Submitted:
12 January 2025
Posted:
13 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus
2.3. Preparation of NPs-Modified Electrodes
2.3.1. Synthesis of Saffron-Conjugated Silver Nanoparticles (AgNPs@Sa)
2.3.2. Preparation of Modified, with Silver Nanoparticles (AgNPs@Sa), Carbon Paste Electrode Surface
2.3.3. Sample Preparation
2.4. Analytical Determination
3. Results and Discussion
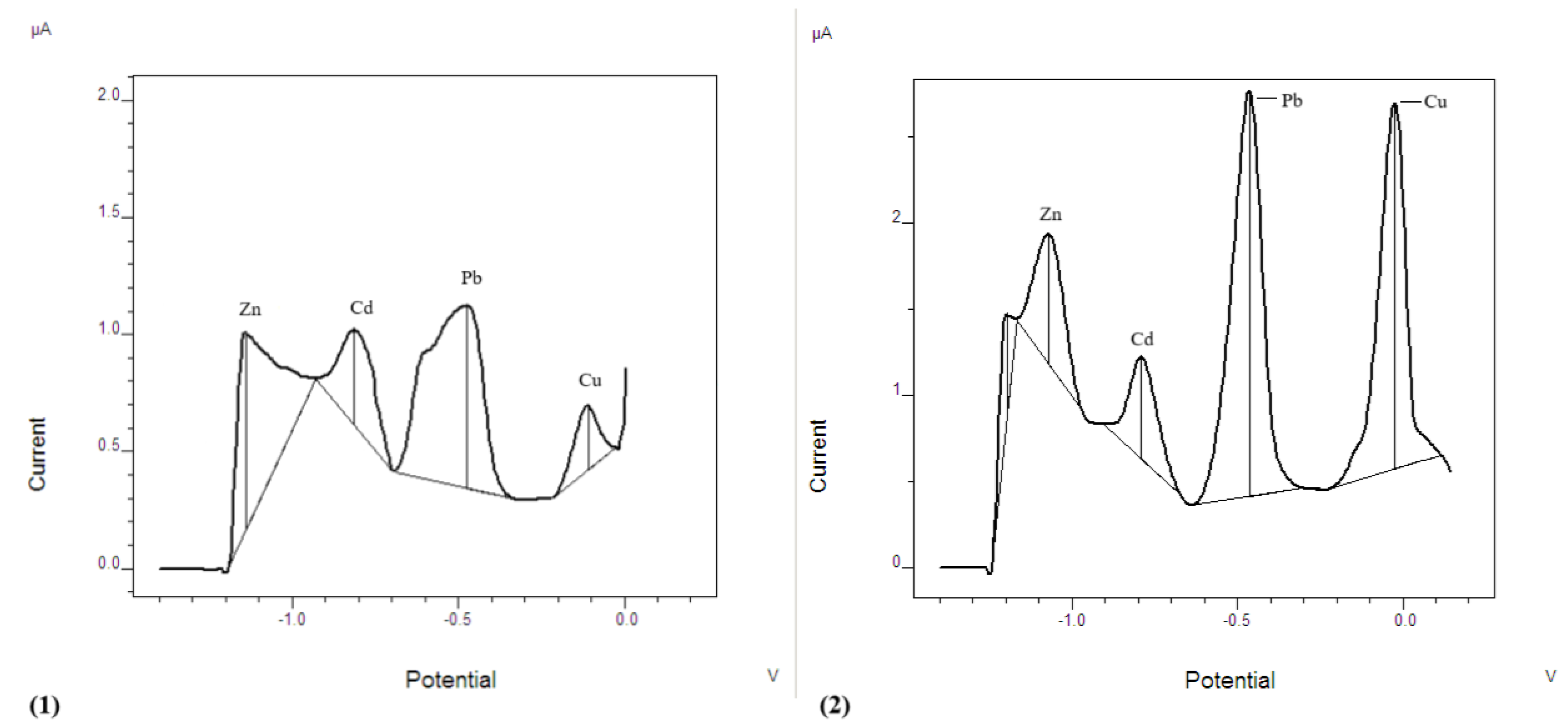
3.1. Comparison of Bare CPE Electrode with Modified AgNPs@Sa-CPE Electrode
3.2. Development of Analytical Methodology
3.2.1. Simultaneous Determination of Cd²⁺, Pb²⁺, Zn²⁺, and Cu²⁺
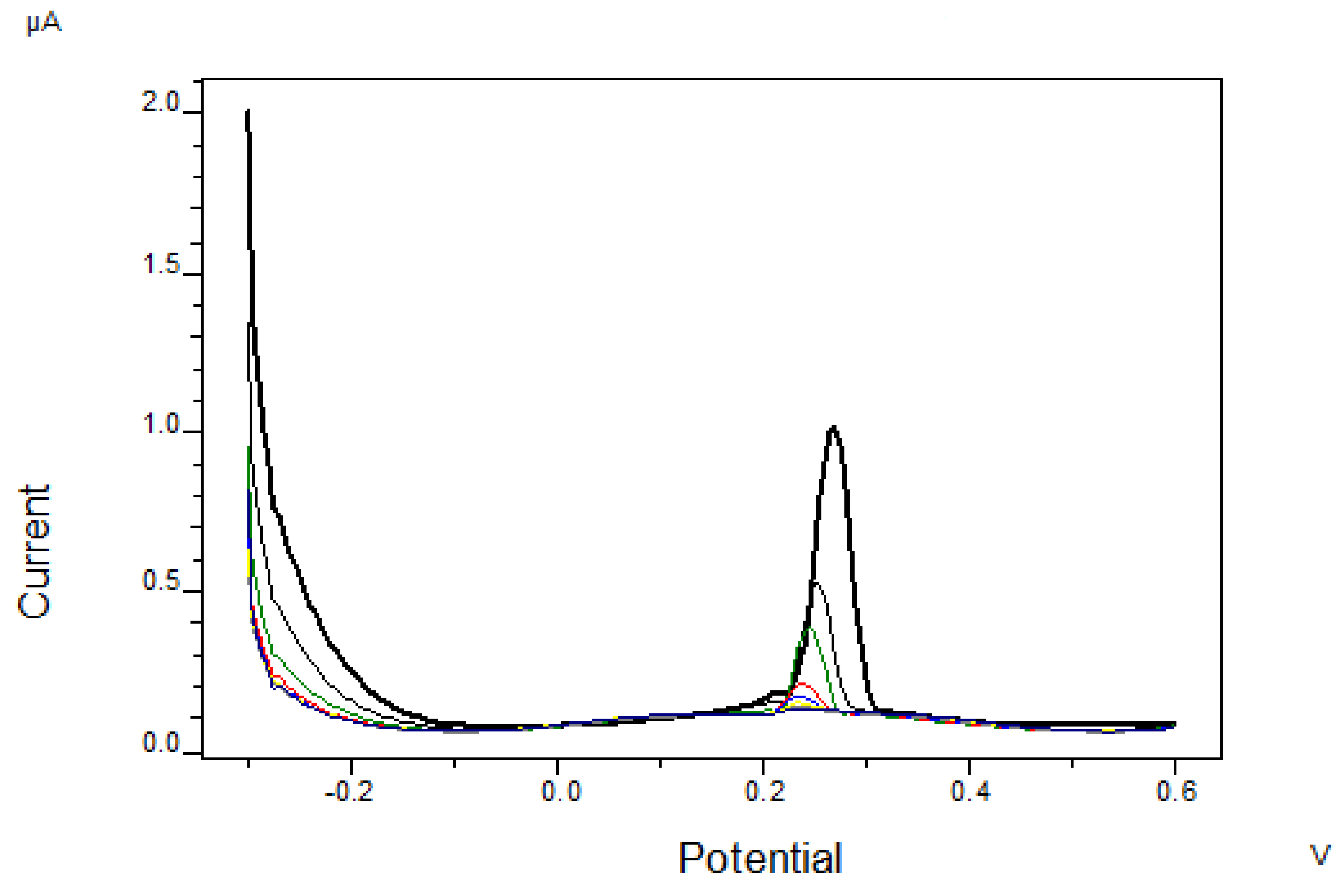
3.2.2. Determination of Chloride
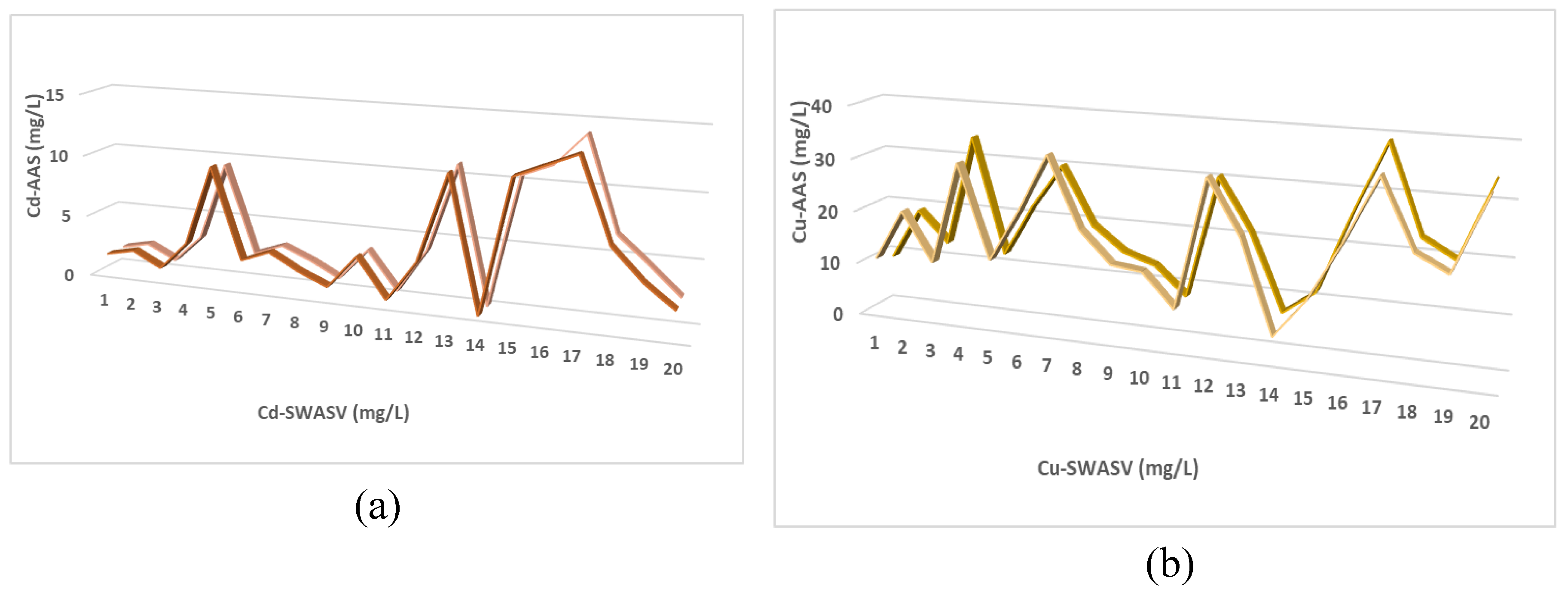
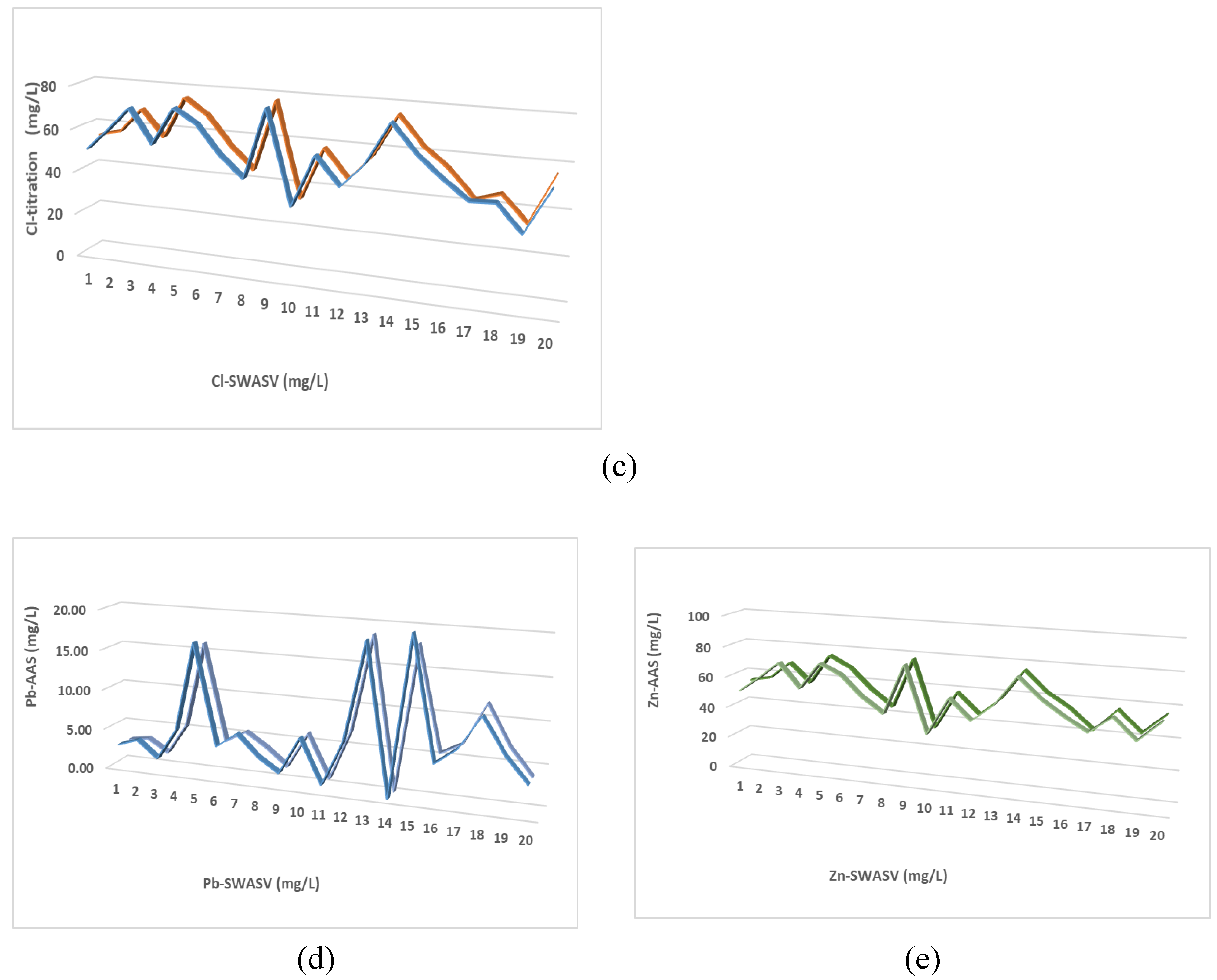
3.3. Analytical Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. Trends in Analytical Chemistry. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Bansal, V.; Lazarides, T.; Kumar, N. Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Ind. Eng. Chem. 2017, 30–43. [Google Scholar] [CrossRef]
- Jafarzadeh, N.; Heidari, K.; Meshkinian, A.; Kamani, H.; Mohammadi, A.A.; Conti, G.O. Non-carcinogenic risk assessment of exposure to heavy metals in underground water resources in Saraven, Iran: Spatial distribution, monte-carlo simulation, sensitive analysis. Environ. Res. 2022, 204, 112002. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Rad, S.S.; Vilas–Boas, J.A.; Khataee, A. A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources. Chemosphere. 2020, 267, 128904. