Submitted:
21 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
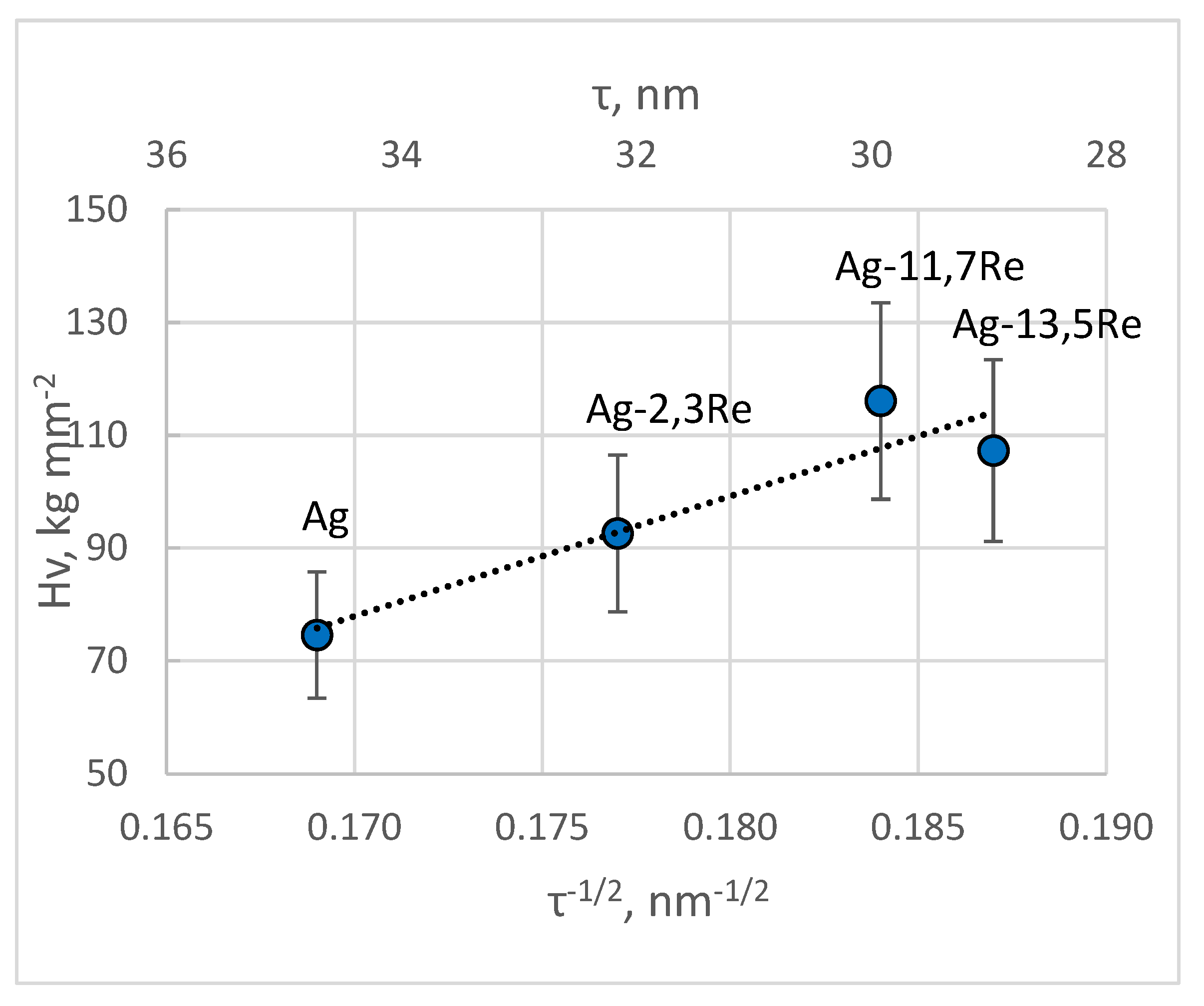
Silver-white, matte, smooth, and durable deposits of silver-rhenium, with thicknesses ranging from 2.0 to 13.7 μm and containing 0.15 to 13.5 wt.% Re, were obtained with a current efficiency of 66-98% from a developed dicyanoargentate-perrhenate bath based on a borate-phosphate-carbonate silver-plating electrolyte. The study was focused on the influence of bath composition, the [Ag(I)]:[ReO4-] ratio, surfactant additives, applied current density, temperature, and stirring, on the alloys composition, structure, morphology, microhardness, adhesion, and porosity. A voltammetric analysis was conducted, considering the influence of ethanolamines on electrode processes. In baths with TEA, coatings similar to a silver matrix with rhenium doped in mass fractions are likely achievable. MEA is recommended due to its process-activating properties. All coatings were nanocrystalline (τ = 28.5 - 35 nm). For deposits containing less than 10 wt.% Re, characteristic silver XRD peaks were observed, while other deposits, additional peaks attributed probably to Re(VII) and Re(VI) oxides. A linear relationship, typical for Hall-Petch plots, was obtained, confirming that grain boundaries play a crucial role in mechanical properties of coatings. The conditions for stable electrochemical synthesis of promising functional Ag-Re coatings of predetermined composition (0.7-1.5 wt.