1. Introduction
The pharmaceutical industry, one of the most influential sectors of the economy, presents a paradox; while it works for the creation, production and sale of medicinal chemicals, it often disposes of toxic waste incorrectly and inefficiently. This situation brings with it numerous damages that severely affect the environment. Drug residues can be found mainly in wastewater, both surface and deep, although they can also be found in soil, air and biota [
1]. The main routes of contamination are closely related to the consumption of drugs, where the metabolites resulting from the physiological metabolism of the drug can be excreted through feces and urine [
2], as well as the disposal of pharmaceutical waste resulting from the technological process itself, and those drugs that have reached their expiration date or have not been consumed.
Pharmaceutical industry produces various toxic waste such as radioactive waste, chemical waste, infectious waste, finished products such as vaccines, expired and/or unused or contaminated drugs [
3]. One of the most polluting are finished pharmaceutical products due to their high production volumes. Steroids, NSAIDs, antidepressants, antibiotics and cytostatics constitute fundamental groups of medicines that can cause accumulation and damage to the environment. Although many of them present a minimal risk due to their low toxicity, it is increasingly evident that they can generate major ecotoxicological effects in the medium at long term. One of the most representative examples is the case of diclofenac, an anti-inflammatory drug that is capable of causing acute and chronic toxicity with long-lasting harmful effects. The presence of a small concentration of diclofenac has been associated with chronic toxicity, endocrine disruption and the development of resistance to pathogens. The consequences are especially worrying in aquatic organisms, since they are subject to multigenerational exposure [
4]. The toxic effect of diclofenac on bacteria, algae, microcrustaceans and fish, are the reason why is the compounds with the greatest acute toxicity in phytoplankton and zooplankton [
5].
There are clinical studies that indicate that diclofenac can cause side effects on the kidney and liver in mammals; it also occurs in vultures, where it causes death due to acute renal failure, visceral gout and the accumulation of uric acid within the internal organs [
6]. Vultures eating remains of animals treated veterinary with diclofenac are poisoned due to the accumulation of the drug [
7]. Different species of vultures on the Asian continent such as (Gyps bangalensis), (Gypsindicus) and (Gyps tenuirostris), were declared endangered due to the bio accumulative effect of diclofenac [
8].
This growing situation regarding drug-related contamination brings with it the development of efficient elimination technologies. In this regard, some of the most significant are those that treat effluents and wastewater. The usual processes focus on oxygenation and separation of solid waste, to subsequently treat wastewater through chemical and biological processes. The latter have the advantage of being more environmentally friendly, highlighting enzymatic treatments in drug detoxification. In this sense, oxidase enzymes such as laccases play a fundamental role by constituting a group of biomolecules capable of catalyzing the oxidation of a wide spectrum of substrates and compounds of a recalcitrant nature. As a consequence of their versatility, in recent decades’ various studies have been reported that highlight the biochemical properties and potential technological applications of these enzymes in bioremediation processes [
9]. However, when enzymes are generally used in waste treatment and bioremediation processes, the possibility of inactivation due to extreme pH conditions and high temperatures must be taken into account. For all the above, the present study aimed to evaluate the catalytic capacity of purified heat-resistant induced laccase from
Curvularia kusanoi L7 in the removal of diclofenac.
2. Results
2.1. Obtaining and Evaluating Heat-Resistant Laccase from Curvularia kusanoi L7
The laccases were purified from their respective cultures by partitioning into three-phase systems. Both in the induced and non-induced cultures, yields of over 80% were obtained with a purification factor of 50 for the non-induced culture and 75 for the induced cultures. The purification is greater in the latter, due to the induction process itself, where a greater production of laccase occurs as a result of the biological interactions between C. kusanoi and T. pleuroticola. In this type of biological interaction, Trichoderma acts as a pathogenic fungus, triggering a physiological defense response in Curvularia, which responds by expressing greater production of enzymes specially laccases, who is related to defense processes by constituting important virulence factors and one of the main defense mechanisms of lignolytic fungi.
