Submitted:
21 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
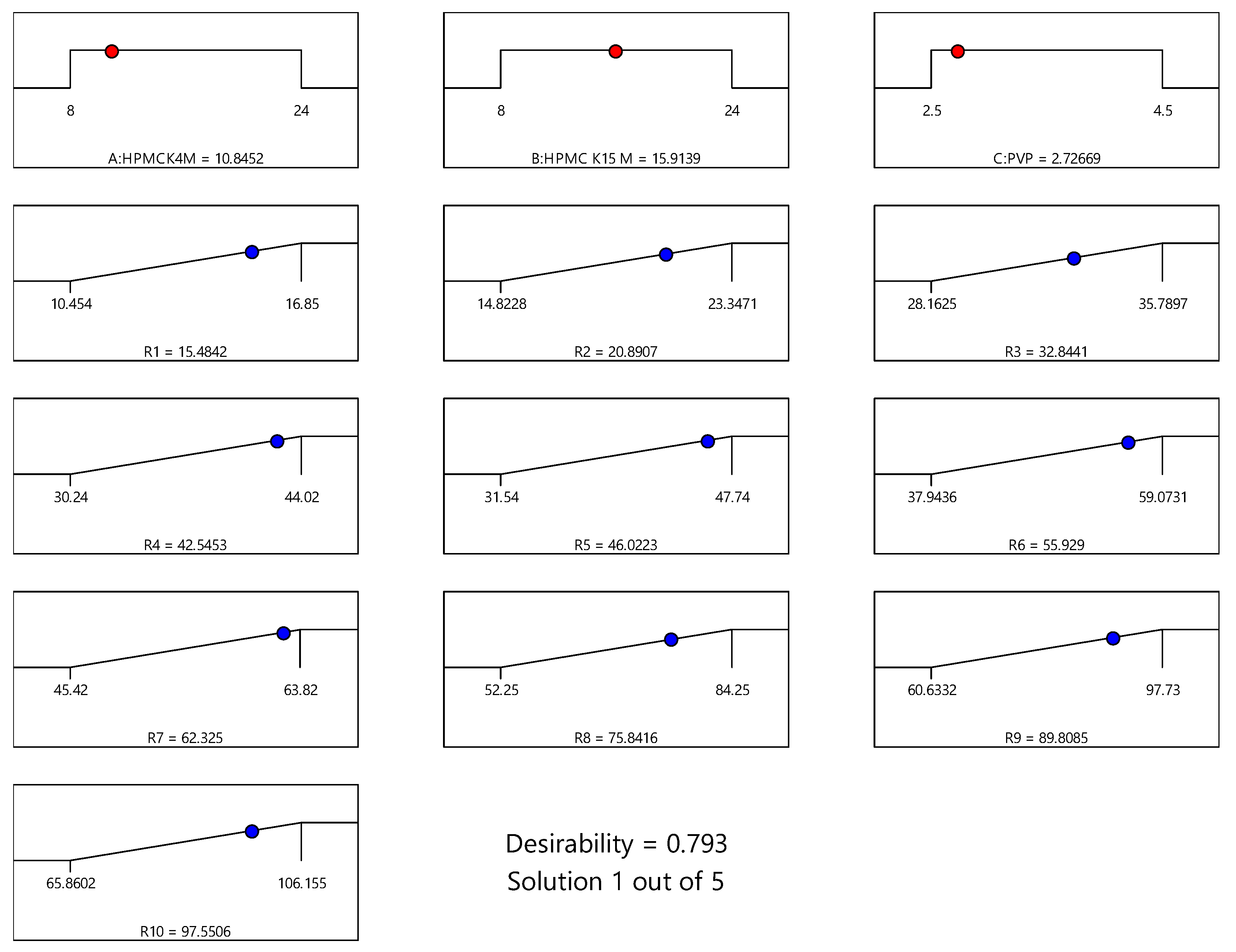
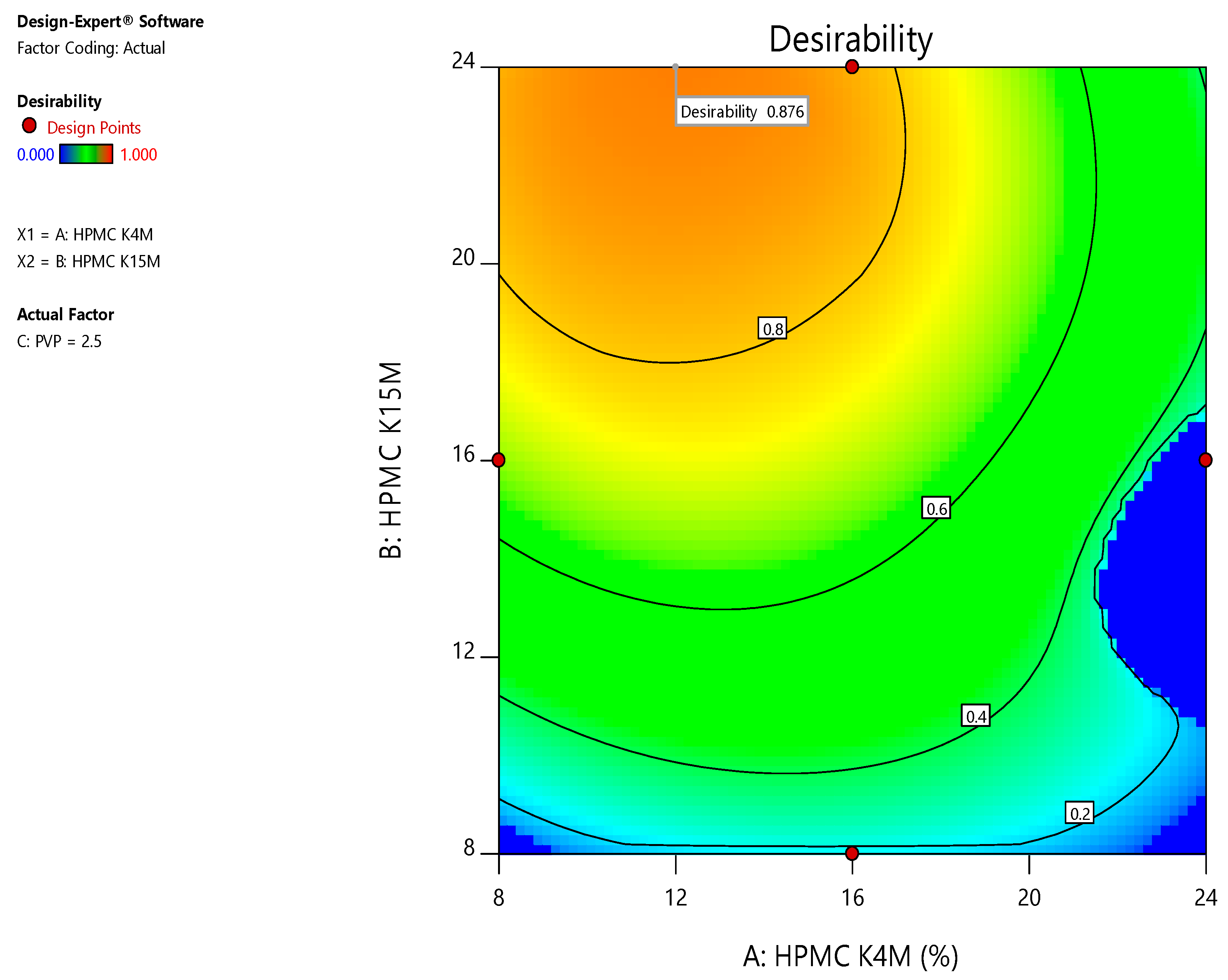
Axitinib is classified as BCS class II by the Biopharmaceutical Classification System (BCS). Axitinib is used to treat renal cancer patients. However, no sustained-release tablets have been documented using the Quality by Design (QbD) method. The aim of the research work was to design sustained release formulations of AXB, using response surface methodology through Box-Behnken statistical design (BBD) by wet granulation technique. The amounts of release retardant polymers investigated were HPMC K4M (X1), HPMC K15M (X2), and Polyvinyl pyrrolidone (PVP) (X3). In vitro cumulative percentage release in 0-24 (h), such as (R1), (R2), (R3), (R4), (R5), (R6), (R7), (R8), (R9), and (R10), are employed as dependent variables. The desirability 0.793 functions were found to be optimized in sustained-release formulations. Finally, the BBD proved valuable in improving the sustained release formulation and determining the impacts of formulation factors. The research finding is to develop the ideal formulation with great strength and long-term release.
