1. Introduction
The Vilsmeier-Haack reaction (VHR) plays a crucial role in modern organic synthesis [1, 2]. It facilitates the introduction of a formyl group into the core of electron-rich aromatic molecules such as phenols, pyrrole [
3], pyrazoles [
4] and other [
5]. Additionally, Vilsmeier reagent (VR) could interact with nonaromatic compound like alkenes, ketones, aldehydes and heterocycles [
6]. Formylopyrroles are important intermediates in the synthesis of pyrrole-containing molecules,
e.g. difluoroboron dyes like BODIPY, BOPHY,
etc [
7]. Using halogenated 2-formylopyrroles enables the preparation of halogenated pyrrole-containing intermediates. Commonly, halogenated 2-formylopyrroles could be obtained by formylation of 2-halopyrroles with VR [
8]. However, yields of this reaction are often low. It is well known, that VHR can lead to the formation of different unexpected products, depending on substrate and reaction conditions. Herein we report the isolation and characterization of (
Z)-1-(3,5-dichloro-2-pyrrolidin)-
N,N-dimethylmethanamine from the reaction mixture of 2-chloropyrrole VHR.
2. Results
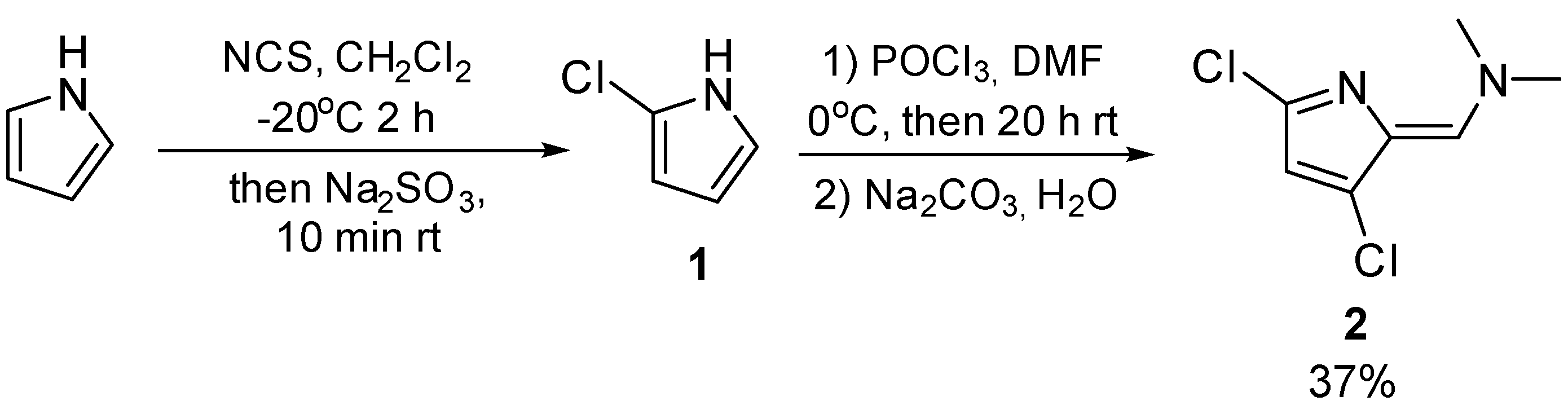
2.1. Synthesis of (Z)-1-(3,5-dichloro-2-pyrrolidin)-N,N-dimethylmethanamine
Typical procedures for 5-chloro-2-formylpyrrole preparation include chlorination of pyrrole and further VHR without purification of 2-chloropyrrole. The reaction yielded a complex mixture of products, from which (
Z)-1-(3,5-dichloro-2-pyrrolidin)-N,N-dimethylmethanamine was isolated by flash-chromatografy with a moderate yield (
Scheme 1).
The obtained product was characterized using 1H and 13C NMR spectroscopy, HRMS and X-ray crystallography. In 1H NMR spectrum singlet signals of methyl groups were observed at 3.31 and 3.66 ppm, singlet signals were also observed at 6.16 (=CHNMe2) and 7.17 ppm (CH of pyrrole fragment). In 13C NMR, all signals of alkene fragments are observed at 113–145 ppm for heterocyclic and vinylidene carbons, and methyl groups gave signals at 40.5 and 47.5 ppm.
