1. Introduction
Citrus, one of the most widely cultivated and economically significant fruit crops worldwide, accounted for an estimated production of 143.8 million tons globally in 2019 [
1]. In Oman, citrus ranks as the fourth most important fruit crop, with extensive cultivation of varieties including lime, sweet lemon, mandarin, orange, shaddock, citron, and grapefruit. These citrus crops are essential to the agricultural economy, particularly in regions like Batinah, Dakhiliyah, and Sharqiyah, where large-scale cultivation occurs [
2]. Juma et.al., [
3] states that the citrus industry in Oman faces several challenges, notably the prevalence of diseases that severely impact crop yield and tree longevity. Over recent decades, biotic factors such as viral and fungal infections have significantly affected citrus production, with witches' broom disease of lime (WBDL) being the most severe. Since the 1980s, WBDL has led to the decline of over half a million lime trees, marking a critical loss to Oman’s citrus sector [
4]. Additionally, citrus tristeza virus (CTV) was detected in Oman in the late 1980s, further threatening the health of citrus trees across the region [
5].
Among these destructive diseases, Huanglongbing (HLB), commonly known as citrus greening disease, is currently one of the most alarming due to its rapid spread and the severe damage it inflicts on infected trees. First reported in China in 1919 [
6], HLB is caused by the phloem-restricted bacterium Candidatus Liberibacter, with three primary pathogenic species identified: Candidatus Liberibacter asiaticus (Las), Candidatus Liberibacter africanus (Laf), and Candidatus Liberibacter americanus (Lam) [
7]. The disease is transmitted by insect vectors, with the Asian citrus psyllid (Diaphorina citri) responsible for the spread of Las and Lam, and the African citrus psyllid (Trioza erytreae) exclusively transmitting Laf.
In Oman, HLB has been reported in acid lime (Citrus aurantiifolia), with infections largely clustered around the Las species [
8]). Typical symptoms include yellowing of leaves, fruit deformation, branch dieback, and tree death. The disease has substantially decreased lime production, presenting a serious threat to the citrus industry. Despite efforts to control HLB, its obligatory nature and reliance on insect vectors complicate management strategies, necessitating advanced detection and prevention techniques [
9].
This study investigates the occurrence of HLB in various citrus varieties housed in the citrus gene bank at Suhar, Oman. By employing molecular detection methods such as PCR and restriction enzyme analysis, we aim to identify the specific Candi-datus Liberibacter species present in symptomatic trees and assess the health status of the primary citrus varieties in Oman’s gene bank. This research marks the first documented report of HLB infection across multiple citrus varieties, including Clementine, Grapefruit, Lime, and Orange, within the region.
2. Problem Statement
The citrus industry in Oman, a crucial component of the agricultural economy, faces severe threats from various plant diseases, with Huanglongbing (HLB), or citrus greening disease, emerging as one of the most destructive. This disease, caused by species of Candidatus Liberibacter bacteria and spread by insect vectors, leads to substantial yield loss, tree decline, and ultimately, death of infected trees. While HLB has been reported in certain lime varieties in Oman, its presence and impact on other citrus types remain largely undocumented. The lack of detailed surveillance and species-specific identification across diverse citrus varieties limits effective disease management and risk assessment within the country.
Given this gap, there is a pressing need to investigate the prevalence of HLB in various citrus varieties beyond lime, particularly within the critical citrus gene bank in Suhar, which serves as a vital resource for citrus propagation in Oman. This study aims to address these challenges by identifying HLB infections in multiple citrus varieties and characterizing the bacterial species involved, providing essential data to inform disease management strategies and protect Oman’s citrus industry.
3. Literature Review
3.1. Overview of Huanglongbing (HLB)
Huanglongbing (HLB), commonly known as citrus greening disease, is one of the most devastating diseases affecting citrus crops globally. It is caused primarily by the phloem-limited bacterium Candidatus Liberibacter asiaticus, which is transmitted by several species of psyllids, particularly Diaphorina citri [
10]. The disease leads to significant yield losses, fruit drop, and a decline in fruit quality, making it a critical threat to the citrus industry [
11]. Recent studies indicate that HLB can also affect the trees’ nutrient dynamics, leading to deficiencies in essential nutrients, which further exacerbates the disease's impact [
12].