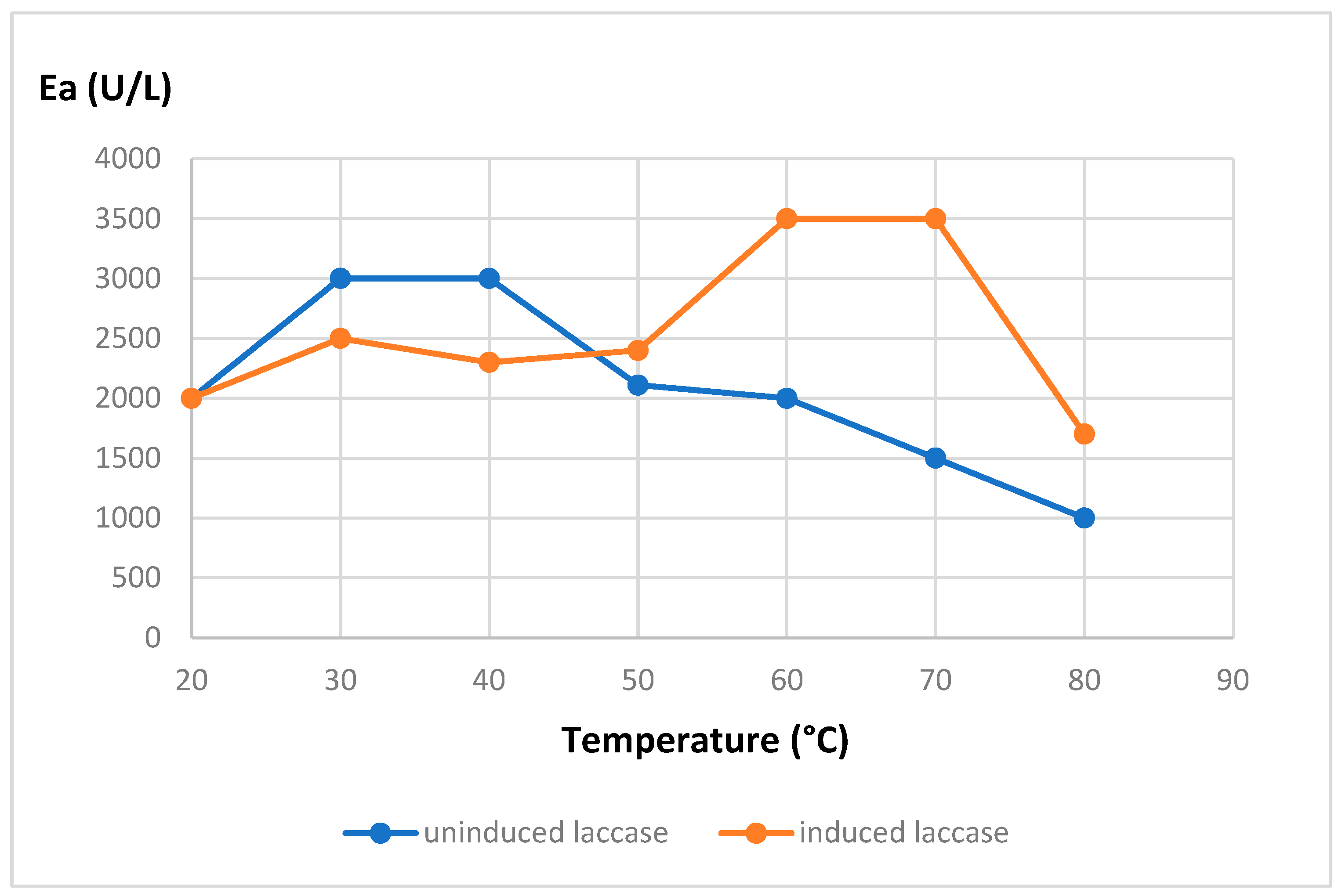
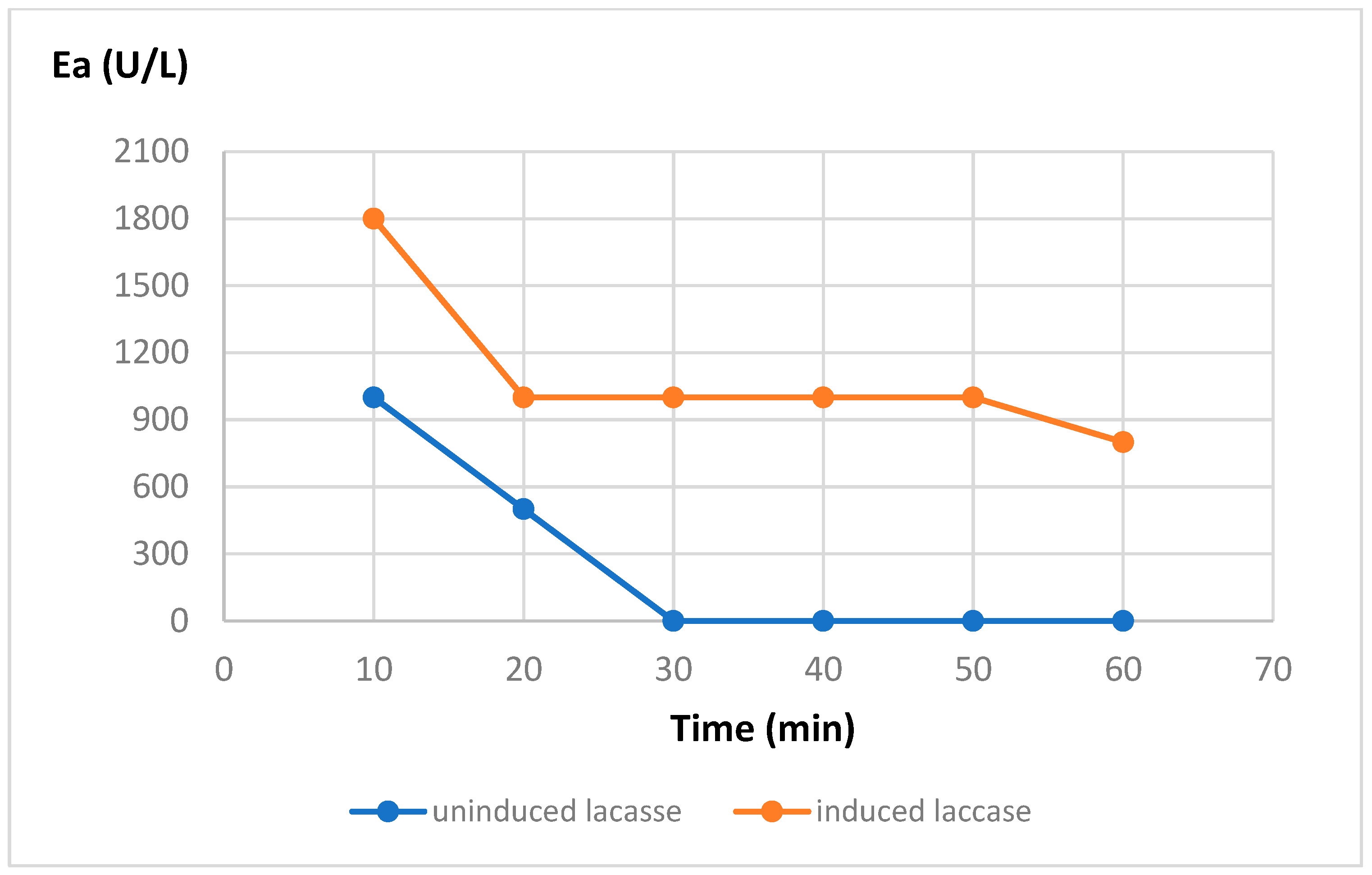
The effect of temperature on the activity and stability of the purified enzymes summarized in
Figure 1 and
Figure 2:
As can be seen in the figures, the induction process significantly increases the temperature range in which the enzyme is active, shifting it from 30-40 to 60-70 °C, and thus allowing the enzyme not only to be active at higher temperatures