Submitted:
21 January 2025
Posted:
21 January 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Phenomenon of Cryogel Synthesis
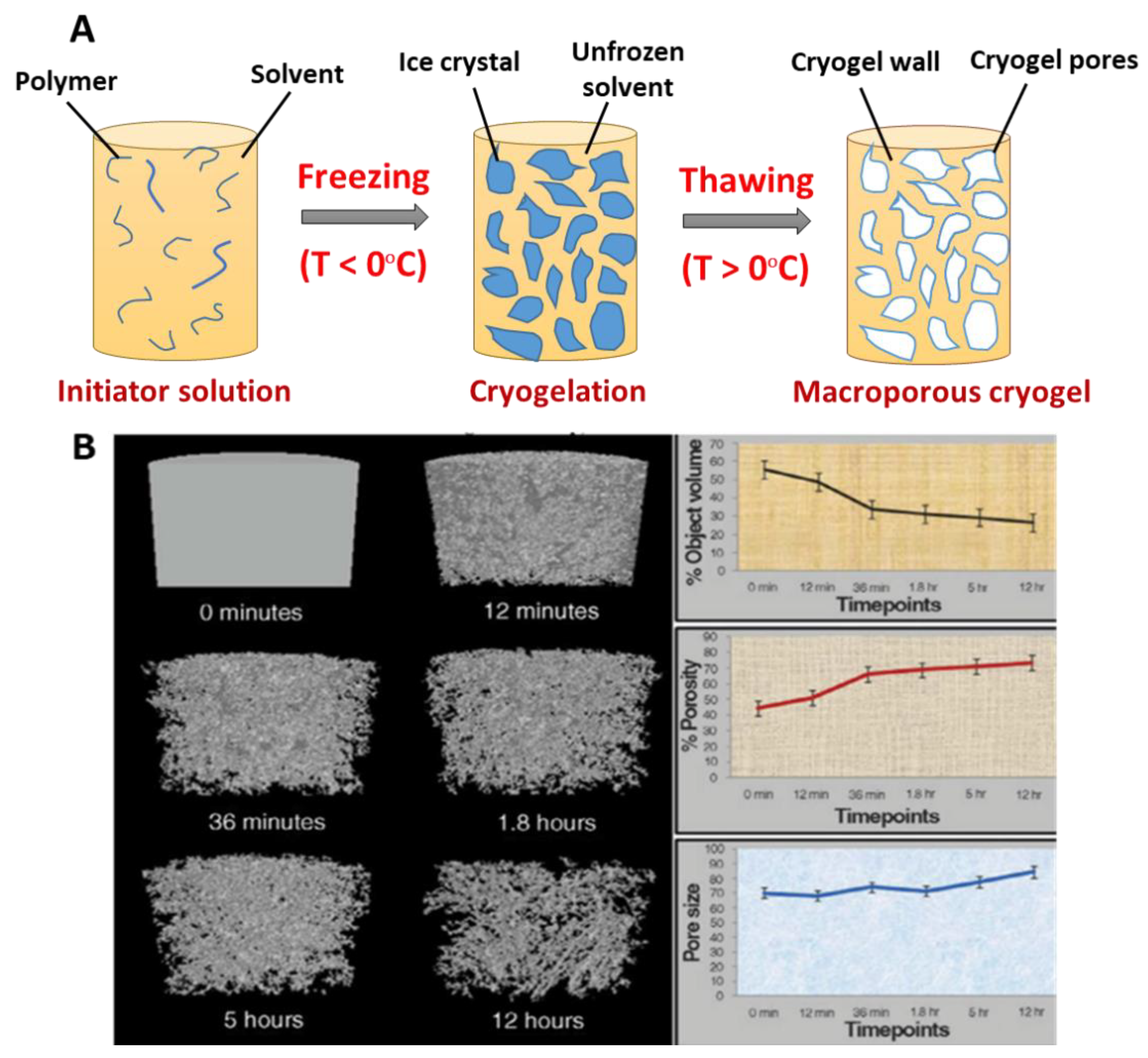
2.1. Freezing of Precursor Solutions and Phase Separation with Ice Formation
2.2. Incubation Under Frozen Condition, Cryo-Concentration and Polymerization
2.3. Thawing and Formation of Interconnected Pore network
3. Crosslinking Mechanisms for Cryogels
4. Factors Affecting Cryotropic Gel Formation
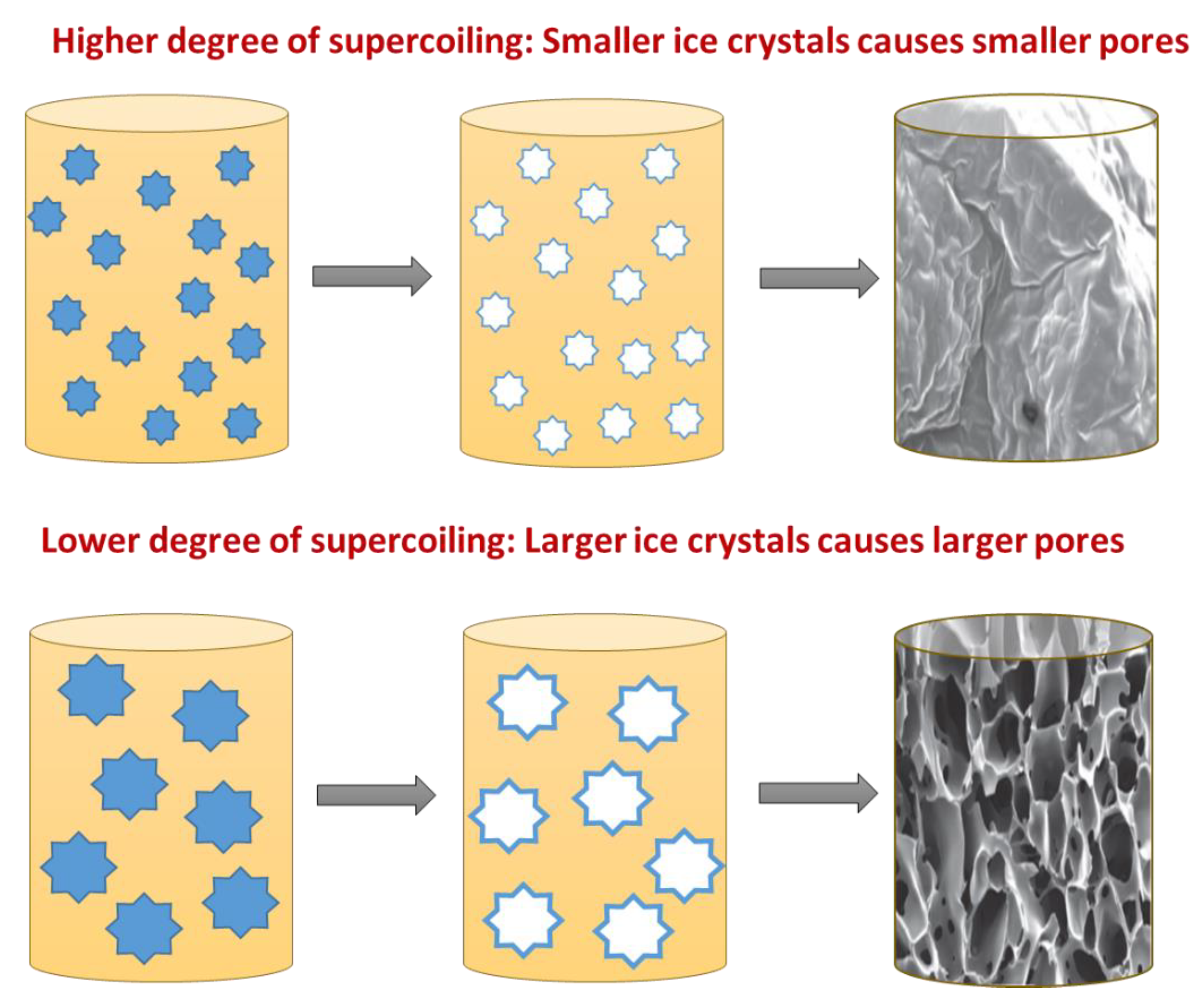
