1. Introduction
Human immunodeficiency virus (HIV) treatment has become increasingly effective, potent, safe, and well-tolerated, particularly with regimens that include integrase strand transfer inhibitors (INSTIs). As a result, HIV infection is now regarded as a communicable chronic disease. The 2024 guidelines from the US Department of Health and Human Services and the European AIDS Clinical Society recommend second-generation integrase strand transfer inhibitors as initial antiretroviral regimens. Examples include bictegravir/emtricitabine/tenofovir alafenamide fumarate (BIC/FTC/TAF) and dolutegravir/lamivudine (DTG/3TC), which are recommended for most treatment-naïve individuals with HIV and as a switch option for those who are virologically suppressed [
1,
2].
Several studies have demonstrated that significant weight gain occurs in some people living with HIV (PWH) after initiating antiretroviral therapy (ART). Greater weight gain was observed in DTG-containing regimens, particularly among Black females in the ADVANCE study conducted in Africa [
3]. In a pooled analysis of eight randomized trials, ART regimens involving INSTIs and TAF appeared to contribute to increased weight gain. Baseline factors such as low CD4 count, high HIV RNA levels, female sex, and Black race were associated with a higher likelihood of weight gain [
4]. Similarly, in the North American AIDS Cohort Collaboration on Research and Design, PWH who initiated INSTI-based regimens—particularly those containing DTG or raltegravir—experienced greater weight gain compared to those on non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapies. Furthermore, among PWH who switched from tenofovir disoproxil fumarate (TDF) to TAF, there was an association with adverse metabolic changes, including weight increases, obesity development, and worsened serum lipid levels, as observed in the Swiss HIV Cohort Study (SHCS) [
5].
Most studies on weight change have been clinical trials conducted primarily in Africa, Europe, and North America, with limited representation from real-world studies in Asia. The DRAGON study aimed to compare weight gain among Asian ART-naive patients initiated on DTG/3TC or BIC/FTC/TAF. Additionally, we report the risk factors associated with significant weight gain (≥10% increase) after a 48-week period.
2. Materials and Methods
This retrospective, observational cohort study was conducted at nine designated hospitals for HIV care in Taiwan and China-mainland. The study period spanned from March 2019 to January 2024 and included treatment-naïve people living with HIV (PWH). Patients were excluded if they were lost to follow-up, transferred to other clinics or hospitals, or deceased. All included patients were followed for a 48-week period.
The primary endpoint was the proportion of participants who experienced a ≥10% increase in weight. Secondary endpoints included achieving undetectable plasma HIV RNA levels (<50 copies/mL) at week 48 after initiating DTG/3TC or BIC/FTC/TAF, evaluated using a snapshot of the per-protocol-exposed population. Additional secondary outcomes included changes in total cholesterol and triglyceride levels over the course of the study. The study received approval from the research ethics committee or institutional review board (registration number: TYGH112029). Patient consent was waived due to the retrospective nature of the study, and all data were analyzed without including personal or sensitive information.
2.1. Data collection and Definition
A standardized case record form was used to collect information on demographics, sexual preferences, body weight, HIV treatment history, and the results of various laboratory investigations. The first weight measurement after the initiation of DTG/3TC or BIC/FTC/TAF, both within 30 days pre- and post-index, was defined as the baseline weight. Follow-up weight measurements were taken every six months, both within 30 days pre- and post-, and were defined as post-weight. Weight change was reported as the absolute change, along with the proportion of patients who experienced weight increases. Laboratory tests included plasma HIV RNA, CD4 lymphocyte count, serum creatinine, liver function tests, lipid profiles, and fasting blood glucose or glycated hemoglobin (HbA1c). These tests were conducted every 3–6 months in accordance with CDC HIV treatment guidelines.
2.2. Statistical Analysis
The distributions of patients' demographics and baseline characteristics were summarized using descriptive statistics. Continuous variables were expressed as medians [IQR], and differences between groups were assessed using the Mann–Whitney test due to their non-normal distribution. Categorical variables were presented as numbers and percentages, and comparisons between groups were performed using the chi-square test or Fisher’s exact test, as appropriate. To adjust for baseline imbalances, a propensity model was generated using inverse probability of treatment weighting (IPTW) [
6]. The inverse probability of being in different groups assigned each individual a weight based on the predicted probability for each group, thereby balancing differences in baseline characteristics between the two groups.
2.3. Analysis and Regression Models
We assessed non-inferiority of BIC/FTC/TAF compared with DTG/3TC using the confidence interval approach for the difference in proportion of patients with virological suppression (HIV RNA of less than 50 copies/mL) at week 48 in the per-protocol-exposed population. A margin of –10% was used to determine non-inferiority.