% Re) were proposed for practical use in power electronics and energy sectors, for manufacturing electrical contacts operating across a wide temperature range. This was realized by deposition from an Ag-rich bath in the area of mixed electrochemical kinetics, at potential values corresponding to the region of half the limiting current: j = 2.5 ‒ 6 mA cm-2, t = 19 - 33°C.
Keywords:
1. Introduction
2. Experimental Section
2.1. Bath Composition and Procedure
2.2. Electrochemical Measurements
2.3. Surface Morphology, Chemical Composition and Crystallographic Structure Characterization
2.4. Microhardness, Adhesion and Porosity Measurements
3. Results and Discussion
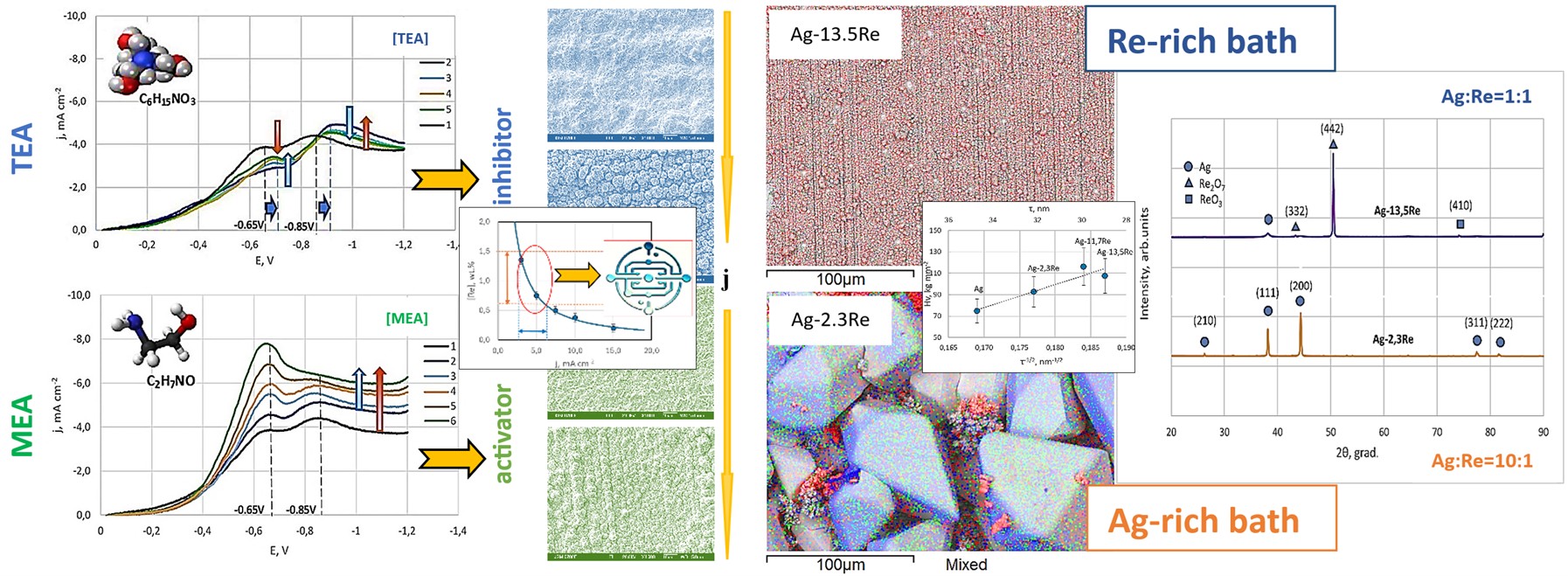
3.1. Voltammetric Studies
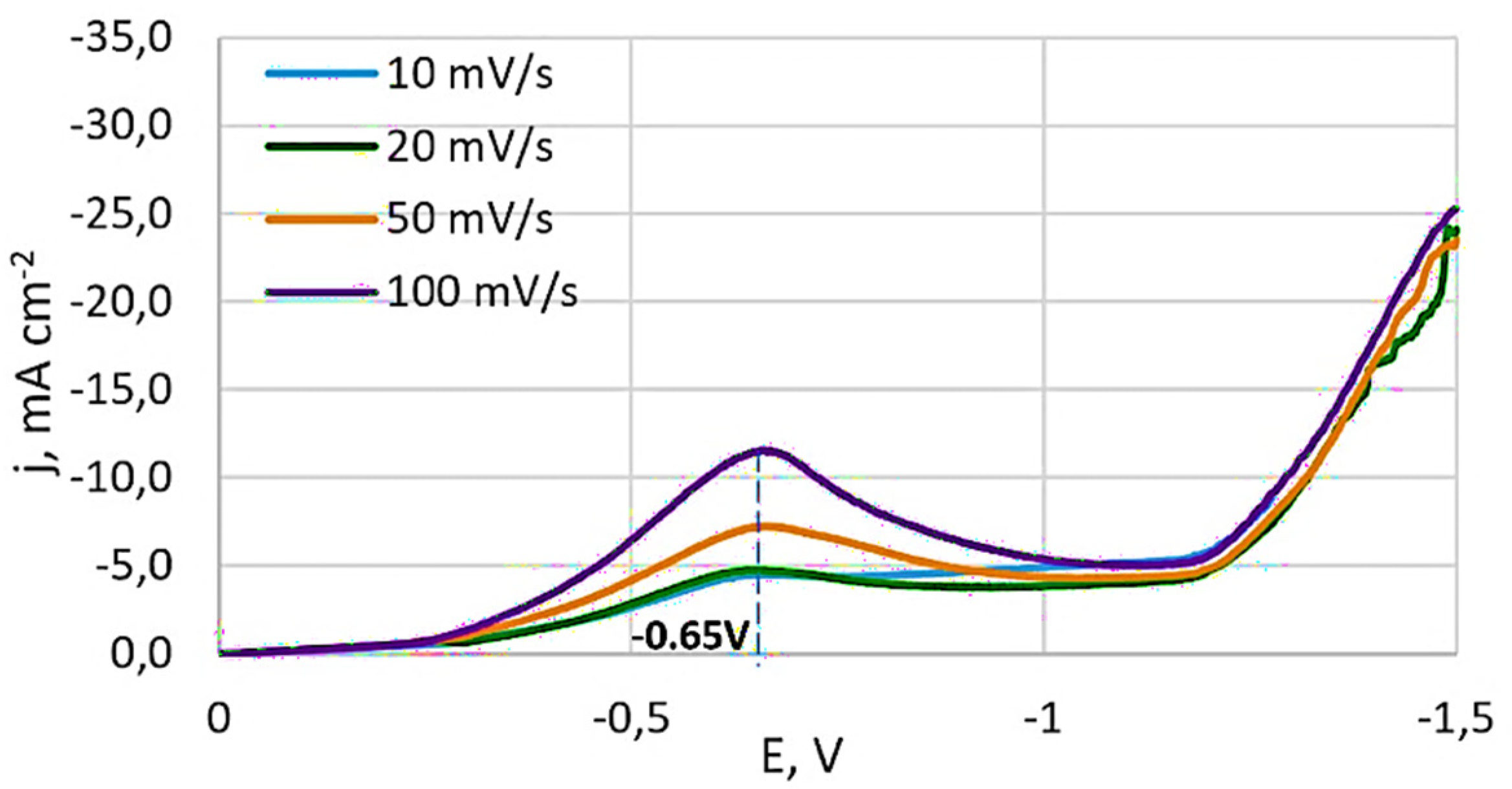
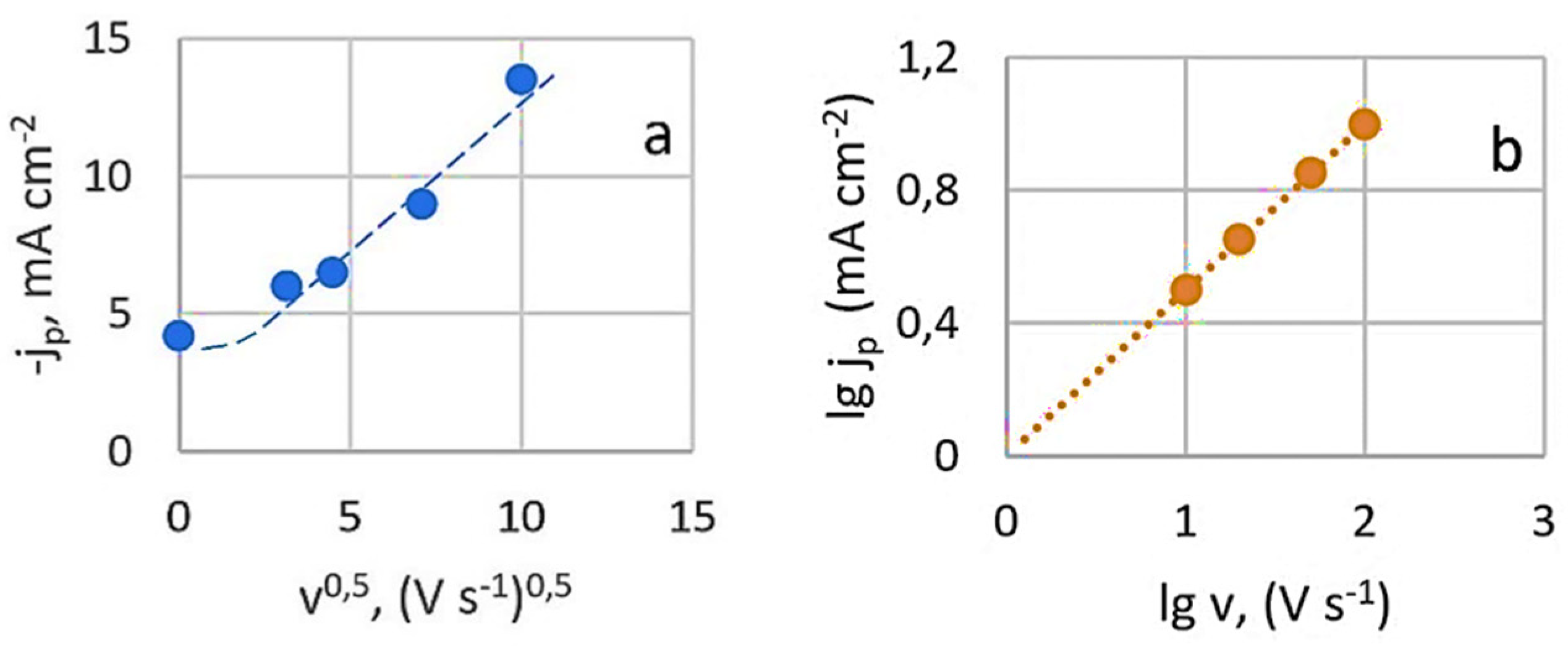
3.1.1. Results of Studies of Stationary Potential
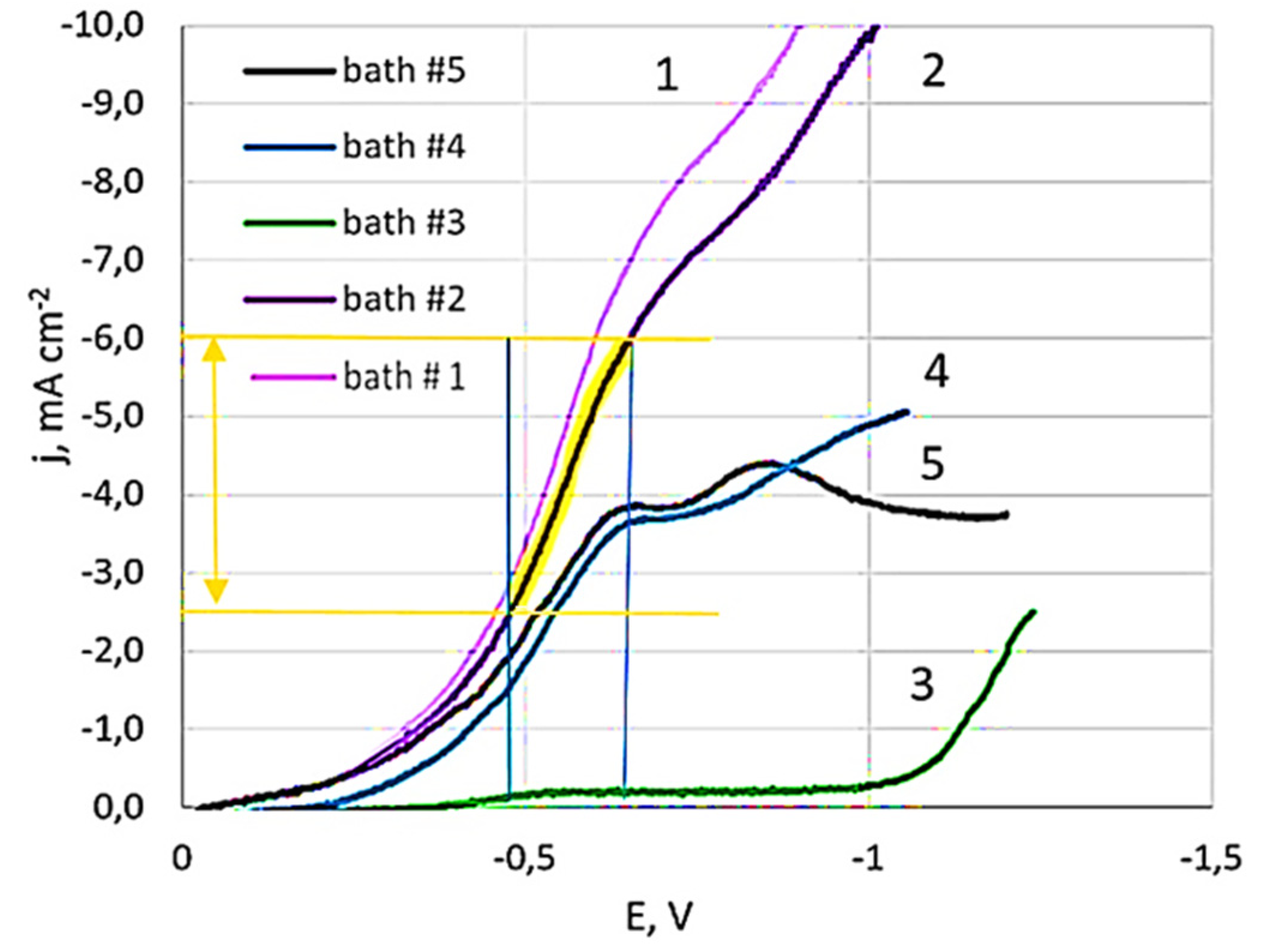
3.1.2. Results of Studies of Potentiodynamic Polarization Curves
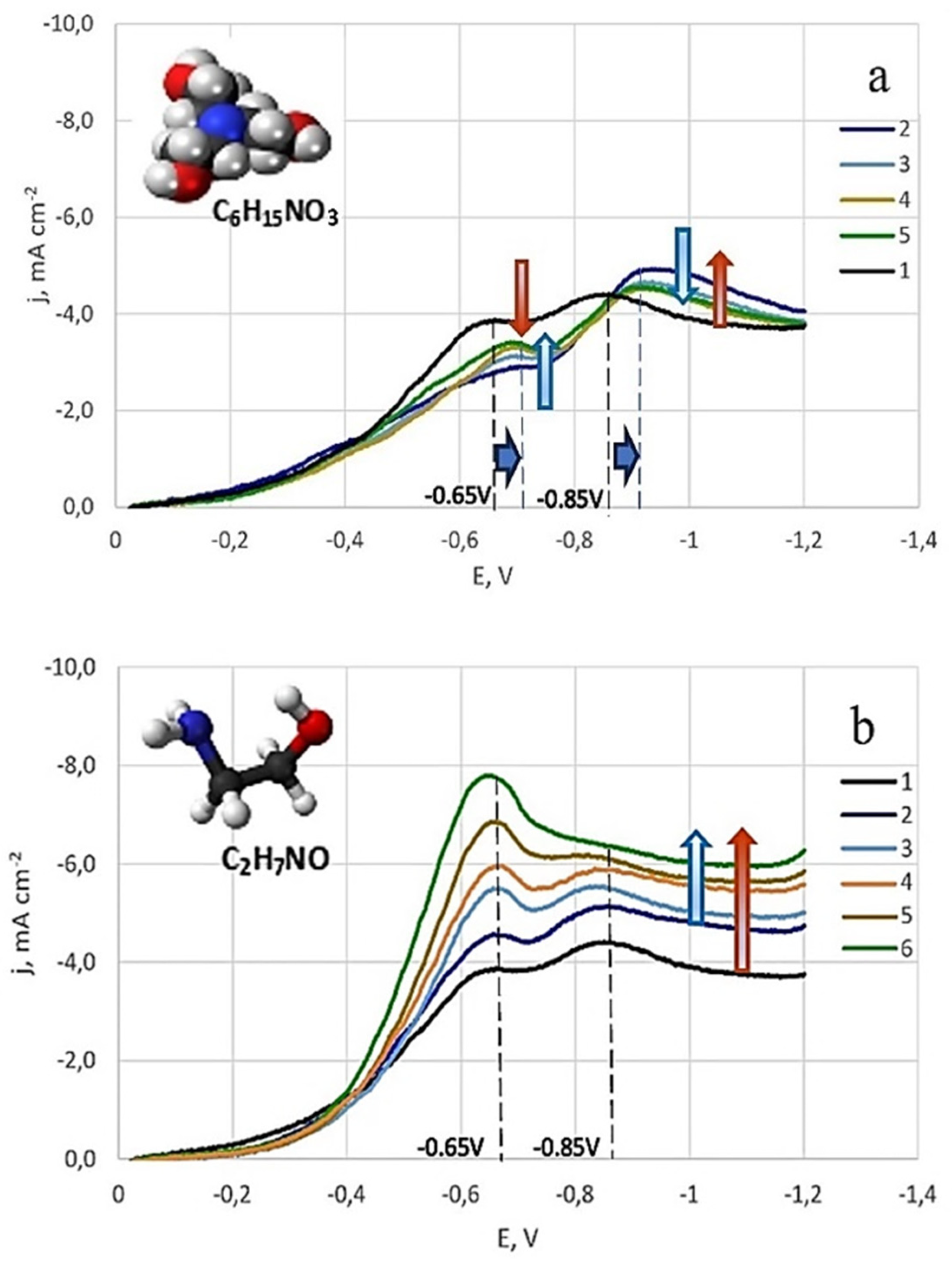
3.1.3. The Effect of Surfactants on the Electrode Process
3.2. Electrodeposition of Ag-Re Coatings
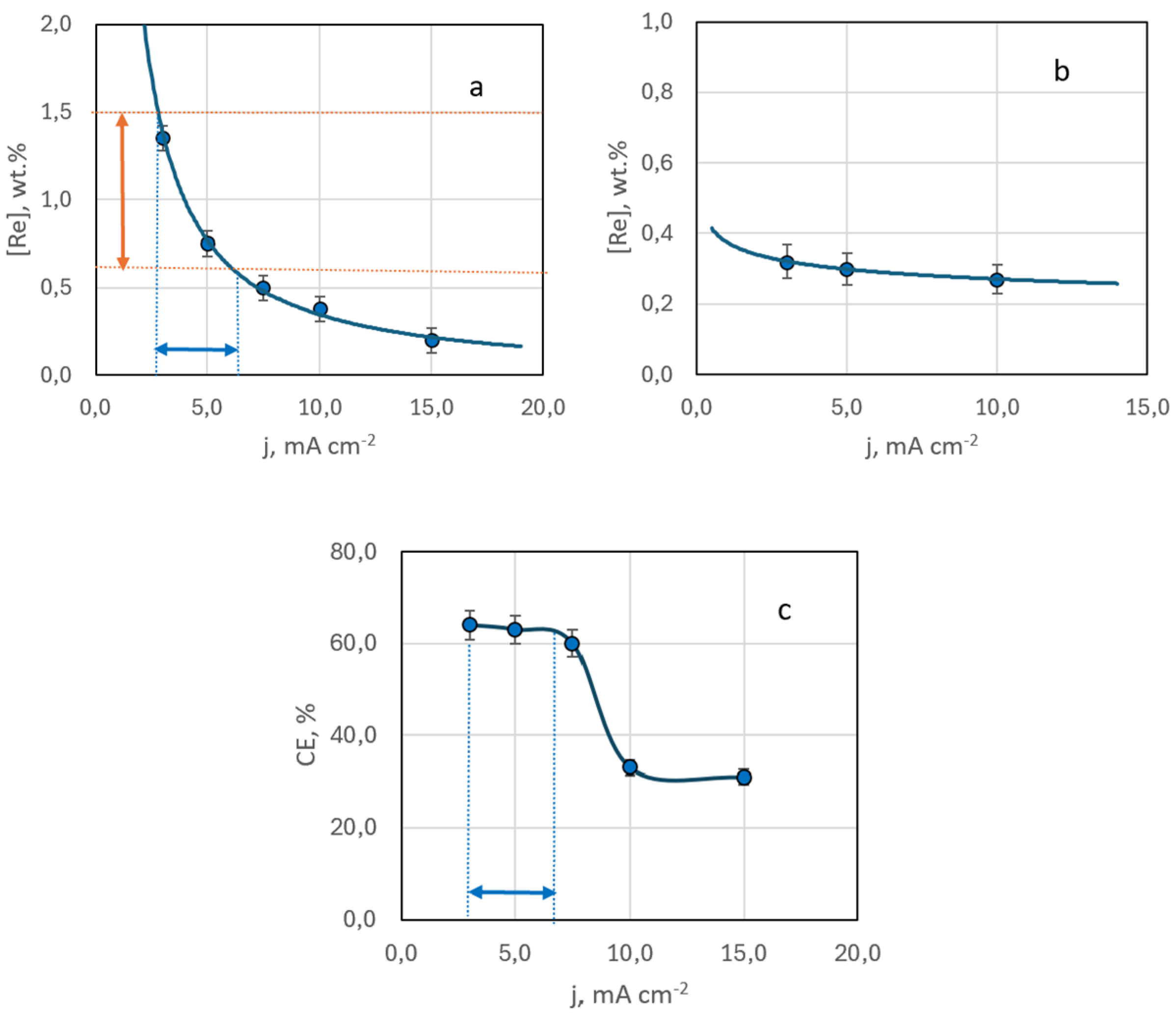
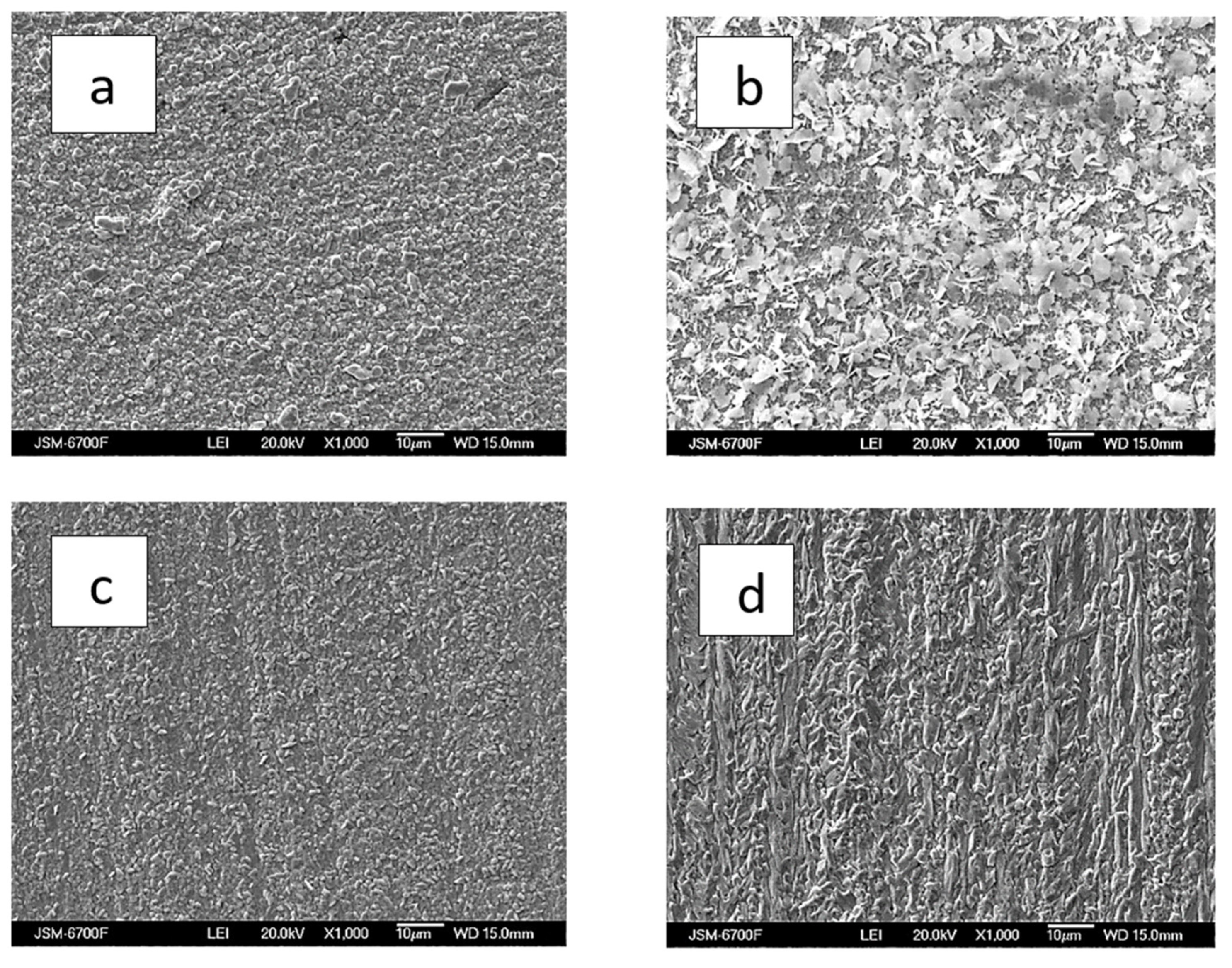
3.2.1. Dependency of Deposit Composition and Morphology on Current Density and Hydrodynamic Electrolysis Condition
