2.1. Study Design and Study Population
The present prospective, observational study was conducted in the Cardiology Department of the “Bagdasar-Arseni” Emergency Hospital in Bucharest, Romania, during an enrollment period of 1 year and 6 months, from May 2021 to October 2022 and following the patients’ evolution after discharge for 12 months, from May 2022 to April 2023. The study was conducted on a sample of 158 patients, over 18 years old and diagnosed with preserved LVEF. The cohort was constituted of patients admitted with a diagnosis of heart failure with preserved ejection fraction, LVEF ≥50 percent.
The follow-up was carried out by phone call in the first year after the discharge, due to the difficulty of follow-up in the cardiology clinic in the context of the recent SARS-COV2 virus pandemic.
The inclusion criteria were: (1) patients over 18 years old; (2) patients of either sex; (3) patients with signs/symptoms of heart failure; (4) patients with NT-proBNP >=300ug/ml; (5) patients with LVEF (left ventricular ejection fraction) >=50% objectified echocardiographically; (6) patients with only signs and symptoms specific to the disease; (7) patients who signed the informed consent and agreed the prospective follow-up and to participate in the evaluation through telephone.
The exclusion criteria were: (1) patients with LVEF < 50%; (2) patients with NT-proBNP < 300ug/ml; (3) patients with severe valvular diseases; (4) patients with severe neuropsychiatric diseases; (5) patients with severe liver, kidney or lung disease; (6) patients with ongoing infection; (7) patients diagnosed with autoimmune diseases or malignancy; (8) patients with moderate and severe anemia; (9) patients with hope of survival less than 1 year; (10) patients who did not sign the informed consent and did not wish to be contacted by phone.
All the patients had venous blood samples collected within 30 minutes from admission. The NT-proBNP level was evaluated through the Elisa method with a PATHFAST compact autoanalyzer.
The LVEF (left ventricular ejection fraction) values were obtained using transthoracic echocardiography performed during the hospitalization. The modified Simpson biplane method was used to calculate the left ventricular end-systolic volumes (LVESVs) and end-diastolic volumes (LVEDVs) from 4 and 2-chamber views. The LV volumes were corrected for body surface areas.
The demographic data considered were age, gender, emergency admission or appointment, NYHA class, normal or arterial hypertension, atrial fibrillation, overweight and obesity. The mean age of the patients enrolled in the study was 72.21, the minimum age was 40 and the maximum age was 93.
Table 1.
Age of the studied population.
Table 1.
Age of the studied population.
| |
Age |
| count = 158 |
|
| mean |
72.21519 |
| std |
10.4524 |
| min |
40 |
| max |
93 |
| 25% |
66 |
| 50% |
72.5 |
| 75% |
80 |
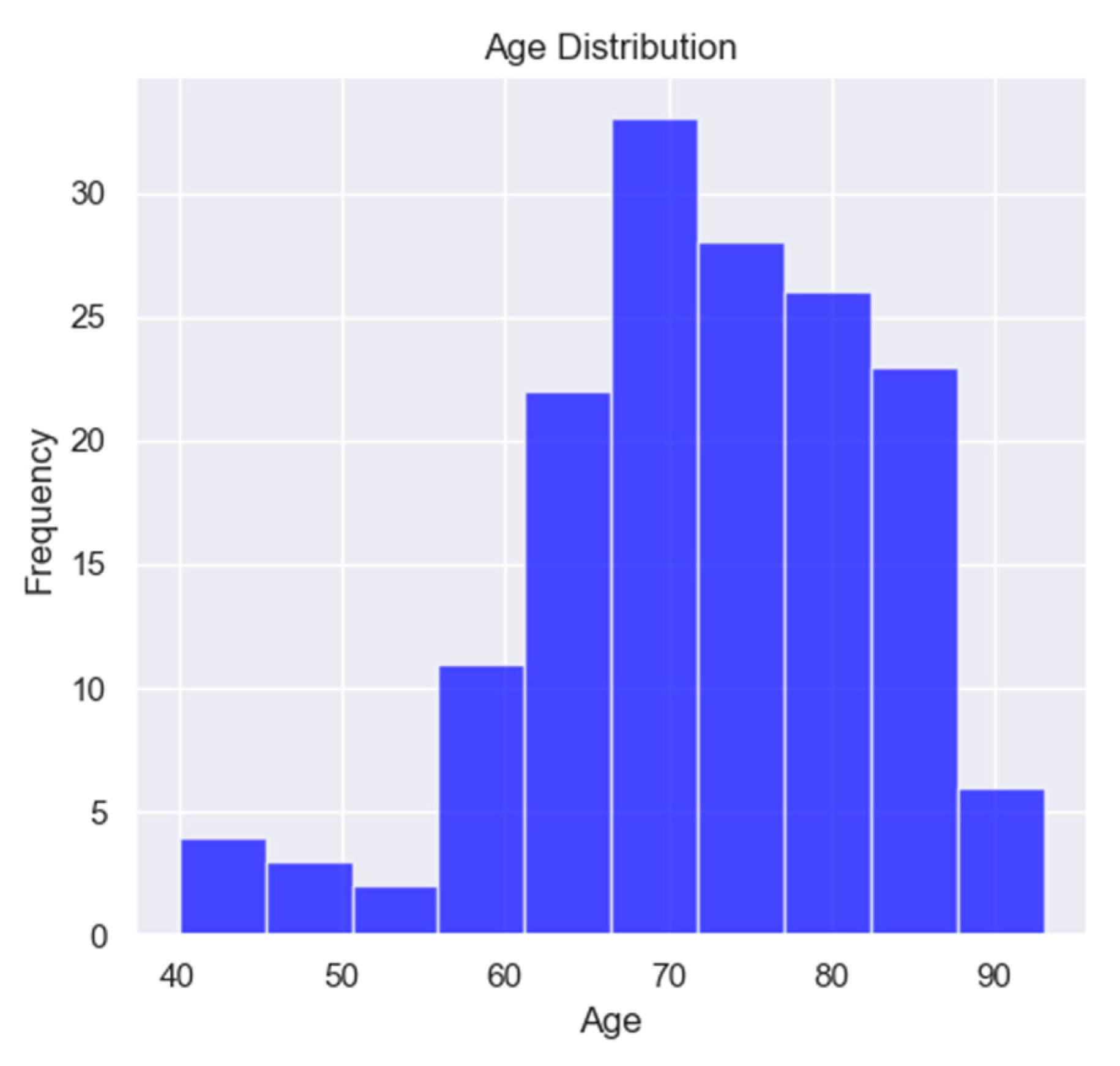
Figure 1.
Age distribution of the studied population.
Figure 1.
Age distribution of the studied population.
In the study, only 9 patients were under 60 years old, suggesting a prevalence of the disease in elderly patients, a characteristic of HFPEF.
Table 2.
Demographic and clinical characteristics of the studied population.
Table 2.
