Submitted:
27 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
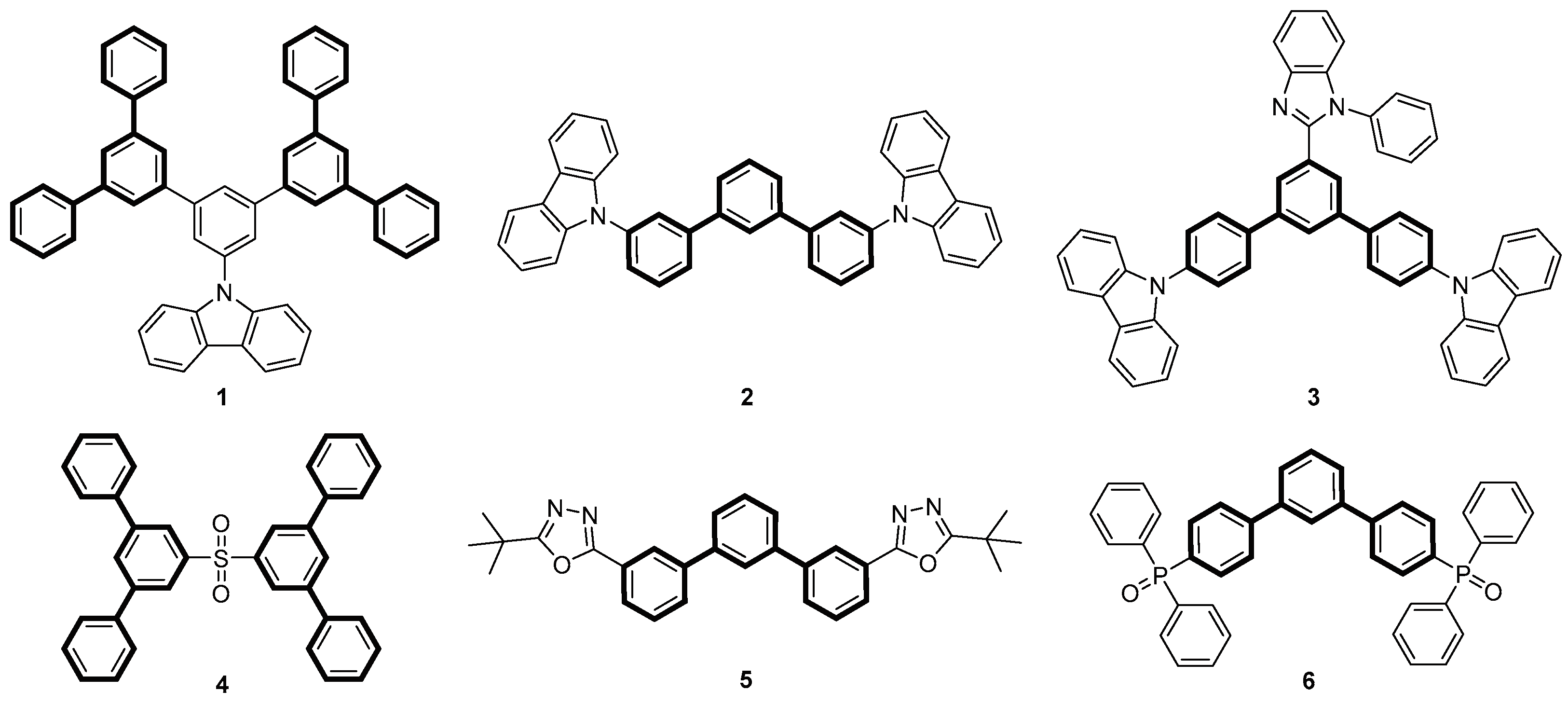
1. Introduction
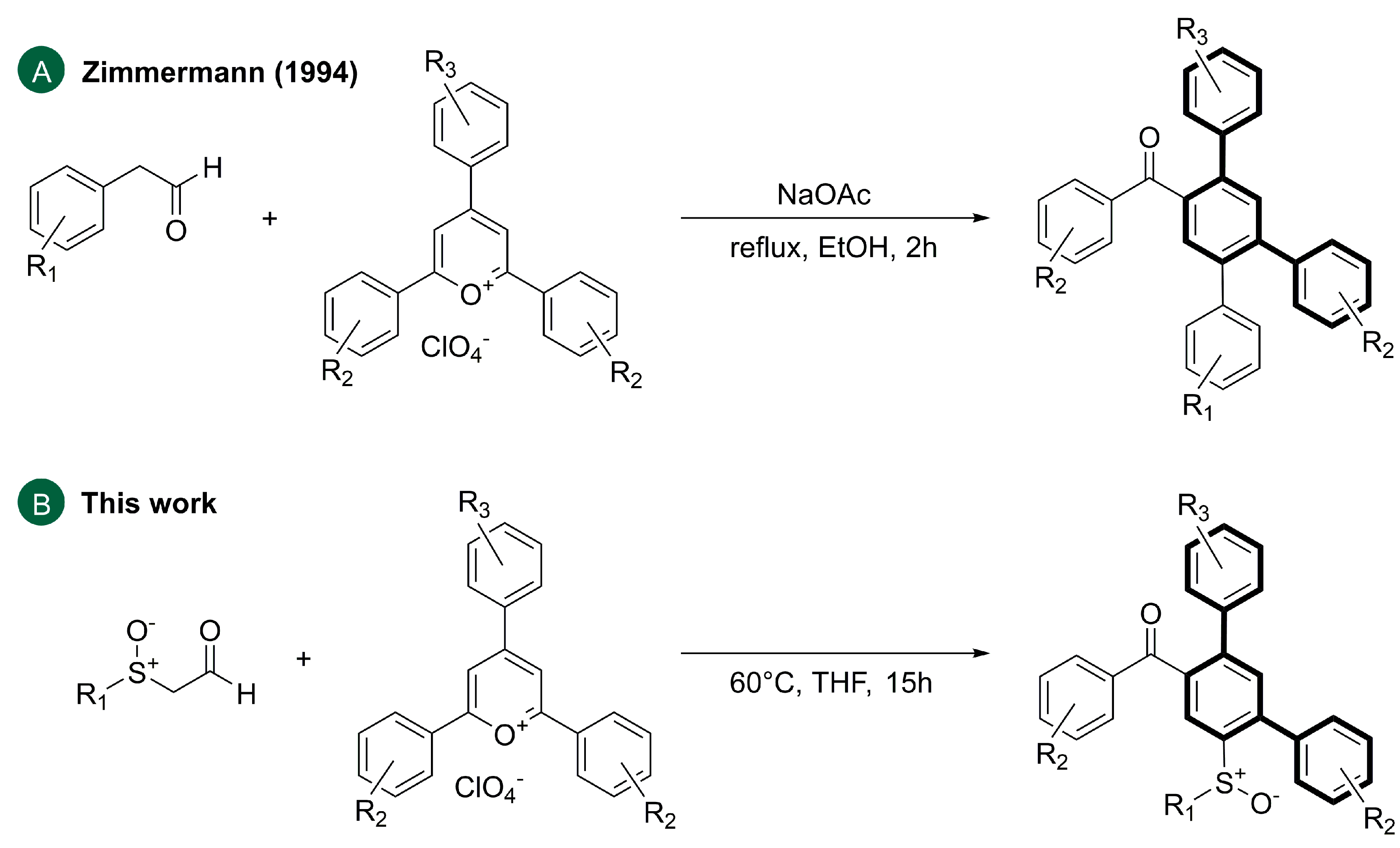
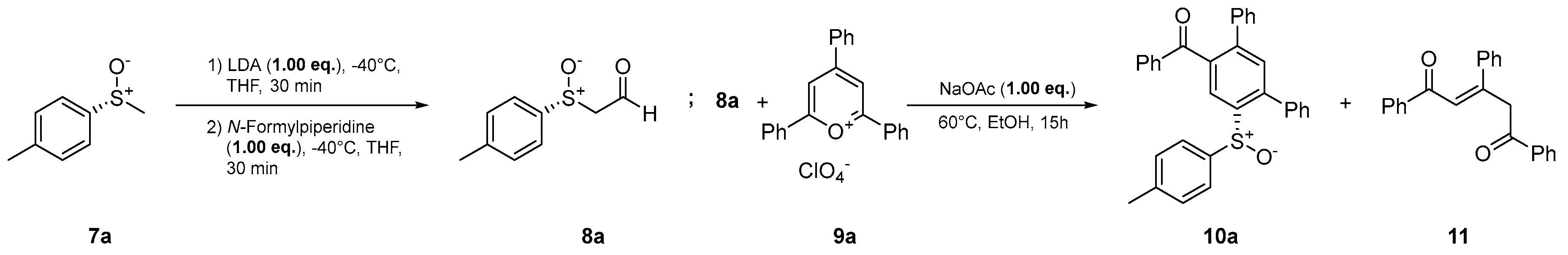
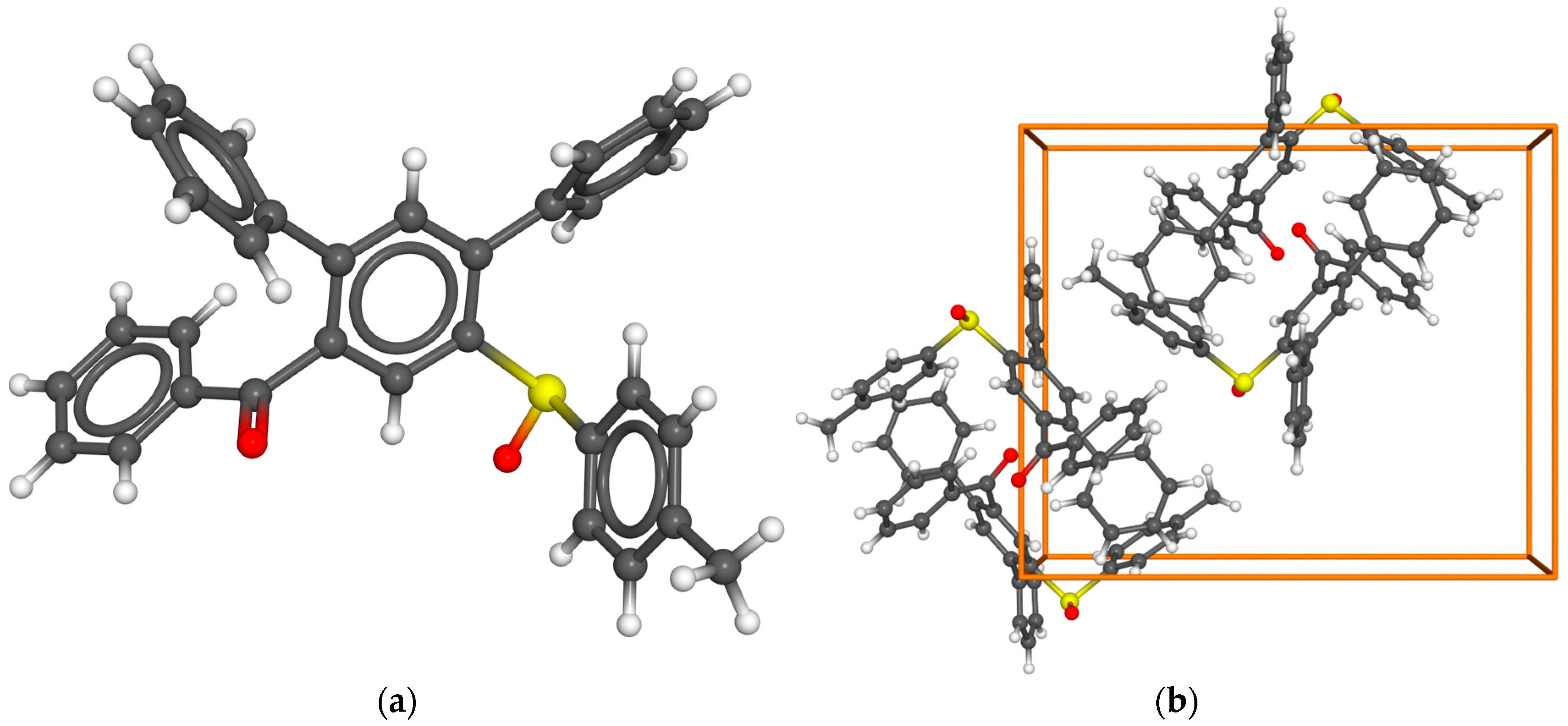
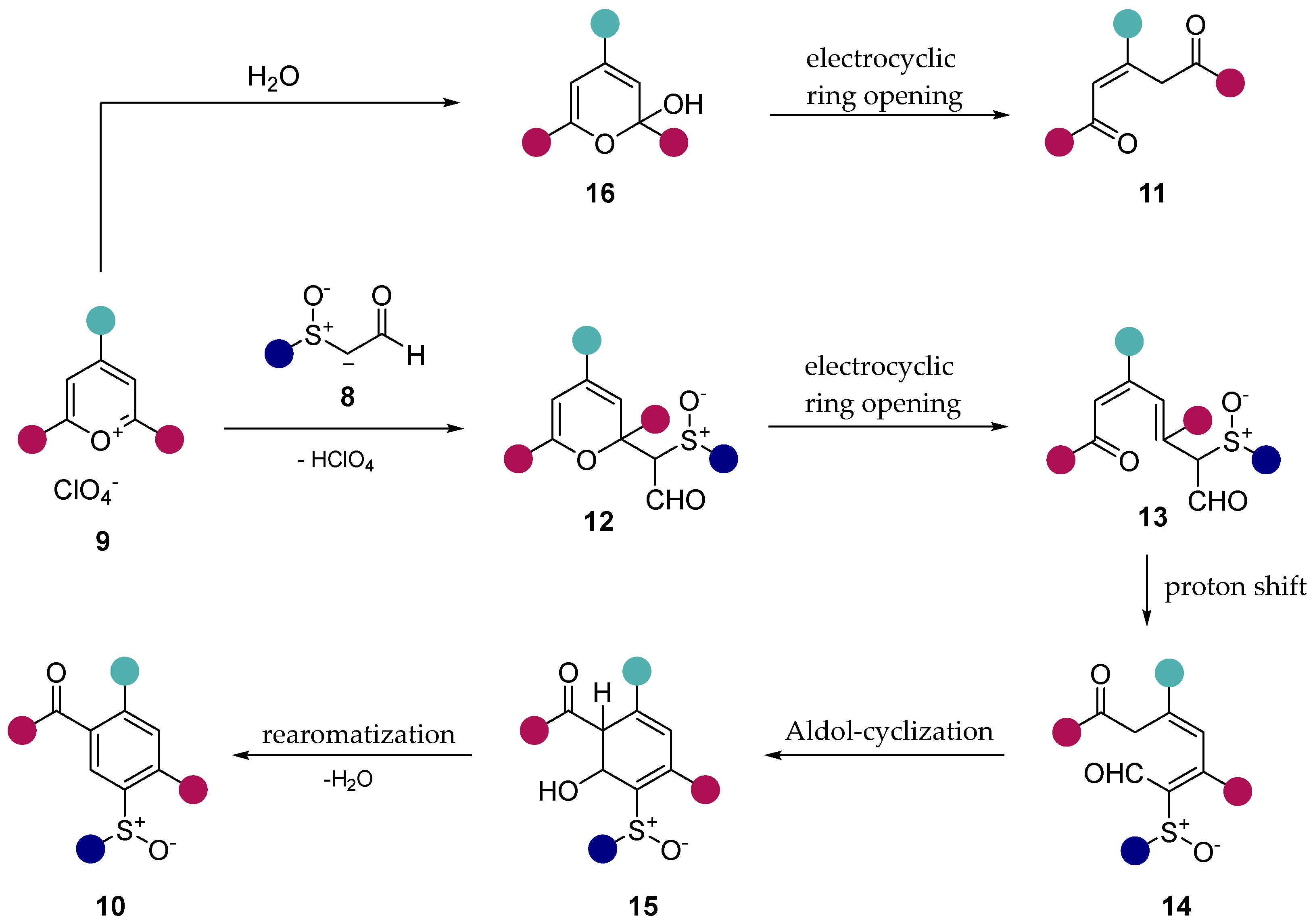
