1. Introduction
Seaweed represents a rich and underexploited source of bioactive compounds with potential applications in nutraceuticals, pharmaceuticals, and functional foods. Among marine macroalgae, brown seaweeds (Phaeophyceae) are particularly notable due to their high content of polyphenols—mainly phlorotannins—alongside complex polysaccharides and minerals [
1].
Despite this, most bioactivity-focused studies have concentrated on a limited number of genera, and significant gaps persist in early-stage evaluations of potential anti-inflammatory properties, as well as in the technological development of functional ingredients, particularly from endemic species such as Durvillaea incurvata, which remains underexplored despite its promising bioactive profile.
Durvillaea incurvata, commonly known as “cochayuyo” in Chile, is a brown seaweed endemic to the southeastern Pacific and traditionally consumed along the Chilean coast. Its documented antioxidant, anti-inflammatory, lipid-lowering, and anti-obesity properties highlight its potential as a source of bioactive compounds for functional applications [
2].
However, translating these biological effects into viable, functional food ingredients requires the development of scalable extraction and stabilization strategies [
3]. Among these, ultrasound-assisted extraction (UAE) has emerged as a green and efficient technique that enhances the recovery of thermolabile compounds by promoting cell disruption and solvent penetration, thereby reducing both solvent consumption and processing time [
4]. Previous work by our group demonstrated that UAE applied to
Durvillaea incurvata produces extracts with superior antioxidant capacity and enzyme-inhibitory activity compared to conventional extraction methods [
5].
To complement these extraction advances, reliable bioactivity screening methods are also essential. Given the high cost and complexity of in vivo assays, in vitro enzymatic tests—such as hyaluronidase inhibition—offer a practical and informative approach for early-stage evaluation of anti-inflammatory potential. This method facilitates the identification of promising candidates prior to engaging in more resource-intensive mechanistic or clinical assessments. While additional assays will be necessary to confirm efficacy under physiological conditions, hyaluronidase inhibition provides a valuable first-line indicator of anti-inflammatory activity at the laboratory scale.
Building on this initial screening, further formulation steps are required to ensure the stability and functionality of the extract in real food systems. Although polyphenolic extracts exhibit promising biological properties, their susceptibility to heat, light, oxygen, and gastrointestinal conditions poses significant challenges for incorporation into food matrices [
6]. To address this limitation, microencapsulation—particularly through spray drying—has been extensively employed in the food industry to improve compound stability, protect bioactivity, and enable controlled release [
7]. Maltodextrin is frequently selected as a wall material due to its high water solubility, low viscosity, and compatibility with food-grade applications [
8]. Moreover, encapsulation enhances dispersibility and can mitigate sensory drawbacks commonly associated with phenolic-rich ingredients.
To optimize the encapsulation process and ensure efficient use of materials and energy, Response Surface Methodology (RSM) offers a powerful statistical tool. RSM allows for the simultaneous evaluation of multiple variables and their interactions, enabling the identification of optimal conditions with a reduced number of experimental trials. It is widely applied in food engineering to model complex processes, such as encapsulation efficiency, antioxidant retention, and bioactive protection [
9]. In this study, we aimed to (1) obtain a polyphenol-rich extract of
Durvillaea incurvata using UAE and evaluate its anti-hyaluronidase activity and cytotoxicity in gastrointestinal cell models and (2) encapsulate the extract using spray drying with maltodextrin as the wall material, optimizing the process via RSM. This study provides foundational evidence supporting the stabilization and
in vitro evaluation of bioactive compounds from seaweed, offering a relevant basis for their potential incorporation into food systems. The insights generated here are expected to guide future work focused on
in vivo validation and food matrix integration, facilitating the development of functional ingredients for health-promoting applications.
2. Materials and Methods
2.1. Materials
The seaweed Durvillaea incurvata was collected from the “Palo Muerto” sector (Latitude: −39.8833 Longitude: −73.5167), Corral commune, Chile. Immediately, they were cleaned with seawater and stored in a box to protect them from light during transport to the lab (all done within 24 hours). In the laboratory, the seaweed was washed with distilled water, cut into cubes of approximately 1 cm3, freeze-dried, and ground to a particle size of approximately 0.5 mm. Samples were then stored (-80 °C) until extraction.
All chemicals and reagents were of analytical grade. Unless stated otherwise, most of them were acquired from Sigma Chemical Co. (Saint Louis, MO, USA).
2.2. Ultrasound-Assisted Extract (UAE) Obtention
The extraction was carried out according to the optimal conditions obtained by Muñoz-Molina et al. [
5]. Briefly, extraction using ethanol 70% (v/v) was carried out for 80 min at 50 °C by using a thermoregulated bath without agitation. An ultrasonic processor (Sonics VCX series, 500 W, 20 kHz, Sonics & Materials Inc.) was used to generate ultrasonic waves every 8 seconds, stimulating the extraction. Once the extraction was finished, the extract was filtered with a 0.45 μm cellulose syringe filter and stored at -80 °C until use.
2.3. Cytotoxicity Assessment of UAE
To corroborate its safety as a food ingredient, the cytotoxicity of UAE was assessed based on the data described by Pacheco et al. [
10], using HT-29 and Caco-2 cells. Propidium iodide (PI) viability assays were performed in 96-well microplates. For each well, 5000 cells (HT-29 or Caco-2) were seeded per well in a volume of 100 µL of RPMI-1640 medium without phenol red, supplemented with 10% FBS and 1% Pen/Stp. The cells were allowed to acclimatize until the next day to allow their adhesion to the plate. Subsequently, the cells were treated with different concentrations of the extract (100, 500, 750, and 1000 µg/mL). Two µL of Dimethylsulfoxide (DMSO) was used as a negative control, and 100 µL of Dimethylformamide (DMF) was used as a positive one. The effects of treatments on cell viability were evaluated at 24 and 48 hours. Once the treatment times were completed, 100 µL of HBSS-Ca2+/PI buffer was added to the wells corresponding to the treatments and negative control, and 2 µL of 500 µM propidium iodide was added to the wells corresponding to the positive control, thus achieving the same final concentration of propidium iodide in all wells. The plate was incubated at 37 °C for 5 minutes, and propidium iodide incorporation into the cells was measured by determining the fluorescence with an emission wavelength of 530 nm and an excitation wavelength of 620 nm in a Varioskan® Flash multiple reader (Thermo Fisher Scientific Inc.). Finally, the percentage of cell viability was calculated with respect to the negative control (average of 3 different experiments).