Demographic and clinical characteristics of the studied population.
| Variable |
Prevalence % |
| Female |
63% |
| Male |
37% |
| Admission Emergency |
72% |
| Admission Appointment |
28% |
| NYHA class I |
1% |
| NYHA class II |
9% |
| NYHA class III |
53% |
| NYHA class IV |
37% |
| Normal blood pressure |
3% |
| Hypertension stage I |
11% |
| Hypertension stage II |
30% |
| Hypertension stage III |
56% |
| Atrial fibrillation |
46% |
| Overweight and obesity |
78% |
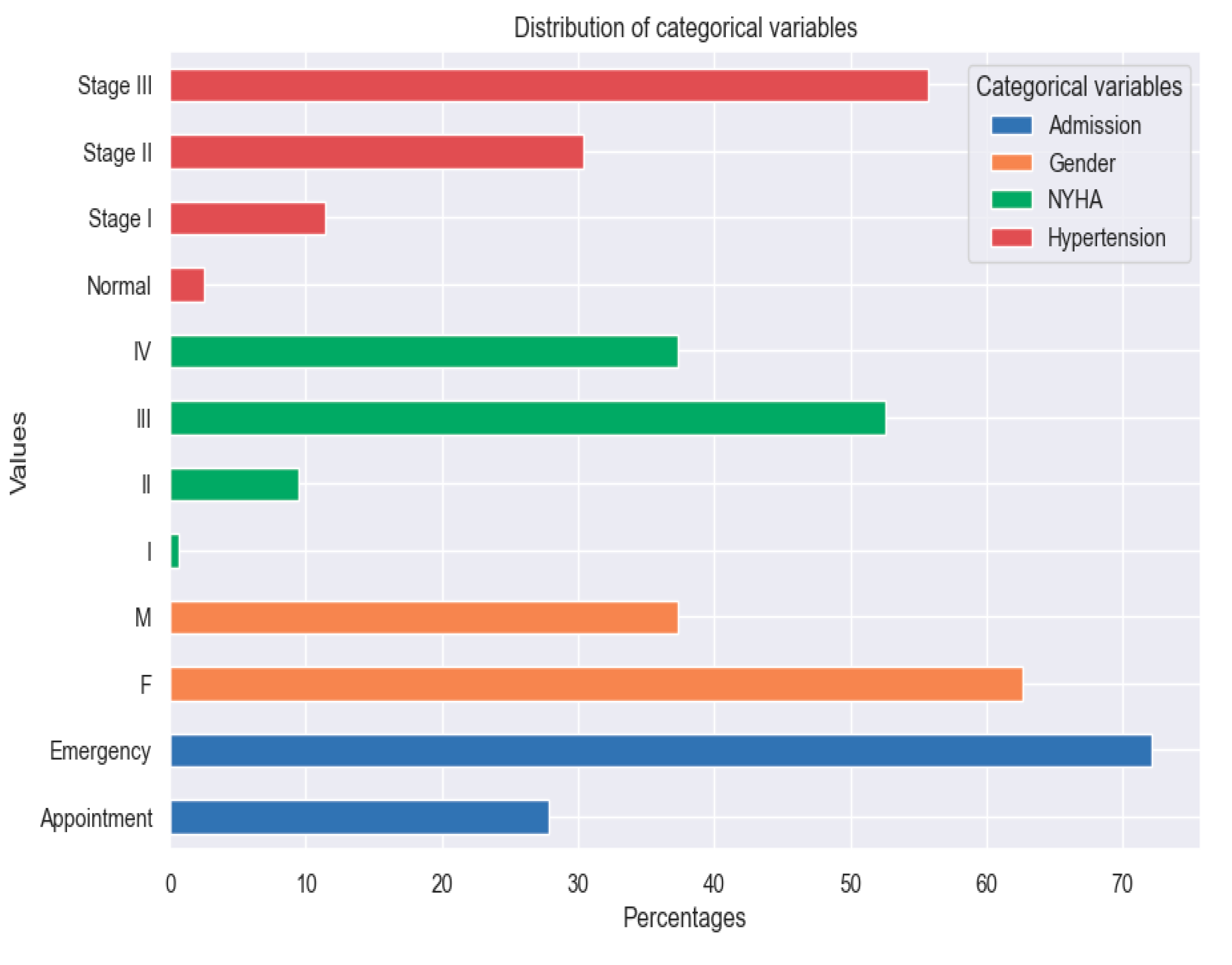
Figure 2.
Distribution of categorical variables.
Figure 2.
Distribution of categorical variables.
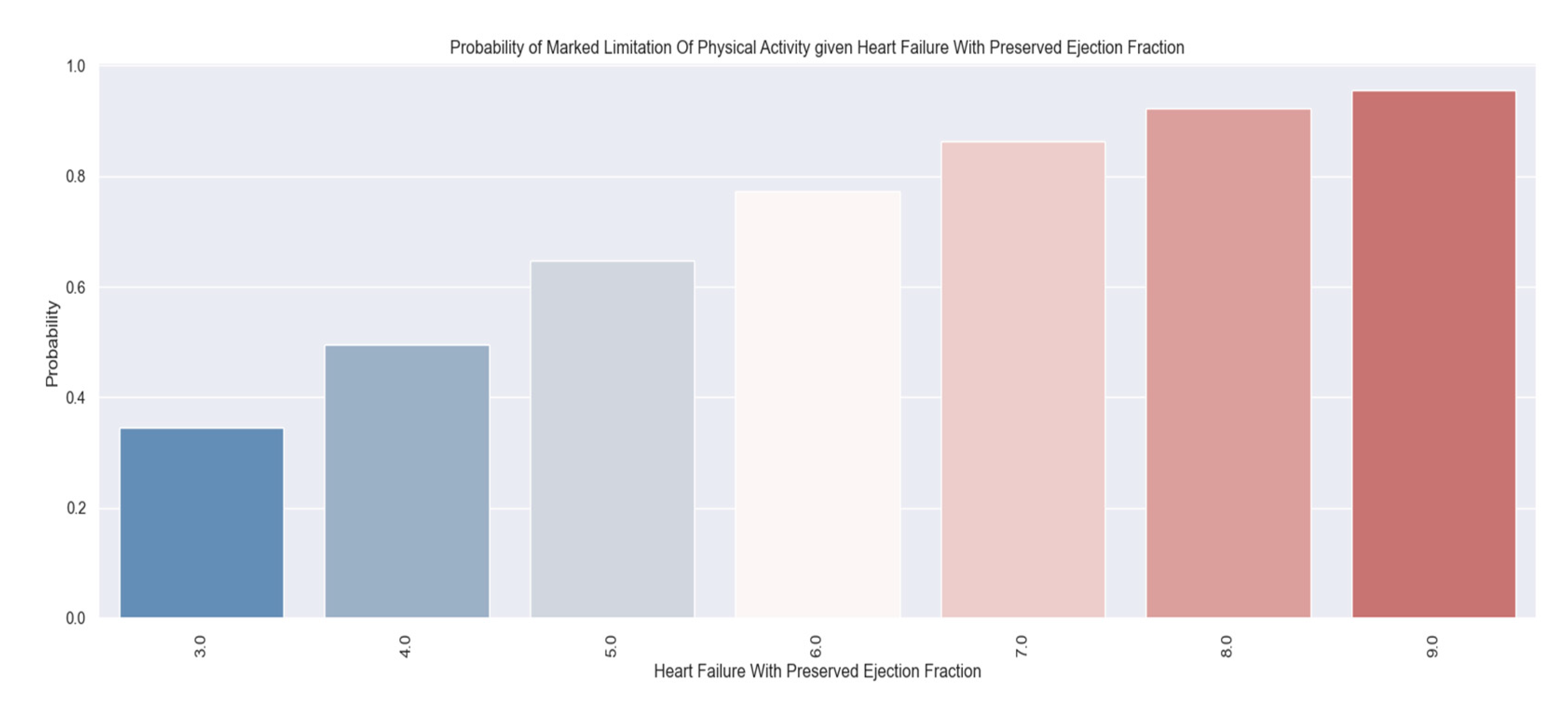
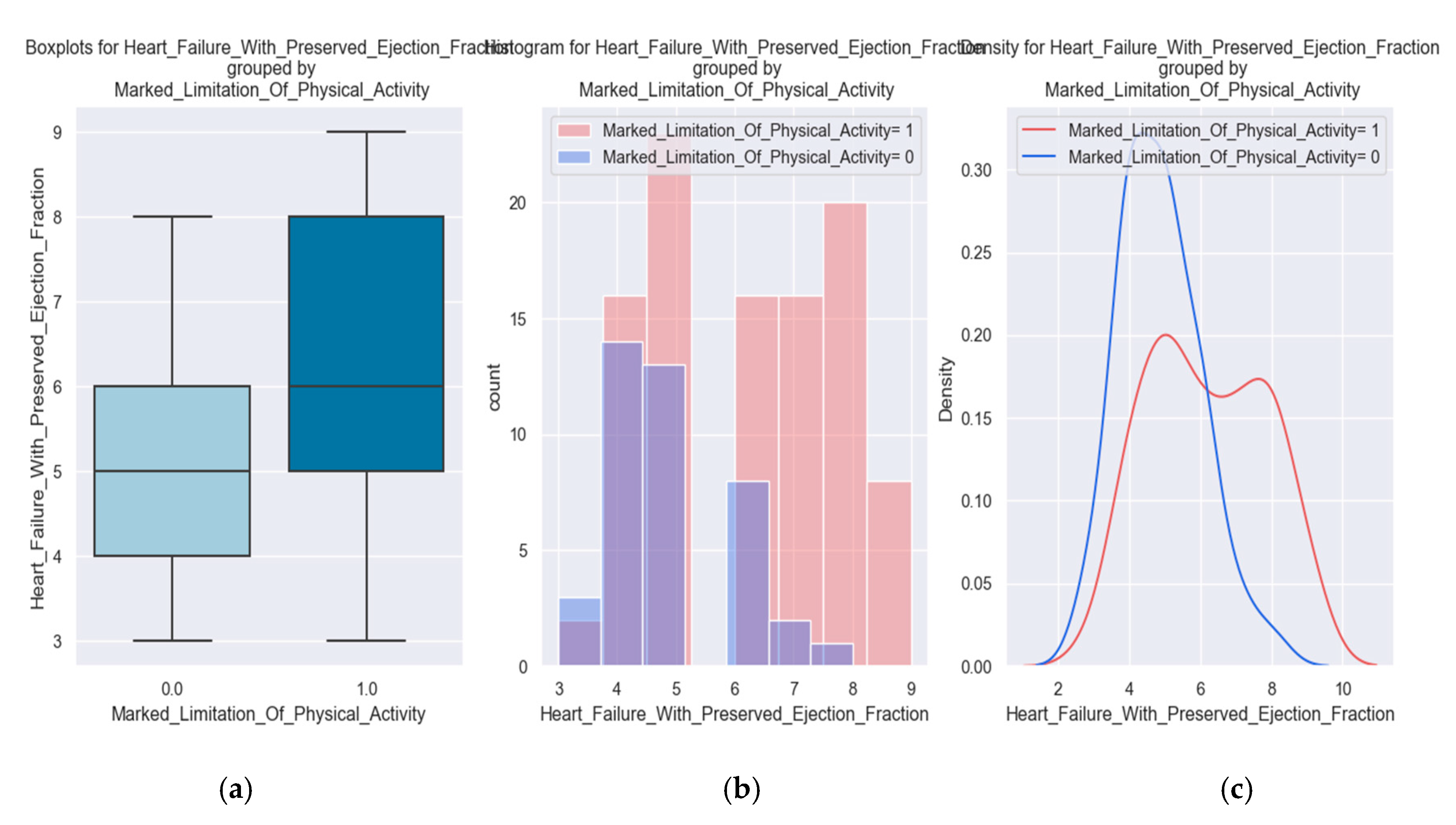
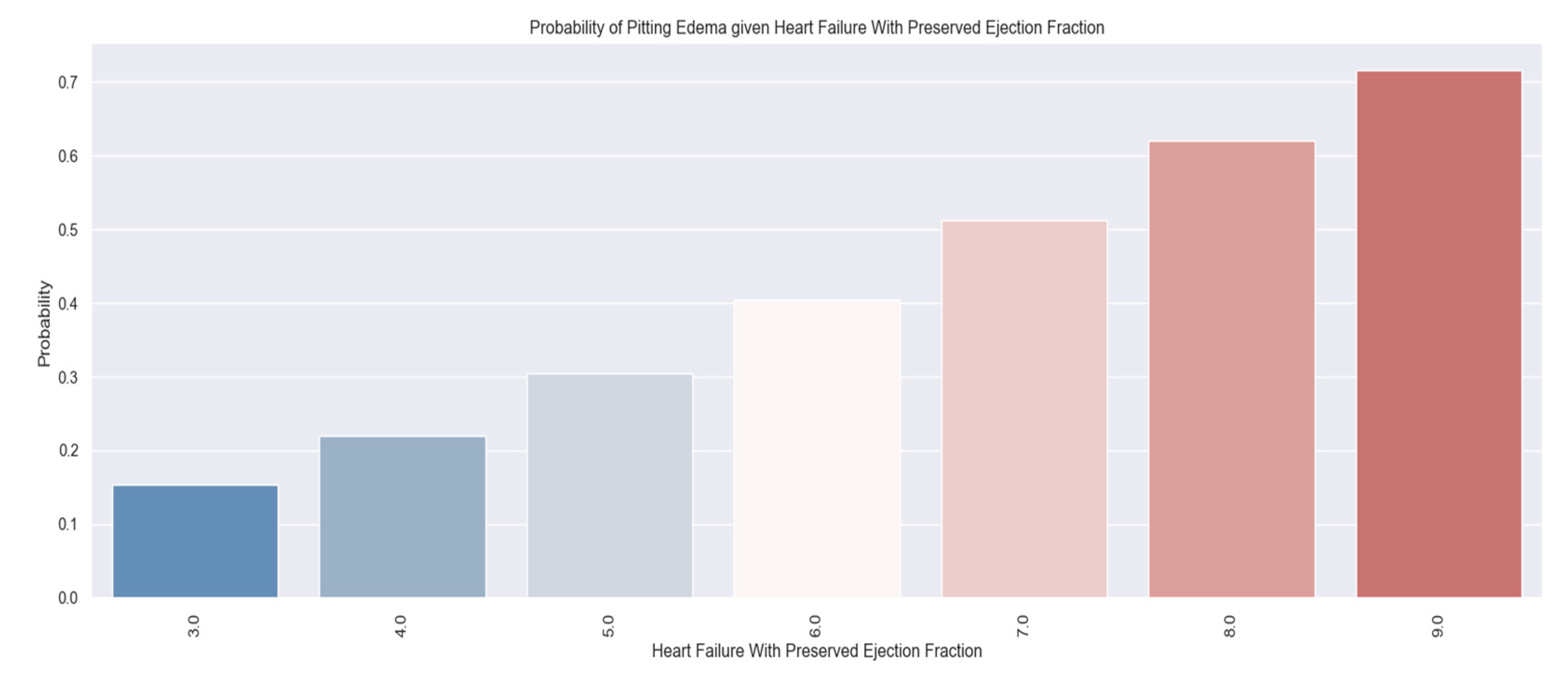
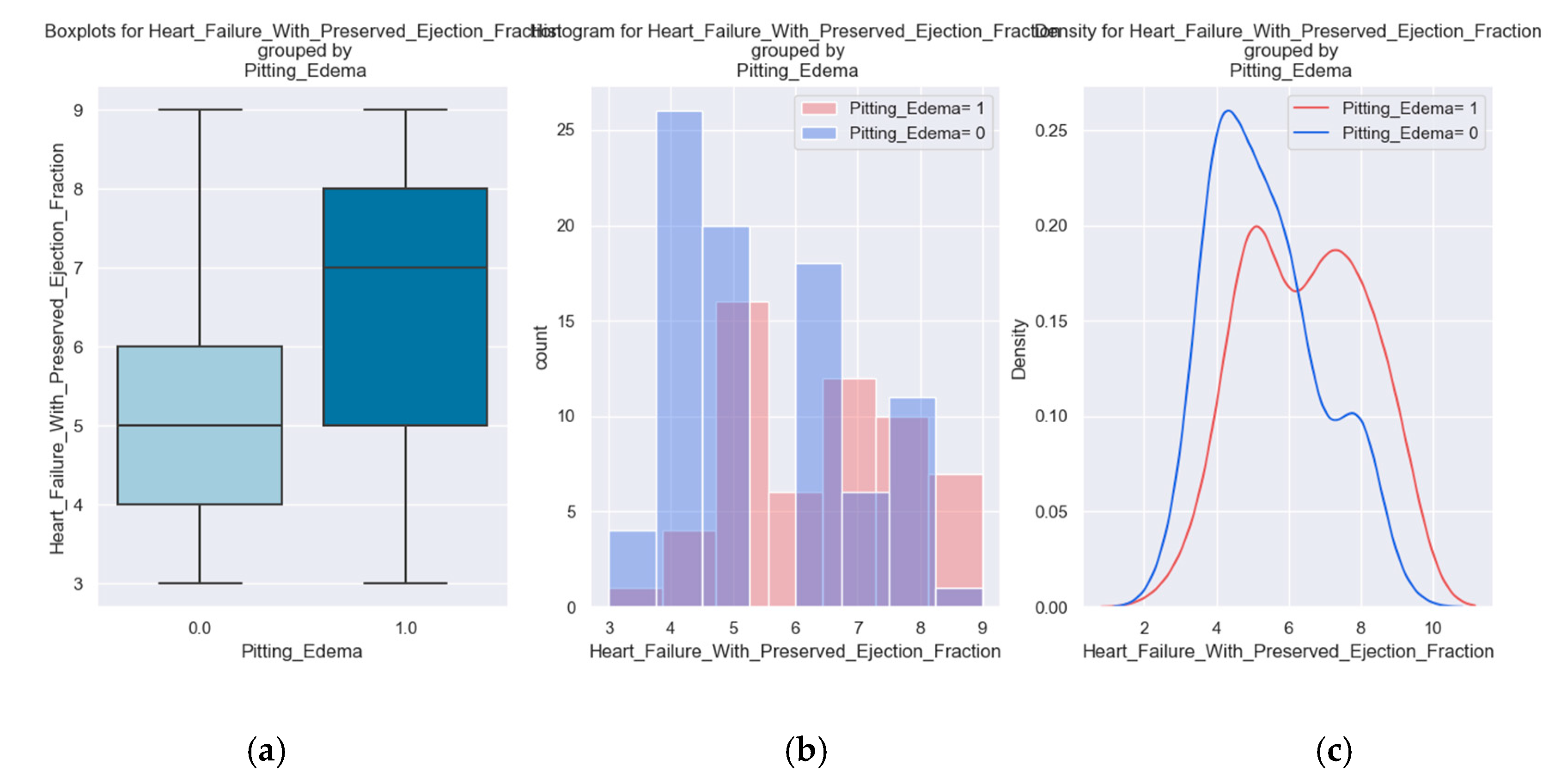
The tracked quality-of-life factors were the following: marked limitation of physical activity, edema, recent fatigue, the ability to exercise, palpitations and sadness.
