Submitted:
18 January 2025
Posted:
21 January 2025
You are already at the latest version
Abstract
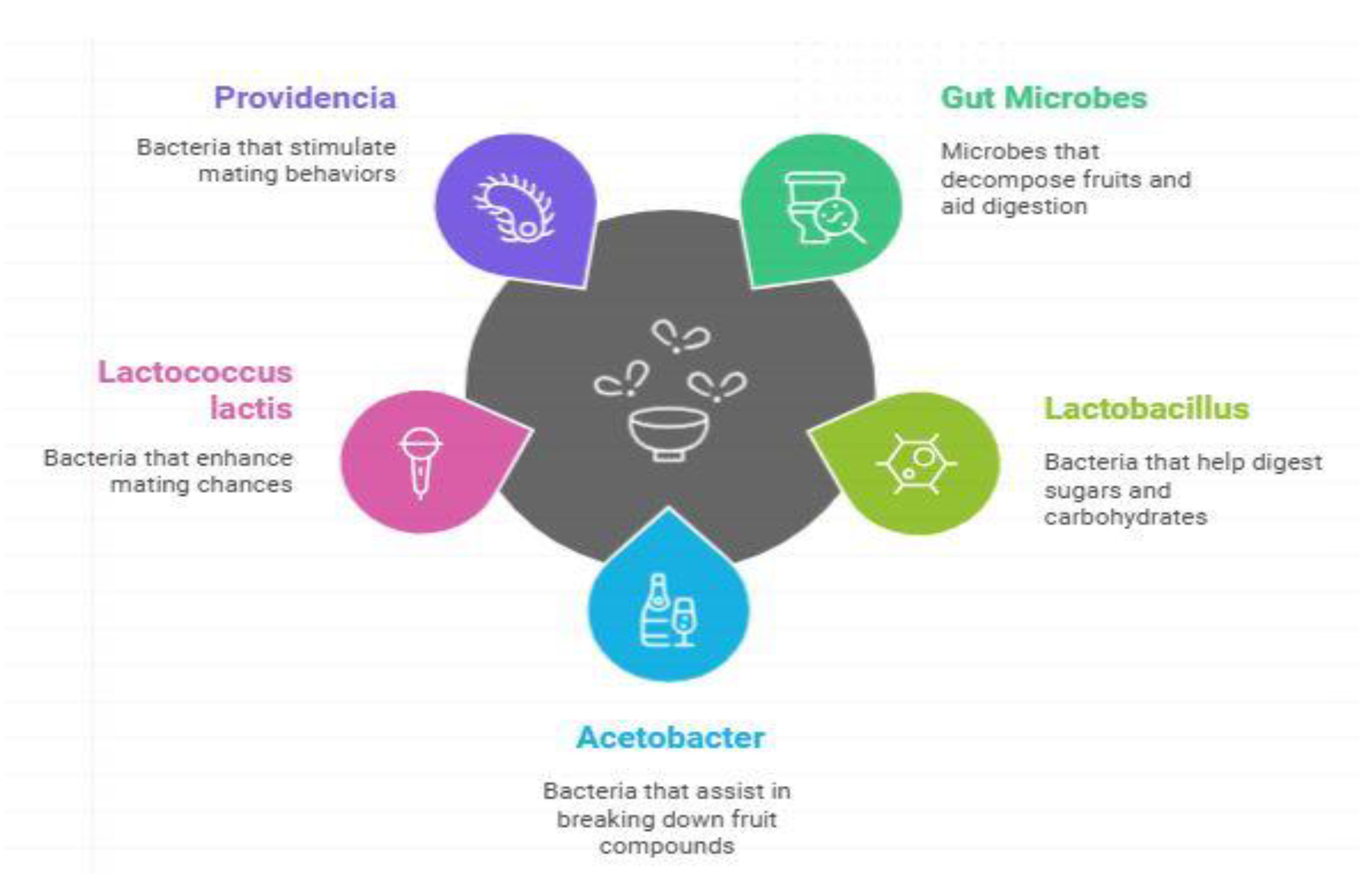
Bactrocera zonata or peach fruit fly, is a quarantined pest and is a major threat to horticultural crops especially in Pakistan and South Asian region. Influence of gut microbiota in determining the biological and behavioral features of B. zonata concerning digestion, immunity, mating, and foraging. Bacterial species which are Enterobacter, Lactobacillus, and Acetobacter are involved in the various aspects of nutritional acquisition, immunology, and fertility through producing volatile metabolites that impacts on sexual activity. It also plays a part in the ecological adaptations of gut microbiota; they put forward new approaches to integrated pest management (IPM). This Integrated pest management deals with microbiome change, pheromones disruption and the use of microbiome boosted baits, which supports the environmental objectives of agriculture. Digital surveillance and monitoring systems can be used to enhance the real-time adoption. IPM strategies such as microbiota manipulation and pheromone intervention present ecological innovative pest control solutions to chemical insecticides. These methods involve using protein based chemicals, microorganisms and mechanical attractants such as methyl eugenol and protein hydrolysate lures. Modern technologies provide higher accuracy and efficacy of these methods: digital video surveillance, and the use of automated equipment in monitoring contribute to suppression of B. zonata. This review predisposes indications of microbiome-targeted approaches towards changing the ‘paradigm of pest control’ in the context of IPM, reducing pesticide reliance, perusing conservation of beneficial insects and indeed nurturing sustainable agriculture. This biological control aims for field testing, gut microbiota manipulation, and targeted microbial management for location dependent pest management solutions for optimization of pest control and to tackle current and emerging issues in agriculture.
Keywords:
Introduction
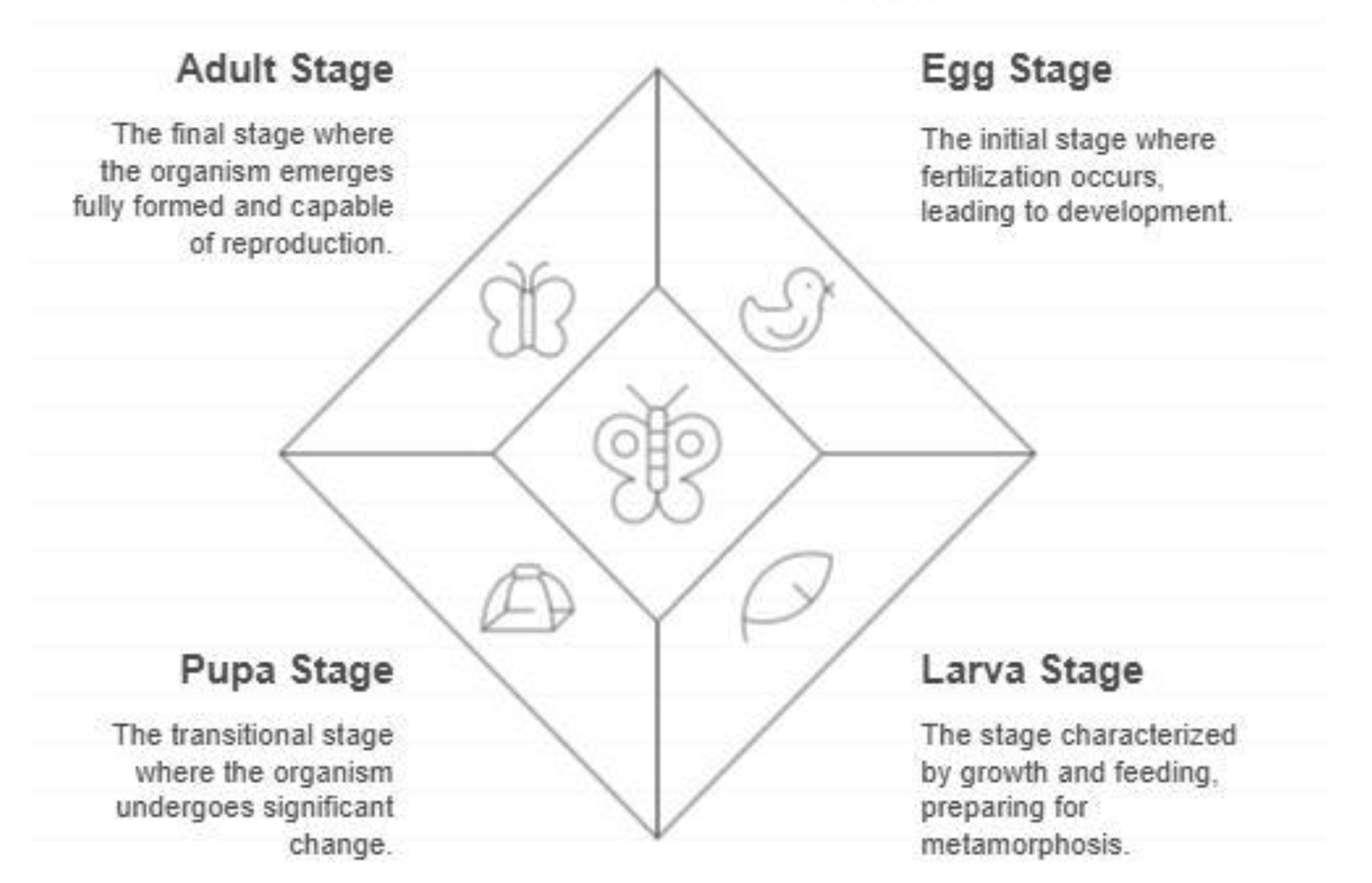
Understanding Life Stages of Drosophila melanogaster
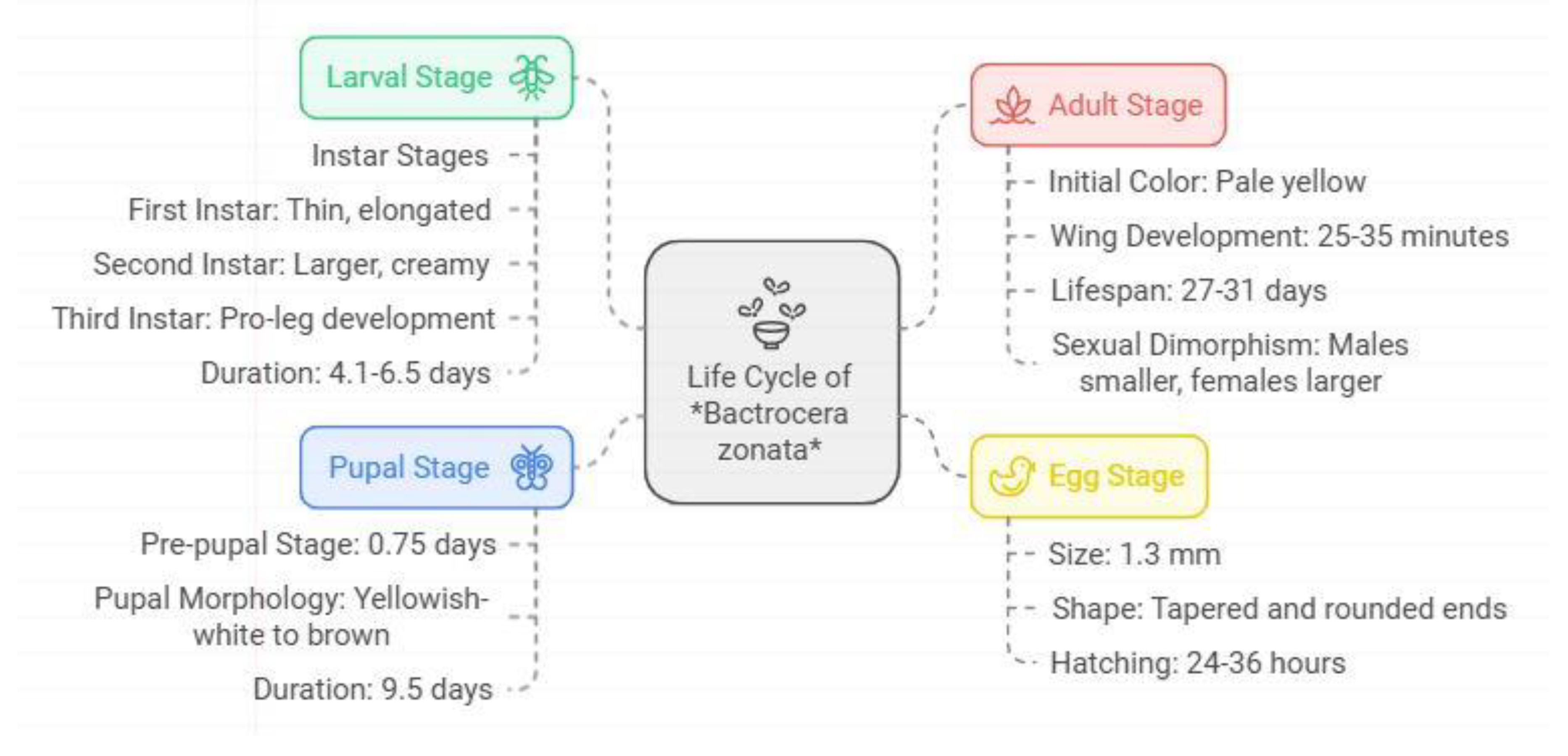
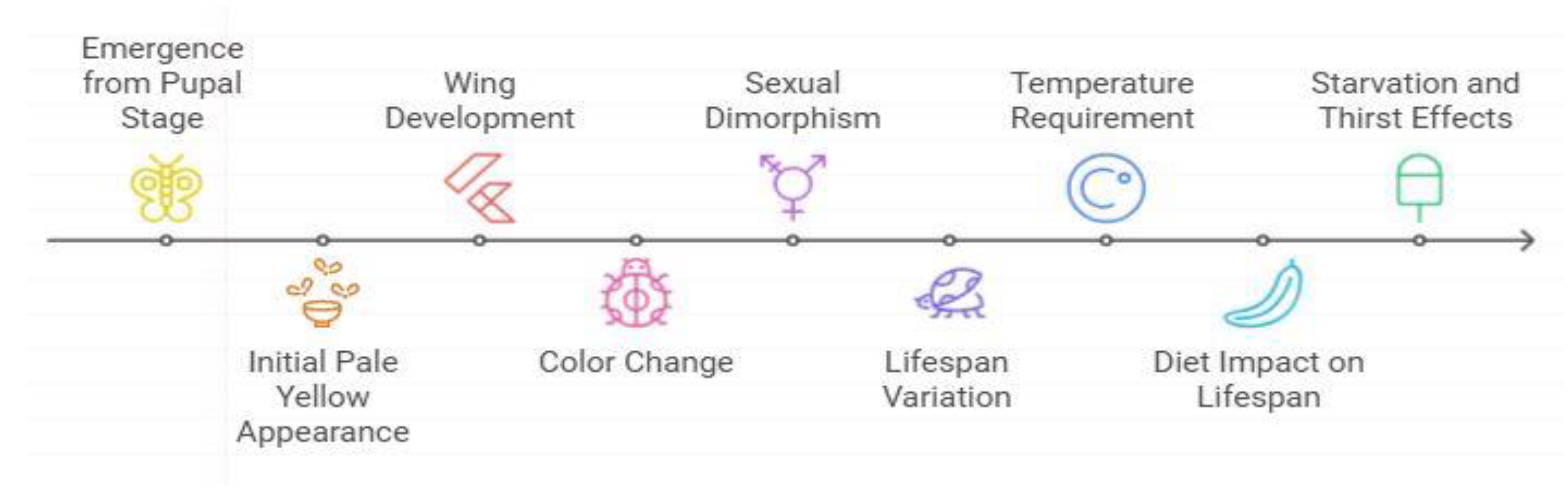
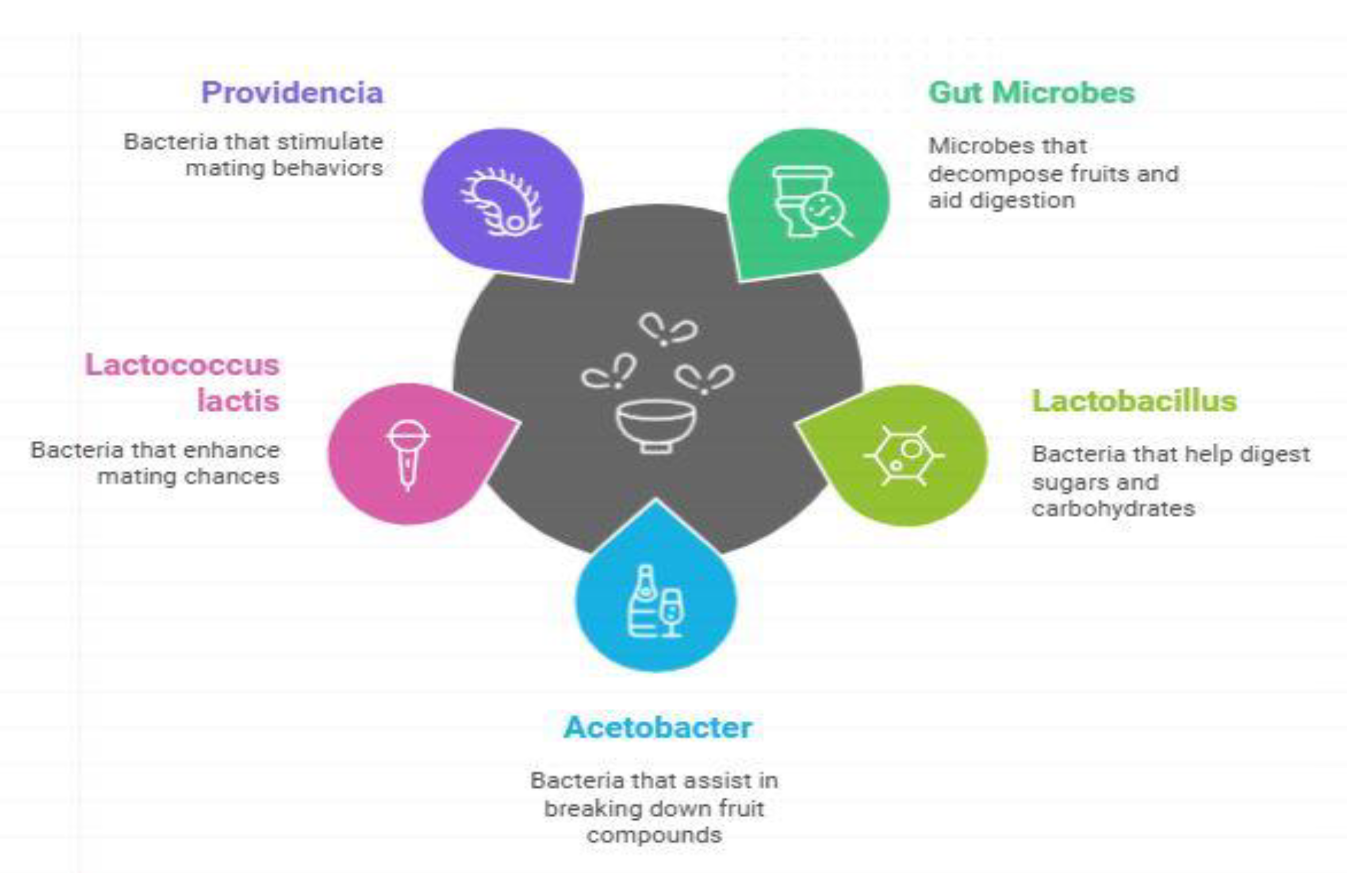
Gut Microbiota Association with Host Microbe Intercation and Response in Drosophila malanogster
| Reference | Years | Key findings | Methodology |
| Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens | (Deng et al. 2021; Hanan et al. 2023). | For digestion, Enterobacter, for immunity Lactobacillus, for reproduction Acetobacter are found to be important gut microbiota in Bactrocera zonata. | Molecular methods including metagenomic sequencing and culturing methods are employed to quantify and characterize the gut bacteria in Bactrocera zonata. These techniques made it possible to separate gut-associated bacteria. |
| Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives | (Liu et al. 2022). | Some gut microbiota improve reproductive output through transforming molecules that act on sexual behaviors | To investigate microbial composition, Scientist used 16S rRNA gene sequencing; for microbiota-derived metabolites, they used metabolomics; and they used behavioral tests to measure reproductive and sexual behaviors. Microbiota-positive and negative models animals were compared to decipher individual microbial contributions. Molecular biology techniques as well as quantified enzyme activity mapped the remaining pathways of metabolite transformation. |
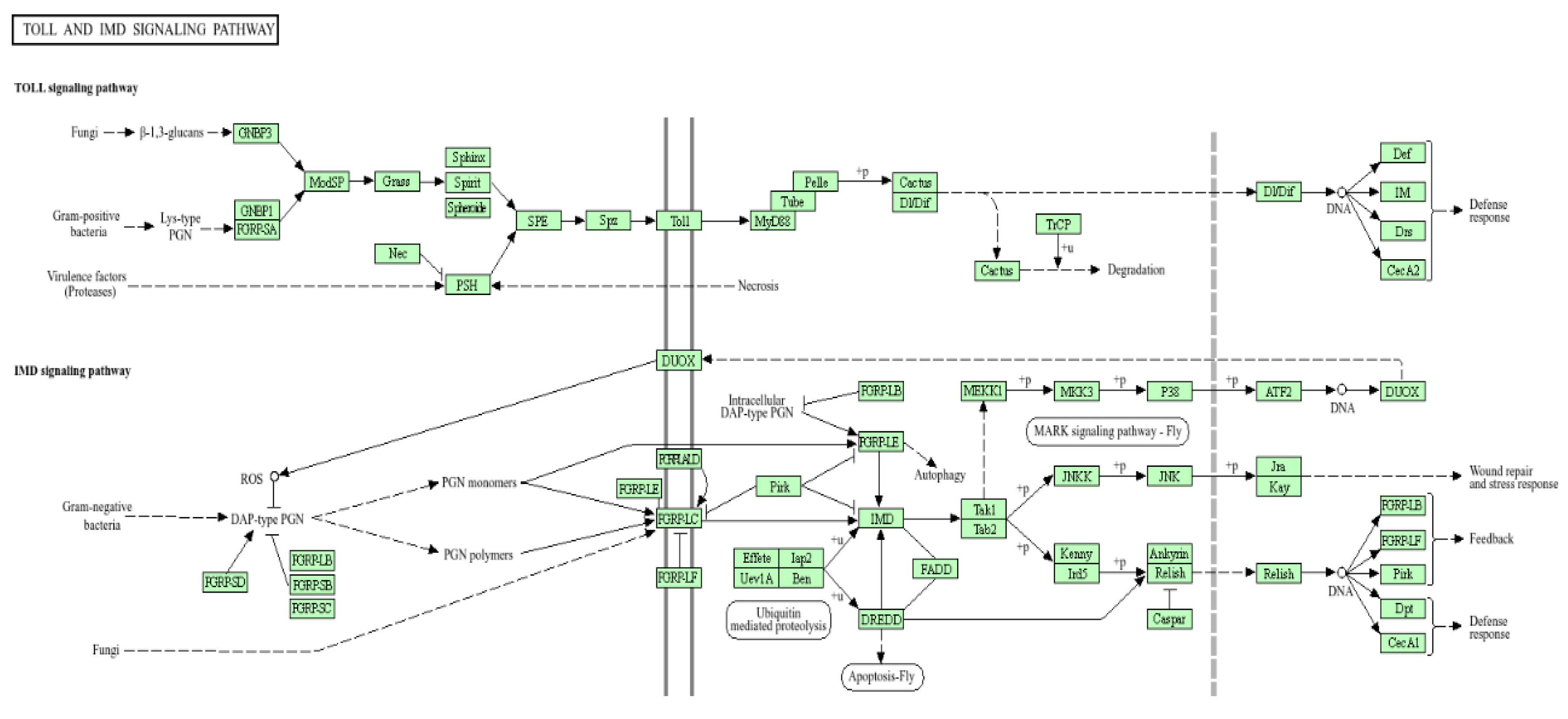
| Combining experimental evolution with next-generation sequencing: a powerful tool to study adaptation from standing genetic variation | (Schlötterer et al. 2021; Takahiro et al. 2007). | Microbial entries influencing the immune pathways like KEGG-Toll and Interrupting Defense like System (IMD) were seen to protect the fruit flies Bactrocera zonata and Drosophila melanogaster from bacterial and fungal infections. | Scientist used traditional Darwin’s process of natural selection with biochemical technology of next-generation sequencing for analyzing adaptation based on standing genetic variation. They examined how microbes impact immune systems through Toll and IMD pathways through genomics from sequences and their immune response assay. These method showed that microbial entries safeguard of fruit flies Drosophila melanogaster from bacterial and fungal diseases. |
| The Microbiota-Gut-Brain Axis | (John et al. 2021; McMullen et al. 2020). | Alteration of gut microbiota was suggested as an effective and eco-friendly pest management, as it lowers the immune response and fertility. | Scientist investigated the microbiota–gut–brain axis and considered the disruption of gut microbiota as an environmental friendly approach toward pest control. Using real-world samples and model organisms, they applied microbiome profiling via 16S rRNA gene sequencing; they also manipulated gut microbial ecosystems and assessed effects on immunity and reproduction. Pest susceptibility and reproductive changes were studied using behavioral and physiological bioassays. |