The estimated propensity score was obtained from the fit of a logistic regression model. This model included the following variables: age, gender, route of transmission, CD4 count, HIV RNA loads, and body mass index (BMI). IPTW was calculated using a logistic regression model of the response on weight gain ≥10% increase at week 48. The weights were 1/e^(X) for individuals in the DTG/3TC group and 1/(1 – e^(X)) for the BIC/FTC/TAF group. All p values were two-sided, and statistical significance was set at a p value <0.05. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC) and SPSS software version 24.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Patient Characteristics
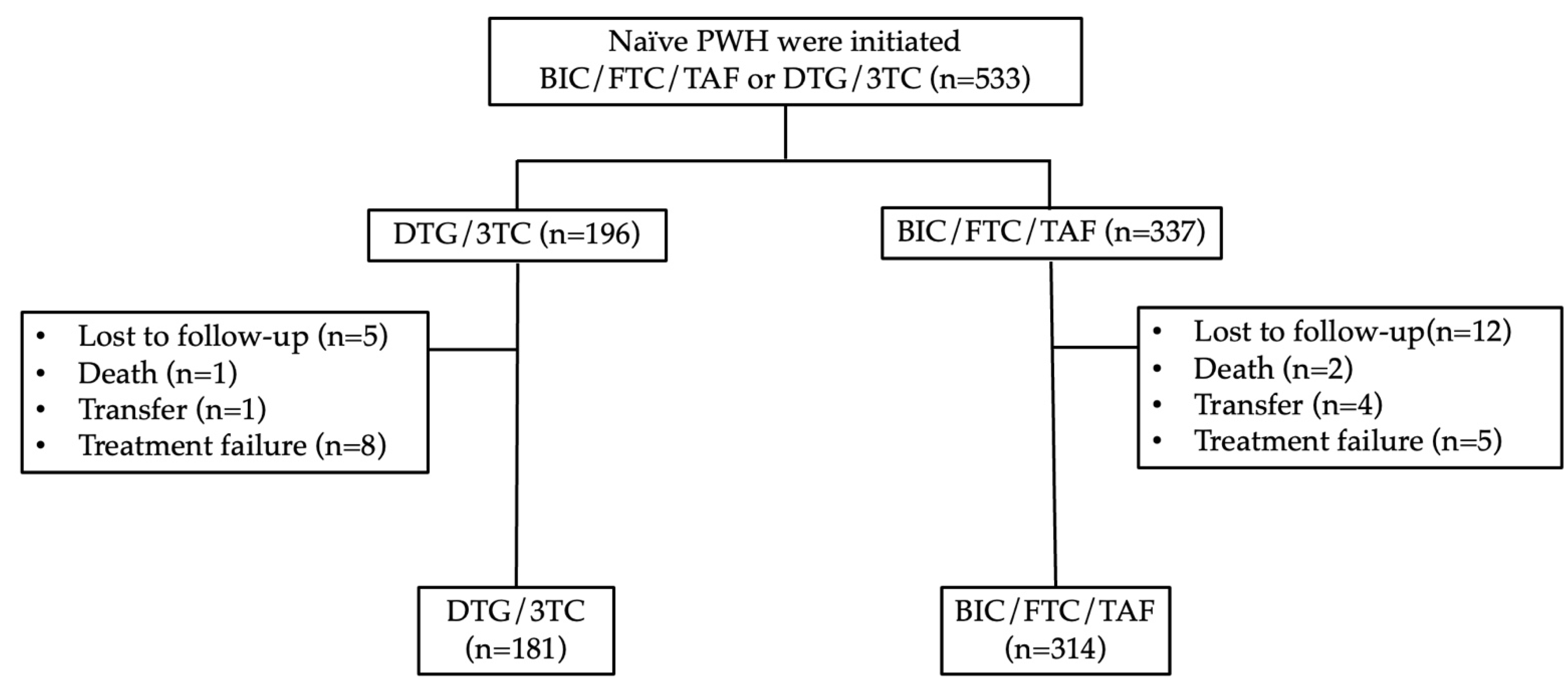
PWH were screened between October 2019 and January 2024. Of the 533 patients screened, 314 met the criteria for inclusion in the BIC/FTC/TAF group, while 181 qualified for the DTG/3TC group. Through week 48, the proportion of patients reporting drug-related adverse events or discontinuation was 8 (4.2%) in the DTG/3TC group and 5 (1.6%) in the BIC/FTC/TAF group.
In the DTG/3TC group, the most common causes of treatment failure were poor compliance (n=3, 1.6%), patient request (n=2, 1.1%), isolation of the M184V mutation (n=1, 0.5%), pruritus (n=1, 0.5%), and increased weight (n=1, 0.5%). In the BIC/FTC/TAF group, the causes were hyperlipidemia (n=2, 0.6%), increased weight (n=1, 0.3%), nausea (n=1, 0.3%), and drug-drug interaction (n=1, 0.3%).
Figure 1.
Flowchart for the inclusion of treatment-experienced patients. Abbreviation: PWH, people living with HIV; BIC/FTC/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG/3TC, dolutegravir/lamivudine.
Figure 1.
Flowchart for the inclusion of treatment-experienced patients. Abbreviation: PWH, people living with HIV; BIC/FTC/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG/3TC, dolutegravir/lamivudine.
The baseline demographics and clinical characteristics of the study population are summarized in
Table 1. The median age was similar between the two groups (34 years, IQR: 28–45 for DTG/3TC and 27–47 for BIC/FTC/TAF,
p=0.72), and the majority were male (92% and 96%, respectively,
p=0.13). The primary route of transmission was men who have sex with men (81% for DTG/3TC and 82% for BIC/FTC/TAF,
p=0.23). The median CD4 count was significantly higher in the DTG/3TC group (302 cells/µL, IQR: 187–440) compared to the BIC/FTC/TAF group (244 cells/µL, IQR: 95–392,
p=0.03), with a greater proportion of patients in the BIC/FTC/TAF group having CD4 counts below 200 cells/µL (42% vs. 27%,
p<0.01). HIV RNA levels were slightly higher in the BIC/FTC/TAF group (4.73 log₁₀ copies/mL, IQR: 4.19–5.44) compared to the DTG/3TC group (4.52 log₁₀ copies/mL, IQR: 3.94–5.22,
p=0.01). Body weight and BMI were similar between the groups (
p=0.39 and
p=0.07, respectively).
