Submitted:
31 December 2024
Posted:
02 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Transformation of EIS Data into Distribution of Relaxation Time
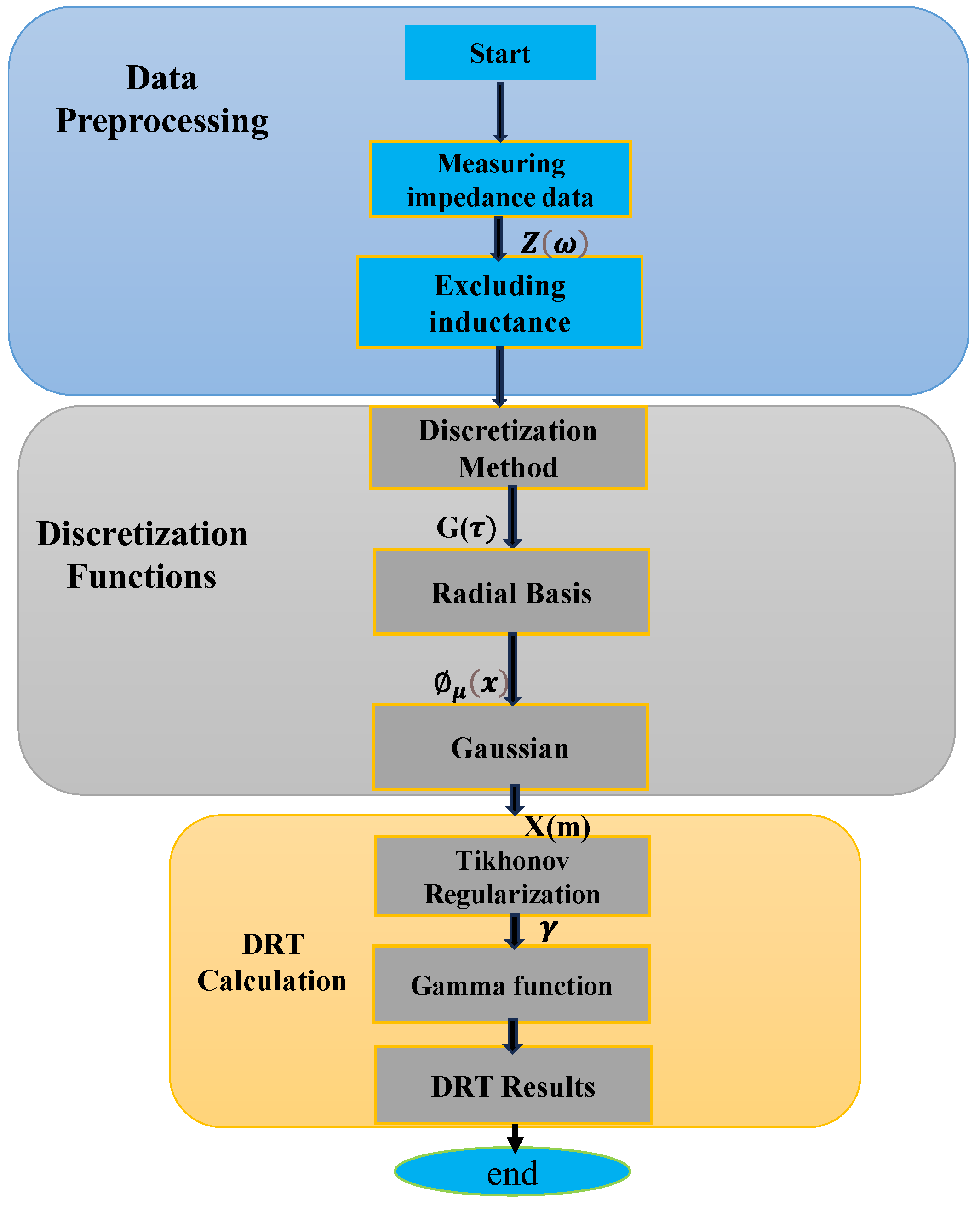
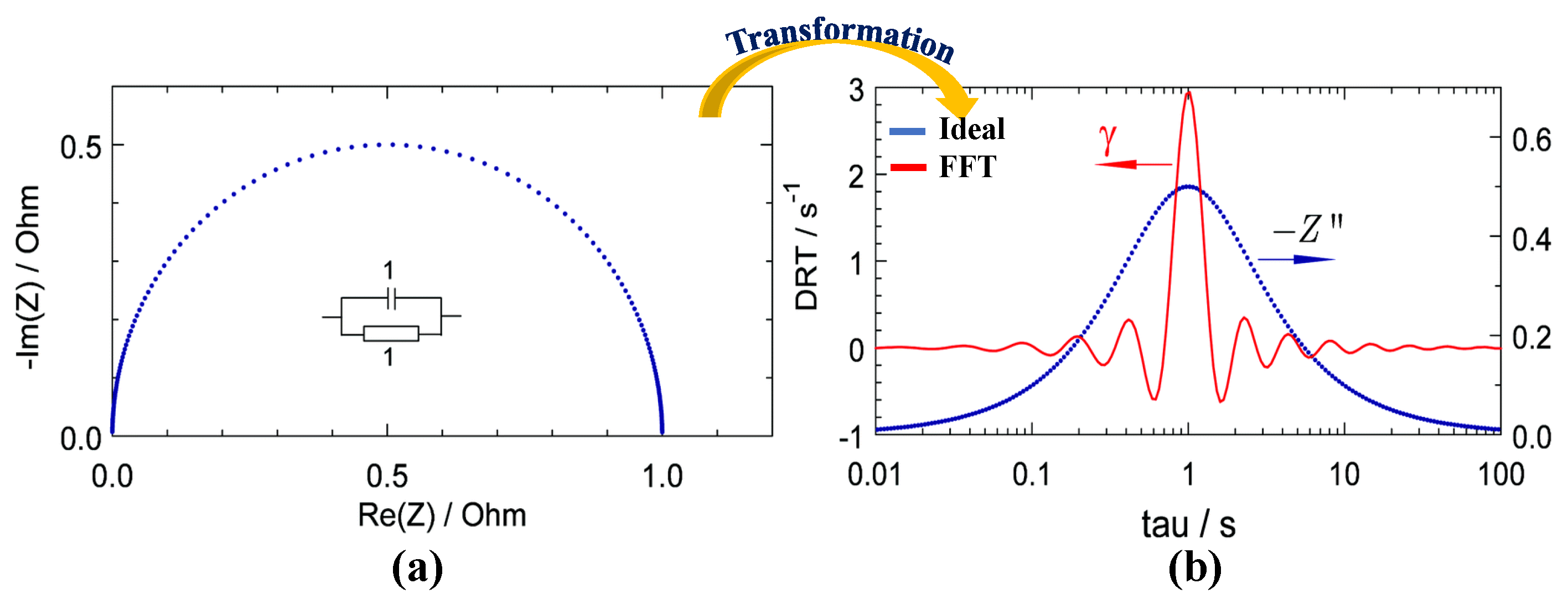
2.1. Workflow of DRT Method
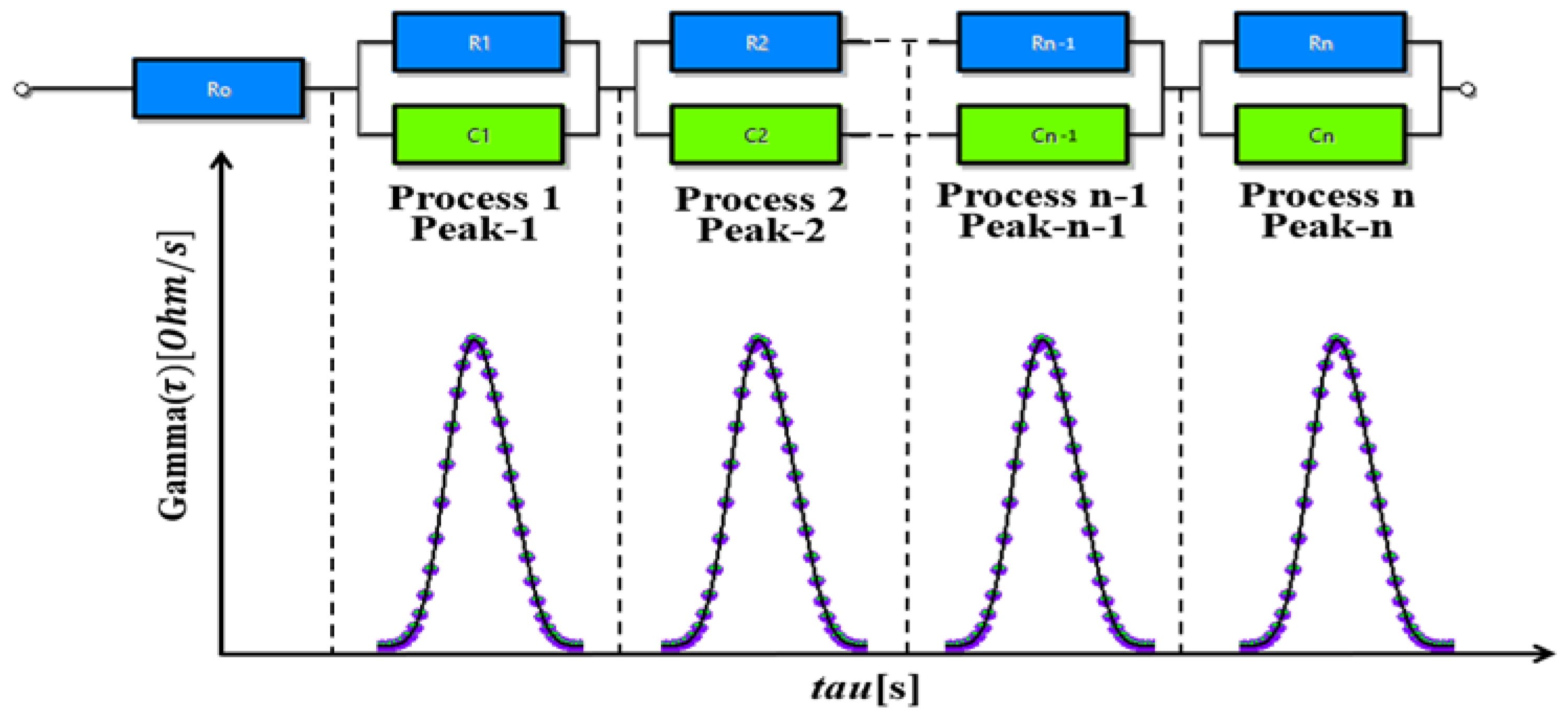
2.2. Derivation of DRT Method
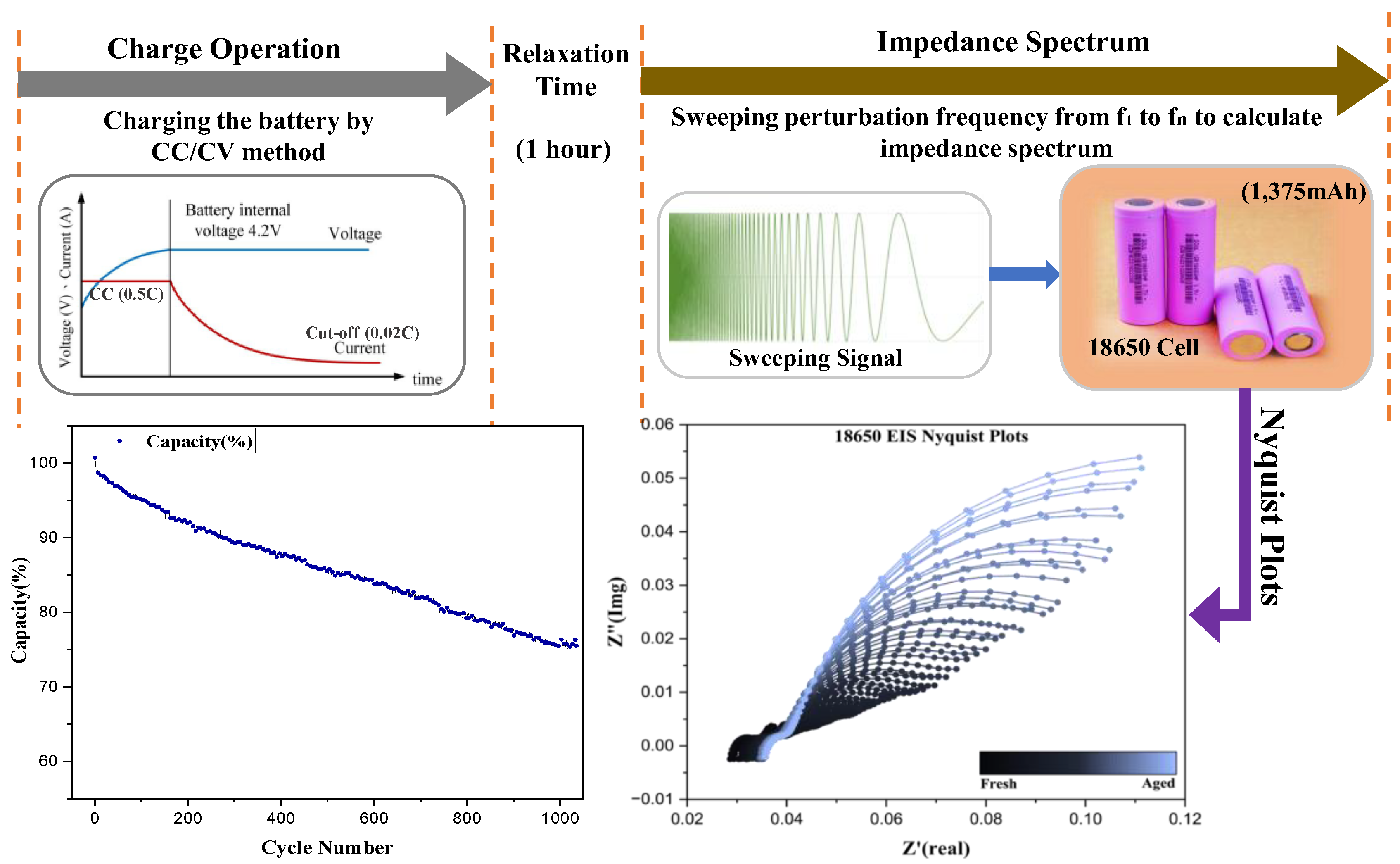
3. Battery Aging Test Method
4. Results and Analysis
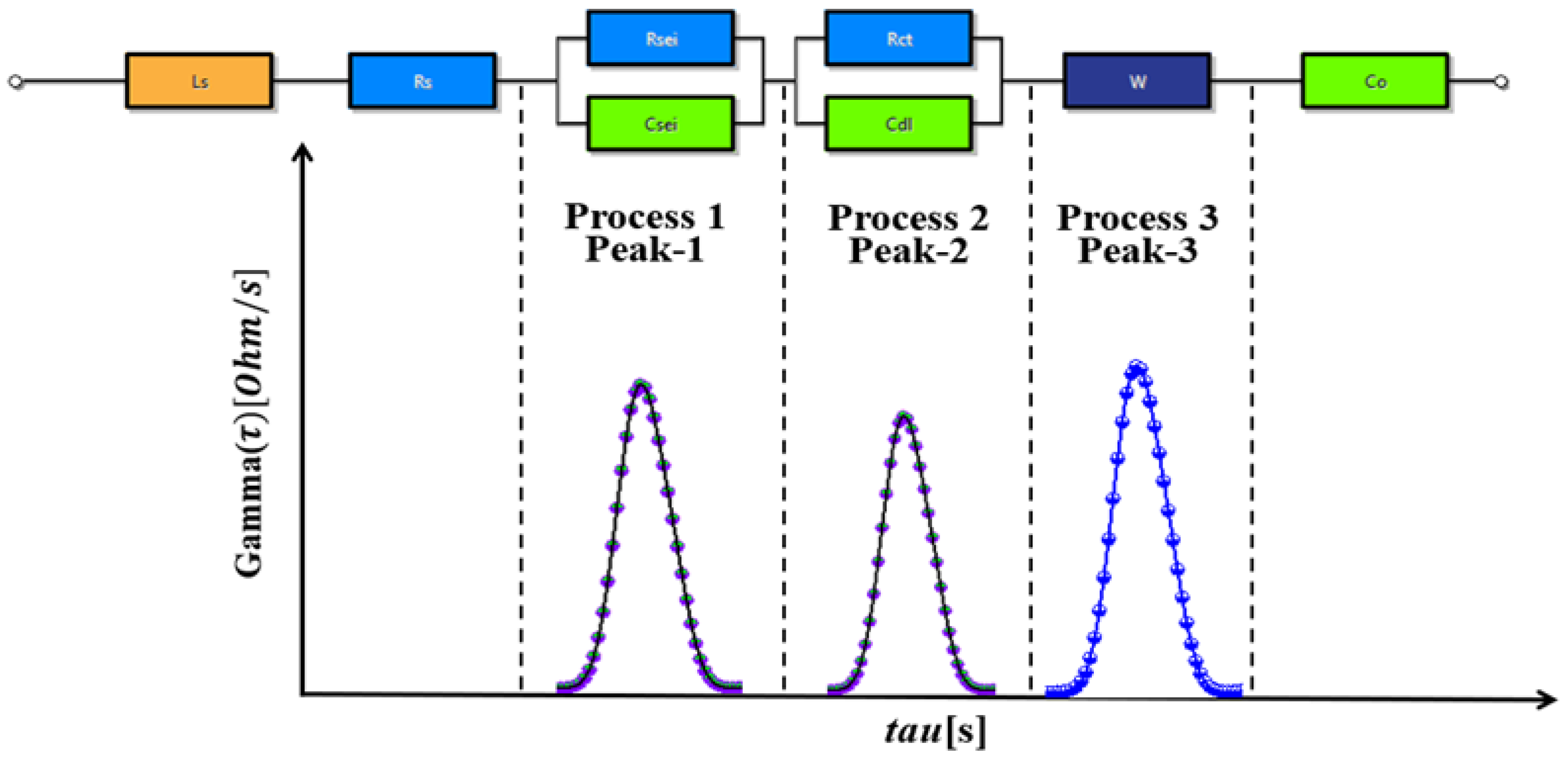
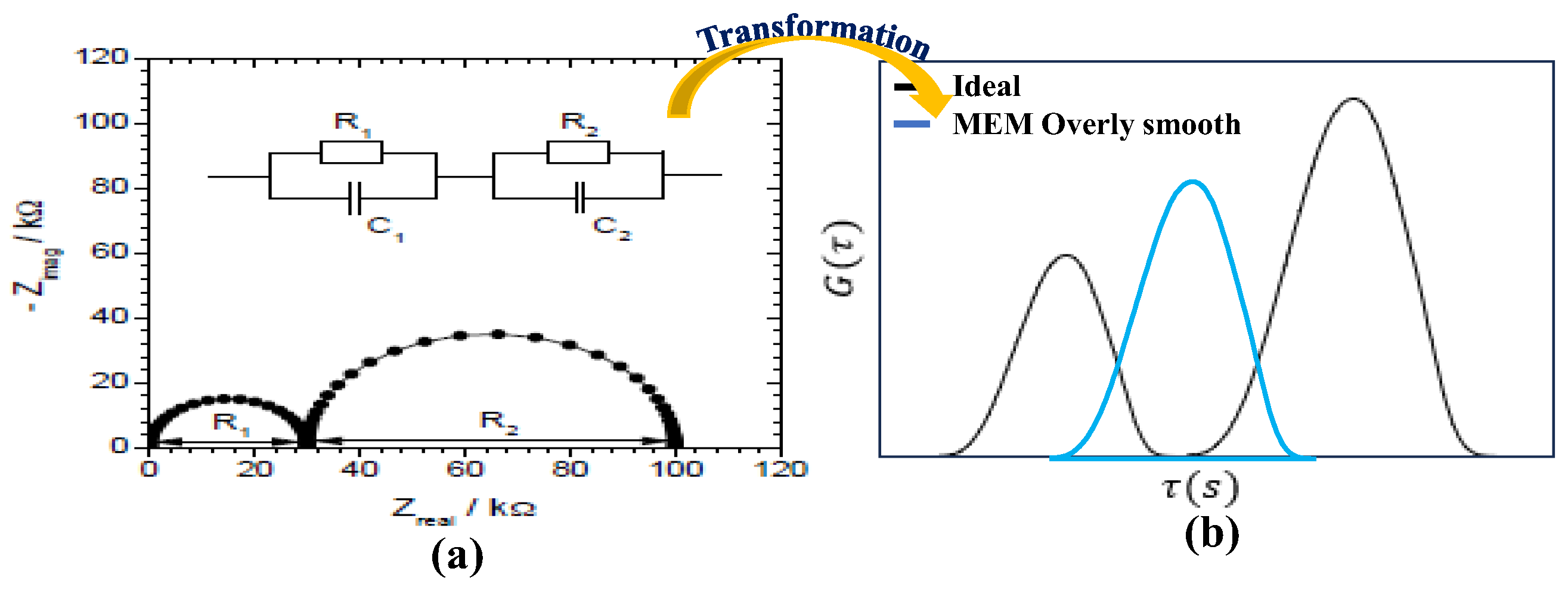
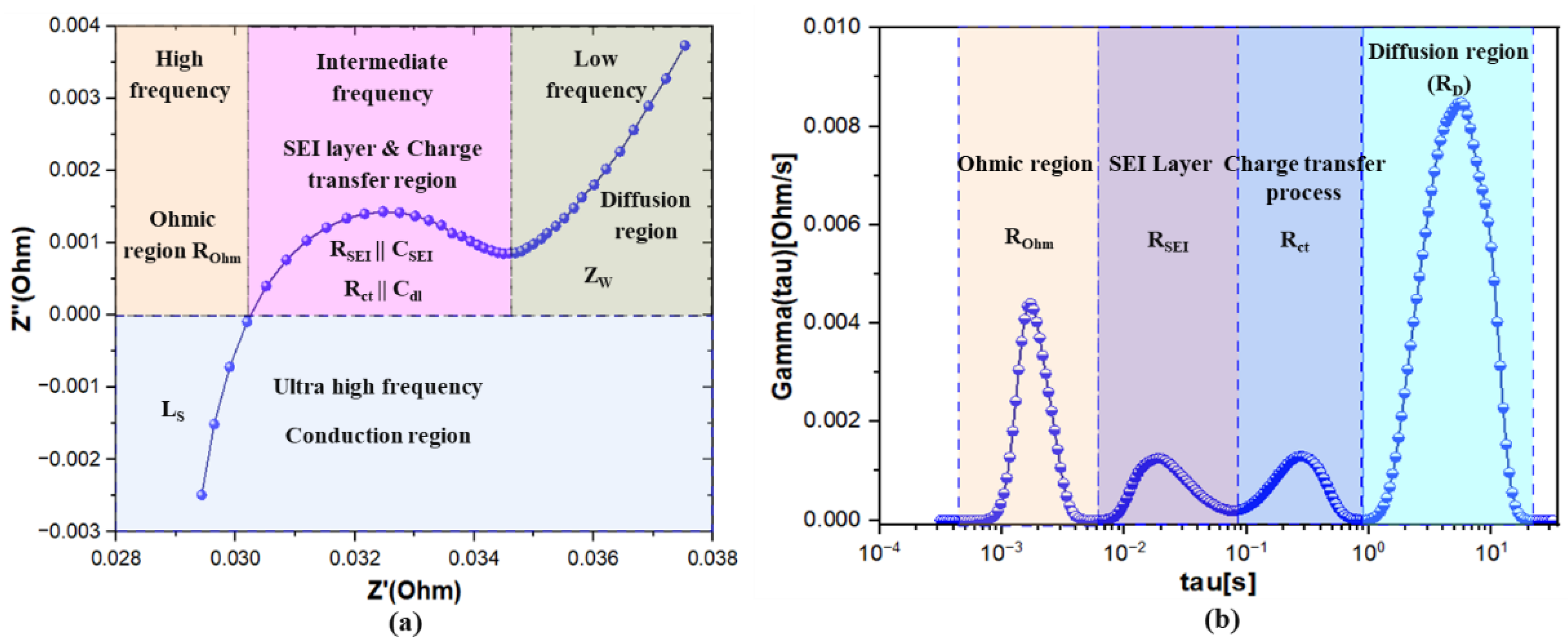
4.1. Correlation Between EIS Nyquist Plots and DRT Plots
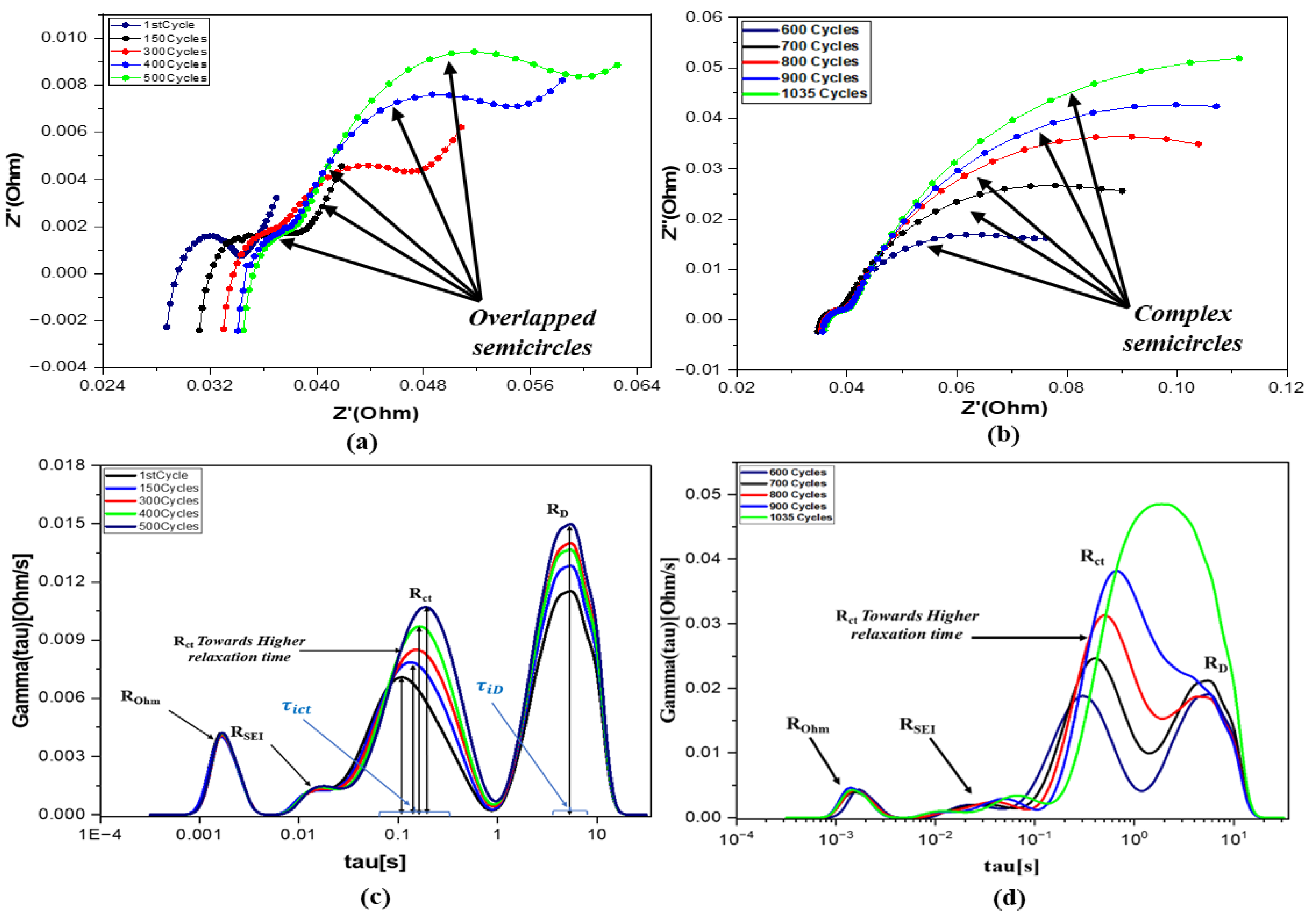
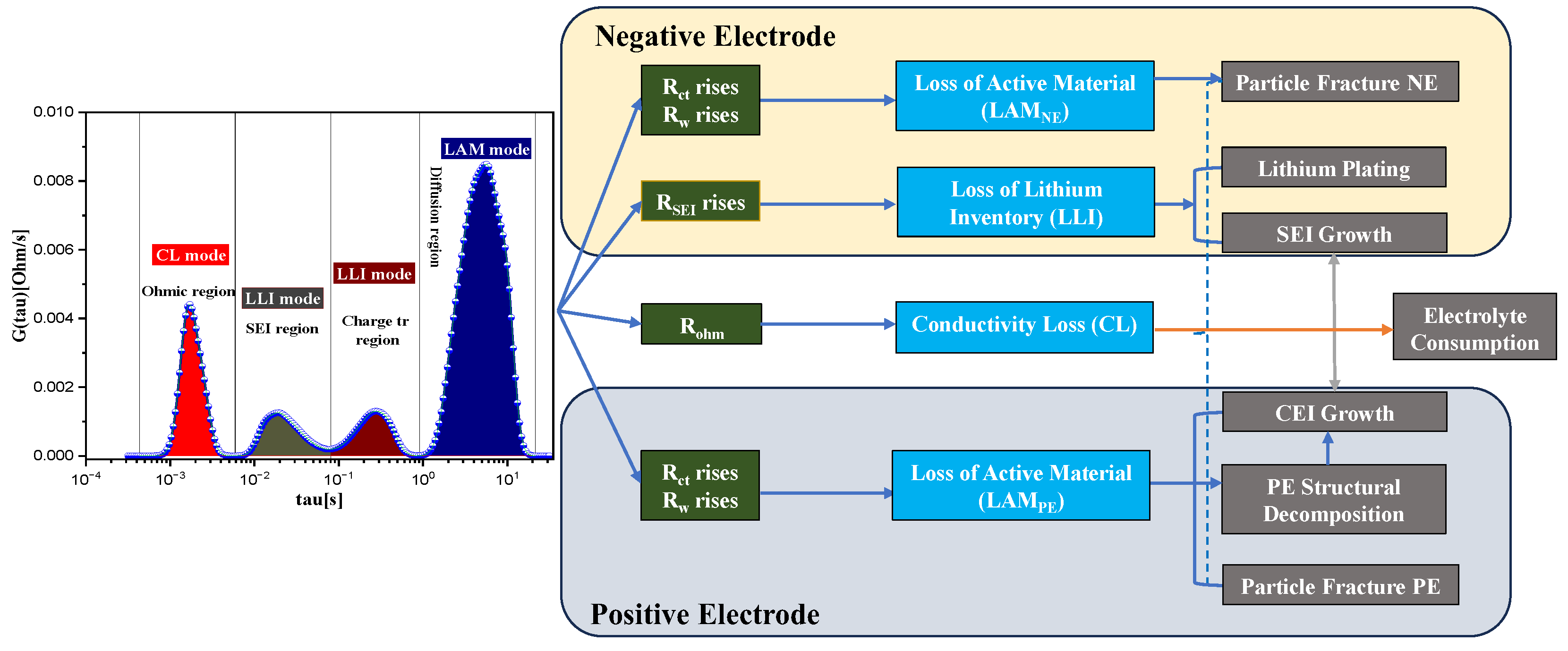
4.2. Detection of the Battery Status Using DRT Plot
5. Conclusions
References
- R. Xiong, L. Li, J. Tian, Towards a smarter battery management system: a critical review on battery state of health monitoring methods, J. Power Sources (2018) 405. [CrossRef]