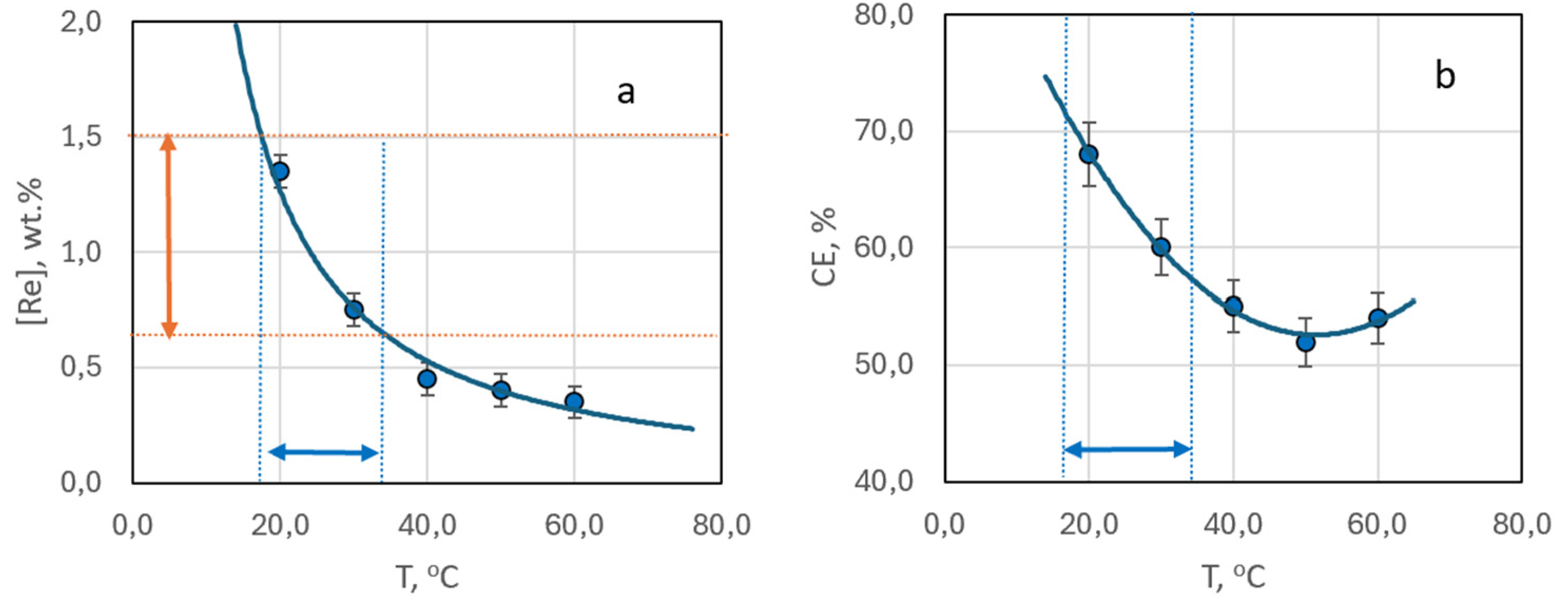
3.2.2. Dependency of Deposit Composition and Morphology on Bath Temperature
3.2.3. Dependency of Deposit Composition and Morphology on Electrolyte Component Ratios
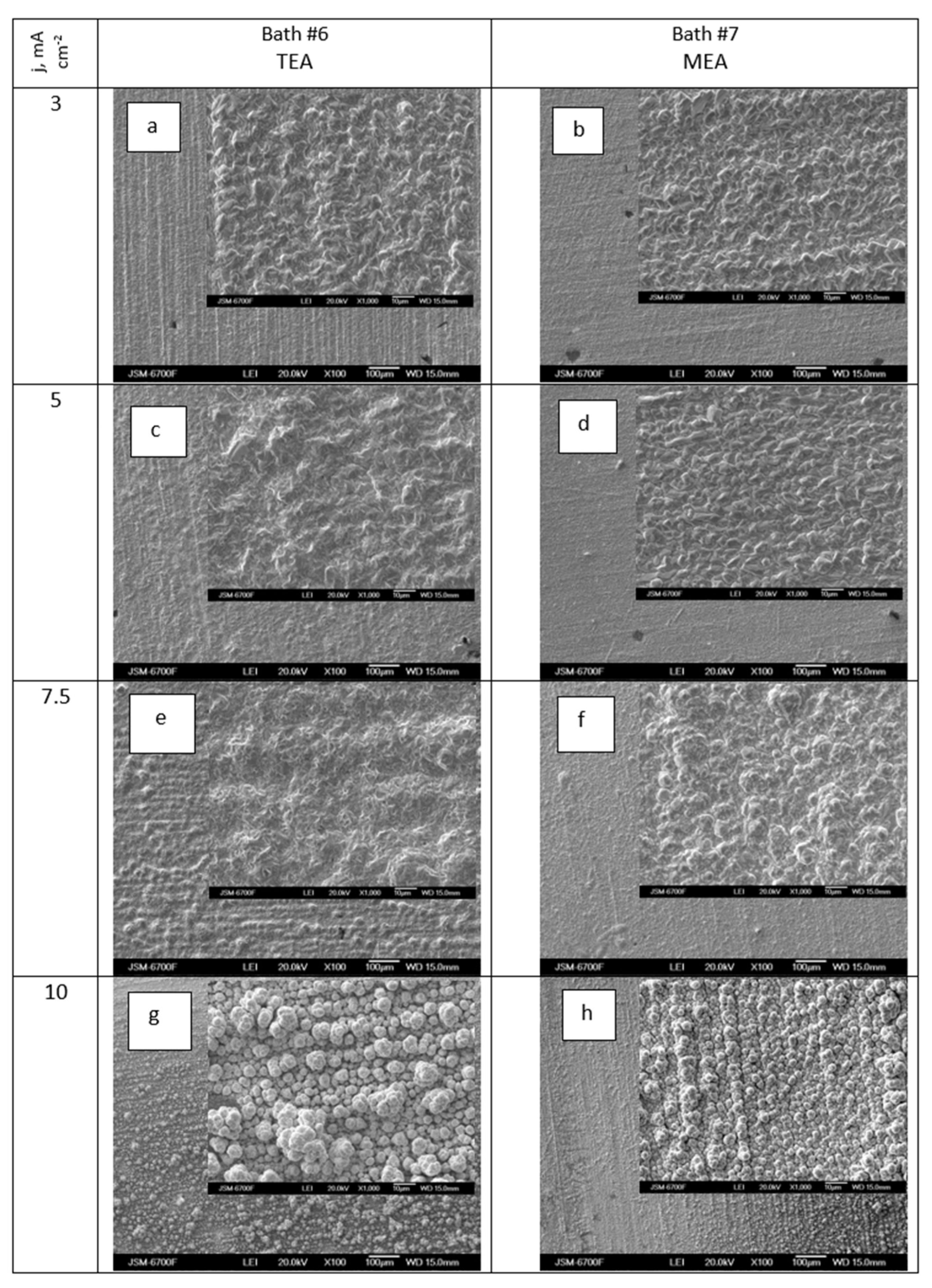
3.2.4. Dependency of Deposit Composition and Morphology on Surfactant Addition
3.2.5. X-ray Phase Analysis
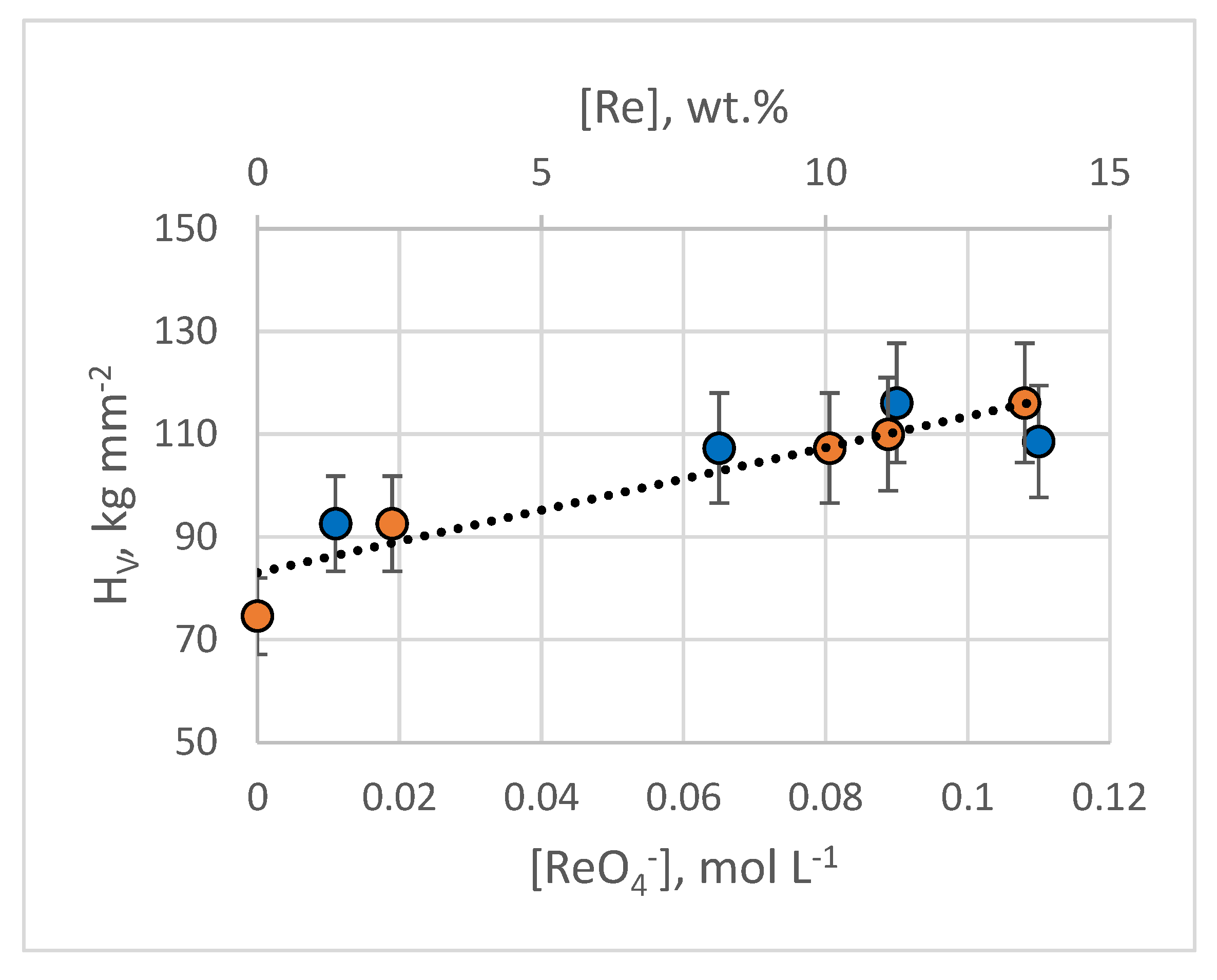
3.2.6. Characterizing Coating Microhardness, Adhesion, and Porosity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silver Based Materials, 2023 https://www.electrical‐contactswiki.com/index.php?title=Silver_Based_Materials>mobileaction=toggle_view_desktop.
- D.S. Cameron, Chemistry, electrochemistry, and electrochemical application | Silver. Editor: Jürgen Garche, Encyclopedia of Electrochemical Power Sources, Elsevier, 2009, pp. 876–882. [CrossRef]
- V. I. Balakai, A.V. Arzumanova, A.V. Starunov, et al. Properties of Electrolytic Silver-Based Alloy. Inorg. Mater. Appl. 2018, 9, 947–953. [CrossRef]
- S. Księżarek, M. Woch, T. Klir, D. Kołacz, M. Karpiński, K. Rudnicki, Environmentally friendly silver-based contact materials: Ores and Non-ferrous Metals 2012, 57, 549–555.
- I. Kuzmar, V. Lanin, N. Pas. Composite electroplated coatings based on silver for electronic products. Technologies in the electronics industry 2006, 6, 58–60. [Google Scholar]