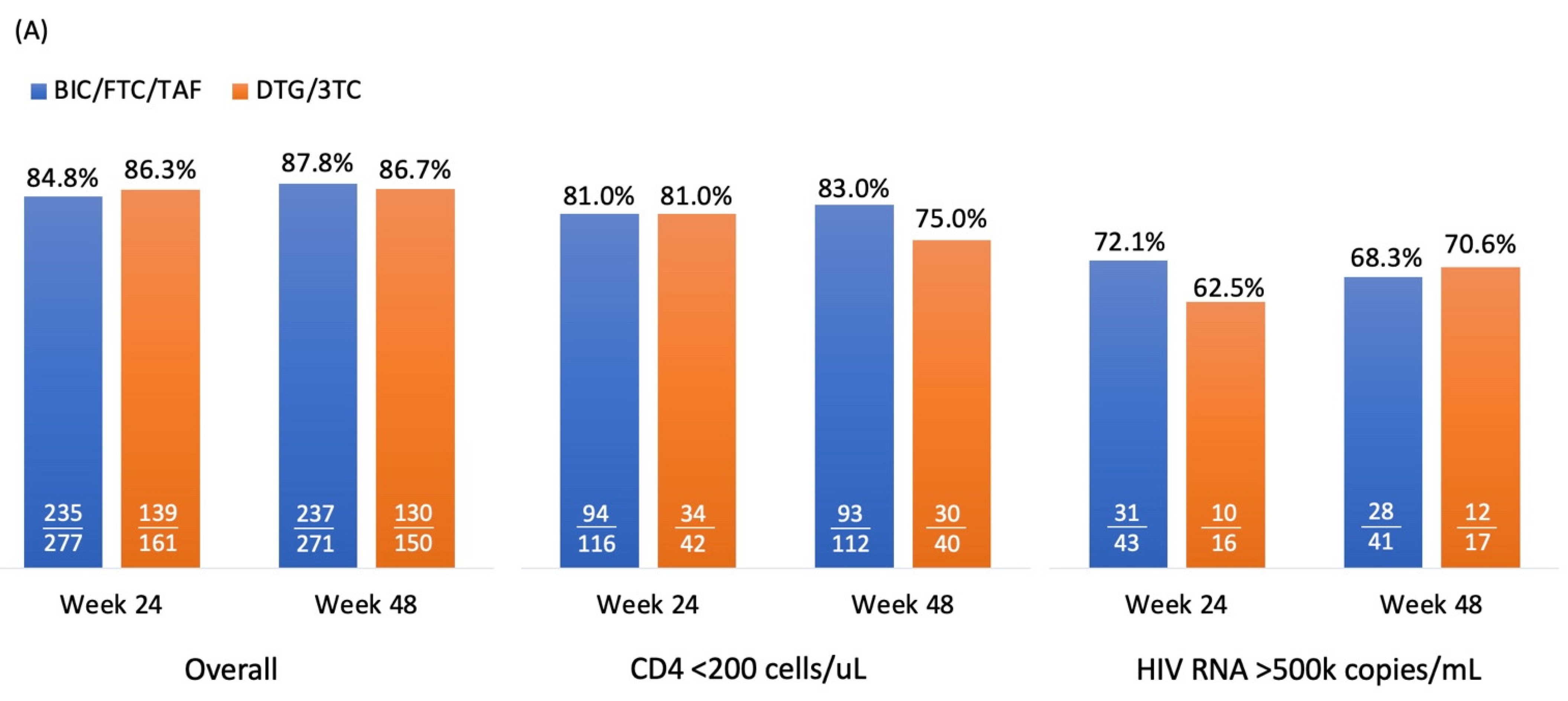
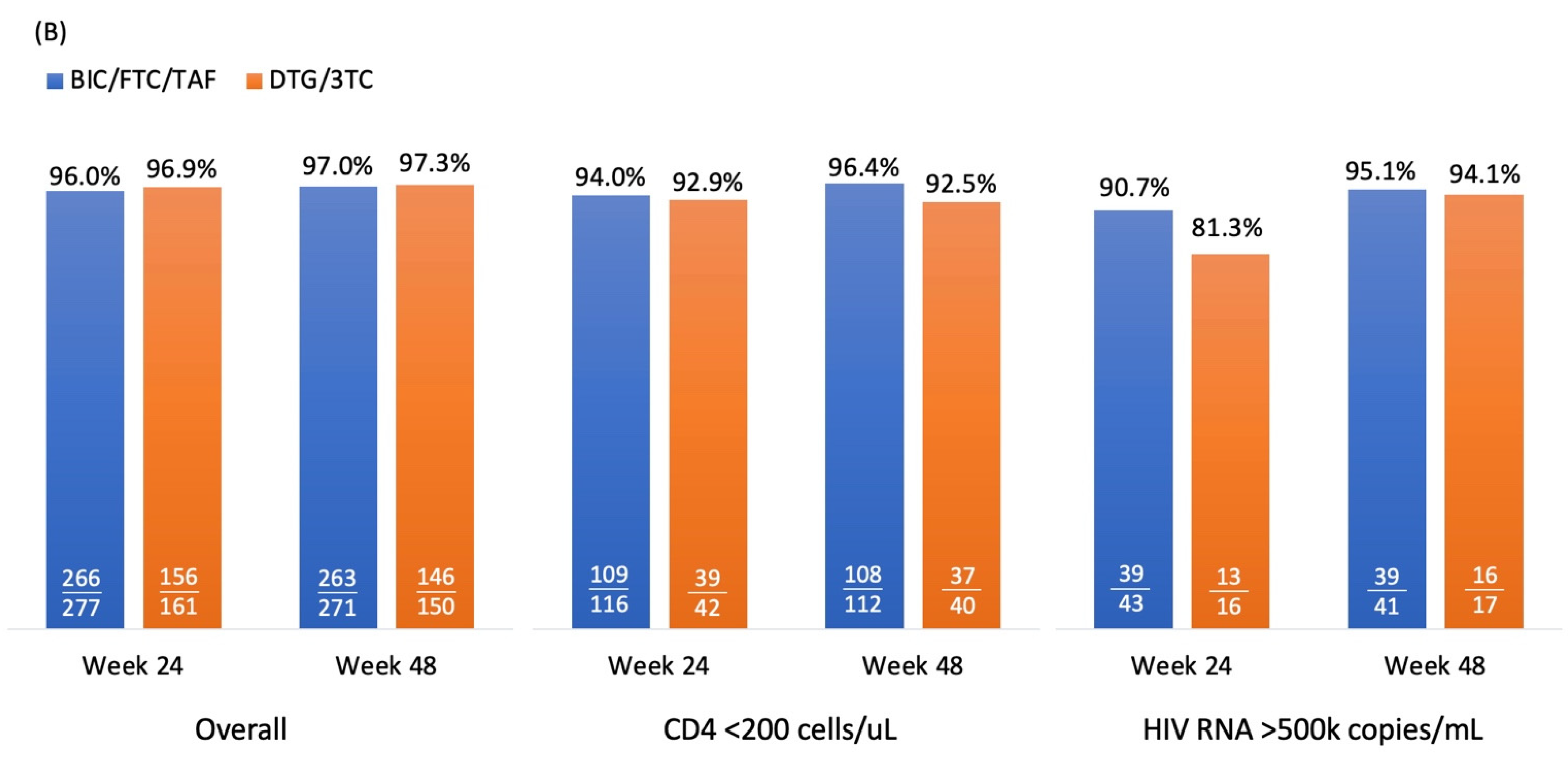
3.2. Virological Effectiveness
Figure 2A and 2B illustrate virologic response rates, measured as the percentage of PWH achieving HIV RNA levels of <50 copies/mL and <200 copies/mL, in the DTG/3TC and BIC/FTC/TAF groups under the
per
protocol-
exposed population. For <50 copies/mL, response rates were high overall, PWH with DTG/3TC and BIC/FTC/TAF achieving 86.3% and 84.8% (difference: 1.5%, 95% CI: -5.4% to 8.4%,
p=0.67), respectively, at week 24, and 86.7% and 87.8% (difference: -1.1%, 95% CI: -7.8% to 5.6%,
p=0.73) at week 48. Among patients with baseline CD4 counts <200 cells/µL, the response rates were similar at week 24 (81.0% for both groups,
p=0.99) but diverged slightly at week 48 (75.0% for DTG/3TC vs. 83.0% for BIC/FTC/TAF,
p=0.27). For patients with HIV RNA levels >500,000 copies/mL at baseline, the response rates were lower but comparable, ranging from 62.5% to 72.1% (
p=0.48) at week 24 and 68.3% to 70.6% (
p=0.86) at week 48.
For <200 copies/mL, response rates were consistently high across both treatment groups. Overall response rates were 96.9% and 96.0% in DTG/3TC and BIC/FTC/TAF group (difference: 0.9%, 95% CI: -2.8% to 