| Selecting aggressiveness to improve biological control agents efficiency | (Royer. P.F et al. 2024; Vargas et al. 2015). | Pest management with bacterial bio-control and protein baits along with pheromones proved its potential for effective population management reducing chemical pesticides use. | Scientist examined the effectiveness of bacterial bio control agents products for biological control pest with special emphasis on protein baits and pest pheromones. This called for field trials in population suppression, laboratory assays in the determination of the aggressiveness of biocontrol agents, and behavioral assays in pheromone attraction. Regarding the approach of crop rotation and integrating the culture of the BT cotton plant, this was shown to possess good prospects in eliminating the use of chemical pesticides. |
| Application of response surface Methodology coupled with Artificial Neural network and genetic algorithm to model and optimize symbiotic interactions between Chlorella vulgaris and Stutzerimonas stutzeri strain J3BG for chlorophyll accumulation | (Salma et al. 2022; International Journal of Entomology, 2023). | Guava and mango fruits were established as the preferred hosts of Bactrocera zonata with guava giving the highest pupal recovery. Most population found in them | They implemented response surface method together with ANNs (artificial neural networks) and genetic algorithms to maximize the relationships’ symbiosis. They also established that Bactrocera zonata mostly breeds on guava and mango, with the highest number of pupae recovered from guava. Population trends and sex/age preferences were assessed using fruit- infestation trials and the number of pupae recovered. |
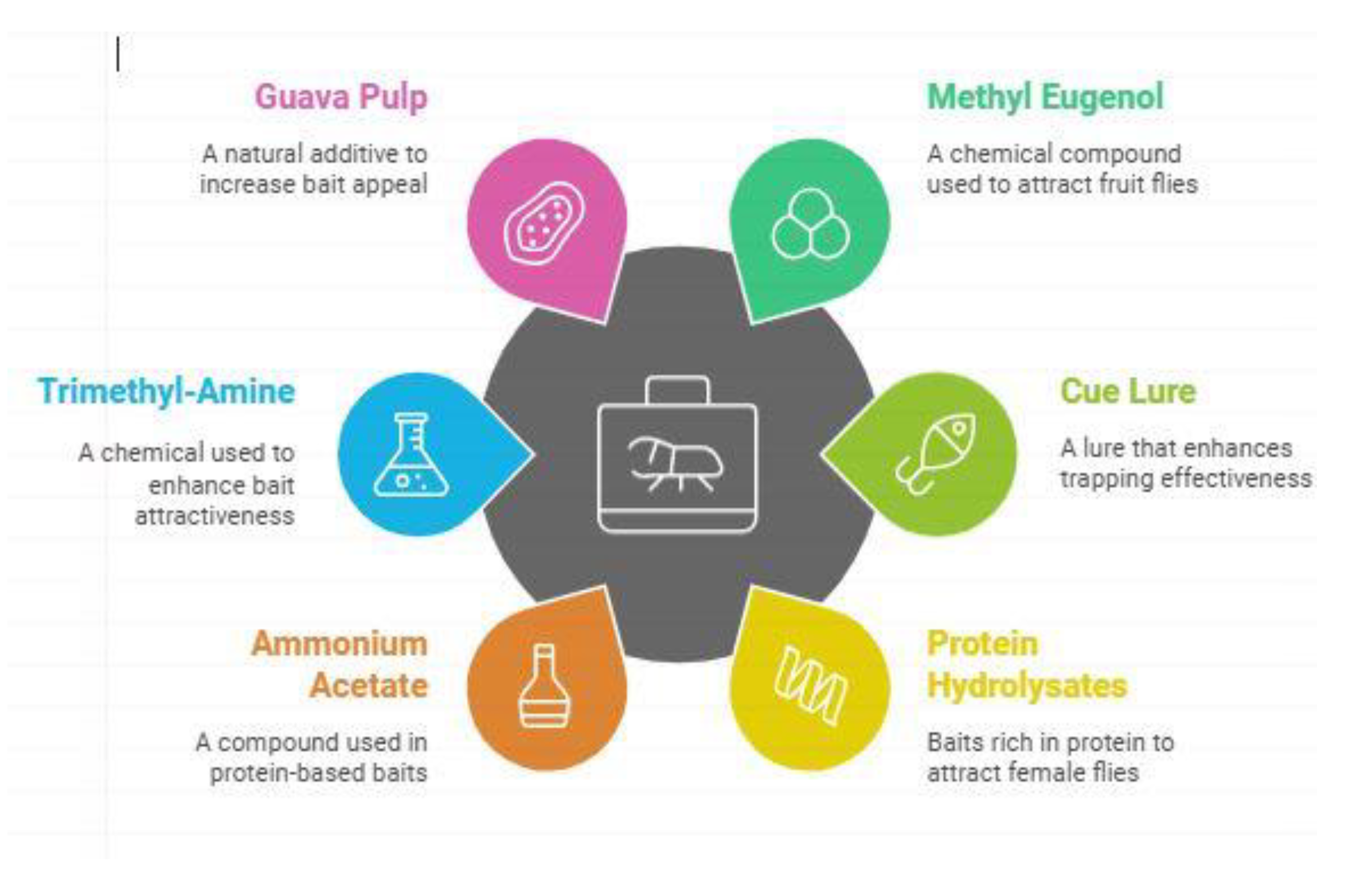
| Olfactory receptors in neural regeneration in the central nervous system | (Royer. P.F et al. 2024; Lillo et al. 2023). | As can be seen for both methyl eugenol and cue lures, innovative trapping techniques are found to increase the effectiveness of fruit fly capture. | Scientist focused on olfactory receptors matter about neural regeneration in the central nervous system. The methodology used by authors which stated thatd examining how methyl eugenol and cue lures, which are modern methods of trapping, improve capturing of fruit flies. |
| Automatic Detection and Monitoring of Insect Pests—A Review | (Lima et al. 2020; | Instant control by using video surveillance and sensor equipped traps are presented as pest populations monitoring tools that provide possibilities of timely correctives. | Scientist used the methods of automatic identification and continuous control of insect pests pointing to video capture and sensor-based luring traps. Their strategy only involved population monitoring devices for pest’s control which ensure that corrective actions are taken immediately. The study brought out how these modern technologies offer sustainable and preventive pest control since pest invasion can be foreseen and checked. |
| Farm field assessments of fruit flies (Diptera: Tephritidae) in Pakistan: distribution, damage and control | (Stonehouse et al. 2019; Jose et al. 2013). | High population of B. zonata in mangoes resulted in yield loss of up to 40% reduced yield and post-harvest losses due to poor refrigeration. | They conducted farm field assessments in Pakistan to evaluate the distribution, damage, and control of fruit flies (Diptera: Tephritidae). They used population and yield data and data on the incidence of Bactrocera zonata on mangoes and found up to 40% reduced yield and high post-harvest losses due to poor cool storage. Field trials were also used also to validate the management techniques which were to be used in practice. |
| Organic management promotes natural pest control through enhanced plant resistance to insects | (Robert et al. 2019). | Hypothesis for affected climate models suggest that habitat suitable for B. zonata could expand by 15–20% by the year 2050, something that calls for increased pest control interventions. | Scientist investigated organizational management as a factor in increasing biological control through plant resistance to pests. Information from climate models was used to predict an increase in favorable locations of about 15–20% by mid-century for Bactrocera zonata. Climate modeling for their habitat-suitability assessment, as well as field survey to assess implementation of pest control measures. |