All the variables used are binary described by the following summary statistics table.
Table 3.
All variables used in the research.
Table 3.
All variables used in the research.
| Variable |
Count |
True |
False |
True Percentage |
| Pitting Edema |
142 |
56 |
86 |
39% |
| Marked Limitation of Physical Activity |
142 |
101 |
41 |
71% |
| Sadness |
142 |
93 |
49 |
65% |
| Ability to Exercise |
142 |
37 |
105 |
26% |
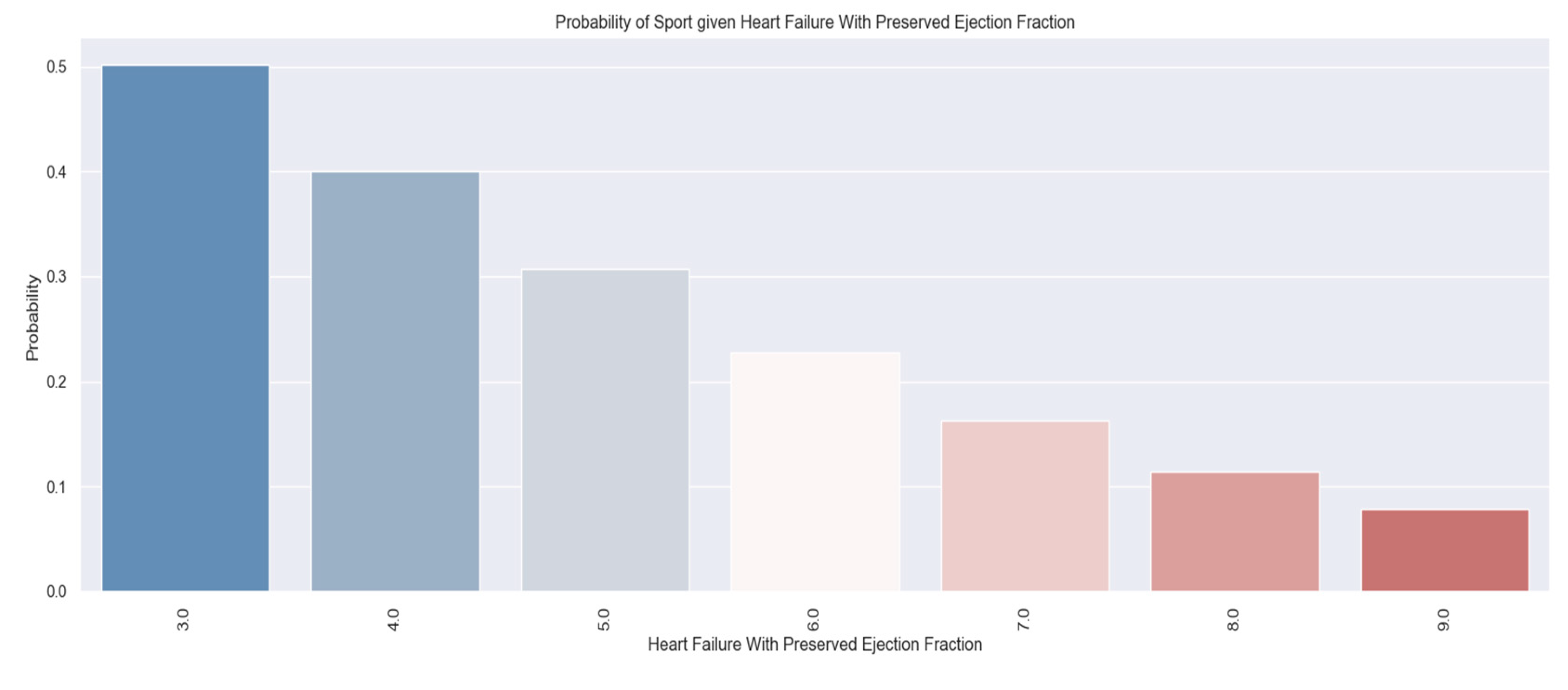
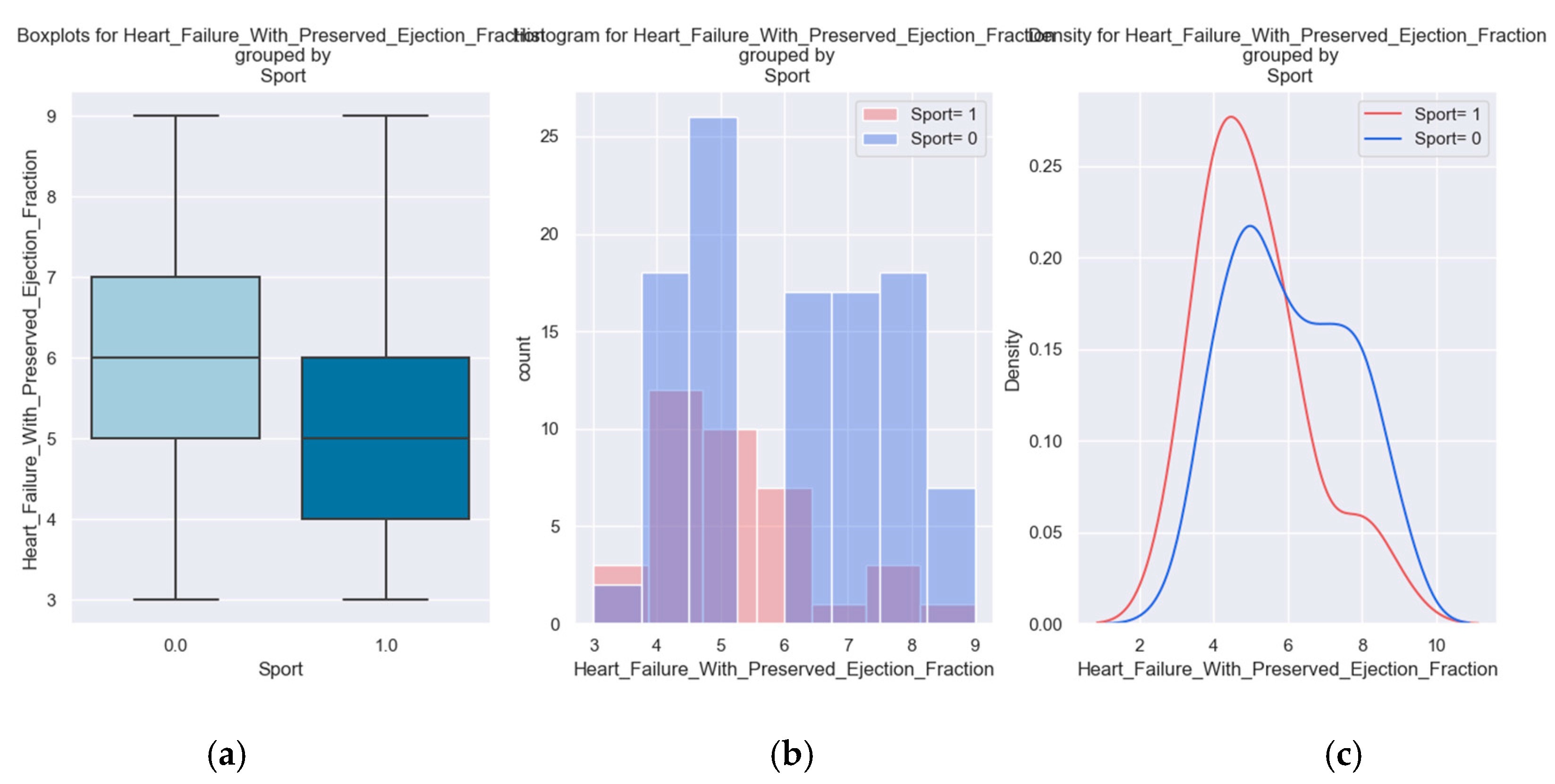