4.6%, p=0.64), respectively at week 24, with similar outcomes at week 48 (97.3% and 97.0%, respectively; difference: 0.3%, 95% CI: -3.0% to 3.6%, p=0.87). Among patients with CD4 counts <200 cells/µL, the response rates were slightly higher in the BIC/FTC/TAF group at week 24 (97.0% vs. 92.9%, p=0.8) and week 48 (96.4% vs. 92.5%, p=0.31). In the subgroup with HIV RNA >500,000 copies/mL, response rates were lower than the overall population but still high, reaching 81.3% and 90.7% (p=0.32) at week 24 and 94.1% and 95.1% (p=0.88) at week 48 for DTG/3TC and BIC/FTC/TAF, respectively.
Overall, both treatment regimens demonstrated high and comparable efficacy in achieving virologic suppression, with BIC/FTC/TAF showing slightly better performance in specific subgroups, particularly among patients with lower CD4 counts and higher baseline viral loads. However, the differences between DTG/3TC and BIC/FTC/TAF were not significant, and the criteria for non-inferiority were met.
By week 24, CD4 counts increased to 240 cells/µL for BIC/FTC/TAF and 238 cells/µL for DTG/3TC. At week 48, the counts further increased to 306 cells/µL and 299 cells/µL, respectively. The differences in CD4 changes between the two regimens were not statistically significant.
