1. Introduction
Indocalamus Nakai (1925: 148), ca. 23 species, is a frutescent genus of the temperate woody bamboos (i.e. the tribe Arundinarieae of Bambusoideae in Poaceae) with important economic and ecological value (Wang and Stapleton, 2006; Guo et al., 2019, 2021). It was characterized by leptomorph rhizomes, solitary branches, persistent culm sheaths, relatively large leaves with distinct transverse veins, racemose or paniculate inflorescences, pedicellate spikelets, three stamens, and two stigmas (Chao et al., 1980; Yang, 1987; Zhao and Yang, 1996; Wang and Stapleton, 2006). The genus was mainly distributed in the southern Yangtze River in China, at elevations of 300–2,400 m under evergreen broad-leaved forests, with culms used for making chopsticks and Chinese brush, and leaves generally used for weaving bamboo hats and wrapping glutinous rice in China and some adjacent countries (Chao et al., 1980; Yang, 1993; Wang and Stapleton, 2006).
Indocalamus Nakai (1925: 148), ca. 23 species, is a frutescent genus of the temperate woody bamboos (i.e. the tribe Arundinarieae of Bambusoideae in Poaceae) with important economic and ecological value (Wang and Stapleton, 2006; Guo et al., 2019, 2021). It was characterized by leptomorph rhizomes, solitary branches, persistent culm sheaths, relatively large leaves with distinct transverse veins, racemose or paniculate inflorescences, pedicellate spikelets, three stamens, and two stigmas (Chao et al., 1980; Yang, 1987; Zhao and Yang, 1996; Wang and Stapleton, 2006). The genus was mainly distributed in the southern Yangtze River in China, at elevations of 300–2,400 m under evergreen broad-leaved forests, with culms used for making chopsticks and Chinese brush, and leaves generally used for weaving bamboo hats and wrapping glutinous rice in China and some adjacent countries (Chao et al., 1980; Yang, 1993; Wang and Stapleton, 2006).
However, molecular phylogenetic studies recently indicated that Indocalamus was not monophyletic because its species were generally clustered into several lineages together with other genera [e.g., Ferrocalamus Hsueh & Keng f. in Keng & Hsueh (1982: 1), Gelidocalamus T. H. Wen (1982: 20), Yushania Keng (1957: 355), and Fargesia Franchet (1893: 1067)] (Zhou et al., 2019; Guo et al., 2019; Qin et al., 2020; Gao et al., 2022). To accelerate the process of taxonomic revision of the genus, taxonomical reassessment on some Indocalamus taxa is not only needed but obligatory. Recently, I. chouzhouensis Yi & Yang (2004: 13) was examined and synonymized with I. emeiensis C. D. Chu & C. S. Chao (1980: 25) (Gao et al., 2022).
In the study, six taxa of Indocalamus, i.e. I. cordatus T. H. Wen et Y. Zou (1991: 18), I. tessellatus (Munro) P. C. Keng (1957: 355), I. multinerus (W. T. Lin et Z. M. Wu) W. T. Lin (2000: 17), I. chebalingensis W. T. Lin (2000: 6), I. guangdongensis H. R. Zhao et Y. L. Yang (1985: 462) and its variety I. guangdongensis var. mollis H. R. Zhao et Y. L. Yang (1985: 462), were re-evaluated. Based on several field works from 2019 to 2021, herbarium specimens, micromorphological characteristics of abaxial leaf epidermis, and the phylogenetic tree by using complete chloroplast genome data, comprehensive investigations were performed, and abovel taxa were treated herein.
2. Materials and Methods
2.1. Morphological Observation
Morphological data were obtained filed investigation and based on the analysis of specimens deposited in the herbaria of Royal Botanical Gardens Kew (K), British Museum of History (BM), Nanjing University (N), Nanjing Forestry University (NFU), South China Agricultural University (CANT), Forestry School of Sichuan Province (SIFS), Sun Yat-Sen University (SYS), and Zhejiang Academy of Forestry (ZJFI), particularly digitalized type specimens at BM and K. Six related species of
Indocalamus were chosen from morphological observation (
Supplementary table S1). Some key features, e.g., culm leaf including sheath, blade, auricles and oral setae, mature culm appendage, node and its longitudinal section, and foliage leaf including sheath, auricles and oral setae, were compared and photographed with the SLR camera EOS 60D with the macro lens EF 50mm f/1.8 STM (Canon, Japan). Specimens were studies in the laboratory under a stereomicroscope. Voucher specimens were deposited in the herbarium of the College of Forestry, Jiangxi Agricultural University, China (JXAU).
2.2. Micromorphology of Abaxial Leaf Epidermis Study
Mature leaves were collected in the field and fixed in formalin-acetic acid-alcohol (FAA).We sampled 6 species (one individual per species except Indocalamus tessellatus) of Indocalamus. We used the middle portion of foliage leaf blades for all samples. Leaves fixed in FAA were cleaned by the ultrasonic cleaner CPX2800H-C (Branson, USA) with clean water and dried at room temperature before mounting on stubs. After gold sputtering with gold powder (3 nm), we photographed the samples using the scanning electron microscope NanoSEM 450 (Nova, USA). Terminology for the epidermis appendages follows Ellis (1979), Zhang et al. (2014) and Leandro et al. (2019). Voucher specimens were deposited in the herbarium of the College of Forestry, Jiangxi Agricultural University, China (JXAU).
2.3. Molecular Phylogeny
2.3.1. Taxon Sampling
Leaf material was collected from living plants from type localities in China, including provinces Chongqing, Jiangxi, and Guangdong. A total of 61 individuals were sampled, including 7 new complete chloroplast genomes of
Indocalamus in this study, 51 complete chloroplast genomes to represent the 12 major clades of the tribe Arundinarieae (
Supplementary Table S1), together with
Bambusa emeiensis Chia & Fung (1980: 211),
Buergersiochloa bambusoides Pilg. (1914: 168) and
Chusquea culeou E. Desv. (1854: 450) as the outgroups, were obtained from NCBI (National Center for Biotechnology Information, USA) and the matrix of Guo et al. (2021). Fresh leaves were collected for all samples and then rapidly dried in silica gel. Voucher specimens were deposited at JXAU.
2.3.2. DNA Extraction, Library Preparation and Data Assembly
Total genomic DNA was extracted from fresh leaves by a modified cetyl-trimethylammonium bromide (CTAB) method (Doyle and Doyle, 1987). Two Illumina paired-end libraries were prepared and sequenced at Novogene Bioinformatics Technology Co. Ltd. (Beijing, China), and 6 GB raw data for each taxon with an average read length of 150 bp were acquired. All contigs of two chloroplast genome sequences were spliced and assembled using SPAdes 3.13.0 (Bankevich et al. 2012). Then, complete chloroplast genomes were annotated using CPGAVAS2 (Shi et al. 2019) and Geneious (Matthew et al. 2012), and finally uploaded to National Center for Biotechnology Information.