2.4. Hyaluronidase Inhibitory Activity of UAE
The assay was performed according to the protocol described by Ling et al. [
11] with slight modifications. First, assay medium (1.00—1.67 U hyaluronidase in 95 μL 20mM sodium phosphate buffer pH 7.0 with 77mM sodium chloride and 0.01% BSA) was preincubated with 5μL of the extract (in DMSO) for 10 min at room temperature (5μL DMSO without extract were used as control). After that, 100 μl hyaluronic acid (0.03% w/v in 300 mM sodium phosphate, pH 5.35) was added, and the mixture was incubated for 45 min at 37 °C. Then, 1 ml acid albumin solution (0.1% bovine serum albumin in 24 mM sodium acetate and 79 mM acetic acid, pH 3.75) was added, and the mixture was incubated for 10 min at room temperature. Finally, the absorbance was measured at 600 nm and the inhibitory activity of UAE was calculated using Equation (1):
Where Ax is the absorbance of the extracted sample, and Ac is the absorbance for the control (DMSO does not have an extract).
The extract was tested at a maximum concentration of 150 μg/mL in the final reaction mixture, and the IC50 value was calculated. The experiment was performed in triplicates and results expressed as mean ±S.D.
2.5. Microencapsulation of UAE
The encapsulation of UAE was performed by spray drying using a B-290 mini spray-dryer (Büchi, Switzerland) and maltodextrin (MD) as the encapsulating agent.
Briefly, 20 g of UAE was mixed at room temperature with MD at different concentrations and water (to make 100 g). The mixture was then homogenized under magnetic stirring for 25–30 min before spray drying. Response surface methodology (RSM) was used to optimize the process, using a Central Composite Design (with four central points) [
9]. The independent variables (see
Table 1) were the inlet temperature (x
1, 150 to 190 °C) and the coating agent concentration in the feeding (x
2, 5 to 20 %). Extract concentration was set at 20 g/100 g for all samples. The dependent variable to be maximized was the encapsulation efficiency (EE). Recovery (R) was also measured, and the constant parameters were air flux, 600 L/h; feeding rate, 1 mL/min; and atomization pressure, 0.5 MPa. The resulting powders were weighed and kept at 4 °C in closed bags protected from light until analysis. The optimal spray drying conditions were obtained using a second-order model (Equation 2) and the Statgraphics Centurion XVI software package.
Where
are the independent variables, whereas
are the regression coefficients (obtained by using the method of least squares),
the encapsulated efficiency (EE), and
the residual error.
2.6. Microparticle Analysis
All the following analyses were performed on the powder obtained under optimal microencapsulation conditions defined above.
2.6.1. Encapsulation Efficiency and Recovery
2.6.1.1. Total Polyphenol
To determination of total polyphenols, the structure of the microcapsule coating material was completely broken by mixing 100 mg of microcapsules and 2 mL of methanol: acetic acid: water (50:8:42 v/v/v), dissolution vortexed (1 min), ultrasonicated for 30 min, and finally centrifuged at 9,000 rpm for 5 min. Phenolic compounds of supernatant were measured according to the Folin-Ciocalteu colorimetric method [
12], and expressed in µg of gallic acid equivalent (GAE) (calibration curve: 1.25 – 20 µg/mL; R
2 = 0.9994).
2.6.1.2. Surface Polyphenol
Four hundred milligrams of microparticles were dispersed in methanol (2 mL) with soft stirring. The dispersion was centrifuged at 2,000 rpm for 5 min. The supernatant is considered the surface fraction of polyphenols in the microparticles. Phenolic compounds were measured according to the Folin-Ciocalteu method, as described previously.
Encapsulation efficiency (EE) and recovery (R) were calculated according to the following equations:
where
corresponds to total polyphenols content in the microparticles powder (µg GAE/g),
corresponds to the polyphenols content in the surface of microparticles powder (µg GAE/g), and
corresponds to the theoretical content of polyphenols in the powder (µg GAE/g) agree with the feed solution content.
2.6.2. Moisture Content
Moisture content in microparticle powder was determined by gravimetry [
13]. One g of microparticles was weighed, placed on a previously weighed Petri dish, and dried in an oven (BE 500, Memmert ®, Schwabach, Germany) at 105 °C for 5 h (until constant weight).
2.6.3. Hygroscopicity
The powder hygroscopicity was determined by gravimetry as follows: about 0.5 g of microparticles were weighed and distributed in watch glasses and placed in a desiccator with a saturated solution of Na
2SO
4 (81% RH) for one week (at room temperature). Hygroscopicity was expressed as g H
2O/ 100 g of dry solids [
14].
2.6.4. Microparticle Size and Shape
Particle size was measured by using a laser scattering particle size distribution analyzer (Partica LA-960, Horiba Scientific, Japan; 650 nm laser diode), where the microparticles were dispersed in ethanol to be analyzed.
Also, microparticle morphology was analyzed by an environmental scanning electron microscope (model EVO MA10, Carl Zeiss, UK), as described previously [
15,
16], with slight modifications. To study the size and shape of microparticles, the powder was placed on a sample holder with the help of an adhesive double-sided carbon tape, then coated in a vacuum coater with a thin layer of gold (EM ACE200, Leica Microsystems, Germany), and observed under high vacuum conditions, with a secondary electron detector (SE1) and an acceleration voltage of 20 kV. An induced fracture on the microparticles was applied to determine the thickness of the shell of the microparticles. The powder was first placed on a copper tape fixed to a specimen slide. Then, the tape was stuck and unstuck by using a second piece of copper tape, achieving to fracture. Finally, samples were coated with gold and observed as described previously. Fractured particles were localized, and shell thickness was measured. All images were saved in TIFF format (1024 x 768 pixels) and analyzed using ImageJ software (National Institute of Health, Bethesda, Maryland, USA,
https://imagej.net).
2.6.5. Differential Scanning Calorimetry (DSC)
Glass transition temperature (
Tg), melting temperature (
Tm) and enthalpy of melting (Δ
Hm), were analyzed by using a differential scanning calorimeter (TA Q20, TA Instruments, New Castle, DE, USA) [
17]. Samples (5–10 mg) were placed in sealed aluminum pans (TA T-Zero capsules) and then loaded onto the equipment. An empty sealed aluminum pan was used as a reference in each analysis and nitrogen was used as a carrier gas (50 mL/min). Thermal analysis was performed from −40 °C to 250 °C at a rate of 10 °C/s. The thermal properties of the samples were analyzed by the resulting heat flow thermograms, using TA Instruments Universal Analysis 2000 software (New Castel, DE, USA). The temperature and heat flow were calibrated using indium and distilled water.
2.6.6. X-Ray Diffraction
X-ray diffractometry was used to corroborate the absence of crystalline structures in the microparticles. An equipment SAXSPoint 2.0 (Anton Paar, Austria) was used with a copper X-ray source (Primus 100, CuKα= 1.54178 Å, 50 kV and 100 μA) and a two-dimensional Eiger R 1 M detector (Dectris, Baden-Daettwil, Switzerland). The microparticle sample was put between a Mylar and Kapton sheet. The diffractograms were obtained at 25 °C, 900 s measurement time, and 115 mm distance between the sample holder and detector.