3.3. Weight Change
Weight changes were compared between the BIC/FTC/TAF and DTG/3TC groups over 48 weeks. At week 24, the mean weight gain was 2.9 kg for BIC/FTC/TAF and 2.2 kg for DTG/3TC (p=0.2). By week 48, weight gain was significantly greater in the BIC/FTC/TAF group compared to the DTG/3TC group (5.1 kg vs. 2.9 kg, p<0.01). Regarding the proportion of participants with a >10% body weight increase, this was consistently higher in the BIC/FTC/TAF group compared to DTG/3TC: 21.7% vs. 12.2% at week 24 (p=0.01), 30.2% vs. 16.5% at week 48 (p<0.01).
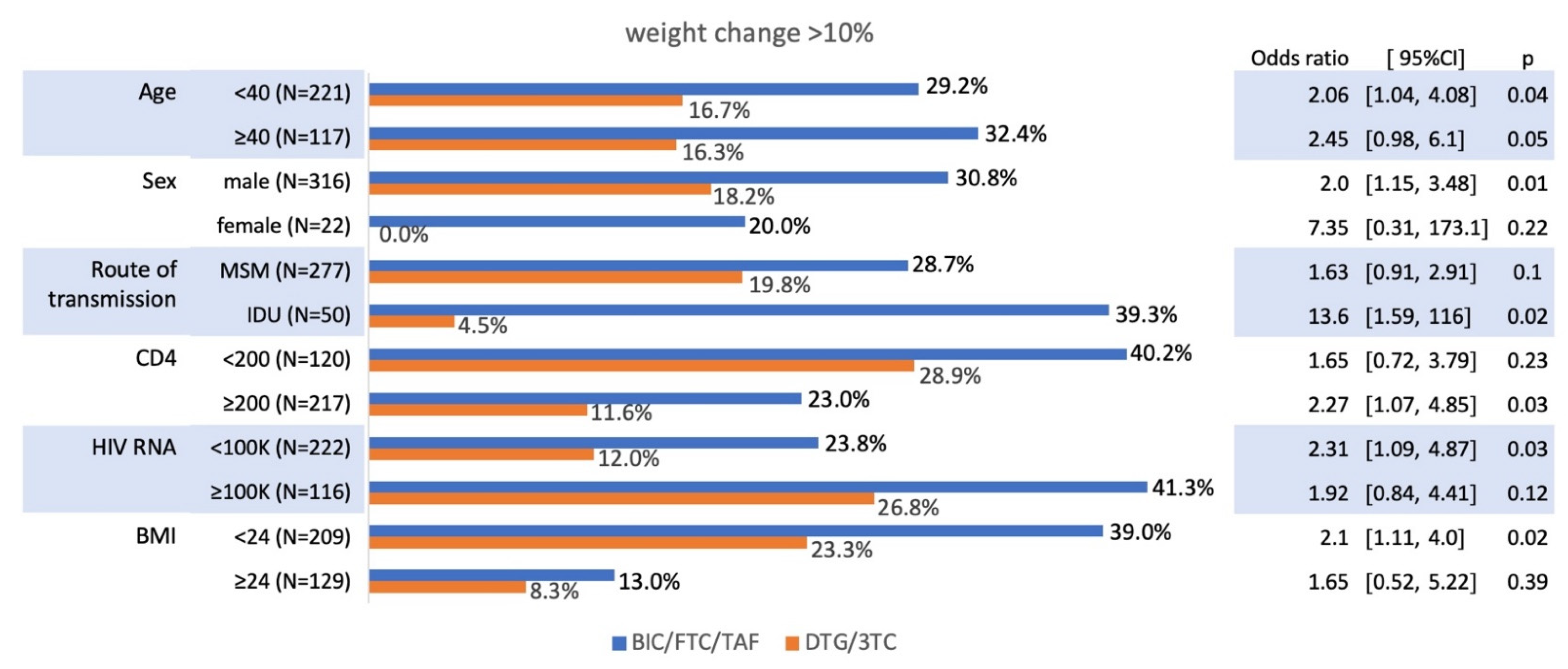
The proportion of PWH experienced a >10% weight increase across various subgroups in the BIC/FTC/TAF and DTG/3TC treatment arms in
Figure 3. Age showed a notable impact, with participants aged ≥40 years in the BIC/FTC/TAF group having a higher proportion of >10% weight gain (32.4%) compared to DTG/3TC (16.3%, odds ratio [OR] 2.45, 95% CI: 0.98–6.1,
p=0.05). Male participants on BIC/FTC/TAF were more likely to experience >10% weight gain (30.8%) than those on DTG/3TC (18.2%, OR 2.0, 95% CI: 1.15–3.48,
p=0.01).
Among participants with CD4 counts ≥200 cells/µL, the BIC/FTC/TAF group had a significantly higher proportion of weight gain (40.2%) compared to DTG/3TC (28.9%, OR 2.27, 95% CI: 1.07–4.85, p=0.03). Similarly, participants with baseline HIV RNA ≥100,000 copies/mL had greater weight gain in the BIC/FTC/TAF group (41.3%) than in the DTG/3TC group (26.8%, OR 2.31, 95% CI: 1.09–4.87, p=0.03). Weight gain differences were also evident based on BMI, with those having BMI <24 showing higher proportions of >10% weight gain in the BIC/FTC/TAF group (39.0%) compared to DTG/3TC (23.3%, OR 2.1, 95% CI: 1.11–4.0, p=0.02).
