Alarcon, M. and C. Cabrera-Vique (2008) Se in food and the human body: a review. Science of the total environment 400(1): 115-141.
- Nestel, P., H. E. Bouis, J. Meenakshi and W. Pfeiffer (2006) Biofortification of staple food crops. The Journal of nutrition 136(4): 1064-1067.
- Neuhierl, B. and A. Böck (1996) "On the Mechanism of Se Tolerance in Se-Accumulating Plants. European Journal of Biochemistry 239(1): 235-238.
- Nève, J. (1996). Se as a risk factor for cardiovascular diseases. Journal of cardiovascular risk 3(1): 42-47.
- Nkansah-Boadu, F. and Baldwin, S.A., (2019) Biological Removal of Se from Mining Influenced Waters: A Critical Review of Challenges. In Proceedings of the IMWA 2019 Conference-Mine Water: Technological and Ecological Challenges.
- Novoselov, S. V., M. Rao, N. V. Onoshko, H. Zhi, G. V. Kryukov, Y. Xiang, D. P. Weeks, D. L. Hatfield and V. N. Gladyshev (2002) Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. The EMBO journal 21(14): 3681-3693.
- Nowack, B., R. Schulin and B. H. Robinson (2006) Critical assessment of chelant-enhanced metal phytoextraction. Environmental Science & Technology 40(17): 5225-5232.
- Ogra, Y., K. Ishiwata, J. R. Encinar, R. Łobiński and K. T. Suzuki (2004) Speciation of Se in Se-enriched shiitake mushroom, Lentinula edodes. Analytical and bioanalytical chemistry 379(5-6): 861-866.
- Ogra, Y., K. Ishiwata, Y. Iwashita and K. T. Suzuki (2005) Simultaneous speciation of Se and sulfur species in selenized odorless garlic (Allium sativum L. Shiro) and shallot (Allium ascalonicum) by HPLC–inductively coupled plasma-(octopole reaction system)-mass spectrometry and electrospray ionization-tandem mass spectrometry. Journal of Chromatography A 1093(1): 118-125.
- Ohlendorf, H. M., R. W. Lowe, P. R. Kelly and T. E. Harvey (1986) Se and heavy metals in San Francisco Bay diving ducks. The Journal of wildlife management: 64-70.
- Parada, J. and J. Aguilera (2007) Food microstructure affects the bioavailability of several nutrients. Journal of food science 72(2): R21-R32.
- Pedrero, Z. and Y. Madrid (2009) Novel approaches for Se speciation in foodstuffs and biological specimens: a review. Analytica chimica acta 634(2): 135-152.
- Peterson, P.J. and Butler, G.W., (1967) Significance of selenocystathionine in an Australian Se-accumulating plant, Neptunia amplexicaulis. Nature, 213(5076): 599-600.
- Pilon, M., J. D. Owen, G. F. Garifullina, T. Kurihara, H. Mihara, N. Esaki and E. A. Pilon-Smits (2003) Enhanced Se tolerance and accumulation in transgenic Arabidopsis expressing a mouse selenocysteine lyase. Plant Physiology 131(3): 1250-1257.
- Pilon-Smits, E. (2005) Phytoremediation. Annu. Rev. Plant Biol. 56: 15-39.
- Pilon-Smits, E. A. and D. L. LeDuc (2009) Phytoremediation of Se using transgenic plants. Curr Opin Biotechnol 20(2): 207-212.
- Pilon-Smits, E. A. H. and C. F. Quinn (2010) Se Metabolism in Plants. Plant Cell Monographs 17: 225−241.
- Pilon-Smits, E.A., Hwang, S., Mel Lytle, C., Zhu, Y., Tai, J.C., Bravo, R.C., Chen, Y., Leustek, T. and Terry, N., 1999. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiology, 119(1), pp.123-132.
- Pilon-Smits, E.A., (2019) On the ecology of Se accumulation in plants. Plants, 8(7), p.197.
- Poblaciones, M., S. Rodrigo, O. Santamaría, Y. Chen and S. McGrath (2014) Agronomic Se biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food chemistry 146: 378-384.
- Poluboyarinov, P.A., Elistratov, D.G. & Moiseeva, I.J. (2020) antitumor activity of selenium and search parameters for its new potentially active derivatives. Russian Journal of Bioorganic Chemistry 46: 989–1003. [CrossRef]
- Potrykus, I. (2009) Lessons from Golden Rice on public sector responsibility and failure. New Biotechnology 25: S321-S322.
- Pray, C. and J. Huang (2008) Biofortification for China: political responses to food fortification and GM technology, interest groups, and possible strategies. Ag Bio Forum. 10(3): 2007.
- Premarathna, L., M. J. McLaughlin, J. K. Kirby, G. M. Hettiarachchi, S. Stacey and D. J. Chittleborough (2012) Selenate-enriched urea granules are a highly effective fertilizer for Se biofortification of paddy rice grain. The Journal of Agricultural and Food Chemistry. 60(23): 6037-6044.
- Prom-u-thai, C., S. Fukai, I. D. Godwin and L. Huang (2007) Genotypic variation of iron partitioning in rice grain. Journal of the Science of Food and Agriculture 87(11): 2049-2054.