Keywords:
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Compatibility Studies
2.2.1. Thermal Analysis
2.2.2. FTIR Analysis
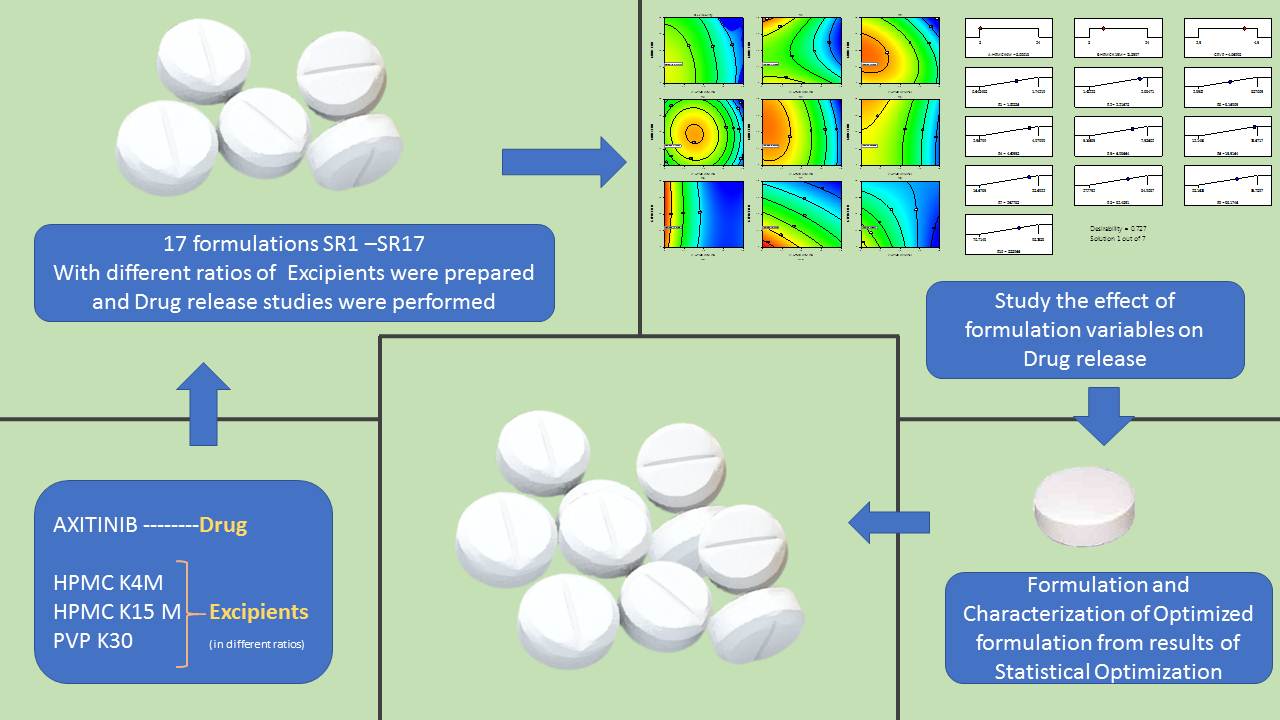
2.3. Experimental Design
2.4. Preparation of Axitinib Sustained Release Tablets
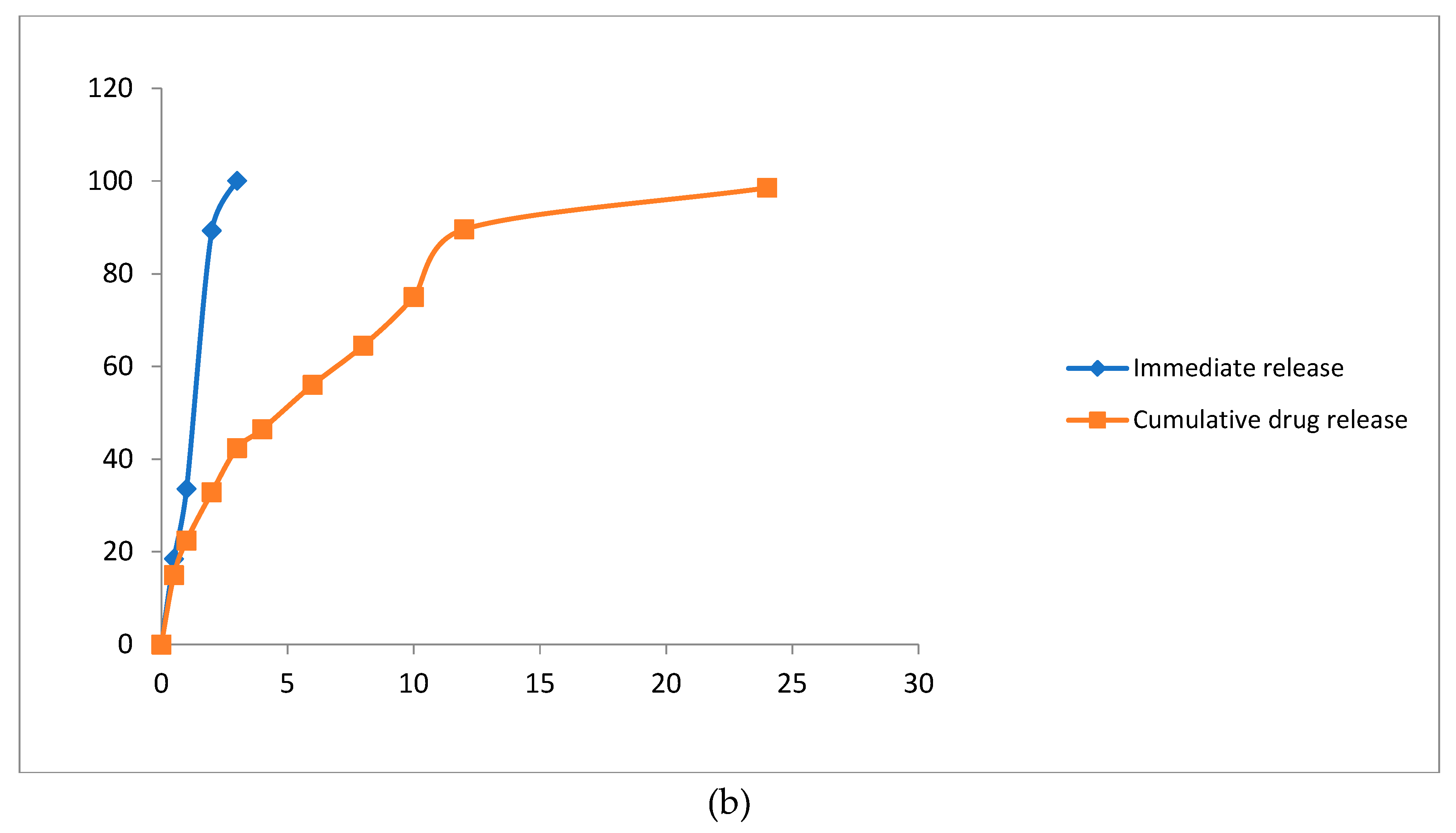
2.5. Preparation of Axitinib Immediate Release Tablets
2.6. In Vitro Drug Release Profile
2.7. LC-MS/MS Analysis
3. Results and Discussion
3.1. Compatibility Studies
3.1.1. Thermal Analysis
3.1.2. FTIR Analysis
3.2. Evaluation of Physical Parameters of Granules and Tablets
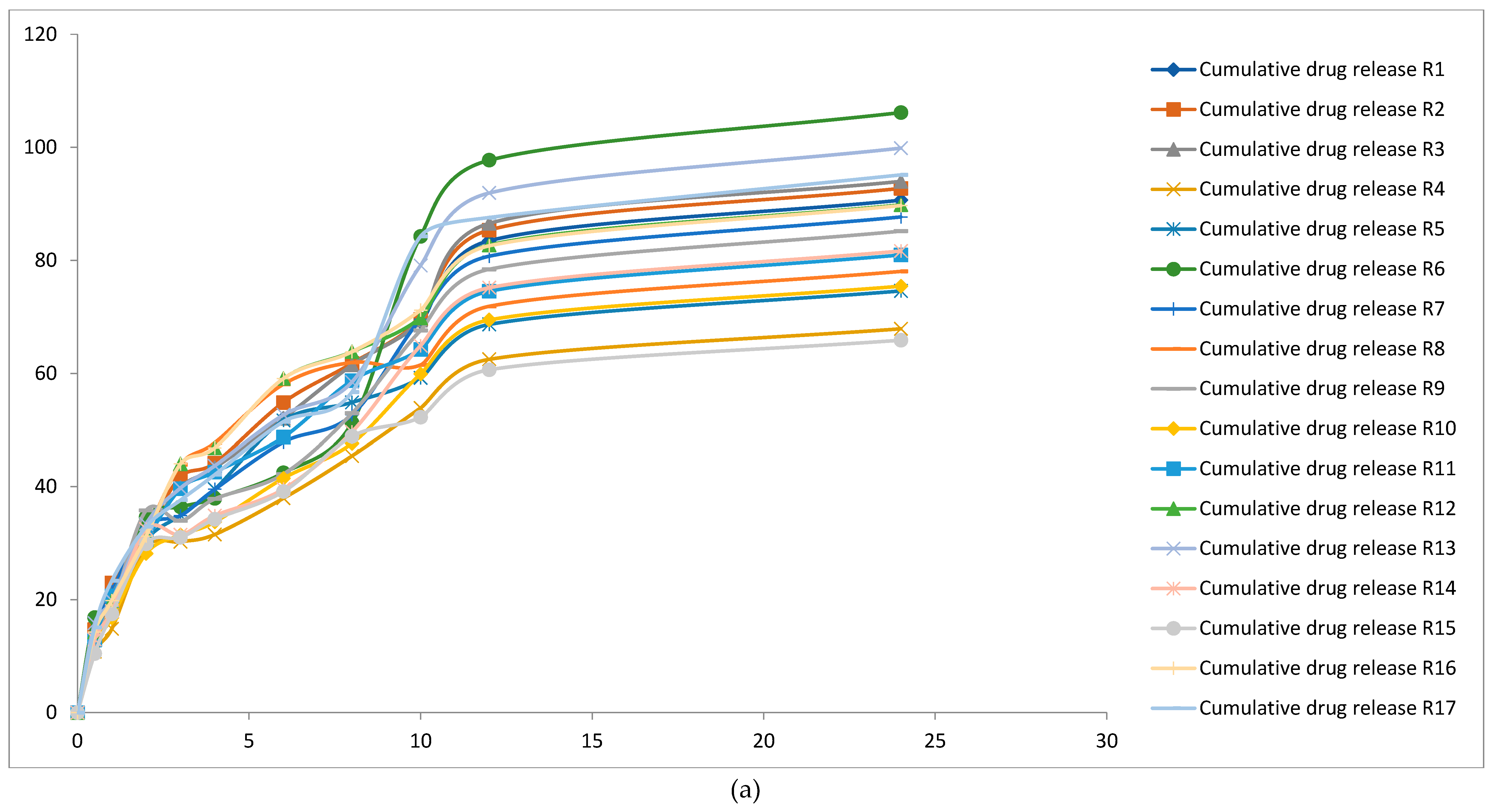
3.3. Effect of Model Independent and Dependent Factors of Dissolution Study
3.4. LC-MS/MS Analysis
4. Conclusions
Funding
Acknowledgements
Conflict of interest
References
- Wilmes LJ, Pallavicini MG, Fleming LM, Gibbs J, Wang D, Li KL, Partridge SC, Henry RG, Shalinsky DR, Hu-Lowe D, Park JW. A novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magnetic resonance imaging. 2007 Apr 1;25(3):319-27. [CrossRef]
- Li KL, Wilmes LJ, Henry RG, Pallavicini MG, Park JW, Hu-Lowe DD, McShane TM, Shalinsky DR, Fu YJ, Brasch RC, Hylton NM. Heterogeneity in the angiogenic response of a BT474 human breast cancer to a novel vascular endothelial growth factor-receptor tyrosine kinase inhibitor: assessment by voxel analysis of dynamic contrast-enhanced MRI. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005 Oct;22(4):511-9. [CrossRef]