- N. V. Bogush, A.A. Khmyl, L.K. Kushner. Composition, structure and functional properties of silver-tungsten composition electrochemistry coatings formed with the help of ultrasound. Doklady BGUIR 2021, 19, 23–31. [Google Scholar] [CrossRef]
- N. V. Bogush, A.A. Khmyl, L.K. Kushner. Structure and physical-mechanical properties of silver-tungsten composite coatings obtained by electrochemical deposition. Doklady BGUIR 2017, 107, 54–61. [Google Scholar]
- D. Kołacz, S. Księżarek, M. Woch. Nanocrystalline Ag-Re Composite as a Potential Material for Electric Contacts Fabrication. Arch. Metall. Mater. 2016, 61, 1847–1852, https://journals.pan.pl/dlibra/publication/120434/edition/104850/content. [Google Scholar] [CrossRef]
- E.U. Turns, (General Dynamics, Ft. Worth, Texas) Industrial Development of the Silver-Rhenium Alloy Plating Process, presented at the 57th National convention of the Arer. Electroplaters Soc. in Montreal, Canada, -25, 1970. 21 June.
- T., Jones. Rhenium plating. Metal Finishing 2003, 101, 86–96. [Google Scholar] [CrossRef]
- T. Jones, Electrodeposition of the Lesser-Known Precious Metals: Osmium, Iridium, Rhodium, Rhenium, Ruthenium, Revised Edition, Finishing Publications Ltd., 2005, 214p.
- Y. N. Sadana, Z.Z. Wang. Electrodeposition of Alloys. XX: Electrodeposition of Gold Rhenium Alloys from Acidic Citrate Solutions and Their Xray Structure. Surface & Coatings Technology 1989, 37, 419–434. [Google Scholar] [CrossRef]