| Health outcomes of 100% orange juice and orange flavored beverage: A comparative analysis of gut microbiota and metabolomics in rats | (John et al. 2020). | It is established that gut microbiota synthesised vitamin B which enhanced reproductive fitness as well as survival under fluctuating environments. | Scientist investigated the effect of 100% orange juice and orange flavored non-juice beverages using gut microbiota and metabolomics in experimental model. For their study, they used 16S rRNA sequencing to characterize gut microbiota, and metabolomic assays to determine vitamin B synthesis capabilities. The experiment proved that the vitamin B obtained from microbiota improved fecundity and overall survivability in changing climate. |
| The micro-eukaryotic community: An underrated component of the mammalian gut microbiota | (Vargas et al. 2015). | Microbial symbiosis works in extending the concept of environment interference as pheromone through microbiota manipulation affect the rate of successful mating. | Scientist focused on investigating the micro eukaryotic community in synthetically constructed mammalian gut microbiota models. Their approach was based on microbiota modulation tests to determine the effects of pheromones on the mating performance. This study showed that microbial symbiosis or microbiota modulation change pheromone emission and successfully influence the chances of reproductive success. |
| Efficacy of lure mixtures in baited traps to attract different fruit fly species in guava and vegetable fields | (Ahmad et al. 2023). | Protein-based baits along with ammonium acetate were able to capture female flies bringing down the overall population as well. | Scientist assessed the effectiveness of lure mixtures in baits used in traps to capture varied fruit fly species in guava and vegetable plantations. Their technique included employment of proteinaceous lures, accompanied with ammonium acetate in cage trapping for collection of female fruit flies. The results showed a significant reduction in the overall fly population, demonstrating the effectiveness of these lure mixtures in pest control. |
| Studies on Biology and Management of Melon fruit fly, Bactrocera cucurbitae (Coquillett) on Cucumber | (Koul & Bhagat et al. 2004). | Interactions of Bactrocera zonata with soil depth and environmental conditions affects the pre-pupal phase very much highlighting species flexibility. | Scientist investigated the biology and control of Bactrocera cucurbitae on cucumber with reference to the physical environment. To this end, their method included determining how the selected factors such as soil depth and environmental conditions influenced the pre-pupal stage of Bactrocera zonata. Environmental parameters were also shown to affect the species’ plasticity and thus the high propensity of the fruit fly to alter its conditions. |
| Meta-analysis of Diets Used in Drosophila Microbiome Research and Introduction of the Drosophila Dietary Composition Calculator (DDCC | (Broderick et al. 2019). | This study shows that gut microbiota Bactrocera zonata adapts the microbiota for survival in various climates to increase the pest’s resistance and dissemination. | Scientist performed a meta-analysis of the diets employed in Drosophila microbiome studies and presented the Drosophila Dietary Composition Calculator. The technique used was diet intervention and microbiota characterization to analyze the change in microbial signatures as a result of the diets. This study highlights how Bactrocera zonata adapts its gut microbiota to survive in varying climates, thereby enhancing the pest’s resistance and facilitating its spread. |
| The scent of royalty: a P450 gene signals reproductive status in a social insect | (Lemaitre & Hoffmann, 2007). | Consequently, tailored biological control of fruit fly mediated by manipulating the immune system in response to microbial stimuli is possible without resort to chemical pesticides. | Scientist explored P450 gene can indicate reproductive condition in social insects while studying the immune function. They conducted genetic and behavior experiments to understand the effects of microbial signals on the immune response. According to this research, there is a possibility of likely developing a Specific organic control of fruit flies through modulation of the host innate immune system upon recognition of microbial associated molecular patterns, thus eliminating the use of chemical sprays. |
| Pesticide handling practices, health risks, and determinants of safety behavior among Iranian apple farmers | (Baghari et al. 2017). | Pheromone enhanced traps were useful for remote attractiveness and sexually competitive suppression to male flies for the MAT. | The researchers have therefore sought to examine the outcomes of applied pesticide handling and the health implications among the Iranian apple growers. They also used their study to evaluate the efficiency of traps with pheromones in control of male fruit flies. The technique used hatching experiments to assess the efficacy of the traps in terms of attraction and the applicability of mating disruption (MAT) in the pest control without the use of insecticides. |