- https://clinicaltrials.gov/ct2/show/NCT00678392.
- Rixe O, Bukowski RM, Michaelson MD, Wilding G. hudes GR, Bolte O, Motzer Rj, Bycott P, Liau Kf, freddo j, Trask PC, Kim S, Rini BI. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975-84. [CrossRef]
- Trask PC, Bushmakin AG, Cappelleri JC, Bycott P, Liau K, Kim S. Health-related quality of life during treatment for renal cell carcinoma: results from a phase II study of axitinib. Acta Oncologica. 2008 Jan 1;47(5):843-51. [CrossRef]
- Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P, Trask P. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. The Lancet. 2008 Jun 21;371(9630):2101-8. [CrossRef]
- Cohen EE, Needles BM, Cullen KJ, Wong SJ, Wade III JL, Ivy SP, Villaflor VM, Seiwert TY, Nichols K, Vokes EE. Phase 2 study of sunitinib in refractory thyroid cancer. Journal of Clinical Oncology. 2008 May 20;26(15_suppl):6025-.
- Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, Pithavala YK, Reich SD, Freddo JL, Wilding G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. Journal of Clinical Oncology. 2005 Aug 20;23(24):5474-83. [CrossRef]
- López-López Á, Godzien J, Soldevilla B, Gradillas A, López-Gonzálvez Á, Lens-Pardo A, La Salvia A, del Carmen Riesco-Martínez M, García-Carbonero R, Barbas C. Oxidized lipids in the metabolic profiling of neuroendocrine tumors–Analytical challenges and biological implications. Journal of Chromatography A. 2020 Aug 16;1625:461233. [CrossRef]
- Huynh HH, Pressiat C, Sauvageon H, Madelaine I, Maslanka P, Lebbé C, Thieblemont C, Goldwirt L, Mourah S. Development and validation of a simultaneous quantification method of 14 tyrosine kinase inhibitors in human plasma using LC-MS/MS. Therapeutic drug monitoring. 2017 Feb 1;39(1):43-54. [CrossRef]
- Guan S, Chen X, Wang F, Xin S, Feng W, Zhu X, Liu S, Zhuang W, Zhou S, Huang M, Wang X. Development and validation of a sensitive LC–MS/MS method for determination of gefitinib and its major metabolites in human plasma and its application in non-small cell lung cancer patients. Journal of pharmaceutical and biomedical analysis. 2019 Aug 5;172:364-71. [CrossRef]
- Fukuda IM, Pinto CF, Moreira CD, Saviano AM, Lourenço FR. Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Brazilian Journal of Pharmaceutical Sciences. 2018 Nov 8;54. [CrossRef]
- Nechita P. Review on polysaccharides used in coatings for food packaging papers. Coatings. 2020 Jun;10(6):566. [CrossRef]