3.4. The Analysis of Risk Factors of Weight Change
By week 48, a total of 338 patients were eligible for inclusion, the BIC/FTC/TAF group (n=205) and the DTG/3TC group (n=133) were analyzed the risk factors of weight change ≥10% increase. Before IPTW, a multivariate logistic regression model revealed no statistically significant difference between the BIC/FTC/TAF and DTG/3TC group, as shown in
Table 2. However, CD4 counts <200 cells/uL (adjusted Odds Ratio [aOR] 1.83, 95% CI: 1.02–3.28,
p=0.04), HIV RNA ≥100,000 copies/mL (aOR 2.03, 95% CI: 1.16–3.58,
p=0.01) and BMI <24 (aOR 3.57, 95% CI: 1.93–6.45,
p<0.01) were identified as significant predictors of a weight change ≥10% increase at week 48 (
Table 2).
After IPTW, BIC/FTC/TAF (aOR 1.72, 95% CI: 1.16–2.54,
p<0.01) emerged as a significant predictor, as shown in
Table 3. Additionally, CD4 counts <200 cells/uL (aOR 2, 95% CI: 1.32–3.03,
p<0.01), HIV RNA ≥100,000 copies/mL (aOR 2.05, 95% CI: 1.37–3.07,
p<0.01) and BMI <24 (aOR 3.51, 95% CI: 2.29–5.37,
p<0.01) remained significant predictors (
Table 3).
3.5. Metabolic Outcomes
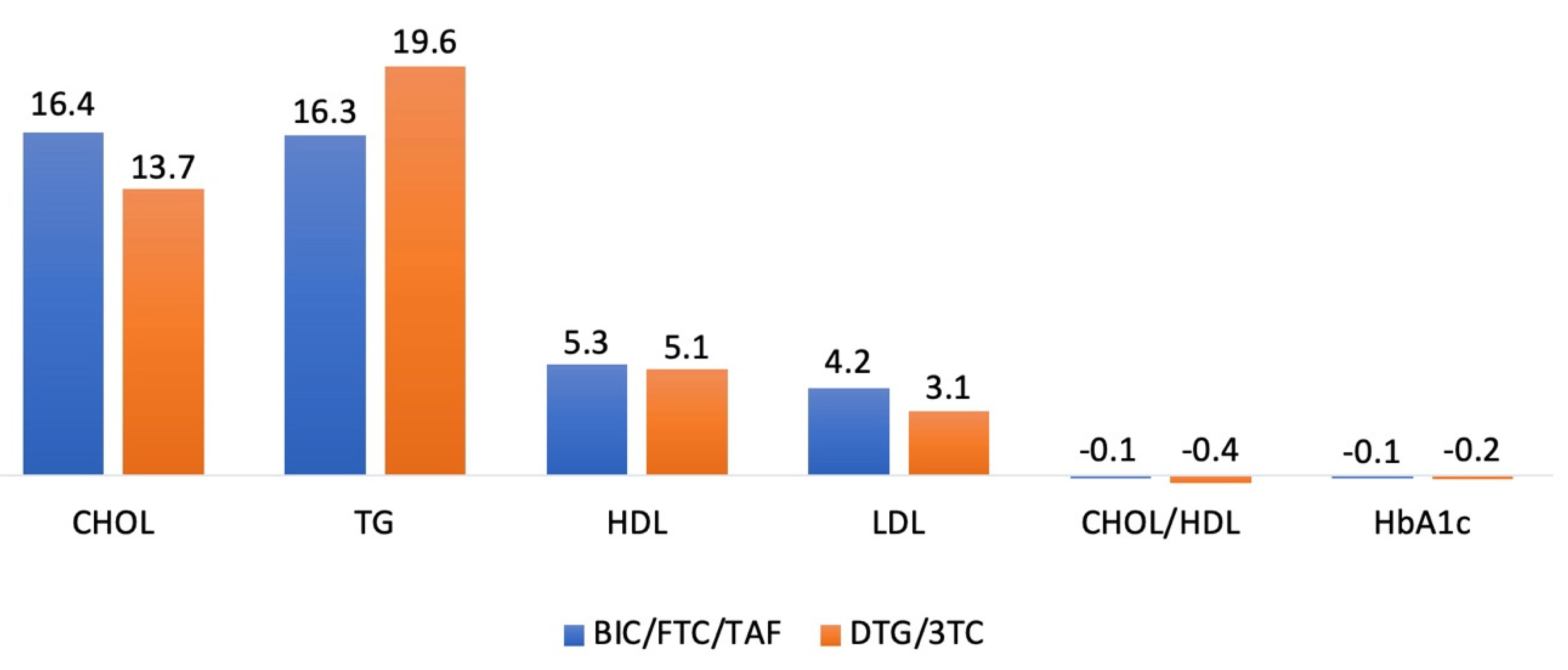
The absolute mean changes in total cholesterol (CHOL), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were 16.4 (± 36.3), 16.3 (± 108.6), 5.3 (± 9.4), and 4.2 (± 28.2) mg/dL, respectively, in the BIC/FTC/TAF group, and 13.7 (± 30.7), 19.6 (± 122.8), 5.1 (± 14.6), and 3.1 (± 24.7) mg/dL, respectively, in the DTG/3TC group. Additionally, the absolute mean changes in the CHOL/HDL ratio and HbA1c were -0.1 (± 1.0) and -0.1% (± 1.0) in the BIC/FTC/TAF group, and -0.4 (± 1.1) and -0.2% (± 0.5) in the DTG/3TC group. Blood lipid parameters did not differ significantly between the groups from baseline to week 48 in
Figure 4.