3.2. Detection and Diagnosis of HLB
Accurate diagnosis of HLB is crucial for effective management strategies. Tradi-tional methods, such as visual inspection and symptom observation, can be mislead-ing due to the asymptomatic nature of infected trees in the initial stages [
13]. Molecu-lar techniques, including Polymerase Chain Reaction (PCR) and DNA sequencing, have become the gold standard for detecting Ca. Liberibacter species in citrus trees[
14]. The study utilized a PCR-based approach targeting the 16S rdna region, aligning with recent findings that emphasize the importance of molecular diagnostics for early detection and surveillance of HLB[
15].
3.3. Distribution and Prevalence of HLB
The geographical distribution of HLB has expanded significantly since its first identification in Florida in 2005 [
16]. Reports indicate that HLB has spread to major citrus-producing regions worldwide, including Asia, Africa, South America, and the Mediterranean [
17]. The prevalence of HLB in the Arabian Peninsula, particularly in Oman, highlights the urgent need for surveillance and management strategies. Recent findings from numerous studies suggest that disease prevalence can reach up to 100% in heavily infected orchards [
18], underscoring the importance of continuous monitoring and early detection.
3.4. Impact on Citrus Production and Management Strategies
HLB significantly impacts citrus production, leading to reduced fruit size and quality, as well as increased production costs due to the need for additional management practices [
19]. Integrated pest management (IPM) strategies are crucial for controlling the vector populations and minimizing the spread of HLB [
20]. Recent studies emphasize the effectiveness of using natural predators of Diaphorina citri and implementing cultural practices that reduce psyllid populations [
21]. The paper under review aligns with these findings, indicating that managing the vector is vital for HLB control.
3.5. Recent Advances in HLB Research
Recent advancements in biotechnology have opened new avenues for HLB management. Genetic studies have identified potential resistance genes in citrus species, which could be leveraged through breeding programs [
22]. Additionally, research into RNA interference (rnai) has shown promise in developing transgenic citrus plants resistant to HLB [
23]. Such innovations are crucial for developing sustainable solutions to combat HLB and its devastating effects on citrus production.
4. Methodology
The study involved the systematic collection of citrus samples from various orchards exhibiting symptoms of Huanglongbing (HLB). Symptomatic and asymptomatic trees were selected based on visual observations of leaf yellowing, stunted growth, and fruit drop. Specific geographic locations were targeted to assess regional differences in HLB prevalence. Samples included leaves and fruit from affected trees, as well as healthy controls from nearby unaffected trees to establish comparative analysis.
The presence of Candidatus Liberibacter asiaticus was detected using Polymerase Chain Reaction (PCR) methods. Leaf samples were processed to extract DNA, followed using specific primers targeting the Liberibacter species. The PCR protocol included temperature cycling parameters that were optimized for maximum sensitivity and specificity. Additionally, quantitative PCR (qpcr) was performed to quantify pathogen loads in infected samples.
Statistical analyses were conducted to compare the prevalence of HLB among different samples. Data was analyzed using software such as R or SPSS, employing statistical tests such as chi-squared tests for categorical data and t-tests or ANOVA for continuous data. Significance was determined at a p-value threshold of <0.05.
The experimental design included a field study layout with randomized sampling to minimize bias. Control groups were established by selecting trees that showed no symptoms of HLB within the same vicinity as the symptomatic trees. Replicates were ensured by collecting multiple samples from different trees within each treatment category.
To ensure the reliability of results, the study included multiple replicates of each sample type and established control groups for comparative purposes. The experimental design allowed for consistent results across different conditions and locations, thus enhancing the overall validity of the findings.
5. Systematic Analysis and Data Collection
5.1. Study Site and Sample Collection
Location: Agricultural Research Station in Suhar, North Al-Batinah Governorate, Oman.
Sample Size: A total of 124 citrus trees were surveyed, including 10 deceased trees.