2.7. Statistical Analysis
For response surface methodology (RSM), STATGRAPHICS Centurion XV software, version 15.2.06 (Old Tavern Rd, The Plains, VA, USA) was used (95% level of confidence). For any means of comparison, data (triplicate) were analyzed by analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison test (p < 0.05) using the same software (STATGRAPHICS).
3. Results and Discussion
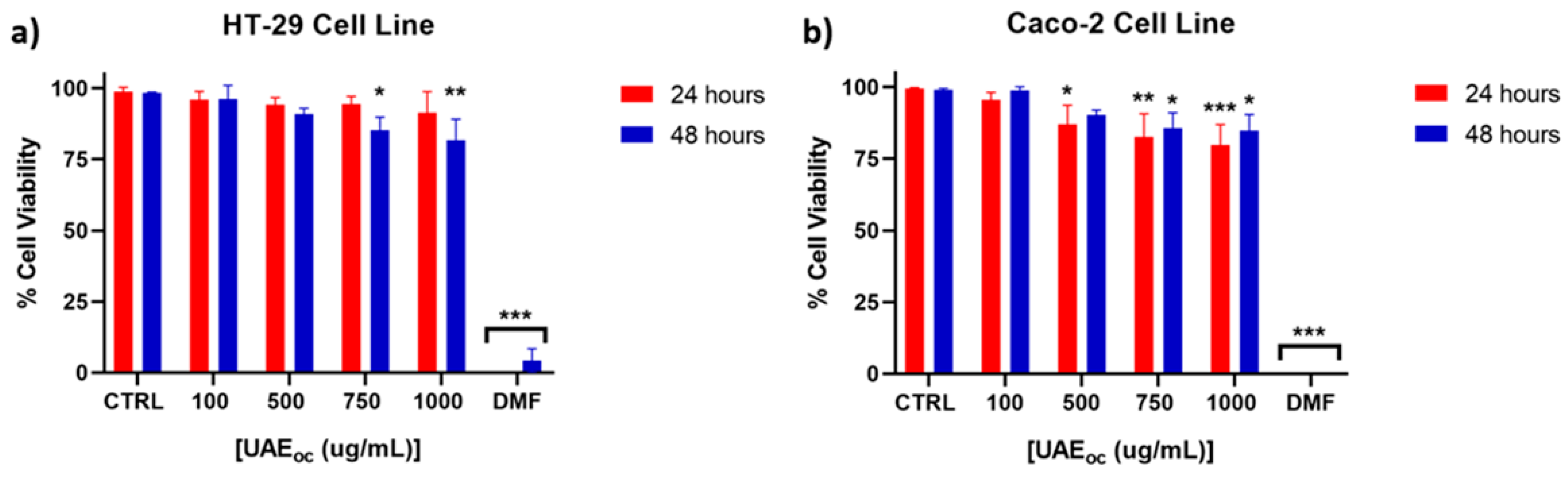
3.1. Cytotoxicity of the UAE
The cytotoxicity of the ultrasound-assisted extract (UAE) was assessed using HT-29 and Caco-2 human epithelial cell lines, both of which are widely employed in in vitro models for intestinal bioavailability and safety evaluation. HT-29 cells represent a model with absorptive and mucus-secreting features, while Caco-2 cells differentiate into enterocyte-like monolayers and are commonly used to simulate intestinal epithelium [
18]. As shown in
Figure 1, cell viability was maintained above the generally accepted cytotoxicity threshold of 75% across most tested concentrations.
In HT-29 cells (
Figure 1a), viability remained unaffected at lower concentrations and declined significantly only at 750 µg/mL after 48 h. A similar trend was observed for Caco-2 cells (
Figure 1b), where significant reductions began at 500 µg/mL. These concentration-dependent effects suggest a relatively low cytotoxic potential, consistent with safety requirements for food-grade bioactives. These findings are in line with Lordan et al. [
19], who observed that brown seaweed extracts reduced cell viability only at concentrations ≥1000 µg/mL.
Pacheco et al. [
10] evaluated the cytotoxicity of ethanolic and acetone extracts (1–1000 µg/mL) from various seaweed species, including
Durvillaea incurvata, using the HT-29 cell line, and reported no significant reduction in cell viability. The divergence from our findings—where reduced viability was observed at higher concentrations after 48 h—may be attributed to differences in extraction methodology. Specifically, Pacheco et al. employed high-pressure liquid extraction (HPLE), a technique that likely yields extracts with lower mannitol content and a distinct polyphenol composition compared to those obtained via ultrasound-assisted extraction (UAE) [
3,
20]. As noted by Quitério et al. [
21], extraction parameters can significantly alter the qualitative and quantitative profile of phenolic compounds. Supporting this, there have been shown that UAE produces a phenolic fingerprint distinct from that of HPLE, which may explain the higher cytotoxicity observed at elevated concentrations in our study [
3].
Regarding the Caco-2 cell line, our results are consistent with those of Lordan et al. [
19], who reported that aqueous and ethanolic extracts from five brown algae species reduced cell viability only at concentrations of 1000 µg/mL, with no effects observed at lower levels (0.1–100 µg/mL). These findings suggest that the cytotoxic effects of seaweed extracts may be concentration-dependent and linked to the abundance and structure of phenolic compounds. Moreover, since both HT-29 and Caco-2 cells originate from colorectal carcinomas, the observed reduction in viability at higher concentrations could reflect potential antiproliferative effects relevant to cancer prevention or therapy rather than cytotoxicity to normal cells.
