Submitted:
06 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
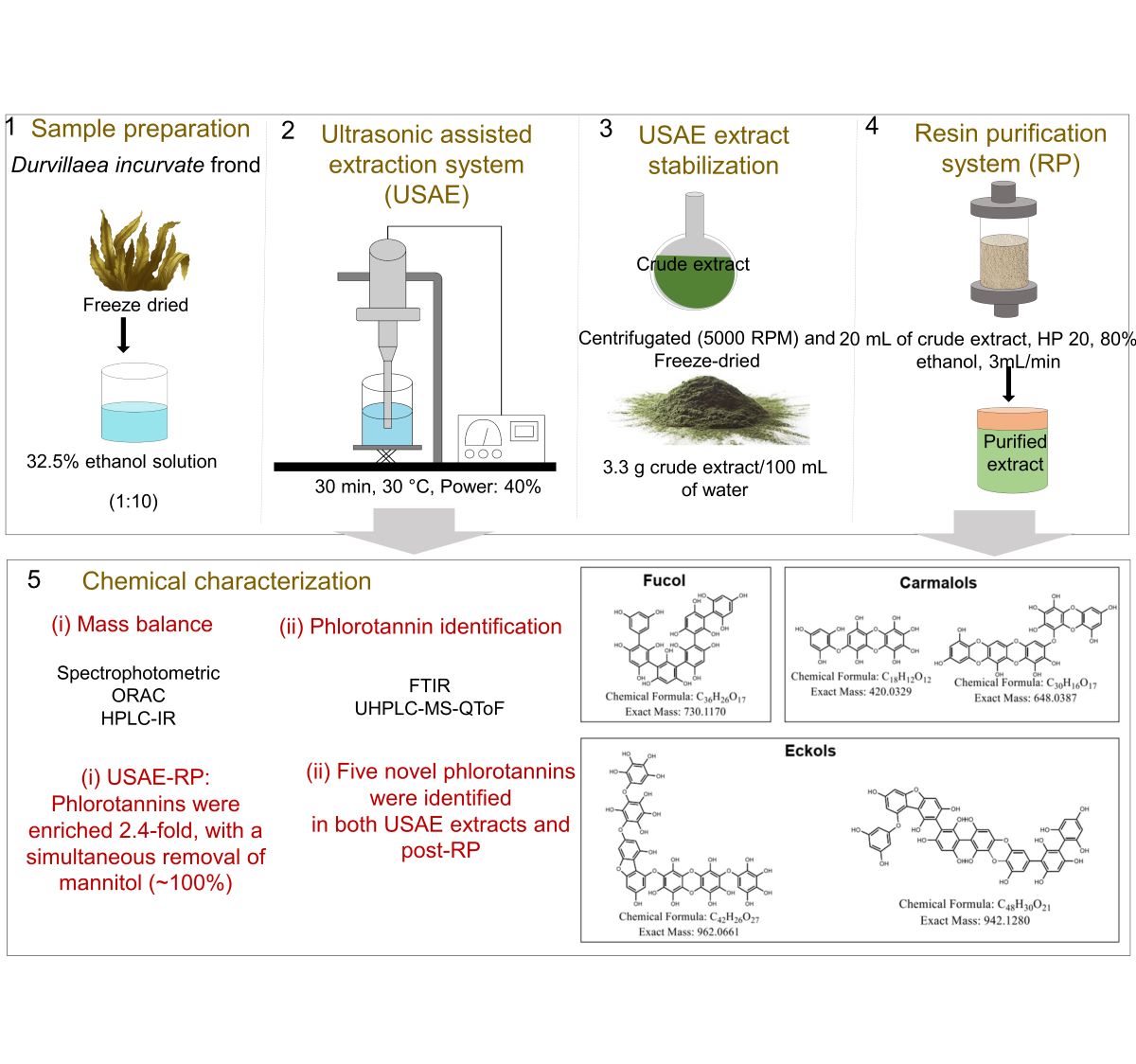
2. Materials and Methods
2.1. Seaweed Material
2.2. Chemicals
2.3. Extraction Techniques
2.3.1. Atmospheric solid-liquid extraction (ASLE)
2.3.2. Ultrasound Assisted Extraction (USAE)
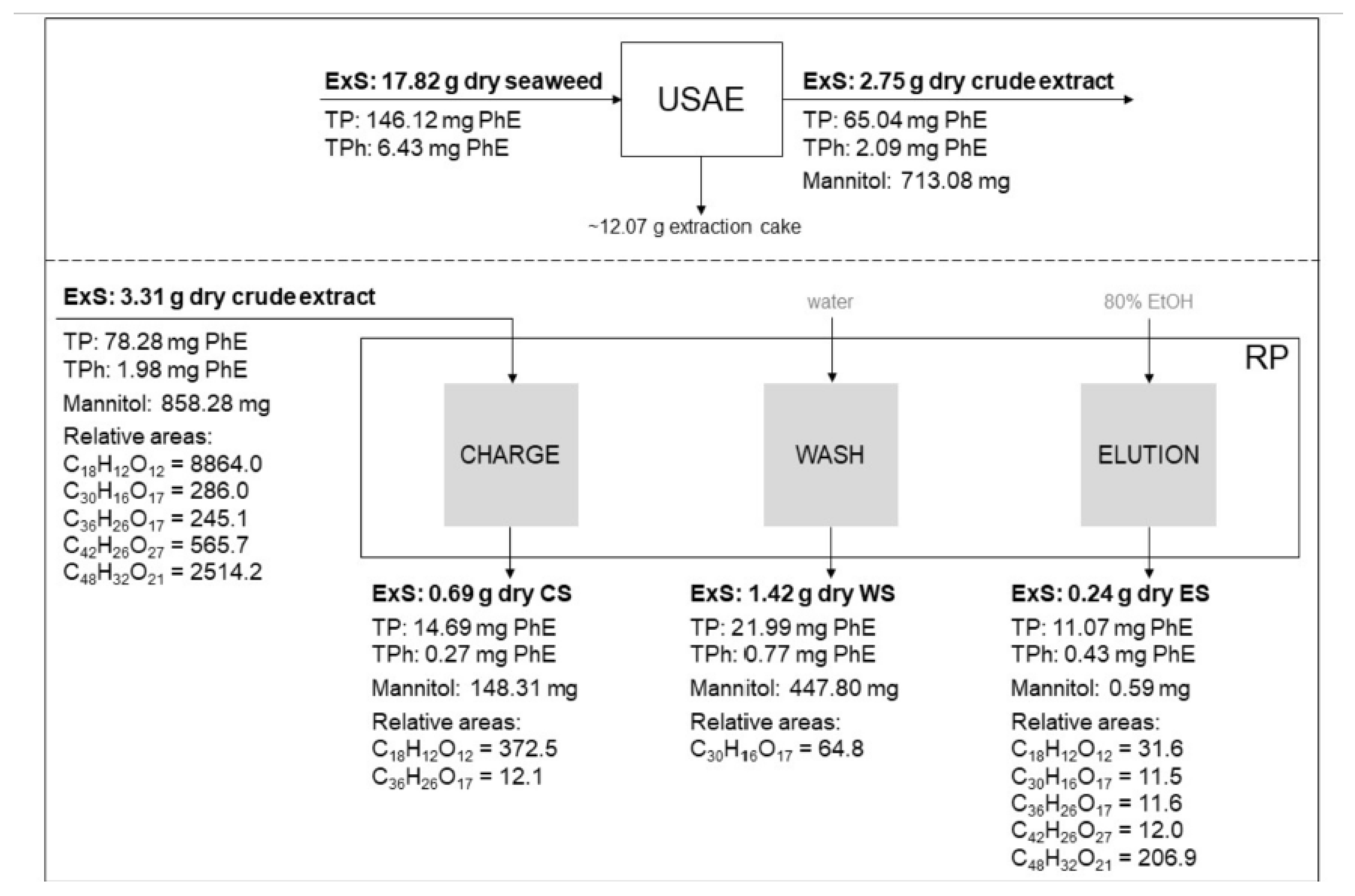
2.4. Resin Purification Process (RP)
2.5. Chemical Analyses
2.5.1. Total Polyphenol Content (TPC)
2.5.2. Total Phlorotannin Content (TPhC)
2.5.3. Antioxidant Capacity (AC): Oxygen Radical Absorbance Capacity Assay (ORAC)
2.5.4. Antioxidant Capacity (AC): DPPH Assay
2.5.5. Fourier Transform Infrared Spectroscopy Analysis of USAE and RP Streams
2.5.6. Mannitol Content
2.5.7. Tentative Identification of Phlorotannins Using a UHPLC Coupled with a QToF Detector
2.6. Statistical Analysis
