Submitted:
23 May 2025
Posted:
26 May 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Discussion

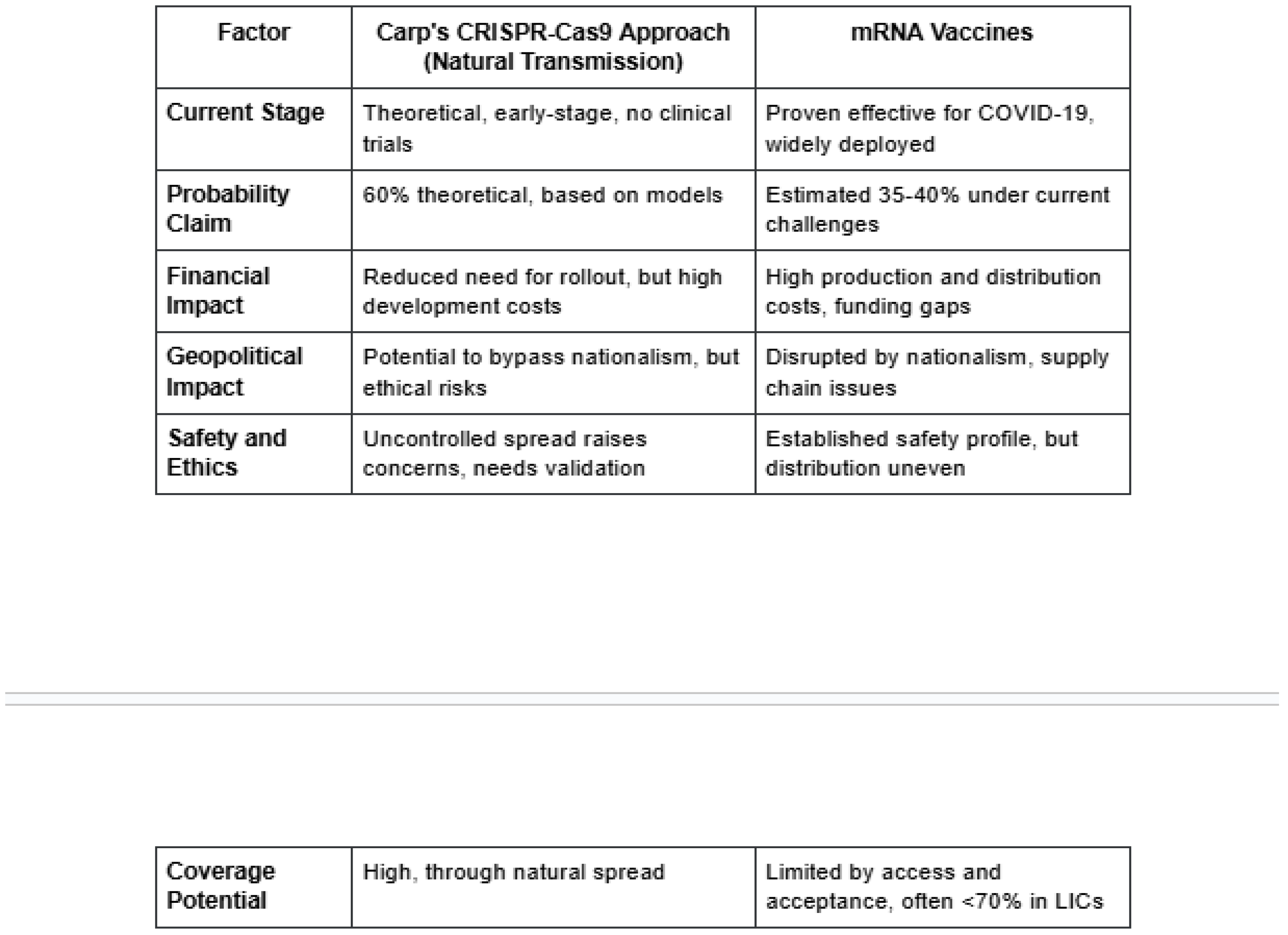

Conclusion
Author’s Note
References
- Koonin, Eugene V.; Dolja, Valerian V.; Krupovic, Mart. The logic of virus evolution. Cell Host & Microbe 2022, 30, 917–929. [Google Scholar] [CrossRef]
- Fensterl, Volker; Chattopadhyay, Saurabh; Sen, Ganes C. No Love Lost Between Viruses and Interferons. Annual Review of Virology 2015, 2, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, P. Biochemistry of Interferons and Their Actions. Annual Review of Biochemistry 1982, 51, 251–282. [Google Scholar] [CrossRef]
- Sen, Ganes C. Biochemical pathways in interferon-action. Pharmacology & Therapeutics 1984, 24, 235–257. [Google Scholar] [CrossRef]
- Martínez, José L. Bacterial pathogens: from natural ecosystems to human hosts. Environmental Microbiology 2012, 15, 325–333. [Google Scholar] [CrossRef]
- Diard, Médéric; Hardt, Wolf-Dietrich. Evolution of bacterial virulence. FEMS Microbiology Reviews 2017, 41, 679–697. [Google Scholar] [CrossRef]
- Alphonse, Noémie; Dickenson, Ruth E.; Odendall, Charlotte. Interferons: Tug of War Between Bacteria and Their Host. Frontiers in Cellular and Infection Microbiology 2021, 11, 624094. [Google Scholar] [CrossRef]
- Daffis, Stephane; Szretter, Kristy J.; Schriewer, Jill; Li, Jianqing; Youn, Soonjeon; Errett, John; Lin, Tsai-Yu; Schneller, Stewart; Zust, Roland; Dong, Hongping; et al. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef]
- Szretter, Kristy J.; Daniels, Brian P.; Cho, Hyelim; Gainey, Maria D.; Yokoyama, Wayne M.; Virgin, Herbert W.; Klein, Robyn S.; Sen, Ganes C.; Diamond, Michael S. 2′-O Methylation of the Viral mRNA Cap by West Nile Virus Evades Ifit1-Dependent and -Independent Mechanisms of Host Restriction In Vivo. PLOS Pathogens 2012, 8, e1002698. [Google Scholar] [CrossRef]
- Diamond, Michael S. IFIT1: A dual sensor and effector molecule that detects non-2′-O methylated viral RNA and inhibits its translation. Cytokine & Growth Factor Reviews 2014, 25, 543–550. [Google Scholar] [CrossRef]
- Menachery, Vineet D.; Debbink, Kari; Baric, Ralph S. Coronavirus non-structural protein 16: Evasion, attenuation, and possible treatments. Virus Research 2014, 194, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Menachery, Vineet D.; Gralinski, Lisa E.; Mitchell, Hugh D.; Dinnon, Kenneth H.; Leist, Sarah R.; Yount, Boyd L.; Graham, Rachel L.; McAnarney, Eileen T.; Stratton, Kelly G.; Cockrell, Adam S.; et al. Middle East Respiratory Syndrome Coronavirus Nonstructural Protein 16 Is Necessary for Interferon Resistance and Viral Pathogenesis. mSphere 2017, 2, e00346–17. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, Craig; Menachery, Vineet D. Coronavirus 2′-O-methyltransferase: A promising therapeutic target. Virus Research 2023, 336, 199211. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, Craig; Lokugamage, Kumari; Vu, Michelle N.; Johnson, Bryan A.; Scharton, Dionna; Plante, Jessica A.; Kalveram, Birte; Crocquet-Valdes, Patricia A.; Sotcheff, Stephanea; Jaworski, Elizabeth; et al. SARS-CoV-2 Uses Nonstructural Protein 16 To Evade Restriction by IFIT1 and IFIT3. Journal of Virology 2023, 97, e0153222. [Google Scholar] [CrossRef]