4. Discussion
This study provides critical insights into weight gain associated with INSTIs, particularly BIC/FTC/TAF, in ART-naïve people living with HIV of Asian origin. The observed significant weight increase, along with the identified risk factors such as lower baseline CD4 counts (<200 cells/µL), higher HIV RNA levels (≥100,000 copies/mL) and lower BMI (<24 kg/m2), highlights the multifactorial nature of ART-related weight gain.
This phenomenon is consistent with prior reports, such as the pooled analysis of randomized clinical trials by Erlandson et al. [
7], which emphasized the role of baseline characteristics and ART components in predicting weight changes following treatment initiation or switch. Moreover, Surial B et al. used data from the Swiss HIV Cohort Study to assess weight changes and metabolic outcomes in PWH receiving stable ART who switched from TDF to TAF [
5]. Their findings were consistent with other studies, demonstrating that TAF leads to a substantially larger increase in weight compared with TDF in PWH initiating or switching ART [
4,
8]. In our study, weight gain was significantly greater in the BIC/FTC/TAF group compared to TAF-free regimen of DTG/3TC (5.1 kg vs. 2.9 kg,
p<0.01).
The ADVANCE study further corroborates our findings, demonstrating that DTG-containing regimens are associated with significant weight gain, particularly among women and those receiving TAF [
3,
9]. Mean weight gain was 8.9 kg in the TAF/
emtricitabine (FTC) + DTG arm, 5.9 kg in the TDF/FTC + DTG arm, and 3.2 kg in the TDF/FTC/efavirenz (EFV) arm at 192 weeks. The metabolic effects observed with TAF, including increased weight and adverse lipid profiles, as documented in the Swiss cohort study, suggest a possible additive or synergistic effect when combined with INSTIs [
5]. However, our study didn’t identify any significant difference between BIC/FTC/TAF and DTG/3TC in terms of blood lipid parameters, possibly due to the short observation period and small sample size.
A strong association was observed between weight gain and the stage of HIV disease, as indicated by low baseline CD4 cell counts and high HIV RNA. These factors were correlated with significant weight gain in our study, consistent with findings from other reports [
10,
11]. This suggests that the return-to-health phenomenon plays a key role in weight gain among PWH initiating ART. While this effect can be beneficial for individuals recovering from advanced HIV disease, it may also contribute to excessive weight gain, particularly in those with early-stage HIV infection or a normal to low baseline BMI (<24 kg/m²).
Notably, our study also addresses a critical gap in the literature by focusing on real-world data from Asia, a region underrepresented in existing research. Prior studies, such as OPERA cohort [
12], have predominantly analyzed populations in North America and Europe, limiting the generalizability of their findings to Asian populations. The differential metabolic responses observed across diverse ethnic groups underscore the importance of region-specific research to guide clinical practice.
Ciccullo A et al. conducted a real-life multicenter cohort study to evaluate the effectiveness of BIC/FTC/TAF and DTG/3TC in treatment-naïve PWH. The findings demonstrate consistently high suppression rates (<50 copies/mL) in both the BIC/FTC/TAF (95.2%) and DTG/3TC (95.9%) groups at week 144, aligning with the outcomes of our study [
13,
14]. Furthermore, our study showed comparable effectiveness in PWH with high baseline viral loads (>500,000 copies/mL) between the two groups, which was also consistent with findings from other studies [
15,
16].
Despite the strengths of this study, including its robust sample size and multicenter design, several limitations warrant consideration. First, the retrospective nature of the study may introduce selection bias and limit causal inferences. Second, the lack of detailed dietary and lifestyle data precludes an analysis of their contributions to weight gain. Third, the study's 48-week follow-up may not capture long-term metabolic changes, as seen in studies with extended follow-up periods, such as the ADVANCE study [
3,
9]. Finally, although IPTW was carried out to balance the baseline and multivariate logistic regression models were used to adjust for confounders, there may still be unidentified or unmeasured confounders that could influence the observed outcomes of weight change.
5. Conclusions
In conclusion, this study adds to the growing body of evidence suggesting that INSTI-based regimens, particularly BIC/FTC/TAF, are associated with significant weight gain in Asian PWH. Given the widespread adoption of these regimens, clinicians should consider individual patient characteristics, such as baseline CD4 count and BMI, when selecting ART. Furthermore, consistently high and comparable effectiveness was observed in both the BIC/FTC/TAF and DTG/3TC groups. Prospective studies with longer follow-up and broader geographic representation are essential to confirm these findings.
Author Contributions
Conceptualization, C.-Y.C. and C.-J.Y.; methodology, C.-Y.C.; software, C.-Y.C. and C.-J.Y.; validation, C.-J.Y., Q.Z., C.-Y.C., and P.M.; formal analysis, C.-Y.C. and C.-J.Y.; investigation, C.-Y.C. and P.M.; resources, W.-H.H.,T.-C.C., M.-S.T., X.Z., M.Z. T.-C.H. and Y.-W.W.; data curation, S.-H.C., Y.-C.L.; writing—original draft preparation, C.-J.Y. and M.-H.H.; writing—review and editing, M.-W.H., C.-P.C.; visualization, S.-H.C.; supervision, C.-Y.C.; project administration, C.-J.Y. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Taoyuan General Hospital (TYGH112029).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and all data were analyzed without personal sensitive information.
Data Availability Statement
All data analyzed or generated during this study are included in the manuscript.