2.7. Relative Mass Balance and Selective Separation Performance Parameters
3. Results and Discussion
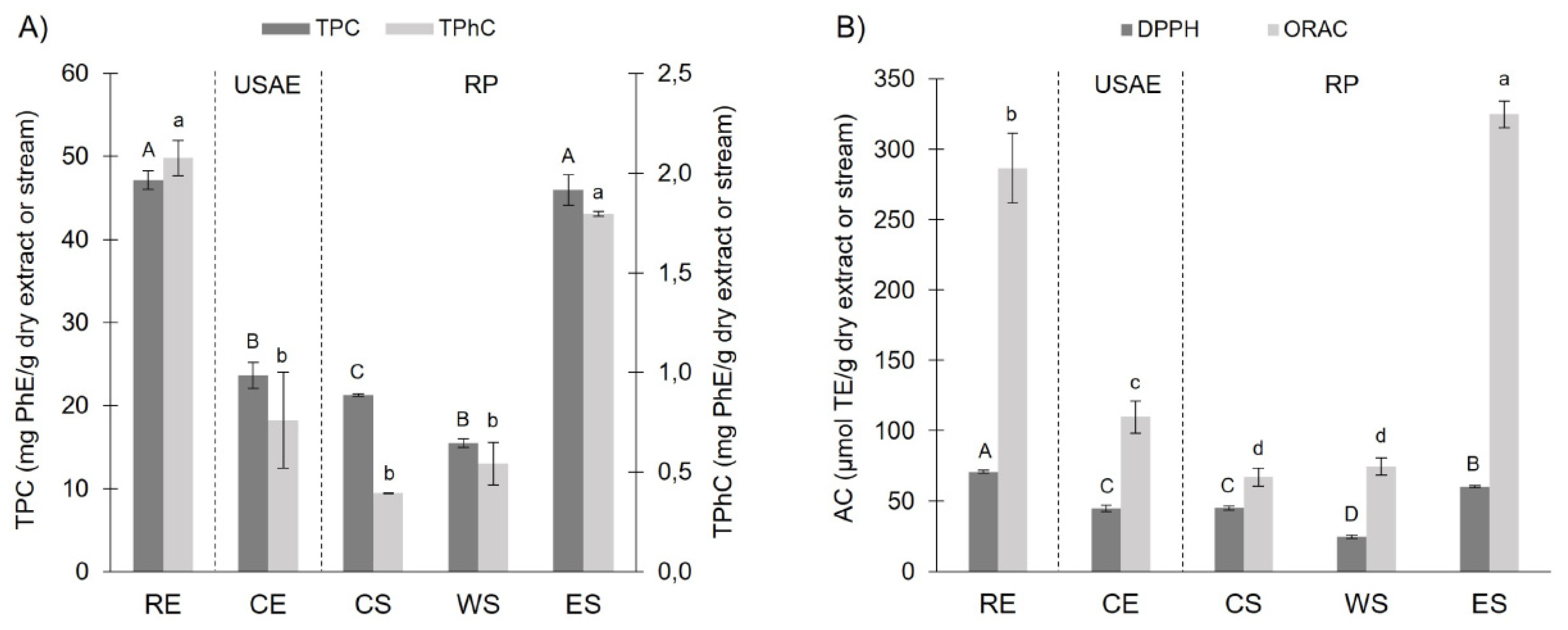
3.1. Spectrophotometric Characterization and Quantification: TPC, TPhC, ACORAC and ACDPPH
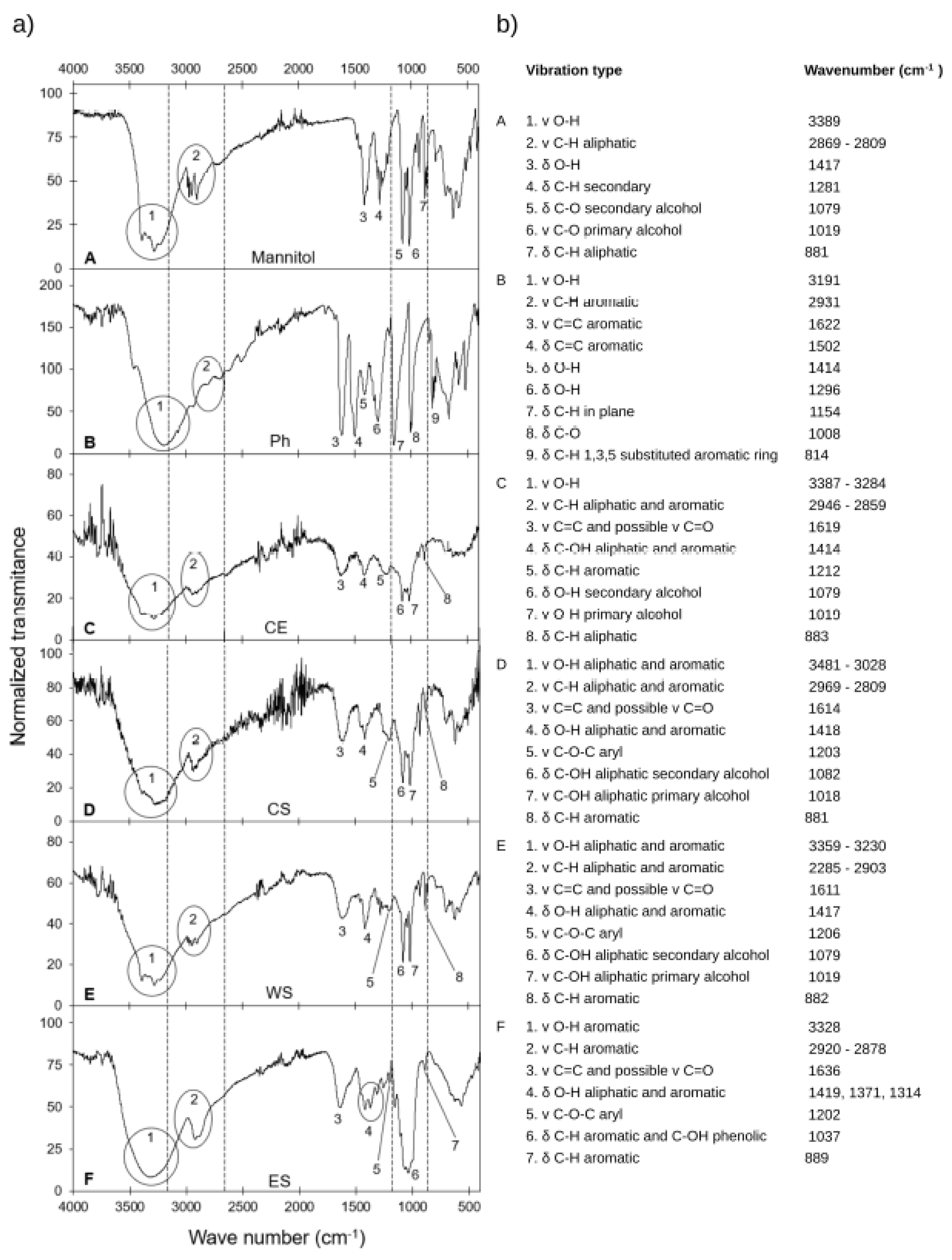
3.2. Fourier Transform Infrared Spectroscopy Analysis
3.3. Mannitol Content
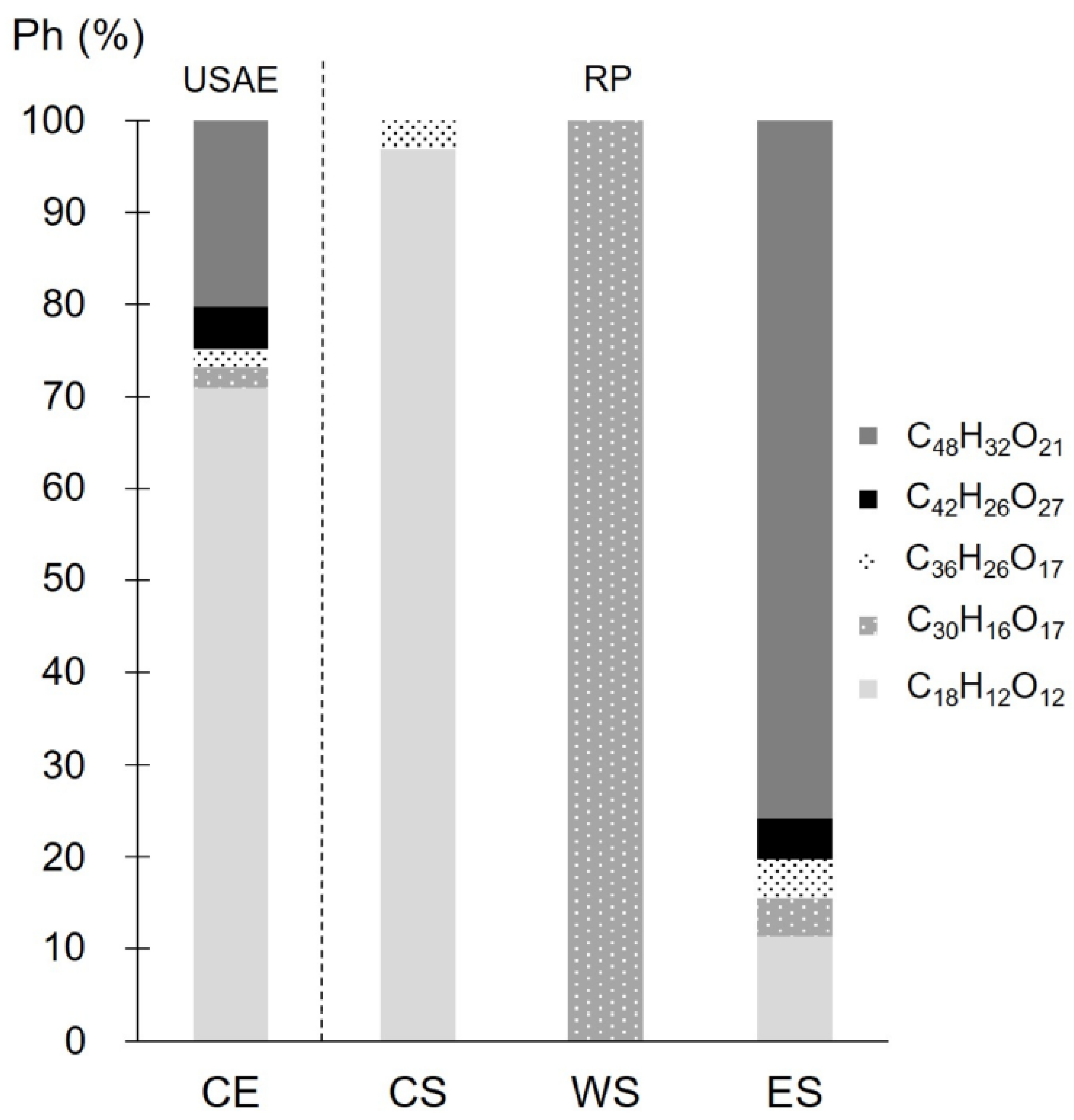
3.4. Tentative Phlorotannins Identification and Determination of Their Relative Abundances by UHPLC-QToF Mass Spectrometry
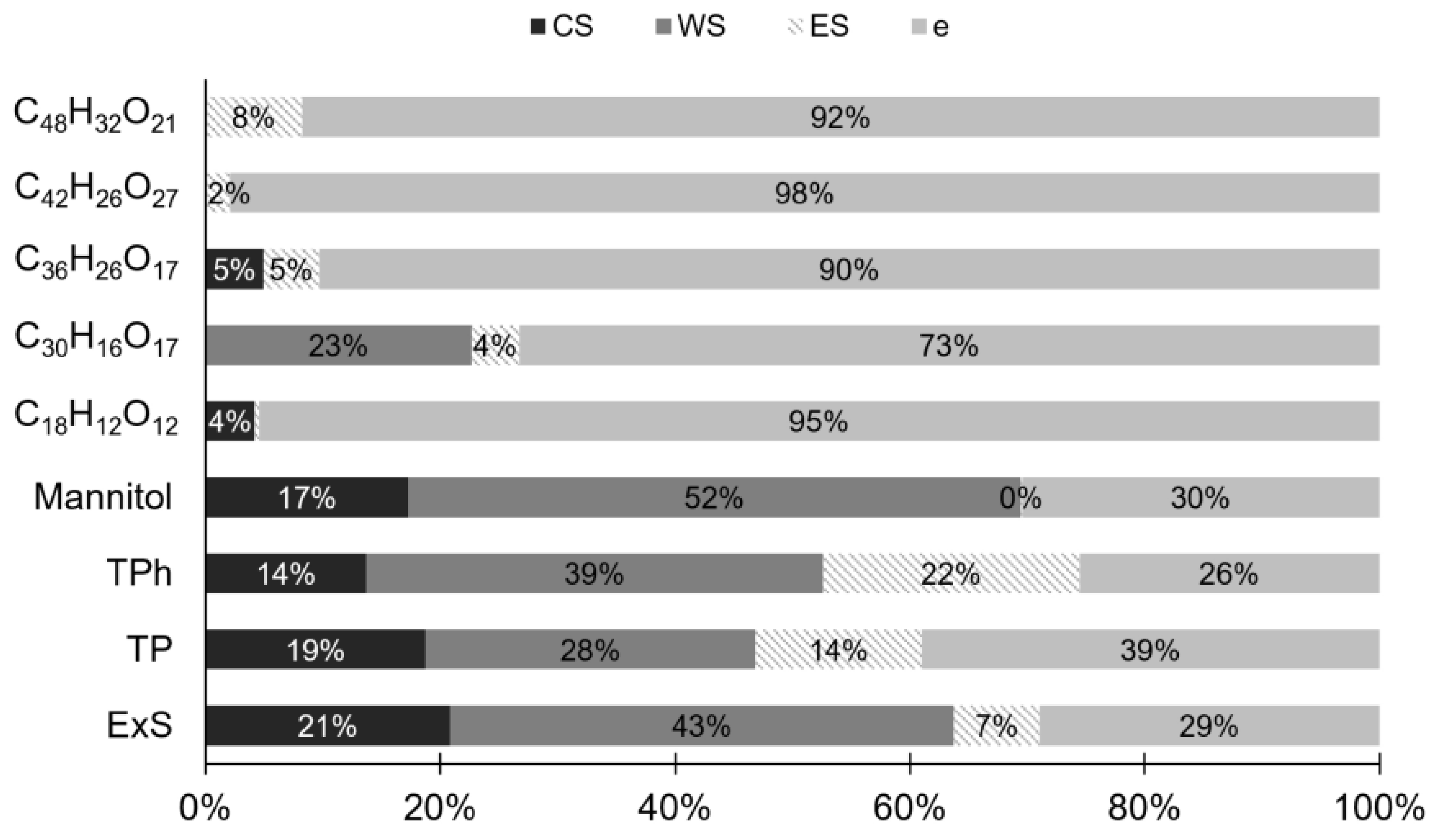
3.5. Process Evaluation: Quality Parameters and RP Mass Balance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Declaration of Competing Interest
References