| Learning experiences in IPM through concise instructional videos | (Thomas et al. 2013). | Microbiome-targeted approaches integrated into existing Integrated Pest Management (IPM) frameworks improve ecological pest control effectiveness | Scientist concerned with learning experiences in Integrated Pest Management (IPM) with the use of instructional videos. They also observed their approach that deals with the inclusion of microbiome-directed tactics into IPM models with help of educational videos. This approach demonstrated that, by integrating a microbiome approach within the framework of IPM, ecological pest control efficacy can be optimized due to increased interactions with microbial natural enemies. |
| Parallel gene expression evolution in natural and laboratory evolved populations | (Schlötterer et al. 2021). | This feature was evident in the Drosophila melanogaster model where similarities in gut microbiota involvement in mating and immune processes assisting in enhanced pest control solutions were also investigated. | Scientist investigated conditionality patterns of gene expression in both nature and laboratory-constructed populations, with Drosophila melanogaster. Some of their approaches used were gene expression profiling of molecules associated with gut microbiota’s role in mating and immune functions. These pathways were seen to have a great potential towards the development of improved pest management systems; the microbiota patterns were demonstrated on how they can be incorporated into the pest management systems. |
| Evolutionary and ecological consequences of gut microbial communities | (Moran et al. 2019). | Specific microbial interactions were associated with fruit fly fitness across indicators of population dynamics, including larval survival rates, reproductive rates and overall population density. | Scientist extended the analysis of evolutionary and ecological aspects of gut microbial consortia. They utilized microbial relationship in the fruit flies, and measuring its impact on the fitness by various factors such as larval viability, reproductive output and population density. The research showed that certain interactions of microorganisms affect population density, which could be of interest to pest management programs. |
| Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber | (Naaz et al. 2020). | The biological control measures of using symbiotic bacteria as a pest control method was found appropriate in pest management while enhancing ecology. | Studied the use of symbiotic bacteria in controlling the Meloidogyne incognita in cucumber. Their approach was to use in vitro, greenhouse and field studies to test the potential of using symbiotic bacteria to manage root-knot nematodes. The study revealed that pest control using biocontrol agents from symbiotic bacteria is a more environmentally friendly method for suppressing pests and supporting the improvement of soil quality and overall species diversification. |
| Automatic detection and identification of brown stink bug, Euschistus servus, and southern green stink bug, Nezara viridula, (Heteroptera: Pentatomidae) using intraspecific substrate-borne vibrational signals | (Mankin et al. 2011). | Real-time pest surveillance technologies such as automatic surveillance systems were suggested for use to allow early intervention. | Scientist created scientifically significant real time pest monitoring technologies (SSRTPM) based on intraspecific substrate borne vibratory signals for the automatic identification of Brown Stink Bug (Euschistus servus) and Southern Green Stink bug (Nezara viridula). The study’s approach was based on the analysis of vibrational signals obtained through computerized monitoring and control systems that enabled early identification of pest likelihood and subsequent pest control. |
| Cultivating sustainable solutions: integrated pest management (ipm) for safer and greener agronomy | (Sharma et al. 2023). | Integrating digital control with an Art-based approach offers up a powerful protective mechanism towards pests. | Scientist emphasized the application of an IPM that was linked with digital control and an art-based strategy to boost the pest defense. The Integrated Pest Management plan used technological techniques of surveillance alongside artistic approaches in addressing the pest issues. This integration provided a viable and revolutionary method of protection and streamlined pest results while encouraging the effective utilization of environmentally friendly methodologies in agronomical ventures. |