- Menachery, Vineet D.; Yount, Boyd L.; Josset, Laurence; Gralinski, Lisa E.; Scobey, Trevor; Agnihothram, Sudhakar; Katze, Michael G.; Baric, Ralph S. Attenuation and Restoration of Severe Acute Respiratory Syndrome Coronavirus Mutant Lacking 2′-O-Methyltransferase Activity. Journal of Virology 2014, 88, 4251–4264. [Google Scholar] [CrossRef]
- Lazear, Helen M.; Schoggins, John W.; Diamond, Michael S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Dowling, Jack W.; Forero, Adriana. Beyond Good and Evil: Molecular Mechanisms of Type I and III IFN Functions. The Journal of Immunology 2022, 208, 247–256. [Google Scholar] [CrossRef]
- Chiale, Carolina; Greene, Trever T.; Zuniga, Elina I. Interferon induction, evasion, and paradoxical roles during SARS-CoV-2 infection*. Immunological Reviews 2022, 309, 12–24. [Google Scholar] [CrossRef]
- Garcia-Del-Barco, Diana; Risco-Acevedo, Daniela; Berlanga-Acosta, Jorge; Martos-Benítez, Frank Daniel; Guillén-Nieto, Gerardo. Revisiting Pleiotropic Effects of Type I Interferons: Rationale for Its Prophylactic and Therapeutic Use Against SARS-CoV-2. Frontiers in Immunology 2021, 12, 655528. [Google Scholar] [CrossRef]
- Felgenhauer, Ulrike; Schoen, Andreas; Gad, Hans Henrik; Hartmann, Rune; Schaubmar, Andreas R.; Failing, Klaus; Drosten, Christian; Weber, Friedemann. Inhibition of SARS–CoV-2 by type I and type III interferons. Journal of Biological Chemistry 2020, 295, 13958–13964. [Google Scholar] [CrossRef]
- Lokugamage, Kumari G.; Hage, Adam; Vries, Maren de; Valero-Jimenez, Ana M.; Schindewolf, Craig; Dittmann, Meike; Rajsbaum, Ricardo; Menachery, Vineet D. Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. Journal of Virology, 2020. [Google Scholar] [CrossRef]
- Shimizu, Jun; Sasaki, Tadahiro; Ong, Guang Han; Koketsu, Ritsuko; Samune, Yoshihiro; Nakayama, Emi E.; Nagamoto, Tetsuharu; Yamamoto, Yuki; Miyazaki, Kazuo; Shioda, Tatsuo; et al. IFN-γ derived from activated human CD4+ T cells inhibits the replication of SARS-CoV-2 depending on cell-type and viral strain. Scientific Reports 2024, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, Alejandro; Vizcarra, Pilar; Quereda, Carmen; Moreno, Ana; Casado, José Luis; group, the CoVEX study. IFN-γ+ cell response and IFN-γ release concordance after in vitro SARS-CoV-2 stimulation. European Journal of Clinical Investigation 2021, 51, e13636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Jian; Liu, Jian; Chen, Zhilu; Peng, Haoran; Zhu, Cuisong; Feng, Daobin; Zhang, Shuye; Zhao, Ping; Zhang, Xiaoyan; Xu, Jianqing; et al. Angiotensin-Converting Enzyme 2 Potentiates SARS-CoV-2 Infection by Antagonizing Type I Interferon Induction and Its Down-Stream Signaling Pathway. mSphere 2022, 7, e0021122. [Google Scholar] [CrossRef]