Citrus Groups: The block consisted of four citrus groups:
Clementine (29 trees)
Grapefruit and Pomelo (12 trees)
Lime (37 trees)
Orange (36 trees)
Sample Collection: Leaves from mature trees showing typical symptoms of Huanglongbing (HLB) were collected. Samples were stored in labelled plastic bags and transported in an ice cooler to the laboratory.
5.2. Sample Preparation and DNA Extraction
Washing: Samples were washed with tap water followed by 70% ethanol to min-imize contamination.
Tissue Processing: Midribs and petioles were removed; five grams of the re-maining leaf tissue were homogenized in liquid nitrogen using sterile mortars and pestles.
DNA Isolation: DNA was extracted using the CTAB method as described by Doyle and Doyle (1987).
5.3. DNA Quality and Quantity Assessment
5.4. Polymerase Chain Reaction (PCR) Amplification
Primers Used: The 16S rdna region was amplified using primers CN265 and CN266, targeting a 448 bp amplicon.
PCR Conditions: The reaction mixture consisted of:
1 μl of DNA template
12.5 μl of 2X Dream Taq polymerase master mix
1 μl of each primer.
Cycling Parameters: The following PCR conditions were utilized:
Initial denaturation: 95 °C for 2 min
35 cycles of:
Denaturation: 94 °C for 30 s
Annealing: 58 °C for 45 s
Extension: 72 °C for 1 min 30 s
Final extension: 72 °C for 10 min
5.5. Gel Electrophoresis
PCR products were analyzed using 1% agarose gel electrophoresis to confirm amplification.
5.6. Restriction Fragment Length Polymorphism (RFLP) Analysis
Restriction Enzyme: PCR products from the CN265/CN266 primers were di-gested with xbai to differentiate between species.
Digestion Conditions: The reaction included:
10 μl of PCR product
7 μl of DNA-free water
2 μl of 10X buffer