| Conspecific and heterospecific pheromones stimulate dispersal of entomopathogenic nematodes during quiescence | (Kaplan et al. 2020). | Some other outstanding strategies such as microbiome target disruption and pheromone interruption have been proposed for being environmentally friendly control methods compared to chemicals molecules. | Scientist examined the effect of conspecific and heterospecific pheromones on triggering the movement of entomopathogenic nematodes during the dormant state. In this work their experimental approach was based on examining how pheromone treatments influenced the motility and spread of the nematodes. Among the proposed approaches such as microbiome dissonance and pheromone interference are potential gentle-green ways of pest control by providing durable solutions. |
| Biological control of human disease vectors: a perspective on challenges and opportunities | (Thomas et al. 2013). | It was stressed that field validation of microbiome-based tools for integrated pest management was essential for their application in the field | Scientist discussed about the biological control of human diseases and possible difficulties and prospects of pest control. In their approach they highlighted the field validation of microbiome-based tools utilized in Integrated Pest Management (IPM). The study highlights that if the application of these tools has to be done practically under a favorable system, field experiments need to be conducted for these tools to identify their efficiency in disease vector and pest control. |
Microbiome in Bactrocera zonata Lifecycle and Control; Understanding of Reproductive Interference and Biological Control
Microbiome-Based Pest Management: Exploiting Gut Microbiota for Environmental Friendly Management of Bactrocera zonata
Innovative Trapping Techniques and Comprehensive Strategies for the Effective Control of Drosophila melanogaster Fruit Flies
| Baits & Lures Names | Species of Fruit Flies | Crossponding Authors |
|---|---|---|
| Protein Hydrolizate | B. Zonata, B. Dorsalius | (Abbas. M. et al. 2021) |
| GF-120 | B. Zonata | (Nisar. N et al. 2020) |
| Methyl.Eugenole | B. Zonata, B. Dorsalius | (Muhammad. K et al. 2021) |
| Methyl.Eugenole | B. Zonata | (Murtaza.K et al. 2012 b) |
| Methyl.Eugenole | B. Zonata, B. Dorsalius | (Abadin et al. 2020) |
| MAT + Methyl.Eugenole | B. Zonata, B. Dorsalius | (Ghanim, M.N et al. 2023) |
| Ammonium Acetate | B. Zonata | (Lillo et al.2023) |
| Trimethylamine + protein hydrolysate (mixture) | B. Zonata, B. Dorsalius | (Royer et al. 2023). |
Global Review of the Extent and Impact of Bactrocera zonata on Fruits Crops with Special Emphasis on Its Economic Loss
Systematic Study of Host Related Activities and Life Cycle Characteristics of Bactrocera zonata
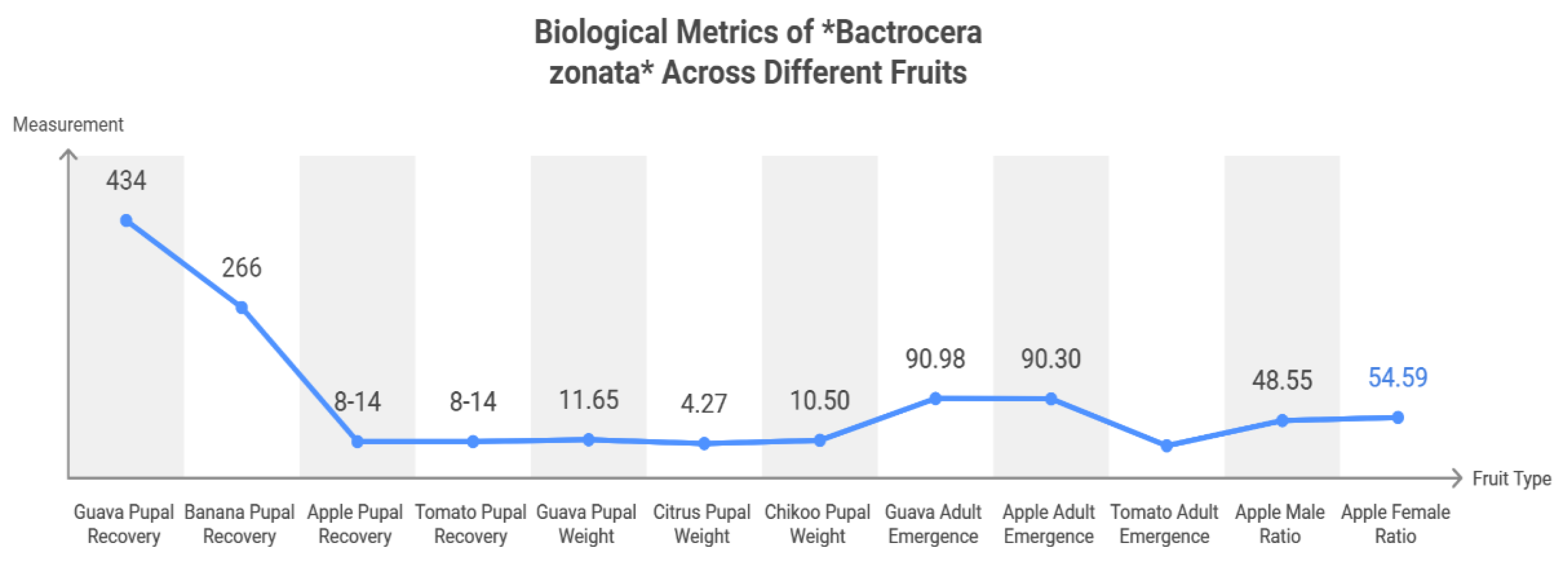
Microbiota-Based Strategies Against Bactrocera zonata with Regard to Integrated Pest Management in Sustainable Agriculture
Future Aspects Outcome & Stratiges to Control Fruit Flies
Conclusion
References
- Ahmad. I., M. Sohail, and K.S.ahmad. Morpho-anatomical determinants of yield potential in Olea europaea L. cultivars belonging to diversified origin grown in semi-arid environments. Jourenl of PLOS one 2023, 23, 10–13. [Google Scholar]
- Ahmad. S., M.Jamil, and Y. Luo.2023. Knockdown of the ecdysone receptor disrupts development and causes mortality in the melon fly, Zeugodacus cucurbitae. Insect Molecular Biology.
- Asif. M., K. Khakwani and H. Hussain. Evaluation of Cotton Genotypes for Agro-Morphological Traits and Resistance to Insect Pests in Faisalabad, Pakistan. Sarhad Journel of Agriculture 2023, 40, 386–394. [Google Scholar]
- Ayaz. B and F. Altunsoy. Effects of heavy metal pollution on population dynamics of another important pollinator insect group: Horseflies (Diptera: Tabanidae). Jol of Biological diversity and conservation 2024, 17, 148–155. [Google Scholar]
- Azam. A and M. Shafique, Agriculture in Pakistan and its Impact on Economy―A Review. International Journal of Advanced Science and Technology 2017, 103, 47–60. [Google Scholar] [CrossRef]
- Baghari. A., R. Kolyaee and G. Faraji. Efficacy of methyl eugenol bait traps for controlling the mango fruit fly Bactrocera zonata (Diptera: Tephritidae). J. Crop Prot 2017, 6, 181–189. [Google Scholar]
- Behar. A., L. J.McCormick and S. J. Perlman. Rickettsia felis Infection in a Common Household Insect Pest, Liposcelis bostrychophila (Psocoptera: Liposcelidae). Applied and environmental Microbiology 2018, 26, 2280–2285. [Google Scholar]
- Ben-Yakir. D., E. hadar, M. Chen. Evaluating insecticides for the control of narcissus flies under field conditions in Israel. Jo. of Phytoparasitica 1997, 25, 93–97. [Google Scholar] [CrossRef]
- Bhagat. D., S.K. Samanta ans S. Bhattacharya. Efficient Management of Fruit Pests by Pheromone Nanogels. Magazine of scientific reports 2013, 12. [Google Scholar]
- Boulahia-Kheder. S., Advancements in management of major fruit flies (Diptera: Tephritidae) in North Africa and future challenges: A review. Journal of Applied Entomology 2021, 145, 939–957. [Google Scholar] [CrossRef]
- Brodeick. N.A., K. F. Raffa and Jo. Handelsman. Chemical modulators of the innate immune response alter gypsy moth larval susceptibility to Bacillus thuringiensis. BMC Microbiology 2010, 129, 1–13. [Google Scholar]