- Qaim, M. and Stein, A.J., 2009. Biofortification of staple crops: how well does it work and what does it cost Ernährungs Umschau, 56(5): 274-280.
- Qaim, M., A. J. Stein and J. Meenakshi (2007) Economics of biofortification. Agricultural Economics 37(s1): 119-133.
- Qin, H.-b., J.-m. Zhu and H. Su (2012) Se fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 86(6): 626-633.
- Quinn, C. F., M. L. Galeas, J. L. Freeman and E. AH (2007) Se: Deterrence, toxicity, and adaptation. Peptides 125: 6160-6164.
- Quinn, C., J. Freeman, M. Galeas, E. Klamper and E. Pilon-Smits (2008) Se protects plants from prairie dog herbivory–implications for the functional significance and evolution of Se hyperaccumulation. Oecologia 155: 267-275.
- Ramos, S. J., Y. Yuan, V. Faquin, L. R. G. Guilherme and L. Li (2011) Evaluation of genotypic variation of broccoli (Brassica oleracea var. italic) in response to Se treatment. Journal of agricultural and food chemistry 59(8): 3657-3665.
- Rayman, M. P. (2000) The importance of Se to human health. The lancet 356(9225): 233-241.
- Rayman, M. P. (2008) Food-chain Se and human health: emphasis on intake. British journal of nutrition 100(02): 254-268.
- Rayman, M. P. (2012) Se and human health. The Lancet 379(9822): 1256-1268.
- Reeves, R. D. and A. J. Baker (2000) Metal-accumulating plants. Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York: 193-229.
- Roberge, M. T., A. J. Borgerding and J. W. Finley (2003) Speciation of Se compounds from high Se broccoli is affected by the extracting solution. Journal of agricultural and food chemistry 51(15): 4191-4197.
- Robinson, B. H., G. Bañuelos, H. M. Conesa, M. W. Evangelou and R. Schulin (2009) The phytomanagement of trace elements in soil. Critical Reviews in Plant Sciences 28(4): 240-266.
- Rosenfeld, I. and O. A. Beath (2013) Se: geobotany, biochemistry, toxicity, and nutrition, Academic Press.
- Schiavon, M., Moro, I., Pilon-Smits, E.A., Matozzo, V., Malagoli, M. and Dalla Vecchia, F., (2012) Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. Aquatic toxicology, 122: 222-231.
- Schiavon, M., M. Pilon, M. Malagoli and E. A. Pilon-Smits (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for Se hyperaccumulation—a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Frontiers in plant science 6: 2.
- Schram, E., Z. Pedrero, C. Cámara, J. W. Van Der Heul and J. B. Luten (2008) Enrichment of African catfish with functional Se originating from garlic. Aquaculture Research 39(8): 850-860.
- Schrauzer, G. N. (2001). Nutritional Se supplements: product types, quality, and safety. Journal of the American College of Nutrition 20(1): 1-4.
- Searchinger, T., R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T.-H. Yu (2008) Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319(5867): 1238-1240.
- Séby, F., Gautier, M.P., Lespes, G. and Astruc, M., (1997) Se speciation in soils after alkaline extraction. Science of the Total Environment, 207(2-3): 81-90.
- Sharma, S., A. Bansal, S. K. Dhillon and K. S. Dhillon (2010) Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant and Soil 329(1-2): 339-348.
- Sharma, S., Bansal, A., Dogra, R., Dhillon, S.K. and Dhillon, K.S., (2011) Effect of organic amendments on uptake of Se and biochemical grain composition of wheat and rape grown on seleniferous soils in northwestern India. Journal of Plant Nutrition and Soil Science, 174(2): 269-275.
- Shen, L., P. V. Dael, L. Luten and H. Deelstra (1996) Estimation of Se bioavailability from human, cow's, goat and sheep milk by an in vitro method. International journal of food sciences and nutrition 47(1): 75-81.
- Shibagaki, N., A. Rose, J. P. McDermott, T. Fujiwara, H. Hayashi, T. Yoneyama and J. P. Davies (2002) Selenate-resistant mutants of Arabidopsis thaliana identify SULTR1; 2, a sulfate transporter required for efficient transport of sulfate into roots. The Plant Journal 29(4): 475-486.
- Sors, T. G., D. R. Ellis and D. E. Salt (2005) Se uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86(3): 373-389.
- Spallholz, J. E., L. M. Boylan and M. Rhaman (2004) Environmental hypothesis: is poor dietary Se intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India. Science of the total environment 323(1): 21-32.
- Stadlober, M., M. Sager and K. J. Irgolic (2001) Effects of selenate supplemented fertilisation on the Se level of cereals—identification and quantification of Se compounds by HPLC–ICP–MS. Food Chemistry 73(3): 357-366.
- Stadtman, T. (1991) Biosynthesis and function of selenocysteine-containing enzymes. Journal of Biological Chemistry 266(25): 16257-16260.
- Staicu, L.C., Van Hullebusch, E.D., Rittmann, B.E. and Lens, P.N., (2017) Industrial Se pollution: sources and biological treatment technologies. Bioremediation of Se contaminated wastewater, pp.75-101.
- Stein, A. J. (2009). Global impacts of human mineral malnutrition. Plant and Soil 335(1-2): 133-154.