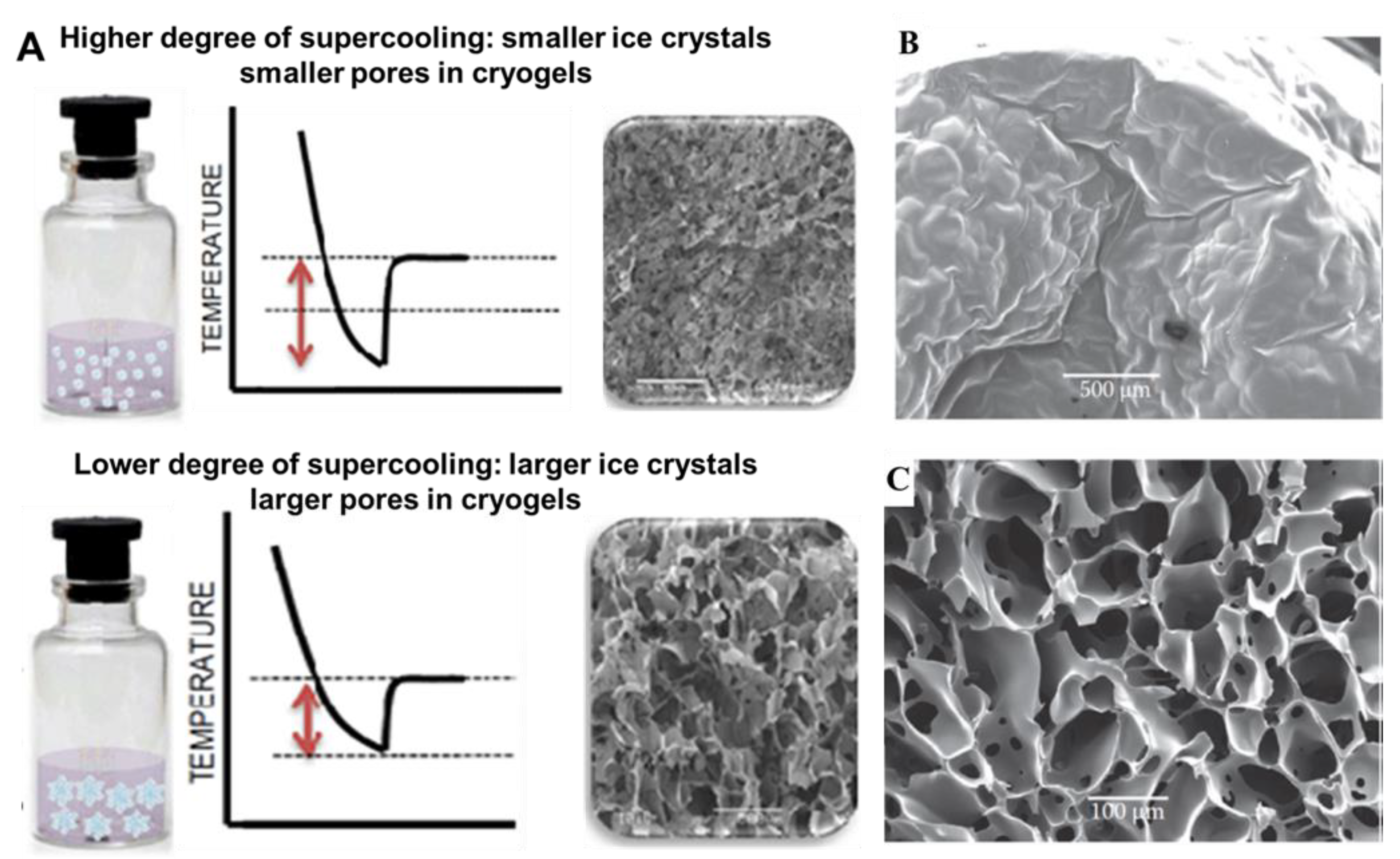
4.1. Ice Nucleation and Crystal Growth
4.2. Effect of Solvent
4.3. Effect of Temperature
4.4. Rate of Thawing
4.5. Effects of Added Solutes
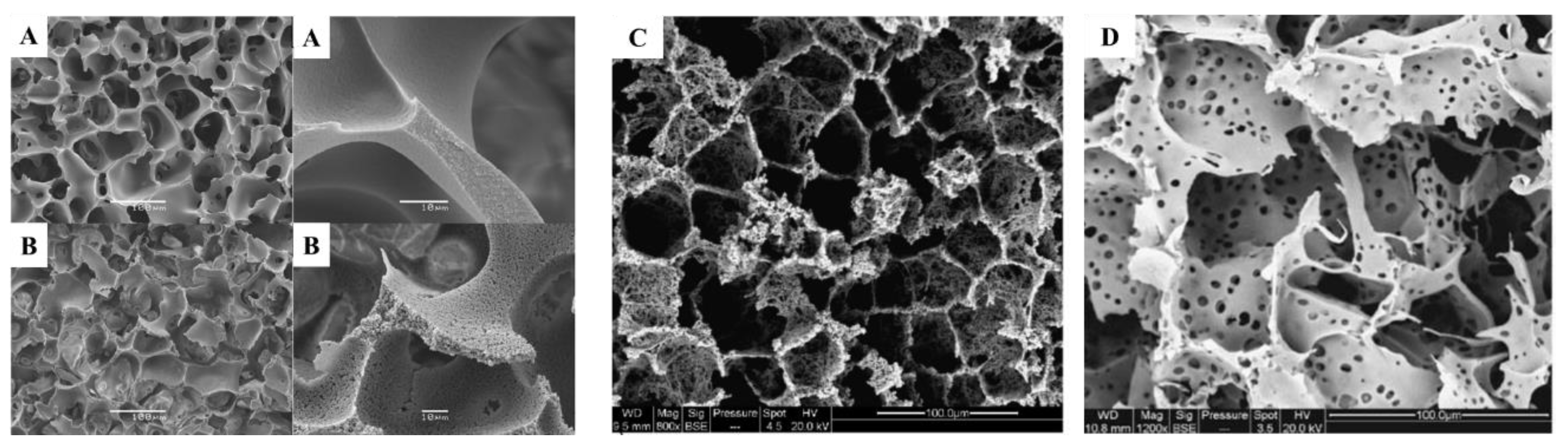
4.6. Precursor Composition
5. Conclusions and future directions
Author Contributions
Funding
Data Availability
Conflicts of Interest
References
- Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14:23-36.
- Petkovich ND, Stein A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chemical Society reviews. 2013;42:3721-39.