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic, and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A.; Buledi, J.A.; Nawaz, M.; Rajpar, D.B.; Shah, Z.H.; Orooji, Y.; Yola, M.L.; Karimi-Maleh, H.; Lin, H.; Solangi, A.R. Identification of heavy metal ions from aqueous environment through gold, silver and copper nanoparticles: An excellent colorimetric approach. Environ. Res. 2022, 205, 112475. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: a review. Microchim. Acta. 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ghorbani, A.; Li, Y.; Pehlivan, N.; Barker, J.; Ding, Y.; Liu, G.; Zargar, M. Responsible mechanisms for the restriction of heavy metal toxicity in plants via the co-foliar spraying of nanoparticles. Agronomy. 2023, 13, 1748. [Google Scholar] [CrossRef]
- Lehmann, A.; Stahr, K. Nature and significance of anthropogenic urban soils. J. Soils Sediments 2007, 7, 247–260. [Google Scholar] [CrossRef]
- Mónok, D.; Kardos, L.; Pabar, S.A.; Kotroczó, Z.; Tóth, E.; Végvári, G. Comparison of soil properties in urban and non-urban grasslands in Budapest area. Soil Use Manag. 2021, 37, 790–801. [Google Scholar] [CrossRef]
- Ariño, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes: A review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Singh, M. Biosensors for heavy metals. Biometals 2005, 18, 121–129. [Google Scholar] [CrossRef]
- Hakonen, A.; Strömberg, N. Fluorescence and naked-eye detection of Pb²⁺ in drinking water using a low-cost ionophore-based sensing scheme. Chemosensors 2018, 6, 51. [Google Scholar] [CrossRef]
- Massadeh, A.M.; Alomary, A.A.; Mir, S.; Momani, F.A.; Haddad, H.I.; Hadad, Y.A. Analysis of Zn, Cd, As, Cu, Pb, and Fe in snails as bioindicators and soil samples near traffic road by ICP-OES. Environ. Sci. Pollut. Res. 2016, 23, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Corr, J.J.; Larsen, E.H. Arsenic speciation by liquid chromatography coupled with ionspray tandem mass spectrometry. J. Anal. At. Spectrom. 