but also to increase its thermal resistance, maintaining its residual enzymatic activity at temperatures of 80°C for more than an hour.
2.2. Evaluation of Diclofenac Removal by Laccase Activity Using UV-Vis Spectral Scanning
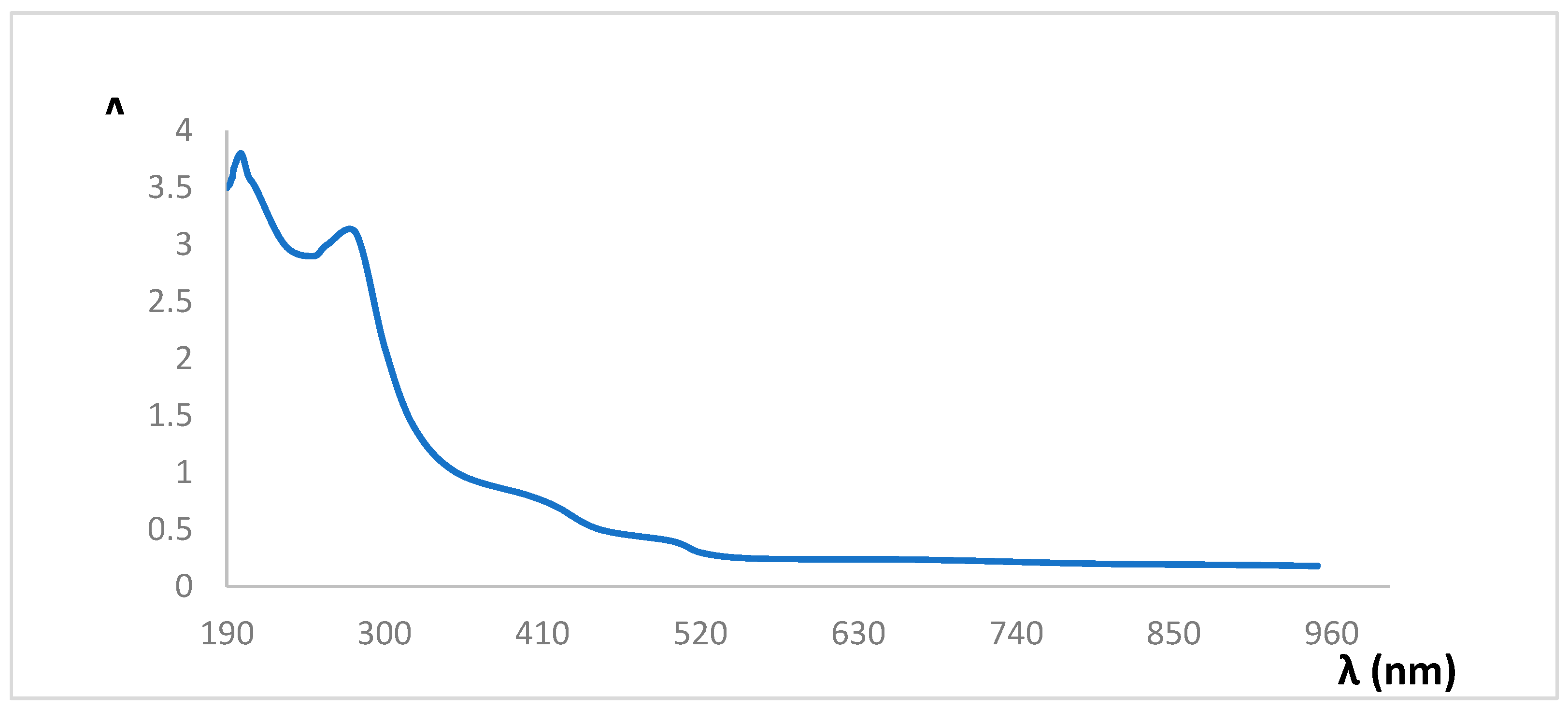
The characteristic spectrum according to the Spectral Scan in the UV-Vis range of Diclofenac (
Figure 3) showed two characteristic bands, one at 200 and another at 280 nm.