- Bueno. E., K.R. Martin, R.A. Raguso and J. G. Mcmullen. Response of Wild Spotted Wing Drosophila (Drosophila suzukii) to Microbial Volatiles. Jol. Of Chemical Ecology 2020, 46, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Cheng. S., R. Lin, C. Yu and R. Sun. Toxic effects of seven pesticides to aphid parasitoid, Aphidius gifuensis (Hymenoptera: Braconidae) after contact exposure. Crop Protection 2021, 145. [Google Scholar]
- Das. K., S. Pramaani and M. Mandi. Monocrotophos Induced Changes in the Life Cycle Parameters of Fruit Fly. Jol of Environmental Science 2024, 3, 6–13. [Google Scholar]
- Deng, Jian-Yu, L, Chen-yi-Hang, Z. Jun-Xiang and F-Wang, Analysis of sex pheromone production and field trapping of the Asian corn borer (Ostrinia furnacalis Guenée) in Xinjiang, China. Journel of Integrative agriculture 2023, 22, 1093–1103. [CrossRef]
- Deutscher. T.A., T. A. Chapman and O.L. Reynold. Tephritid-microbial interactions to enhance fruit fly performance in sterile insect technique programs. BMC microbiology 2019, 19, 2–14. [Google Scholar]
- Dominiak. B.C. Components of a systems approach for the management of Queensland fruit fly Bactrocera tryoni (Froggatt) in a post dimethoate fenthion era. Jol. Of crop protection 2019, 116, 56–67. [Google Scholar] [CrossRef]
- Doherty. E.M., R.L. Meagher and A.G. Dela.. Turfgrass Cultivar Diversity Provides Associational Resistance in the Absence of Pest Resistant Cultivars. Journel of Environmental Entomology 2019, 48, 623–632. [Google Scholar] [CrossRef]
- Diller. J. G. P., F. Huftlein and C. Laforsch. Allelochemical run-off from the invasive terrestrial plant Impatiens glandulifera decreases defensibility in Daphnia. Scientific report 2023, 13, 1207–1213. [Google Scholar] [CrossRef]
- Diller. Y., A. Shamsian and D. Nestel. A real-time remote surveillance system for fruit flies of economic importance: sensitivity and image analysis. Journel of Pest science 2023, 96, 611–622. [CrossRef]
- Dion. W.A., T. E. Steenwinkel and T. Werner. From Aedes to Zeugodacus: a review of dipteran body coloration studies regarding evolutionary developmental biology, pest control, and species discovery. Magzine of Current Opinion in Genetics & Development 2021, 69, 35–41. [Google Scholar]
- Doharey. R.K., Antim and M.Kumar. Adoption Level of Rural Women about Storage Structure and Practices for Storing Grains. Journel of Asian Journal of Agricultural Extension, Economics & Sociology 2022, 20, 352–359. [Google Scholar]
- Engel. P and N.A. Moran. The gut microbiota of insects – diversity in structure and function. FEMS microbiology review 2013, 37, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Fezza. T., T. E. Shell and C. Nicholas. Less is more: Fewer attract-and-kill sites improve the male annihilation technique against Bactrocera dorsalis (Diptera: Tephritidae). Jol. PLOS one 2024, 21, 10–19. [Google Scholar]
- El-AFify, AH.R.M. Shareef and N.M. Ghanim. Seasonal Activity of Bactrocera zonata (Saunders) and Ceratitis capitata in a Navel Orange Orchard in Dakahlia, Egypt. Arab Journal of Plant Protection 2023, 41, 98–104. [Google Scholar] [CrossRef]
- Elkelany. M.N., M. M.Elbokl and N. M.Ghanim. Modifications in Male Annihilation Technique for Attracting Bactrocera zonata Under Field Conditions. Egyptian Academic Journel of biological Science (A. Entomology) 2024, 17, 13–24. [Google Scholar] [CrossRef]
- Gogi. M. D., M. J. Nisar, B. Atta and M. Iqbal. Pathogenicity of fungal and bacterial bioinsecticides against adult peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae) admixed with adult diet under controlled conditions. Egyptian Academic Journel of biological Science 2021, 31, 1–20. [Google Scholar]
- Gogi. M. D., A. Nawaz and M. Sufiyan. Comparative bio-efficacy of nuclear polyhedrosis virus (NPV) and Spinosad against American bollworm, Helicoverpa armigera (Hubner). Biological Control and Crop Protection 2019, 63, 227–282. [Google Scholar]
- Gupta. R., P. Malik, R.Rani and R. Solanki. Recent progress on nanoemulsions mediated pesticides delivery: Insights for agricultural sustainability. Plant Nano Biology 2024, 8, 73–100. [Google Scholar]
- Ganie, S.A. Rehman, S.A., Nisar, T., Paray, M.A., Bano, P. and Khurshid, R. Fruit fly management and control strategies: A review. Biopestic. Int. 2022, 18, 89–100. [Google Scholar]
- Gupta. S., A. Radhakrishnan, G. Lin and H. Sinhna. Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype. Journel of National Library of Medicine 2015, 11, 1–23. [Google Scholar]
- Gogi. M.D., M. J. Nisar, B. Atta and M. Iqbal. Pathogenicity of fungal and bacterial bioinsecticides against adult peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae) admixed with adult diet under controlled conditions. Egyptian Academic Journel of biological Science 2021, 31, 1–20. [Google Scholar]
- Haider. F.U., S. A. Cheema and M. Farooq, Impact of cover crops in improving agro-ecosytems including sustainable weed suppression – a review. Pak. J. Weed Sci. Res 2019, 25, 47–62. [Google Scholar]