- Chen W, Gan L, Huang J. Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial. Biomimetics (Basel, Switzerland). 2023;8.
- Sai H, Tan KW, Hur K, Asenath-Smith E, Hovden R, Jiang Y, et al. Hierarchical porous polymer scaffolds from block copolymers. Science. 2013;341:530-4.
- Loh QL, Choong C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng Part B-Re. 2013;19:485-502.
- Ma, PX. Scaffolds for tissue fabrication. Mater Today. 2004;7:30-40.
- Górnicki T, Lambrinow J, Golkar-Narenji A, Data K, Domagała D, Niebora J, et al. Biomimetic Scaffolds—A Novel Approach to Three Dimensional Cell Culture Techniques for Potential Implementation in Tissue Engineering. Nanomaterials. 2024;14:531.
- Çimen D, Özbek MA, Bereli N, Mattiasson B, Denizli A. Injectable Functional Polymeric Cryogels for Biological Applications. Biomedical Materials & Devices. 2024:1-11.
- Andres M, Robertson E, Hall A, McBride-Gagyi S, Sell S. Controlled pore anisotropy in chitosan-gelatin cryogels for use in bone tissue engineering. Journal of Biomaterials Applications. 2024:08853282231222324.
- Xiang Y, Yan J, Bao X, Gleadall A, Sun T. Investigation of cell infiltration and colonization in 3D porous scaffolds via integrated experimental and computational strategies. Journal of Biotechnology. 2024;382:78-87.
- Garg T, Singh O, Arora S, Murthy RSR. Scaffold: A Novel Carrier for Cell and Drug Delivery. Crit Rev Ther Drug. 2012;29:1-63.
- Shih TY, Blacklow SO, Li AW, Freedman BR, Bencherif S, Koshy ST, et al. Injectable, Tough Alginate Cryogels as Cancer Vaccines. Adv Healthc Mater. 2018;7.
- Mi HY, Jing X, Turng LS. Fabrication of porous synthetic polymer scaffolds for tissue engineering. J Cell Plast. 2015;51:165-96.
- Pelin G, Sonmez M, Pelin C-E. The Use of Additive Manufacturing Techniques in the Development of Polymeric Molds: A Review. Polymers. 2024;16:1055.
- Chen D, Yang B, Yang C, Wu J, Zhao Q. Macroporous Hydrogels Prepared By Ice Templating: Developments And Challenges. Chinese Journal of Chemistry. 2023;41:3082-96.
- Hollister, SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518-24.
- Deville, S. Ice-templating, freeze casting: Beyond materials processing. J Mater Res. 2013;28:2202-19.
- Deville S, Maire E, Lasalle A, Bogner A, Gauthier C, Leloup J, et al. Influence of Particle Size on Ice Nucleation and Growth During the Ice-Templating Process. J Am Ceram Soc. 2010;93:2507-10.
- Deville S, Saiz E, Nalla RK, Tomsia AP. Freezing as a path to build complex composites. Science. 2006;311:515-8.
- Deville S, Saiz E, Tomsia AP. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials. 2006;27:5480-9.
- Joukhdar H, Seifert A, Jüngst T, Groll J, Lord MS, Rnjak-Kovacina J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Advanced materials (Deerfield Beach, Fla). 2021;33:e2100091.
- Kumar A, Mishra R, Reinwald Y, Bhat S. Cryogels: Freezing unveiled by thawing. Mater Today. 2010;13:42-4.
- Saylan Y, Denizli A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels. 2019;5.
- Shur YL, Jorgenson MT. Patterns of permafrost formation and degradation in relation to climate and ecosystems. Permafrost Periglac. 2007;18:7-19.
- Lozinsky, V.I. Polymeric cryogels as a new family of macroporous and supermacroporous materials for biotechnological purposes. Russian Chemical Bulletin. 2008;57:1015-32.
- Joukhdar H, Seifert A, Jüngst T, Groll J, Lord MS, Rnjak-Kovacina J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Advanced Materials. 2021;33:2100091.
- Kirsebom H, Mattiasson B, Galaev IY. Building macroporous materials from microgels and microbes via one-step cryogelation. Langmuir : the ACS journal of surfaces and colloids. 2009;25:8462-5.
- Zhang H, Lee JY, Ahmed A, Hussain I, Cooper AI. Freeze-align and heat-fuse: microwires and networks from nanoparticle suspensions. Angewandte Chemie. 2008;47:4573-6.
- Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B. Polymeric cryogels as promising materials of biotechnological interest. Trends in Biotechnology. 2003;21:445-51.
- Attwater J, Wochner A, Pinheiro VB, Coulson A, Holliger P. Ice as a protocellular medium for RNA replication. Nat Commun. 2010;1.
- Liu RH, Orgel LE. Efficient oligomerization of negatively-charged beta-amino acids at -20 degrees C. J Am Chem Soc. 1997;119:4791-2.
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Uspekhi Khimii. 2002;71:559-85.
- Kirsebom H, Rata G, Topgaard D, Mattiasson B, Galaev IY. Mechanism of Cryopolymerization: Diffusion-Controlled Polymerization in a Nonfrozen Microphase. An NMR Study. Macromolecules. 2009;42:5208-14.
- Pincock, R.E. Reactions in Frozen Systems. Accounts Chem Res. 1969;2:97-&.
- Bruice TC, Butler AR. Ionic Reactions in Frozen Aqueous Systems. Federation proceedings. 1965;24:S45-9.
- Lozinsky VI, Plieva FM, Galaev IY, Mattiasson B. The potential of polymeric cryogels in bioseparation. Bioseparation. 2001;10:163-88.
- Carvalho BMA, Da Silva SL, Da Silva LHM, Minim VPR, Da Silva MCH, Carvalho LM, et al. Cryogel Poly(acrylamide): Synthesis, Structure and Applications. Sep Purif Rev. 2014;43:241-62.
- Kirsebom H, Topgaard D, Galaev IY, Mattiasson B. Modulating the porosity of cryogels by influencing the nonfrozen liquid phase through the addition of inert solutes. Langmuir. 2010;26:16129-33.
- Lozinsky VI, Vainerman ES, Titova EF, Belavtseva EM, Rogozhin SV. Study of Cryostructurization of Polymer Systems.4. Cryostructurization of the System - Solvent Vinyl Monomer Divinyl Monomer Initiator of Polymerization. Colloid and Polymer Science. 1984;262:769-74.
- Gusev DG, Lozinsky VI, Bakhmutov VI. Study of Cryostructurization of Polymer Systems.10. H-1-Nmr and H-2-Nmr Studies of the Formation of Cross-Linked Polyacrylamide Cryogels. European Polymer Journal. 1993;29:49-55.
- Lozinsky VI, Domotenko LV, Zubov AL, Simenel IA. Study of cryostructuration of polymer systems.12. Poly(vinyl alcohol) cryogels: Influence of low-molecular electrolytes. Journal of Applied Polymer Science. 1996;61:1991-8.
- Tripathi A, Kathuria N, Kumar A. Elastic and macroporous agarose-gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. Journal of Biomedical Materials Research Part A. 2009;90a:680-94.
- Kathuria N, Tripathi A, Kar KK, Kumar A. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 2009;5:406-18.
- Dainiak MB, Galaev IY, Kumar A, Plieva FM, Mattiasson B. Chromatography of living cells using supermacroporous hydrogels, cryogels. Adv Biochem Eng Biot. 2007;106:101-27.