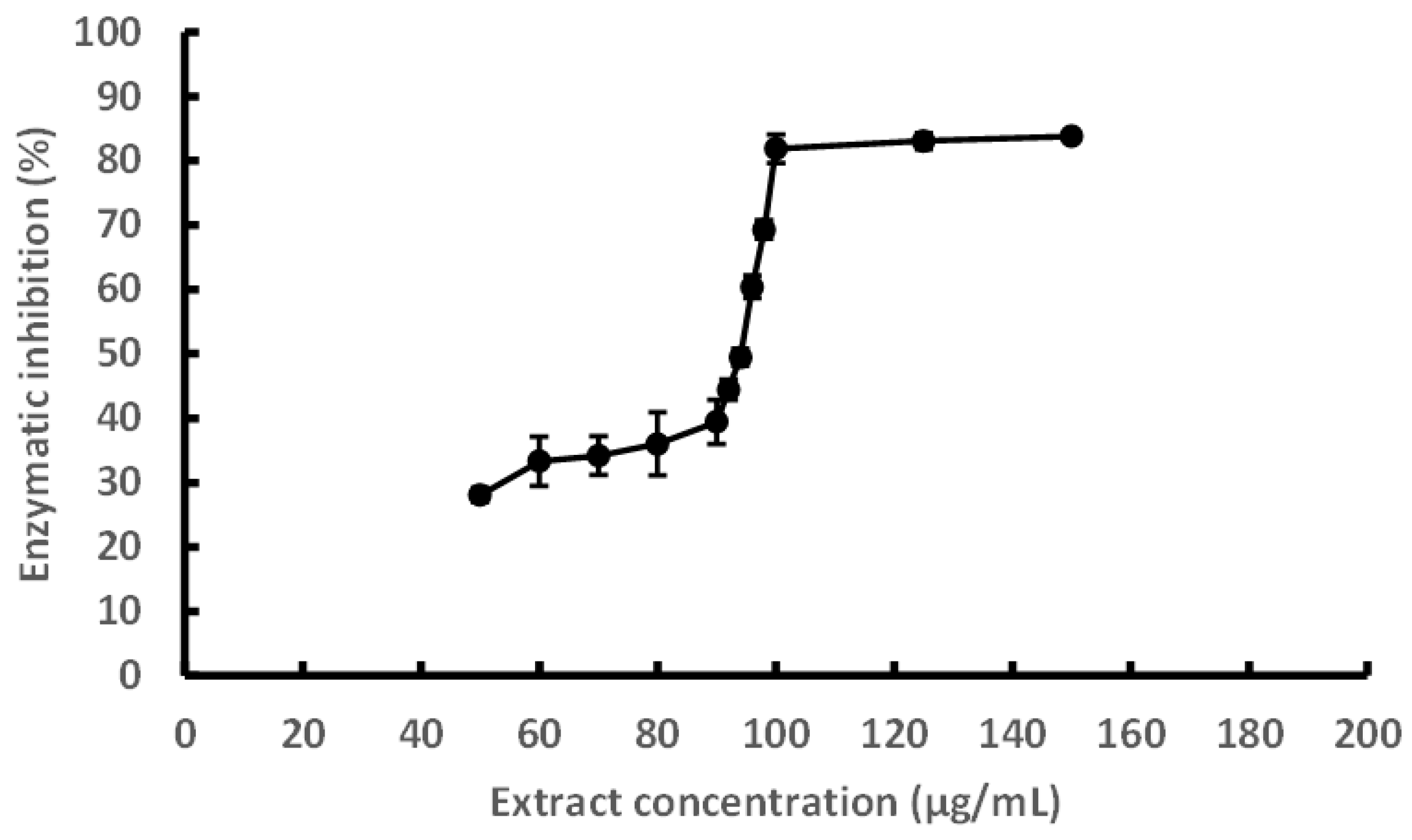
3.2. Inhibition of Hyaluronidase by UAE
The inhibitory effect of the UAE on hyaluronidase activity was evaluated for the first time and is presented in
Figure 2. The extract exhibited a dose-dependent inhibition, with an IC₅₀ value of 93.6 μg/mL and maximal inhibition exceeding 80% at concentrations above 100 μg/mL. This activity is likely attributable to polyphenolic compounds commonly found in brown algae, such as phlorotannins (e.g., phloroglucinol, eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol), which have demonstrated affinity for hyaluronidase and are proposed to act via competitive inhibition mechanisms. Previous studies have also suggested that phlorotannins with higher molecular weights tend to exert stronger inhibitory effects on this enzyme [
22,
23].
Hyaluronidase catalyzes the depolymerization of hyaluronic acid, a key glycosaminoglycan of the extracellular matrix, and plays a role in inflammation, cancer progression, and allergic reactions [
24]. The inhibition of this enzyme has been associated with anti-inflammatory and antiallergic effects and is frequently used as an in vitro proxy for preliminary anti-inflammatory screening. In this context, hyaluronidase inhibition allows for rapid, cost-effective evaluation of anti-inflammatory potential at the laboratory scale, enabling the identification of promising extracts before advancing toward more complex mechanistic or
in vivo studies.
Comparable inhibitory effects have been observed in other brown seaweed species. For instance, there have been reported that ethanolic extracts of
Scytosiphon sp. exhibited anti-hyaluronidase activity with an IC₅₀ of 0.67 mg/mL, which was superior to the conventional anti-allergic drug disodium cromoglycate (IC₅₀ = 1.13 mg/mL) and similar to epigallocatechin gallate (EGCG, IC₅₀ = 0.56 mg/mL), a well-known polyphenolic compound [
25].
Although the present study did not include compound-level identification, previous reports indicate that
D. incurvata extracts contain multiple bioactives beyond phlorotannins, including alginates, fucoidans, and carotenoids such as fucoxanthin, which have also been linked to anti-inflammatory mechanisms [
26,
27]. Further research is needed to elucidate the specific compounds responsible for the observed effects and their interactions with hyaluronidase.
In light of the broader biological activities described for
D. incurvata, including anti-diabetic, neuroprotective, and antihypertensive potential [
5], this species remains a promising candidate for developing functional ingredients. However, a more comprehensive evaluation involving mechanistic cellular assays (e.g., cytokine modulation, NF-κB pathway inhibition), bioaccessibility under gastrointestinal conditions, and compound characterization will be required to validate its application in food and health contexts fully.