1 μl of xbai enzyme.
Incubation: Samples were incubated at 37 °C for 16 hours and analyzed on 2% agarose gel stained with ethidium bromide.
5.7. Further Confirmation of Infection
A representative sample from each citrus group was amplified using primers OI1 and OI2c, followed by digestion with xbai.
5.8. Sequencing and Bioinformatics Analysis
Sequencing: The 1,160 bp amplicon from CN265/CN266 was sequenced (Mac-rogen, Korea).
Bioinformatics Tools: NCBI-BLAST tool and MEGA 11 software were used for sequence analysis and alignment.
5.9. Statistical Analysis
Infection rates were calculated for each citrus group based on the number of infected samples relative to the total tested samples.
6. Results and discussion
In this study, a total of 114 citrus trees were tested for Huanglongbing (HLB) at the Agricultural Research Station in Suhar, where various citrus species had been cultivated for the past 20 years.
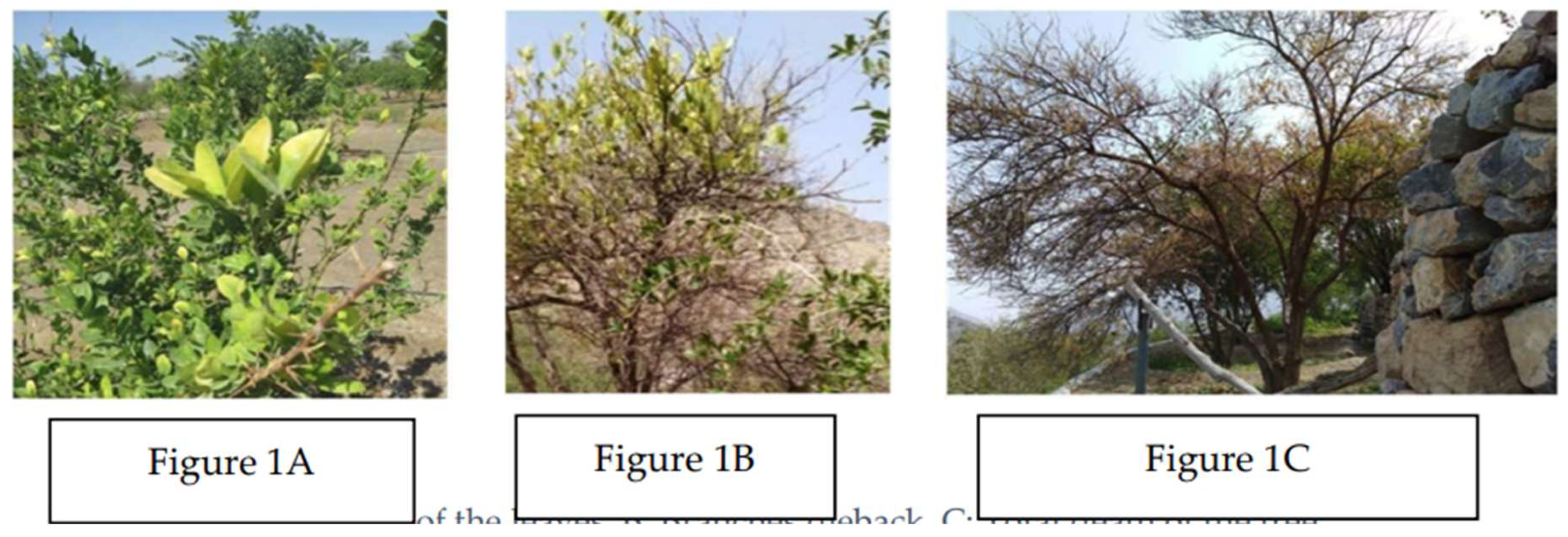
All infected trees exhibited typical HLB symptoms, including leaf yellowing(
Figure 1A), small and deformed fruit, and dieback(
Figure 1B), which correspond to the known effects of HLB on citrus health. This decline in tree vitality, especially noted in Lime, points to the potential for significant impacts on citrus production in the affected areas. In the late stages of the disease, tree decline, and mortality were observed(
Figure 1C), further emphasizing the urgent need for effective disease management interventions.
The sample set included ten deceased trees, with four distinct citrus groups repre-sented: Clementine (29 trees), Grapefruit and Pomelo (12 trees), Lime (37 trees), and Orange (36 trees) (
Table 1).
6.1. Infection Rates and Symptoms