- Hannan. A., A. Basitand D. Qiu, Anti-insect activity of a partially purified protein derived from the entomopathogenic fungus Lecanicillium lecanii (Zimmermann) and its putative role in a tomato defense mechanism against green peach aphid. Jounrel of invertebrate pathology. 2023, 170, 107–282. [Google Scholar]
- Hoffmann. C and W. Entling. Single and combined effects of Drosophila suzukii and Drosophila melanogaster on sour rot development in viticulture. Journal of Applied Entomology 2019, 144, 153–160. [Google Scholar]
- Hollingsworth. R.G., L. F. Aristizbal and S.P. Arthurs. Incorporating Beauveria bassiana Into an Integrated Pest Management Plan for Coffee Berry Borer in Hawaii. Journel of Frontiers in sustainable food system 2020, 22. [Google Scholar]
- Johan. M.A., I. Bankloe, and P. Lalit. Relevance of Advanced Plant Disease Detection Techniques in Disease and Pest Management for Ensuring Food Security and Their Implication: A Review. American Journal of Plant Sciences 2023, 14, 1260–1295. [Google Scholar] [CrossRef]
- John. G McMullenII., G. Pters-Schulze and A. E. Douglas. How gut microbiome interactions affect nutritional traits of Drosophila melanogaster. Jol of experimental Biology 2020, 223, 1–16. [Google Scholar]
- Johan. M.A., I. Bankloe, and P. Lalit. Relevance of Advanced Plant Disease Detection Techniques in Disease and Pest Management for Ensuring Food Security and Their Implication: A Review. American Journal of Plant Sciences 2023, 14, 1260–1295. [Google Scholar] [CrossRef]
- Jose. C..F., Farm field assessments of fruit flies (Diptera: Tephritidae) in Pakistan: Distribution, damage and control. Integrated Pest Management: Sustainable Approach to Crop Protection 2020, 21, 661–669. [Google Scholar]
- Kaara. S., I.Ozgen and M. Yegul. A new apricot pest: Omaloplia labrata (Burmeister) (Coleoptera: Scarabaeidae: Sericini). Journal of Entomology and Zoology Studies 2024, 12, 01–03. [Google Scholar] [CrossRef]
- Kaplan. M and Y. Bayram. occurrence, population development, infestation and damage caused by olive fly (Bactrocera olea gmel.) (diptera: tephritidae) inolive orchards of mardin province, turkey. he journal of animal & plant sciences 2021, 31, 610–616. [Google Scholar]
- Khan. F.Z.A., S.A. Manzoor and S.V. Joseph. Drivers of farmers’ intention to adopt integrated pest management: a case study of vegetable farmers in Pakistan. Ecological Society of America 2021, 12, 12–38. [Google Scholar]
- Koul. O., Phytochemicals and Insect Control: An Antifeedant Approach. Journel of Taylor & Francis online 2008, 27, 1–24. [Google Scholar]
- Lanjar. A.G., A. W. Solangi and A. Bukero. Insecticide Application Tactics to Suppress Population of Drosicha Mangiferae (Green). EUROPEAN ACADEMIC RESEARCH 2014, 8, 11182–11396. [Google Scholar]
- Laskar. N., K. Das and C.P. Nath. Dissipation Kinetics and Residue Distribution of Imazethapyr in Urdbean (Vigna mungo (L.) Hepper) and Urdbean Field soil and its Effect on soil Microbial Population. Bulletin of Environmental Contamination and Toxicology 2024, 8, 173–196. [Google Scholar]
- Lello. F., M. Dido, M. Mkiramweni and Joseph, Fruit fly automatic detection and monitoring techniques: A review. Smart Agriculture technology 2023, 5, 100–294. [Google Scholar]
- Lemaitre. B., N.A. Broderick. Gut-associated microbes of Drosophila melanogaster. Jol of Taylor & Francis online 2012, 4, 307–321. [Google Scholar]
- Lillo. G., C. Caila, D. Cice and S. Camposeo. Blooming Phenograms, Pollen Production, and Pollen Quality during Storage of Pistachio Cultivars in New Mediterranean Growing Areas. Jol of MDPI 2024, 13, 1–13. [Google Scholar]
- Lillo. P., M.D.M. Delgadoand M. A. Porcel. Quality of Organic Amendments Drives Agroecosystem Multifunctionality and Soil Micro-Food Web Short-Term Responses to Organic Matter Inputs. Jourenl of SSRN 2024, 55, 610–619. [Google Scholar]
- Lillo-Saavedra. M., A. Espinoza-Salgado and D. Rivera. Early Estimation of Tomato Yield by Decision Tree Ensembles. Jol. Of agriculture MDPI 2022, 12, 22–28. [Google Scholar]
- Lima. M.C.., M. E.Damascena and C.O.G.Bazzo. Automatic Detection and Monitoring of Insect Pests—A Review. Journel of Analysis Techn in agriculture 2020, 10, 161–169. [Google Scholar]
- Liu. Y.., yang-Geng and T. Jiang. Pest suppression services and dietary niche differentiation of bats in Chinese smallholder farming systems: implications for integrated pest management. Jol of Pest Sciences 2024, 97, 1587–1603. [Google Scholar]
- Murtaza. G., M. Razman, and H. Bilal. Monitoring of fruit fly, Bactrocera zonata (Diptera: Tephritidae) population by installing traps in mango orchard Bahawalnagar, Pakistan. Journal of Applied Research in Plant Sciences 2024, 2, 147–151. [Google Scholar]
- Mac-Cormack. K. 2016. Enhancing the monitoring and trapping of protected crop pests by incorporating LED technology into existing traps. Library and University Collections (L&UC). 185-197.