- Velasco MV, Ford JL, Rowe P, Rajabi-Siahboomi AR. Influence of drug: hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. Journal of Controlled Release. 1999 Jan 1;57(1):75-85. [CrossRef]
- Heng PW, Chan LW, Easterbrook MG, Li X. Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets. Journal of Controlled Release. 2001 Sep 11;76(1-2):39-49. [CrossRef]
- Yi S, Wang J, Lu Y, Ma R, Gao Q, Liu S, Xiong S. Novel hot melt extruded matrices of hydroxypropyl cellulose and amorphous felodipine–plasticized hydroxypropyl methylcellulose as controlled release systems. AAPS PharmSciTech. 2019 Aug;20(6):1-4. [CrossRef]
- Cascone S, Lamberti G, Titomanlio G, d’Amore M, Barba AA. Measurements of non-uniform water content in hydroxypropyl-methyl-cellulose based matrices via texture analysis. Carbohydrate polymers. 2014 Mar 15;103:348-54. [CrossRef]
- Barba AA, d’Amore M, Chirico S, Lamberti G, Titomanlio G. Swelling of cellulose derivative (HPMC) matrix systems for drug delivery. Carbohydrate Polymers. 2009 Oct 15;78(3):469-74. [CrossRef]
- Riekes MK, Kuminek G, Rauber GS, de Campos CE, Bortoluzzi AJ, Stulzer HK. HPMC as a potential enhancer of nimodipine biopharmaceutical properties via ball-milled solid dispersions. Carbohydrate polymers. 2014 Jan 2;99:474-82. [CrossRef]