| Palpitations |
158 |
59 |
99 |
37% |
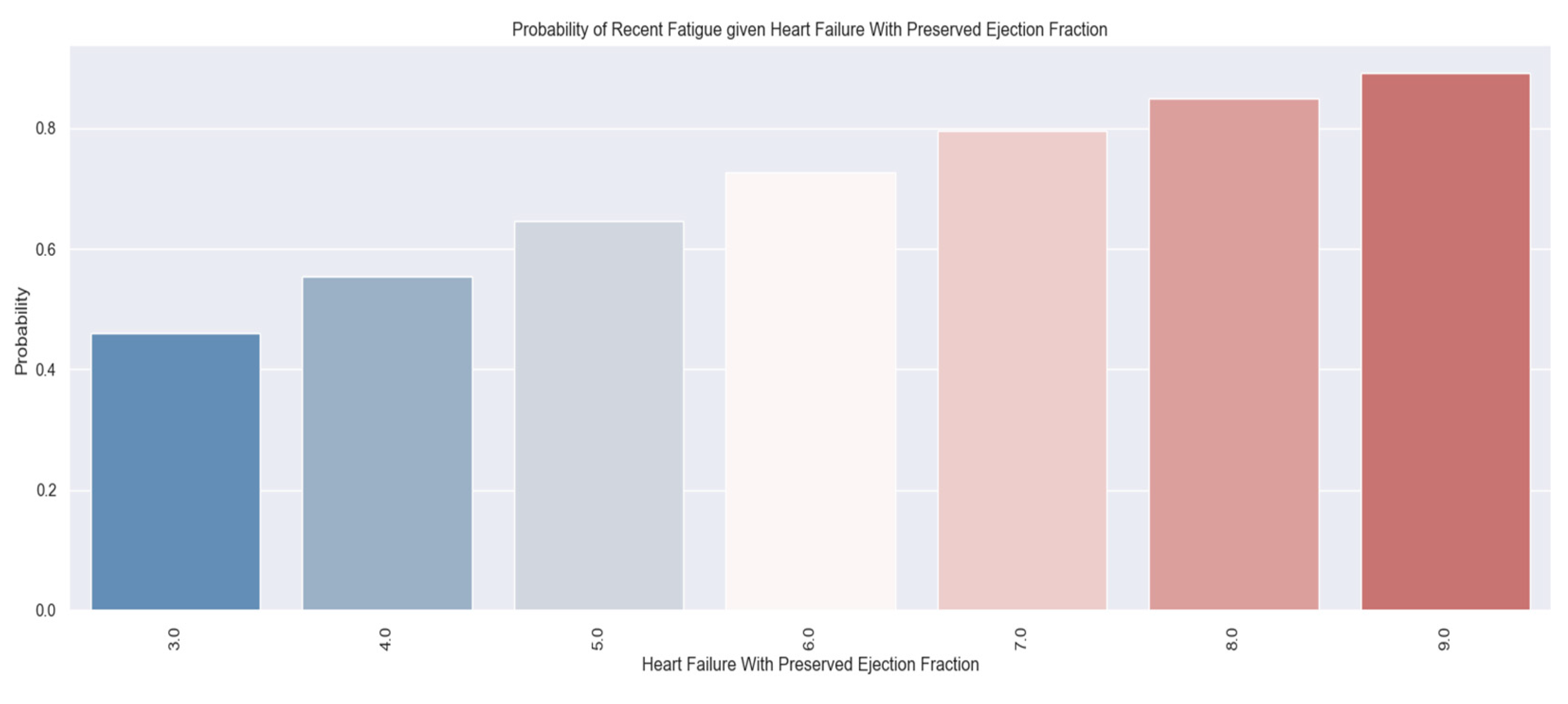
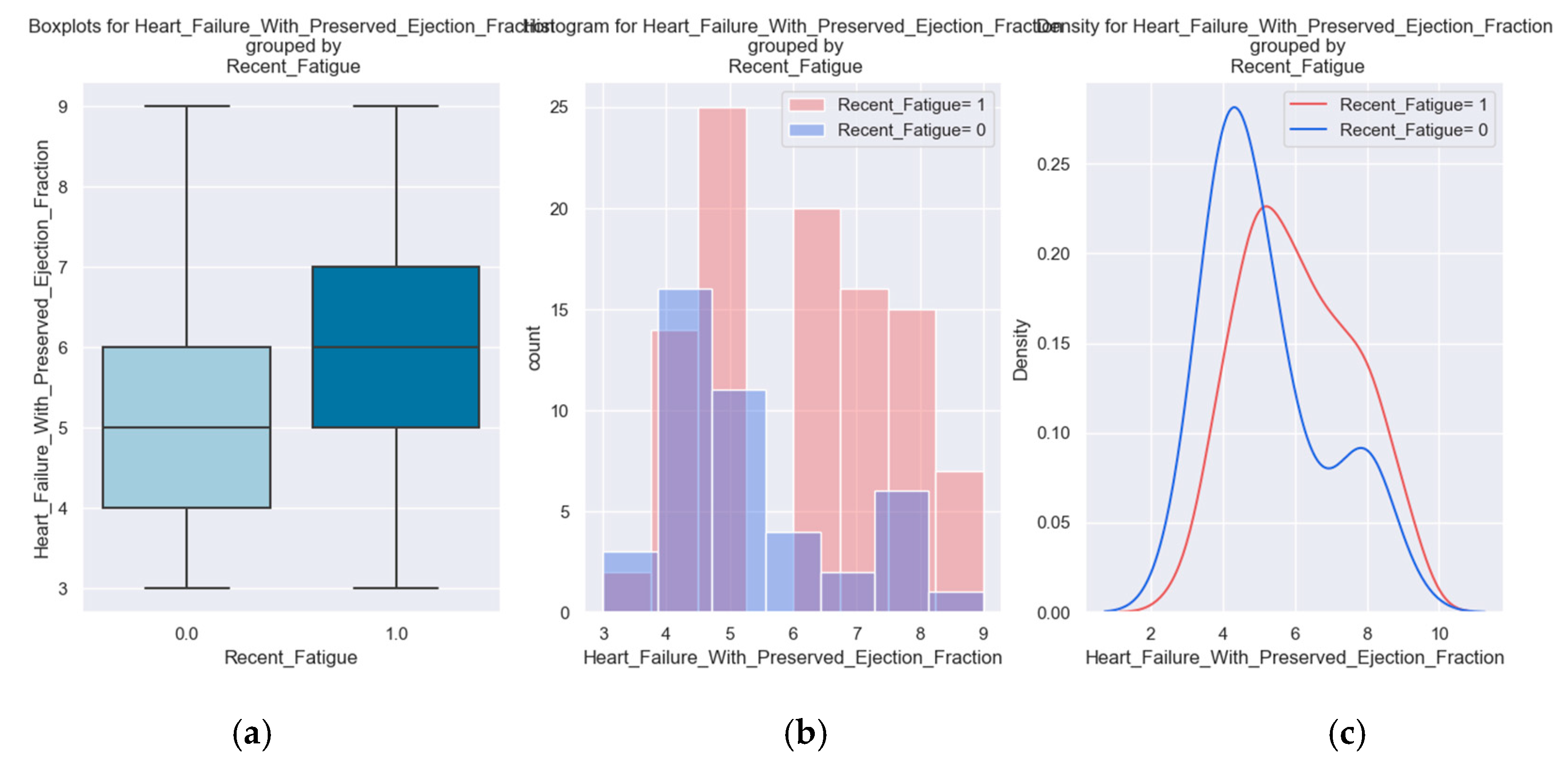
| Recent Fatigue |
142 |
99 |
43 |
70% |
These variables were chosen to describe patients' quality of life because they have a direct impact on lifestyle and show the symptoms and limitations caused by heart failure decompensation. For example, the presence of pitting edema, marked limitation of physical activity and fatigue at regular exertion indicate an exacerbation