These results are in agreement with those obtained by Correia (2018) [
7], who found two bands associated with this drug, one at 220 nm and another at 280 nm. In his study, a good signal was also evident, with a stable baseline, at λ above 270 nm, while at values close to 220 nm, the baseline presents a lot of noise despite the fact that signals with higher absorbance values are obtained. The first band is considered to correspond to the aromatic ring that contains the caboxyl group that forms the bond with the metal by the non-bonded electrons in the oxygen atom. Other authors have found the maximum absorption for the Diclofenac standard near 276 nm [
10,
11].
These results confirm that the compound under study presents absorption maxima in the ultraviolet range, especially in the 270-290 nm interval. The differences that may be found in the maximum absorption at a fixed wavelength value are mainly due to the particular conditions of the spectroscopy equipment [
12]. Tapia [
13], studied the UV-Vis spectrum of Diclofenac in methanolic solution, carried out a scan in the ultraviolet zone (200 to 400 nm) with which he obtained the maximum absorption at 274 nm.
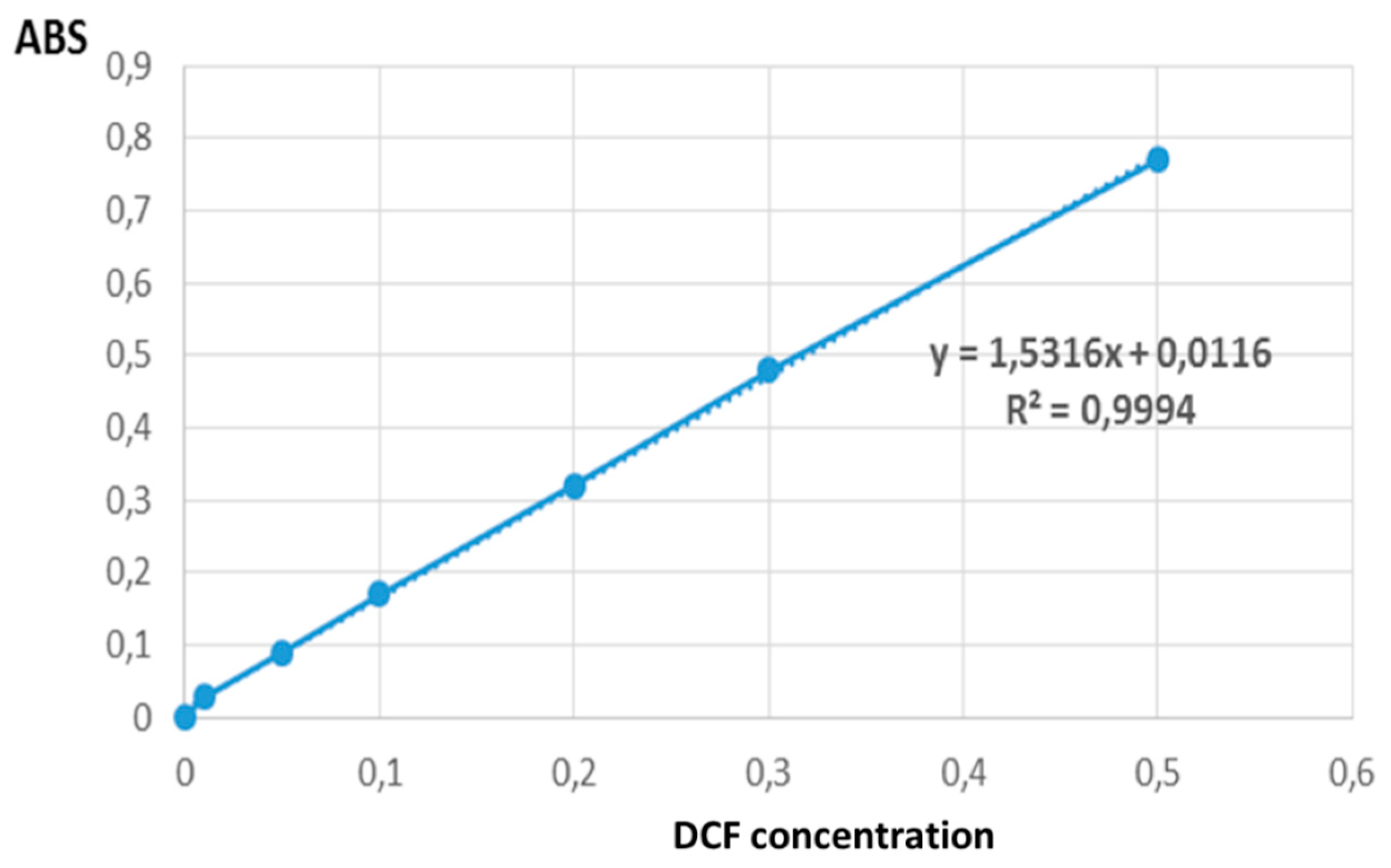
From the maximum absorption of the compound detected at 280 nm, the calibration curve was made as shown in
Figure 4.