- Stolz, J.F., Basu, P., Santini, J.M. and Oremland, R.S., (2006) Arsenic and Se in microbial metabolism. Annu. Rev. Microbiology, 60:107-130.
- Stroud, J., M. Broadley, I. Foot, S. Fairweather-Tait, D. Hart, R. Hurst, P. Knott, H. Mowat, K. Norman and P. Scott (2010) Soil factors affecting Se concentration in wheat grain and the fate and speciation of Se fertilisers applied to soil. Plant and soil 332(1-2): 19-30.
- Sun, G.-X., X. Liu, P. N. Williams and Y.-G. Zhu (2010) Distribution and translocation of Se from soil to grain and its speciation in paddy rice (Oryza sativa L.). Environmental science & technology 44(17): 6706-6711.
- Sura-de Jong, M., Reynolds, R.J., Richterova, K., Musilova, L., Staicu, L.C., Chocholata, I., Cappa, J.J., Taghavi, S., van der Lelie, D., Frantik, T. and Dolinova, I., (2015) Se hyperaccumulators harbor a diverse endophytic bacterial community characterized by high Se resistance and plant growth promoting properties. Frontiers in plant science, 6, p.113.
- Szpunar, J., R. Lobinski and A. Prange (2003) Hyphenated techniques for elemental speciation in biological systems. Applied spectroscopy 57(3): 102A-112A.
- Tan, J., W. Wang, D. Wang and S. Hou (1994) Adsorption, volatilization, and speciation of Se in different types of soils in China. Se in the Environment: 47-68.
- Tapiero, H., D. Townsend and K. Tew (2003) The antioxidant role of Se and seleno-compounds. Biomedicine & pharmacotherapy 57(3): 134-144.
- Terry, N., A. Zayed, M. De Souza and A. Tarun (2000) Se in higher plants. Annual review of plant biology 51(1): 401-432.
- Thavarajah, D., P. Thavarajah, A. Wejesuriya, M. Rutzke, R. P. Glahn, G. F. Combs and A. Vandenberg (2011) The potential of lentil (Lens culinaris L.) as a whole food for increased Se, iron, and zinc intake: preliminary results from a 3 year study. Euphytica 180(1): 123-128.
- Thiry, C., A. Ruttens, L. De Temmerman, Y.-J. Schneider and L. Pussemier (2012) Current knowledge in species-related bioavailability of Se in food. Food Chemistry 130(4): 767-784.
- Thomson, C. (2004) Assessment of requirements for Se and adequacy of Se status: a review. European Journal of Clinical Nutrition 58(3): 391-402.
- Thornton, I., D. Kinniburgh, G. Pullen and C. Smith (1983) Geochemical aspects of Se in British soils and implications to animal health [Toxicity and deficiency diseases; Great Britain]. Trace substances in environmental health: proceedings of University of Missouri's annual conference (USA).
- Turakainen, M., H. Hartikainen and M. M. Seppänen (2004) Effects of Se treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. Journal of agricultural and food chemistry 52(17): 5378-5382.
- United Republic of Tanzania (2020) National Biofortification Guidelines. Ministry of Agriculture, May 2020. https://www.kilimo.go.tz/uploads/dasip/English_version_Final_new2.pdf, Retrieved on Oct 30, 2023.
- Unnevehr, L. J. (1986). Consumer demand for rice grain quality and returns to research for quality improvement in Southeast Asia. American Journal of Agricultural Economics. 68(3): 634-641.
- Vamerali, T., M. Bandiera, P. Lucchini, N. M. Dickinson and G. Mosca (2014) Long-term phytomanagement of metal-contaminated land with field crops: integrated remediation and biofortification. European Journal of Agronomy. 53: 56-66.
- Van Huysen, T., S. Abdel-Ghany, K. L. Hale, D. LeDuc, N. Terry and E. A. Pilon-Smits (2003) Overexpression of cystathionine-γ-synthase enhances Se volatilization in Brassica juncea. Planta 218(1): 71-78.
- Vonderheide, A. P., K. Wrobel, S. S. Kannamkumarath, C. B'Hymer, M. Montes-Bayón, C. Ponce de León and J. A. Caruso (2002) "Characterization of Se species in Brazil nuts by HPLC-ICP-MS and ES-MS. Journal of agricultural and food chemistry 50(20): 5722-5728.
- Wang, D., Dinh, Q.T., Thu, T.T.A., Zhou, F., Yang, W., Wang, M., Song, W. and Liang, D., (2018) Effect of Se-enriched organic material amendment on Se fraction transformation and bioavailability in soil. Chemosphere, 199: 417-426.
- Wang, S., D. Liang, D. Wang, W. Wei, D. Fu and Z. Lin (2012) Se fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Science of the total Environment 427: 159-164.
- Wang, X. and K. Taniguchi (2003) Does better nutrition enhance economic growth? The economic cost of hunger. Nutrition intake and economic growth. Studies on the cost of hunger. FAO, Rome.
- Wang, Z. and Y. Gao (2001). Biogeochemical cycling of Se in Chinese environments. Applied Geochemistry 16(11): 1345-1351.
- Wang, Z., S. Xie and A. Peng (1996) Distribution of Se in soybean samples with different Se concentration. Journal of Agricultural and Food Chemistry 44(9): 2754-2759.