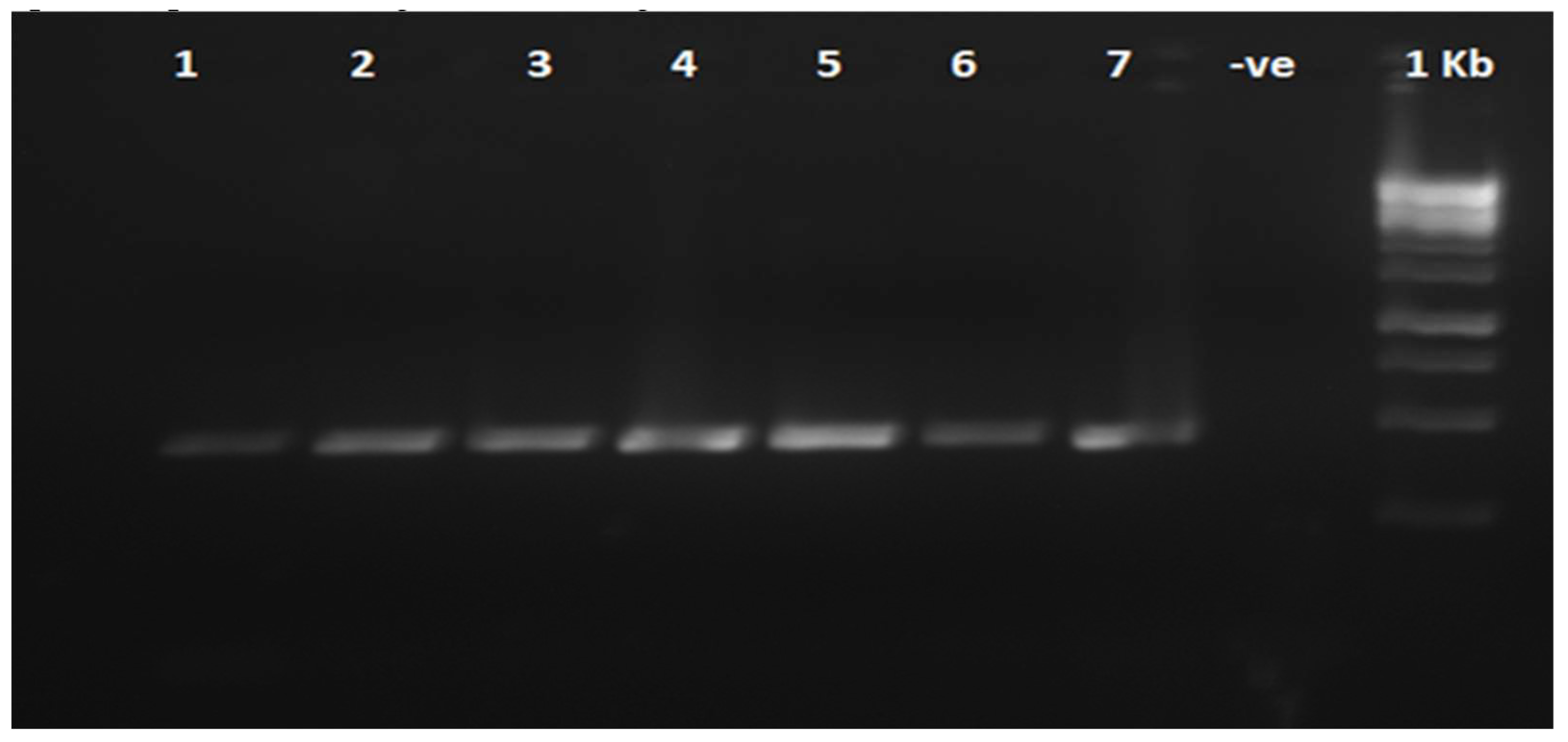
The results indicated that 21 trees, or approximately 18.4% of the total tested samples, were infected with HLB, as confirmed by PCR amplification of the 448 bp region using primers CN265 and CN266(see
Figure 2). The infection rates varied significantly among the citrus groups, with the highest prevalence observed in Clementine (27.6%), followed by Orange (22.2%) and Lime (10.8%). Grapefruit and Pomelo displayed the lowest infection rate at 8.8%. These findings align with previous studies indicating that certain citrus varieties are more susceptible to HLB, highlighting the need for species-specific management strategies.
6.2. Molecular Characterization of the Pathogen
To confirm the presence of Candidatus Liberibacter asiaticus (Las), a partial 16S rdna region was amplified using the primers CN265/CN266produce 448 bp PCR products (
Figure 2)
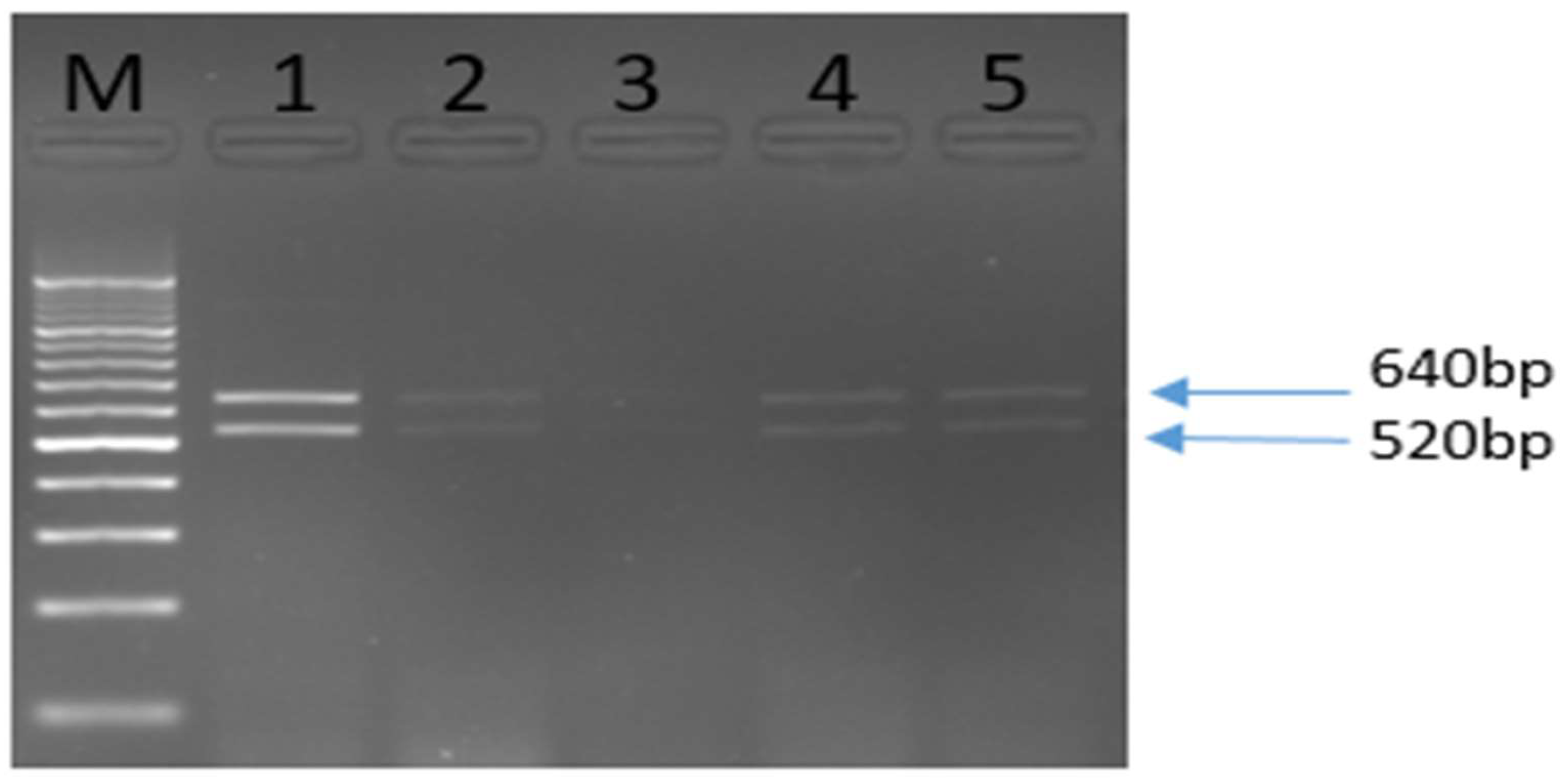
This outcome was supported by the additional confirmation provided by the OI1/OI2c primers, which generated a 1,160 bp amplicon that was similarly digested to yield the expected fragments for Las. The resulting 1,160 bp PCR products underwent restriction enzyme digestion with xbai, which produced two fragments of approximately 640 bp and 520 bp, confirming the presence of the Las strain in the infected samples(see Fig. 3).
Sequencing of the amplified products from the CN265/CN266 primers further corroborated the identity of the pathogen, as the derived sequences showed over 99.5% identity with previously documented Omani isolates (GQ369792 and GU074017) in the genbank database. These results not only confirm the presence of Las but also suggest the need for ongoing monitoring of HLB in Oman, especially considering the high infection rates observed in certain citrus varieties.