- Nart V, Franca MT, Anzilaggo D, Riekes MK, Kratz JM, de Campos CE, Simões CM, Stulzer HK. Ball-milled solid dispersions of BCS Class IV drugs: Impact on the dissolution rate and intestinal permeability of acyclovir. Materials Science and Engineering: C. 2015 Aug 1;53:229-38. [CrossRef]
- Jain AK, Söderlind E, Viridén A, Schug B, Abrahamsson B, Knopke C, Tajarobi F, Blume H, Anschütz M, Welinder A, Richardson S. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. Journal of controlled release. 2014 Aug 10;187:50-8. [CrossRef]
- Pani NR, Nath LK. Development of controlled release tablet by optimizing HPMC: Consideration of theoretical release and RSM. Carbohydrate polymers. 2014 Apr 15;104:238-45. [CrossRef]
- Yang Y, Chang S, Bai Y, Du Y, Yu DG. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydrate Polymers. 2020 Sep 1;243:116477. [CrossRef]
- Tak JW, Gupta B, Thapa RK, Woo KB, Kim SY, Go TG, Choi Y, Choi JY, Jeong JH, Choi HG, Yong CS. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box–Behnken design. AAPS PharmSciTech. 2017 May;18(4):1125-34. [CrossRef]
- Thapa P, Jeong SH. Effects of formulation and process variables on gastroretentive floating tablets with a high-dose soluble drug and experimental design approach. Pharmaceutics. 2018 Sep;10(3):161.