- Watkinson, J.H. (1981) Changes of blood selenium in New Zealand adults with time and importation of Australian wheat. The American Journal of Clinical Nutrition. 34(5): 936-942.
- Welch, R. M. (2002) Breeding strategies for biofortified staple plant foods to reduce micronutrient malnutrition globally. The Journal of nutrition. 132(3): 495S-499S.
- Whanger, P. (2002) Selenocompounds in plants and animals and their biological significance. Journal of the American College of Nutrition. 21(3): 223-232.
- Whanger P. D. (2004) Selenium and its relationship to cancer: an update. British Journal of Nutrition. 91(1):11-28. doi:10.1079/BJN20031015.
- White, P. J. and M. R. Broadley (2009) Biofortification of crops with seven mineral elements often lacking in human diets--iron, zinc, copper, calcium, magnesium, Se and iodine. New Phytol. 182(1): 49-84.
- White, P. J., M. R. Broadley, H. C. Bowen and S. E. Johnson (2007) Se and its relationship with sulfur. Sulfur in plants an ecological perspective, Springer: 225-252.
- White, P., H. Bowen, P. Parmaguru, M. Fritz, W. Spracklen, R. Spiby, M. Meacham, A. Mead, M. Harriman and L. Trueman (2004) Interactions between Se and Sulphur nutrition in Arabidopsis thaliana. Journal of Experimental Botany 55(404): 1927-1937.
- WHO (2002) The world health report 2002: reducing risks, promoting healthy life, World Health Organization.
- WHO (2009) Global health risks: mortality and burden of disease attributable to selected major risks, World Health Organization.
- Williams, P. N., E. Lombi, G.-X. Sun, K. Scheckel, Y.-G. Zhu, X. Feng, J. Zhu, A.-M. Carey, E. Adomako and Y. Lawgali (2009). Se characterization in the global rice supply chain. Environmental science & technology. 43(15): 6024-6030.
- World Health Organization, 2006. Guidelines on food fortification with micronutrients. World Health Organization. https://iris.who.int/bitstream/handle/10665/43412/9241594012_eng.pdf, Retrieved on October 31, 2023.
- Wu, Z., G. S. Banuelos, Z. Q. Lin, Y. Liu, L. Yuan, X. Yin and M. Li (2015). Biofortification and phytoremediation of Se in China. Front Plant Science 6: 136.
- Xue, T., H. Hartikainen and V. Piironen (2001). Antioxidative and growth-promoting effect of Se on senescing lettuce. Plant and Soil. 237(1): 55-61.
- Yang, G., S. Wang, R. Zhou and S. Sun (1983). Endemic Se intoxication of humans in China. The American journal of clinical nutrition. 37(5): 872-881.
- Yang, X. E., W. R. Chen and Y. Feng (2007). Improving human micronutrient nutrition through biofortification in the soil-plant system: China as a case study. Environ Geochem Health. 29(5): 413-428.
- Yläranta, T. (1985). Increasing the Se content of cereal and grass crops in Finland. Academic dissertation, Agricultural Research Centre, Institute of Soil Science, Jokioinen. Yliopistopaino: Helsinki.
- Yoshimoto, N., H. Takahashi, F. W. Smith, T. Yamaya and K. Saito (2002). Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. The Plant Journal 29(4): 465-473.
- Yuan, L., X. Yin, Y. Zhu, F. Li, Y. Huang, Y. Liu and Z. Lin (2012). Se in plants and soils, and selenosis in Enshi, China: implications for Se biofortification. Phytoremediation and biofortification, Springer: 7-31.
- Zayed, A. M. and N. Terry (1992). Se volatilization in broccoli as influenced by sulfate supply. Journal of Plant Physiology. 140(6): 646-652.
- Zayed, A., C. M. Lytle and N. Terry (1998). Accumulation and volatilization of different chemical species of Se by plants. Planta 206(2): 284-292.
- Zhang, J. (2009). "Evaluation of nanotoxicity of foods and drugs: Biological roperties of red elemental Se at nano size (nano-Se) in vitro and in vivo. Nanotoxicity, from in vivo and in vitro models to health risks.97-114.
- Zhang, L., P. F. Byrne and E. A. Pilon-Smits (2006). Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytologist. 170(1): 33-42.
- Zhang, Y. and J. N. Moore (1996). Se fractionation and speciation in a wetland system. Environmental science & technology 30(8): 2613-2619.
- Zhang, Z., Xiong, Y., Chen, H. and Tang, Y., 2020. Understanding the composition and spatial distribution of biological selenate reduction products for potential Se recovery. Environmental Science: Water Research & Technology, 6(8): 2153-2163.
- Zhao, F. J. and S. P. McGrath (2009). Biofortification and phytoremediation. Curr Opin Plant Biol 12(3): 373-380.
- Zhao, F.-J., Y. Su, S. Dunham, M. Rakszegi, Z. Bedo, S. McGrath and P. Shewry (2009). Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. Journal of cereal science 49(2): 290-295.
- Zhou, X., Li, Y. and Lai, F., 2018. Effects of different water management on absorption and accumulation of Se in rice. Saudi Journal of Biological Sciences, 25(6): 1178-1182.
- Zhu, Y. G., E. A. Pilon-Smits, F. J. Zhao, P. N. Williams and A. A. Meharg (2009). Se in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14(8): 436-442.