6.3. Implications for Disease Management
The study highlights the critical importance of monitoring and managing HLB within the region. The presence of HLB-positive trees necessitates immediate actions, including the removal of affected trees from the citrus gene bank to prevent further spread of the disease. Moreover, replanting with scientifically produced disease-free seedlings in net-protected areas can significantly mitigate the risk of HLB resurgence.
To control the insect vector Diaphorina citri, known to transmit Las, integrated pest management strategies should be adopted. This may include the application of chemical pesticides or exploring biological control options. Furthermore, given the potential presence of the alternative strain Candidatus Liberibacter africanus (Laf) in the region, further surveys should be conducted to determine the distribution of both strains and their respective insect carriers, particularly Trioza erytreae.
7. Conclusion
The findings from this study confirm the presence of Huanglongbing (HLB) in various citrus species at the Agricultural Research Station in Suhar, Oman, with a notable infection rate of 18.4%. The molecular characterization identified Candidatus Liberibacter asiaticus (Las) as the causative agent, demonstrating significant implications for citrus production in the region. The symptoms observed in infected trees, such as leaf yellowing and fruit deformation, emphasize the destructive potential of HLB. The study provides essential insights into the status of HLB in Omani citrus, indicating an urgent need for initiative-taking disease management measures to mitigate its impact.