of the disease. These patients should be medically reassessed as soon as possible.
The inability to exercise daily is a parameter that is associated with the limitations of the disease and contributes to the unfavorable evolution.
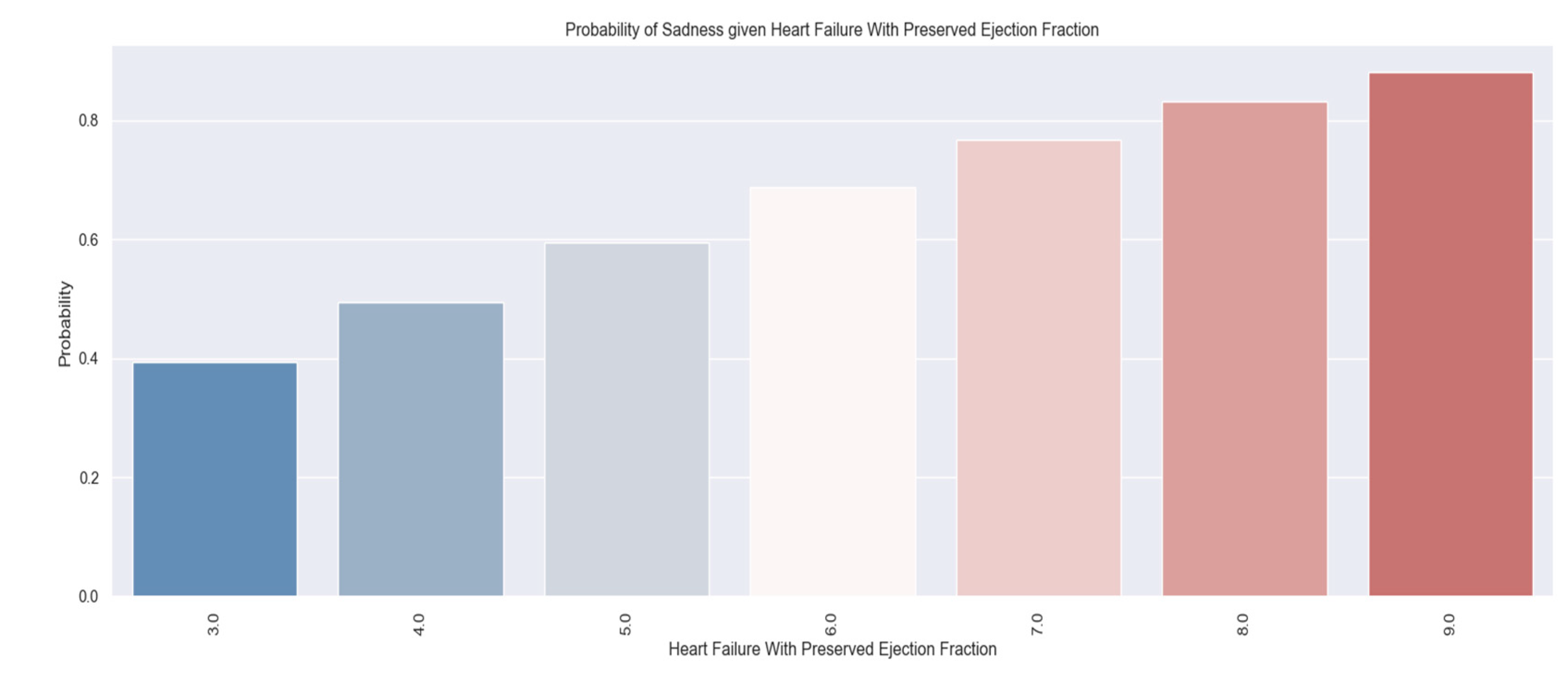
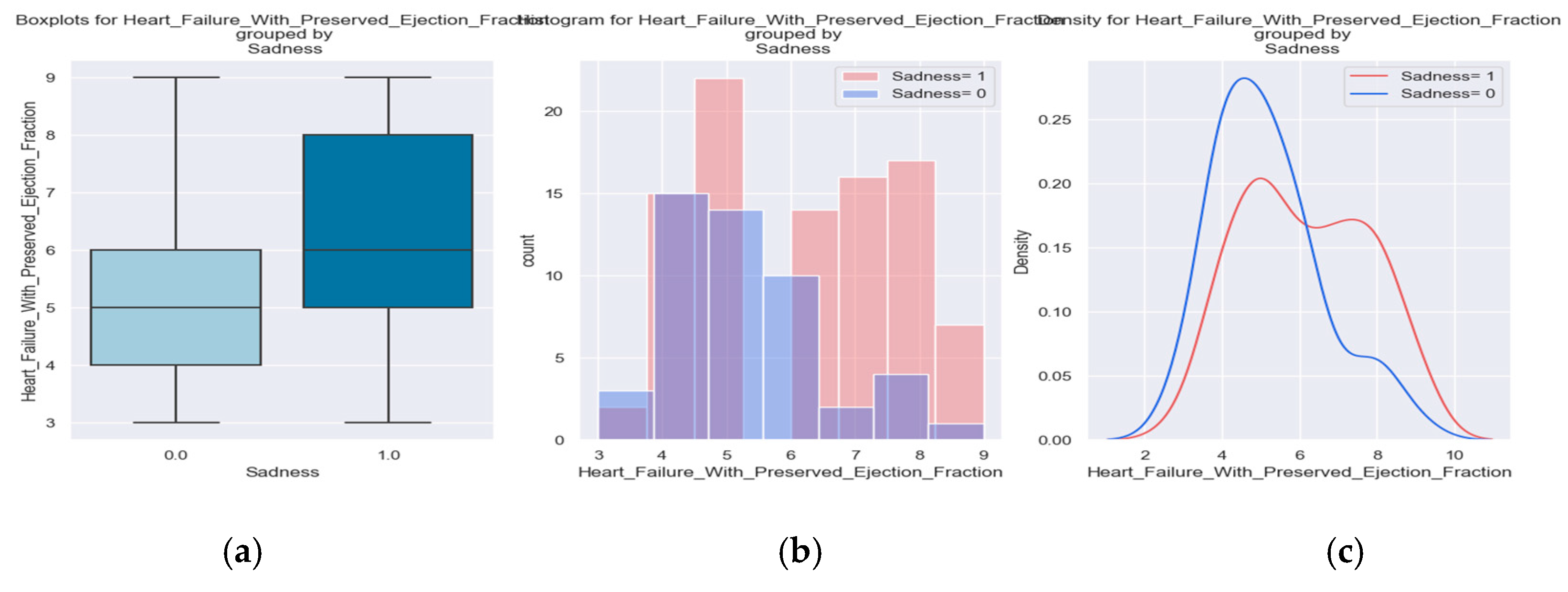
Illness means physical and mental limitations so we wanted to identify also which patients were sad and could benefit from psycho-emotional support.