- Busnadiego, Idoia, Sonja Fernbach, Marie O. Pohl, Umut Karakus, Michael Huber, Alexandra Trkola, Silke Stertz, and Benjamin G. Hale. 2020. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio, 11. [CrossRef]
- Goletti, Delia; Petrone, Linda; Manissero, Davide; Bertoletti, Antonio; Rao, Sonia; Ndunda, Nduku; Sette, Alessandro; Nikolayevskyy, Vladyslav. The potential clinical utility of measuring severe acute respiratory syndrome coronavirus 2-specific T-cell responses. Clinical Microbiology & Infection 2021, 27, 1784–1789. [Google Scholar] [CrossRef]
- Tovey, Michael G.; Lallemand, Christophe. Safety, Tolerability, and Immunogenicity of Interferons. Pharmaceuticals 2010, 3, 1162–1186. [Google Scholar] [CrossRef]
- Meyts, Isabelle; Casanova, Jean-Laurent. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. European Journal of Immunology 2021, 51, 1039–1061. [Google Scholar] [CrossRef]
- Zhang, Qian, Daniela Matuozzo, Jérémie Le Pen, Danyel Lee, Leen Moens, Takaki Asano, Jonathan Bohlen, Zhiyong Liu, Marcela Moncada-Velez, Yasemin Kendir-Demirkol, and et al. 2022. Recessive inborn errors of type I IFN immunity in children with COVID-19 pneumonia. The Journal of Experimental Medicine, 219. [CrossRef]
- Abolhassani, Hassan; Landegren, Nils; Bastard, Paul; Materna, Marie; Modaresi, Mohammadreza; Du, Likun; Aranda-Guillén, Maribel; Sardh, Fabian; Zuo, Fanglei; Zhang, Peng; et al. Inherited IFNAR1 Deficiency in a Child with Both Critical COVID-19 Pneumonia and Multisystem Inflammatory Syndrome. Journal of Clinical Immunology 2022, 42, 471–483. [Google Scholar] [CrossRef]
- Su, Helen C.; Jing, Huie; Zhang, Yu; Casanova, Jean-Laurent. Interfering with Interferons: A Critical Mechanism for Critical COVID-19 Pneumonia. Annual Review of Immunology 2023, 41, 561–585. [Google Scholar] [CrossRef]
- Jafarzadeh, Abdollah; Nemati, Maryam; Saha, Bhaskar; Bansode, Yashwant D.; Jafarzadeh, Sara. Protective Potentials of Type III Interferons in COVID-19 Patients: Lessons from Differential Properties of Type I- and III Interferons. Viral Immunology 2021, 34, 307–320. [Google Scholar] [CrossRef]
- Sorrentino, Leonardo; Silvestri, Valentina; Oliveto, Giuseppe; Scordio, Mirko; Frasca, Federica; Fracella, Matteo; Bitossi, Camilla; D’auria, Alessandra; Santinelli, Letizia; Gabriele, Lucia; et al. Distribution of Interferon Lambda 4 Single Nucleotide Polymorphism rs11322783 Genotypes in Patients with COVID-19. Microorganisms 2022, 10, 363. [Google Scholar] [CrossRef]
