Submitted:
17 January 2025
Posted:
20 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Methods
2.2. Molecular Dynamics Simulations
3. Results and Discussion
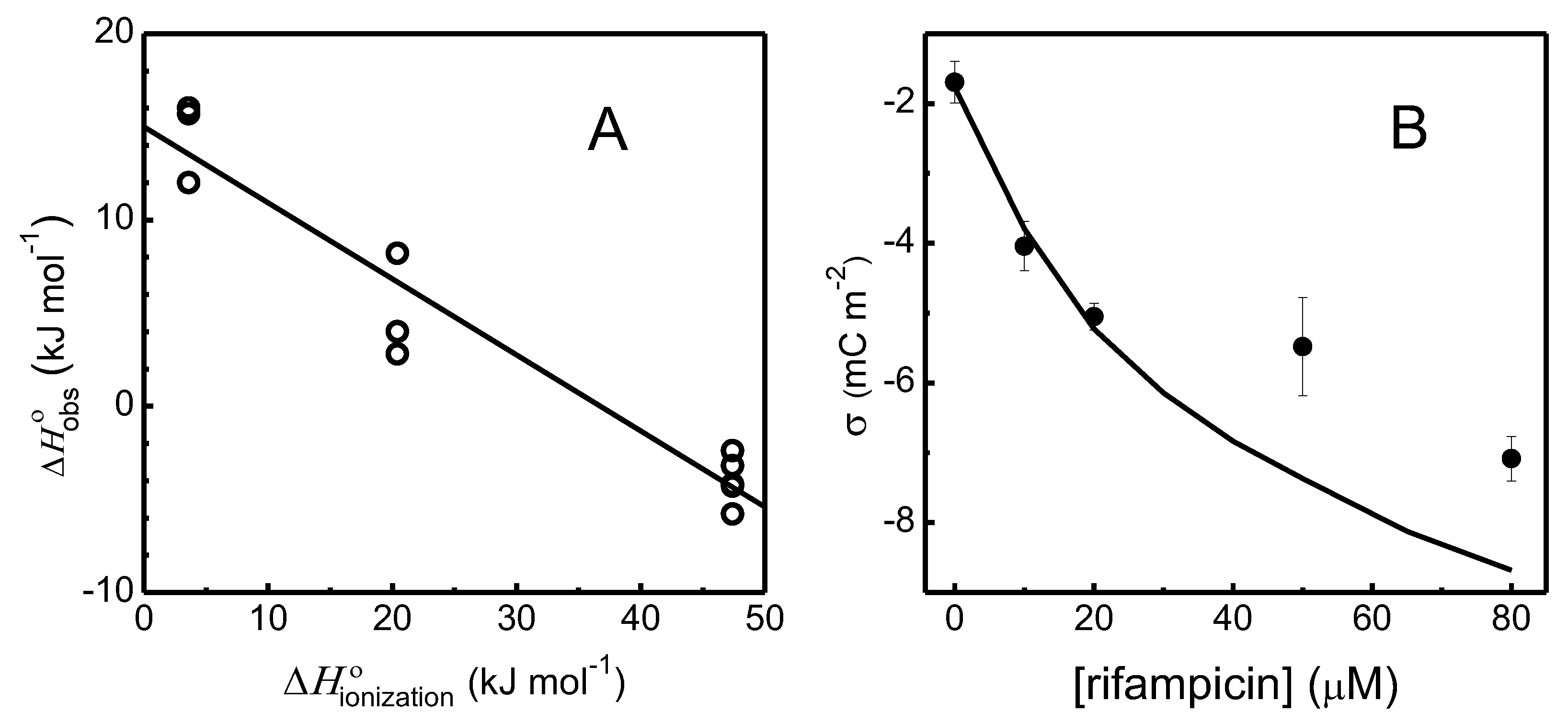
3.1. Experimental Results for the Interaction of Rifampicin with POPC Bilayers
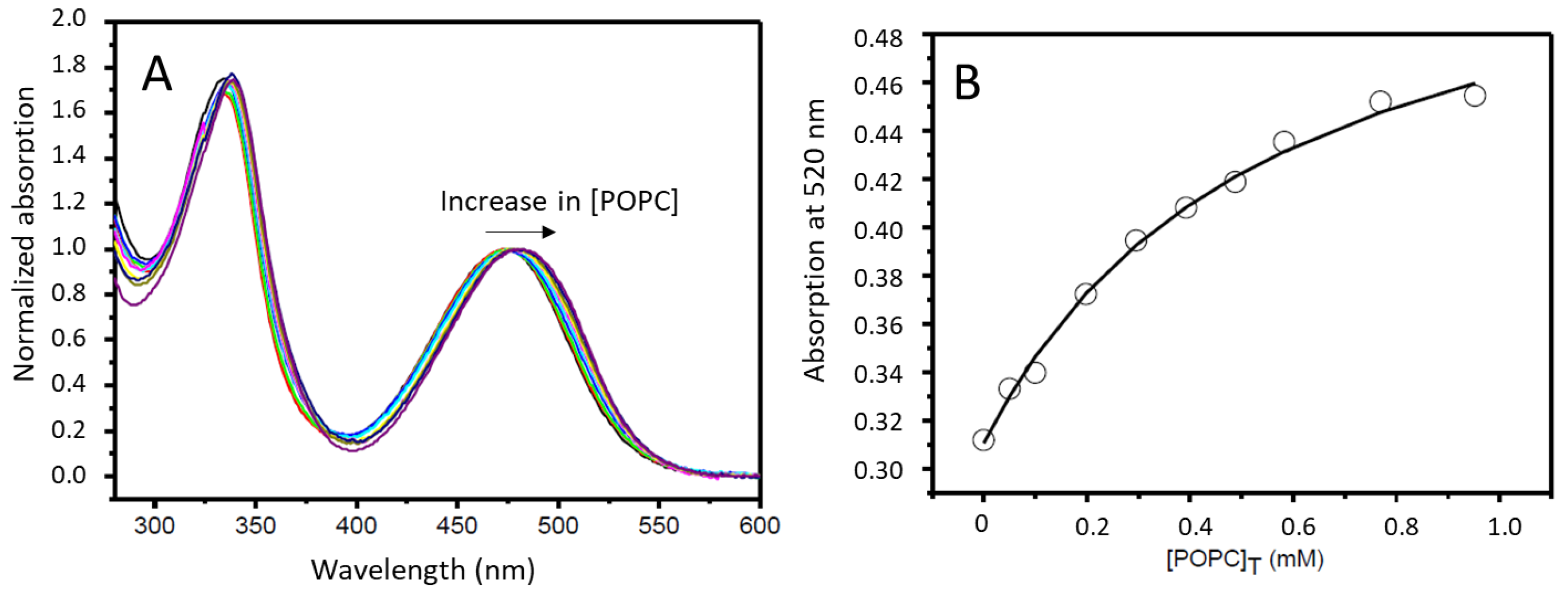
3.1.1. Association of Rifampicin with POPC LUVs
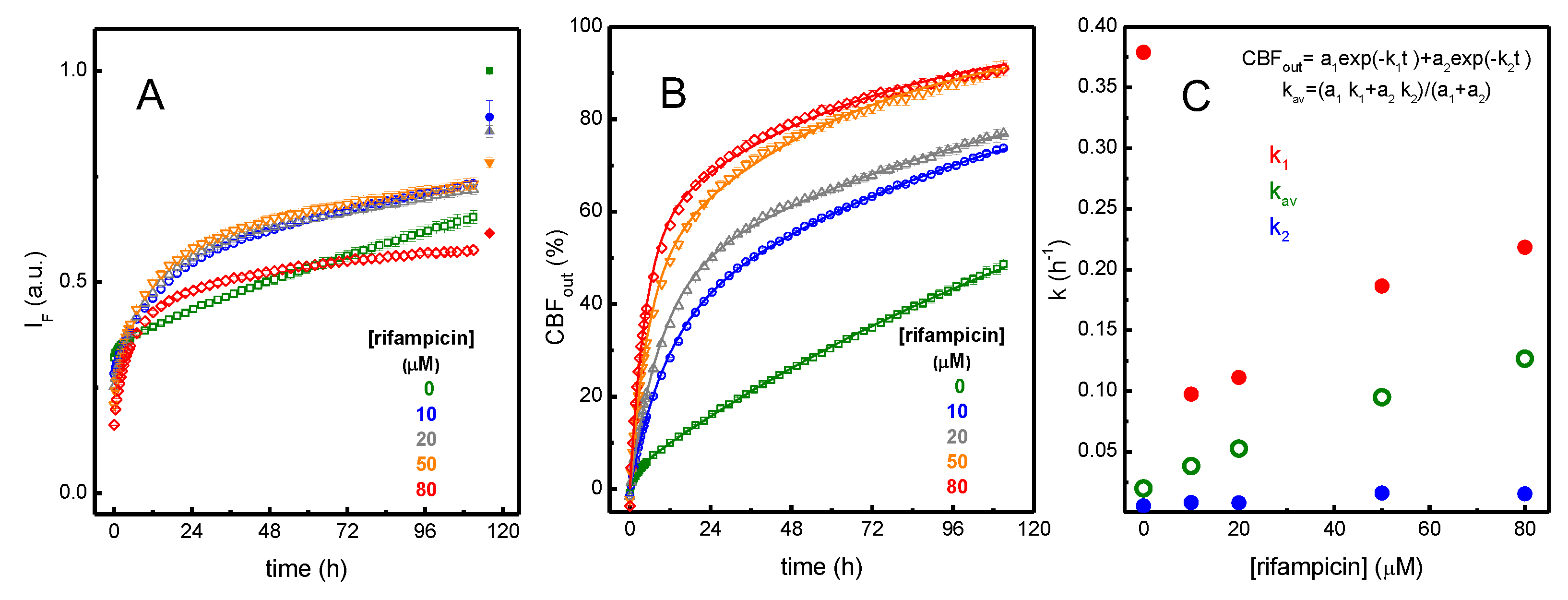