- Lin TW, Hsu SH. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Adv Sci (Weinh). 2020;7:1901388.
- Lin T-W, Hsu S-h. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Advanced Science. 2020;7:1901388.
- Yetiskin B, Okay O. High-strength silk fibroin scaffolds with anisotropic mechanical properties. Polymer. 2017;112:61-70.
- Yetiskin B, Okay O. High-strength and self-recoverable silk fibroin cryogels with anisotropic swelling and mechanical properties. International Journal of Biological Macromolecules. 2019;122:1279-89.
- Cheng Q-P, Hsu S-h. A self-healing hydrogel and injectable cryogel of gelatin methacryloyl-polyurethane double network for 3D printing. Acta Biomaterialia. 2023;164:124-38.
- Bilici C, Altunbek M, Afghah F, Tatar AG, Koc B. Embedded 3D Printing of Cryogel-Based Scaffolds. ACS Biomater Sci Eng. 2023;9:5028-38.
- Zhang X, Hang Y, Ding Z, Xiao L, Cheng W, Lu QJB. Macroporous silk nanofiber cryogels with tunable properties. J Am Chem Soc. 2022;23:2160-9.
- Petrov PD, Tsvetanov CB. Cryogels via UV Irradiation. In: Okay O, editor. Polymeric Cryogels: Macroporous Gels with Remarkable Properties. Cham: Springer International Publishing; 2014. p. 199-222.
- Reichelt S, Abe C, Hainich S, Knolle W, Decker U, Prager A, et al. Electron-beam derived polymeric cryogels. Soft Matter. 2013;9:2484-92.
- Haleem A, Chen S, Pan J, Weidong H. Gamma radiation induced synthesis of double network hydrophilic cryogels at low pH loaded with AuNPs for fast and efficient degradation of Congo red. Journal of Hazardous Materials Advances. 2023;10:100299.
- Lozinsky VI, Sakhno NG, Damshkaln LG, Bakeeva IV, Zubov VP, Kurochkin IN, et al. Study of cryostructuring of polymer systems: 31. Effect of additives of alkali metal chlorides on physicochemical properties and morphology of poly(vinyl alcohol) cryogels. Colloid J+. 2011;73:234-43.
- Rodionov IA, Grinberg NV, Burova TV, Grinberg VY, Lozinsky VI. Cryostructuring of polymer systems. Proteinaceous wide-pore cryogels generated by the action of denaturant/reductant mixtures on bovine serum albumin in moderately frozen aqueous media. Soft Matter. 2015;11:4921-31.
- Cai Z, Tang Y, Wei Y, Wang P, Zhang H. Physically Cross-Linked Hyaluronan-Based Ultrasoft Cryogel Prepared by Freeze–Thaw Technique as a Barrier for Prevention of Postoperative Adhesions. Biomacromolecules. 2021;22:4967-79.
- Cai Z, Zhang F, Wei Y, Zhang H. Freeze–Thaw-Induced Gelation of Hyaluronan: Physical Cryostructuration Correlated with Intermolecular Associations and Molecular Conformation. Macromolecules. 2017;50:6647-58.
- Lozinsky VI, Damshkaln LG, Brown R, Norton IT. Study of cryostructuring of polymer systems. XIX. On the nature of intermolecular links in the cryogels of locust bean gum. Polymer International. 2000;49:1434-43.
- Lozinsky VI, Damshkaln LG, Brown R, Norton IT. Study of cryostructuration of polymer systems. XXI. Cryotropic gel formation of the water-maltodextrin systems. Journal of Applied Polymer Science. 2002;83:1658-67.
- Soradech S, Williams AC, Khutoryanskiy VV. Physically cross-linked cryogels of linear polyethyleneimine: influence of cooling temperature and solvent composition. Macromolecules. 2022;55:9537-46.
- Takei T, Danjo S, Sakoguchi S, Tanaka S, Yoshinaga T, Nishimata H, et al. Autoclavable physically-crosslinked chitosan cryogel as a wound dressing. J Biosci Bioeng. 2018;125:490-5.
- Dragan ES, Dinu MV, Ghiorghita CA. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels2022.
- Pita-López ML, Fletes-Vargas G, Espinosa-Andrews H, Rodríguez-Rodríguez R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. European Polymer Journal. 2021;145:110176.
- Berillo D, Mattiasson B, Kirsebom H. Cryogelation of Chitosan Using Noble-Metal Ions: In Situ Formation of Nanoparticles. Biomacromolecules. 2014;15:2246-55.
- Takei T, Danjo S, Sakoguchi S, Tanaka S, Yoshinaga T, Nishimata H, et al. Autoclavable physically-crosslinked chitosan cryogel as a wound dressing. Journal of bioscience and bioengineering. 2018;125:490-5.
- Jain E, Kumar A. Designing supermacroporous cryogels based on polyacrylonitrile and a polyacrylamide-chitosan semi-interpenetrating network. Journal of biomaterials science Polymer edition. 2009;20:877-902.
- Srivastava A, Kumar A. Synthesis and characterization of a temperature-responsive biocompatible poly(N-vinylcaprolactam) cryogel: a step towards designing a novel cell scaffold. J Biomater Sci Polym Ed. 2009;20:1393-415.
- Jain E, Damania A, Shakya AK, Kumar A, Sarin SK, Kumar A. Fabrication of macroporous cryogels as potential hepatocyte carriers for bioartificial liver support. Colloid Surface B. 2015;136:761-71.
- Hwang Y, Sangaj N, Varghese S. Interconnected macroporous poly(ethylene glycol) cryogels as a cell scaffold for cartilage tissue engineering. Tissue Eng Part A. 2010;16:3033-41.
- Jain A, Bajpai J, Bajpai AK, Mishra A. Thermoresponsive cryogels of poly(2-hydroxyethyl methacrylate-co-N-isopropyl acrylamide) (P(HEMA-co-NIPAM)): fabrication, characterization and water sorption study. Polymer Bulletin. 2020;77:4417-43.
- Tonta MM, Sahin ZM, Cihaner A, Yilmaz F, Gurek A. Synthesis of Polyacrylamide-Based Redox Active Cryogel Using Click Chemistry and Investigation of Its Electrochemical Properties. ChemistrySelect. 2021;6:12644-51.
- Dragan ES, Dinu MV, Ghiorghita CA, Lazar MM, Doroftei F. Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin. Molecules2021.
- Sedlačík T, Nonoyama T, Guo H, Kiyama R, Nakajima T, Takeda Y, et al. Preparation of Tough Double- and Triple-Network Supermacroporous Hydrogels through Repeated Cryogelation. Chemistry of Materials. 2020;32:8576-86.
- Jain E, Srivastava A, Kumar A. Macroporous interpenetrating cryogel network of poly(acrylonitrile) and gelatin for biomedical applications. Journal of Materials Science: Materials in Medicine. 2009;20:173-9.
- Dispinar T, Van Camp W, De Cock LJ, De Geest BG, Du Prez FE. Redox-Responsive Degradable PEG Cryogels as Potential Cell Scaffolds in Tissue Engineering. Macromolecular Bioscience. 2012;12:383-94.
- Kumar A, Srivastava A. Cell separation using cryogel-based affinity chromatography. Nature Protocols. 2010;5:1737-47.