3.3. Optimization of UAE Microencapsulation Conditions
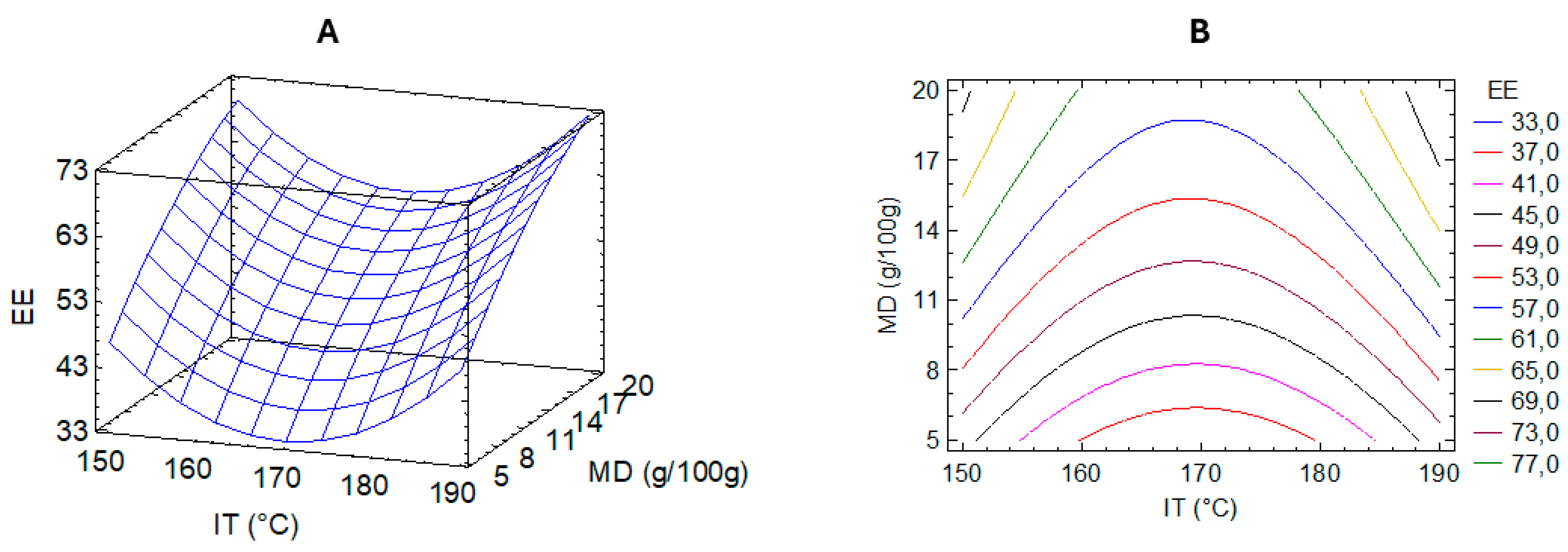
To improve the stability and potential applicability of the UAE as a bioactive food ingredient, microencapsulation via spray drying was optimized using Response Surface Methodology (RSM). This statistical approach enables modeling nonlinear interactions between process variables and allows for efficient experimental design in complex systems. In this case, RSM was employed to evaluate the effects of two critical parameters—inlet temperature (IT) and maltodextrin concentration (MD)—on encapsulation efficiency (EE), defined as the percentage of total polyphenols successfully retained within the microparticles.
Table 2 summarizes the EE results for each experimental run in the central composite design. A second-order polynomial regression model was fitted to the data, yielding the following equation:
This model showed a high coefficient of determination (R² = 0.9555; adjusted R² = 0.9183), indicating an adequate fit and predictive capability. ANOVA confirmed the model's significance (p < 0.01), validating its use in identifying optimal encapsulation conditions.
The response surface and contour plots (
Figure 3) indicated that both low and high inlet temperatures produced relatively high EE values, suggesting a non-linear relationship between thermal input and polyphenol retention. This outcome may reflect the complex balance between drying kinetics, solvent evaporation rates, and thermal degradation. High temperatures may enhance droplet drying and encapsulation shell formation, minimizing polyphenol losses due to surface exposure. However, excessive heat may degrade sensitive compounds, consistent with the observed variability at the upper-temperature range.
The optimal predicted encapsulation conditions were IT = 198.28 °C and MD = 23.11 g/100 g, with a projected EE of 88.97%. Validation experiments under these conditions yielded an actual EE of 72.7% ± 1.2%, which, although lower than predicted, is consistent with prior findings for polyphenol encapsulation using maltodextrin [
8]. These results confirm the robustness of the model while highlighting inherent process variability, likely due to polyphenol volatility and heterogeneity of extract composition.
In addition to EE, extract recovery (R)—defined as the ratio of total phenolic content in the powder to the amount initially fed into the dryer—was 45.9% ± 2.4% under optimal conditions. This value aligns with previous reports where brown algae extracts were encapsulated using maltodextrin, obtaining R values between 32% and 50% [
28]. Lower recovery rates are typically associated with forming fine particles prone to sticking on chamber walls or escaping collection systems.
This study’s RSM model prioritized encapsulation efficiency (EE) as the primary response variable, offering a valuable framework for optimizing spray-drying conditions. While effective for evaluating the influence of inlet temperature and maltodextrin concentration, future work would benefit from a multi-response optimization approach. Including additional criteria such as antioxidant capacity, retention of hyaluronidase inhibition, and process cost-efficiency would enable a more comprehensive assessment of encapsulation performance and enhance its industrial relevance.
3.4. Microparticles Analysis
The microparticles produced under optimal spray-drying conditions exhibited physicochemical properties consistent with stable formulations suitable for storage and handling. The resulting powder showed a moisture content of 3.4 ± 0.1%, water activity of 0.193 ± 0.004, and hygroscopicity of 30.3 ± 0.4 g/100 g. Particle size analysis revealed an average diameter of 19.17 ± 2.79 μm, falling within the typical range for food-grade microencapsulated products. These values are comparable to those reported for maltodextrin-based seaweed microparticles and suggest good physical stability under appropriate storage conditions [
28]. Low water activity (<0.3) is particularly relevant for preventing microbial growth and maintaining the functional integrity of encapsulated bioactives. The hygroscopicity value indicates moderate moisture affinity, which supports the need for proper packaging to avoid clumping or degradation during storage. Particle size and morphology, in turn, influence powder dispersibility, solubility, and reconstitution behavior—key parameters for future incorporation into food systems [
29].