- Mankin. R. W, B. Rohde and S. McNeill. Vibrational duetting mimics to trap and disrupt mating of the devastating Asian citrus psyllid insect pest. 170th Meeting of the Acoustical Society of America 2016, 26, 16–25.
- Manoukis. N.C., R.I. Vargas and T. E.Shelly. A field test on the effectiveness of male annihilation technique against Bactrocera dorsalis (Diptera: Tephritidae) at varying application densities. Jol of PLOS One 2019, 17, 2–14. [Google Scholar]
- Masood. S., M. Usman, A. Javid and M. F. H. Ferodosi. Control of insect pests and yield improvement in brinjal by plant extracts. INT. J. BIOL. BIOTECH. 2023, 20, 329–335. [Google Scholar]
- McMullem. John.G., G.Peters-Schulze and A.E. Douglas. How gut microbiome interactions affect nutritional traits of Drosophila melanogaster. Jol. of Experimental Biology 2020, 223, 13–22. [Google Scholar]
- Mir. M.S., A. Saxena and S.A. Mir. Role of Intercropping in Sustainable Insect-Pest Management: A Review. International Journal of Environment and Climate Change 2022, 12, 3390–3403. [Google Scholar]
- Mir. M.S. Salim and Saxena. Role of Intercropping in Sustainable Insect-Pest Management: A Review. Journel of International Journal of Environment and Climate Change 2023, 12, 3390–3403. [Google Scholar]
- Moran. P.J., J. J. Miskella and J. D. Madsen. Toxicity of herbicides used for control of waterhyacinth in the California Delta towards the planthopper Megamelus scutellaris released for biological control. Biocontrol Science and Technology 2023, 23. [Google Scholar]
- Murtaza. G., Fazlullah, T. Ahmad and M. Ramzan. Biology and Morphology of Bactrocera dorsalis and Bactrocera zonata on Guava under Laboratory Conditions. Indian Journel of pure aand applied biosciences 2021, 9, 180–185. [Google Scholar]
- Naaz. H., J.A. Siddiqui, R. Fan and M. Hafeez. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Frontiers in Physiology 2023, 22, 20–23. [Google Scholar]
- Napitupulu. T. P., Antagonistic fungal volatiles as potential biocontrol countermeasure for microbial postharvest fruit diseases. Egyptian Journal of Biological Pest Control 2023, 33, 92–100. [Google Scholar]
- Ndlela. S., S. N and S.A. Mohamed. Important alien and potential native invasive insect pests of key fruit trees in Sub-Saharan Africa: advances in sustainable pre- and post-harvest management approaches. Jol of CABI agriculture and Biosciences 2022, 3, 2–46. [Google Scholar]
- Rabha. A., D.K. Sharma, C. Baruah and A. N. Das. Impact of Corn Meal and Wild Yeast Media on Drosophila Diversity in the Assam-Meghalaya Rolling Terrain. Journel of entomological society of iran 2024, 44, 329–348. [Google Scholar] [CrossRef]
- Rasouli. F., H. Abbasipour and A. Rezazadeh. Evaluation of the chinaberry Melia azedarach extract against the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae) in vitro. Revista de la Sociedad Entomológica Argentina 2024, 83, 1–8. [Google Scholar]
- Rehman. M.F and M. M. Khan. Application of nanopesticides and its toxicity evaluation through Drosophila model. ioprocess and Biosystems Engineering 2024, 47, 1–22. [Google Scholar] [CrossRef]
- Robert. K., P. Parsons and R. Ross. A survey on wireless sensor network technologies in pest management applications. Jol. Of discover applied sciences 2020, 28, 19–34. [Google Scholar]
- Royer. J.E.,C. Mille, S. Cazeres and D.G. Mayer. soeugenol, a More Attractive Male Lure for the Cue-Lure-Responsive Pest Fruit Fly Bactrocera curvipennis (Diptera: Tephritidae: Dacinae), and New Records of Species Responding to Zingerone in New Caledonia. Journel of Economic entomology 2019, 112, 1502–1507. [Google Scholar] [CrossRef]
- Royer. P, F. Dumont and E. Lucas. May biocontrol agents artificially selected for their aggressiveness improve crop protection. Journel of pest sciences 2024, 10, 20–24. [Google Scholar]
- Schlotterer. C., M. Lirakis and M. Dolezal. Redefining reproductive dormancy in Drosophila as a general stress response to cold temperatures. Journel of Insect Physiology 2018, 107, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sharma. A., S.Raminiwas, D. Singh. Efficacy of Artificial diets on biological characteristics of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) in India. international Journal of Tropical Insect Science 2023, 43, 1691–1700. [Google Scholar] [CrossRef]
- Sheng-Kai. H, C.Schlotterer. Reproductive isolation arises during laboratory adaptation to a novel hot environment. Jol of genome Biology 2024, 141, 2–17. [Google Scholar]
- Shichao. Y., F. Luo, Y. Zhang and L.H. Jin. Drosophila Innate Immunity Involves Multiple Signaling Pathways and Coordinated Communication Between Different Tissues. Jornel of Frontiers in immunology 2022, 13, 53–95. [Google Scholar]
- Shield. G.F. Incipient Speciation and Additional Diversity within the Simulium Arcticum Complex of Black Flies (Diptera: Simuliidae). The American Midland Naturalist 2014, 172, 1–13. [Google Scholar] [CrossRef]
- Shivay. Y.S.., and R. Prasad. Enhanced Efficiency Fertilizers or Slow-Release and NI/UI Blended Nitrogen Fertilizers. Indian Journel of fertilize 2021, 17, 316–321. [Google Scholar]
- Smith. D.J., M. Helmy and N D.Lindley, 2022. The transformation of our food system using cellular agriculture: What lies ahead and who will lead it. Trends in food science and technology 2021, 127, 368–376. [Google Scholar]
- Srivastava. R and A.C. Shukla. Fusarium pallidoroseum: A potential entomopathogenic agent for the biological management of Aphis gossypii. Journel of Applied and Natural Science 2021, 13, 775–785. [Google Scholar]
- Stonehouse. J., R. Mahmood and D. Huggett. Farm field assessments of fruit flies (Diptera: Tephritidae) in Pakistan: distribution, damage and control. Crop Protection 2002, 21, 661–669. [Google Scholar] [CrossRef]
- Susanna. D and D. Pratiwi. Current status of insecticide resistance in malaria vectors in the Asian countries: a systematic review. National Center of Biotechnology Informatio (NIH) 2022, 12, 10–20. [Google Scholar]
- Tait. G., S. Mermer, D. Stockton and J. Lee. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. Journel of economic Entomology 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Takahiro. H.,T. Kikuchi and T.Fukatsu. Obligate symbiont involved in pest status of host insect. Proceeding of the royal of royal society 2007, 20, 6–20. [Google Scholar]
- Toledo. J., M. Brenda. Moran-Aceves and P. Liedo. Can Entomopathogenic Nematodes and Their Symbiotic Bacteria Suppress Fruit Fly Pests? A Review. Jol. Of Microorganism 2023, 23, 11–16. [Google Scholar]
- Ullah. U-Naeem and M. Ramzan. Insect Pests of Cotton Crop and Management Under Climate Change Scenarios. Journal of Enviornmental climate, plants and Vegetation growth 2020, 367–396.
- Vargas. R.I., J. C. Pinero and L. Leblanc. An Overview of Pest Species of Bactrocera Fruit Flies (Diptera: Tephritidae) and the Integration of Biopesticides with Other Biological Approaches for Their Management with a Focus on the Pacific Region. Jol of Insecta 2015, 6, 297–318. [Google Scholar]
- Vargas. R.I., J.C. Pinero and L. Leblanc. An Overview of Pest Species of Bactrocera Fruit Flies (Diptera: Tephritidae) and the Integration of Biopesticides with Other Biological Approaches for Their Management with a Focus on the Pacific Region. Magazine of Multidisciplinary Digital Publishing Institute 2015, 6, 297–318. [Google Scholar]
- Verma. N and A Bhardwaj. Biosensor Technology for Pesticides—A review. Journel of apllied Biocehmistry 2015, 175, 3093–3119. [Google Scholar]
- Zain-Ul-Aabdin. A., N. Blaoch, R.M. Memon and N.H. Khhro. Population Fluctuations of Bactrocera Species (Diptera: Tephritidae) in Guava and Mango Orchards at Different Climatic Conditions of Sindh. Pakistan Journal of Zoology 2023, 55, 37–42. [Google Scholar]
- Zhang. Q.F., John G. & Mc. Mullen II. Succinate: a microbial product that modulates Drosophila nutritional physiology. Journel of insect sciences 2023, 29, 315–318. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