- Davidson-Rozenfeld G, Chen X, Qin Y, Ouyang Y, Sohn YS, Li Z, et al. Stiffness-Switchable, Biocatalytic pH-Responsive DNA-Functionalized Polyacrylamide Cryogels and their Mechanical Applications. Advanced Functional Materials. 2024;34:2306586.
- Dragan ES, Cocarta AI. Smart Macroporous IPN Hydrogels Responsive to pH, Temperature, and Ionic Strength: Synthesis, Characterization, and Evaluation of Controlled Release of Drugs. Acs Appl Mater Inter. 2016;8:12018-30.
- Okten NS, Tanc B, Orakdogen N. Design and molecular dynamics of multifunctional sulfonated poly (dimethylaminoethyl methacrylate)/mica hybrid cryogels through freezing-induced gelation. Soft Matter. 2019;15:7043-62.
- Persson P, Baybak O, Plieva F, Galaev IY, Mattiasson B, Nilsson B, et al. Characterization of a continuous supermacroporous monolithic matrix for chromatographic separation of large bioparticles. Biotechnology and Bioengineering. 2004;88:224-36.
- Uygun M, Senay RH, Avcibasi N, Akgol S. Poly(HEMA-co-NBMI) Monolithic Cryogel Columns for IgG Adsorption. Appl Biochem Biotech. 2014;172:1574-84.
- Bakhshpour M, Topcu AA, Bereli N, Alkan H, Denizli A. Poly (Hydroxyethyl Methacrylate) immunoaffinity cryogel column for the purification of human immunoglobulin M. Gels. 2020;6:4.
- Hwang YS, Sangaj N, Varghese S. Interconnected Macroporous Poly(Ethylene Glycol) Cryogels as a Cell Scaffold for Cartilage Tissue Engineering. Tissue Engineering Part A. 2010;16:3033-41.
- Hwang YS, Zhang C, Varghese S. Poly(ethylene glycol) cryogels as potential cell scaffolds: effect of polymerization conditions on cryogel microstructure and properties. J Mater Chem. 2010;20:345-51.
- Ferraraccio LS, Russell J, Newland B, Bertoncello P. Poly (ethylene glycol)(PEG)-Cryogels: a novel Platform towards Enzymatic Electrochemiluminescence (ECL)-based Sensor Applications. Electrochimica Acta. 2024:144007.
- Srivastava A, Jain E, Kumar A. The physical characterization of supermacroporous poly(N-isopropylacrylamide) cryogel: Mechanical strength and swelling/de-swelling kinetics. Materials Science and Engineering A. 2007;464:93-100.
- Salar Amoli M, Anand R, EzEldeen M, Geris L, Jacobs R, Bloemen V. Development of 3D Printed pNIPAM-Chitosan Scaffolds for Dentoalveolar Tissue Engineering. Gels 2024, 10, 140. 2024.
- Haleem A, Wu F, Ullah M, Saeed T, Li H, Pan J. Chitosan functionalization with vinyl monomers via ultraviolet illumination under cryogenic conditions for efficient palladium recovery from waste electronic materials. Separation and Purification Technology. 2024;329:125213.
- Stoyneva V, Momekova D, Kostova B, Petrov P. Stimuli sensitive super-macroporous cryogels based on photo-crosslinked 2-hydroxyethylcellulose and chitosan. Carbohyd Polym. 2014;99:825-30.
- Chatterjee S, Ghosal K, Kumar M, Mahmood S, Thomas S. A detailed discussion on interpenetrating polymer network (IPN) based drug delivery system for the advancement of health care system. Journal of Drug Delivery Science and Technology. 2023;79:104095.
- Thönes S, Kutz LM, Oehmichen S, Becher J, Heymann K, Saalbach A, et al. New E-beam-initiated hyaluronan acrylate cryogels support growth and matrix deposition by dermal fibroblasts. International journal of biological macromolecules. 2017;94:611-20.
- Madaghiele M, Salvatore L, Demitri C, Sannino A. Fast synthesis of poly(ethylene glycol) diacrylate cryogels via UV irradiation. Materials Letters. 2018;218:305-8.
- Georgiev GL, Trzebicka B, Kostova B, Petrov PD. Super-macroporous dextran cryogels via UV-induced crosslinking: synthesis and characterization. Polymer International. 2017;66:1306-11.
- Barrow M, Zhang H. Aligned porous stimuli-responsive hydrogels via directional freezing and frozen UV initiated polymerization. Soft Matter. 2013;9:2723-9.
- Thomas AM, Shea LD. Cryotemplation for the rapid fabrication of porous, patternable photopolymerized hydrogels. Journal of Materials Chemistry B. 2014;2:4521-30.
- Serex L, Braschler T, Filippova A, Rochat A, Béduer A, Bertsch A, et al. Pore Size Manipulation in 3D Printed Cryogels Enables Selective Cell Seeding. Advanced Materials Technologies. 2018;3:1700340.
- Bilici Cid, Altunbek M, Afghah F, Tatar AG, Koç B. Embedded 3D printing of cryogel-based scaffolds. ACS Biomaterials Science & Engineering. 2023;9:5028-38.
- Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, et al. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci USA 2012;109:19590-5.
- Singh D, Zo SM, Kumar A, Han SS. Engineering three-dimensional macroporous hydroxyethyl methacrylate-alginate-gelatin cryogel for growth and proliferation of lung epithelial cells. Journal of Biomaterials Science, Polymer Edition. 2013;24:1343-59.
- Berillo D, Volkova N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran. Journal of materials science. 2014;49:4855-68.
- Carballo-Pedrares N, López-Seijas J, Miranda-Balbuena D, Lamas I, Yáñez J, Rey-Rico A. Gene-activated hyaluronic acid-based cryogels for cartilage tissue engineering. Journal of Controlled Release. 2023;362:606-19.
- Bayir, E. Comparative evaluation of alginate-gelatin hydrogel, cryogel, and aerogel beads as a tissue scaffold. Sakarya University Journal of Science. 2023;27:335-48.
- Mancino R, Caccavo D, Barba AA, Lamberti G, Biasin A, Cortesi A, et al. Agarose Cryogels: Production Process Modeling and Structural Characterization. Gels. 2023;9:765.
- Şeker Ş, Aral D, Elçin AE, Murat EY. Biomimetic mineralization of platelet lysate/oxidized dextran cryogel as a macroporous 3D composite scaffold for bone repair. Biomedical Materials. 2024;19:025006.
- Reichelt S, Becher J, Weisser J, Prager A, Decker U, Moller S, et al. Biocompatible polysaccharide-based cryogels. Mat Sci Eng C-Mater. 2014;35:164-70.
- Kumakura, M. Preparation method of porous polymer materials by radiation technique and its application. Polym Advan Technol. 2001;12:415-21.
- Reichelt S, Prager A, Abe C, Knolle W. Tailoring the structural properties of macroporous electron-beam polymerized cryogels by pore forming agents and the monomer selection. Radiat Phys Chem. 2014;94:40-4.
- Tonta MM, Sahin ZM, Cihaner A, Yilmaz F, Gurek A. Synthesis of Polyacrylamide-Based Redox Active Cryogel Using Click Chemistry and Investigation of Its Electrochemical Properties. ChemistrySelect. 2021;6:12644-51.
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angewandte Chemie International Edition. 2001;40:2004-21.