- Zahid, Warisha; Farooqui, Nida; Zahid, Nida; Ahmed, Khalid; Anwar, Muhammad Faraz; Rizwan-Ul-Hasan, Syed; Hussain, Azhar R; Sarría-Santamera, Antonio; Abidi, Syed Hani. Association of Interferon Lambda 3 and 4 Gene SNPs and Their Expression with COVID-19 Disease Severity: A Cross-Sectional Study. Infection and Drug Resistance ume 2023, 16, 6619–6628. [Google Scholar] [CrossRef] [PubMed]
- Fang, Michelle Z.; Jackson, Sarah S.; O'Brien, Thomas R. IFNL4: Notable variants and associated phenotypes, Gene 2019, 730, 144289. [Google Scholar] [CrossRef] [PubMed]
- Akusjärvi, Sara Svensson; Zanoni, Ivan. Yin and yang of interferons: lessons from the coronavirus disease 2019 (COVID-19) pandemic. Current Opinion in Immunology 2024, 87, 102423. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, Trine H. Human genetics of SARS-CoV-2 infection and critical COVID-19. Clinical Microbiology & Infection 2022, 28, 1417–1421. [Google Scholar] [CrossRef]
- Romeih, Marwa; Mahrous, Mary R; Kassas, Mohamed El. Incidental radiological findings suggestive of COVID-19 in asymptomatic patients. World Journal of Radiology 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Orchansky, P; Rubinstein, M; Sela, I. Human interferons protect plants from virus infection. Proceedings of the National Academy of Sciences 1982, 79, 2278–2280. [Google Scholar] [CrossRef]
- Malik, Aniko E.; Issekutz, Thomas B.; Derfalvi, Beata. The Role of Type III Interferons in Human Disease. Clinical & Investigative Medicine 2021, 44, E5–E18. [Google Scholar] [CrossRef]
- Mesev, Emily V.; LeDesma, Robert A.; Ploss, Alexander. Decoding type I and III interferon signalling during viral infection. Nature Microbiology 2019, 4, 914–924. [Google Scholar] [CrossRef]
- Rojas, José M.; Alejo, Alí; Martín, Verónica; Sevilla, Noemí. Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway. Cellular and Molecular Life Sciences 2020, 78, 1423–1444. [Google Scholar] [CrossRef]
- Takaoka, Akinori; Yanai, Hideyuki. Interferon signalling network in innate defence. Cellular Microbiology 2006, 8, 907–922. [Google Scholar] [CrossRef]
- Tian, Yu; Wang, Ming-Li; Zhao, Jun. Crosstalk between Autophagy and Type I Interferon Responses in Innate Antiviral Immunity. Viruses 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M. A. G.; Ribaudo, Michael; Guo, Ju-Tao; Barik, Sailen. Identification of Interferon-Stimulated Gene Proteins That Inhibit Human Parainfluenza Virus Type 3. Journal of Virology 2016, 90, 11145–11156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Xiang; Michal, Jennifer J.; Zhang, Lifan; Ding, Bo; Lunney, Joan K.; Liu, Bang; Jiang, Zhihua. Interferon Induced IFIT Family Genes in Host Antiviral Defense. International Journal of Biological Sciences 2013, 9, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Loevenich, Simon; Malmo, Jostein; Liberg, Ann Magritt; Sherstova, Tatyana; Li, Youxian; Rian, Kristin; Johnsen, Ingvild B.; Anthonsen, Marit W. Cell-Type-Specific Transcription of Innate Immune Regulators in response to HMPV Infection. Mediators of Inflammation 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Loevenich, Simon, Alix S. Spahn, Kristin Rian, Victor Boyartchuk, and Marit Walbye Anthonsen. 