1996, 11, 1215. [Google Scholar] [CrossRef]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometries. Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Buffle, J.; Tercier-Waeber, M.L. Voltammetric environmental trace-metal analysis and speciation: From laboratory to in situ measurements. Trends Anal. Chem. 2005, 24, 172–191. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, Y.Z.; Li, P.H.; Xiao, X.Y.; Jiang, M.; Li, S.S.; Liu, W.Q. Electrochemical spectral methods for trace detection of heavy metals: a review. Trends Anal. Chem. 2018, 106, 139–150. [Google Scholar] [CrossRef]
- Zhou, L.; Sekar, S.; Chen, J.; Lee, S.; Kim, D.Y.; Manikandan, R. A polyrutin/AgNPs coated GCE for simultaneous anodic stripping voltammetric determination of Pb(II) and Cd(II) ions in environmental samples. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129082. [Google Scholar]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today 2010, 5, 128–142. [Google Scholar] [CrossRef]
- Aragay, G.; Merkoçi, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd(II) and Pb(II) in environment and food based on shuttle-like α-Fe₂O₃ nanoparticles decorated β-Bi₂O₃ microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Sharma, P.; Ganguly, M.; Doi, A.; Sahu, M. Recent advances in the application of copper nanocluster and copper nanoparticle in water for heavy metals detection: A review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100970. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S.; Malav, L.C. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches - A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of heavy metals on plant growth: an overview. Contaminants in Agriculture: Sources, Impacts and Management, 2020; 79–101. [Google Scholar]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: new roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride in soil: From nutrient to soil pollutant. Environ. Exp. Bot. 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Yang, C.; Chu, C.; Tan, X. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Mohamed, E.; Ren, J.; Tao, L.; Mala, A. Effectiveness of attapulgite modified by chlorides on speciation and environmental risk of heavy metals in soil. Int. J. Environ. Sci. Technol. 2024, 21, 6713–6732. [Google Scholar] [CrossRef]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial monitoring of arsenic and heavy metals in the Almyros area, Central Greece. Statistical approach for assessing the sources of contamination. Environ. Monit. Assess. 2015, 187, 399. [Google Scholar] [CrossRef]
- Golia, E.E.; Aslanidis, P.-S.C.; Papadimou, S.G.; Androudi, M.; Tsiropoulos, N.G. Assessment of remediation of soils, moderately contaminated by potentially toxic metals, using different forms of carbon (charcoal, biochar, activated carbon). Impacts on contamination, metals availability and soil indices. Sustainable Chem. Pharm. 2022, 28, 100724. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, N.; Golia, E.E. Preliminary investigation of the use of Silybum marianum (L.) Gaertn. as a Cd accumulator in contaminated Mediterranean soils: the relationships among cadmium (Cd) soil fractions and plant Cd content. Euro-Mediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, M.; Zheng, Y.; Han, Y.; Liu, H.; Shen, J. A micro sample pretreatment technique combined with ion chromatography and its application in the determination of polyvinyl chloride. J. Chromatogr. A 2023, 1690, 463778. [Google Scholar] [CrossRef] [PubMed]