2. Results and Discussion
3. Conclusion
4. Materials and Methods
4.1. General Methods
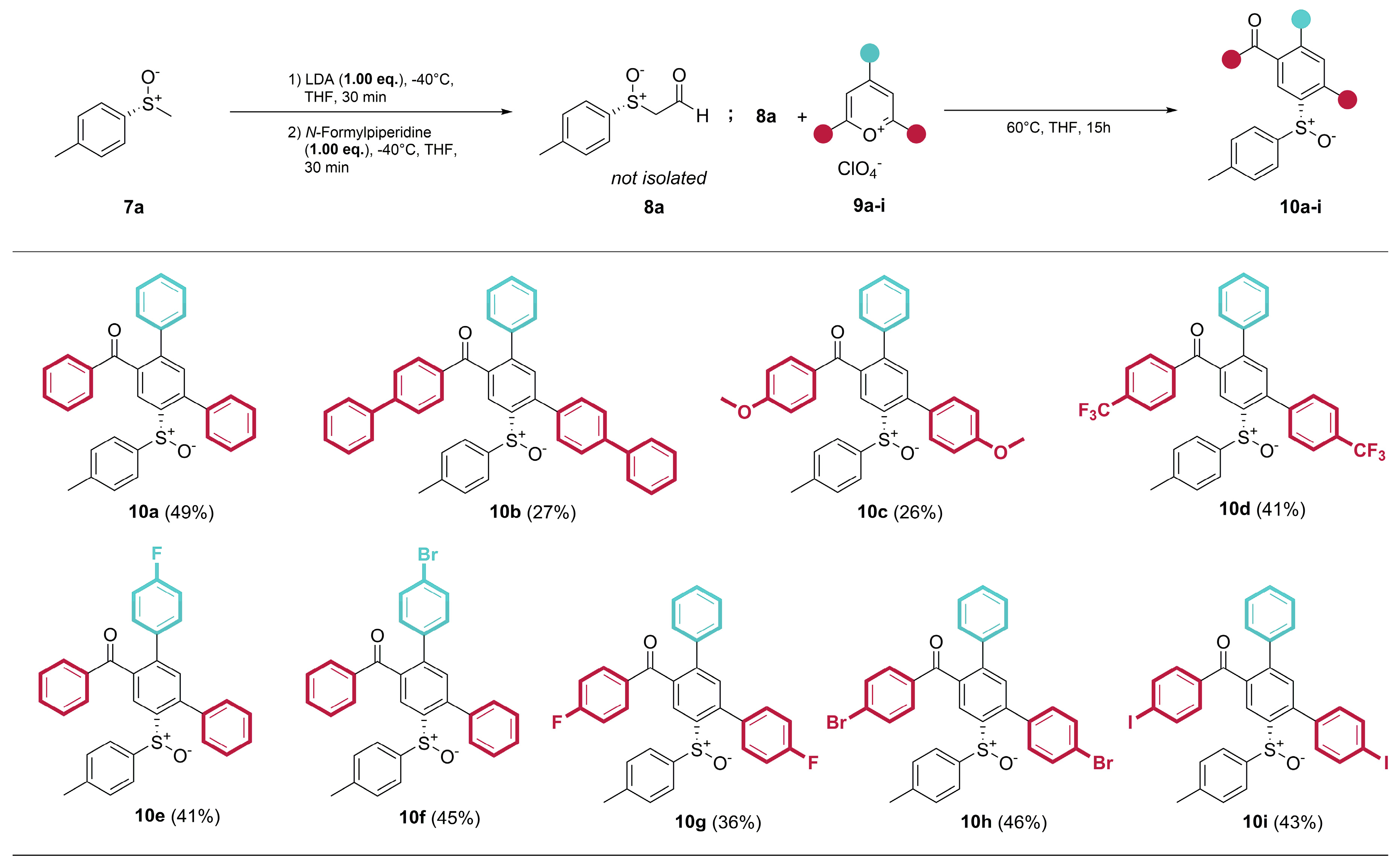
4.2. General procedure for the synthesis of the ring transformation products
- (R)-Phenyl(6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)methanone (10a) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 49% as an off-white solid. mp 186°C. Rf= 0.22 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1669, 1041, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.30 (s, 1H), 7.77 – 7.71 (m, 2H), 7.52 – 7.40 (m, 4H), 7.39 – 7.26 (m, 7H), 7.25 – 7.16 (m, 3H), 7.07 – 7.02 (m, 2H), 7.00 – 6.94 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 143.6q, 143.0q, 142.2q, 141.7q, 141.4q, 138.9q (2C), 137.4q, 137.0q, 133.4, 132.4, 130.2, 129.7, 129.6, 129.0, 128.7, 128.7, 128.5, 128.5, 128.1, 125.7, 124.6, 21.5 ppm. MS (ESI) (m/z): 473.16 [M+H]+, 495.14 [M+Na]+, 511.11 [M+K]+, 945.31 [2M+H]+, 967.29 [2M+Na]+, 983.26 [2M+K]+. HRMS (ESI) (m/z): Calcd for C32H24O2S [M+H]+ 473.15698; Found 473.15715. Anal. Calc. for C32H24O2S: C 81.33; H 5.12. Found C 81.32; H 5.34. = +28.27° (c 0.53; acetone).