2021. Human Metapneumovirus Induces IRF1 via TANK-Binding Kinase 1 and Type I IFN. Frontiers in Immunology, 12. [CrossRef]
- Tanaka, Yukie; Morita, Naoko; Kitagawa, Yoshinori; Gotoh, Bin; Komatsu, Takayuki. Human metapneumovirus M2-2 protein inhibits RIG-I signaling by preventing TRIM25-mediated RIG-I ubiquitination. Frontiers in Immunology 2022, 13, 970750. [Google Scholar] [CrossRef]
- Hastings, Andrew K.; Erickson, John J.; Schuster, Jennifer E.; Boyd, Kelli L.; Tollefson, Sharon J.; Johnson, Monika; Gilchuk, Pavlo; Joyce, Sebastian; Williams, John V. Role of Type I Interferon Signaling in Human Metapneumovirus Pathogenesis and Control of Viral Replication. Journal of Virology 2015, 89, 4405–4420. [Google Scholar] [CrossRef]
- Hoogen, Bernadette G. van Den; Boheemen, Sander van; Rijck, Jonneke de; Nieuwkoop, Stefan van; Smith, Derek J.; Laksono, Brigitta; Gultyaev, Alexander; Osterhaus, Albert D. M. E.; Fouchier, Ron A. M. Excessive production and extreme editing of human metapneumovirus defective interfering RNA is associated with type I IFN induction. Journal of General Virology 2014, 95, 1625–1633. [Google Scholar] [CrossRef]
- Schoggins, John W. Interferon-Stimulated Genes: What Do They All Do? Annual Review of Virology 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Su, Jianguo. The discovery of type IV interferon system revolutionizes interferon family and opens up a new frontier in jawed vertebrate immune defense. Science China Life Sciences 2022, 65, 2335–2337. [Google Scholar] [CrossRef]
- Pang, An Ning; Chen, Shan Nan; Liu, Lan Hao; Li, Bo; Song, Jing Wei; Zhang, Shan; Nie, P. IFN-υ and its receptor subunits, IFN-υR1 and IL10RB in mallard Anas platyrhynchos. Poultry Science 2024, 103, 103673. [Google Scholar] [CrossRef]
- Chen, Shan Nan; Li, Bo; Gan, Zhen; Wang, Kai Lun; Li, Li; Pang, An Ning; Peng, Xue Yun; Ji, Jia Xiang; Deng, Yu Hang; Li, Nan; et al. Transcriptional Regulation and Signaling of Type IV IFN with Identification of the ISG Repertoire in an Amphibian Model, Xenopus laevis. The Journal of Immunology 2023, 210, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, Dan; Puckett, Lindsay; Petrovic, Sanja; Xia, Weiming; Chen, Guiquan; Vega, Jose; Dembinsky-Vaknin, Adi; Shen, Jie; Plante, Martin; Burt, David S.; et al. A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Annals of Neurology 2008, 63, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, Dan; Maron, Ruth; Burt, David S.; Weiner, Howard L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears β-amyloid in a mouse model of Alzheimer disease. Journal of Clinical Investigation 2005, 115, 2423–2433. [Google Scholar] [CrossRef]