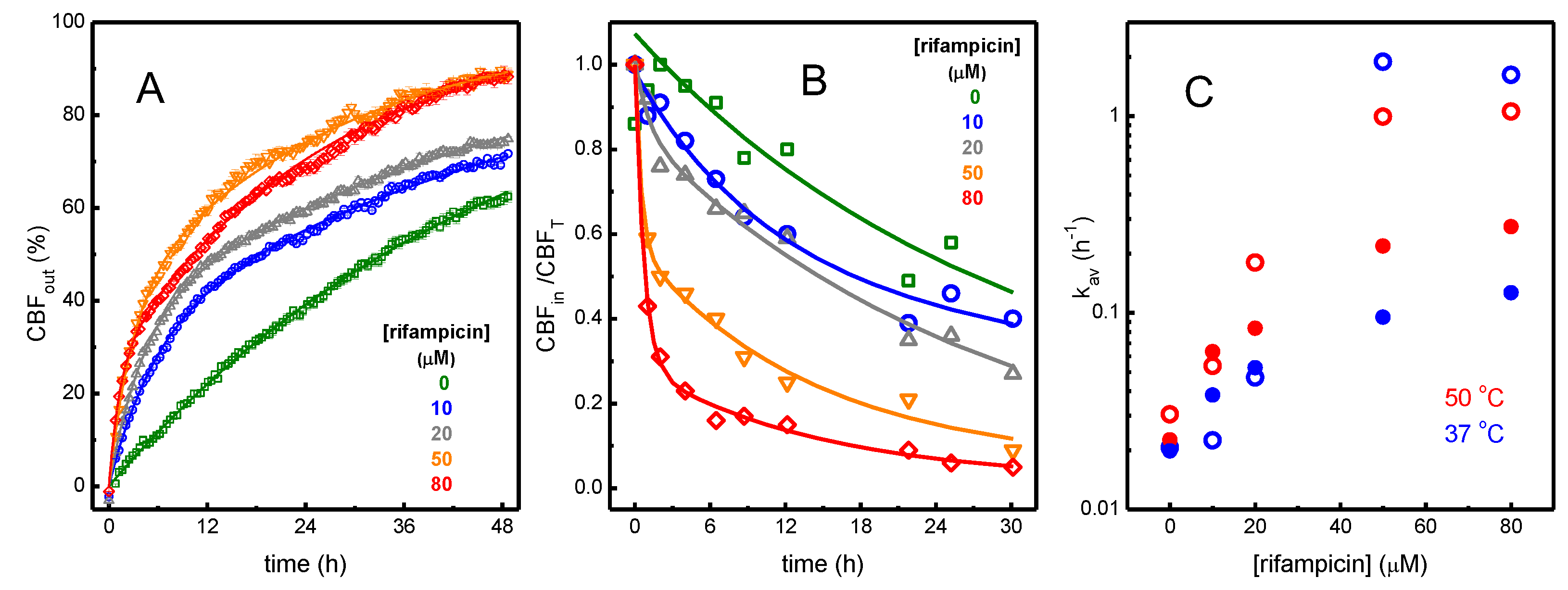
3.1.2. Perturbation of the Membrane Barrier Properties by Rifampicin
3.2. Molecular Dynamics Simulation of the Interaction of Rifampicin with POPC Bilayers
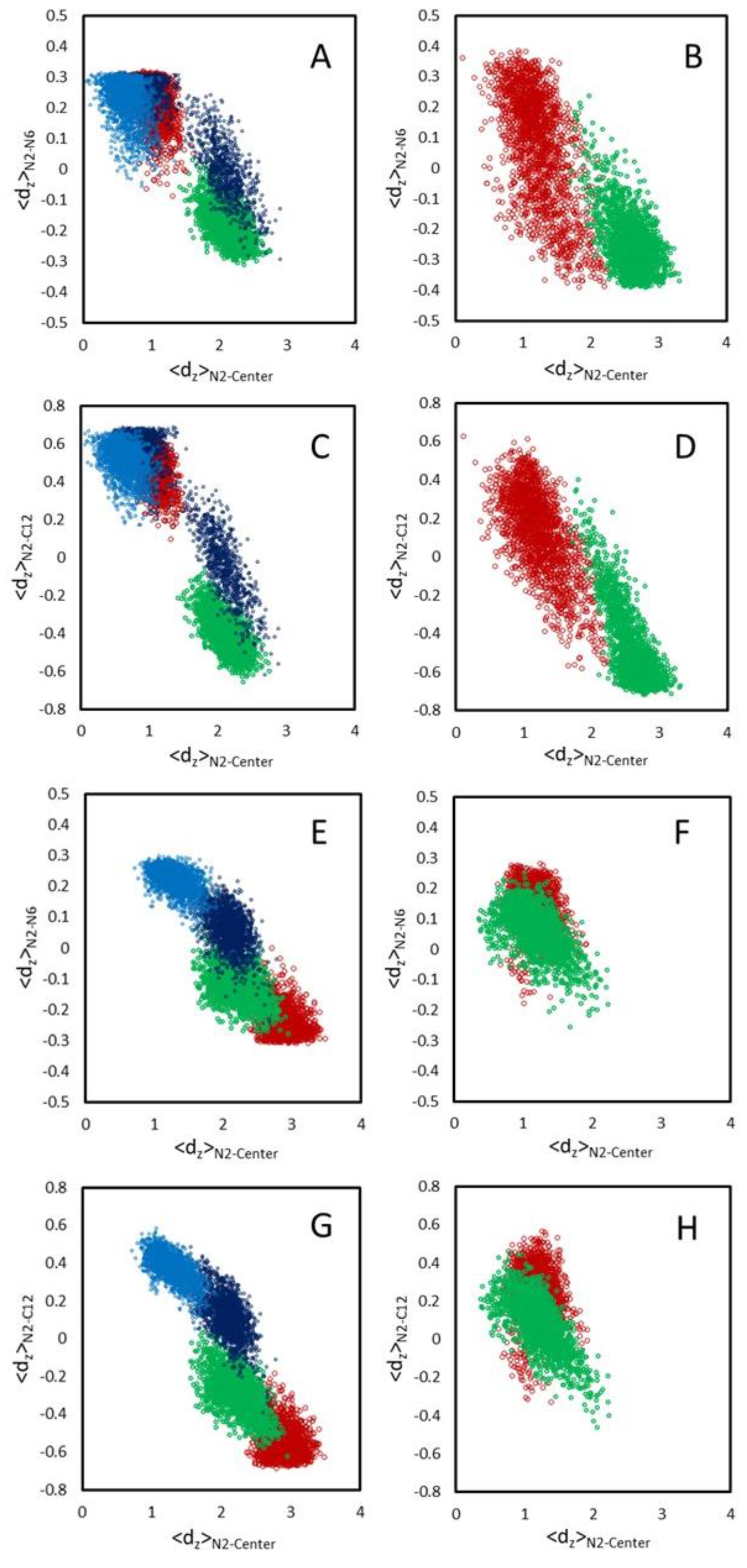
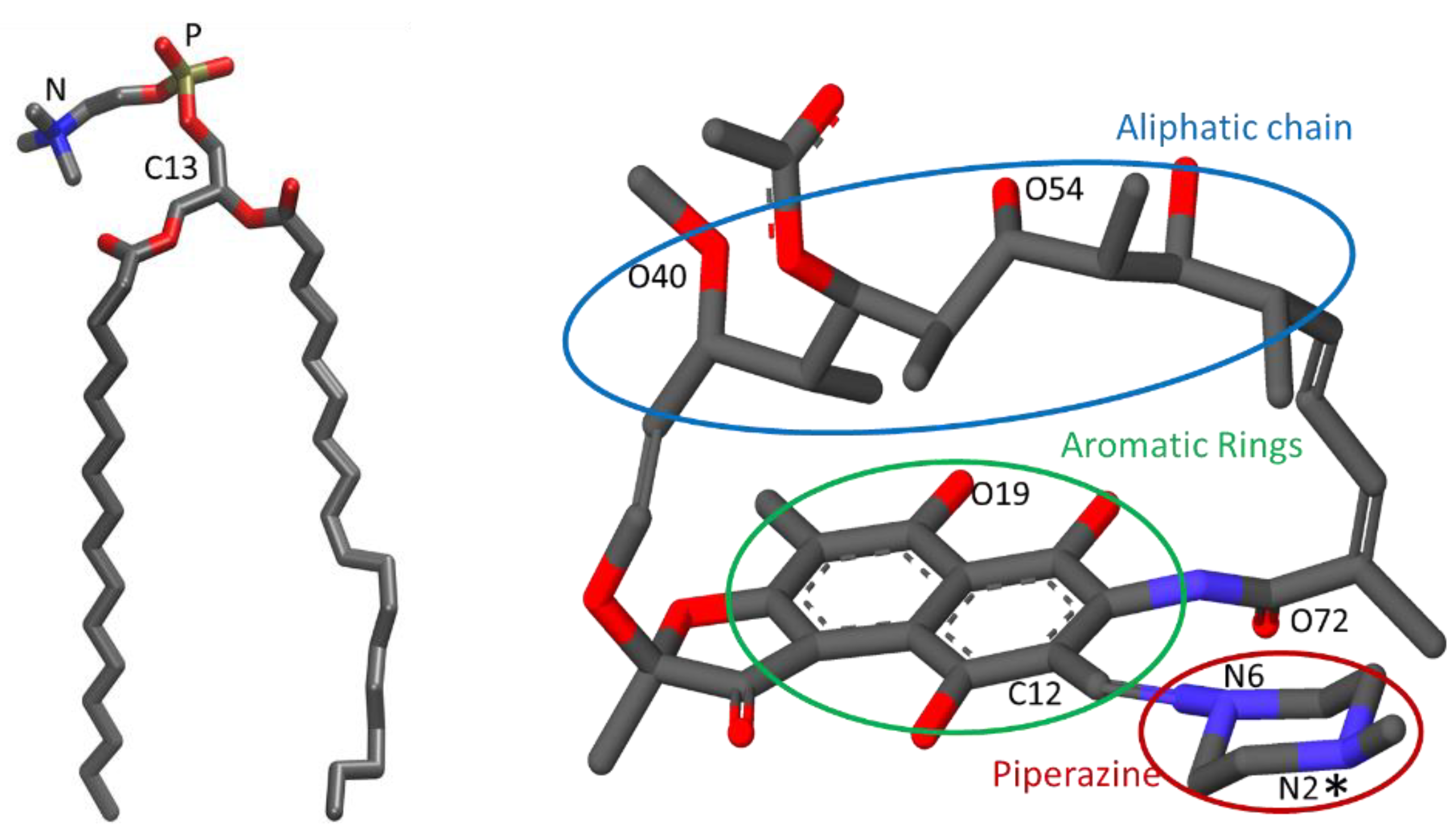
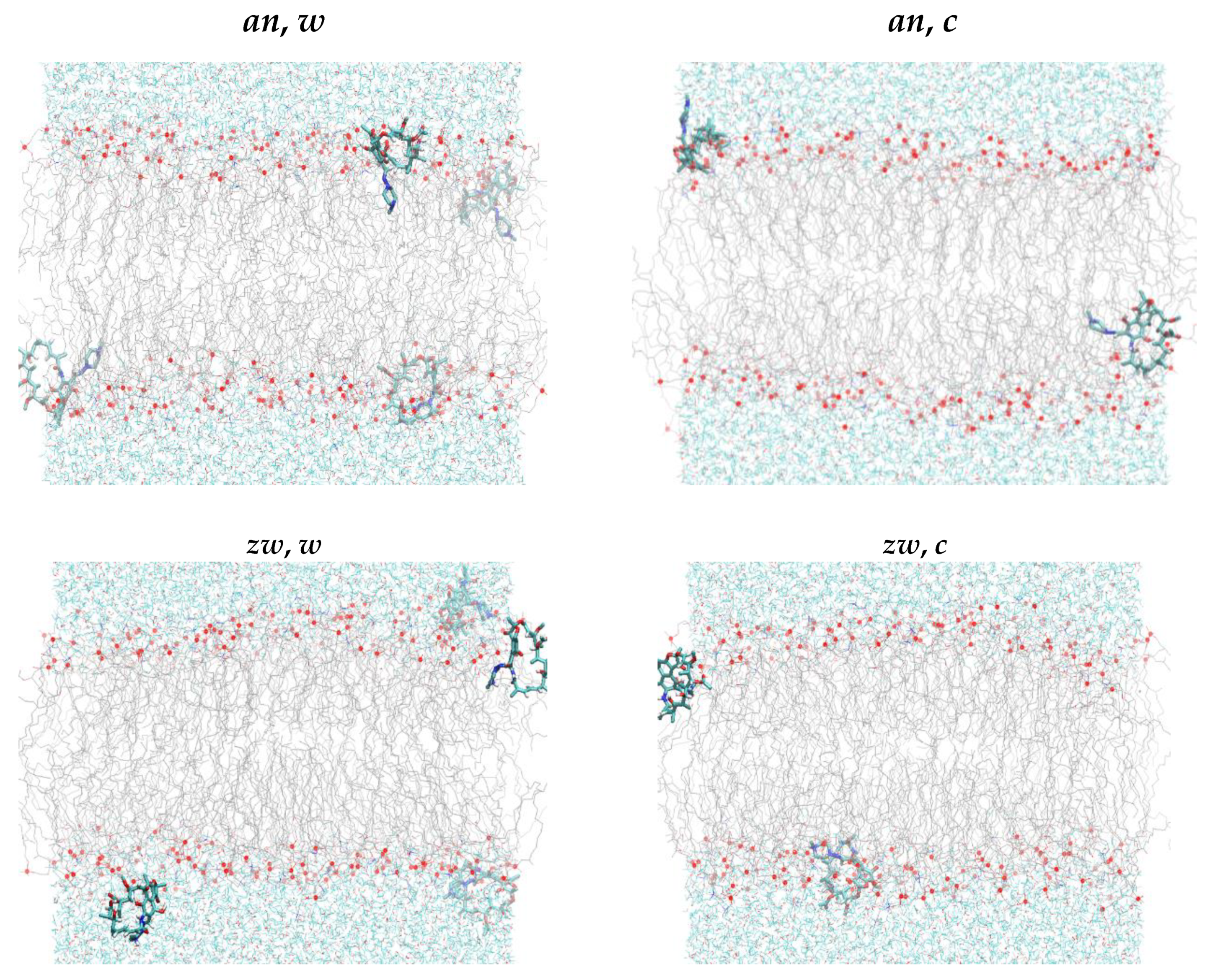
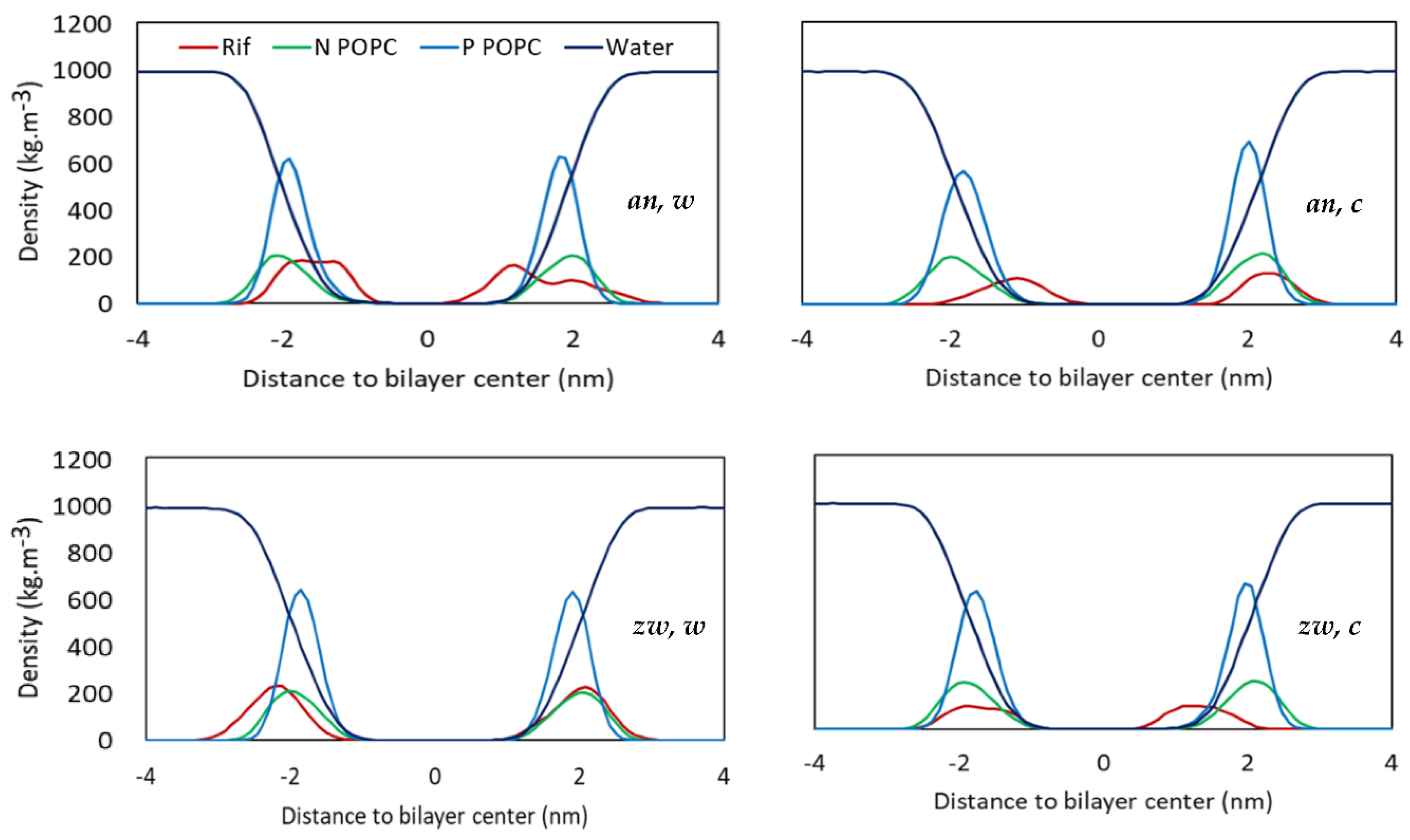