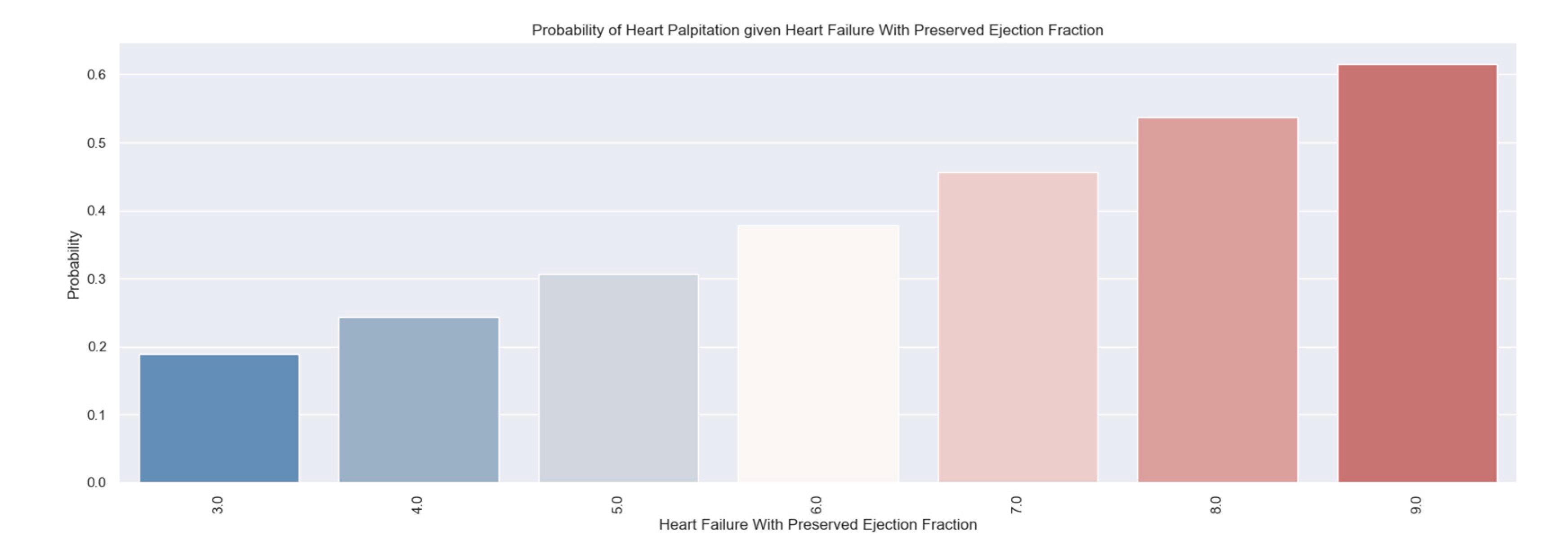
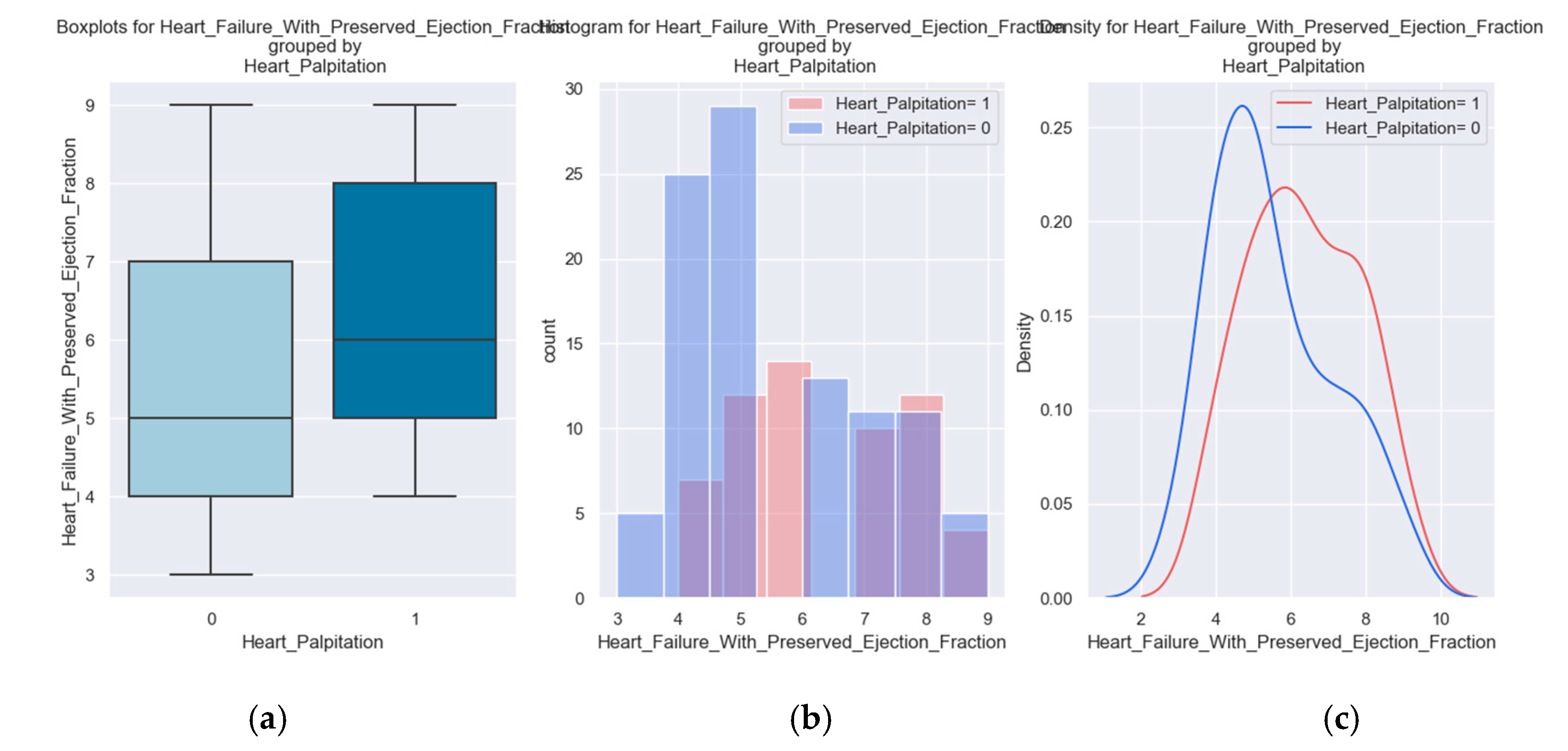
We considered it useful to use some parameters from the hospitalization of the patients to determine if there is a relationship between them and the H2FPEF score. One variable was the presence of palpitations. This parameter is a symptom of the disease and at the same time contributes to the course of the disease. It suggests a possible cause - atrial fibrillation - which must be investigated and treated if it exists.