Calibration includes the selection of a model to estimate the parameters that allow determining the linearity of the curve and, consequently, the capacity of an analytical method to obtain results that are directly proportional to the concentration of the compound in a sample, within a certain working interval. Linearity is a fundamental requirement of the calibration curve. To demonstrate linearity, it is necessary to meet certain acceptance criteria in which this linearity can be statistically justified. As the main acceptance criterion for linearity from the calibration curve, the coefficient of determination R
2 ˃ 0.98 is considered [
14]. The calibration curve presented in figure 2 shows linearity with an R
2 = 0.9994, since as mentioned above the value of the coefficient of determination must be above 0.98. Soto (2007) [
15], used UV-Vis spectrophotometry as a method for quantifying Diclofenac. In the study, different levels of drug concentration were evaluated, reaching linearity values similar to the present study.
Using the curve obtained, the percentages of drug removal after enzymatic treatment with the enzyme laccase were calculated. The results of the study are summarized in the
Table 1:
The results obtained show a high enzymatic degradation of the drug. It is important to note that these values were reached after 1 hour of incubation. If these results are compared with those reported by Lobos (2020) [
16], who achieved a degradation of 64.7% after 48 hours of enzymatic treatment, the present research stands out for being more effective and faster.
2.3. HPLC Evaluation of Diclofenac Removal by Laccase Activity
Diclofenac degradation by laccase activity were verified by HPLC. The retention time (Rt) for the drug pattern was 5.7 minutes, similar to the study of sunlight degradation of drugs and their evaluation by HPLC [
10], where a Rt of 5.64 minutes was obtained for DCF.
The removal levels of Diclofenac, calculated by the ratio between the areas of the pattern and the area of the injected samples, are presented in
Table 2.
As can be seen in the table, the removal of the drug is slightly higher than that obtained by UV-Vis determination. This may be associated with the fact that HPLC determinations are more sensitive and capable of detecting minor variations with a higher degree of specificity. This chromatographic method for determining Diclofenac is very effective and is used in Cuba as a method to evaluate the quality of this medicine in pharmaceutical laboratories.
3. Discussion
Among the most commonly used pharmaceutical compounds, analgesics and non-steroidal anti-inflammatory drugs constitute one of the most widely consumed in the world [
17]. These are ones of the most studied and detected in wastewater treatment plants and the ones that stand out the most in ecotoxicological studies, after antibiotics [
18]. In the specific case of diclofenac, its concentration does not decrease in biotransformation tests under aerobic and anaerobic conditions, which explains its incomplete elimination in wastewater treatment plants [
19]. For these reasons, it is vital to achieve processes that allow the complete elimination of this drug
Similar studies have been carried out by various authors, Lobos (2020) [
16] used the direct biodegradation of the drug by the fungi
Trametes hirsutas and
Trametes versicolor (large producers of laccase enzymes), unlike the present work where only purified heat-resistant laccase where used more efficiently. On the other hand, the concentrations of Diclofenac used in their study (0.1 mg/L) were lower than those used in this research. This comparison shows the ability of pure enzymes to act more specifically on their substrate, without the presence of other substances that could act as inhibitors of the enzymatic degradation process. Urrea et al. (2008) [
20], also used the fungus
Trametes versicolor in the degradation of DCF and obtained complete degradation of the drug after 4 hours of exposure to the fungus. This author suggests that the result of the research could be associated with changes that may have occurred in the culture medium or in the amount of initial mycelium used as inoculum. Changes in the culture medium may imply that the enzymes involved in the degradation of the drug are produced due to the secondary metabolism of the fungus. These enzymes may be increased or decreased depending on the presence or absence of certain compounds in the medium. Compared to this study, although complete degradation of Diclofenac was achieved after 4 hours, in the present investigation more than 70% of the drug was degraded in one hour for all cases. It should also be noted that the concentrations of DCF used were also higher than those used by this author [
16]. Fuad et al. (2015) [
21] used laccase and manganese peroxidase enzymes for the degradation of DCF, obtained from 9 days of cultivation of
Trametes maxima and
Pleuretus spp. These authors obtained results above 85% degradation after one hour, for a DCF concentration of 0.002 mg/mL. This result compared with Urrea et al (2008) [
20] and Lobos (2020) [
16], are superior, however, they are still far below those achieved in the present investigation.
Laccases are versatile enzymes capable of oxidizing compounds of similar chemical nature. In this regard, Auriol et al. (2008) [
22], used purified laccase to remove estrogens from effluents of synthetic and real treatment plants. These authors managed to completely remove the drugs from the water, which required a dose of 20 U/mL of enzyme. The system behaved similarly for both synthetic and real water.