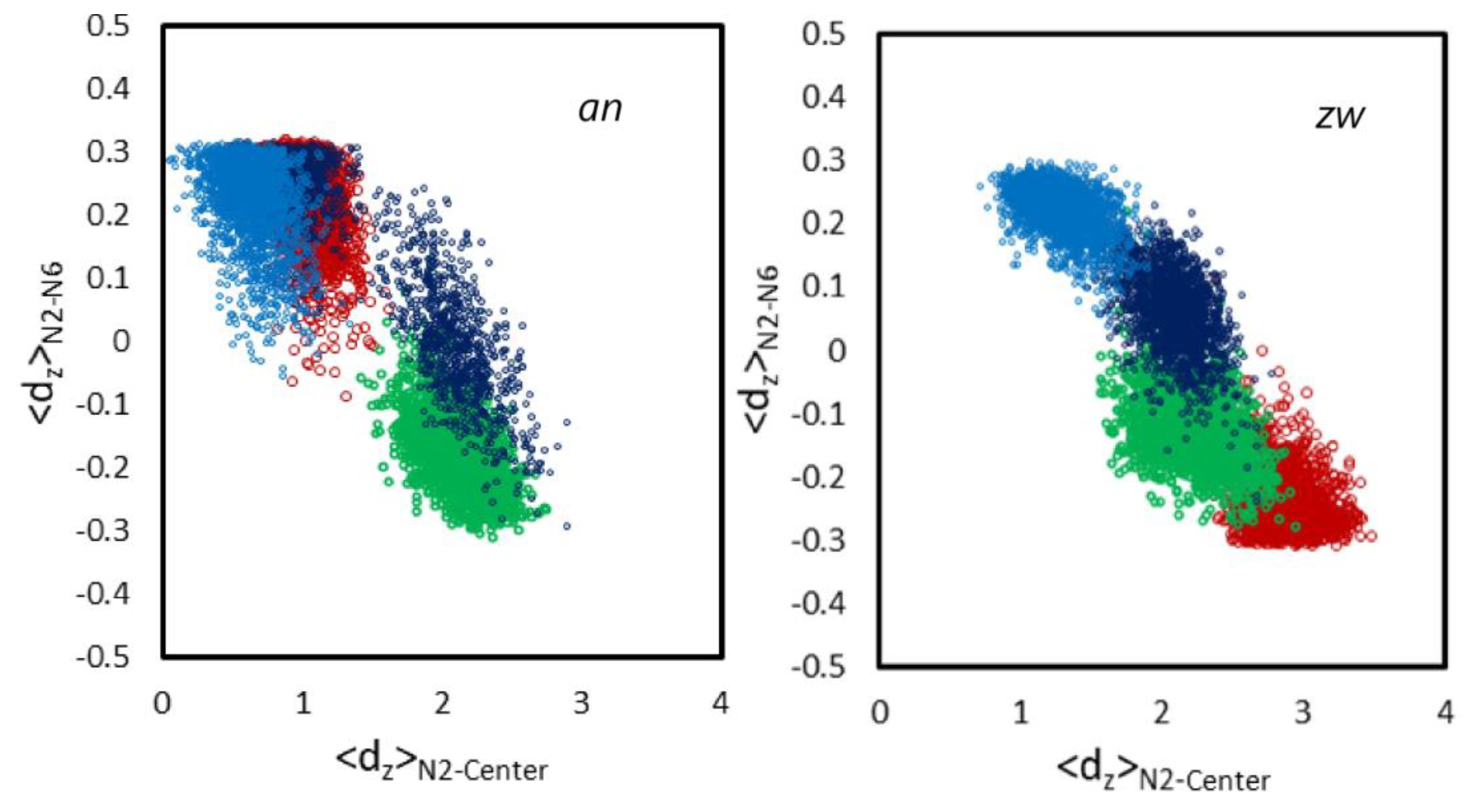
3.2.1. Location and Orientation
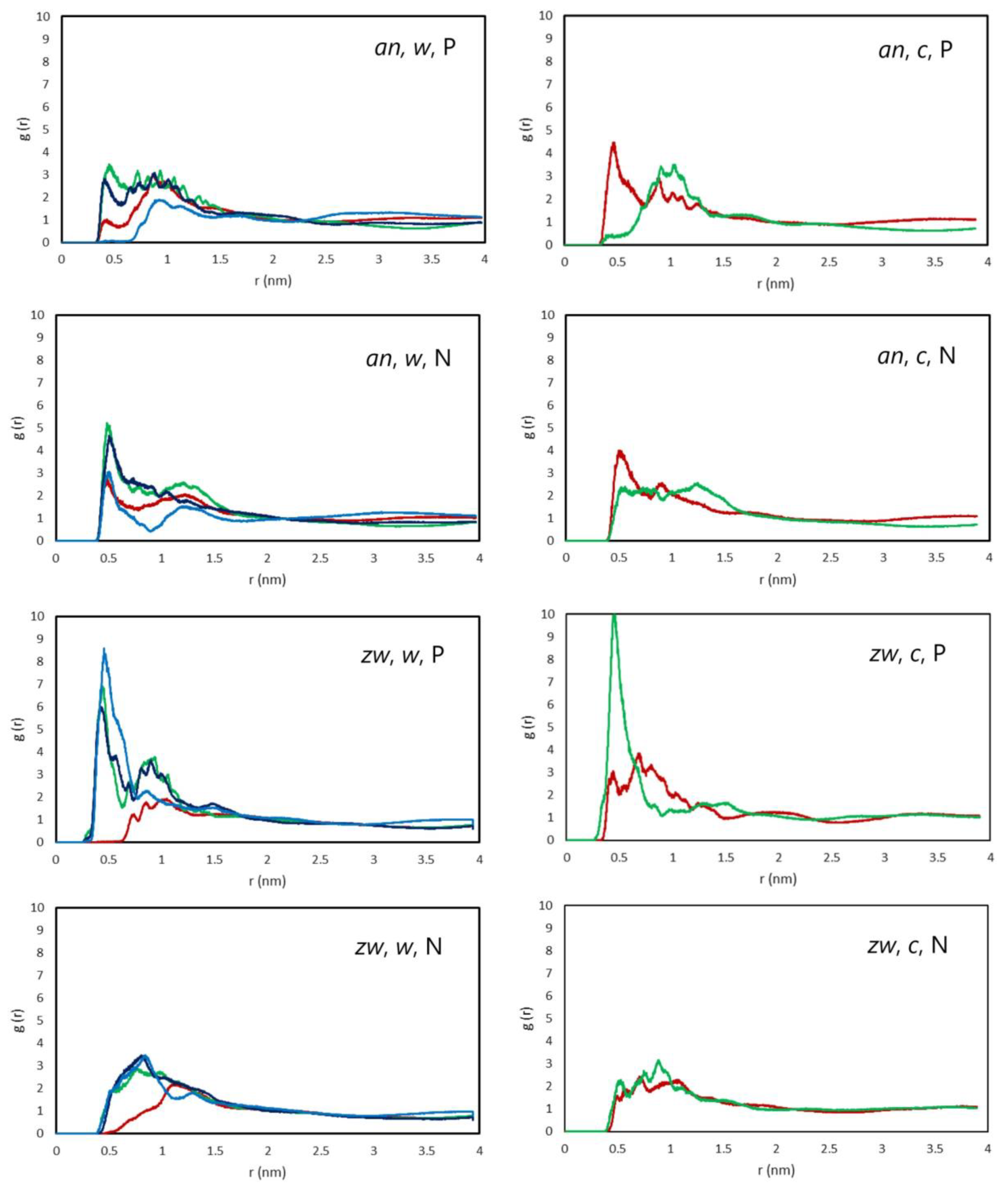
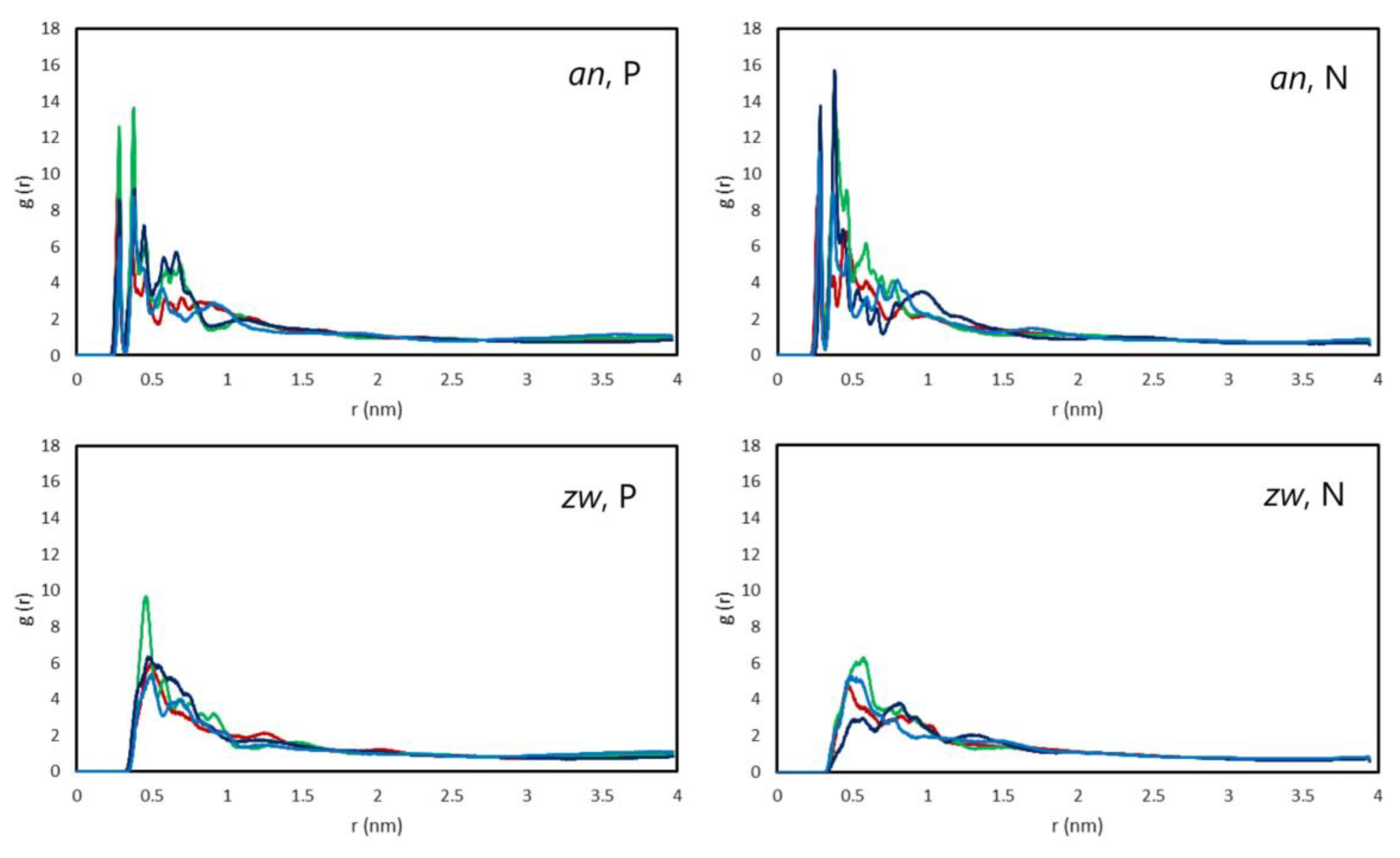
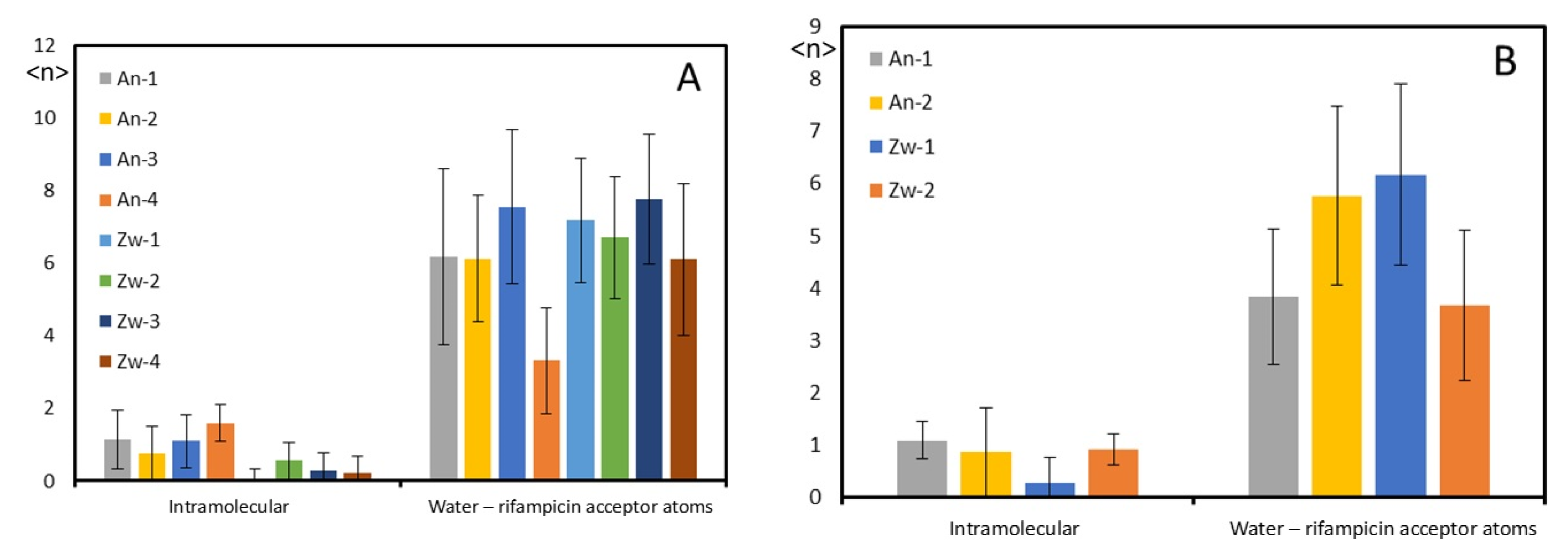
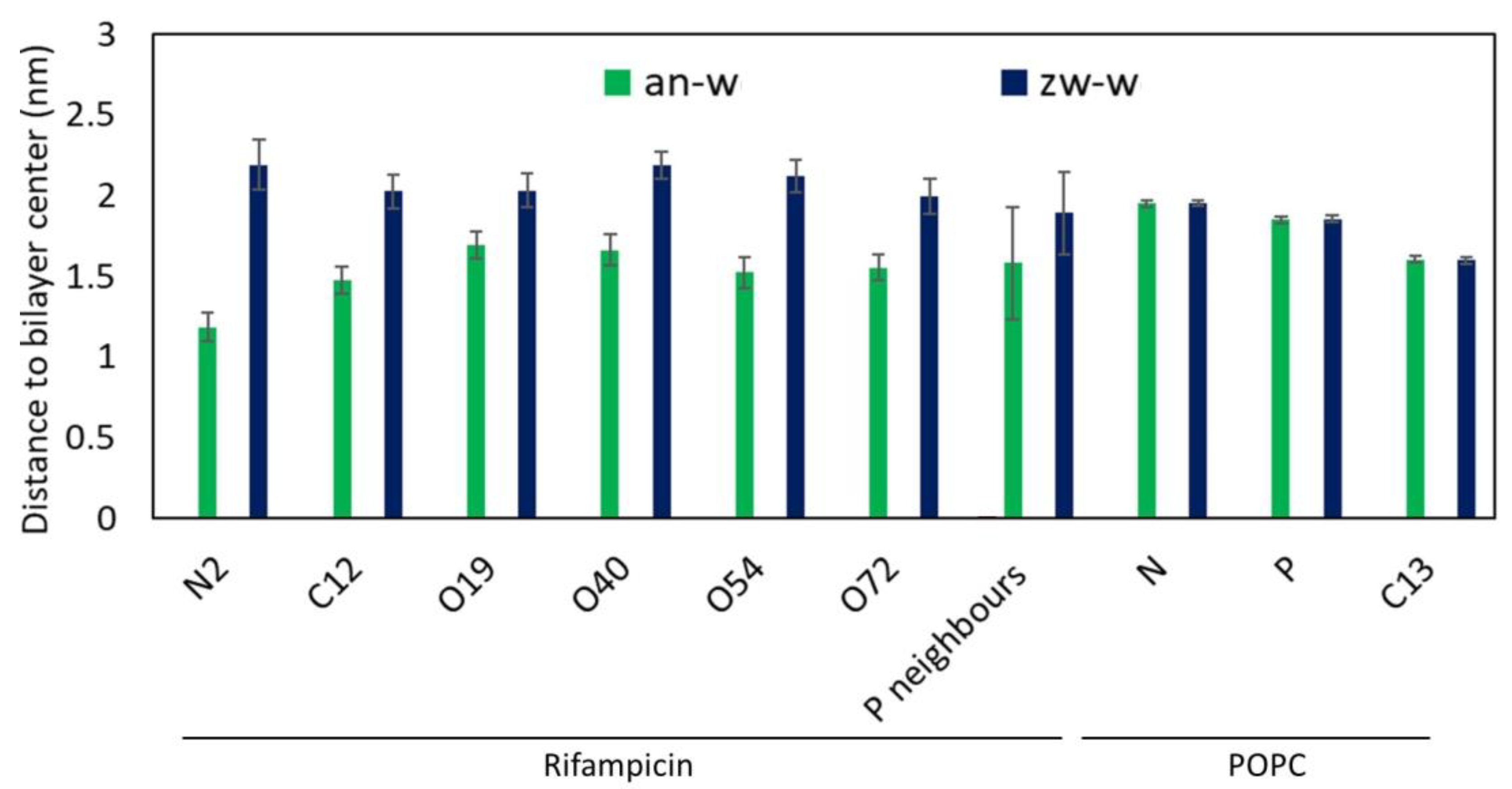
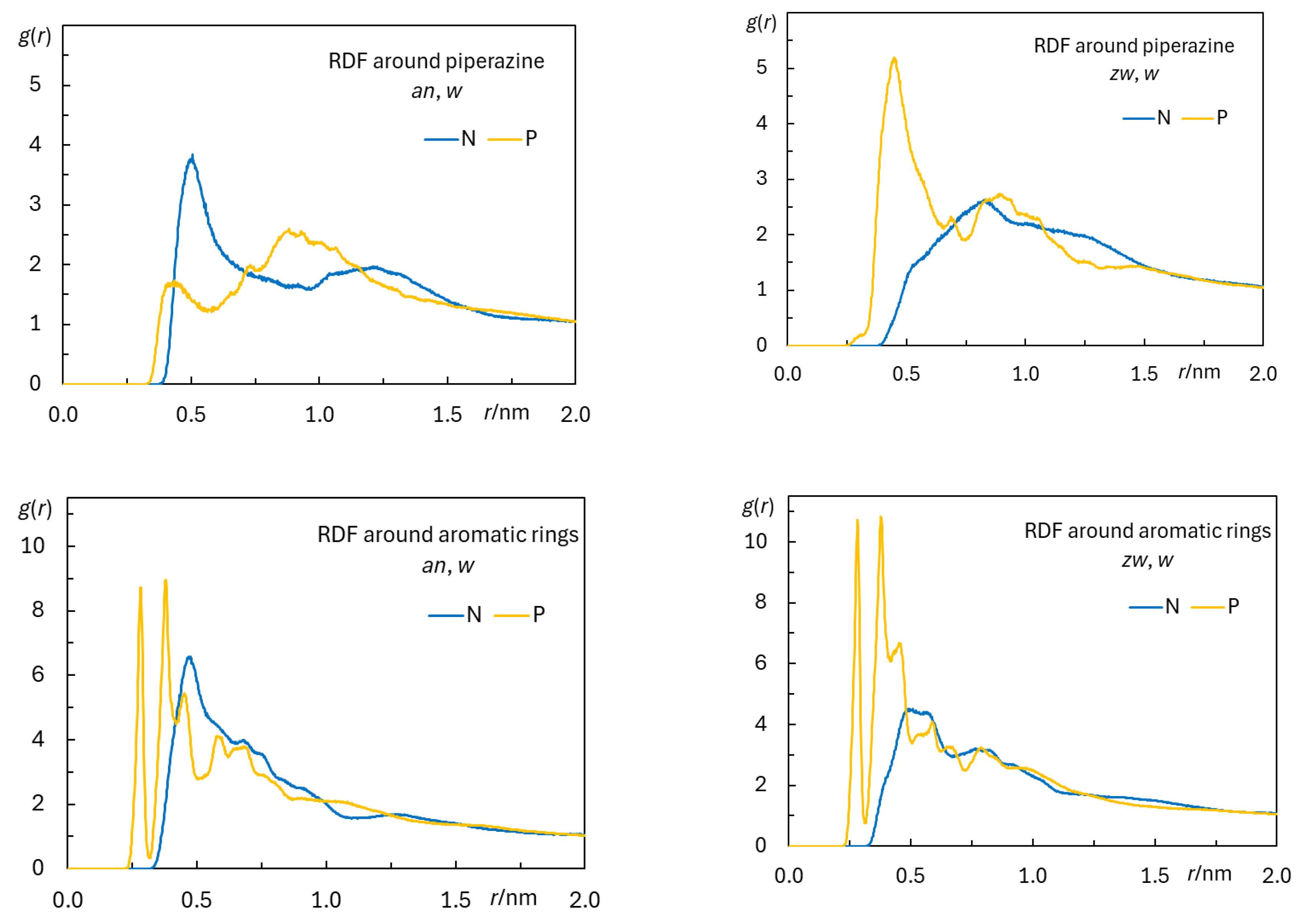
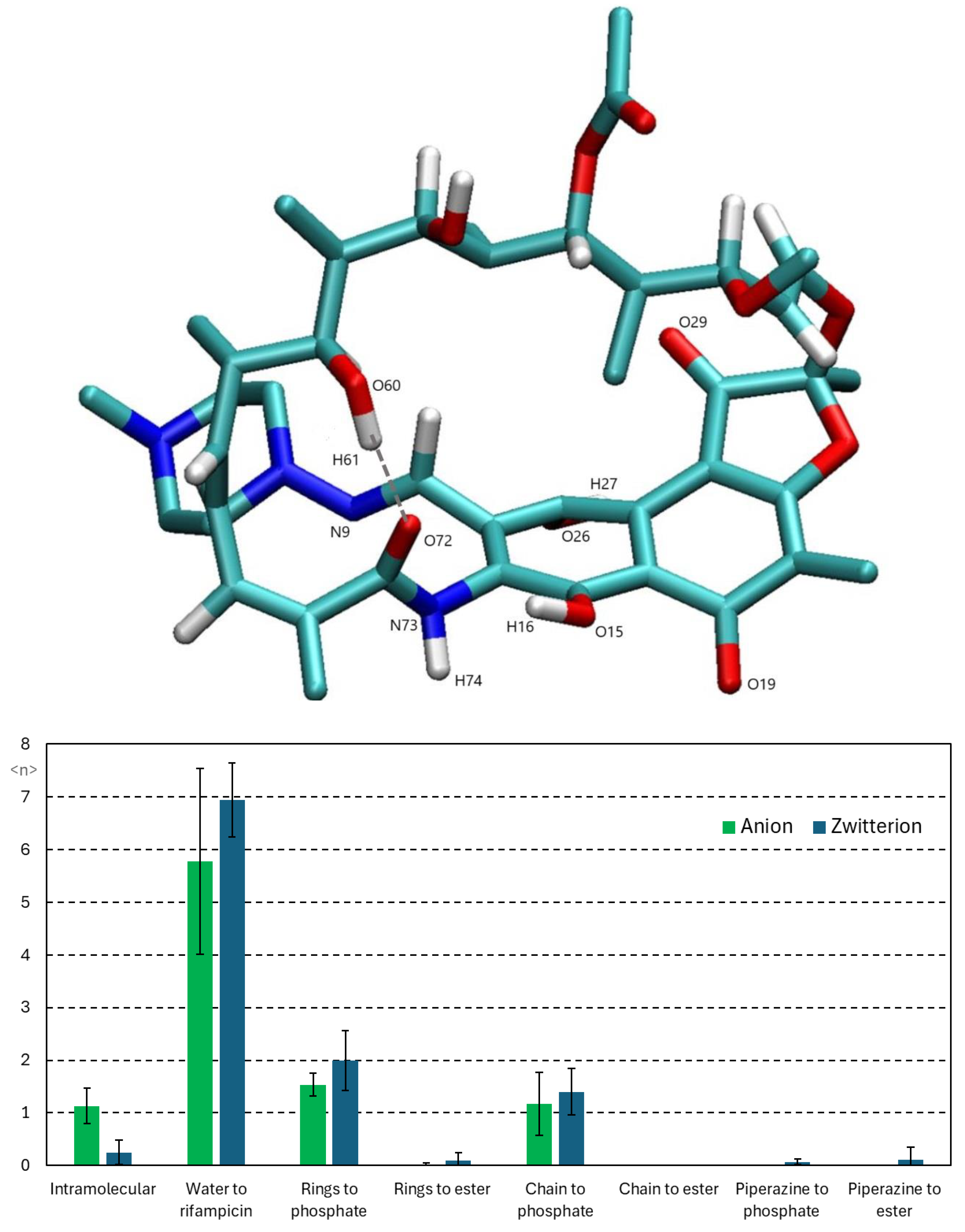
3.2.2. Interactions Between Rifampicin and Lipid Groups
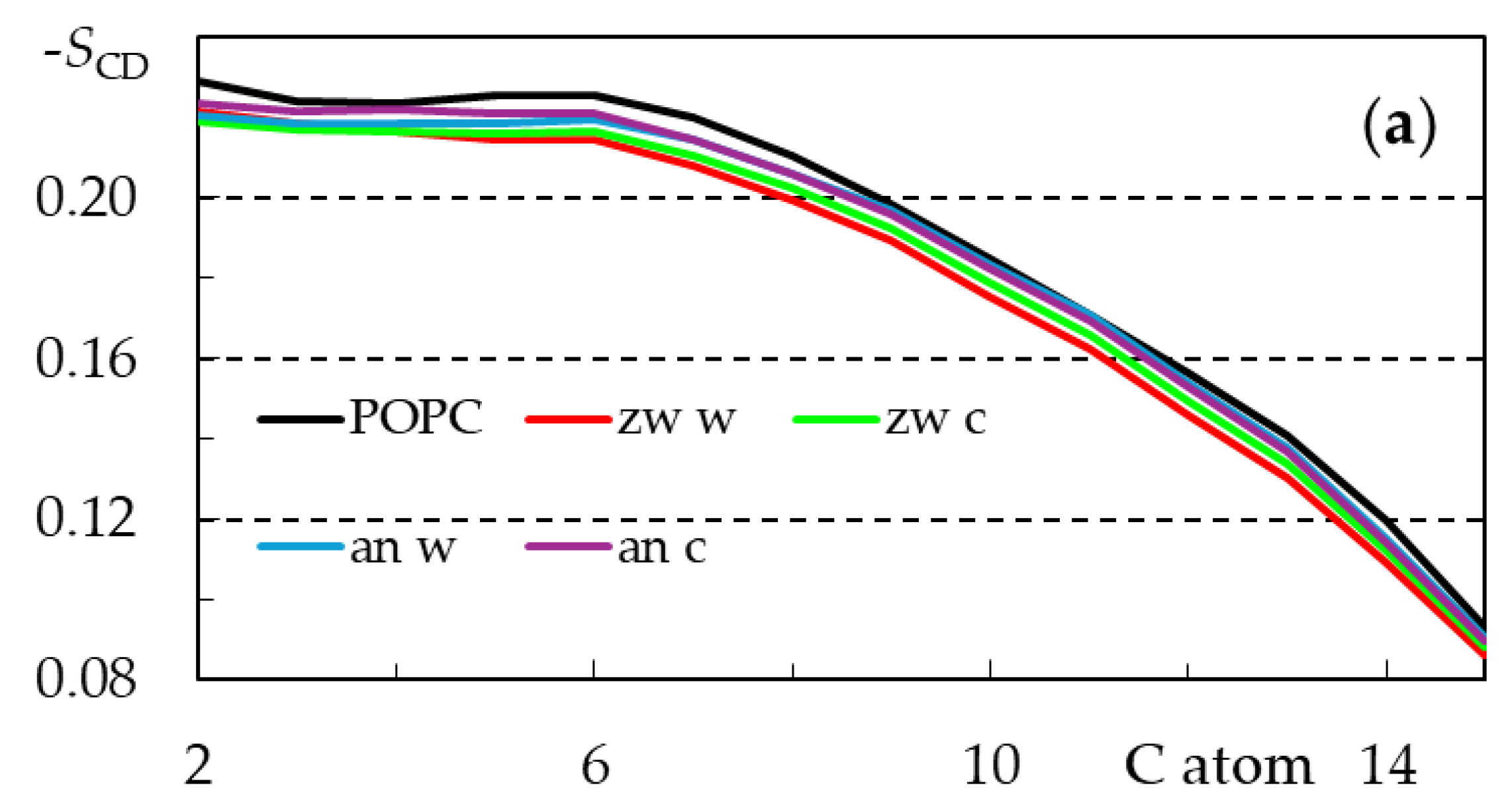