Below, we have the distribution of the independent variable used in this research, the H2FPEF score, a specific indicator of heart failure with preserved ejection fraction.
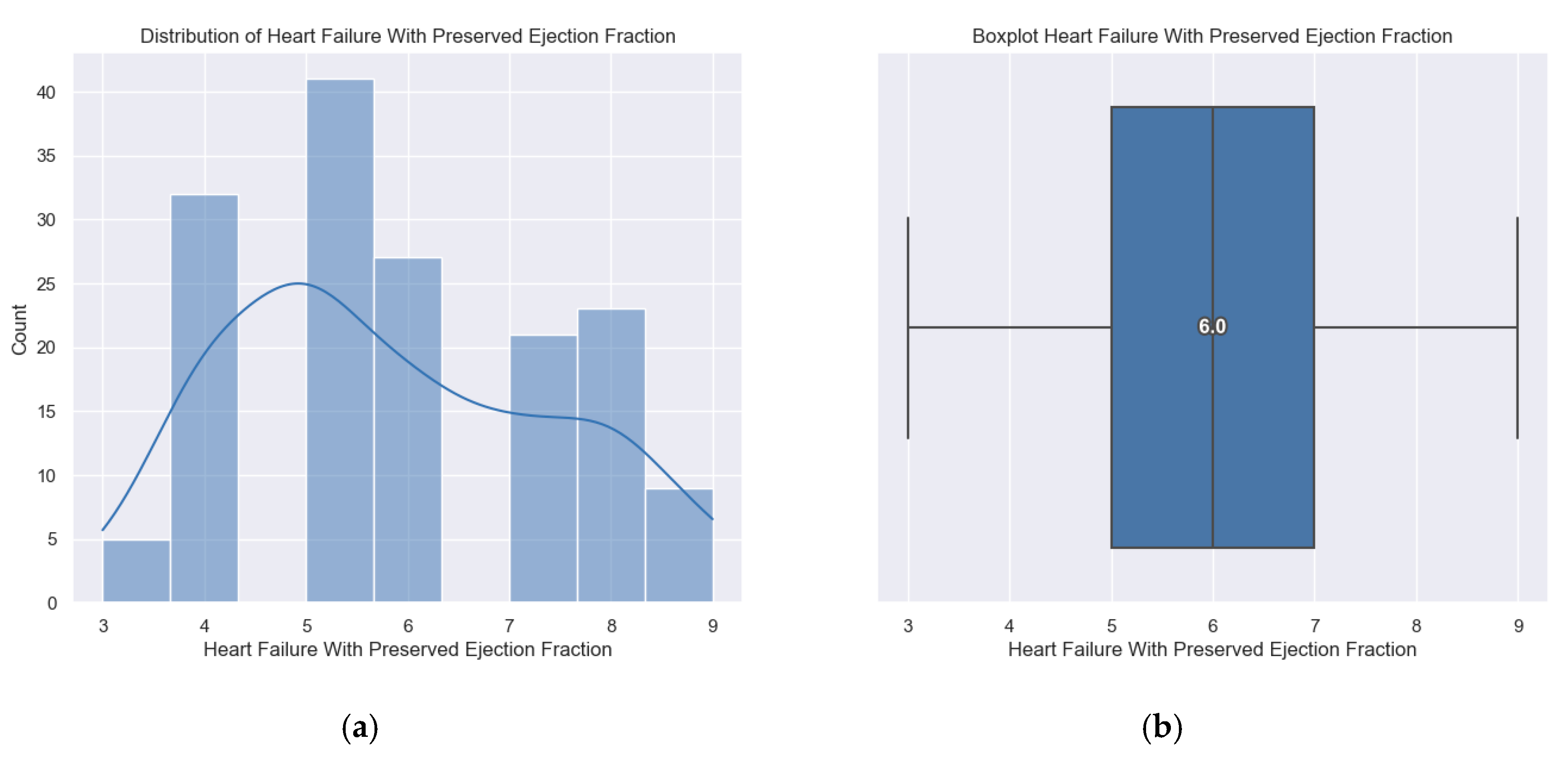
Figure 3.
(a, b) Distribution of the H2FPEF score.
Figure 3.
(a, b) Distribution of the H2FPEF score.
The patients enrolled in the study had a minimum H2FPEF score of 3 points, a maximum score of 9 points and the average score was 6 points.
Quality of life data was obtained through a telephone interview in which patients answered the questions below. The average duration of a phone call was 15 minutes. Also, the patients received indications regarding lifestyle optimization, about precipitating factors, about the need for periodic monitoring and about a new clinical reassessment, for symptomatic patients.
It should be mentioned that 16 patients died, representing 10% of the total number. The main cause was HF for 10 patients, stroke for 4 patients and neoplasia diagnosed after discharge for 2 patients.
We mention that for the variables obtained from the answers provided during the phone interview (marked limitation of physical activity, pitting edema, recent fatigue, ability to exercise, palpitations and sadness) we recorded and analyzed data for only 142 patients. The other 16 patients died within the first year after discharge.
During the first year of follow-up, 31% of patients had at least one readmission for heart failure worsening.
The questions of the phone interview were:
In the last month, have you breathed harder during physical efforts?
Do you have pitting edemas?
Are you feeling more tired lately?
Do you manage to exercise minimum 30 min/day, minimum 5 days/week?
Are you sad? Is your sadness caused by the cardiovascular disease?
Do you have palpitations?
Have you been admitted to a cardiology ward in the last year? If the answer was Yes, the reason for hospitalization was also specified.
We mention that only the answers that were related to the evolution of heart failure were considered.
We tried to find a correlation between the answers provided and the value of the H2FPEF score to be able to predict which patients are at higher risk and thus be able to make an early identification of the vulnerable ones. These patients require closer follow-up through frequent check-ups with a general practitioner or cardiologist. They and their families should be medically educated about the precipitating factors and the signs and symptoms of the disease’s decompensation to get to the doctor faster.
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (protocol code PO-35-F-03, October 1st, 2021).