- Crowell AD, FitzSimons TM, Anslyn EV, Schultz KM, Rosales AM. Shear Thickening Behavior in Injectable Tetra-PEG Hydrogels Cross-Linked via Dynamic Thia-Michael Addition Bonds. Macromolecules. 2023;56:7795-807.
- Smith, G.P. Chemical and Proteolytic Modification of Antibodies. Making and Using Antibodies. 2006:195-260.
- Lin TW, Hsu Sh. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Advanced Science. 2020;7:1901388.
- Lin K-T, Ma R, Wang P, Xin J, Zhang J, Wolcott MP, et al. Deep eutectic solvent assisted facile synthesis of lignin-based cryogel. Macromolecules. 2018;52:227-35.
- Sezen S, Thakur VK, Ozmen MM. Highly effective covalently crosslinked composite alginate cryogels for cationic dye removal. Gels. 2021;7:178.
- Chambre L, Maouati H, Oz Y, Sanyal R, Sanyal A. Thiol-Reactive Clickable Cryogels: Importance of Macroporosity and Linkers on Biomolecular Immobilization. Bioconjugate Chemistry. 2020;31:2116-24.
- Ciolacu D, Rudaz C, Vasilescu M, Budtova T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydrate polymers. 2016;151:392-400.
- Fukumori T, Nakaoki T. Significant improvement of mechanical properties for polyvinyl alcohol film prepared from freeze/thaw cycled gel. Open Journal of Organic Polymer Materials. 2013;2013.
- Mao S, Onggowarsito C, Feng A, Zhang S, Fu Q, Nghiem LD. A cryogel solar vapor generator with rapid water replenishment and high intermediate water content for seawater desalination. Journal of Materials Chemistry A. 2023;11:858-67.
- Tripathi A, Kumar A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromolecular bioscience. 2011;11:22-35.
- da Silva LP, Cerqueira MT, Sousa RA, Reis RL, Correlo VM, Marques AP. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta biomaterialia. 2014;10:4787-97.
- Elsherbiny DA, Abdelgawad AM, El-Naggar ME, El-Sherbiny RA, El-Rafie MH, El-Sayed IE-T. Synthesis, antimicrobial activity, and sustainable release of novel α-aminophosphonate derivatives loaded carrageenan cryogel. International Journal of Biological Macromolecules. 2020;163:96-107.
- Meena LK, Raval P, Kedaria D, Vasita R. Study of locust bean gum reinforced cyst-chitosan and oxidized dextran based semi-IPN cryogel dressing for hemostatic application. Bioactive Materials. 2018;3:370-84.
- Zhang H, Zhang F, Wu J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. Reactive and Functional Polymers. 2013;73:923-8.
- Kolosova OY, Karelina PA, Vasil'ev VG, Grinberg VY, Kurochkin II, Kurochkin IN, et al. Cryostructuring of polymeric systems. 58. Influence of the H2N-(CH2)n-COOH–type amino acid additives on formation, properties, microstructure and drug release behaviour of poly(vinyl alcohol) cryogels. Reactive and Functional Polymers. 2021;167:105010.
- Okamoto A, Miyoshi T. A BIOCOMPATIBLE GEL OF HYALURONAN. In: Kennedy JF, Phillips GO, Williams PA, editors. Hyaluronan: Woodhead Publishing; 2002. p. 285-92.
- Luan T, Wu L, Zhang H, Wang Y. A study on the nature of intermolecular links in the cryotropic weak gels of hyaluronan. Carbohyd Polym. 2012;87:2076-85.
- Rajasekaran R, Dutta A, Ray PG, Seesala VS, Ojha AK, Dogra N, et al. High fibroin-loaded silk-PCL electrospun fiber with core–shell morphology promotes epithelialization with accelerated wound healing. Journal of Materials Chemistry B. 2022;10:9622-38.
- Kadakia PU, Jain E, Hixon KR, Eberlin CT, Sell SA. Sonication induced silk fibroin cryogels for tissue engineering applications. Materials Research Express. 2016;3:055401.
- Chen H, Hao B, Ge P, Chen S. Highly stretchable, self-healing, and 3D printing prefabricatable hydrophobic association hydrogels with the assistance of electrostatic interaction. Polymer Chemistry. 2020;11:4741-8.
- Yuan T, Cui X, Liu X, Qu X, Sun J. Highly tough, stretchable, self-healing, and recyclable hydrogels reinforced by in situ-formed polyelectrolyte complex nanoparticles. Macromolecules. 2019;52:3141-9.
- Dragan ES, Dinu MV, Ghiorghita CA. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels. 2022;8.
- Jonidi Shariatzadeh F, Solouk A, Bagheri Khoulenjani S, Bonakdar S, Mirzadeh H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids and Surfaces B: Biointerfaces. 2021;203:111725.
- Wang M, Hu J, Ou Y, He X, Wang Y, Zou C, et al. Shape-recoverable hyaluronic acid–waterborne polyurethane hybrid cryogel accelerates hemostasis and wound healing. ACS applied materials & interfaces. 2022;14:17093-108.
- Plieva F, Xiao HT, Galaev IY, Bergenstahl B, Mattiasson B. Macroporous elastic polyacrylamide gels prepared at subzero temperatures: control of porous structure. J Mater Chem. 2006;16:4065-73.
- Memic A, Colombani T, Eggermont LJ, Rezaeeyazdi M, Steingold J, Rogers ZJ, et al. Latest Advances in Cryogel Technology for Biomedical Applications. Advanced Therapeutics. 2019;2:1800114.
- Van Rie J, Declercq H, Van Hoorick J, Dierick M, Van Hoorebeke L, Cornelissen R, et al. Cryogel-PCL combination scaffolds for bone tissue repair. Journal of Materials Science: Materials in Medicine. 2015;26:1-7.
- Hwang Y, Zhang C, Varghese S. Poly(ethylene glycol) cryogels as potential cell scaffolds: effect of polymerization conditions on cryogel microstructure and properties. Journal of Materials Chemistry. 2010;20:345-51.
- Zhang X, Yan H, Xu C, Dong X, Wang Y, Fu A, et al. Skin-like cryogel electronics from suppressed-freezing tuned polymer amorphization. Nature Communications. 2023;14:5010.
- Lozinsky VI, Damshkaln LGJJoAPS. Study of cryostructuration of polymer systems. XVII. Poly (vinyl alcohol) cryogels: Dynamics of the cryotropic gel formation. 2000;77:2017-23.
- Tuncaboylu DC, Okay O. Hierarchically macroporous cryogels of polyisobutylene and silica nanoparticles. Langmuir : the ACS journal of surfaces and colloids. 2010;26:7574-81.
- Zhang XZ, Wang FJ, Chu CC. Thermoresponsive hydrogel with rapid response dynamics. J Mater Sci Mater Med. 2003;14:451-5.
- Zhang XZ, Chu CC. Thermosensitive PNIPAAm cryogel with superfast and stable oscillatory properties. Chemical communications. 2003:1446-7.
- Zhang XZ, Chu CC. Synthesis of temperature sensitive PNIPAAm cryogels in organic solvent with improved properties. J Mater Chem. 2003;13:2457-64.
- Tripathi A, Kathuria N, Kumar A. Elastic and macroporous agarose–gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009;90:680-94.
- Ak F, Oztoprak Z, Karakutuk I, Okay O. Macroporous Silk Fibroin Cryogels. Biomacromolecules. 2013;14:719-27.