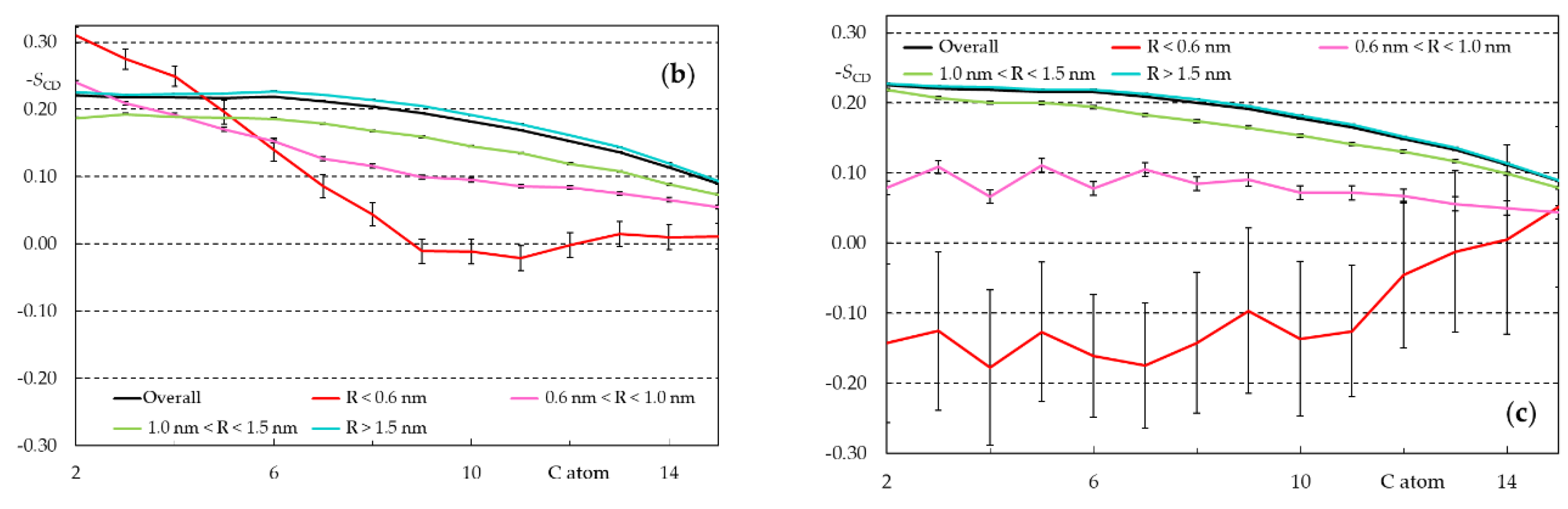
3.2.3. Rifampicin-Induced Bilayer Perturbation
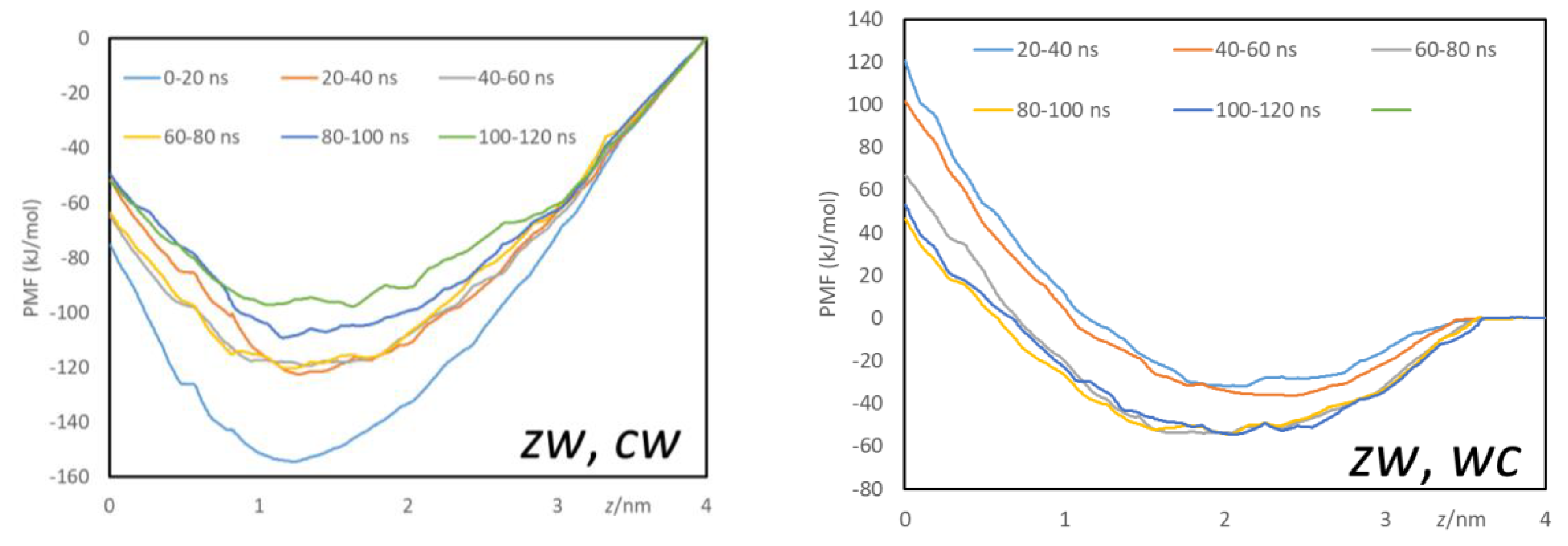
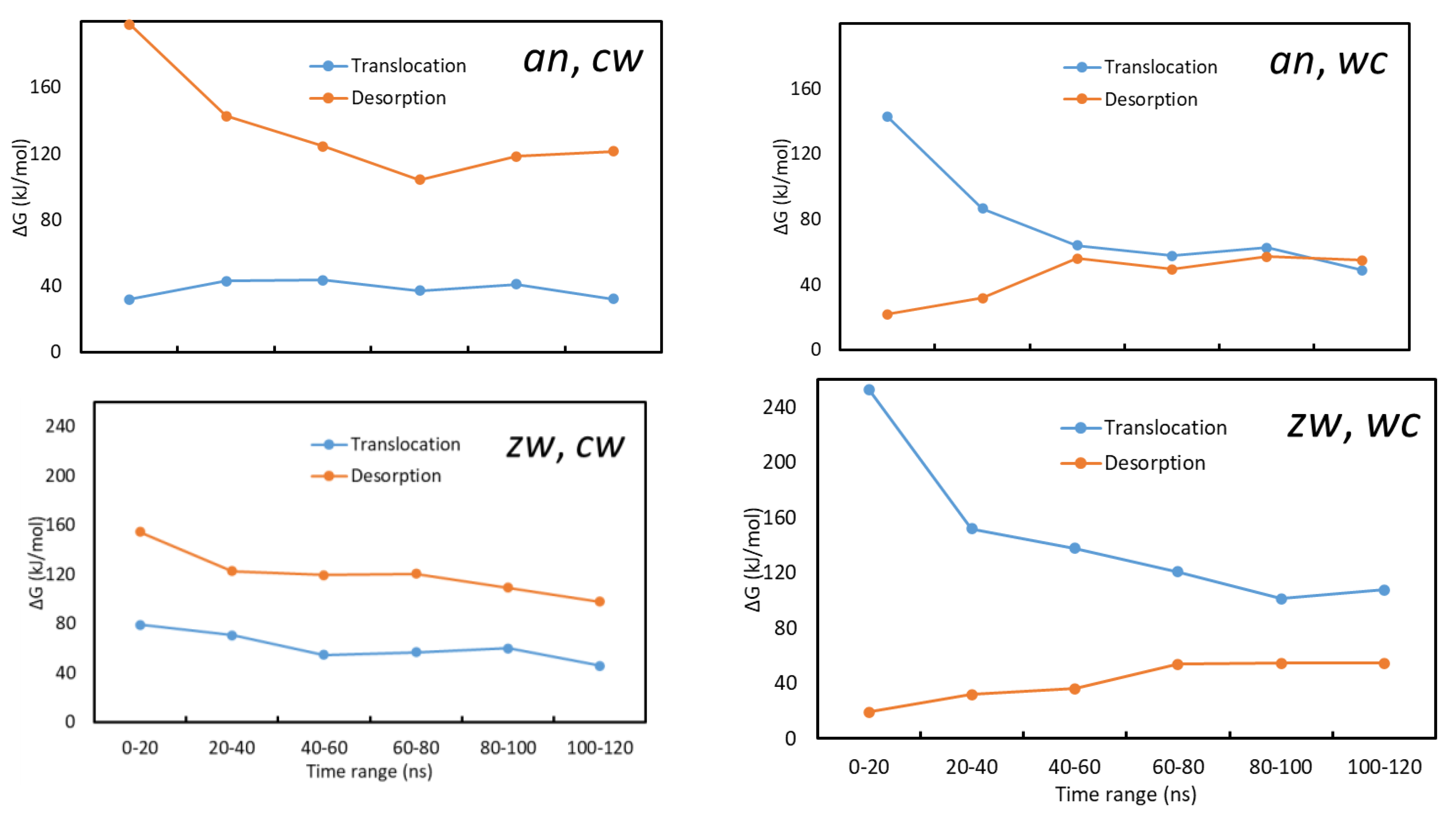
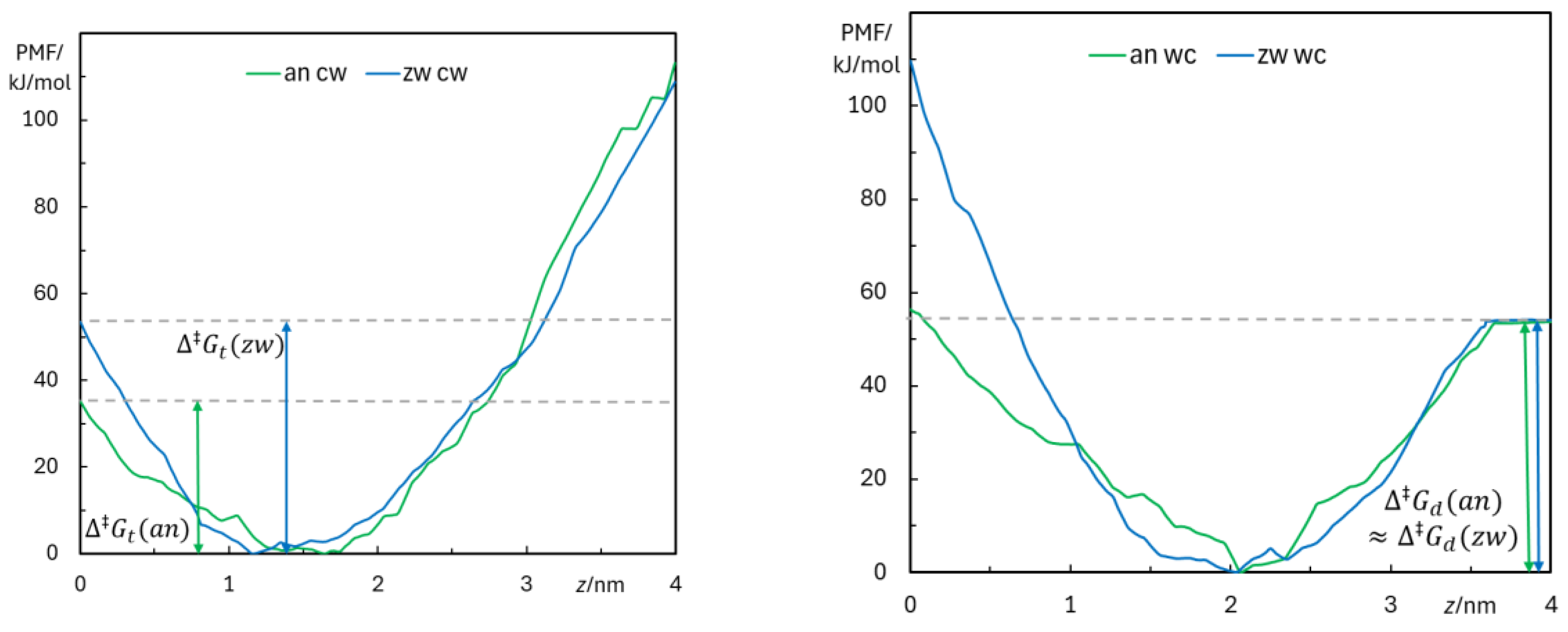
3.2.4. Free Energy Profiles from Umbrella Sampling MD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A - Complementary Experimental Data and Analysis