2.2. XRD studies
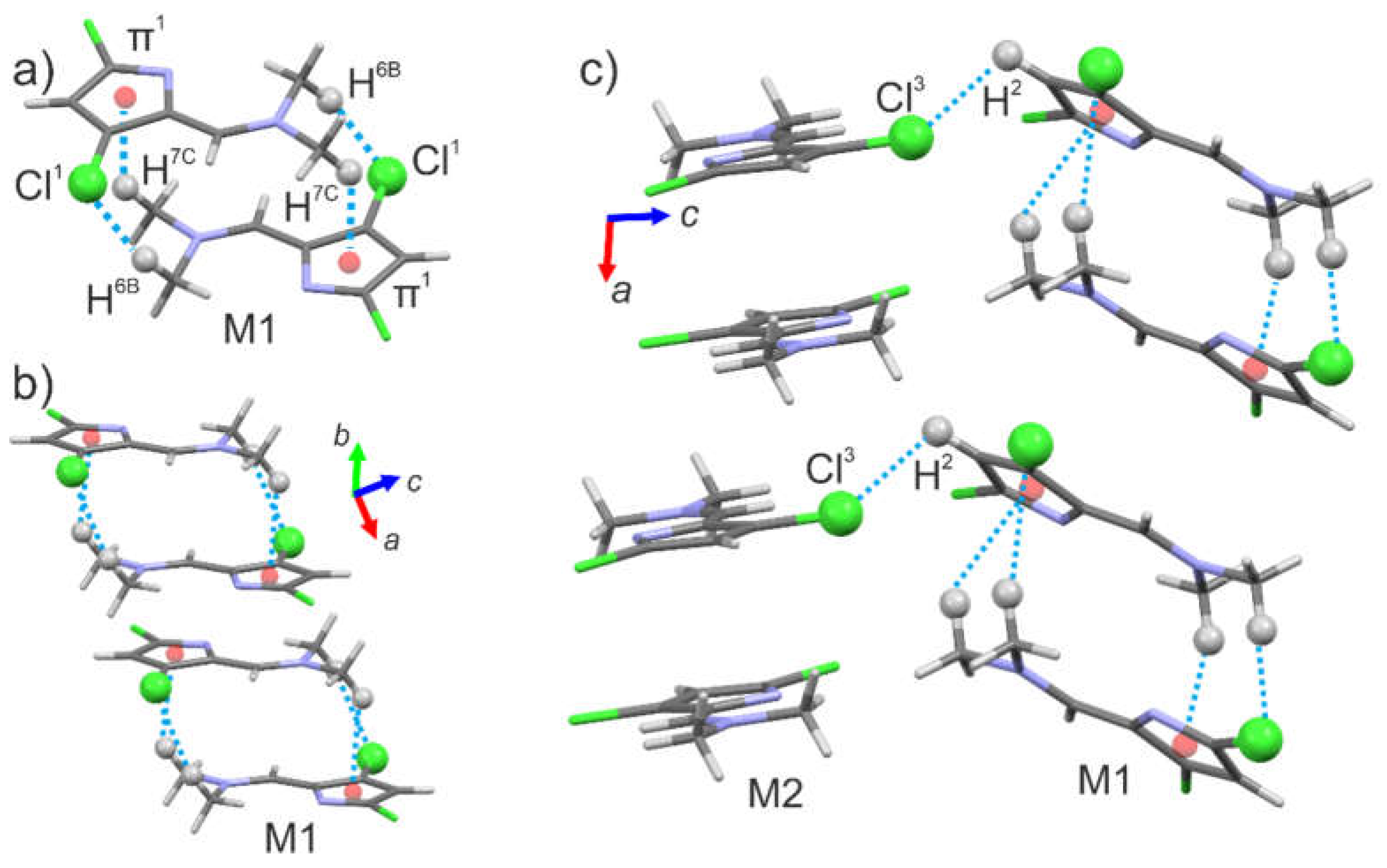
Single crystal X-ray studies were performed. Obtained substance in crystal has monoclinic
P2
1/
n space group with two molecules in the asymmetric unit M1 and M2. The fragment of the structure with corresponding labeling scheme is shown in
Figure 1a. The conformation of molecules is planar with intramolecular C-H···N interactions (C7-H···N2 (d
H···N2 = 2.20 Å) and C13-H···N4 (d
H···N4 = 2.22 Å)).