- Plieva F, Huiting X, Galaev IY, Bergenståhl B, Mattiasson B. Macroporous elastic polyacrylamide gels prepared at subzero temperatures: control of porous structure. Journal of Materials Chemistry. 2006;16:4065-73.
- Srivastava A, Jain E, Kumar A. The physical characterization of supermacroporous poly (N-isopropylacrylamide) cryogel: mechanical strength and swelling/de-swelling kinetics. Materials Science and Engineering: A. 2007;464:93-100.
- Zhang X-Z, Chu C-C. Synthesis of temperature sensitive PNIPAAm cryogels in organic solvent with improved properties. Journal of Materials Chemistry. 2003;13:2457-64.
- Lozinsky VI, Damshkaln LG, Shaskol'skii BL, Babushkina TA, Kurochkin IN, Kurochkin II. Study of cryostructuring of polymer systems: 27. Physicochemical properties of poly(vinyl alcohol) cryogels and specific features of their macroporous morphology. Colloid J+. 2007;69:747-64.
- Lozinsky VI, Damshkaln LG. Study of cryostructuration of polymer systems. XVII. Poly(vinyl alcohol) cryogels: Dynamics of the cryotropic gel formation. Journal of Applied Polymer Science. 2000;77:2017-23.
- Lozinsky VI, Ivanov RV, Kalinina EV, Timofeeva GI, Khokhlov AR. Redox-initiated radical polymerisation of acrylamide in moderately frozen water solutions. Macromolecular Rapid Communications. 2001;22:1441-6.
- Lozinsky VI, Vainerman ES, Korotaeva GF, Rogozhin SV. Study of Cryostructurization of Polymer Systems.3. Cryostructurization in Organic Media. Colloid and Polymer Science. 1984;262:617-22.
- Gun'ko VM, Savina IN, Mikhalovsky SV. Cryogels: Morphological, structural and adsorption characterisation. Advances in colloid and interface science. 2013;187:1-46.
- Plieva FM, Andersson J, Galaev IY, Mattiasson B. Characterization of polyacrylamide based monolithic columns. J Sep Sci. 2004;27:828-36.
- Ozmen MM, Dinu MV, Dragan ES, Okay O. Preparation of Macroporous Acrylamide-Based Hydrogels: Cryogelation Under Isothermal Conditions. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry. 2007;44:1195-202.
- Tanaka R, Hatakeyama T, Hatakeyama H. Formation of locust bean gum hydrogel by freezing-thawing. Polymer International. 1998;45:118-26.
- Kumar A, Rodriguez-Caballero A, Plieva FM, Galaev IY, Nandakumar KS, Kamihira M, et al. Affinity binding of cells to cryogel adsorbents with immobilized specific ligands: effect of ligand coupling and matrix architecture. Journal of Molecular Recognition. 2005;18:84-93.
- Lozinsky VI, Damshkaln LG, Bloch KO, Vardi P, Grinberg NV, Burova TV, et al. Cryostructuring of polymer systems. XXIX. Preparation and characterization of supermacroporous (spongy) agarose-based cryogels used as three-dimensional scaffolds for culturing insulin-producing cell aggregates. Journal of Applied Polymer Science. 2008;108:3046-62.
- Lozinsky VI, Zubov AL, Savina IN, Plieva FM. Study of cryostructuration of polymer systems. XIV. Poly(vinyl alcohol) cryogels: Apparent yield of the freeze-thaw-induced gelation of concentrated aqueous solutions of the polymer. Journal of Applied Polymer Science. 2000;77:1822-31.
- Lozinsky VI, Zubov AL, Kulakova VK, Titova EF, Rogozhin SV. Study of Cryostructurization of Polymer Systems.9. Poly(Vinyl Alcohol) Cryogels Filled with Particles of Cross-Linked Dextran Gel. Journal of Applied Polymer Science. 1992;44:1423-35.
- Lozinsky VI, Zubov AL, Makhlis TA. Entrapment of Zymomonas mobilis cells into PVA-cryogel carrier in the presence of polyol cryoprotectants. Immobilized Cells: Basics and Applications. 1996;11:112-7.
- Lozinsky VI, Savvichev AS, Tumansky BL, Nikitin DI. Some microorganisms during their entrapment in PAAG act as ''biological accelerators'' in how they affect the gel-formation rate. Immobilized Cells: Basics and Applications. 1996;11:118-25.
- Lozinsky VI, Zubov AL, Titova EF. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization.2. Entrapped cells resemble porous fillers in their effects on the properties of PVA-cryogel carrier. Enzyme and Microbial Technology. 1997;20:182-90.
- Lozinsky VI, Damshkaln LG, Kurochkin IN, Kurochkin II. Study of cryostructuring of polymer systems: 25. The influence of Surfactants on the properties and structure of gas-filled (Foamed) poly(vinyl alcohol) cryogels. Colloid J+. 2005;67:589-601.
- Lozinskii VI, Savina IN. Study of cryostructuring of polymer systems: 22. Composite poly(vinyl alcohol) cryogels filled with dispersed particles of various degrees of hydrophilicity/hydrophobicity. Colloid J+. 2002;64:336-43.
- Savina IN, Mattiasson B, Galaev IY. Graft polymerization of acrylic acid onto macroporous polyacrylamide gel (cryogel) initiated by potassium diperiodatocuprate. Polymer. 2005;46:9596-603.
- Savina IN, Lozinskii VI. Study of cryostructuring of polymer systems: 23. Composite poly(vinyl alcohol) cryogels filled with dispersed particles containing ionogenic groups. Colloid J+. 2004;66:343-9.
- Savina IN, Hanora A, Plieva FM, Galaev IY, Mattiasson B, Lozinsky VI. Cryostructuration of polymer systems. XXIV. Poly(vinyl alcohol) cryogels filled with particles of a strong anion exchanger: Properties of the composite materials and potential applications. Journal of Applied Polymer Science. 2005;95:529-38.
- Savina IN, Hanora A, Plieva FM, Galaev IY, Mattiasson B, Lozinsky VI. Cryostructuration of polymer systems. XXIV. Poly (vinyl alcohol) cryogels filled with particles of a strong anion exchanger: Properties of the composite materials and potential applications. Journal of applied polymer science. 2005;95:529-38.
- Kolosova OY, Ryzhova AS, Chernyshev VP, Lozinsky VI. Study of Cryostructuring of Polymer System. 65. Features of Changes in the Physicochemical Properties of Poly(vinyl alcohol) Cryogels Caused by the Action of Aqueous Solutions of Amino Acids of General Formula H2N–(CH2)n–COOH. Colloid Journal. 2023;85:930-42.
- Jain E, Srivastava A, Kumar A. Macroporous interpenetrating cryogel network of poly(acrylonitrile) and gelatin for biomedical applications. J Mater Sci Mater Med. 2009;20 Suppl 1:S173-9.
- Lozinsky VI, Bakeeva IV, Presnyak EP, Damshkaln LG, Zubov VP. Cryostructuring of polymer systems. XXVI. Heterophase organic-inorganic cryogels prepared via freezing-thawing of aqueous solutions of poly(vinyl alcohol) with added tetramethoxysilane. Journal of Applied Polymer Science. 2007;105:2689-702.
- Carvalho BMA, Da Silva SL, Da Silva LHM, Minim VPR, Da Silva MCH, Carvalho LM, et al. Cryogel poly (acrylamide): synthesis, structure and applications. Separation & Purification Reviews. 2014;43:241-62.