- Cao, Weiping; Kim, Jin Hyang; Reber, Adrian J.; Hoelscher, Mary; Belser, Jessica A.; Lu, Xiuhua; Katz, Jacqueline M.; Gangappa, Shivaprakash; Plante, Martin; Burt, David S.; et al. Nasal delivery of Protollin-adjuvanted H5N1 vaccine induces enhanced systemic as well as mucosal immunity in mice. Vaccine 2017, 35, 3318–3325. [Google Scholar] [CrossRef]
- Chabot, Sophie; Brewer, Angela; Lowell, George; Plante, Martin; Cyr, Sonya; Burt, David S.; Ward, Brian J. A novel intranasal Protollin™-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine 2005, 23, 1374–1383. [Google Scholar] [CrossRef]
- Kosmaoglou, Maria; Schwarz, Nele; Bett, John S.; Cheetham, Michael E. Molecular chaperones and photoreceptor function. Progress in Retinal and Eye Research 2008, 27, 434–449. [Google Scholar] [CrossRef]
- Roy, Shreya; Nagrale, Prachi. Encoding the Photoreceptors of the Human Eye. Cureus 2022, 14, e30125. [Google Scholar] [CrossRef]
- Munita, Jose M., and Cesar A. Arias. 2016. Mechanisms of Antibiotic Resistance. Microbiology Spectrum, 4. [CrossRef]
- Blazquez, Jesus; Oliver, Antonio; Gomez-Gomez, Jose-Maria. Mutation and Evolution of Antibiotic Resistance: Antibiotics as Promoters of Antibiotic Resistance? Current Drug Targets 2002, 3, 345–349. [Google Scholar] [CrossRef]
- Handa, Vishal L.; Patel, Bhoomi N.; Bhattacharya, Arpita; Kothari, Ramesh K.; Kavathia, Ghanshyam; Vyas, B. R. M. A study of antibiotic resistance pattern of clinical bacterial pathogens isolated from patients in a tertiary care hospital. Frontiers in Microbiology 2024, 15, 1383989. [Google Scholar] [CrossRef]
- Riggs, Arthur D. Making, Cloning, and the Expression of Human Insulin Genes in Bacteria: The Path to Humulin. Endocrine Reviews 2020, 42, 374–380. [Google Scholar] [CrossRef]
- Ferrer-Miralles, Neus; Domingo-Espín, Joan; Corchero, José Luis; Vázquez, Esther; Villaverde, Antonio. Microbial factories for recombinant pharmaceuticals. Microbial Cell Factories 2009, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Spadiut, Oliver; Capone, Simona; Krainer, Florian; Glieder, Anton; Herwig, Christoph. Microbials for the production of monoclonal antibodies and antibody fragments. Trends in Biotechnology 2014, 32, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gomes, Antonio Milton Vieira; Carmo, Talita Souza; Carvalho, Lucas Silva; Bahia, Frederico Mendonça; Parachin, Nádia Skorupa. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Yanyan; Li, Xiaowei; Chen, Xin; Nielsen, Jens; Petranovic, Dina; Siewers, Verena. Expression of antibody fragments in Saccharomyces cerevisiae strains evolved for enhanced protein secretion. Microbial Cell Factories 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Wang, Hanchen; Fu, Tianfan; Du, Yuanqi; Gao, Wenhao; Huang, Kexin; Liu, Ziming; Chandak, Payal; Liu, Shengchao; Katwyk, Peter Van; Deac, Andreea; et al. Scientific discovery in the age of artificial intelligence. Nature 2023, 620, 47–60. [Google Scholar] [CrossRef]