3.4.1. Microparticle Morphology by Scanning Electron Microscope (SEM)
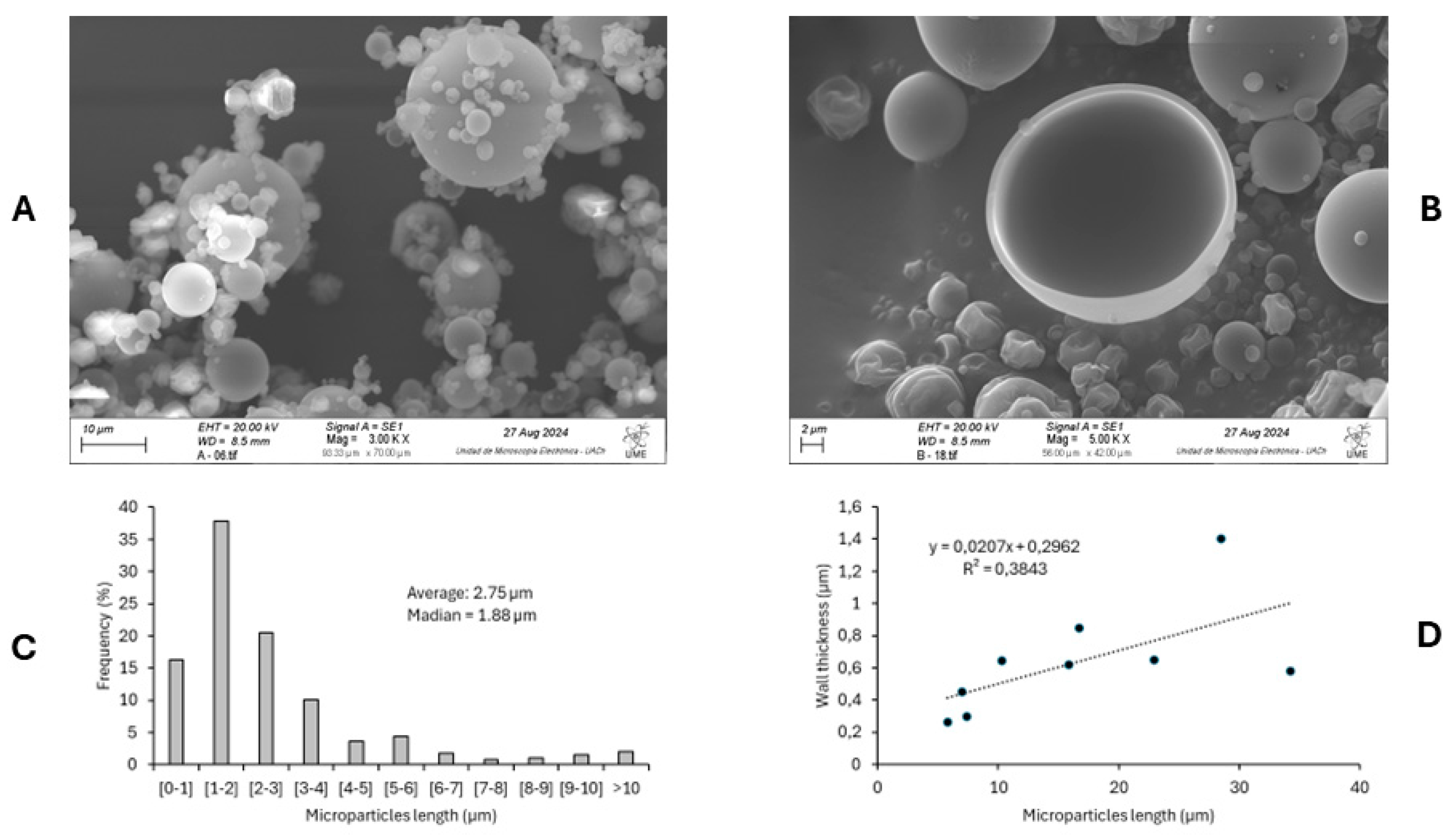
The microparticles' morphology under optimal encapsulation conditions was analyzed using scanning electron microscopy (SEM). Representative micrographs (
Figure 4A) revealed that the spray-dried particles exhibited predominantly spherical shapes with smooth surfaces and minimal visible fissures, which indicate effective encapsulation. The absence of structural collapse and the presence of intact spherical morphology suggest that the maltodextrin concentration and inlet temperature selected were appropriate for microcapsule formation [
30].
Agglomeration was observed to be limited and primarily occurred around larger particles, likely due to interactions during particle recirculation in the drying chamber. Although often considered undesirable, controlled agglomeration may improve solubility and dispersion by enhancing wettability and preventing the segregation of powder components [
31].
The particle size distribution, presented in
Figure 4C, shows that approximately 80% of particles measured less than 4 µm in diameter, with a mean diameter of 2.75 µm and a median of 1.88 µm. Despite most falling within the sub-10 µm range, some particles reached sizes greater than 30 µm. This size distribution aligns with previous studies using maltodextrin for seaweed extract encapsulation, and it has significant implications for powder solubility and bulk properties [
28].
A transparent encapsulation shell was observed in fractured particles (
Figure 4B), with wall thickness ranging from ~0.2 µm to ~1.4 µm. Wall thickness tended to increase with particle size. Still, it plateaued around 1 µm for particles larger than 15 µm (
Figure 4D). These results confirm the successful formation of microcapsules and are consistent with prior reports using maltodextrin as wall material [
16].
Together, these findings reinforce the adequacy of maltodextrin for stabilizing ultrasound-assisted seaweed extracts.
3.4.2. Differential Scanning Calorimetry (DSC) of the Microparticles
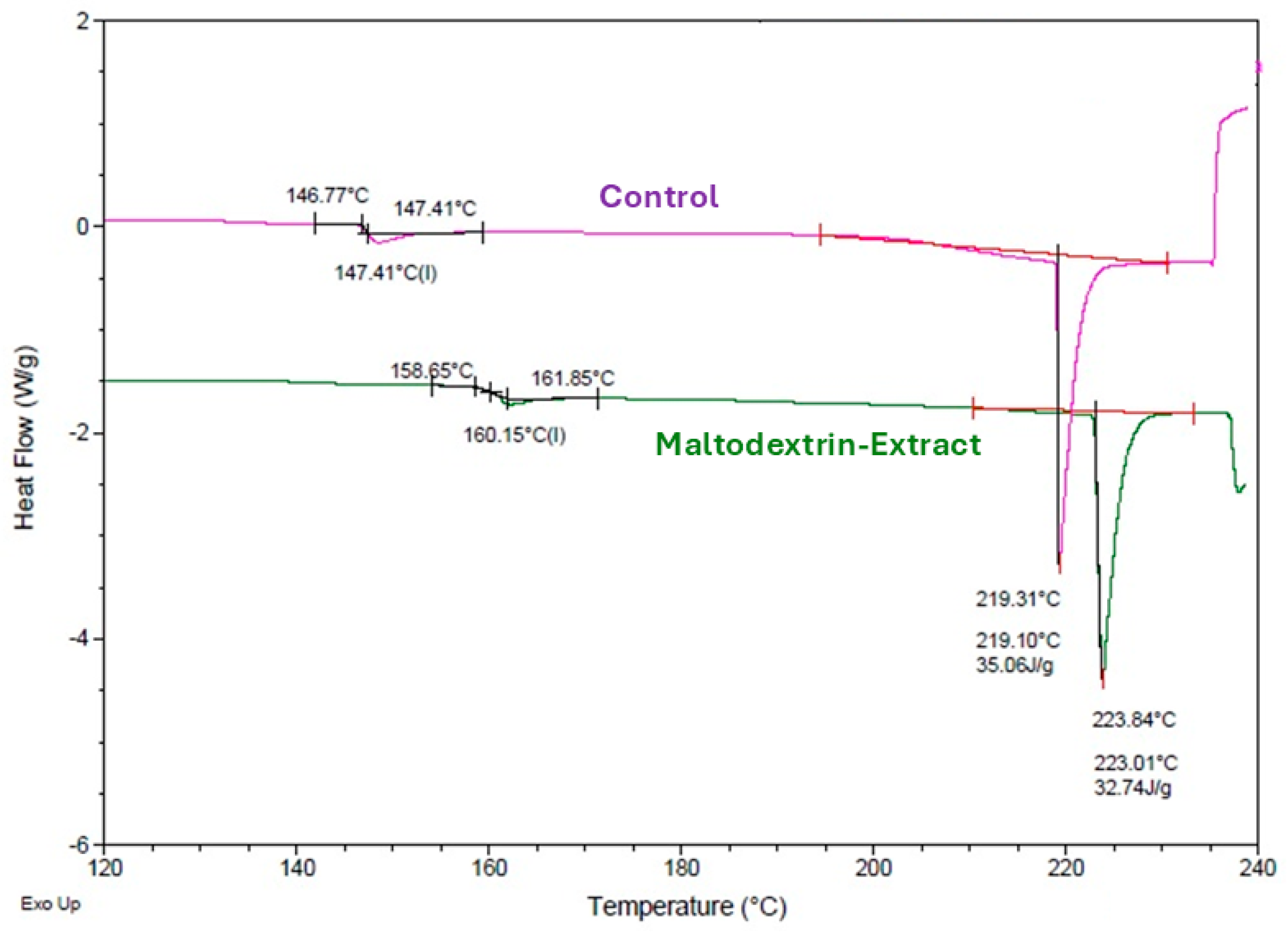
DSC thermograms (
Figure 5) of microcapsules with and without extract showed no thermal transitions below 100 °C, indicating physical stability under typical storage conditions. A glass transition temperature (Tg) of 160.15 °C for the encapsulated sample was recorded, higher than that of the empty maltodextrin particles. This increase suggests molecular interactions between extract constituents and maltodextrin, such as hydrogen bonding or increased cross-link density, contributing to structural rigidity [
32].