- Podorozhko EA, Buzin MI, Golubev EK, Shcherbina MA, Lozinsky VI. A Study of Cryostructuring of Polymer Systems. 59. Effect of Cryogenic Treatment of Preliminarily Deformed Poly (vinyl alcohol) Cryogels on Their Physicochemical Properties. Colloid Journal. 2021;83:634-41.
- Konstantinova NR, Lozinsky VI. Cryotropic gelation of ovalbumin solutions. Food Hydrocolloids. 1997;11:113-23.
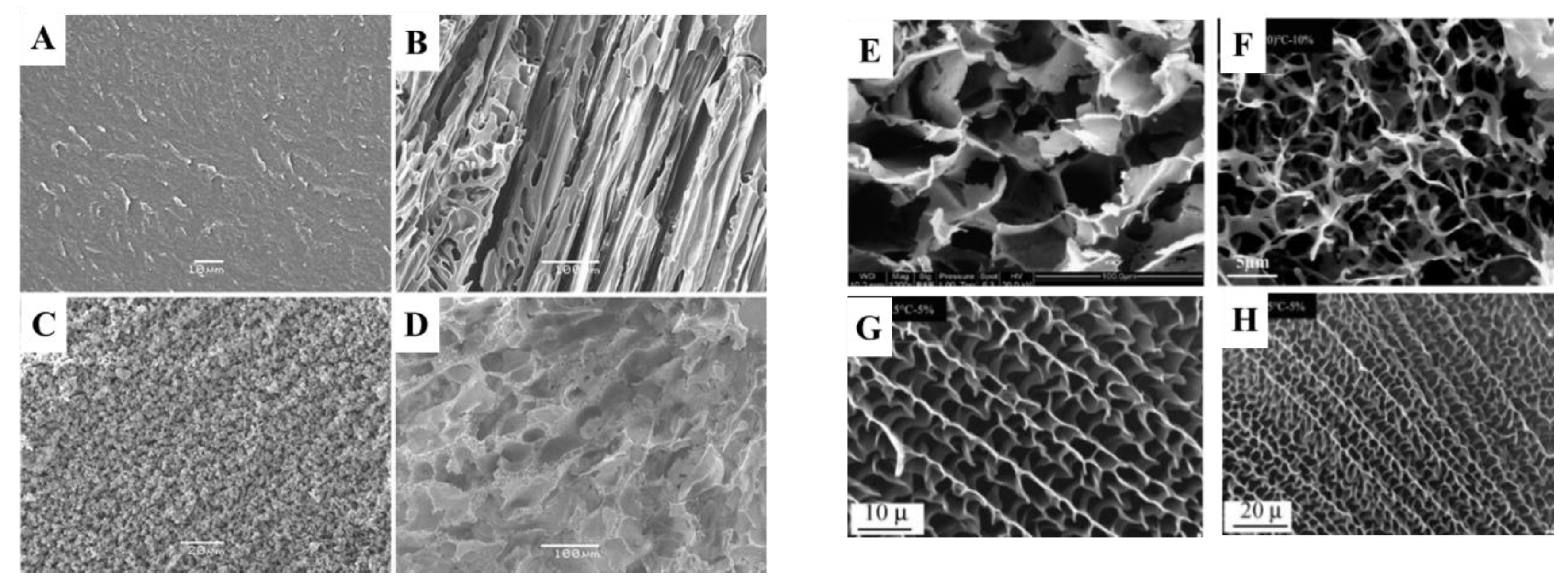
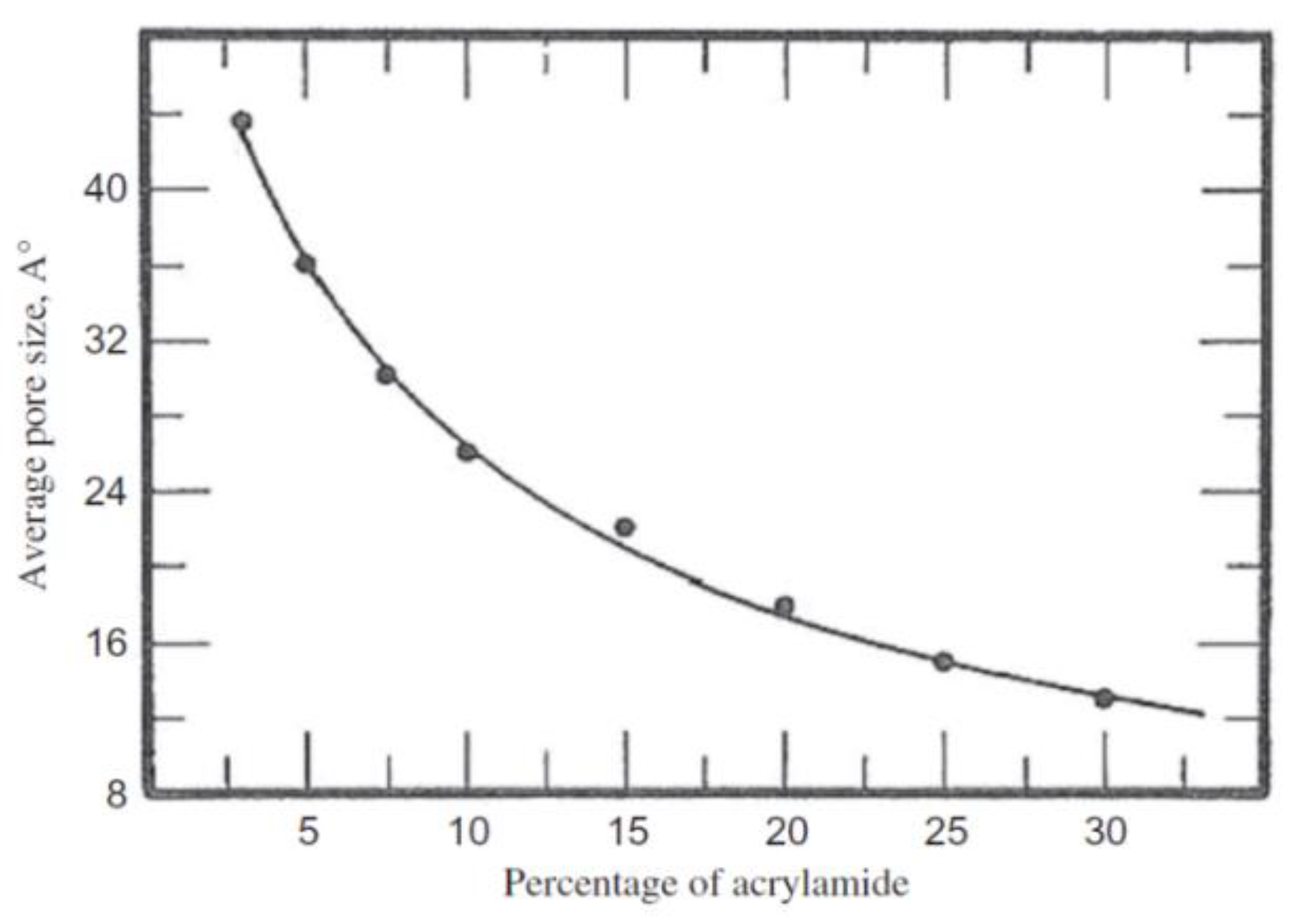
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