- Y. Nguyen, J. Yoon, J. Shin. Electrodeposition of Pd-Ag alloy for electrical contacts. Surface engineering 2022, 38, 633–640. [Google Scholar] [CrossRef]
- V.V. Povetkin, S.V. Skifsky, E.V. Koreshkova, Electrolyte for deposition of copper-rhenium alloy, Patent: RU 2 234 560 C1. Published: 2004.06.27 https://patenton.ru/patent/RU2234560C1.
- D. Kołacz, S. D. Kołacz, S. Księżarek. Nanocrystalline Ag-Re composite as a potential material for electric contacts fabrication. Conference paper of the 27th International Conference on Electrical Contacts, June 22 – 26, 2014, Dresden, Germany. pp. 451–456. https://ieeexplore.ieee.org/document/6857211.
- S. Księżarek, M. Woch, D. Kołacz. Progress in fabrication technology of silver-based contact materials with particular account of the Ag-Re and AgSnO2Bi2O3 composites. Proceedings of the 26th International Conference on Electrical Contacts and the 4th International Conference on Reliability of Electrical Products and Electrical Contacts on -17, 2012, Beijing China, pp.186-193. DOI: 10.2478/amm-2014-0083. https://core.ac.uk/download/pdf/53096993.pdf. [CrossRef]
- F. Hauner, D. F. Hauner, D. Jeannot, J. Pinard, AgFe and AgFe2O3 Contact Materials for Voltage Switcher: 19-th International Conference on Electric Contact Phenomena: Nurnberg 1998: pp. 317–323.