- Bujes-Garrido, J.; Izquierdo-Bote, D.; Heras, A.; Colina, A.; Arcos-Martínez, M.J. Determination of halides using Ag nanoparticles-modified disposable electrodes. A first approach to a wearable sensor for quantification of chloride ions. Anal. Chim. Acta 2018, 1012, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in Electrochemical Detection Methods for Measuring Contaminants of Emerging Concerns. Electrochem. Sci. Adv. 2021, 2, e2100184. [Google Scholar] [CrossRef]
- Ali, Z.; Ullah, R.; Tuzen, M.; Ullah, S.; Rahim, A.; Saleh, T.A. Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: A review. Trends Environ. Anal. Chem. 2023, 37, e00187. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Zhang, L.; Xu, J.; Wang, H.; Wang, C.; Geng, T. Amperometric sensing of hydrogen peroxide using a glassy carbon electrode modified with silver nanoparticles on poly(alizarin yellow R). Microchim. Acta 2011, 173, 135–141. [Google Scholar] [CrossRef]
- Hassan, K.M.; Elhaddad, G.M.; AbdelAzzem, M. Voltammetric determination of cadmium(II), lead(II) and copper(II) with a glassy carbon electrode modified with silver nanoparticles deposited on poly(1,8-diaminonaphthalene). Microchim. Acta 2019, 186, 440. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Amin, H.M.A. Silver nanoparticles modified electrodes for electroanalysis: An updated review and a perspective. Microchem. J. 2022, 175, 107166. [Google Scholar] [CrossRef]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Disposable sensor for electrochemical