8. Future Work
Future research should focus on the following key areas:
Enhanced Surveillance: Conduct comprehensive surveys to determine the geographical spread of HLB and the presence of its insect vectors, specifically Trioza erytreae and Diaphorina citri, in various citrus-growing regions of Oman.
Vector Management: Develop integrated pest management strategies that combine chemical and biological control measures to combat the insect vectors responsible for HLB transmission.
Disease Management Protocols: Establish and promote protocols for the eradication of infected trees, followed by replanting with scientifically produced, disease-free seedlings in protected environments.
Genetic Studies: Investigate the genetic diversity of the HLB strains present in Oman to understand their evolutionary dynamics and potential virulence factors.
Public Awareness and Training: Implement training programs for farmers and stakeholders to raise awareness about HLB, its symptoms, and best practices for management and prevention.
By addressing these areas, future studies can contribute significantly to controlling HLB and safeguarding citrus production in Oman.
Author Contributions
Conceptualization, Mohammed Al Sadrani; methodology, Ahmed Al Fahdi and Ali Al Adawi; software, not applicable; validation, Mohammed Al Sadrani, Ahmed Al Fahdi, and Ramalingam Dharmalingam; formal analysis, Ahmed Al Fahdi and Ali Al Adawi; investigation, Mohammed Al Sadrani; resources, Mohammed Al Sadrani; data curation, not applicable; writing—original draft preparation, Saif Al Kaabi and Ramalingam Dharmalingam; writing—review and editing, Raied Abou Kubaa and Ramalingam Dharmalingam; visualization, not applicable; supervision, Mohammed Al Sadrani; project administration, Mohammed Al Sadrani; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Data Availability Statement
The data supporting the reported results in this study are available upon request from the corresponding or first author.
Acknowledgments
We acknowledge the support of the laboratory team for their assistance with sample collection and molecular analyses specially Ms. Rahma Al Mqbali and Ms.Muna ALJabri.
Conflicts of Interest
The authors declare no conflicts of interest..
Abbreviations
The following abbreviations are used in this manuscript:
| HLB |
Huanglongbing |
| CTV |
Citrus tristeza virus |
| WBDL |
Witches' broom disease of lime |
| PCR |
Polymerase chain reaction |
| DNA |
Deoxyribonucleic acid |
| CTAB |
Cetyltrimethylammonium bromide |
References
- FAO, “Statistical Yearbook World Food and Agriculture 2020 ,” Rome, 2020.
- S. A. I. Al Zadjali, “Knowledge Diffusion, Pesticide Related Decision-Making and the Farming Community of Northern Sultanate of Oman,” 2014.
- J. S. K. Al-Anbari, “Understanding constraints to the development of the agricultural sector in Oman: an application of the theory of planned behaviour,” 2017.
- S. Noorizadeh, M. Golmohammadi, A. Bagheri, and A. Bertaccini, “Citrus industry: Phytoplasma-associated diseases and related challenges for Asia, America and Africa,” Crop Prot., vol. 151, p. 105822, Jan. 2022. [CrossRef]
- J. M. Bove And M. Garnier, “Major Diseases And Pathogens Of Citrus In The Mediterranean Region And Western Asia: Today And Tomorrow,” Phytopathol. Union, P. 1, 1997.
- K.-H. Lin and K.-H. Lin, “The citrus huang lung bin (greening) disease in China,” in Rehabilitation of Citrus Industry in the Asia Pacific Region. Proc. Asia Pacific International Conference on Citriculture, Chiang Mai, Thailand, 1990, vol. 4, no. 10, pp. 1–26.