- D. Kolacz, S. Ksiezarek, P. Borkowski. The Influence of Mechanical Alloying and Plastic Consolidation on the Resistance to Arc Erosion of the Ag-Re Composite Contact Material. Materials 2021, 14, 3297. [Google Scholar] [CrossRef]
- P. Borkowski, E. P. Borkowski, E. Walczuk, K. Frydman, D. Wójcik-Grzybek, Switching properties of contacts made of silver-tungsten and silver-tungsten-rhenium composite; Proceedings of the 59th IEEE Holm Conference on Electrical Contacts; Newport, RI, USA. ; pp. 197–206. 22–25 September 2013. [Google Scholar] [CrossRef]
- S. Księżarek, D. Kołacz, M.Czepelak, Z. Śmieszek, K. Marszowski, Technological aspects of plastic working of the Ag-Re(5, 8 and 10) wt % composite contact material: Metallurgy – Metallurgical engineering news. Hutnik–Wiadomości Hutnicze 2011, 8, 633–636, https://journals.pan.pl/dlibra/publication/120434/edition/104850/content. [CrossRef]
- B.V. Farmakovsky, E.A. B.V. Farmakovsky, E.A. Somkova, O.S. Sergeeva, M.A. Yurkov, D.A. Tochenyuk, R.Yu. Bystrov, A.S. Semenov, T.V. Peskov, D.A. Gerashchenkov, Silver-based alloy for nanostructured coatings. 2009. Patent RU 2350673. https://patenton.ru/patent/RU2350673C1.pdf.
- Bersirova, S.A. Kochetova, O.O. Bondar, Synthesis and characterization of electrolytic Ag-Re coatings, Proceedings of the “2nd International Research and Practice Conference “Nanoobjects & Nanostructuring”(N&N-2022)”,23-25 September 2022,Lviv, pp.57-58. https://drive.google.com/file/d/1TROnAqz8nft88hJ9adnHajWc_JEpGWa9/view.
- T.G. Shibleva, V.V. T.G. Shibleva, V.V. Povetkin, T.E. Ivanova, Electrolyte for deposition of silver-rhenium alloy, Patent RU 2 459 017 C1. Application: 2011119376/02, 2011.05.13 Published: 2012.08.20 https://patenton.ru/patent/RU2459017C1. 2459. [Google Scholar]
- Ivanov, A.F.; Kruglikov, S.S.; Mikhailova, A.N.; Ivanova, G.D.; Goncharov, V.K. , Electrolyte for coating silver-rhenium alloys. Copyright certificate RUS No. 627188. Application No. 2473785 dated 04/01/1977. Published: 05.10.1978. https://patenton.ru/patent/SU627188A1.
- Ivanov, A.F.; Ginzberg, S.A. , Electrolyte for the deposition of silver-based alloys, Patent: SU 528356 A1. Application: 2111560, 1974.12.26. Published: 1976.09.15 https://patenton.ru/patent/SU528356A1.
- Khmyl, A.A.; Kushner, L.K.; Kuzmar, I. Peculiarities of the formation of functional coatings silver - rhenium oxides / A. A. Khmyl [et al.] // Creation of new and improvement of existing technologies and equipment for applying galvanic and their replacement coatings: materials of the 5th Republican Scientific and Technical Seminar, Minsk, December 22-23, 2015 / Belarusian State Technological University. [editorial team: I. M. Zharsky (chief editor), O. B. Dormeshkin, A. A. Chernik]. - Minsk: BSTU, 2015; 4–7. [Google Scholar]
- Turns, E.W.; Head, J.W.; Hoffman, H.C.; Porter, A.C. Process for lubricating a bearing surface, U.S. patent 3,342,708, Sept. 19, 1967.
- Cao, H.; Chai, D.; Wu, L.; Zhen, G. Communication — A Mechanistic Study on Electrodeposition of Rhenium from Acidic Solution of Ammonium Perrhenate. Journal of The Electrochemical Society 2017, 164, D825. [Google Scholar] [CrossRef]
- Bersirova, S. Byk, V. Kublanovsky, Electrodeposition of silver. Monograph.; Kyiv: Medinform. 2013, 168 p. http://www.ionc.kiev.ua/deposite/Ag_electrodeposition.pdf.
- Bersirova, V. Kublanovsky, V. Emelianov. Electrodeposition of functional silver coatings from borate-phosphate-carbonate baths. Chemija 2003, 14, 16–21. [Google Scholar]
- Bersirova, V. Kublanovsky, H. Cesiulis, Electrochemical Formation of Functional Silver Coatings: Nanostructural Peculiarities. ECS Transactions 2013, 50, 155–163. [Google Scholar] [CrossRef]
- Bersirova, A. Krolikowski, V. Kublanovsky, Deposition conditions and corrosion characteristics of galvanic silver coatings for microelectronics. Ochrona Korozja 2002, 11A, 149–152. [Google Scholar]
- G. K. Burkat, I.V. Safronova, Electrodeposition of silver from dicyanoargenate electrolyte in the presence of nanocarbon additives. Modern problems of science and education 2014, 2, 1–8. [Google Scholar]
- Bersirova, V. Kublanovskii, Crystalline Roughness as a Morphological Characteristic of the Surface of Electroplated Silver Coatings. Russian Journal of Applied Chemistry 2009, 82, 1944–1948. [Google Scholar] [CrossRef]
- Bersirova, V. Kublanovsky, S. Bersirov, Modeling the Composition of the Pre-Cathode Layer in Dicyanoargentate Buffer Electrolyte without Excess Ligand Proceedings of the 12th International Conference Nanomaterials: Application & Properties ’2022 NAP-2022 (September 11–16, 2022) Krakow, Poland. – 2022. [CrossRef]
- Bersirova, V. Kublanovsky, Electrochemical Behavior of Silver in Dicyanoargentate Electrolytes. Proceedings of the 7th International Conference Nanomaterials: Application & Properties ’2017 NAP-2017 (September 10–15, 2017) Zatoka, Odesa region , Ukraine, 2017. https://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=8190157.
- Bersirova, G. Tsesiulis, V. Kublanovskii, Selective electrochemical dissolution of silver coatings on copper and its alloys. Russian Journal of Applied Chemistry 2009, 82, 1222–1225. [Google Scholar] [CrossRef]
- Bersirova, V.S. Kublanovsky, Electrolytic Nickel-rhenium alloys: synthesis, structure and corrosion properties. Materials Science 2019, 54, 506–511. [Google Scholar] [CrossRef]
- Bersirova, V.S. Kublanovsky, S.V. Bik, Electrodeposition of Ni-Re alloys from sulfamate and citrate electrolytes. Ukrainian Chemistry Journal 2017, 83, 110–116. [Google Scholar]
- Y. S. Yapontseva, V.S. Kublanovsky, O.A. Vyshnevskyi, Electrodeposition of CoMoRe alloys from a citrate electrolyte. Journal of Alloys and Compounds 2018, 766, 894–901. [Google Scholar] [CrossRef]
- V. Zhulikov, Yu. Gamburg, Eelectrodeposition of rhenium and its alloys. Russian Journal of Electrochemistry 2016, 52, 847–857. [Google Scholar] [CrossRef]
- Naor, A.; Eliaz, N.; Gileadi, E. Electrodeposition of Alloys of Rhenium with Iron-Group Metals from Aqueous Solutions. Journal of the electrochemical society 2010, 157, D422. [Google Scholar] [CrossRef]
- Rhenium. Term paper. File archive of students Studfile. https://studfile.net/preview/2474240/page:3/.
- J. R. DePew, T.L. Larson, Electrodeposition of rhenium for wear resistance. A preliminary report. Plating 1970, 475–478. [Google Scholar]
- Influence of electrolyte composition on the structure and properties of composite electrochemical coatings based on silver / V.K. Brantsevich [and others] // Materials of the 48th scientific conference of graduate students, undergraduates and students. Minsk, 7–11 May, 2012, pp. 120–121.
- L.I. Antropov, Theoretical Electrochemistry. Mir Publisher, Moscow, 1972.
- M. Grall, Electrochemical study of chemical properties in ethanolamine and its mixtures with water (Commissariat a l’Energie Atomique, Fontenay-aux-Roses (France). Centre d’Etudes Nucleaires) CEA Fontenay-aux-Roses, 92 (France), 1964. https://inis.iaea.org/search/search.aspx?orig_q=RN:36065597.