determination of chloride ions. Talanta 2016, 155, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.C.; Karastogianni, S.; Girousi, S. Development of an Electrochemical Sensor Using a Modified Carbon Paste Electrode with Silver Nanoparticles Capped with Saffron for Monitoring Mephedrone. Sensors 2022, 22, 1625. [Google Scholar] [CrossRef] [PubMed]
- Karastogianni, S.; Paraschi, I.; Girousi, S. Electrochemical sensing of the maple syrup urine disease biomarker valine, using saffron-silver nanoparticles. Biosens. Bioelectron: X 2022, 12, 100275. [Google Scholar] [CrossRef]
- Karastogianni, S.; Girousi, S. A novel electrochemical bioimprinted sensor of butyl paraben on a modified carbon paste electrode with safranine-o capped to silver nanoparticles. Int. J. Curr. Res. 2017, 9, 61118–61124. [Google Scholar]
- Abd El-Aziz, A.R.M.; Gurusamy, A.; Alothman, M.R.; Shehata, S.M.; Hisham, S.M.; Alobathani, A.A. Silver nanoparticles biosynthesis using Saussurea costus root aqueous extract and catalytic degradation efficacy of safranin dye. Saudi J. Biol. Sci. 2021, 28, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Sarakatsanou, C.; Karastogianni, S.; Girousi, S. Preparation of a glassy carbon electrode modified with saffron conjugated silver nanoparticles for the sensitive and selective electroanalytical determination of amoxicillin in urine samples. Anal. Methods 2023, 15, 4572–4581. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS 11466. Environment Soil Quality, ISO Standards Compendium, Geneva, Switzerland, 1994; replaced by ISO/DIS 11465:2020, Sludge, treated biowaste, soil and waste—Determination of dry residue or water content and calculation of the dry matter fraction on a mass basis.