2.3.3. Data Matrices and Phylogenetic Analyses
To understand the phylogenetic relationships among 5 species and 1 variant within the
Indocalamus, we selected and reannotated 51 complete chloroplast genomes to represent the 12 major clades of the tribe Arundinarieae, together with 3 outgroups (
Supplementary table S1).
Maximum Likelihood (ML) and Bayesian Inference (BI) analyses were performed to infer the phylogenetic relationships. ML analyses with rapid bootstrapping and 1000 replications (IQ-TREE; Nguyen et al. 2014) were conducted by using complete chloroplast sequences under the TVM+I+G model as recommended by jModelTest v2.1.4 (Darriba et al. 2012). With the same model, BI analyses were conducted in MrBayes v3.2.6 (Ronquist et al. 2012) using 2 runs of 4 chains each, and run for ten million generations with the first 5,000 trees discarded as burn-in.
3. Results and Discussion
3.1. Morphological Comparison
Species in Indocalamus are similar in some vegetative characters. Both have hollow culms, persistent culm sheathes, with the solitary branches sometimes early as thick as the main culms, and large leaves with distinct transverse veins. We found other vegetative characters that provide useful information for indentification in the field. Taxa in Indocalamus have some characters are stable in field populations, such as appurtenances on infranodal regions of young culms and culm sheaths, the relationship between blades and the main culms, the shape and size of sheath auricles , and the number of leaves on the ultimate branches. The morphological characteristics of the six species are compared and analyzed as follows.
Indocalamus guangdongensis var.
mollis H. R. Zhao et Y. L. Yang was described by Huiru Zhao and Yaling Yang, and distinguished from
I.
guangdongensis mainly with a row of densely short-villi below the midrib side of leaves (Zhao et Yang, 1985). After studying specimens from both regions and field trips to type localities (
Figure 2), we can conclude that the differences concerning the morphological characteristics of the two species are significant. The most obvious difference is the shape and form of the blade on the culm sheaths. The former has recurved, subulate, or linear blades (
Figure 3 J), and the latter has erect, ovate-lanceolate blades (
Figure 3 D). Moreover, The former has abaxially white pubescent on one side of the midrib or extending to the leaf base (
Figure 3 L), and the latter is glabrous (
Figure 3 F). The hairs on the mature culm are complete different, the former is hairless (
Figure 3 G), and the latter densely covered with white-short tomentose (
Figure 3 A). Leaf sheathes are also different.
I.
guangdongensis var.
mollis is without oral setae, leaf sheaths are smooth and hairless (
Figure 3 K), and
I.
guangdongensis is the opposite (
Figure 3 E). Therefore, there are significant differences in morphological characteristics between the two species.
Indocalamus cordatus Wen et Y. Zou distinguished from other
Indocalamus species mainly with a cordate leaf blade base (
Figure 4 A & B), and both sides are asymmetrical along the midrib (Wen, 1991). After observing the paratypes of
I.
cordatus, the character of leaf base cordate is not stable (
Figure 4 C). After observing the image of type and neotype specimens (
Figure 5 A & B), the mentioned differences fall within the variability encompassed by
I.
tessellatus. We investigated 3 groups of
Indocalamus tessellatus in Hunan, Chongqing and Jiangxi provinces, and carried out a further morphological comparison for
I.
tessellatus and
I.
cordatus. In this study, we selected the populations of Jiangxi Province with relatively complete morphological characteristics for comparison herein. Some key characters showed no essential morphological differences between the above two species. Annual mature sheathes are densely black-brown strigose (
Figure 6 B & H). The culm infranodal region is covered with white-short tomentose (
Figure 6 C & I). Lanceolate blade is erect and variable in size (
Figure 6 D & J). Auricles and oral setae absent, very short ligule, with brown hirtellous (
Figure 6 E & K). Leaf sheaths leathery, glabrous; auricles and oral setae rare or scarce (
Figure 6 F & L).
From the time of its original description until the present work,
Indocalamus multinerus had been consistently unaccepted in the previous studies, including the
Flora of China (2006); The
Iconographia Bambusoidearum Sinicarum (2008) placed
I.
multinerus in the incertae sedis taxon, but the
World Checklist of Bamboos and Rattans (2017) accepted it. Besides, It had synonymized with
I.
longiauritus Hand.-Mazz. (Xia et al., 2009). After examining type specimens (
Figure 7 A & B) kept at CANT, we found that culm sheaths and leaf sheaths, including blades, auricles, and oral setae, showed no essential morphological differences between
I.
multinerus and
I.
chebalingensis. To figure out whether
Indocalamus multinerus and
I.
chebalingensis are conspecific, we investigated the type localities, and carried out a further morphological comparison for them (
Figure 8). The results of some key characters showed no essential morphological differences between the above two species. Annual culms uniformly distributed with pressed white fur-felt (
Figure 8 B & I). Culm sheaths are both with dense reddish-brown bristles on the basal of young culms (
Figure 8 A & H), ovate-lanceolate blades are erect and hugging culms, and the smaller falcate auricles with the radiating oral setae (
Figure 8 C & J). Mature culms are nearly solid with a wall of about 3 mm in thickness and spongy filler (
Figure 8 F, 8 G & 8 M, 8 N). Besides, both of the two species have three or four leaves on the ultimate branches with weak falcate auricles and radiating oral setae (
Figure 8 D & K).
3.2. Micromorphological Comparison of Abaxial Leaf Epidermis
As a result of the scarcity of flowering collections, bamboo species identification mainly depends on vegetative features, and leaf epidermal micromorphology has proven to be useful in bamboo taxonomy (Zhang et al. 2014; Leandro et al. 2019).
The stomatal apparatuses, papillae and various hairs usually observed on the abaxial epidermis. In the same species, the characters of the rows of the stomatal apparatus between the veins, the number of papillae around the stomatal apparatus, and the categories of hairs, are no essential differences. The 6 species and 8 groups micromorphology of the abaxial leaf epidermis are analysised as follows.
The abaxial leaf blade epidermis of
Indocalamus guangdongensis var.
mollis looks messier, densely papillae are distributed ruleless (
Figure 9 G), and
I.
guangdongensis is very sparse, only have eight to ten elongated papillae overarch the stomatal apparatus (
Figure 9 H). The former with more bristles, curved microhair, and inconspicuous silica bodies, and the latter’s bristles are sparse, microhairs are straight and flat, and silica bodies are distributed in the costal and intercostal regions significant. As a result,
I.
guangdongensis var.
mollis has entirely different micromorphological characteristics of leaf epidermis from
I.
guangdongensis.