- H. Cesiulis, O. Bersirova, A. Valiuniene, I., Prosycevas, G. Baltrunas, Structure and Morphology of Silver Electrodeposits. Materials Science (Medziagotyra) 2004, 10, 142–146. [Google Scholar]
- C. A. Majid, M.A. Hussain Structural transformations in polycrystalline rhenium trioxide. Phw. Chem. Solids 1995, 56, 255–259. [Google Scholar] [CrossRef]
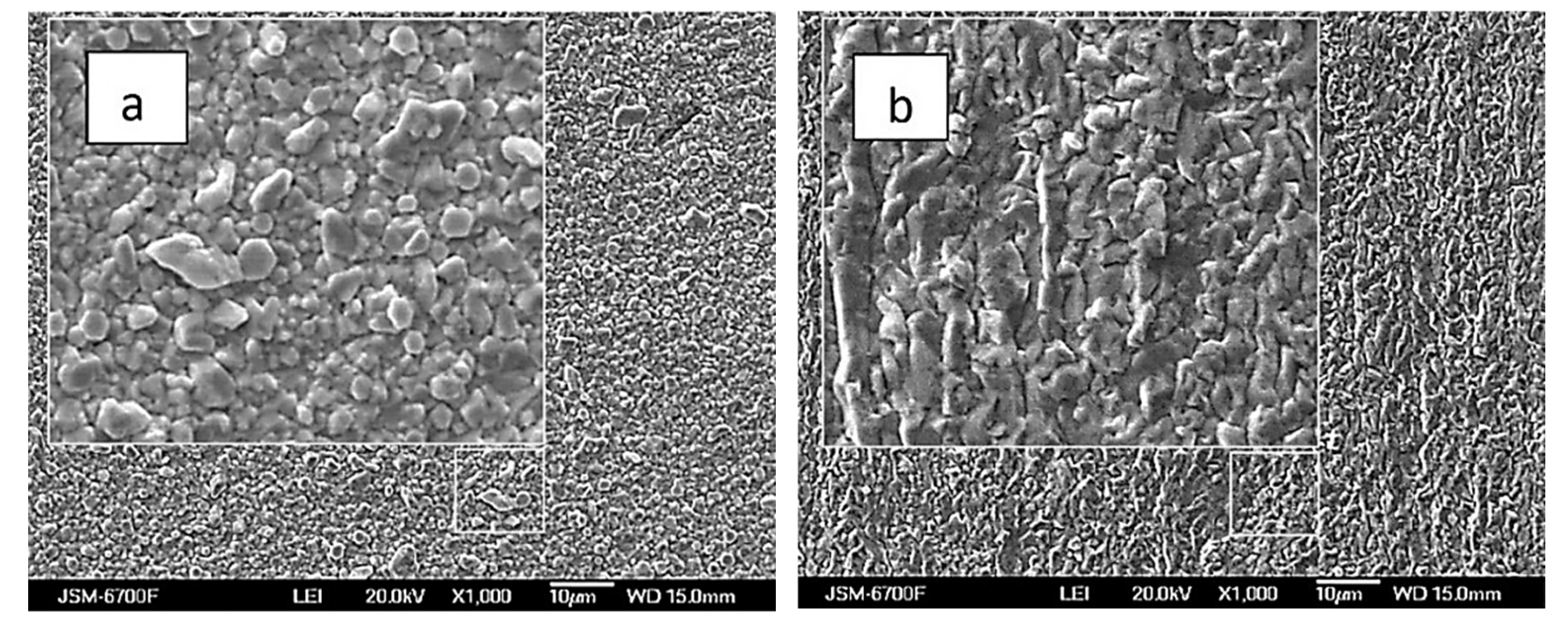
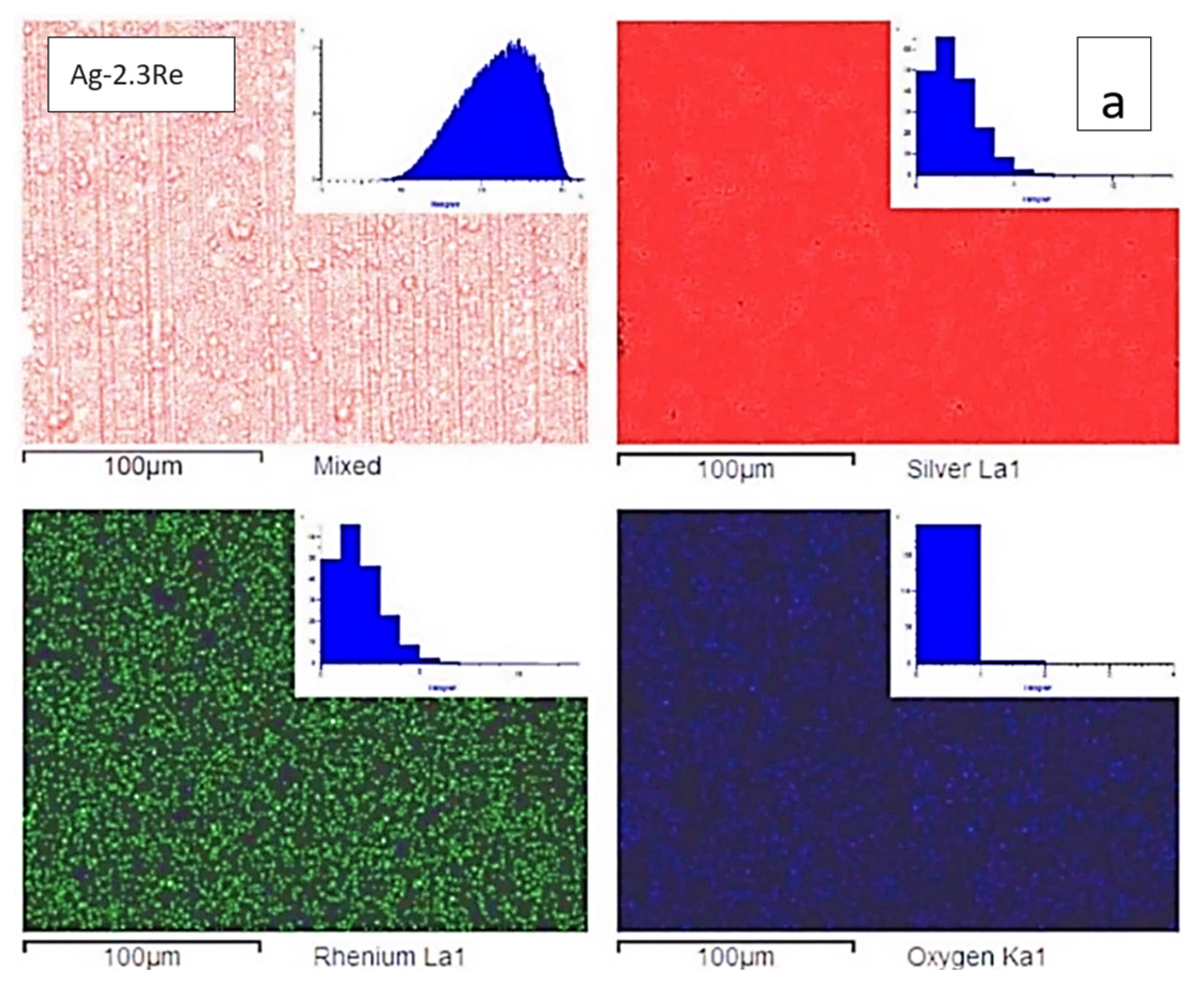
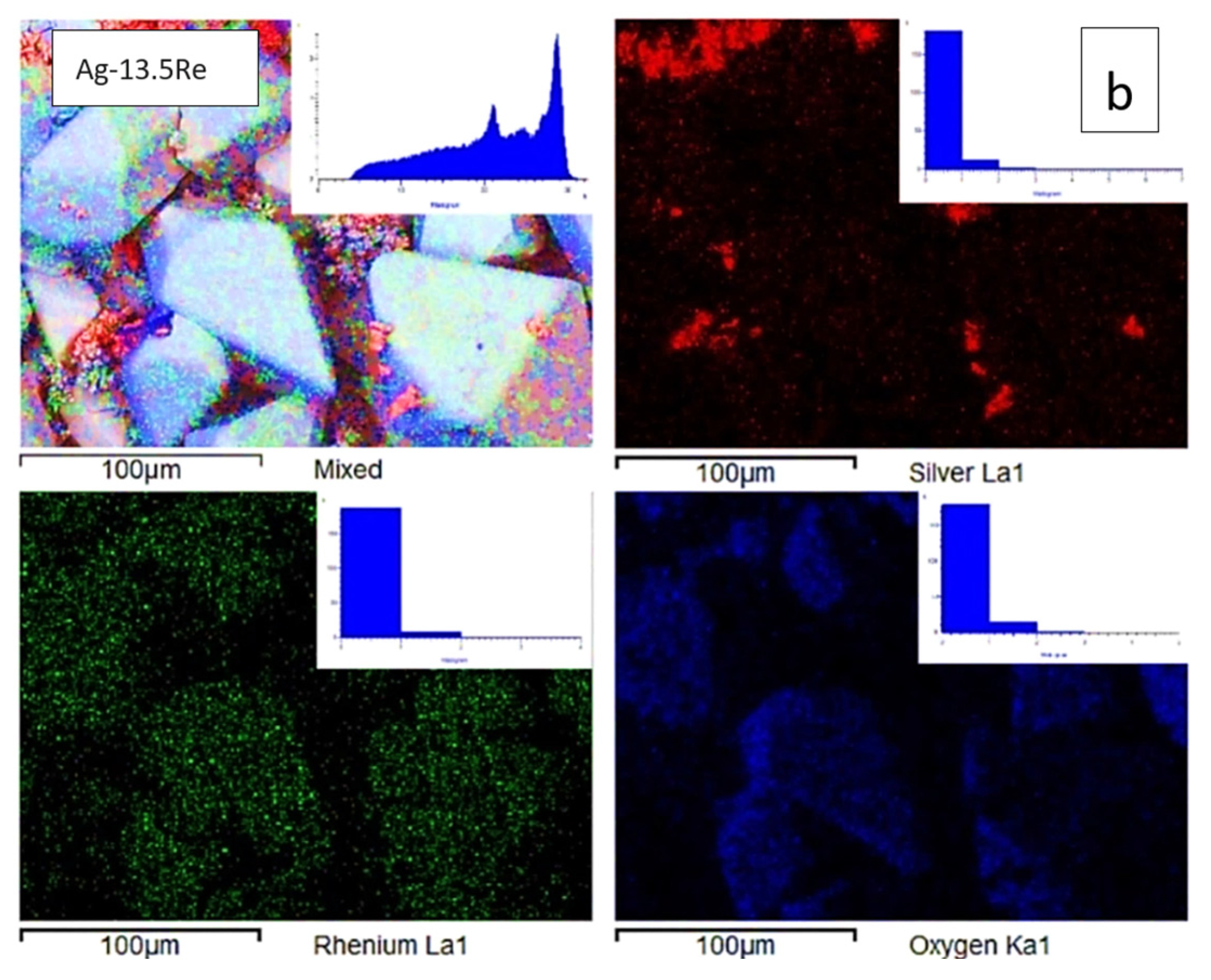
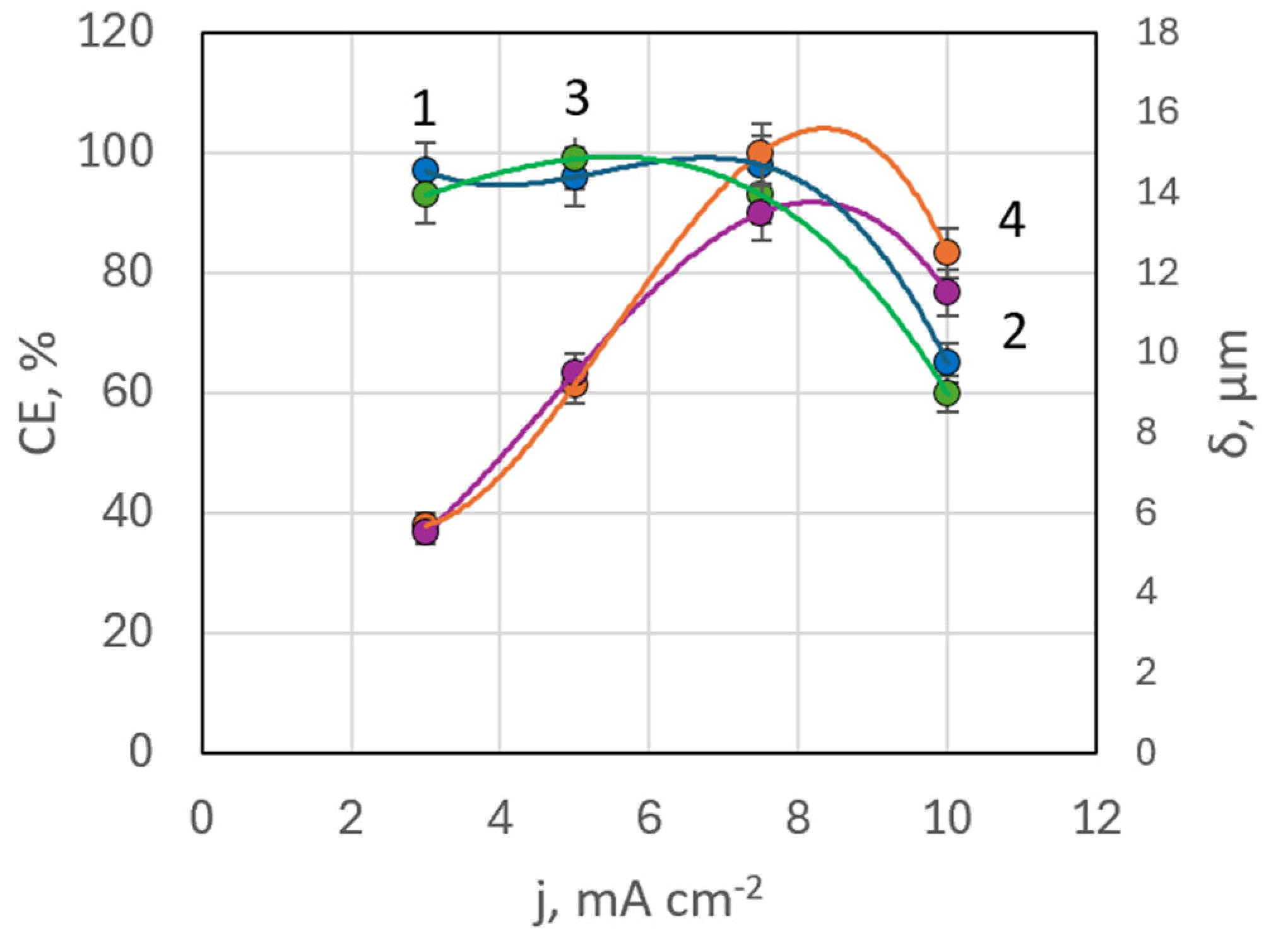


| Bath,(mol L−1) | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 |
|---|---|---|---|---|---|---|---|---|---|---|
| NH4ReO4 | - | 0.011 | 0.022 | 0.0022 | - | - | - | - | - | - |
| KReO4 | - | - | - | - | 0.055 | 0.055 | 0.055 | 0.065 | 0.09 | 0.11 |
| KAg(CN)2 | 0,11 | 0,11 | 0,0044 | 0,022 | 0,11 | 0,11 | 0,11 | 0.044 | 0.009 | 0.011 |
| KH2PO4 | 0.05 | 0.05 | 0.002 | 0.01 | 0.05 | 0.05 | 0.05 | 0.025 | 0.014 | 0.005 |
| K2HPO4 | 0.3 | 0.3 | 0.01 | 0.06 | 0.3 | 0.3 | 0.3 | 0.15 | 0.085 | 0.03 |
| K2CO3 | 0.025 | 0.025 | 0.001 | 0.005 | 0.025 | 0.025 | 0.025 | 0.001 | 0.0055 | 0.0025 |
| H3BO3 | 0.2 | 0.2 | 0.007 | 0.04 | 0.2 | 0.2 | 0.2 | 0.1 | 0.05 | 0.02 |
| C2H7NO (MEA) | - | - | - | - | - | 0.033; 0.066; 0.099; 0.132; 0.160 |
- | - | - | - |
| C6H15NO3 (TEA) | - | - | - | - | - | - | 0.015; 0.030; 0.045; 0.060 | - | - | - |
| [Ag+]:[ReO4-] ratio |
- | 10:1 | 1:5 | 10:1 | 2:1 | 2:1 | 2:1 | 1: 1.5 | 1:10 | 1:10 |
| pH (at 20oC) |