M1 molecules form dimer connected by C6-H···Cl1 and C7-H···π1 interactions (π1 is N2-C1-C2-C3-C4 ring), dimers are packing into chain along a-axis (Fig. 2a and b). M2 molecules also form dimers and chains along a-axis, but intermolecular interactions are absent. Chains M1 and M2 are connected to each other by C2-H···Cl3 interactions (Fig. 2c).
3. Materials and Methods
All chemicals were purchased from commercial sources and used without additional purification unless otherwise noted. The progress of reactions was monitored by thin-layer chromatography (TLC) on Sorbfil Silica 60 F254 on aluminum sheets with UV visualization. Column chromatography was performed by using 100–200 mesh silica gel. NMR spectra were recorded on Bruker Avance-400 (400.13 MHz for
1H, 100.62 MHz for
13C) spectrometer; chemical shifts of
1H and
13C{
1H} are provided in ppm, with solvent signals serving as the internal standard (
1H = 7.24 ppm and
13C = 77.16 ppm for CDCl
3). The masses of molecular ions were determined by high-resolution mass spectrometry (HRMS) by means of a DFS Thermo Scientific instrument (EI, 70 eV). X-ray diffraction data for compound was collected at 296 K using a Bruker KAPPA APEX II diffractometer with graphite monochromated MoK
α radiation. Absorption corrections were applied using SADABS [
9]. The structure was solved by SHELXT [
10]. Refinement was carried out by the full-matrix least-squares technique with SHELXL [
10] using OLEX2 [
11] software. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were calculated at idealized positions according to the riding model.
Synthesis of 2-chloro-1H-pyrrole (1).
5.17 ml (75 mmol) of pyrrole was added to 200 ml of dry CH2Cl2. The solution was cooled to -20oC. Then, to the cooled mixture a 10.46 g (78 mmol) of NCS was added. The obtained mixture was stirred for 10 min and was kept at the temperature -20oC for 2 hour. A 9.87 g (78 mmol) of Na2SO3 was added, the resulting mixture was stirred for 10 min. After that, 150 ml water and 100 ml CH2Cl2 were added, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (3 × 50 mL). The combined organic phase was washed with water (3 × 100 mL), dried over Na2SO4, filtered and evaporated under reduced pressure at room temperature. The product was used without purification.
1H NMR (δ, 300.13 MHz, CDCl3): 6.11 (m, 1H, NH-CH=CH), 6.21 (t, 1H, J = 3.4 Hz, NH-CCl=CH), 6.66 (m, 1H, NH-CH=CH), 8.08 (s, 1H, NH).
Synthesis of (Z)-1-(3,5-dichloro-2H-pyrrol-2-ylidene)-N,N-dimethylmethanamine (2).
To an ice-cooled solution of 4.86 mL (52 mmol) POCl3 a 4.03 mL (52 mmol) DMF was added dropwise an Ar atmosphere. After the addition, 70 ml of dry CH2Cl2 was added, and then a solution of 4.8 g (47 mmol) 2-chloro-1H-pyrrole in 50 ml dry CH2Cl2 was added dropwise while cooling to 0°C in an Ar atmosphere. The resulting mixture was stirred for 20 hours rt. After that, solution of 25 g Na2CO3 in 100 ml water was added, and the mixture was stirred for 50 min. Then, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 50 mL). The combined organic phase was washed with water (3 × 100 mL), dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel, eluent: EtOAc : Hex = 1 : 1. Light yellow solid. Yield = 2.27 g (37%).