An endothermic peak at approximately 220 °C, attributed to the melting and gelatinization of maltodextrin, was also observed. The higher temperature of this transition in the encapsulated sample reinforces the notion of improved thermal stability conferred by the interaction between polyphenolic compounds and the wall material [
33].
3.4.3. X-Ray Diffraction of the Microparticles
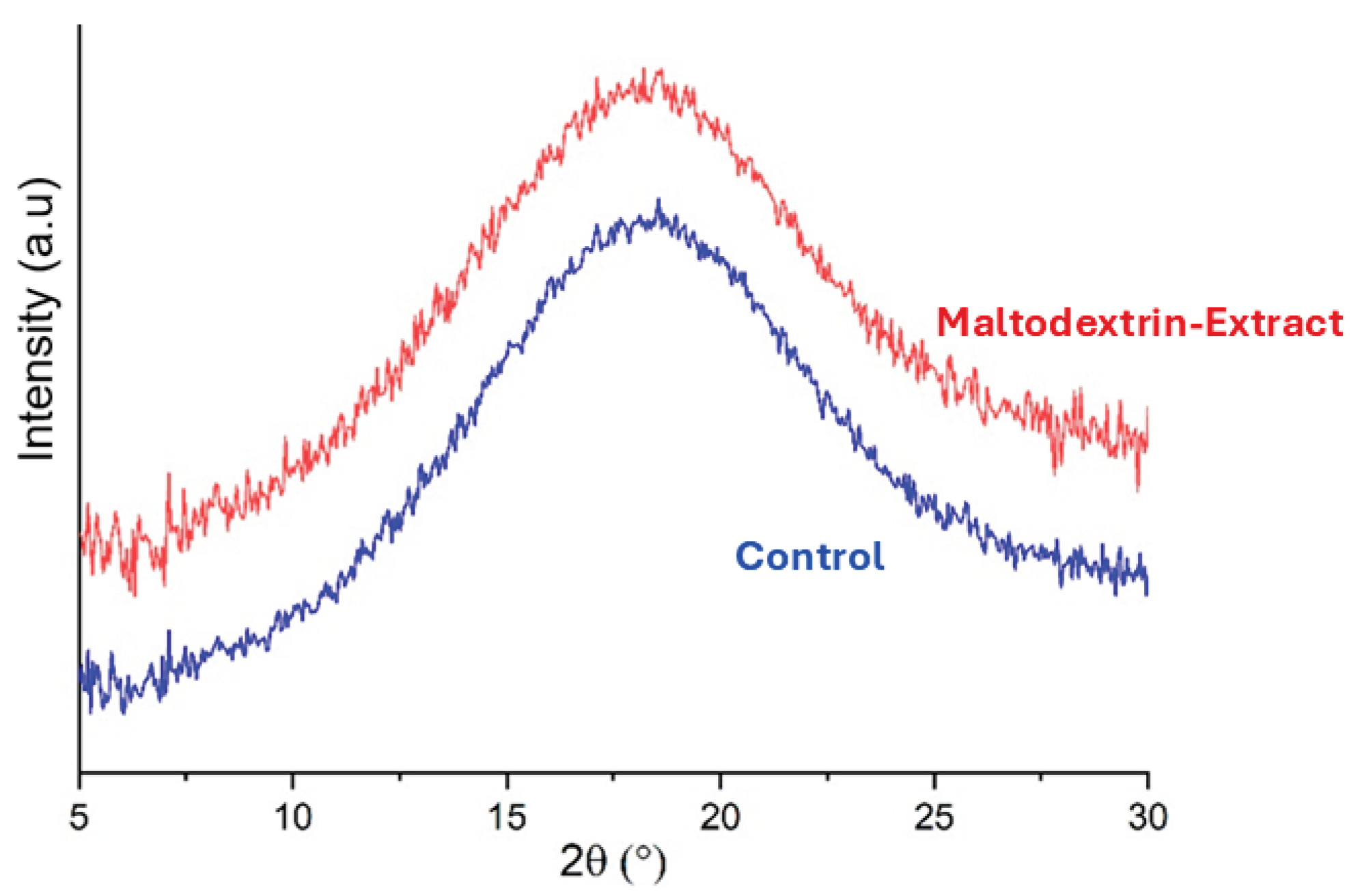
X-ray diffraction analysis (
Figure 6) confirmed the amorphous nature of the spray-dried microparticles. Both extract-loaded and empty microparticles showed broad halos without distinct crystalline peaks, suggesting molecular-level dispersion of the bioactive compounds within the maltodextrin matrix. This amorphous state is desirable, as it enhances solubility and facilitates controlled release in aqueous systems [
34,
35]. The absence of crystallinity also implies that the encapsulation process did not induce phase separation or aggregation of the polyphenols. This further supports the suitability of maltodextrin for stabilizing bioactive seaweed extracts.