Acknowledgments
We are grateful to Shu-Ting, Gan for providing the statistical consulting services from the Division of Biostatistics, Department of Research and Development, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan, Taiwan, R.O.C.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HIV |
human immunodeficiency virus |
| PWH |
people living with HIV |
| INSTIs |
integrase strand transfer inhibitors |
| DTG/3TC |
dolutegravir/lamivudine |
| BIC/FTC/TAF |
bictegravir/emtricitabine/tenofovir alafenamide fumarate |
| TDF |
tenofovir disoproxil fumarate |
| ART |
antiretroviral therapies |
| NNRTIs |
non-nucleoside reverse transcriptase inhibitors |
| SHCS |
Swiss HIV Cohort |
| HbA1c |
glycated hemoglobin |
| IPTW |
inverse probability of treatment weighting |
| IQR |
interquartile range |
| IDU |
injection drug user |
| aOR |
adjusted odds ratio |
| CHOL |
total cholesterol |
| TG |
triglyceride |
| HDL |
high-density lipoprotein |
| LDL |
low-density lipoprotein |
| BMI |
body mass index |
| FTC |
emtricitabine |
| EFV |
efavirenz |
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2024. Available at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination?view=full#:~:text=Initial%20Combination%20Antiretroviral%20Regimens% 20for%20People%20with%20HIV; accessed October 18, 2024.
- European AIDS Clinical Society. EACS guidelines version 12.1. October 2024. Available at: https://eacs.sanfordguide.com/; accessed November 14, 2024.
- Sokhela, S.; Venter, W.D.F.; Bosch, B.; Woods, J.; McCann, K.; Akpomiemie, G.; Chandiwana, N.; Mashabane, N.; Tembo, A.; Simmons, B.; et al. Final 192-week efficacy and safety results of the advance trial, comparing 3 first-line antiretroviral regimens. Open. Forum. Infect. Dis. 2024, 11, ofae007. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Surial, B.; Mugglin, C.; Calmy, A.; Cavassini, M.; Günthard, H.F.; Stöckle, M.; Bernasconi, E.; Schmid, P.; Tarr, P.E.; Furrer, H.; et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann. Intern. Med. 2021, 174, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Li, L.; Greene, T. Propensity score weighting analysis and treatment effect discovery. Stat. Methods. Med. Res. 2019, 28, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Carter, C.C.; Melbourne, K.; Brown, T.T.; Cohen, C.; Das, M.; Esser, S.; Huang, H.; Koethe, J.R.; Martin, H.; et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin. Infect. Dis. 2021, 73, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Venter, W.D.F.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Venter, W.D.F.; Sokhela, S.; Simmons, B.; Moorhouse, M.; Fairlie, L.; Mashabane, N.; Serenata, C.; Akpomiemie, G.; Masenya, M.; Qavi, A.; et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020, 7, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Bakal, D.R.; Coelho, L.E.; Luz, P.M.; Clark, J.L.; De Boni, R.B.; Cardoso, S.W.; Veloso, V.G.; Lake, J.E.; Grinsztejn, B. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J. Antimicrob. Chemother 2018, 73, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.; Ofotokun, I.; McComsey, G.A.; Brown, T.T.; Moser, C.; Sugar, C.A.; Currier, J.S. Changes in waist circumference in HIV-infected individuals initiating a raltegravir or protease inhibitor regimen: effects of sex and race. Open. Forum. Infect. Dis 2018, 5, ofy201. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, K.; Bourgi, K.; Rebeiro, P.F.; Palella, F.; Moore, R.D.; Altoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J.; Horberg, M.A.; et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J. Int. AIDS. Soc. 2020, 23, e25484. [Google Scholar] [CrossRef] [PubMed]
- Ciccullo, A.; Baldin, G.; Cervo, A.; Moschese, D.; Lagi, F.; Cossu, M.V.; Grimaldi, A.; Giacomelli, A.; Rusconi, S.; Sterrantino, G.; et al. Comparing the long-term effectiveness and safety of dolutegravir/lamivudine versus bictegravir/emtricitabine/tenofovir alafenamide fumarate as first-line regimens: a real-life multicentre cohort. J. Antimicrob. Chemother 2024, dkae392. [Online ahead of print]. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lin, Y.C.; Chen, C.P.; Cheng, S.H.; Chang, S.Y.; Ku, S.Y.; Cheng, C.Y. Assessment of risk factors for virological nonsuppression following switch to dolutegravir and lamivudine, or bictegravir, emtricitabine, and tenofovir alafenamide fumarate in a real-world cohort of treatment-experienced adults living with HIV. PLoS. One. 2024, 19, e0314003. [Google Scholar] [CrossRef] [PubMed]
- Benson, P.; Kuretski, J.; Donovan, C.; Harper, G.; Merrill, D.; Metzner, A.A.; Mycock, K.; Wallis, H.; Brogan, A.P.; Patarroyo, J.; Oglesby, A. Real-world effectiveness of dolutegravir/lamivudine in people with hiv-1 in test-and-treat settings or with high baseline viral loads: TANDEM study subgroup analyses. Infect. Dis. Ther. 2024, 13, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Rao, M.; Chen, W.; Cai, K.; Zhang, L.; Xu, L.; Sun, L.; Liu, X.; He, Y.; Wang, H. Dolutegravir plus lamivudine dual-drug regimen in treatment-naive hiv-1-infected patients with high-level viral load: preliminary data from the real world. J. Acquir. Immune. Defic. Syndr. 2022, 91, S16–S19. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).