A1 – Protons Exchanged with the Buffer Due to Rifampicin Association with the LUVs
A2 – Changes in LUVs Zeta Potential Due to Association of Rifampicin
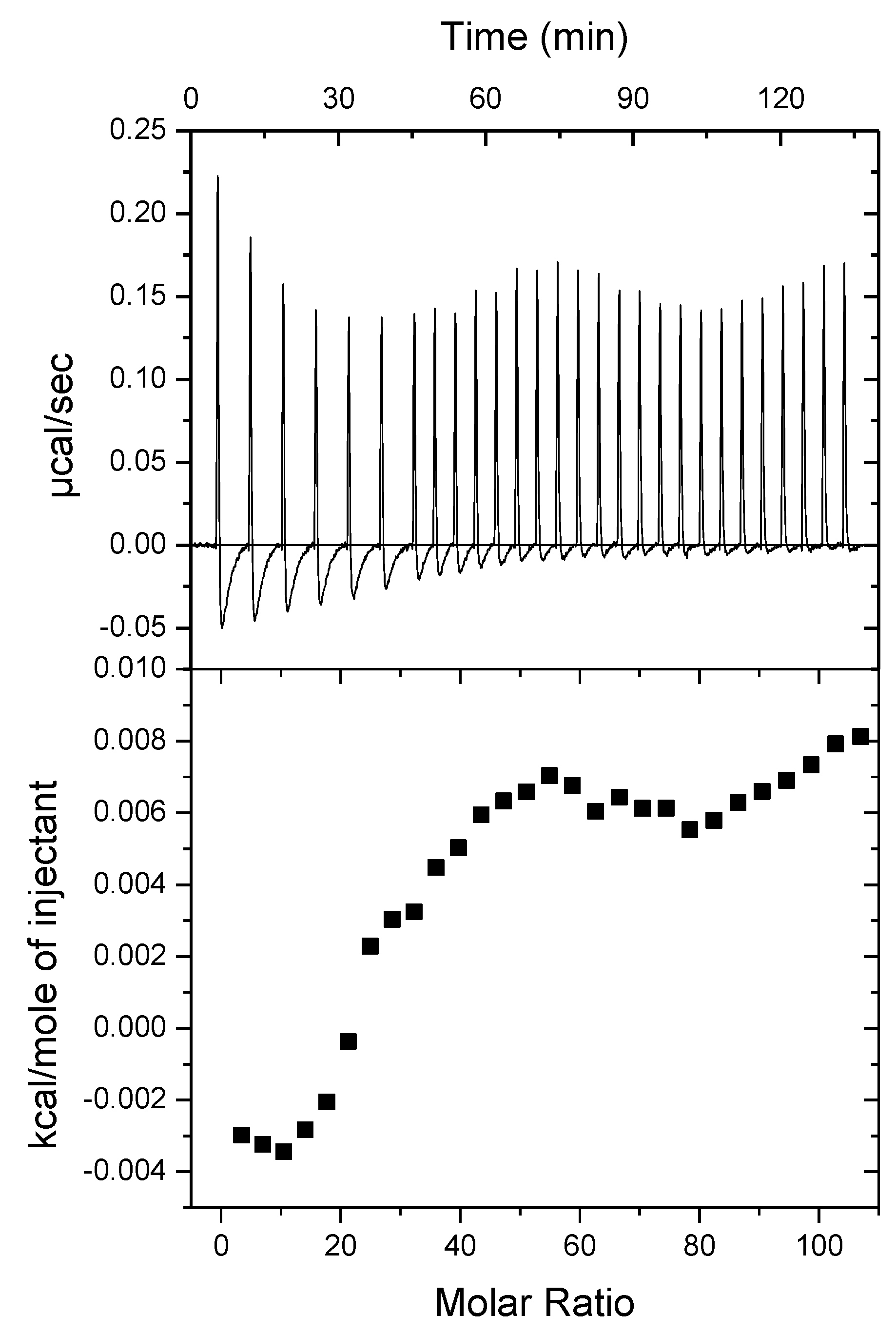
A3 – Thermograms Obtained by ITC for the Addition of POPC LUVs to 100 µM Rifampicin
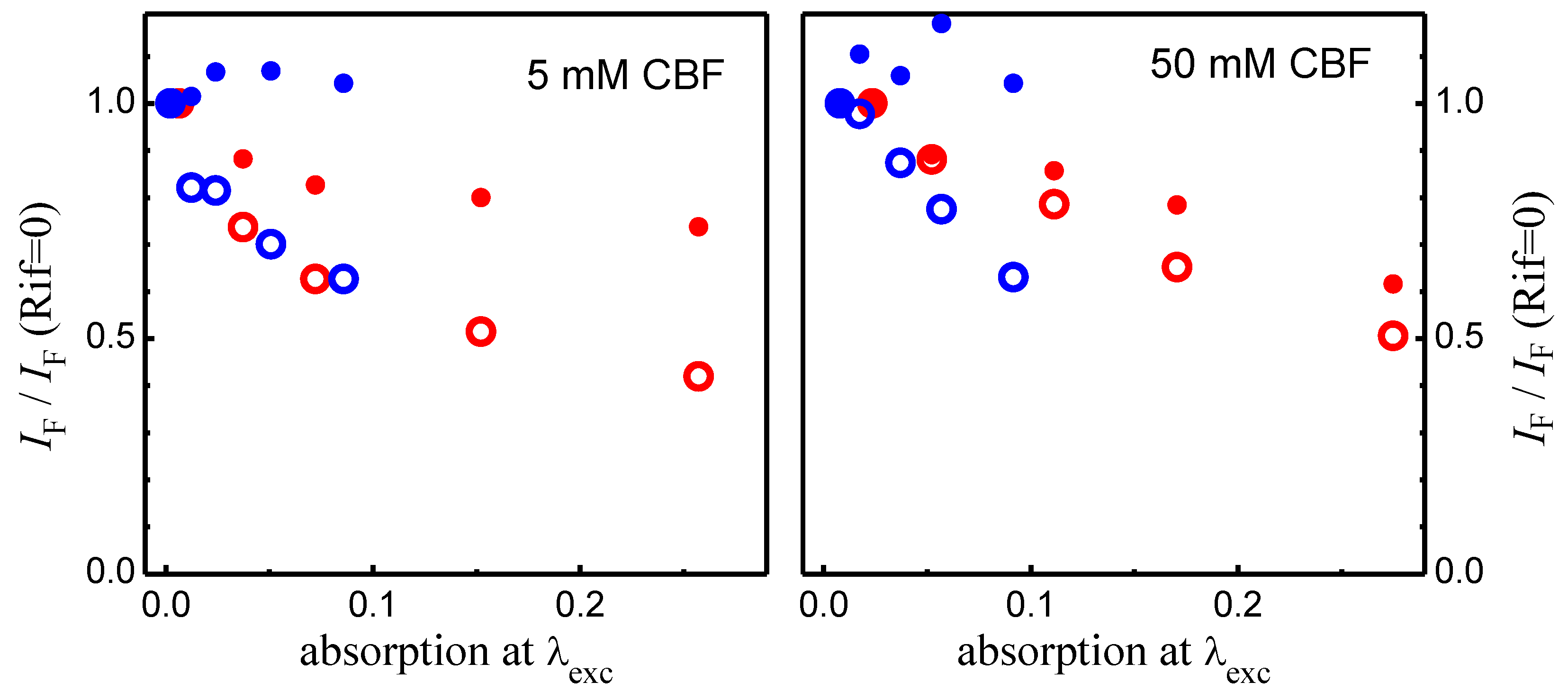
A4 – Calculation of the Extent of CBF Leakage from the Fluorescence Intensity
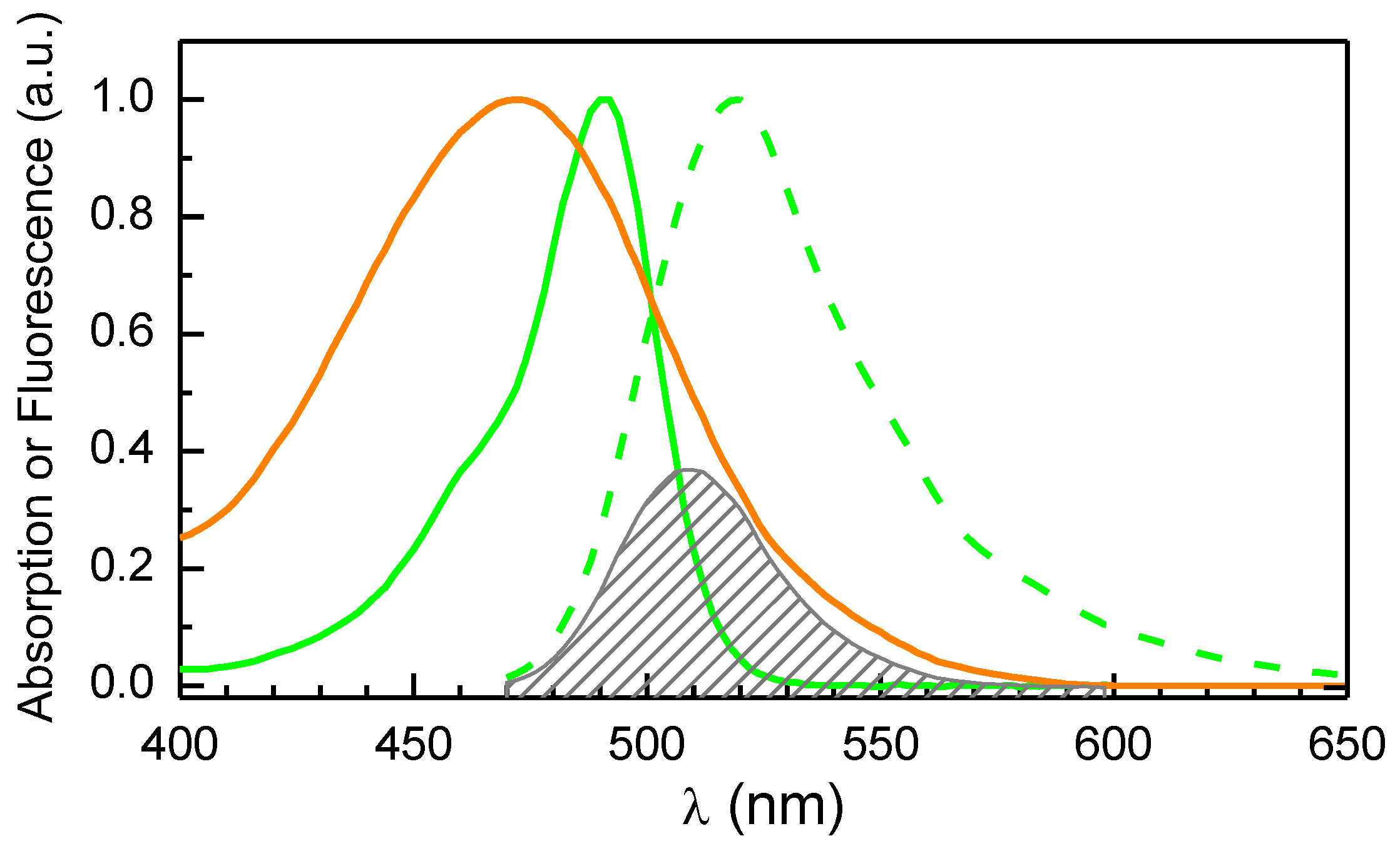