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA - J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Perez-Correa, J.R. Polyphenols of Carménère Grapes. Mini-Reviews Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M. Antioxidant Potential and Polyphenol Content of Selected Food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from brown algae: a review on their antioxidant mechanisms and applications in oxidative stress-mediated diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: a randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Bahar, B.; O’doherty, J.V.; Smyth, T.J.; Ahmed, A.M.; Sweeney, T. A cold water extract of Fucus vesiculosus inhibits lipopolysaccharide (LPS) induced pro-inflammatory responses in the porcine colon ex-vivo model. Innov. Food Sci. Emerg. Technol. 2016, 37, 229–236. [Google Scholar] [CrossRef]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of low glycemic response foods using polyphenols from seaweed. J. Funct. Foods 2019, 56, 33–39. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’doherty, J.; O’donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Erpel, F.; Mariotti-Celis, M.S.; Parada, J.; Pedreschi, F.; Pérez-Correa, J.R. Pressurized Hot Liquid Extraction with 15% v/v Glycerol-Water as An Effective Environment-Friendly Process to Obtain Durvillaea incurvata and Lessonia spicata Phlorotannin Extracts with Antioxidant and Antihyperglycemic Potential. Antioxidants 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Ferreira-Anta, T.; Torres, M.D.; Vilarino, J.M.; Dominguez, H.; Flórez-Fernández, N. Green Extraction of Antioxidant Fractions from Humulus lupulus Varieties and Microparticle Production via Spray-Drying. Foods 2023, 12, 3881. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yang, R.; Zhang, Y.; Chen, Y.; Jiang, C.; Li, X. Synergistic Therapeutic Effects of D-Mannitol–Cerium–Quercetin (Rutin) Coordination Polymer Nanoparticles on Acute Lung Injury. Molecules 2024, 29, 2819. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Villain, F. Free radical scavenging properties of mannitol and its role as a constituent of hyaluronic acid fillers: a literature review. Int. J. Cosmet. Sci. 2017, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Camilo, C.; Mateos, R.; Pérez-Correa, J.R. A macroporous resin purification process to obtain food-grade phlorotannin-rich extracts with α-glucosidase inhibitory activity from Chilean brown seaweeds: An UHPLC-MS profiling. Food Chem. 2022, 402, 134472. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- J.J. Ojeda, M. Dittrich, Fourier Transform Infrared Spectroscopy for Molecular Analysis of Microbial Cells, in: 2012: pp. 187–211. [CrossRef]
- Gheda, S.; Hamouda, R.A.; Naby, M.A.; Mohamed, T.M.; Al-Shaikh, T.M.; Khamis, A. Potent Effect of Phlorotannins Derived from Sargassum linifolium as Antioxidant and Antidiabetic in a Streptozotocin-Induced Diabetic Rats Model. Appl. Sci. 2023, 13, 4711. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, A.L.; Jarratt, G.; Haritos, V.S. Analysis of the fate of valuable bioactive polyphenols during commercial winemaking and their partitioning into wastes for valorization. Clean. Waste Syst. 2023, 5. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carménère Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef] [PubMed]
- AOAC International, Official Methods of Analysis of AOAC International, 20th ed., 2020.