- Zimmermann, R. and M. Qaim (2004). Potential health benefits of Golden Rice: a Philippine case study. Food Policy 29(2): 147-168.
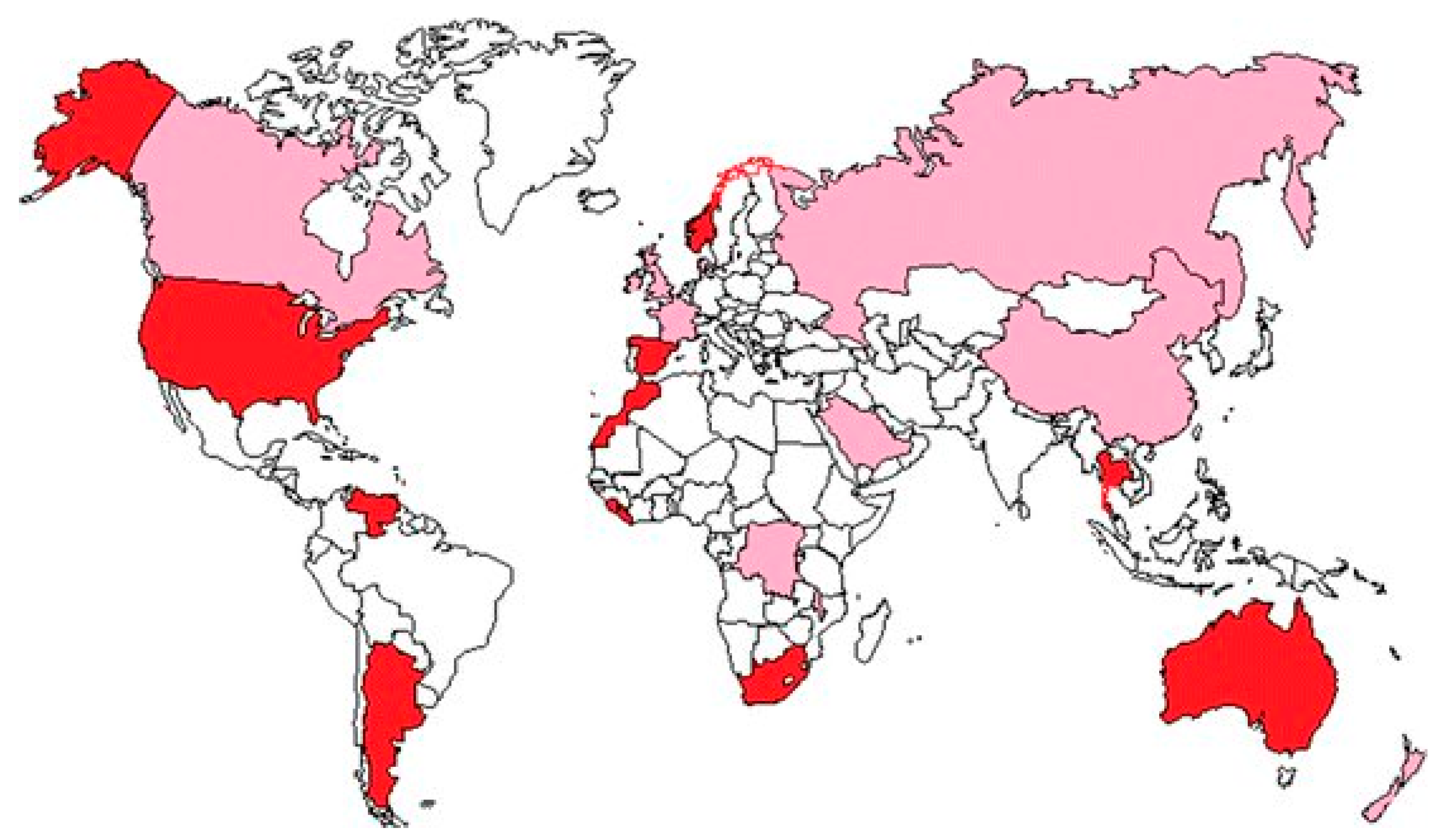
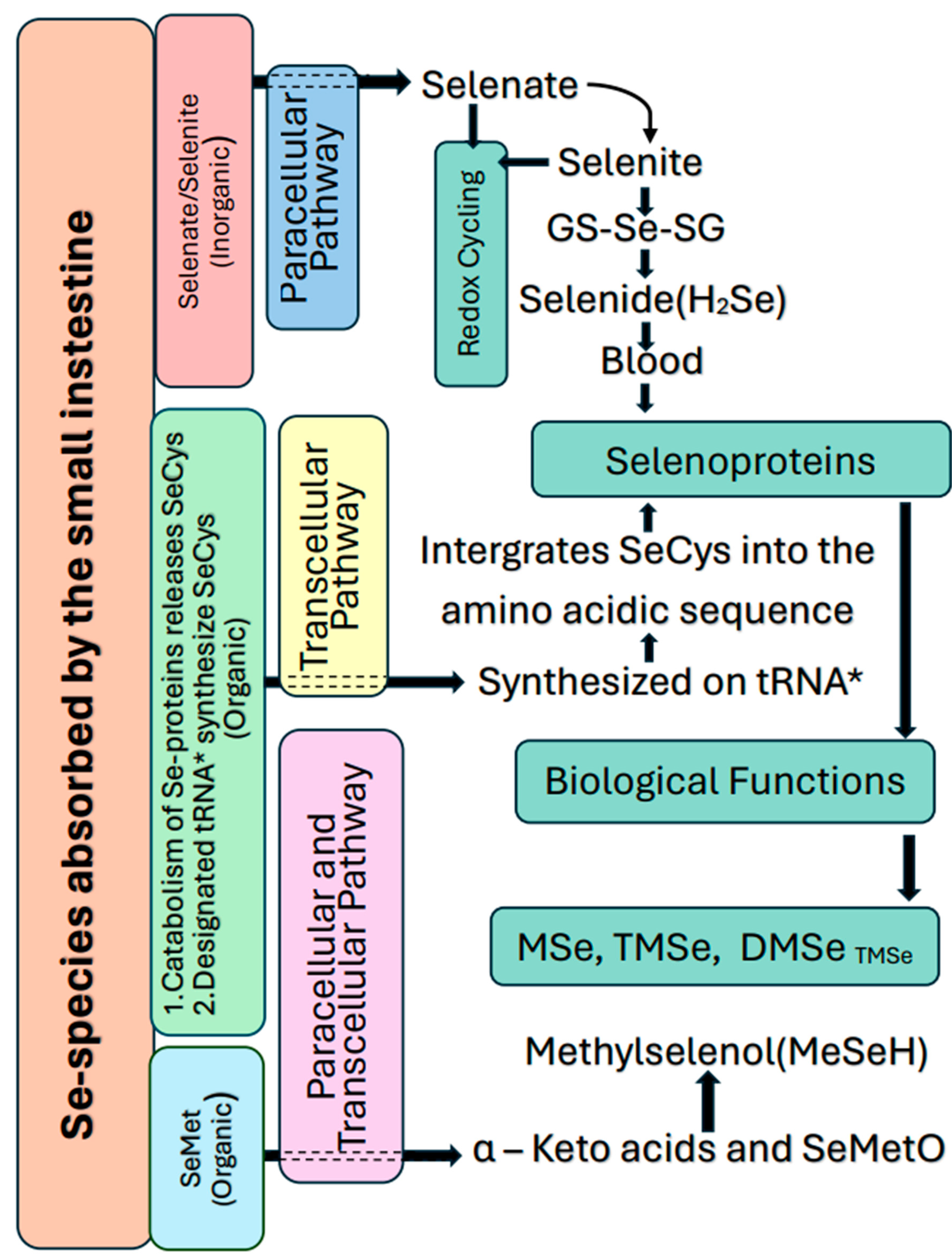
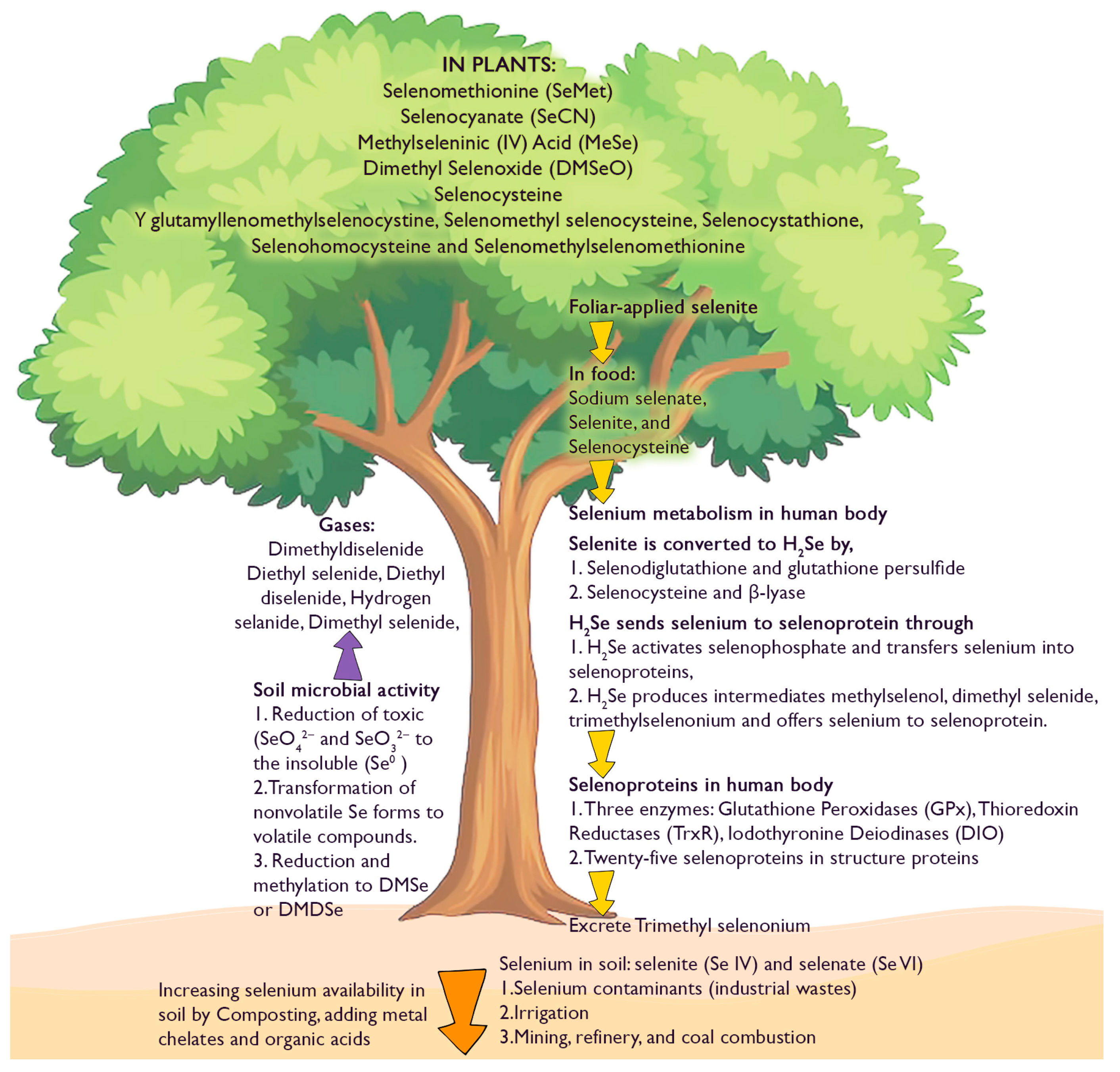
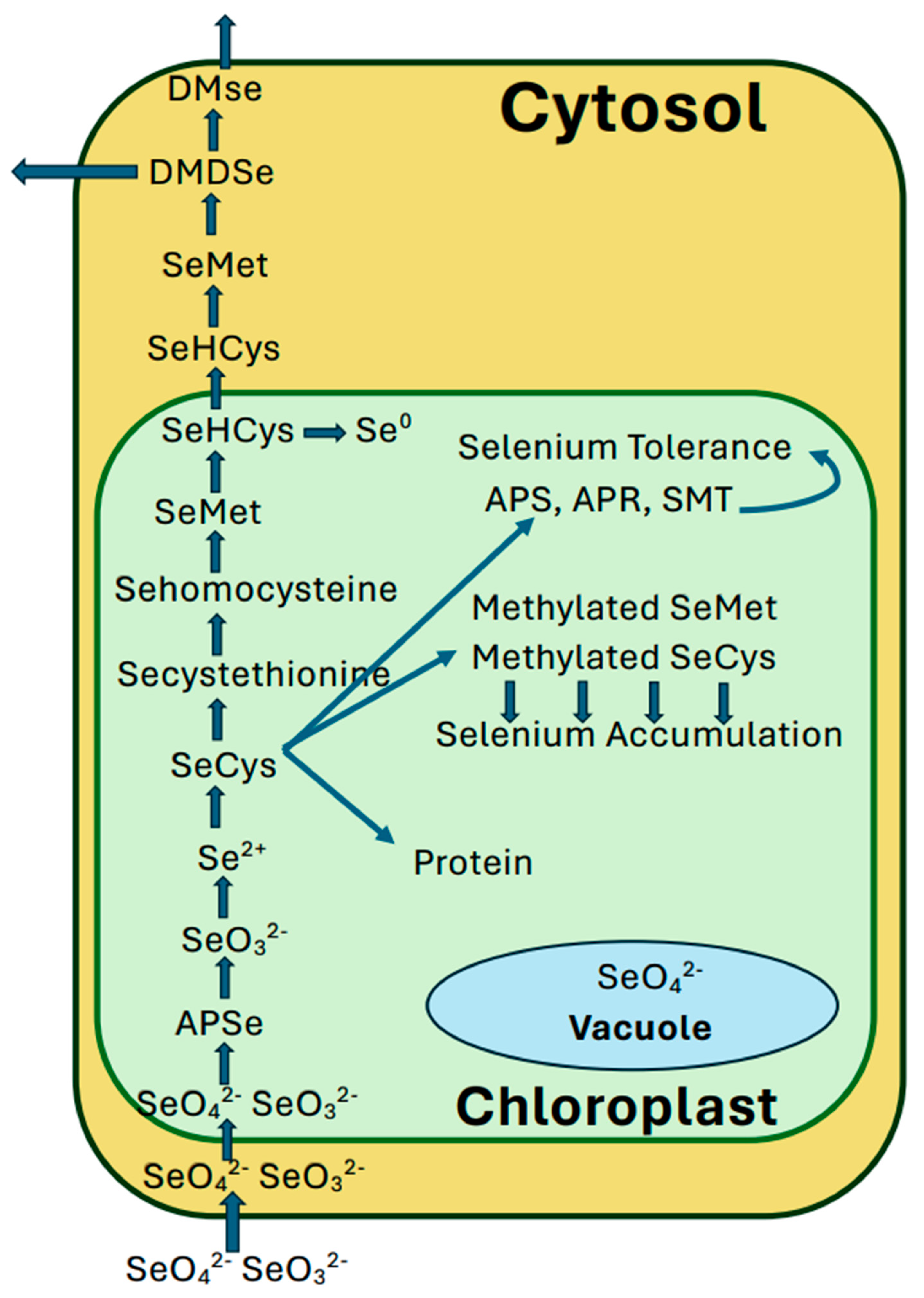
| Se in biological compound | Properties | Reference |
| Glutathione peroxidase (GPx) | Se containing enzyme, protecting cells from oxidative damage | Parada and Aguilera 2007; Thiry et al. 2012 |
| Thioredoxin reductases (TrxR) | Maintaining the cellular redox balance, important for various cellular processes, including DNA synthesis, repair, and cell growth. | Thiry et al. 2012; Lu and Holmgren 2014 |
| Iodothyronine deiodinases (DIO) | Responsible for the conversion of thyroid hormones. | Lu and Holmgren 2014 |
| Selenoprotein | Free-radical scavenging activity in the human body with cancer preventive properties | Clark et al. 1996; Rayman 2000; McKenzie et al. 2001; Abdulah et al. 2005 |
| Methylselenol | An anticancer agent | Abdulah et al. 2005 |
| Methylselenocysteine and selenomethionine | More anti-carcinogenic and more bioavailable compound** | Rayman 2008; Brummell et al. 2011 |