- X. Feng, M. Ouyang, X. Liu, L. Lu, Y. Xia, X. He, Thermal runaway mechanism of lithium ion battery for electric vehicles: a review, Energy Storage Mater. 10 (2018). [CrossRef]
- Birkl CR, Roberts MR, Mcturk E, et al. Degradation diagnostics for lithium ion cells. J Power Sources 2017;341:373e86. [CrossRef]
- Sohaib, M., & Choi, W. (2024). Investigation of the Aging Phenomena in Lithium-Ion Batteries Using Distribution of Relaxation Time Analysis.
- Bloom I, Cole BW, Sohn JJ, et al. An accelerated calendar and cycle life study of Li-ion cells. J Power Sources 2001;101(2):238e47. [CrossRef]
- Wright RB, Christophersen JP, Motloch CG, et al. Power fade and capacity fade resulting from cycle-life testing of Advanced Technology Development Program lithium-ion batteries. J Power Sources 2003;119e121:865e9. [CrossRef]
- Belt J, Utgikar V, Bloom I. Calendar and PHEV cycle life aging of high-energy, lithium-ion cells containing blended spinel and layered-oxide cathodes. J Power Sources 2011;196(23):10213e21. [CrossRef]
- Pop V, Bergveld HJ, Regtien PPL, et al. Battery aging and its influence on the electromotive force. J Electrochem Soc 2007;154(8):A744e50. [CrossRef]
- Sarasketa-Zabala E, Aguesse F, Villarreal I, et al. Understanding lithium inventory loss and sudden performance fade in cylindrical cells during cycling with deep-discharge steps. J Phys Chem C 2015;119(2):896e906. [CrossRef]
- X. Wang, X. Wei, Q. Chen, J. Zhu and H. Dai, "Lithium-ion battery temperature on-line estimation based on fast impedance calculation", J. Energy Storage, vol. 26, Dec. 2019. [CrossRef]
- R. Al Nazer, V. Cattin, P. Granjon, M. Montaru and M. Ranieri, "Broadband identification of battery electrical impedance for HEVs", IEEE Trans. Veh. Technol., vol. 62, no. 7, pp. 2896-2905, Sep. 2013. [CrossRef]