In the scientific literature other methods for the degradation of diclofenac are referred to, among which the following stand out: Advanced oxidation with UV light/H
2O
2, and ozone. Vogna et al. (2008) [
23], used this method and achieved a removal of 32% by ozonation and 39% by UV/H2O2 treatment, after 90 minutes of the process. Taking into account these results, the present investigation is much more efficient in terms of % removal and overall process time. García et al. (2015) [
4] evaluated the degradation of ibuprofen, caffeine, Diclofenac and salicylic acid by heterogeneous photocatalysis. The study showed degradation percentages between 20 and 40% for DCF. In all cases, a tendency towards greater degradation was observed at lower drug doses. This method belongs to the group of advanced oxidation processes, as does the enzymatic treatment, the latter with higher DCF degradation results according to the present investigation.
Another method developed for the degradation of DCF is the synthesis of graphene nanocomposites and palladium and silver nanoparticles, using cochineal extract as a reducing agent [
24]. These authors achieve a degradation percentage of 60%. Ravina et al. (2002) [
25], studied the mineralization of Diclofenac through the Fenton reaction in a concentric photoreactor, where he managed to achieve the total mineralization of this compound. However, this method is complex and requires the necessary infrastructure that makes its use as a routine method more expensive.
The difficult degradation of Diclofenac is a worldwide problem. In this regard, Correia and Marcano (2015) [
26] evaluated the presence and elimination of pharmaceutical compounds in wastewater plants. Their study and review of more than 100 scientific journals showed that the group of analgesics and NSAIDs present high percentages of removal (over 65%) except for the case of DCF with only 38%. The term removal in the articles studied by Correia and Marcano (2015) [
26] refers to the conversion of the pharmaceutical compound into another different compound, so it is not clear whether the drug was mineralized or structurally altered. They reported that DCF presents a low biodegradability due to the presence of two chlorine atoms in its structure and a medium adsorption in sludge. If these results are compared with those obtained in the present investigation, the great difference that exists between the percentages of degradation can be observed. For these reasons, enzymatic treatment with
C. kusanoi L7 heat-resistant induced laccase could be considered a more effective method to treat residues of this drug.
On the other hand, the heat-resistant nature of Curvularia kusanoi L7 laccase is a very important aspect to point out which gives it added value by eliminating one of the most important problems when working with enzymes, which is thermal denaturation. The usefulness of heat-resistant enzymes in different industrial processes is an example of efficiency, since the high costs that working with enzymes often entails are offset by the durability and stability of this type of product. Similarly, obtaining active and stable enzymes to use as biotechnological tools constitutes one of the most promising emerging clean solutions and one of the greatest exponents of green chemistry and sustainability in the conception of more efficient processes with low or zero carbon emissions. Enzymatic biocatalysis are environmentally friendly technologies and an inseparable part of the three pillars of the bioeconomy: the production of food and feed, the obtaining of bioproducts (preferably with added value) and the production of bioenergy. The importance of using enzymes lies in the opportunity to achieve economic growth and at the same time guarantee the safety of biological resources and their effective and sustainable use. And if at the same time they are used in the detoxification of the environment it is a win-win in every sense.
4. Materials and Methods
4.1. Microorganisms
In this research, two strains of Ascomycete fungi were used, both belonging to the culture collection of the Cuban Institute of Animal Science. Curvularia kusanoi L7 and Trichoderma pleuroticola, identified by molecular biology and their nucleotide sequences deposited in Genbank with accession numbers KY795957 and MK992922, respectively.
4.2. Obtaining and Evaluating Heat-Resistant Laccase from Curvularia kusanoi L7
3 cm
2 of the fresh culture of
Curvularia kusanoi L7 in PDA were taken and inoculated in Erlenmeyer flask with 3 g of wheat bran in 100 ml of citrate buffer (100 mM, pH 5.5) and incubated in an orbital shaker at 120 rpm for a period of 7 days at 25°C. The induction of heat-resistant laccase was obtained by confronting the 48-hour-old
C. kusanoi cultures with a
Trichoderma pleuroticola inoculum of 1x10
7 CFU/g of substrate. At the end of the fermentation, the cultures were centrifuged (4ºC, 10,000 rpm, 5 min) and the enzymatic extract was stored [
27].
The crude enzymatic extract resulting from the fermentation where purified using the partition methodology in three phasic systems [
28]. The crude extract was saturated to 80% with ammonium sulfate and tert-butanol 1.0:1.0 (v/v). Once the three phases appeared, only the intermediate phase was reserved, which was resuspended in phosphate buffer pH 7. The protein concentration was determined with a standard curve of bovine serum albumin (50-0.01 mg/mL) [
29].The enzyme yield and the purification factor of the process were calculated [
28].
To evaluate the purified laccase enzymes from both induced and uninduced cultures, their catalytic activity against syringaldazine (5 mM in ethanol) as a substrate was determined. The oxidation reaction was monitored in kinetic mode for 1 minute under aerobic conditions at 530 nm. A unit of laccase activity (U) was considered as the amount of enzyme that catalyzes the transformation of 1.0 μmol of syringaldazine per minute [
30]. All analyses were performed in triplicate.