A5 – Spectral Overlap Between CBF and Rifampicin
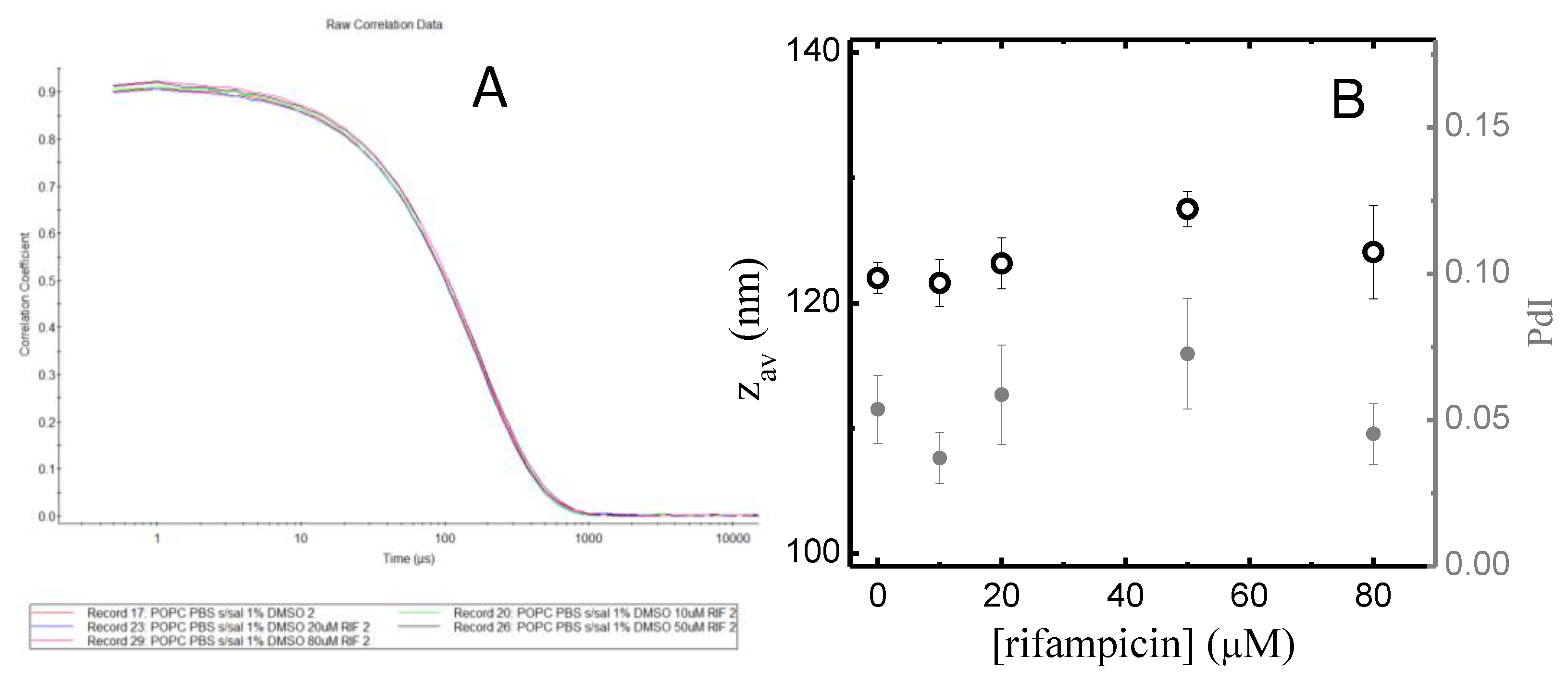
A6 – Size of the POPC LUVs in the Absence and Presence of Rifampicin
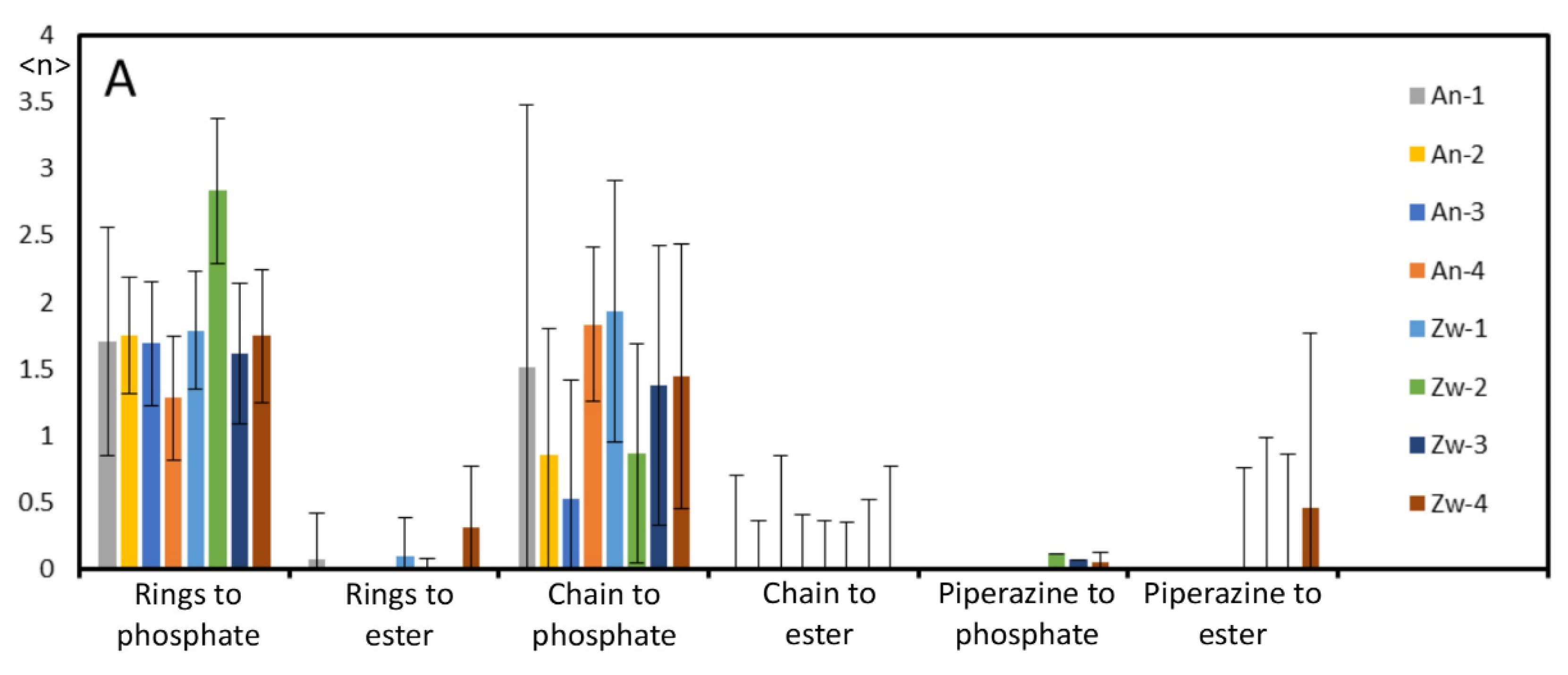
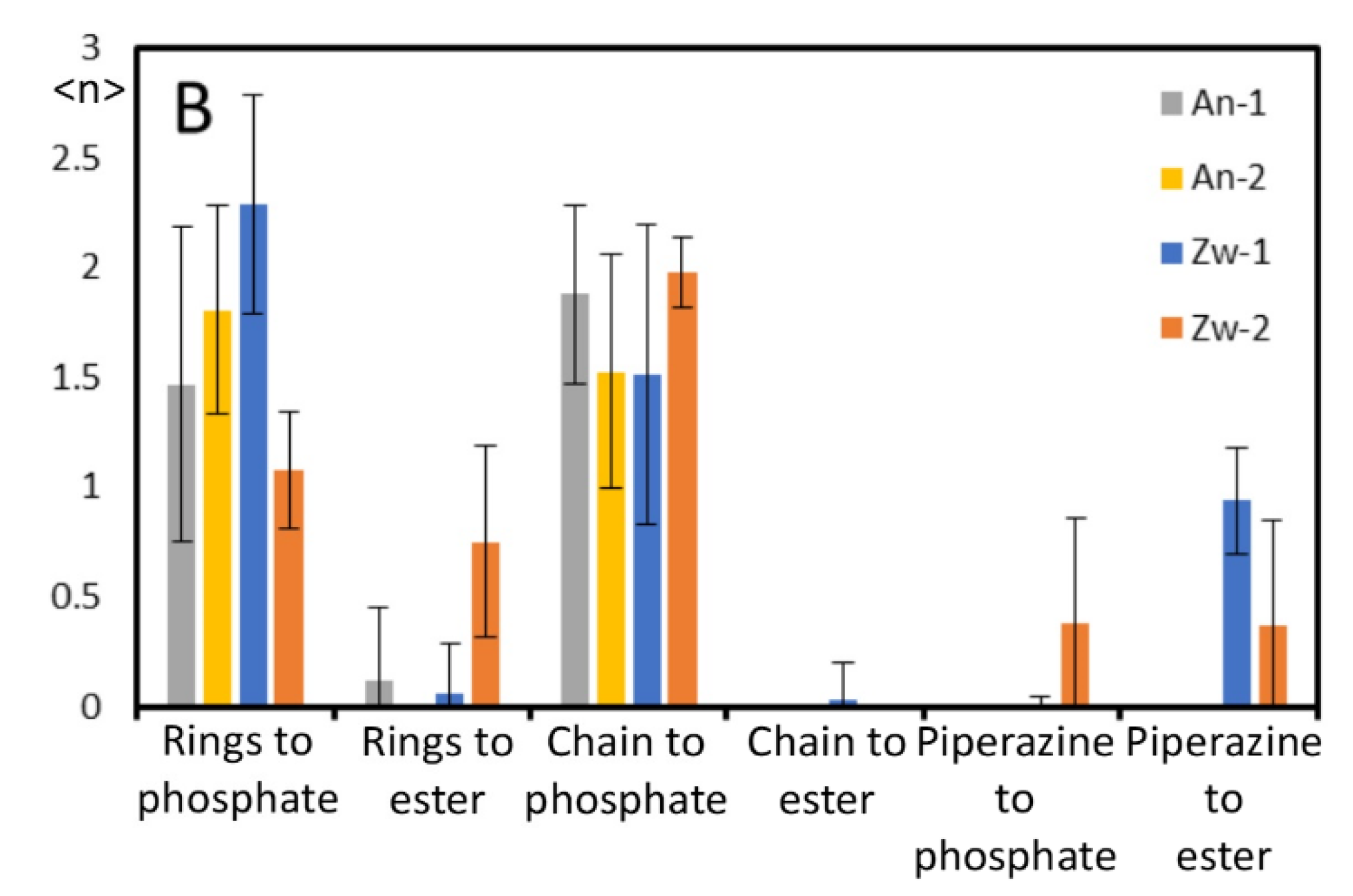
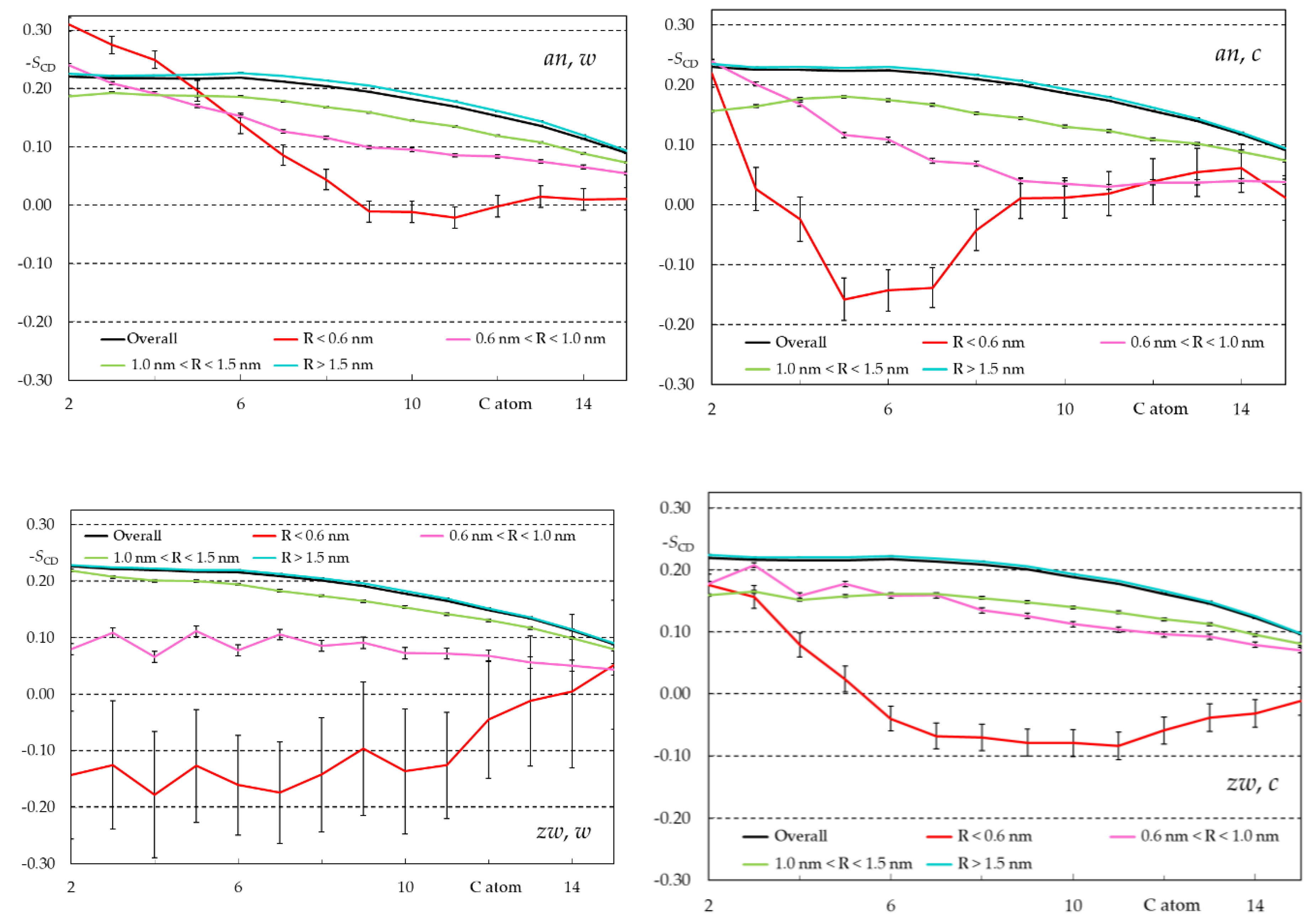
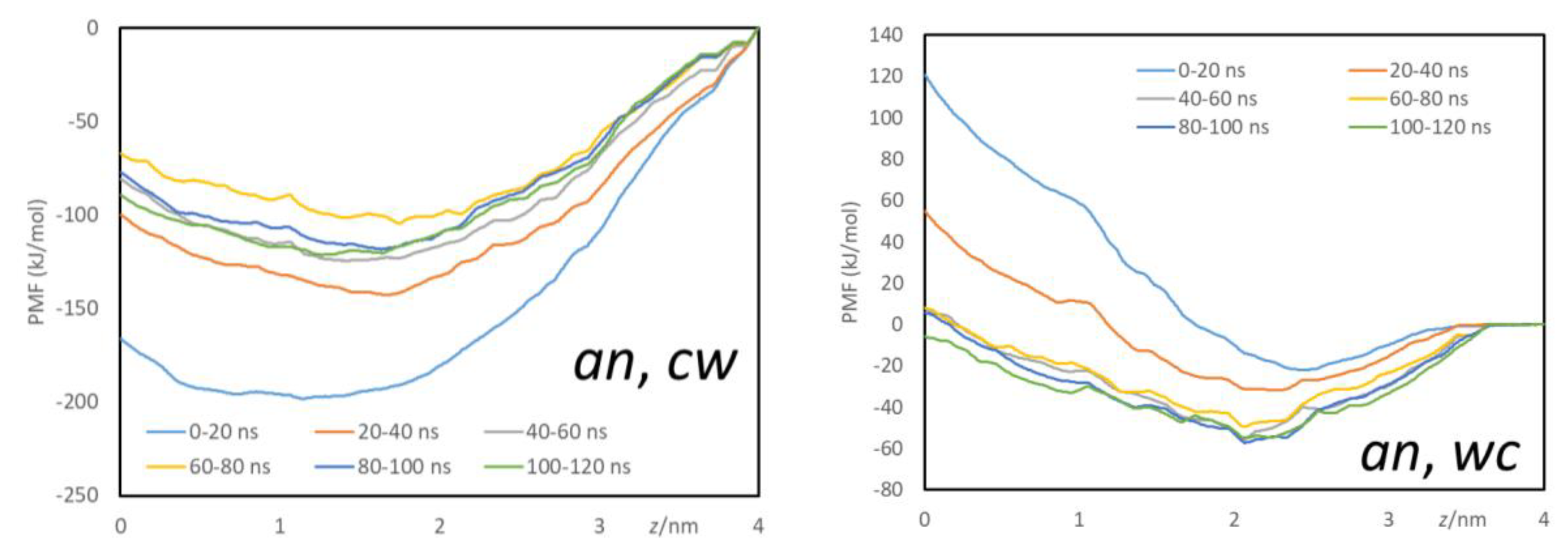
Appendix B - Complementary MD Simulation Results and Analysis.