- Singh B, Saini G, Vyas M, Verma S, Thakur S. Optimized chronomodulated dual release bilayer tablets of fexofenadine and montelukast: quality by design, development, and in vitro evaluation. Future Journal of Pharmaceutical Sciences. 2019 Dec;5(1):1-20. [CrossRef]
- Vo AQ, Zhang J, Nyavanandi D, Bandari S, Repka MA. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydrate Polymers. 2020 Oct 15;246:116519. [CrossRef]



| Independent Variables | Levels | |||
| Low% | High% | |||
| HPMC K4M | X1 | 8 | 24 | |
| HPMC K15M | X2 | 8 | 24 | |
| PVP K 30 | X3 | 2.5 | 4.5 | |
| Factor 1 | Factor 2 | Factor 3 | Drug Release | |||||||||||
| Std | Run | A:HPMCK4M | B:HPMC K15 M | C:PVP | 30 Minutes | 1 hours | 2 hours | 3 hours | 4 hours | 6 hours | 8 hours | 10 hours | 12 hours | 24 hours |
| 17 | 1 | 16 | 16 | 3.5 | 14.390 | 21.381 | 31.084 | 39.84 | 43.34 | 52.021 | 61.48 | 69.12 | 83.465 | 90.660 |
| 13 | 2 | 16 | 16 | 3.5 | 14.72 | 22.952 | 31.812 | 41.74 | 44.16 | 54.833 | 61.78 | 69.56 | 85.376 | 92.736 |
| 16 | 3 | 16 | 16 | 3.5 | 14.92 | 21.381 | 31.084 | 39.84 | 43.34 | 52.021 | 61.48 | 69.42 | 86.536 | 93.996 |
| 4 | 4 | 24 | 24 | 3.5 | 10.775 | 14.822 | 29.241 | 30.24 | 31.54 | 37.943 | 45.42 | 53.87 | 62.495 | 67.882 |
| 6 | 5 | 24 | 16 | 2.5 | 11.84 | 19.411 | 30.528 | 34.94 | 39.54 | 51.680 | 54.86 | 59.2 | 68.672 | 74.592 |
| 1 | 6 | 8 | 8 | 3.5 | 16.85 | 19.350 | 34.617 | 36.42 | 37.93 | 42.444 | 51.24 | 84.25 | 97.73 | 106.15 |
| 11 | 7 | 16 | 8 | 4.5 | 13.92 | 21.420 | 32.842 | 34.86 | 39.54 | 47.847 | 52.66 | 69.6 | 80.736 | 87.696 |
| 10 | 8 | 16 | 24 | 2.5 | 12.39 | 20.626 | 31.688 | 43.88 | 47.74 | 58.084 | 61.94 | 61.5 | 71.862 | 78.057 |
| 9 | 9 | 16 | 8 | 2.5 | 13.52 | 18.567 | 35.789 | 33.98 | 37.84 | 42.223 | 52.94 | 67.6 | 78.416 | 85.176 |
| 8 | 10 | 24 | 16 | 4.5 | 11.975 | 16.766 | 28.162 | 31.45 | 33.75 | 41.463 | 47.64 | 59.875 | 69.455 | 75.442 |
| 3 | 11 | 8 | 24 | 3.5 | 12.85 | 20.643 | 30.594 | 39.62 | 42.58 | 48.722 | 58.72 | 64.25 | 74.53 | 80.955 |
| 14 | 12 | 16 | 16 | 3.5 | 14.26 | 19.819 | 31.248 | 44.02 | 46.84 | 59.073 | 63.82 | 69.82 | 82.708 | 89.838 |
| 5 | 13 | 8 | 16 | 2.5 | 15.85 | 19.836 | 33.394 | 39.74 | 43.68 | 52.660 | 58.48 | 79.1 | 91.93 | 99.855 |
| 12 | 14 | 16 | 24 | 4.5 | 12.96 | 17.975 | 32.478 | 31.48 | 34.86 | 39.566 | 49.64 | 64.9 | 75.168 | 81.648 |
| 2 | 15 | 24 | 8 | 3.5 | 10.454 | 17.475 | 29.885 | 31.02 | 34.24 | 39.141 | 48.86 | 52.25 | 60.633 | 65.860 |
| 15 | 16 | 16 | 16 | 3.5 | 14.24 | 19.819 | 31.248 | 44.02 | 46.84 | 59.073 | 63.82 | 71.2 | 82.592 | 89.712 |
| 7 | 17 | 8 | 16 | 4.5 | 15.1 | 23.347 | 32.915 | 37.62 | 42.12 | 51.452 | 56.7 | 84.25 | 87.58 | 95.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