| Crop | Prominent Se compounds | Reference |
| Garlic, onion, and leeks, Broccoli | Selenomethyl cysteine, methyl selenocysteine (MeSeCys), | Beilstein et al. 1991; Ogra et al. 2005; Avila et al. 2013 |
| Brassica and Allium | and Glu-MeSeCys |
Beilstein et al. 1991; Ogra et al. 2005; Whanger 2002; Rayman 2008 |
| Indian mustard | Selenomethionine(SeMet) SeOMet, | Grant et al. 2004 |
| Broccoli sprout | MeSeCys and glucosinolates | Avila et al. 2013 |
| Arabidopsis thaliana | Selenite | Beilstein et al. 1991; Kahakachchi et al. 2004; Pilon-Smits and Quinn 2010 |
| Wheat, barley, and rye | SeMet | Stadlober et al. 2001, Poblaciones et al. 2014 |
| Brazil nuts | SeMet | Vonderheide et al. 2002 |
| Shiitake mushrooms | SeMet | Ogra et al. 2004 |
| Rice | SeMet (Dominant), MeSeCys, SeCys | Beilstein et al. 1991; Li et al. 2010 |
| Radish | MeSeCys and -glutamyl S MeSeCys | Combs and Gray 1998; Tapiero et al. 2003 |
| Valency | Remarks | Reference |
| Se (0) | Produced by microbial activities | (Chen et al. 2009; Mishra et al. 2011). |
| Produced by the physical reduction of both Se (+4) and Se (+6) becomes oxidized forms | Chen et al. 2009; Mishra et al. 2011 | |