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- M.S. Mariotti-Celis, P.R. Rivera-Tovar, N.L. Huamán-Castilla, J.R. Pérez-Correa, Purification and fractionation of crude seaweed extracts by adsorption-desorption processes, in: J.R. Pérez-Correa, R. Mateos, H. Domínguez (Eds.), Marine Phenolic Compounds: Science and Engineering, 2023: pp. 187–215.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLOS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ismail, M.M.; El Zokm, G.M.; El Sikaily, A.M.; Selim, A.I.; Ismail, G.A. Chemodiversity and bioactivity assessment of phlorotannins from some Phaeophyta species from the Red Sea. J. Appl. Phycol. 2023, 35, 1769–1788. [Google Scholar] [CrossRef]
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Maqui (Aristotelia chilensis (Mol.) Stuntz) and murta (Ugni molinae Turcz): Native Chilean sources of polyphenol compounds. Mini-Reviews Org. Chem. 2019, 16, 261–276. [Google Scholar] [CrossRef]
- N. Muñoz-Molina, J. N. Muñoz-Molina, J. Parada, M. Simirgiotis, R. Montecinos-González, Potential of Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound Assisted Extraction against Aging Related Diseases, (2023). [CrossRef]
- Rivera-Tovar, P.R.; Torres, M.D.; Camilo, C.; Mariotti-Celis, M.S.; Domínguez, H.; Pérez-Correa, J.R. Multi-response optimal hot pressurized liquid recovery of extractable polyphenols from leaves of maqui (Aristotelia chilensis [Mol.] Stuntz). Food Chem. 2021, 357, 129729. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Yeo, M.; Jung, W.-K.; Kim, G. Fabrication, characterisation and biological activity of phlorotannin-conjugated PCL/β-TCP composite scaffolds for bone tissue regeneration. J. Mater. Chem. 2012, 22, 3568–3577. [Google Scholar] [CrossRef]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of Mannitol Extracts From Brown Macro Algae by