Micromorphology of the abaxial leaf blade epidermis can separate these two species adequately.
By scanning electron microscopy, micromorphological features of the abaxial leaf epidermis of 3 groups of
I.
tessellatus (
Figure 9 C, D & E) and the type locality of
I.
cordatus (
Figure 9 F) were investigated. Eight to ten elongated papillae overarch the stomatal apparatus, and short papillae are densely present. Macro-hairs and micro-hairs are few in numbers and scattered and distributed in the intercostal region. Saddle-shaped silica bodies are non-significant, and distributed in the costal and intercostal areas. As a result,
I.
cordatus has exactly the same micromorphological characteristics of leaf epidermis as
I.
tessellatus.
After comparing the micromorphological features of the abaxial leaf epidermis between the two species, we can conclude that the shape and size of the stomatal apparatuses, papillae, micro-hairs, saddle-shaped silica bodies, and prickles are not significant (
Figure 9 A & E). One to two rows of stomatal apparatuses are usually distributed between veins. Eight to ten elongated papillae lay the stomatal apparatus, and one or two short papillae are sporadically present on the base of elongated papillae. Micro-hairs are distributed in the intercostal region and with equal lengths of the basal cells and apical cells (apical cells are sleazy and brittle). Saddle-shaped silica bodies are randomization distributed in the coastal regions, as well as thick and short prickles. Morphological and micromorphological results showed that there were no significant differences between them.
3.3. Molecular Phylogenetic Relationships
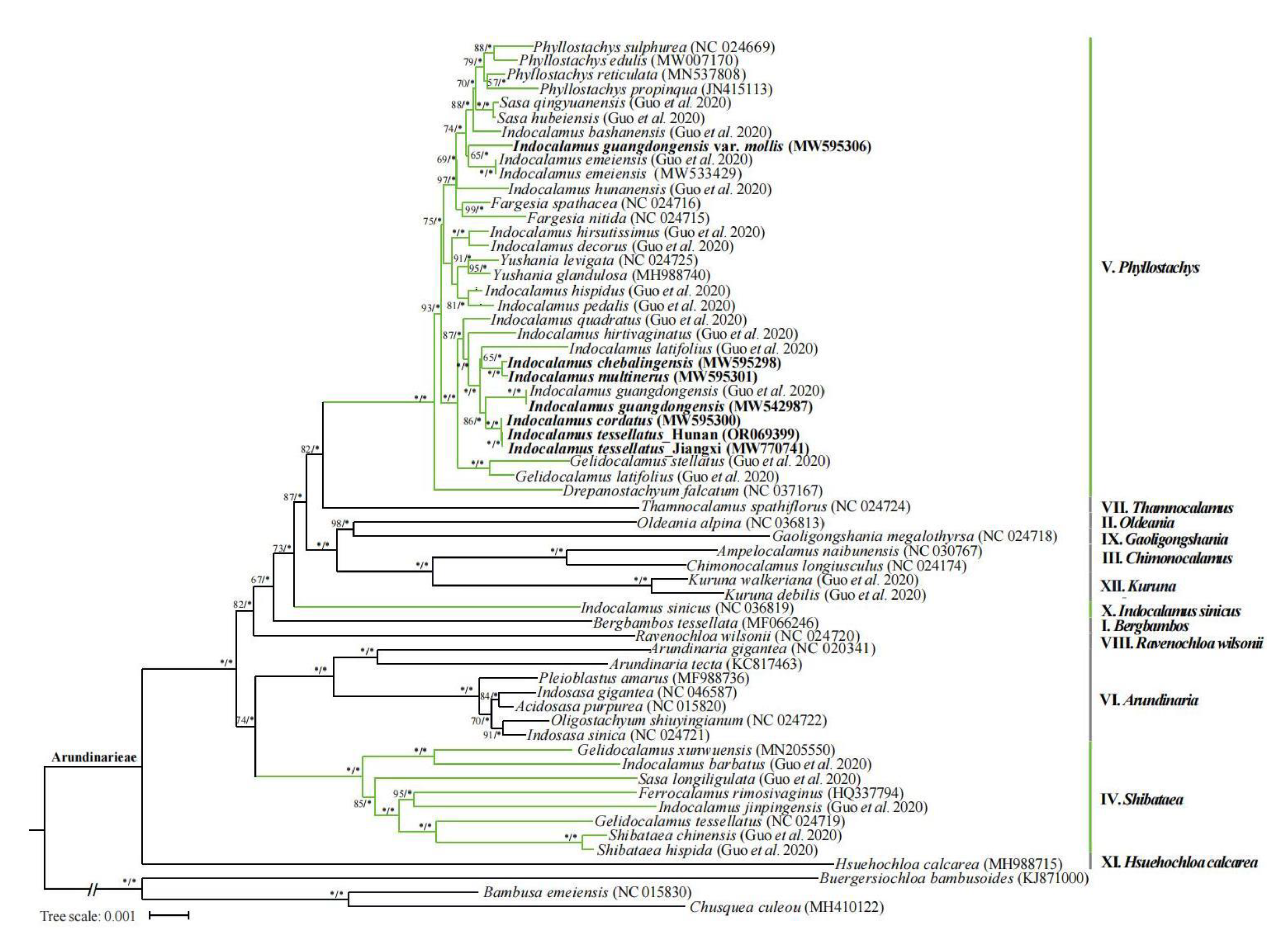
In the phylogenetic tree, this study involved 6 species and 8 groups of Indocalamus are clustered together into the Phyllostachys clade (Ⅴ), which is consistent with most Indocalamus taxa.
Indocalamus guangdongensis var.
mollis and
I.
guangdongensis are clustered together with a distant clade, which shows a lower level of homology between the two species (
Figure 10). The molecular phylogenetic relationships don’t support the two species as the same taxa. Therefore,
Indocalamus guangdongensis var.
mollis is should be a separate species.
Indocalamus cordatus and
I.
tessellatus,
I.
multinerus and
I.
chebalingensis are clustered together into the same clade with almost negligible branch length, which shows a high level of homology between these species (bootstrap value 100% in the ML analysis and posterior probability 1.0 in the BI analysis) (
Figure 10). The molecular phylogenetic relationships support that
Indocalamus cordatus is the same as
I.
tessellatus,
I.
multinerus is the same as
I.
chebalingensis, and the former taxa should be merged with the latter.