7.00 | 7.65 | 7.80 | 7.60 | 7.21 | 8.78 (0.16M MEA) |
8.15 (0.06M TEA) |
8.18 |
8.42 |
8.49 |
| Bath | #1 | #2 | #3 | #4 | #5 | #6 | #7 |
|---|---|---|---|---|---|---|---|
| EAg, V (Ag/AgCl) | +0.200 | -0.022 | -0.122 | +0.024 | +0.007 | +0.170 | +0.110 |
| EPt, V (Ag/AgCl) | -0.0040 | -0.0145 | -0.030 | +0.004 | +0.004 | +0.150 | +0.0038 |
| Sample | Intensities Ihkl of diffraction peaks (in arbitrary units) | |||
| I111 | I200 | I311 | I222 | |
| Ag | 55 | 100 | 3 | 0.6 |
| Ag-2.3Re | 62 | 100 | 2 | 0.7 |
| Ag-11.7Re | 19.5 | 100 | 4 | 0.9 |
| Ag-13.5Re | 100 | ~0 | ~0 | ~0 |
| Bath #1 |
Bath #2 [Ag+]:[ReO4-]=10:1 |
Bath #9 [Ag+]:[ReO4-]=1:1.5 |
Bath #10 [Ag+]:[ReO4-]=1:10 |
||
| (111) | Θ, deg | 19,07 | 19,06 | 19,06 | - |
| dhkl, Å | 2,3567 | 2,3579 | 2,3579 | - | |
| a0, Å | 4,0770 | 4,0791 | 4,0791 | - | |
| (200) | Θ, deg | 22,16 | 22,15 | 22,14 | - |
| dhkl, Å | 2,0413 | 2,0422 | 2,0431 | - | |
| a0, Å | 4,0826 | 4,0844 | 4,0862 | - | |
| (311) | Θ, deg | 38,72 | 38,74 | 38,74 | - |
| dhkl, Å | 1,2309 | 1,2318 | 1,2318 | - | |
| a0, Å | 4,0824 | 4,0853 | 4,0853 | - | |
| (222) | Θ, deg | 40,62 | 40,62 | 40,62 | - |
| dhkl, Å | 1,1827 | 1,1827 | 1,1824 | - | |
| a0, Å | 4,0969 | 4,0969 | 4,0959 | - | |
| (442) | Θ, deg | - | - | - | 25.22 |
| dhkl, Å | - | - | - | 1.8071 | |
| a0, Å | - | - | - | 3.3202 | |
| (332) | Θ, deg | - | - | - | 21.66 |
| dhkl, Å | - | - | - | 2.0861 | |
| a0, Å | - | - | - | 2.2484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