- Kolluri, Sheela; Lin, Jianchang; Liu, Rachael; Zhang, Yanwei; Zhang, Wenwen. Machine Learning and Artificial Intelligence in Pharmaceutical Research and Development: a Review. The AAPS Journal 2022, 24, 1–10. [Google Scholar] [CrossRef]
- Messeri, Lisa; Crockett, M. J. Artificial intelligence and illusions of understanding in scientific research. Nature 2024, 627, 49–58. [Google Scholar] [CrossRef]
- Şahin, Mehmet Fatih; Topkaç, Erdem Can; Dogan, Cagri; Şeramet, Serkan; Özcan, Rıdvan; Akgül, Hacı Murat; Yazici, Cenk Murat. STILL USING ONLY CHATGPT? THE COMPARISON OF FIVE DIFFERENT ARTIFICIAL INTELLIGENCE CHATBOTS’ ANSWERS TO THE MOST COMMON QUESTIONS ABOUT KIDNEY STONES. Journal of Endourology 2024, 38, 1172–1177. [Google Scholar] [CrossRef]
- Plotkin, S. A., & Mortimer, E. A. (Eds.). (2008). Vaccines (5th ed.). Philadelphia, PA: Saunders Elsevier.
- Lakoff, George, and Mark Johnson. 2003. Metaphors We Live By. [CrossRef]
- Hauser, David J.; Fleming, Megan E. Mother Nature’s Fury: Antagonist Metaphors for Natural Disasters Increase Forecasts of Their Severity and Encourage Evacuation. Science Communication 2021, 43, 570–596. [Google Scholar] [CrossRef]
- Vigh, J. L. (2010). Formation of the Hurricane Eye (Doctoral dissertation). Retrieved from https://www.researchgate.net/publication/270703003_Formation_of_the_Hurricane_Eye.
- Bradley, Isabel. L’Œil du cyclone: Disaster and ‘wakeful’ modes of perception in Maximin and Glissant. Francosphères 2023, 12, 141–157. [Google Scholar] [CrossRef]
- Simondon, G. (2005). L’individuation à la lumière des notions de forme et d’information. Grenoble, France: Éditions Jérôme Millon.
- Sun Tzu. (1971). The Art of War (L. Giles, Trans.). Oxford, UK: Oxford University Press.
- Bachelard, G. (1994). The Poetics of Space (M. Jolas, Trans.). Boston, MA: Beacon Press.
- Tauber, A. I. (1994). The Immune Self: Theory or Metaphor? Cambridge, UK: Cambridge University Press.
- Carp, T. N. (2025). Recent Human Metapneumovirus Outbreak in East Asia: The Time to Shift Immunological Gears is Now. [CrossRef]
- Carp, T. N. (2025). Why Creating Transmissible Microbial Interferon Factories May Bring a Promise of a “Golden Era” in Future Human and Animal Health. Preprints. [CrossRef]
- Carp, T. N. (2024). Calibrating Human Immunity in the Context of Advanced Microbial Evolution and Self-Camouflaging. Preprints. [CrossRef]
- Brodrick, M. (2023, October 27). The Eye of the Hurricane. Open Health Policy. Retrieved from https://www.openhealthpolicy.com/p/the-eye-of-the-hurricane.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