4. Taxonomic Treatment
Based on the morphological, micromorphological and molecular evidence, we here propose a new combination and two new synonyms of Indocalamus in China.
1. Indocalamus mollis (H. R. Zhao et Y. L. Yang) L. Q. Gao, W. G. Zhang et G. Y. Yang, comb. nov.
Basionym:Indocalamus guangdongensis var. mollis H. R. Zhao et Y. L. Yang in Acta Phytotaxon. Sin. 23 (6): 462. 1985.
Type: CHINA. Hunan: Yongzhou, Shuangpai, Yangming Shan, 14 May 1977, Z.P.Wang 77018 (holotype: N).
Description: Rhizomes leptomorph. Culms 0.9–2.2 m tall, 0.5–1.1 cm in diameter, hollow; internodes terete, 10–19 cm long, young culm puberulous, infranodal region white and light-brown velutinous, glabrescent when old; wall 1.5–2.5 mm thick; supranodal ridge prominently slight or flat; intranodes 4–7 mm long. Culm sheaths tardily deciduous, oblong, thinly leathery or thickly papery, subequal to internodes, densely reddish-brown strigose and aducous, margin densely reddish-brown ciliate; auricles not developed; oral setae none or several, caducous; ligule subtruncate, ca. 1–1.5 mm high, densely pubescent; blades recurved, subulate or linear. Foliage leaves 2–4 per ultimate branch; leaf sheaths smooth and hairless, margin without ciliate; leaf auricles not developed; oral setae none; ligules truncate, 2–3 mm tall, margin without ciliate; blades oblong-lanceolate, 24.0–47.0 × 5.0–9.0 cm, adaxially glabrous, abaxially white pubescent along one side of midrib and extending to leaf base; longitudinal veins 8–14 pairs, base cuneate, apex long-acuminate, margin entire. Inflorescence unknown.
Additional specimens examined: —CHINA. Guangxi: Gongcheng, Y.K.Li 402992 (N); Hunan: Yongzhou, Shuangpai, Yangming Shan, Y.Tsiang et S.H.Chen 711 (SYS!); ibid., Y.Tsiang et S.H.Chen 484 (SYS!); ibid., L.Q.Gao et al. GXYM2014 (JXAU!); Hubei: Changyang, Duzhenwan, S.Y.Wang 660 (N).
2. Indocalamus tessellatus (Munro) Keng f., Acta Phytotaxon. Sin. 6(4): 355. 1957.
箬竹 (Ruo Zhu) Figs. 1, 4–5
Indocalamus cordatus Wen et Y. Zou, J. Bamboo Res. 10(1): 18~19. f. 3. 1991. Syn. Nov.—Type: CHINA. Jiangxi, Duchang, Dagang reservoir, 10 Sep 1982, Zou Yuan 286 (paratype: ZJFI!; NFU!); Duchang, Dagang, Fengshuxia, 2 Jun 1990, T. H. Wen et S. Jin 90661 (holotype: ZJFI), syn. nov.
Type: UK. London, Kew bamboo Garden (cultivated, introduced from China), 18 Aug 1998, Stapleton 1122 (neotypes, designated by Stapleton (2000), K! [K000912169, K000912170, K000912171, K000912172]).
Additional specimens examined: —CHINA. Hunan: Ningyuan, Jiuyi Mountain, alt. 1350 m, 4 Sep. 1979, B.M.Yang 06329 (NYA!); Liuyang, Yangming Mountain, alt. 1050 m, 27 Aug. 2020, L.Q.Gao 2001 (JAXU!); Shimen, Jiashan town, alt. 650 m, 30 Aug. 2020, L.Q.Gao 2004 (JAXU!); Jiangxi: Xiushui, alt. 600 m, 13 Aug. 1994, C.M.Tan 94755 (PE!); ibid., alt. 800 m, 30 Apr. 1994, C.M.Tan 94342 (PE!); ibid., alt. 500, 5 Jun. 2016, C.M.Tan 1606985 (JJF!); Jing’an, alt. 150, 30 Jul. 2013, H.G.Ye et al. LXP10-31 (IBSC!); Fujian: Jiangle, alt. 600 m, 20 Sep. 1991, Longxi expedition 2515 (PE!); ibid., alt. 700, 16 Sep. 1991, Longxi expedition 2183 (PE!); Longyan, alt. 1185 m, 18 Jul. 2017, X.F.Zeng ZXF29072 (CZH!); ibid., alt. 929 m, 1 Jun. 2009, H.Z.Guo et al. 1822 (XMBG!); Zhejiang: Hangzhou, May 1980, Yao et Meng 80013 (HZ!); Anhui: Huoshan, alt. 200, 25 Sep. 1983, M.B.Deng 81671 (NAS!); Hubei: Jingshan, alt. 303, 6 Sep. 2005, J.Q.Wu et X.X.Yin 6736 (HIB!).
3. Indocalamus chebalingensis W. T. Lin, J. Bamboo Res. 19(1): 6. f. 1. 2000.
Indocalamus multinerus (W. T. Lin et Z. M. Wu) W. T. Lin, J. Bamboo Res. 19(4): 17. f. 1. 2000. Syn. Nov.
Basionym:Arundinaria multinervis W. T. Lin et Z. M. Wu, J. South China Agr. Univ. 11(3):48. f. 3. 1990. Type: CHINA. Guangdong, Shixing, Chebaling, 300 m, 25 June 1989, Z. M. Wu 55876 (Syntypes: CANT! [CANT00002992, CANT00002993, CANT00002994, CANT00002995, CANT00002997, CANT00002998, CANT00002999]).
Type: CHINA. Guangdong, Shixing, Chebaling, 1 July 1989, C. L. Zhang 56102 (lectotype, designated here: CANT! [CANT00002933]; isolectotypes: CANT! [CANT00002930, CANT00002931, CANT00002934]).
Additional specimens examined: — CHINA. Guangdong: Shixing, Chebaling, alt. 336 m, 26 Aug. 2020, L. Q. Gao et al. GDSX02 (JXAU!); ibid., alt. 384 m, 26 Aug. 2020, L. Q. Gao et al. GDSX02 (JXAU!); Hunan: Yongzhou, Dong’an, alt. 592 m, 27 Aug. 2020, L. Q. Gao et al. HN2032 (JXAU!).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: Liqin Gao. Investigation: Liqin Gao, Yonglong Li. Formal analysis: ChengkunWang and Jianqing Wang. Funding acquisition: Guangyao Yang and Wengen Zhang. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [31960335] and Key R & D Planned Projects of Jiangxi Province [20192BBF60015].