To determine the effect of temperature on enzymatic activity and enzyme stability, purified laccases where preincubated in a thermostatic bath (Thermo Scientific, EEUU) at 30, 40, 50, 60, 70, and 80◦C for 1 h, taking 100 µl aliquots every 10 min and determining activity as described above. The reaction mixture without substrate was used as a blank, preincubated as well for 1 min at the different temperatures used in the study.
4.3. Evaluation of Diclofenac Removal by Heat-Resistant Laccase Using UV-Vis Spectral Scanning
A standard solution of 1 mg/mL Diclofenac sodium (Sigma-Aldrich, ≥ 98% purity) in ethanol was prepared [
31]. To determine the Diclofenac removed level, the working wavelength was previously selected by means of a spectral scan between 190 and 950 nm. At the wavelength where the maximum absorption of the compound was found, the calibration curve was read, plotting OD (optical density) vs C (concentration in mg/mL). The calibration curve was made with the Diclofenac standards in the concentration range between 0.01-0.5 mg/mL. Each determination was performed in triplicate. The degradation study was performed by incubating 100 µL of enzyme and 900 µL of Diclofenac solution at 60 ºC for a final reaction volume of 1 mL and an incubation time of 1 hour. In this study, the degradative effect of the enzyme was also evaluated against different levels of drug concentration (0.5, 0.3, 0.1 mg/mL). The removal percentage was calculated according to equation 1, Where: Ci and Cf correspond to the initial and final concentrations of the drug, respectively. The Diclofenac-enzyme mixture was used as a control before the degradation process. Each determination was performed in triplicate.
Likewise, the degradation products of the diclofenac removal assay by laccase activity described above were also analyzed by HPLC.
4.4. High Performance Liquid Chromatography (HPLC) Evaluation of Diclofenac Removal by Laccase Activity
The level of Diclofenac removal was verified by HPLC according to following chromatographic conditions: Column C8 Tecknokroma, Mobile phase flow: 1.0 mL/min, UV detector at 254 nm, Injected volume: 10 µL, Mobile phase: Solution A and Methanol (34:66). For the preparation of the mobile phase, the solution A was prepared using: 1.04g of
x 2
in 1L of H
2O and pH 2.5 adjusted with
. The chromatographic profile of the Diclofenac standard (pattern) was determined at the concentrations used in the previous test and its removal (%) by laccase activity was evaluated using equation 2, Where: C: Diclofenac concentration (%), As: area of the sample peak, and Ap: area of the pattern peak.
5. Conclusions
Although the degradation of diclofenac is a complex process, the use of purified heat-resistant fungal laccase from Curvularia kusanoi strain L7 allows an exhaustive degradation of this drug, making this enzymatic treatment an interesting option to scale up and evaluate in treatment systems for other waste and compounds of a similar nature.
Author Contributions
Conceptualization, L.S, M.A.; investigation, M.A., L.S., CF; methodology, L.S., M.A., CF; data curation, L.S.; formal analysis, M.A., L.S.; writing—original draft preparation, L.S., M.A. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Data Availability Statement
The microorganism used in the study has its sequences deposited to GenBank under accession number KY795957.
Conflicts of Interest
The authors declare no conflicts of interest
References
- INFAC. Fármaco-contaminación. Impacto Ambiental de los Medicamentos. 2016; 24(10): 1-2. Disponible en: http://www.osakidetza.euskadi.eus/cevime.
- Silva y Bonora. Impacto ambiental de los medicamentos y su regulación en Brasil. Revista Cubana de Salud Pública. 2014, 40, 268–273. [Google Scholar]
- Lozano, C. (2017). Industria Farmacéutica, entre la cura y la enfermedad: contaminación en la Ciudad de México. Colegio Alemán Alexander von Humboldt Plantel Sur. Ciudad de México. Disponible en: http://vinculacion.dgire.unam.mx/.
- García, M.A.; Contreras, A.; Aguilera, M.G.; Ruiz EA y Morales, M.A. Manejo de residuos de fármacos: una breve revisión. Rev. Int. Contam. Ambie. 2021, 37, 329–344. [Google Scholar] [CrossRef]
- Ferrari, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotox. Environ. Safe. 2003, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry MJ, I.; Arshad, M.; Mahmood, S.; Ali, A.; Khan, A.A. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Correia de Soto A. Comportamiento Ambiental del Ibuprofeno y el Diclofenaco en Suelos Venezolanos [ tesis de doctorado en Ingeniería, área: ambiente]. Universidad de Carabobo; 2018. URI: http://hdl.handle.net/123456789/5021.
- Pizarro, Y.; Ordoñez, J.; Mackliff, C.; Medina, A.; Segura, M. Ecofarmacovigilancia y la determinación del diclofenaco sódico mediante electro análisis. Rev Ciencia UNEMI. 2019, 12, 54–63. [Google Scholar] [CrossRef]
- Aghaee, M.; Salehipour, M.; Rezaei, S.; Mogharabi-Manzari, M. Bioremediation of organic pollutants by laccase-metal–organic framework composites: A review of current knowledge and future perspective. Bioresource Technology 2024, 131072. [Google Scholar] [CrossRef]
- Stuttgart: Deutscher Apotheker Verlag, 2016.Santibañez S. Determinación de la cinética de degradación de Diclofenaco, Ibuprofeno y su mezcla, a temperatura ambiente [tesis de grado en Químico Farmacéutico Biólogo]. Universidad Autónoma del Estado de México; 2014.