- D. Y-p, “4.12 Genetic Diversity of Candidatus Liberibacter asiaticus and Ca. L. Americanus Based on Sequence Variations of Their rrna Operon,” 2008. [CrossRef]
- P. Donkersley, F. W. S. Silva, C. M. Carvalho, A. M. Al-Sadi, and S. L. Elliot, “Biological, environmental and socioeconomic threats to citrus lime production,” J. Plant Dis. Prot. 2018 1254, vol. 125, no. 4, pp. 339–356, Mar. 2018. [CrossRef]
- A. Al Fahdi et al., “Characterization of Huanglongbing disease associated with acid lime (Citrus aurantifolia Swingle) in Oman,” J. Plant Pathol., vol. 100, no. 3, pp. 419–427, Oct. 2018. [CrossRef]
- D. K. Ghosh, M. Motghare, and S. Gowda, “Citrus Greening : Overview of the Most Severe Disease of Citrus,” Adv. Agric. Res. Technol. J. N, 2018.
- D. Djeddour and I. Rwomushana, “The Asian Citrus Greening Disease (Huanglongbing): Evidence Note on Invasiveness and Potential Economic Impacts for East Africa,” 2021. [CrossRef]
- H. Fielder et al., “A Synoptic Review of Plant Disease Epidemics and Outbreaks Published in 2022,” Phytopathology, vol. 114, no. 8, pp. 1717–1732, Aug. 2024. [CrossRef]
- J. Menezes, R. Dharmalingam, and P. Shivakumara, “HLB Disease Detection in Omani Lime Trees Using Hyperspectral Imaging Based Techniques,” Commun. Comput. Inf. Sci., vol. 2027 CCIS, pp. 67–81, 2024. [CrossRef]
- F. Morán, M. Herrero-Cervera, S. Carvajal-Rojas, and E. Marco-Noales, “Real-time on-site detection of the three ‘Candidatus Liberibacter’ species associated with HLB disease: a rapid and validated method,” Front. Plant Sci., vol. 14, p. 1176513, Jun. 2023. [CrossRef]
- M. Mubeen et al., “Innovative strategies for characterizing and managing huanglongbing in citrus,” World J. Microbiol. Biotechnol., vol. 40, no. 11, p. 342, Nov. 2024. [CrossRef]
- X. Martini, K. Malfa, L. L. Stelinski, F. B. Iriarte, and M. L. Paret, “Distribution, Phenology, and Overwintering Survival of Asian Citrus Psyllid (Hemiptera: Liviidae), in Urban and Grove Habitats in North Florida,” J. Econ. Entomol., vol. 113, no. 3, pp. 1080–1087, Jun. 2020. [CrossRef]
- A. Urbaneja, T. G. Grout, S. Gravena, F. Wu, Y. Cen, and P. A. Stansly, “Citrus pests in a global world,” The Genus Citrus, pp. 333–348, Jan. 2020. [CrossRef]
- D. Greco, A. Aprile, L. De Bellis, and A. Luvisi, “Diseases Caused by Xylella fastidiosa in Prunus Genus: An Overview of the Research on an Increasingly Widespread Pathogen,” Front. Plant Sci., vol. 12, p. 712452, Aug. 2021. [CrossRef]
- S. Li, F. Wu, Y. Duan, A. Singerman, and Z. Guan, “Citrus Greening: Management Strategies and Their Economic Impact,” hortscience, vol. 55, no. 5, pp. 604–612, May 2020. [CrossRef]
- A. Gomes Garcia et al., “The importance of Integrated Pest Management to flatten the huanglongbing (HLB) curve and limit its vector, the Asian citrus psyllid,” Entomol. Gen., vol. 42, pp. 349–359, 2022. [CrossRef]
- K. S. Bhairavi, S. Mazumder, and S. Deka, “Recent advances in organic pest management in citrus,” Adv. Org. Farming Crop Prod. Manag., Pp. 515–532, Nov. 2024. [CrossRef]
- T. Yin et al., “Genome-wide identification, characterization, and expression profile of NBS-LRR gene family in sweet orange (Citrus sinensis),” Gene, vol. 854, p. 147117, Feb. 2023. [CrossRef]
- J. Hang, D. Zhang, P. Chen, J. Zhang, and B. Wang, “Classification of plant leaf diseases based on improved convolutional neural network,” Sensors (Switzerland), vol. 19, no. 19, Oct. 2019. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).