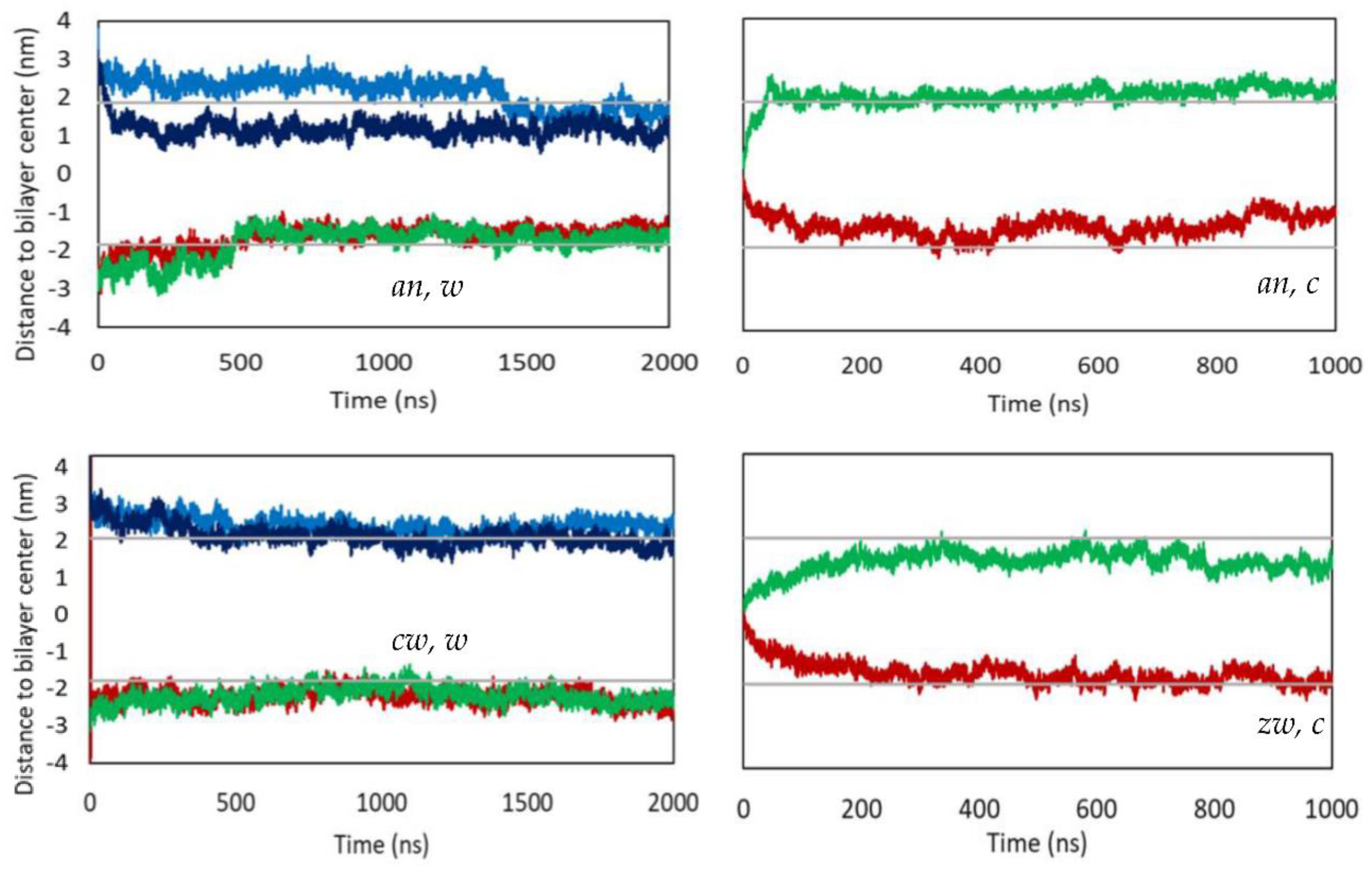
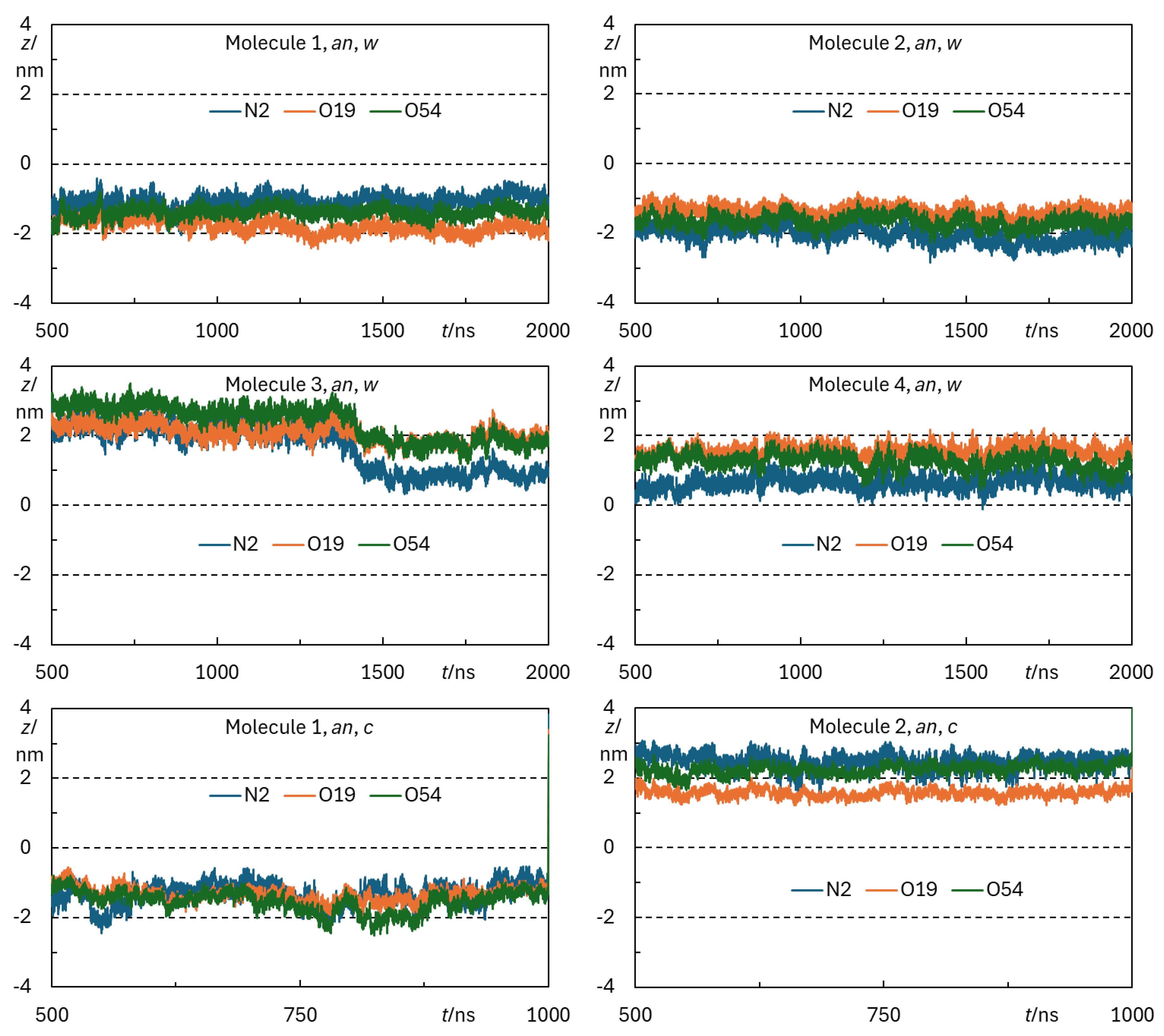
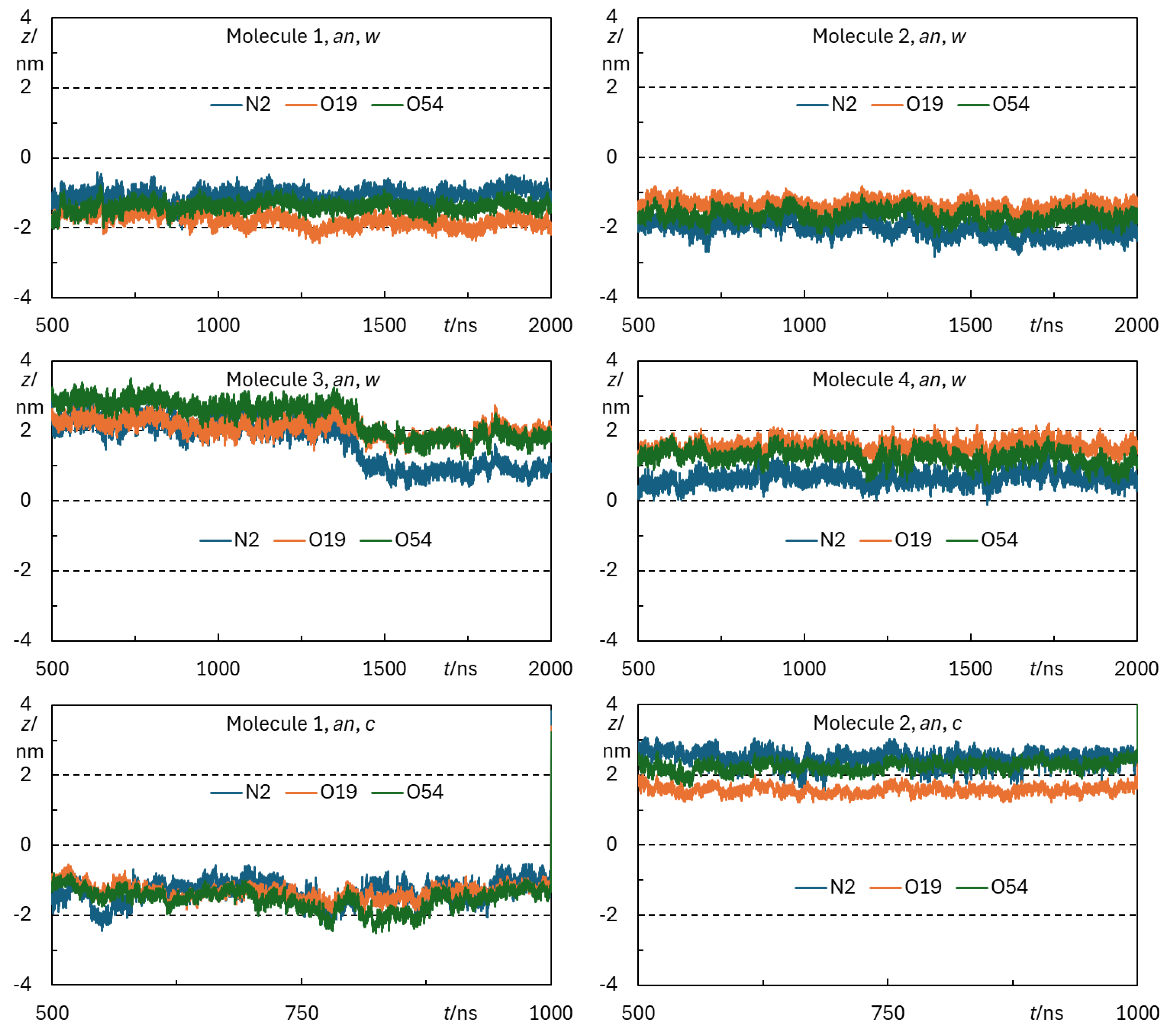
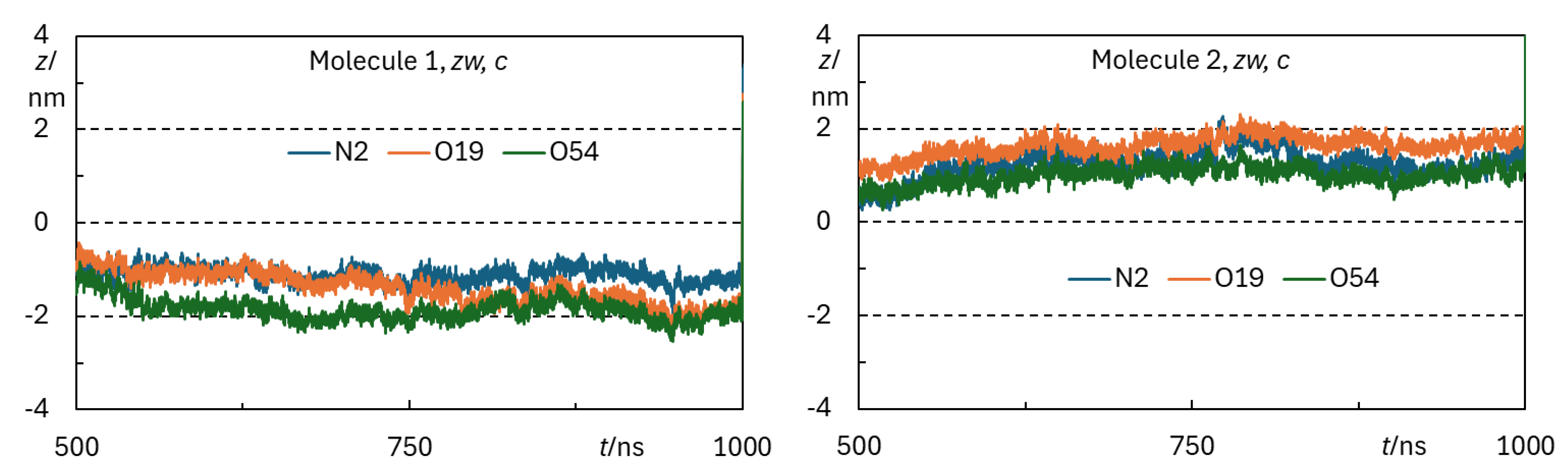
References
- Smith, D.; Artursson, P.; et al. Passive Lipoidal Diffusion and Carrier-Mediated Cell Uptake Are Both Important Mechanisms of Membrane Permeation in Drug Disposition. Mol. Pharm. 2014, 11(6), 1727–1738. [Google Scholar] [CrossRef]
- Matsson, P.; Doak, B.C.; et al. Cell permeability beyond the rule of 5. Adv. Drug Delivery Rev. 2016, 101, 42–61. [Google Scholar] [CrossRef]
- Doak, B.C.; Over, B.; et al. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chemistry & Biology 2014, 21(9), 1115–1142. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23(1–3), 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Delivery Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wieske, L.H.E.; Atilaw, Y.; et al. Going Viral: An Investigation into the Chameleonic Behaviour of Antiviral Compounds. Chemistry-a European Journal 2022, 29(8), e202202798. [Google Scholar] [CrossRef]
- Ermondi, G.; Vallaro, M.; et al. Rifampicin as an example of beyond-rule-of-5 compound: Ionization beyond water and lipophilicity beyond octanol/water. Eur. J. Pharm Sci. 2021, 161. [Google Scholar] [CrossRef]
- Kramer, S.D.; Aschmann, H.E.; et al. When barriers ignore the "rule-of-five". Adv. Drug Delivery Rev. 2016, 101, 62–74. [Google Scholar] [CrossRef]
- ChemAxon Marvin Sketch 22.9.0. 2022. http://www.chemaxon.com. http://www.chemaxon.com.