- Yogesh, J.; Ratendra, K.; Teotia, U. In Vitro Evaluation of Release Modifying Potential of Prunus persica Gumin Matrix Tablets. Research Journal of Pharmaceutical Dosage Forms and Technology. 2014, 6, 18–12. [Google Scholar]
- Santos, M. 2006. Análisis y distribución de principios activos farmacológicos en los procesos convencionales de depuración de aguas residuales urbanas. Tesis de doctorado. España, Departamento de Química Analítica, Universidad de Sevilla.
- Tapia, W. Determinación del diclofenaco por análisis instrumental en medicamentos de marca (tesis de diploma). Universidad nacional de Callao. Callao, 2015.
- Dosal MA y Villanueva, M. Curvas de calibración en los métodos analíticos. Marzo, 2008. Disponible en: https://epaginapersonal.unam.mx/app/webroot/files/5404/Antologiadedocumentosdeapoyo(1507y1602)_8593.
- Soto, ME. Evaluación del mecanismo de liberación de diclofenaco sódico desde una matriz hidrofílica a base de alginato de sodio (tesis de grado). Universidad de Chile. Valdivia-Chile, 2007.
- Lobos, CC. Biotransformación y/o degradación de Dcilofenaco por hongos con actividad lignolítica (tesis de diploma). Universidad de Concepción. Chile, 2020. Disponible en: https://repositorio.udec.
- Conaghan, P. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatology International 2012, 32, 1491–1502. [Google Scholar] [CrossRef]
- Santos, H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue, C.; Montenegro, M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. Journal of Hazardous Materials 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Lahti, M.; Oikari, A. Microbial transformation of pharmaceuticals naproxen, bisoprolol, and diclofenac in aerobic and anaerobic environments. Arch. Environ. Con. Tox. 2011, 61, 202–210. [Google Scholar] [CrossRef]
- Urrea, M.E.; Trujillo, M.; Vicent, T. Caminal ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2008, 74, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Fuad AE y col. (2015). Estudio de la correlación entre actividades enzimáticas de lacasa y manganeso peroxidasa con la remoción de fármacos. 2º Congreso Nacional AMICA 2015. Disponible en: https://www.researchgate.net/publication/317336418.
- Auriol, M.; Filali-Meknassi, Y.; Adams, C.; Tyagu, R.; Noguerol, R.; Piña, B. Removal of estrogenic activity of natural and synthetic hormones form municipal wastewater: Efficiency of horseadish peroxidase and laccase from Trametes versicolor. Chemosphere 2008, 70, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; D’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/ H2O2 and ozone; Wat. Res. 2004, 38, 414–422. [Google Scholar]
- Oñate M, Ambar S. Degradación de Carbamazepina y Diclofenaco bajo luz solar y artificial utilizando nanocompuestos de paladio y plata sintetizados con extracto de cochinilla [trabajo de titulación a la obtención de título de ingeniería en biotecnología]. Universidad de las Fuerzas Armadas. Sangolquí, 2018. Disponible en: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=http://repositorio.espe.edu.ec/bitstream/21000/15381/1/T-ESPE-040565.pdf&ved=2ahUKEwisodvO7-GIAxVISzABHQk8O50QFnoECBkQAQ&usg=AOvVaw0NzJkXCUOXYxrOq-rCuSxs.
- Ravina, M.; Campanella, L.; Kiwi, J. Accelerated mineralization of the drug Diclofenac via Fenton reactions in a concentric photo-reactor. Wat. Res. 2002, 36, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Correia, A. & Marcano L. (2015). Presence and elimination of pharmaceutical compounds in wastewater treatment plants. Worldwide review and national perspective. Boletín de malariología y salud ambiental. Vol. LV (1): 1-18. Disponible en: http://iaes.edu.ve/iaespro/ojs/index.php/bmsa/article/view/113/55.
- Wang, L. Y., Cheng, G. N y May, A. S. (2014). Fungal solid-state fermentation and various methods of enhancement in cellulases production. Biomass and Bioenergy, 67, pp. 319-338. [CrossRef]
- Alberto, M., Valiño, E.C., Ayala, M., Folch, J.L., Sánchez-Cruz, R. (2019). Cellulolytic and ligninolytic potential of new strains of fungi for the conversion of fibrous substrates. Biotechnology Research and Innovation, 3 (1), p. 177-186. [CrossRef]
- Bradford, Marion M. "A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding." Analytical biochemistry 72.1-2 (1976): 248-254.Perna, V., Agger, J. W., Holck, J. y Meye, A. S. (2018). Multiple Reaction Monitoring for quantitative laccase kinetics by LC-MS. Scientific Reports, 8, pp. 8114. [CrossRef]
- Perna, V., Agger, J. W., Holck, J. y Meye, A. S. (2018). Multiple Reaction Monitoring for quantitative laccase kinetics by LC-MS. Scientific Reports, 8, pp. 8114. [CrossRef]
- United States Pharmacopeial Convention. USP 40 - NF 35 The United States Pharmacopeia and National Formulary 2017. 40ª rev. (USP), 35ª ed. (NF).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).