4. Conclusions
UAE of Durvillaea incurvata yielded a polyphenol-rich extract that exhibited no cytotoxic effects in HT-29 and Caco-2 cell lines at concentrations up to 750 μg/mL, supporting its preliminary safety for food-related applications. In addition, the extract showed significant in vitro anti-inflammatory potential through hyaluronidase inhibition, reinforcing its promise as a bioactive candidate for further investigation. To enhance its stability and applicability, the extract was successfully microencapsulated using spray drying with maltodextrin, producing microparticles with favorable morphological, thermal, and structural properties. The combination of spherical shape, low water activity, amorphous matrix, and increased glass transition temperature indicates improved compound protection and suitability for incorporation into food systems. Collectively, these findings support the feasibility of using UAE and encapsulation as sustainable strategies to develop stabilized seaweed-derived ingredients, paving the way for future studies on bioavailability, gastrointestinal stability, and application in functional food matrices.
Author Contributions
Conceptualization, J.P and N.M-M.; methodology, J.P., A.Z., and P.R.; validation, N.M-M. and C.C.; formal analysis, J.P. and N.M-M.; investigation, N.M-M., C.C., and P.R.; resources, J.P., A.Z., and P.R.; writing—original draft preparation, N.M-M. and J.P.; writing—review and editing, M.S.M-C.; visualization, J.P.; supervision, J.P.; project administration, J.P. and M.S.M-C.; funding acquisition, J.P. and M.S.M-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID-Chile through the FONDECYT Regular, grants numbers 1241497 and 1220097, and the FOVI grant 240263.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
This research was funded by ANID-Chile through the FONDECYT Regular grants 1241497, and 1220097, and the FOVI grant 240263. We thank Jorge Rivas for his help with the cochayuyo sample collection. We thank the SEM technical support of the Electron Microscopy Unit, a core facility - Zeiss Reference Center of Universidad Austral de Chile.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23(3), 543–597. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Yans, C.; Boscán-González, A.; Duran, P.; Parra-Soto, S.; Angarita, L. Durvillaea antarctica: a seaweed for enhancing immune and cardiometabolic health and gut microbiota composition modulation. Int. J. Mol. Sci. 2023, 24(13), 10779. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Contreras-Contreras, G.; Rivas-Reyes, P.I.; Pérez-Jiménez, J.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S. Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants 2025, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29(6), 3161–3173. [Google Scholar] [CrossRef]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods 2024, 13(2), 0–10. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Figueiredo, J. de A.; Oliveira, N.L.; Botrel, D.A.; Mattoso, L.H.C. Devel-opment of alginate/pectin microcapsules by a dual process combining emulsification and ultrasonic gelation for encapsulation and controlled release of anthocyanins from grapes (Vitis labrusca L.). Food Chem. 2022, 391. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45(7), 1386–1394. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Sampaio, F.C.; de Faria, J.T.; Pitangui, C.G.; Lovaglio, F.; Casazza, A.A.; Converti, A.; Perego, P. Optimization of spray drying microencapsulation of olive pomace polyphenols using response surface methodology and artificial neural network. Lwt 2018, 93, 220–228. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18(7), 1–12. [Google Scholar] [CrossRef]
- Ling, S.K.; Tanaka, T.; Kouno, I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by β-glucosidase in the presence of amino acids. Biol. Pharm. Bull. 2003, 26(3), 352–356. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16(3), 144–158. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. 18th edn. Association of Official Analytical Chemists 2005, Arlington, VA, USA.
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65(7), 1248–1252. [Google Scholar] [CrossRef]
- Bernal-Millán, M.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried microencapsulation of oregano (Lippia graveolens) polyphenols with maltodextrin enhances their stability during in vitro digestion. J. Chem. 2022, 8740141. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Palacios-González, E.; Lartundo-Rojas, L.; Garibay-Febles, V.; Yáñez-Fernández, J.; Hernandez-Sanchez, H.; Gutiérrez-López, G.; Alamilla-Beltran, L. Microstructural properties and distribution of components in microparticles obtained by spray-drying. J. Food Eng. 2015, 152, 105–112. [Google Scholar] [CrossRef]
- Shi, Q.; Lin, W.; Zhao, Y.; Zhang, P. Thermal characteristics and state diagram of Penaeus vannamei meat with and without maltodextrin addition. Thermochim. Acta 2015, 616, 92–99. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; ... Wichers, H. The impact of food bioactives on health: in vitro and ex vivo models 2015. [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Paul Ross, R. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141(3), 2170–2176. [Google Scholar] [CrossRef]
- Erpel, F.; Mariotti-Celis, M.S.; Parada, J.; Pedreschi, F.; Pérez-Correa, J.R. Pressurized hot liquid extraction with 15% v/v glycerol-water as an effective environment-friendly process to obtain durvillaea incurvata and lessonia spicata phlorotannin extracts with antioxidant and antihyperglycemic potential. Antioxidants 2021, 10(7). [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20(11), 677. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37(6), 703–709. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS n: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10(12), 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: a biological and therapeutic perspective. Curr. Med. Chem. 2009, 16(18), 2261–2288. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The anti-allergic activity of polyphenol extracted from five marine algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Arunkumar, K.; Nalluri, M.; Anjana, K.; Mohan, G.; Raja, R. Fucoxanthin as antioxidant, anti-hyaluronidase and cytotoxic agent: potential of brown seaweeds decoction for tea supplement. J. Food Meas. Charact. 2023, 17(4), 3980–3989. [Google Scholar] [CrossRef]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Sivagnanam, S.P.; Park, J.S.; Cho, Y.J.; Chun, B.S. Effect of wall materials on the spray drying encapsulation of brown seaweed bioactive compounds obtained by subcritical water extraction. Algal Res. 2021, 58, 102381. [Google Scholar] [CrossRef]
- Neves, M.A.; Hashemi, J.; Prentice, C. Development of novel bioactives delivery systems by micro/nanotechnology. Curr. Opin. Food Sci. 2015, 1, 7–12. [Google Scholar] [CrossRef]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.; Pinheiro, A.; Januário, M.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocolloids 2013, 33, 199–206. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Deroanne, C.; Paquot, M.; Fougnies, C.; Lambrechts, J.C.; Blecker, C.S. Characterization of the physical state of spray-dried inulin. Food Biophys. 2007, 2, 83–92. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray drying of nopal mucilage (Opuntia ficus-indica): Effects on powder properties and characterization. Carbohydr. Polym. 2010, 81(4), 864–870. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Palma-Rodríguez, H.M.; Alvarez-Ramírez, J.; Vargas-Torres, A. Using Modified Starch/Maltodextrin Microparticles for Enhancing the Shelf Life of Ascorbic Acid by the Spray-Drying Method. Starch – Stärke 2018, 70, 1700323. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Ng, W.K.; Tan, R.B. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).