Data Availability Statement
Acknowledgments
We are grateful to the curators and officers of CANT, N, NJU, SIFS, SYS, and ZJFI for the permission to use their scanned images of specimens and research facilities. Thanks to Jiancheng Zhao (ZJFI), Lin Yang (SIFS), Xuehong Ma (NFU), Mingxuan Zhen (CANT), and Yanjun Pang (N) for facilitating some types of material from the respective herbaria, and also to two anonymous reviewers for the valuable comments.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
-
Bankevich, A; 455–477: , Nurk, S., Antipov, D., et al., 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 (5).
- Chao, Q.S., Chu, Z.D., 1981. New taxa and combinations of Bambusoideae in China. J. Nanjing Technol. Coll. For. Prod. 3, 33–44.
- Chao, Q.S., Chu, Z.D., Hsiung, W.Y., 1980. A revision of some genera and species of Chinese bamboos. Acta Phytotaxon. Sin. 18 (1), 24–26.
- Chia, L.Z., Fung, X.L., 1980. On the validity of the genera Sinocalamus McClure and Lingnania McClure. Acta Phytotaxon. Sin. 18 (2), 211–216.
-
Darriba, D; 772: , Taboada, G.L., Doallo, R. et al., 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Method. 9.
- Desvaux, É., 1854. Gramineae Chilenses, Historia de Chile, Flora of Chilena, Paris: Claudio Gay 6, 450, t. 83.
- Doyle, J.J., Doyle, J.L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15.
- Ellis, R.P., 1979. A procedure for standardizing comparative leaf anatomy in the Poaceae. II The epidermis as seen in surface view. Bothalia 12, 641–671.
- Franchet, M.A. Fargesia, nouveau genre de Bambuseés de la Chine. Bull. Mens. Soc. Linn. Paris 1893, 2, 1067–1069. [Google Scholar]
- Gao, L.Q., Li, Y.L., Guo, C.C., et al., 2022. Indocalamus chongzhouensis (Poaceae: Bambusoideae), a new synonym of I. emeiensis: evidence from morphology and complete chloroplast genome data. Phytotaxa, 542(1): 53–63.
- Guo, C., Guo, Z.H., Li, D.Z., 2019. Phylogenomic analyses reveal intractable evolutionary history of a temperate bamboo genus (Poaceae: Bambusoideae). Plant Divers. 41, 213–219.
- Guo, C., Ma, P.F., Yang, G.Q., et al., 2021. Parallel ddRAD and Genome Skimming Analyses Reveal a Radiative and Reticulate Evolutionary History of the Temperate Bamboos. Syst. Biol. 70 (4): 756–773.
- Keng, P.C. & Hsueh, J.R., 1982. Ferrocalamus.
- Keng, P.C., 1957. One new genus and two new species of Chinese bamboos. Acta Phytotaxon. Sin. 6 (4), 355–360.
- Keng, Y.L., 1959. Flora Illustrata Plantarum Primarum Sinicarum (Gramineae), Version 1. Beijing: Sci. Press 13–21.
- Leandro, T.D., Scatena, V.L., Clark, L.G., 2019. Comparative leaf blade anatomy and micromorphology in the systematics and phylogeny of Bambusoideae (Poaceae: Poales). Bot. J. Linn. Soc. 192 (1): 165–183.
- Lin, W.T., 2000. A new species and two new combinations of Bambusoideae. J. Bamboo Res. 19 (1), 6–8.
- Lin, W.T., 2000. Two new species and one new combinations of Bambusoideae. J. Bamboo Res. 19 (4), 1–2, 17.
- McClure, F.A., 1941. On some new and imperfectly known species of Chinese bamboos. Sunyatsenia 6, 32–35.
- Nakai, T. 1925. Two new genera of Bambusaceae, with special remarks on the related genera growing in eastern Asia. J. Arnold Arboretum 6, 145–153.
- Nguyen, L.T, Schmidt, H.A, Haeseler, A. et al., 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 (1): 268–274.
- Pilger, R., 1914. Neue und weniger bekannte Gramineen aus papuasien. Bot. Jahrb. Syst. 52: 168.
- Qin, Q.M., Tong, Y.H., Zheng, X.R., et al., 2020. Sinosasa (Poaceae: Bambusoideae), a new genus from China. Taxon 70 (1): 1–21.
- Ronquist, F., Teslenko, M., van der Mark, P., et al., 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542.
- Shi, L.C., Chen, H.M., Jiang, M., et al., 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucl. Acid. Res. 47 (W1): 65–73.
- Vorontsova, M.S., Clark, L.G., Dransfield, J., et al., 2017. World Checklist of Bamboos and Rattans[M]. Beijing: Sci. Press 106–110.
- Wang, Z.P., Stapleton, C. 2006. Indocalamus. In: Wu, Z.Y., Raven P.H., Hong, D.Y. (eds.), Flora of China. Beijing: Sci. Press; St. Louis: Mo. Botan. Gard. Press 22, 135–143.
- Wen, T.H. 1982. A new genus and some new species of Bambusoideae from China. J. Bamboo Res. 1 (1): 20–45.
- Wen, T.H., 1991. Some ideas on the taxonomy of several Bambusoideae taxonss. J. Bamboo Res. 10 (1), 11–25.
- Xia, N.H., Lin, R.S., Wang, R.H., 2009. Taxonomic notes of Bambusoideae (Poaceae) from Guangdong. J. Trop. Subtrop. Bot. 17(4), 351–354.
- Yang, Y.L. 1987. A revision of the genus Indocalamus of Bambusoideae from the world (Ⅰ). J. Nanjing Univ. (Nat. Sci.) 23 (3): 453–462.
- Yang, Y.L. 1993. The endemic genus of China: geographical distribution of Indocalamus Nakai. J. Plant Resour. Environ 2 (1): 41–44.
- Yi, T.P., Shi, J.Y., Ma, L.S., et al., 2008. Iconographia Bambusoidearum Sinicarum[M]. Beijing: Sci. Press 688–712, 728.
- Yi, T.P., Yang L. 2004. A new species of Indocalamus Nakai from western Sichuan of China. J. Bamboo Res. 23: 13–15.
- Zhang, Y.X., Zeng, C.X., Li, D.Z., 2014 Scanning electron microscopy of the leaf epidermis in Arundinarieae (Poaceae: Bambusoideae): evolutionary implications of selected micromorphological features. Bot. J. Linn. Soc. 176 (1), 46–65.
- Zhao, H.R., Yang, Y.L., 1985. New taxa and new combinations of Indocalamus from China. Acta Phytotaxon. Sin. 23 (6), 460–465.