1H NMR (δ, 300.13 MHz, CDCl3): 3.36 (s, 3H, Me1), 3.72 (s, 3H, Me2), 6.21 (s, 1H, Cy C-H), 7.22 (s, 1H, -C=CH-NMe2).
13C NMR (δ, 100.6 MHz, CDCl3): 40.5, 47.5, 113.1, 126.3, 131.8, 144.2, 144.4.
HRMS (EI, m/z): calculated for C7H835Cl2N2 - 190.0059, found - 190.0063
X-ray Structure Determination: crystal data for C7H8Cl2N2, M = 191.05 g mol–1, colourless elongated prism, crystal dimensions 1.00 × 0.62 × 0.18 mm, monoclinic, space group P21/n, a = 7.3574(3), b = 15.7753(6), c = 15.4311(6) Å, β = 100.599(2)°, V = 1760.46(12) Å3, Z/Z’ = 8/2, Dcalc = 1.442 g cm–3, R1 = 0.037, wR2 = 0.109, Rint = 0.036, S = 1.05. Data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2360994.
4. Conclusions
In summary, (Z)-1-(3,5-dichloro-2-pyrrolidin)-N,N-dimethylmethanamine was obtained as side product of 2-chloropyrrole formylation with Vilsmeier reagent.
Author Contributions
Conceptualization, A.V.; methodology, A.V.; investigation, D.R. and A.S.; writing—original draft preparation, A.V., D.R. and A.S.; writing—review and editing, A.V.; supervision, A.V.;. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by Novosibirsk Institute of Organic Chemistry programs № 1021052605821-9 and №122040800263-6 (X-ray studies).
Data Availability Statement
The X-Ray data are available in a publicly accessible repository.
Acknowledgments
Authors thank Multi-access Chemical Center in Novosibirsk Institute of Organic Chemistry for NMR and HRMS studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neena, N.; Chaudhri, V.; Singh, F. V.; China, H.; Dohi, T. Synthetic Utility of the Vilsmeier–Haack Reagent in Organic Synthesis Synlett 2023, 34, 777-792.
- Su, W.; Weng, Y.; Jiang, L.; Yang, Y.; Zhao, L.; Chen, Z.; Li, Z.; Li, J. (2010). Recent Progress in the Use of Vilsmeier-Type Reagents. Org. Prep. Proced. Int. 2010,; 42, 503–555.
- 2-Pyrrolealdehyde. Org. Synth. 1956, 36, 74. [CrossRef]
- Badalyan, K. S.; Akopyan, A. E.; Attaryan, H. S.; Asratyan, G. V. Vilsmeier-Haack formylation of 1H-pyrazoles Russ. J. Gen. Chem. 2014, 84, 793–795. [Google Scholar] [CrossRef]
- Chahal, M.; Dhillon, S.; Rani, P.; Kumari, G.; Aneja, D.K.; Kinger, M. Unravelling the Synthetic and Therapeutic Aspects of Five, Six and Fused Heterocycles Using Vilsmeier–Haack Reagent. RSC Adv. 2023, 13, 26604–26629. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Stanforth, S. P. The Vilsmeier reaction of non-aromatic compounds. In Organic Reactions; Jones, G., Stanforth, S. P., Eds.; John Wiley: New York, 2000; Volume 56, p 355. [Google Scholar]
- Yu, C.; Hao, E.; Sun, Y.; Jiao, L. Recent Advances in Highly Fluorescent Hydrazine-Inserted Pyrrole-Based Diboron-Anchoring Fluorophores: Synthesis and Properties. Synlett 2023, 35, 37–54. [Google Scholar] [CrossRef]
- Cordell, G. A. 2-Halopyrroles. Synthesis and chemistry. J. Org. Chem. 1975, 40, 3161–3169. [Google Scholar] [CrossRef]
- Sheldrick, G. M. SADABS, Program for area detector adsorption correction, Institute for Inorganic Chemistry, University of Goettingen: Goettingen, Germany, 1996.
- Sheldrick, G. M. , Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C., 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. , OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).