| Insoluble, immobile and poorly bioavailable | Kulp and Pratt 2004; Lenz and Lens 2009 | |
| Very common and found everywhere at the concentration range of 0.01 to 2.0 mg kg–1 | (Fordyce 2005). | |
| Elemental Se is prominent in anaerobic soils | Elrashidi et al. 1987 | |
| Se (+2) Selenide or Se2- |
Very slightly mobile in acid soils, forms stable minerals and organic compounds. The selenide minerals produced after biotic reduction, and selenide minerals are bioavailable. Major species: HSe−, H2Se° |
Kulp and Pratt 2004; Lenz and Lens 2009; Kabata-Pendias, and Szteke, 2015 |
| Selenide is prominent in anaerobic soils | Elrashidi et al. 1987; Wu et al. 2015 | |
| Se (+4) Selenite (SeO32-) Hydro selenite (HSeO4) |
The most toxic valence state organic matter and on Fe-, Mn- or Al oxidizes. Easily absorbed hydrous sesquioxides and organic matter Bound with oxides and oxyhydroxides of such metals is dissolved by microorganisms at later stages Major species: HSeO4–, SeO23– Selenite becomes predominant in acidic to neutral soils |
He et al. 2010; Wu et al. 2015 Wang et al. 2012; Kabata-Pendias, and Szteke, 2015; Elrashidi et al. 1987 |