- Geilfus, C.M.; Ludwig-Müller, J.; Bárdos, G.; Zörb, C. Early response to salt ions in maize (Zea mays L.). J. Plant Physiol. 2018, 220, 173–180. [Google Scholar] [CrossRef]
- Grim, R.J. Catalytic Activity of an Intermetallic Compound of Cadmium and Copper in the Vapor-phase Reduction of Nitrobenzene. J. Phys. Chem. 1942, 46, 464–469. [Google Scholar] [CrossRef]
- Tibbetts, D.F.; Davis, J.; Compton, R.G. Sonoelectroanalytical detection of lead at a bare copper electrode. Fresenius’ J. Anal. Chem. 2000, 368, 412–414. [Google Scholar] [CrossRef]
- Lahari, S.A.; Amreen, K.; Dubey, S.K.; Ponnalagu, R.N.; Goel, S. Optimized porous carbon-fibre microelectrode for multiplexed, highly reproducible and repeatable detection of heavy metals ions in real water samples. Environ. Res. 2023, 220, 115192. [Google Scholar] [CrossRef]
- Lee, P.M.; Chen, Z.; Li, L.L.; Liu, E. Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochim. Acta 2015, 174, 207–214. [Google Scholar] [CrossRef]
- Yıldız, C.; Bayraktepe, D.E.; Yazan, Z.; Önal, M. Bismuth nanoparticles decorated on Na-montmorillonite-multiwall carbon nanotube for simultaneous determination of heavy metal ions-electrochemical methods. J. Electroanal. Chem. 2022, 910, 116205. [Google Scholar] [CrossRef]
- Antunović, V.; Ilić, M.; Baošić, R.; Jelić, D.; Lolić, A. Synthesis of MnCo₂O₄ nanoparticles as modifiers for simultaneous determination of Pb(II) and Cd(II). PLoS ONE 2019, 14, e0210904. [Google Scholar] [CrossRef]
- Lalmalsawmi, J.; Zirlianngura, T.; Tiwari, D.; Lee, S.M.; Kim, D.J. Indigenously synthesized nanocomposite materials: Use of nanocomposite as novel sensing platform for trace detection of Pb⁺. J. Electroanal. Chem. 2021, 897, 115578. [Google Scholar] [CrossRef]
- Keramari, V.; Papadimou, S.G.; Golia, G.G.; Girousi, S. Bismuth Film along with dsDNA-Modified Electrode Surfaces as Promising (bio)Sensors in the Analysis of Heavy Metals in Soils. Biosensors 2024, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Fiore, L.; Massoud, R.; Cortese, C.; Moscone, D.; Palleschi, G.; Arduini, F. Low-cost and reagent-free paper-based device to detect chloride ions in serum and sweat. Talanta 2018, 179, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Hua, Y.; Liu, M.; Li, S.; Yin, M.; Lv, X.; Wang, H. Highly selective electroanalysis for chloride ions by conductance signal outputs of solid-state AgCl electrochemistry using silver-melamine nanowires. Sens. Actuators B: Chem. 2019, 300, 127058. [Google Scholar] [CrossRef]
- Abbas A; Amin HM. Silver nanoparticles modified electrodes for electroanalysis: An updated review and a perspective. Microchemical Journal 2022, 175, 107166.
- Chatziathanasiou, E.; Liava, V.; Golia, E.E.; Girousi, S. Analytical Applications of Voltammetry in the Determination of Heavy Metals in Soils, Plant Tissues, and Water—Prospects and Limitations in the Co-Identification of Metal Cations in Environmental Samples. Analytica 2024, 5, 358–383. [Google Scholar] [CrossRef]
|
Detection limit (mg L-1) |
Metals for detection | ||||||
| R.S.D.s (%) | Cd | Pb | Zn | Cu | |||
| Nanoparticles (NPs) | Ref. | AgNPs@Sa | LOD | 0.191 | 0.223 | 0.361 | 0.213 |
| sr % | 6.1 | 5.4 | 3.3 | 6.6 | |||
| [52] | AuNPs | LOD | 1.126 | 1.419 | - | 0.966 | |
| sr % | 2.69 | 1.79 | - | 1.22 | |||
| [53] | SnNPs | LOD | 0.63 | 0.60 | - | 0.52 | |
| sr % | 6.1 | 7.4 | - | 2.5 | |||
| [54] | BiNPs | LOD | 0.097 | 0.008 | 0.707 | 0.157 | |
| sr % | 5.76 | 2.07 | 7.89 | 0.43 | |||
| [55] | MnCO2O4NPs | LOD | 0.79 | 1.67 | - | - | |
| sr % | 3.46 | 7.68 | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