- S. Gantenbein, M. Weiss and E. Ivers-Tiffée, "Impedance based time-domain modeling of lithium-ion batteries: Part I", J. Power Sources, vol. 379, pp. 317-327, Mar. 2018. [CrossRef]
- Dubarry M, Truchot C, Liaw BY. Synthesize battery degradation modes via a diagnostic and prognostic model. J Power Sources 2012;219:204e16. [CrossRef]
- Encinas-Sanchez ´ V, de Miguel MT, Lasanta MI, García-Martín G, P´erez FJ. Electrochemical impedance spectroscopy (EIS): an efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol Energy Mater Sol Cells 2019;191:157–63. [CrossRef]
- Sakita AMP, Della Noce R, Lavall RL. Potential-dependent electrochemical impedance spectroscopy as a powerful tool for evaluating supercapacitor electrode performance. J Electrochem Soc 2021;168:080525. [CrossRef]
- Han S Bin, Oh H, Lee WY, Won J, Chae S, Baek J. On-line eis measurement for highpower fuel cell systems using simulink real-time. Energies 2021;14. [CrossRef]
- Magar HS, Hassan RYA, Mulchandani A. Electrochemical impedance spectroscopy (Eis): principles, construction, and biosensing applications. Sensors 2021;21. [CrossRef]
- Nara H, Yokoshima T, Osaka T. Technology of electrochemical impedance spectroscopy for an energy-sustainable society. Curr Opin Electrochem 2020;20: 66–77. [CrossRef]
- Ivers-Tiffée, E., & Weber, A. (2017). Evaluation of electrochemical impedance spectra by the distribution of relaxation times. Journal of the Ceramic Society of Japan, 125(4), 193-201. [CrossRef]
- Weiß, Alexandra, et al. "Distribution of relaxation times analysis of high-temperature PEM fuel cell impedance spectra." Electrochimica Acta 230 (2017): 391-398. [CrossRef]
- Hörlin, Tom. "Deconvolution and maximum entropy in impedance spectroscopy of noninductive systems." Solid State Ionics 107.3-4 (1998): 241-253. [CrossRef]
- Boukamp, Bernard A. "Derivation of a Distribution Function of Relaxation Times for the (fractal) Finite Length Warburg." Electrochimica acta 252 (2017): 154-163. [CrossRef]