- Online, D. Rifampicin. https://go.drugbank.com/drugs/DB01045 (accessed 4 October 2024).
- Avdeef, A.; Kansy, M. "Flexible-Acceptor" General Solubility Equation for beyond Rule of 5 Drugs. Mol. Phar, 2020; 17, 10, 3930–3940. [Google Scholar] [CrossRef]
- Gallo, G.G.; Radaelli, P.; Rifampin. Analytical Profiles of Drug Substances 1976, 5, 468–509.
- Hermann, K.F.; Neuhaus, C.S.; et al. Kinetics of lipid bilayer permeation of a series of ionisable drugs and their correlation with human transporter-independent intestinal permeability. Eur. J. Pharm Sci. 2017, 104, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Samelo, J.; Mora, M.J.; et al. Partition of Amphiphilic Molecules to Lipid Bilayers by ITC: Low-Affinity Solutes. ACS Omega 2017, 2(10), 6863–6869. [Google Scholar] [CrossRef]
- Wiener, M.C.; White, S.H. Structure of a Fluid Dioleoylphosphatidylcholine Bilayer Determined by Joint Refinement of X-ray and Neutron-Diffraction Data.3. Complete Structure. Biophys. J. 1992, 61(2), 434–447. [Google Scholar] [CrossRef]
- Martins, P.T.; Velazquez-Campoy, A.; et al. Kinetics and Thermodynamics of Chlorpromazine Interaction with Lipid Bilayers: Effect of Charge and Cholesterol. JACS 2012, 134(9), 4184–4195. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, A.; Almeida, P.F.F. Kinetics of dye efflux and lipid flip-flop induced by delta-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, alpha-helical peptides. Biochemistry 2004, 43(27), 8846–8857. [Google Scholar] [CrossRef]
- Barbet, J.; Machy, P.; et al. Weak Acid-Induced Release of Liposome-Encapsulated Carboxyfluorescein. BBA 1984, 772(3), 347–356. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.J.; Hub, J.S. MemGen: a general web server for the setup of lipid membrane simulation systems. Bioinformatics 2015, 31(17), 2897–2899. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Eichenberger, A.P.; et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. Biophys. Lett. 2011, 40(7), 843–856. [Google Scholar] [CrossRef]
- Poger, D.; Van Gunsteren, W.F.; et al. A New Force Field for Simulating Phosphatidylcholine Bilayers. J. Comput. Chem. 2010, 31(6), 1117–1125. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; et al. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Pullman, B., Ed.; Springer: Dordrecht, 1981; Vol. 14. [Google Scholar]
- Malde, A.K.; Zuo, L.; et al. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. Journal of Chemical Theory and Computation 2011, 7(12), 4026–4037. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; et al. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. Journal of Chemical Theory and Computation 2018, 14(11), 5834–5845. [Google Scholar] [CrossRef]
- Barca, G.M.J.; Bertoni, C.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152(15). [Google Scholar] [CrossRef]
- Perdew, J.P. DENSITY-FUNCTIONAL APPROXIMATION FOR THE CORRELATION-ENERGY OF THE INHOMOGENEOUS ELECTRON-GAS. Phys. Rev. B 1986, 33(12), 8822–8824. [Google Scholar] [CrossRef]
- Becke, A.D.; Density-functional thermochemistry, I.I.I. The role of exact exchange. J. Chem. Phys. 1993, 98(7), 5648–5652. [Google Scholar] [CrossRef]
- Singh, U.C.; Kollman, P.A. AN APPROACH TO COMPUTING ELECTROSTATIC CHARGES FOR MOLECULES. J. Comput. Chem. 1984, 5(2), 129–145. [Google Scholar] [CrossRef]
- Besler, B.H.; Merz, K.M.; et al. ATOMIC CHARGES DERIVED FROM SEMIEMPIRICAL METHODS. J. Comput. Chem. 1990, 11(4), 431–439. [Google Scholar] [CrossRef]
- Lindahl, E.; Abraham, M.J.; et al. GROMACS 2019.4 Source Code. 2019, 2019. [Google Scholar]
- Abraham, M.J.; Murtola, T.; et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1 (Supplement C), 19–25. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29(7), 845–854. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; et al. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation 2008, 4(3), 435–447. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single-Crystals - A New Molecular-Dynamics Method. Journal of Applied Physics 1981, 52(12), 7182–7190. [Google Scholar] [CrossRef]
- Nose, S. A UNIFIED FORMULATION OF THE CONSTANT TEMPERATURE MOLECULAR-DYNAMICS METHODS. J. Chem. Phys. 1984, 81(1), 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics - Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31(3), 1695–1697. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. SETTLE - AN ANALYTICAL VERSION OF THE SHAKE AND RATTLE ALGORITHM FOR RIGID WATER MODELS. J. Comput. Chem. 1992, 13(8), 952–962. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; et al. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18(12), 1463–1472. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; et al. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103(19), 8577–8593. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. NON-PHYSICAL SAMPLING DISTRIBUTIONS IN MONTE-CARLO FREE-ENERGY ESTIMATION - UMBRELLA SAMPLING. J. Comput. Phys. 1977, 23(2), 187–199. [Google Scholar] [CrossRef]
- Hub, J.S.; de Groot, B.L.; et al. g_wham-A Free Weighted Histogram Analysis Implementation Including Robust Error and Autocorrelation Estimates. Journal of Chemical Theory and Computation 2010, 6(12), 3713–3720. [Google Scholar] [CrossRef]
- Kumar, S.; Bouzida, D.; et al. THE WEIGHTED HISTOGRAM ANALYSIS METHOD FOR FREE-ENERGY CALCULATIONS ON BIOMOLECULES.1. THE METHOD. J. Comput. Chem. 1992, 13(8), 1011–1021. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Martins, P.A.T.; et al. Interaction of Bile Salts with Model Membranes Mimicking the Gastrointestinal Epithelium: A Study by Isothermal Titration Calorimetry. Langmuir 2015, 31(33), 9097–9104. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Kishore, N.; et al. Thermodynamic Quantities for the Ionization Reactions of Buffers. J. Phys. Chem. Ref. Data 2002, 31(2), 231–370. [Google Scholar] [CrossRef]
- Fukada, H.; Takahashi, K. Enthalpy and heat capacity changes for the proton dissociation of various buffer components in 0.1 M potassium chloride. Protein. Struct. Funct. Genet. 1998, 33(2), 159–166. [Google Scholar] [CrossRef]
- Moreno, M.J.; Bastos, M.; et al. Partition of amphiphilic molecules to lipid bilayers by isothermal titration calorimetry. Anal. Biochem. 2010, 399(1), 44–47. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S. Electrostatic Potentials at Membrane-Solution Interfaces. In Current Topics in Membranes and Transport; Bronner, F., Kleinzeller, A., Eds.; Academic Press, 1977; Vol. 9, pp. 71–144. [Google Scholar]
- Matos, C.; de Castro, B.; et al. Zeta-Potential Measurements as a Tool to Quantify the Effect of Charged Drugs on the Surface Potential of Egg Phosphatidylcholine Liposomes. Langmuir 2004, 20(2), 369–377. [Google Scholar] [CrossRef]
- Winiski, A.P.; Eisenberg, M.; et al. Fluorescent-Probes of Electrostatic Potential 1-Nm from the Membrane-Surface. Biochemistry 1988, 27(1), 386–392. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.; Gresalfi, T.; et al. Adsorption of Mono-Valent Cations to Bilayer Membranes Containing Negative Phospholipids. Biochemistry 1979, 18(23), 5213–5223. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.A.L.; Coreta-Gomes, F.M.; et al. Synthesis and Characterization of a Lipidic Alpha Amino Acid: Solubility and Interaction with Serum Albumin and Lipid Bilayers. J. Phys. Chem. B 2013, 117(13), 3439–3448. [Google Scholar] [CrossRef]
- Rooney, E.K.; Lee, A.G. BINDING OF HYDROPHOBIC DRUGS TO LIPID BILAYERS AND TO THE (CA2++MG2+)-ATPASE. BBA 1983, 732(2), 428–440. [Google Scholar] [CrossRef] [PubMed]
- Rooney, E.K.; East, J.M.; et al. INTERACTION OF FATTY-ACIDS WITH LIPID BILAYERS. BBA 1983, 728(2), 159–170. [Google Scholar] [CrossRef]
- Kucerka, N.; Nieh, M.P.; et al. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. BBA-Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.N.; Freire, J.M.; et al. Quantitative analysis of molecular partition towards lipid membranes using surface plasmon resonance. Scientific Reports 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Yoshikami, S.; et al. LIPOSOME-CELL INTERACTION - TRANSFER AND INTRACELLULAR RELEASE OF A TRAPPED FLUORESCENT MARKER. Science 1977, 195(4277), 489–492. [Google Scholar] [CrossRef] [PubMed]
- Missner, A.; Pohl, P. 110 Years of the Meyer-Overton Rule: Predicting Membrane Permeability of Gases and Other Small Compounds. Chemphyschem 2009, (9–10), 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Knutson, J.R. Mechanism of Fluorescence Concentration Quenching of Carboxyfluorescein in Liposomes - Energy-Transfer to Nonfluorescent Dimers. Anal. Biochem. 1988, 172(1), 61–77. [Google Scholar] [CrossRef] [PubMed]
- Kibblewhite, J.; Drummond, C.J.; et al. Lipoidal Eosin and Fluorescein Derivatives as Probes of the Electrostatic Characteristics of Self-Assembled Surfactant Water Interfaces. J. Phys. Chem. 1989, 93(21), 7464–7473. [Google Scholar] [CrossRef]
- Loura, L.M.S. Simple Estimation of Forster Resonance Energy Transfer (FRET) Orientation Factor Distribution in Membranes. IJMS 2012, 13(11), 15252–15270. [Google Scholar] [CrossRef] [PubMed]
- Bani-Yaseen, A.D.; Hammad, F.; et al. On the Photophysicochemical Properties of Selected Fluoroquinolones: Solvatochromic and Fluorescence Spectroscopy Study. J. Fluoresc. 2013, 23(1), 93–101. [Google Scholar] [CrossRef] [PubMed]
- Odehnalová, K.; Balouch, M.; et al. Liposomal Copermeation Assay Reveals Unexpected Membrane Interactions of Commonly Prescribed Drugs. Mol. Phar 2024, 21(6), 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Silvander, M.; Johnsson, M.; et al. Effects of PEG-lipids on permeability of phosphatidylcholine/cholesterol liposomes in buffer and in human serum. Chem. Phys. Lipids 1998, 97(1), 15–26. [Google Scholar] [CrossRef] [PubMed]
- Heerklotz, H. Membrane stress and permeabilization induced by asymmetric incorporation of compounds. Biophys. J. 2001, 81(1), 184–195. [Google Scholar] [CrossRef]
- Jimenez, D.G.; Vallaro, M.; et al. Molecular properties, including chameleonicity, as essential tools for designing the next generation of oral beyond rule of five drugs. Admet Dmpk 2024. [Google Scholar] [CrossRef]
- Neves, M.C.; Filipe, H.A.L.; et al. Interaction of Bile Salts With Lipid Bilayers: An Atomistic Molecular Dynamics Study. Frontiers in Physiology 2019, 10, 393. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreno, M.J.; et al. How To Tackle the Issues in Free Energy Simulations of Long Amphiphiles Interacting with Lipid Membranes: Convergence and Local Membrane Deformations. J. Phys. Chem. B 2014, 118(13), 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, N.; Simões, G.M.; et al. Interactions between Rhodamine Dyes and Model Membrane Systems—Insights from Molecular Dynamics Simulations. Molecules 2022, 27(4), 1420. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