Thermophilic Clostridia. Front. Microbiol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, S. Suitability of Sugar Alcohols as Antidiabetic Supplements: A Review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Milman, B.L. General principles of identification by mass spectrometry. TrAC Trends Anal. Chem. 2015, 69, 24–33. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.S.; Wakayama, M.; Ichihara, K.; Sakurai, K.; Ashino, Y.; Kadowaki, R.; Soga, T.; Tomita, M. Metabolome profiling of various seaweed species discriminates between brown, red, and green algae. Planta 2019, 249, 1921–1947. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Vergara-Salinas, J.; Pérez-Correa, J.; Lienqueo, M. Purification of phlorotannins from Macrocystis pyrifera using macroporous resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef] [PubMed]
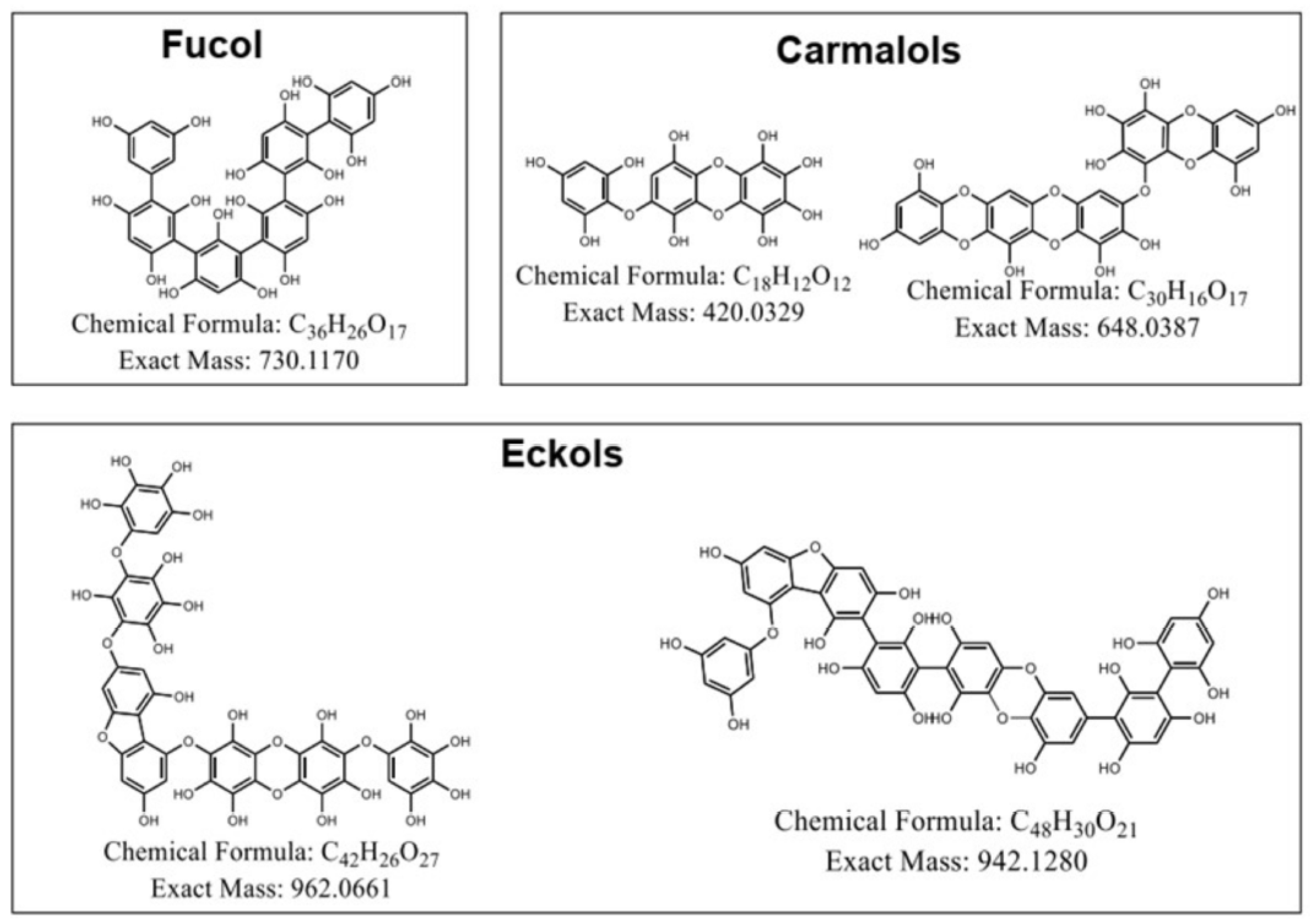
|
Polymerization degree |
Molecular formula |
Theoretical [M-H]- (m/z) | Observed [M-H]- (m/z) | Error (ppm) | MS1 (m/z) | MS2 (m/z) |
| Trimer | C18H11O12 (Carmalol) | 419,0251 | 419,0241 | 2,3 | 389 (-30), 243(-PGU+H2O+2H+), 116 (PGU + H+) | N.D. |
| Pentamer | C30H15O17 (Carmalol) |
647,0309 | 647,0368 | -9,1 | 535(111), 389 (PGU + H2O + 2H+), 243 (PGU + H2O + 2H+), 116 (PGU + H+) |
116, 100(-O) |
| Hexamer | C36H25O17 (Fucol) | 729,1092 | 729,1088 | 0,5 | 681 (47), 535 (-PGU+H2O+2H+), 389 (-PGU+H2O+2H+), 243 (-PGU+H2O+2H+), 116 (PGU + H+) |
A) 116, 100 (-O) |
| Heptamer | C42H25O27 (Eckol) |
961,0583 | 961,0578 | 0,6 | 815(145), 681 (134), 535 (-PGU+H2O+2H+), 389 (-PGU+H2O+2H+), 243 (-PGU+H2O+2H+), 116 (PGU + H+) |
N.D. |
| Octamer | C48H31O21 (Eckol) |
943,1358 | 943,1305 | 5,6 | 791 (151), 501 (-2PGU+H2O+2H+), 389 (112), 243 (-PGU+H2O+2H+), 116 (PGU + H+) | A) 791, 225 (566), 167 (60), 81 (84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