- Boukamp, Bernard A., and Aurélie Rolle. "Analysis and application of distribution of relaxation times in solid state ionics." Solid state ionics 302 (2017): 12-18. [CrossRef]
- J. A. A. Qahouq and Z. Xia, "Single-perturbation-cycle online battery impedance spectrum measurement method with closed-loop control of power converter", IEEE Trans. Ind. Electron., vol. 64, no. 9, pp. 7019-7029, Sep. 2017.
- S. K. Dam and V. John, "High-resolution converter for battery impedance spectroscopy", IEEE Trans. Ind. Appl., vol. 54, no. 2, pp. 1502-1512, Mar. 2018.
- Gaberˇsˇcek M. Understanding Li-based battery materials via electrochemical impedance spectroscopy. Nat Commun 2021;12:19–22.
- Shafiei Sabet P, Warnecke AJ, Meier F, Witzenhausen H, Martinez-Laserna E, Sauer DU. Non-invasive yet separate investigation of anode/cathode degradation of lithium-ion batteries (nickel–cobalt–manganese vs. graphite) due to accelerated aging. J Power Sources 2020;449:227369.
- R. Xiong, L. Li and J. Tian, "Towards a smarter battery management system: A critical review on battery state of health monitoring methods", J. Power Sources, vol. 405, pp. 18-29, Nov. 2018. [CrossRef]
- Li X, Ahmadi M, Collins L, Kalinin SV. Deconvolving distribution of relaxation times, resistances and inductance from electrochemical impedance spectroscopy via statistical model selection: exploiting structural-sparsity regularization and data-driven parameter tuning. Electrochim Acta 2019;313:570–83. [CrossRef]
- Huang, Jake & Sullivan, Neal & Zakutayev, Andriy & O’Hayre, Ryan. (2023). How reliable is distribution of relaxation times (DRT) analysis? A dual regression-classification perspective on DRT estimation, interpretation, and accuracy. Electrochimica Acta. 443. 141879. [CrossRef]
- Zhu, J., Knapp, M., Liu, X., Yan, P., Dai, H., Wei, X., & Ehrenberg, H. (2020). Low-temperature separating lithium-ion battery interfacial polarization based on distribution of relaxation times (DRT) of impedance. IEEE Transactions on Transportation Electrification, 7(2), 410-421. [CrossRef]
- Sohaib, M., & Choi, W. (2024). A Novel Aging Diagnosis Method Using Distribution of Relaxation Time.
- Zhang, Y., Tang, Q., Zhang, Y., Wang, J., Stimming, U., & Lee, A. A. (2020). Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nature communications, 11(1), 1706. [CrossRef]
- Pender, J. P., Jha, G., Youn, D. H., Ziegler, J. M., Andoni, I., Choi, E. J., ... & Mullins, C. B. (2020). Electrode degradation in lithium-ion batteries. ACS nano, 14(2), 1243-1295. [CrossRef]
| Property | Value |
|---|---|
| Chemistry | Nickel Manganese Cobalt |
| Type | 18650 |
| Capacity. max | 2,850 mAh |
| Nominal voltage | 3.65 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