| Selenite is available in acidic soils. Acid soluble fraction of Se (VI) is approximately 44% | Elrashidi et al. 1987; Wang et al. 2012 | |
| Selenite increases arsenic in rice | Hu et al. 2014 | |
| Se (+6) Selenate (SeO42-), SeO |
The dominant species that are highly and slowly absorbed by the plants. Alkaline soils solubilize Se (+6). Highly water soluble and highly bioavailable. Not adsorbed on hydrous sesquioxides (mainly Fe2O3·H2O) major species: SeO42– Selenate is the predominant in oxidized and alkaline soils |
Elrashidi et al. 1987; Séby et al., 1997; Se speciation in soils after alkaline extraction. Science of the Total Environment, 207(2-3), pp.81-90. Dhillon and Dhillon 2009 Kabata-Pendias, and Szteke, 2015 |
| Selenate species are available in aerobic neutral or alkaline soils | Wang et al. 2012 | |
| Under submerged or waterlogged conditions, selenate is converted to elemental Se, selenite or selenide | Mikkelsen et al. 1989b; Jayaweera and Biggar 1996 | |
| Se (+6) prevents arsenic penetration into rice roots | Hu et al. 2014 | |
| Acid-soluble fraction of Se (IV) is approximately 15% | Séby et al., 1997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