- Zhao, H.R., Yang, Y.L., 1996. Bambusoideae. In: Keng, P.C. & Wang, Z.P. (eds.), Flora Reipublicae Popularis Sinicae. Beijing: Sci. Press 9 (1), 676–704.
- Zhou, Y., Zhang, Y.Q., Hou, X.Q., et al., 2019. Phylogeny of Fargesia (Poaceae: Bambusoideae) and infrageneric adaptive divergence inferred from three cpDNA and nrITS sequence data. Plant Syst. Evol. 305: 61–75.
Figure 2.
Holotype of Indocalamus guagndongensis (Z. P. Wang 780025, N!).
Figure 2.
Holotype of Indocalamus guagndongensis (Z. P. Wang 780025, N!).
Figure 3.
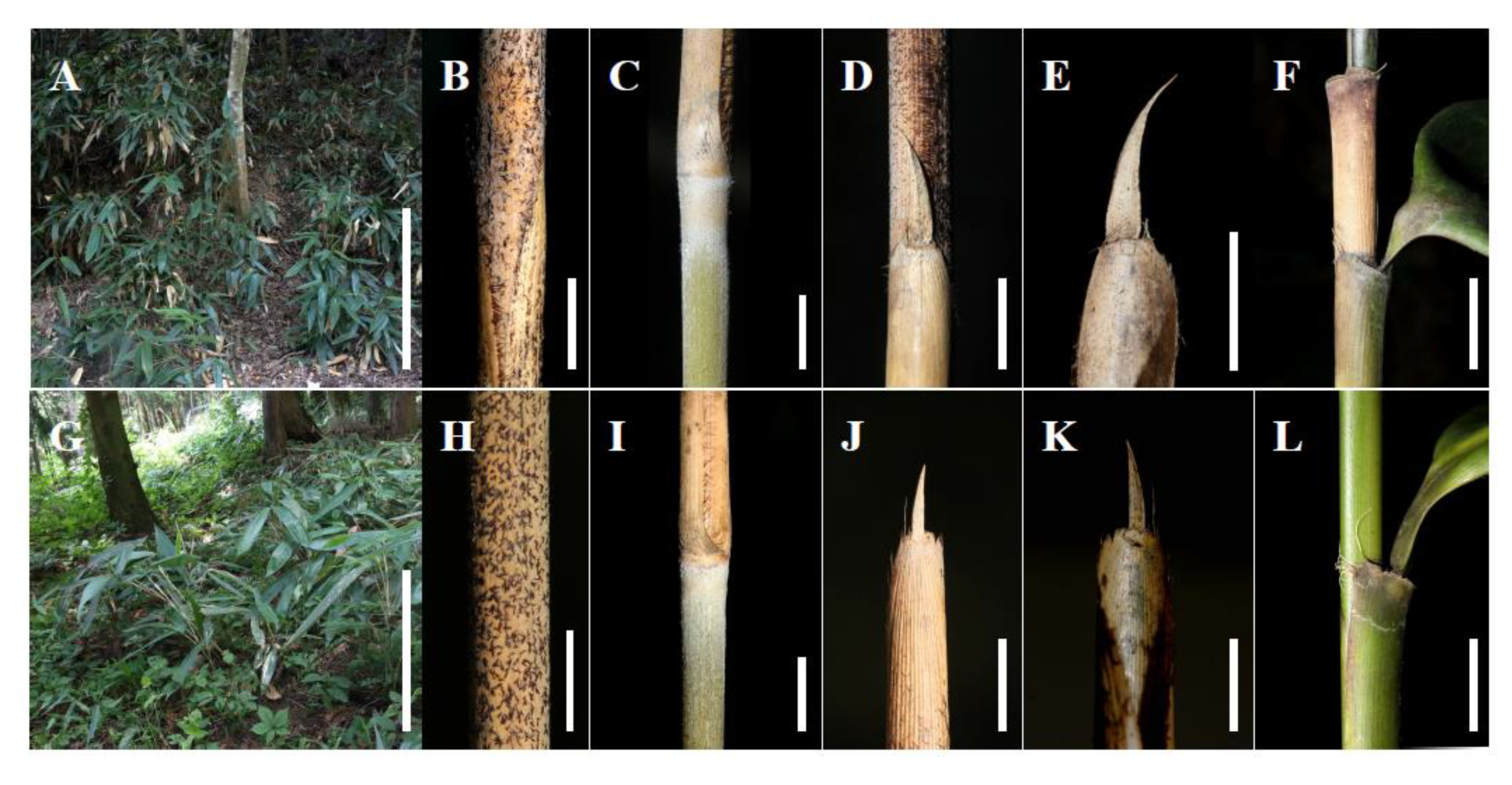
Morphological comparison between Indocalamus guangdongensis (A–F) and I. guangdongensis var. mollis (G–L). A and G. Part of annual mature culm, showing appendages on the surface of the culm; B and H. Part of biennial mature culm, showing branch, supranodal ridge; C and I. Part of annual mature culm, showing basal warty setae on culm sheath and appendages on the infranodal region; D and J. Blade; E and K. Part of leaf sheath, showing ligule and oral setae; F and L. Part of annual mature blade, showing leaf base, back, and hair. (Scale bars=1 cm).
Figure 3.
Morphological comparison between Indocalamus guangdongensis (A–F) and I. guangdongensis var. mollis (G–L). A and G. Part of annual mature culm, showing appendages on the surface of the culm; B and H. Part of biennial mature culm, showing branch, supranodal ridge; C and I. Part of annual mature culm, showing basal warty setae on culm sheath and appendages on the infranodal region; D and J. Blade; E and K. Part of leaf sheath, showing ligule and oral setae; F and L. Part of annual mature blade, showing leaf base, back, and hair. (Scale bars=1 cm).
Figure 4.
Indocalamus cordatus. A. The holotype ink diagram; B-C. Paratypes (Y. Zou 286, ZJFI! and NF!). (Scale bars=1 cm).
Figure 4.
Indocalamus cordatus. A. The holotype ink diagram; B-C. Paratypes (Y. Zou 286, ZJFI! and NF!). (Scale bars=1 cm).
Figure 5.
Indocalamus tessellatus. A. Type (Munro s.n., BM000959209 [image!]); B. Neotype (Stapleton 1122, K000912169 [image!]). (Scale bars=1 cm).
Figure 5.
Indocalamus tessellatus. A. Type (Munro s.n., BM000959209 [image!]); B. Neotype (Stapleton 1122, K000912169 [image!]). (Scale bars=1 cm).
Figure 6.