- (R)-[1,1’-biphenyl]-4-yl(4’-(p-tolylsulfinyl)-[1,1’:3’,1’’:4’’,1’’’-quaterphenyl]-6’-yl)methanone (10b) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 27% as an off-white solid. mp 116°C. Rf= 0.24 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1598, 754, 695. 1H NMR (500 MHz, Chloroform-d): δ 8.34 (s, 1H), 7.85 (d, J = 8.5 Hz, 2H), 7.73 – 7.64 (m, 4H), 7.63 – 7.57 (m, 4H), 7.55 – 7.50 (m, 2H), 7.50 – 7.44 (m, 3H), 7.42 (d, J = 8.1 Hz, 4H), 7.34 (d, J = 6.6 Hz, 2H), 7.28 – 7.20 (m, 3H), 7.06 (s, 4H), 2.32 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.7q, 146.1q, 143.7q, 143.0q, 141.9q, 141.8q, 141.6q, 141.4q, 140.3q, 139.9q, 139.0q, 138.9q, 136.4q, 135.8q, 132.5, 130.8, 130.1, 129.8, 129.1, 129.0, 129.0, 128.6, 128.4, 128.1, 127.9, 127.4, 127.4, 127.2, 127.2, 125.7, 124.7, 21.5 ppm. MS (ESI) (m/z): 625.21 [M+H]+, 647.20 [M+Na]+, 1249.43 [2M+H]+, 1271.41 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C44H32O2S [M+H]+ 625.21958; Found 625.21987. Anal. Calc. for C44H32O2S: C 84.58; H 5.16. Found C 84.28; H 5.27. = -30.94° (c 0.48; acetone).
- (R)-(4-methoxy-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(4-methoxyphenyl)methanone (10c) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 26% as a yellow solid. mp 188°C. Rf= 0.06 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1594, 1248, 1169, 1026. 1H NMR (500 MHz, Chloroform-d): δ 8.24 (s, 1H), 7.76 (d, J = 9.0 Hz, 2H), 7.37 (s, 1H), 7.32 (m, 2H), 7.30 – 7.21 (m, 5H), 7.09 (d, J = 8.3 Hz, 2H), 7.05 (d, J = 8.3 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 9.0 Hz, 2H), 3.92 (s, 3H), 3.87 (s, 3H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 195.8q, 163.8q, 160.0q, 143.4q, 142.8q, 141.7q, 141.6q, 141.5q, 139.1q, 139.0q, 132.6, 132.5, 130.9, 130.1q, 129.9q, 129.7, 128.9, 128.5, 128.0, 125.6, 124.5, 114.1, 113.8, 55.6, 55.5, 21.5 ppm. MS (ESI) (m/z): 533.17 [M+H]+, 555.16 [M+Na]+, 1065.35 [2M+H]+, 1087.33 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C34H28O4S [M+H]+ 533.17811; Found 533.17854. Anal. Calc. for C34H28O4S: C 76.67; H 5.30. Found C 76.33; H 5.12. = +15.19° (c 0.25; acetone).