Morphological comparison between Indocalamus cordatus (A–F) and I. tessellatus (G–L). A and G. Habitat and habit; B and H. Culm sheath, showing basal warty setae; C and I. Part of annual culm, showing white felt wool infranodal region; D and J. Sheath auricle and oral setae; E and K. Blade and ligule; F and L. Part of ultimate leafy branch, showing leaf sheath, micro auricles, and oral setae. (Scale bars=1 cm).
Figure 6.
Morphological comparison between Indocalamus cordatus (A–F) and I. tessellatus (G–L). A and G. Habitat and habit; B and H. Culm sheath, showing basal warty setae; C and I. Part of annual culm, showing white felt wool infranodal region; D and J. Sheath auricle and oral setae; E and K. Blade and ligule; F and L. Part of ultimate leafy branch, showing leaf sheath, micro auricles, and oral setae. (Scale bars=1 cm).
Figure 7.
A. Lectotype of Indocalamus chebalingensis (C. L. Zhang 56102, CANT!); B. Syntype of I. multinerus (Z. M. Wu 55876, CANT!). (Scale bars=1 cm).
Figure 7.
A. Lectotype of Indocalamus chebalingensis (C. L. Zhang 56102, CANT!); B. Syntype of I. multinerus (Z. M. Wu 55876, CANT!). (Scale bars=1 cm).
Figure 8.
Morphological comparison between Indocalamus chebalingensis (A–G) and I. multinerus (H–N). A and H. Part of annual young culm, showing white felt wool infranodal region; B and I. Part of annual mature culm, showing white powdery short hair; C and J. Blade, ligule, sheath auricle, and oral setae; D and K. Part of ultimate leafy branch, showing leaf sheath, auricles, and oral setae; E and L. Part of mature culm, showing branch; F and M. Part of mature culm, cross section; G and N. Part of mature culm, longitudinal section. (Scale bars=1 cm).
Figure 8.
Morphological comparison between Indocalamus chebalingensis (A–G) and I. multinerus (H–N). A and H. Part of annual young culm, showing white felt wool infranodal region; B and I. Part of annual mature culm, showing white powdery short hair; C and J. Blade, ligule, sheath auricle, and oral setae; D and K. Part of ultimate leafy branch, showing leaf sheath, auricles, and oral setae; E and L. Part of mature culm, showing branch; F and M. Part of mature culm, cross section; G and N. Part of mature culm, longitudinal section. (Scale bars=1 cm).
Figure 9.
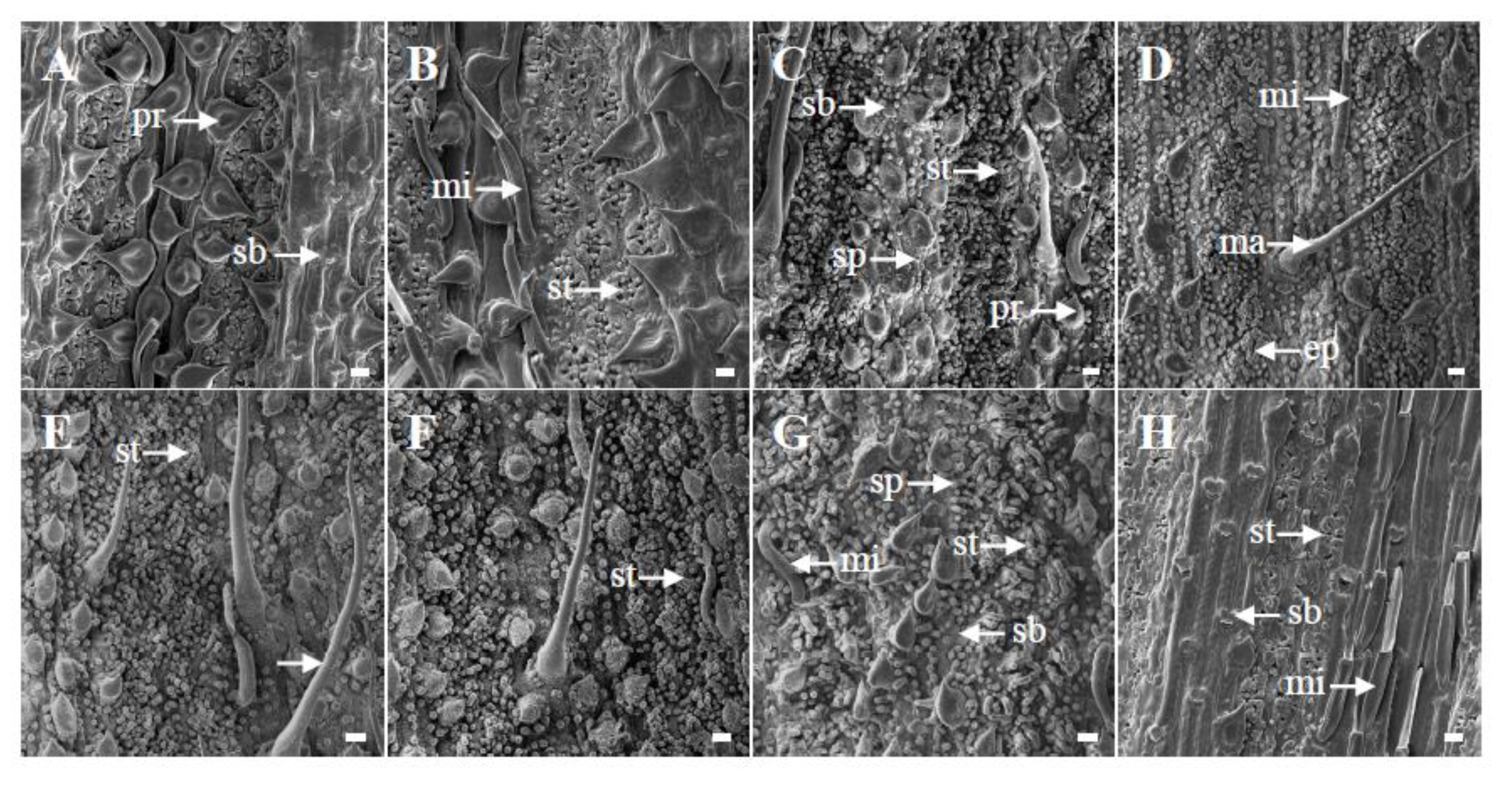
Micromophology of leaf abaxial epidermis under SEM. A. Indocalamus chebalingensis (Chebaling, Guangdong, China); B. I. multinerus (Chebaling, Guangdong, China); C. I. tessellatus (Daweishan, Hunan, China); D. I. tessellatus (Xiushan, Chongqing, China); E. I. tessellatus (Nanchang, Jiangxi, China); F. I. cordatus (DuChang, Jiangxi, China); G. I. guangdongensis var. mollis (Yangmingshan, Hunan, China); H. I. guangdongensis (Futang, Guangdong, China). Abbreviations: ep, elongated papillae; sp, short papillae; ma, macrohairs; mi, microhairs; pr, prickles; sb, silica bodies; st, stomatal apparatuses. (Scale bar =10 μm).