- (R)-(6’-(p-tolylsulfinyl)-4-(trifluoromethyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(4-(trifluoromethyl)phenyl)methanone (10d) Prepared from 300 mg (1.95 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 41% as an off-white solid. mp 91°C. Rf = 0.47 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1672, 1322, 1064. 1H NMR (500 MHz, Chloroform-d): δ 8.34 (s, 1H), 7.78 (d, J = 8.1 Hz, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 8.1 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.38 (s, 1H), 7.23 (m, 5H), 7.07 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 2.32 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.0q, 143.9q, 143.4q, 142.3q, 141.2q, 140.9q, 140.7q, 139.6q, 138.7q, 138.3q, 134.5q (d, 2JCF = 33 Hz), 132.3, 131.0q (d, 2JCF = 33 Hz), 130.2, 130.0 (2C), 129.0, 128.8, 128.5 , 125.9, 125.7 (q, 3JCF = 3.9 Hz), 125.5 (q, 3JCF = 3.9 Hz), 125.3, 124.0q (1JCF = 271 Hz), 123.5q (1JCF = 271 Hz), 21.5 ppm. 19F NMR (471 MHz, Chloroform-d) δ -62.6, -63.2 ppm. MS (ESI) (m/z): 609.13 [M+H]+, 631.11 [M+Na]+, 647.09 [M+K]+, 1217.26 [2M+H]+, 1239.24 [2M+Na]+, 1255.21 [2M+K]+. HRMS (ESI) (m/z): Calcd for C34H22F6O2S [M+H]+ 609.13175; Found 609.13177. Anal. Calc. for C34H22F6O2S: C 67.10; H 3.64. Found C 67.17; H 3.66. = +8.70° (c 0.53; Aceton).
- (R)-(4’’-fluoro-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10e) Prepared from 600 mg (3.98 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 41% as an off-white solid. mp 164°C. Rf = 0.20 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1667, 1039. 1H NMR (500 MHz, Chloroform-d): δ 8.29 (s, 1H), 7.77 – 7.69 (m, 2H), 7.56 – 7.48 (m, 1H), 7.46 – 7.41 (m, 3H), 7.40 – 7.34 (m, 2H), 7.33 (s, 1H), 7.32 – 7.28 (m, 2H), 7.27 – 7.22 (m, 2H), 7.04 (d, J = 8.0 Hz, 2H), 7.00 – 6.94 (m, 2H), 6.94 – 6.88 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 162.6q (d, 1JCF = 248.0 Hz), 143.1q, 142.5q, 142.3q, 141.8q, 141.3q, 138.8q, 137.3q, 136.9q, 135.0q (d, 4JCF = 3.3 Hz), 133.6, 132.4, 130.7 (3JCF = 8.2 Hz), 130.2, 129.8, 129.6, 128.8, 128.6, 128.5, 126.9, 125.7, 124.6, 115.6 (d, 2JCF = 21.6 Hz), 21.5 ppm. 19F NMR (471 MHz, Chloroform-d) δ -113.8 ppm. MS (ESI) (m/z): 491.15 [M+H]+, 513.13 [M+Na]+, 529.10 [M+K]+, 981.29 [2M+H]+, 1003.28 [2M+Na]+, 1019.24 [2M+K]+. HRMS (ESI) (m/z): Calcd for C32H23FO2S [M+H]+ 491.14756; Found 491.14762. Anal. Calc. for C32H23FO2S: C 78.34; H 4.73. Found C 78.46; H 4.96. = +29.52° (c 0.49; acetone).
- (R)-(4’’-bromo-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10f) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 46% as a white solid. mp 167°C. Rf = 0.21 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1660, 1046. 1H NMR (500 MHz, Chloroform-d): δ 8.29 (s, 1H), 7.77 – 7.72 (m, 2H), 7.59 – 7.50 (m, 1H), 7.49 – 7.34 (m, 7H), 7.32 (s, 1H), 7.31 – 7.27 (m, 2H), 7.19 – 7.11 (m, 2H), 7.04 (d, J = 8.0 Hz, 2H), 6.99 – 6.89 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.9q, 143.4q, 142.4q, 142.3q, 141.8q, 141.2q, 138.7q, 137.9q, 137.2q, 136.9q, 133.7, 132.3, 131.7, 130.5, 130.2, 129.8, 129.6, 128.8, 128.8, 128.6, 125.6, 124.6, 122.6q, 21.5 ppm. MS (ESI) (m/z): 551.07 [M+H]+, 573.05 [M+Na]+, 1101.13 [2M+H]+, 1123.11 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C32H23BrO2S [M+H]+ 551.06749; Found 551.06752. Anal. Calc. for C32H23BrO2S: C 69.69; H 4.20. Found C 69.21; H 3.99. = +25.77° (c 0.50; acetone).