Figure 9.
Micromophology of leaf abaxial epidermis under SEM. A. Indocalamus chebalingensis (Chebaling, Guangdong, China); B. I. multinerus (Chebaling, Guangdong, China); C. I. tessellatus (Daweishan, Hunan, China); D. I. tessellatus (Xiushan, Chongqing, China); E. I. tessellatus (Nanchang, Jiangxi, China); F. I. cordatus (DuChang, Jiangxi, China); G. I. guangdongensis var. mollis (Yangmingshan, Hunan, China); H. I. guangdongensis (Futang, Guangdong, China). Abbreviations: ep, elongated papillae; sp, short papillae; ma, macrohairs; mi, microhairs; pr, prickles; sb, silica bodies; st, stomatal apparatuses. (Scale bar =10 μm).
Figure 10.
Phylogeny of Arundinarieae inferred from maximum likelihood (ML) analysis based on complete chloroplast genomes of 61 representative bamboos. Branches indicate 11 accepted lineages of Arundinarieae (I–XI). Numbers associated with branches indicate the bootstrap value and the posterior probability, respectively. Asterisks indicate 100% bootstrap support or 1.0 posterior probability.
Figure 10.
Phylogeny of Arundinarieae inferred from maximum likelihood (ML) analysis based on complete chloroplast genomes of 61 representative bamboos. Branches indicate 11 accepted lineages of Arundinarieae (I–XI). Numbers associated with branches indicate the bootstrap value and the posterior probability, respectively. Asterisks indicate 100% bootstrap support or 1.0 posterior probability.
Figure 1.
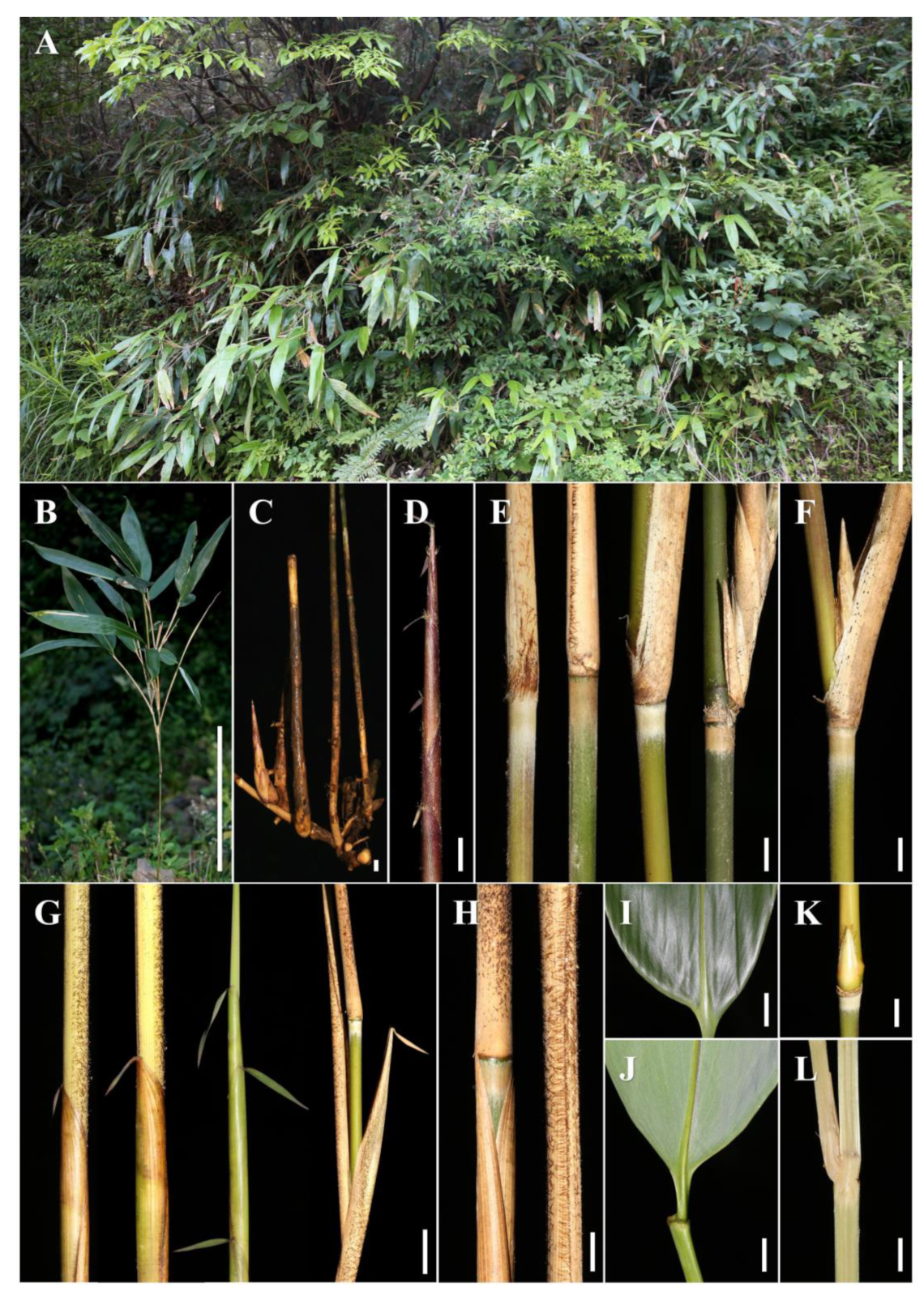
Indocalamus mollis. A and B. Habitat and plants; C and D. Rhizome and new shoot; E. Infranodal region of mature culm; F. Branch; G and H. Culm sheath, blade and ligule; I and J. Leaf; K. Branching node and bud; L. Longitudinal section of branching node. Scale bars=1 m [A & B]; 1 cm [C–L].
Figure 1.
Indocalamus mollis. A and B. Habitat and plants; C and D. Rhizome and new shoot; E. Infranodal region of mature culm; F. Branch; G and H. Culm sheath, blade and ligule; I and J. Leaf; K. Branching node and bud; L. Longitudinal section of branching node. Scale bars=1 m [A & B]; 1 cm [C–L].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).