- (R)-(4-fluoro-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(4-fluorophenyl)methanone (10g) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 36% as an off-white solid. mp 190°C. Rf = 0.26 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1663, 1594, 1226. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.77 – 7.64 (m, 2H), 7.29 – 7.17 (m, 7H), 7.10 (t, J = 8.6 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 6.99 (t, J = 8.6 Hz, 4H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 195.6q, 165.9q (d, 1JCF = 256.1 Hz), 163.12q (d, 1JCF = 248.9 Hz), 143.59q, 143.20q, 142.11q, 141.33q, 141.11q, 138.83q, 138.69q, 133.5q (d, 4JCF = 5.46 Hz), 133.4q (d, 4JCF = 5.95 Hz), 132.7 (d, 3JCF = 9.5 Hz), 132.52, 131.4 (d, 3JCF = 8.1 Hz), 129.92, 128.96, 128.68, 128.33, 125.82, 124.77, 115.8 (d, 2JCF = 22.15 Hz), 115.7 (d, 2JCF = 21.91 Hz), 21.56 ppm. 19F NMR (471 MHz, Chloroform-d) δ -104.2, -112.5 ppm. MS (ESI) (m/z): 509.14 [M+H]+, 531.12 [M+Na]+, 1017.27 [2M+H]+, 1039.25 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C32H22F2O2S [M+H]+ 509.13813; Found 509.13831. Anal. Calc. for C32H22F2O2S: C 75.57; H 4.36. Found C 75.66; H 4.38. = +28.14° (c 0.50; acetone).
- (R)-(4-bromo-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(4-bromophenyl)methanone (10h) Prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 46% as an off-white solid. mp 235°C. Rf = 0.41 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1667, 1039. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.60 – 7.51 (m, 4H), 7.47 (d, J = 8.5 Hz, 2H), 7.33 (s, 1H), 7.23 (s, 5H), 7.18 (d, J = 8.5 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 7.03 (d, J = 8.3 Hz, 2H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): 196.1q, 143.7q, 143.1q, 142.2q, 141.2q, 141.0q, 138.7q, 138.5q, 136.2q, 135.7q, 132.3, 131.9, 131.8, 131.5, 131.2, 129.9, 128.9, 128.8q, 128.7, 128.4, 125.7, 124.9, 123.2q, 21.5 ppm. MS (ESI) (m/z): 628.98 [M+H]+, 650.96 [M+Na]+, 666.93 [M+K]+, 1256.95 [2M+H]+, 1278.93 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C32H22Br2O2S [M+H]+ 628.97800; Found 628.97792. Anal. Calc. for C32H22Br2O2S: C 60.97; H 3.52. Found C 60.89; H 3.66. = -19.41° (c 0.50; acetone).
- (R)-(4-iodo-6’-(p-tolylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(4-iodophenyl)methanone (10i) Prepared from 300 mg (1.95 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield 43% as an off-white solid. mp 234°C. Rf= 0.36 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1659, 1038, 956. 1H NMR (500 MHz, Chloroform-d): δ 8.25 (s, 1H), 7.81 – 7.75 (m, 2H), 7.73 – 7.66 (m, 2H), 7.43 – 7.36 (m, 2H), 7.33 (s, 1H), 7.23 (m, 5H), 7.09 (d, J = 8.0 Hz, 2H), 7.07 – 7.00 (m, 4H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.2q, 143.6q, 142.9q, 142.0q, 141.2q, 140.9q, 138.5q, 138.4q, 137.8, 137.7, 136.7, 136.1, 132.1, 131.2, 131.1, 129.8, 128.8, 128.6, 128.3, 125.6, 124.8, 101.7q, 94.7q, 21.4 ppm. MS (ESI) (m/z): 724.95 [M+H]+, 746.93 [M+Na]+. HRMS (ESI) (m/z): Calcd for C32H22I2O2S [M+H]+ 724.95027; Found 724.95074. Anal. Calc. for C32H22I2O2S: C 53.06; H 3.06. Found C 53.04 ; H 2.99. = -32.45° (c 0.26; acetone).
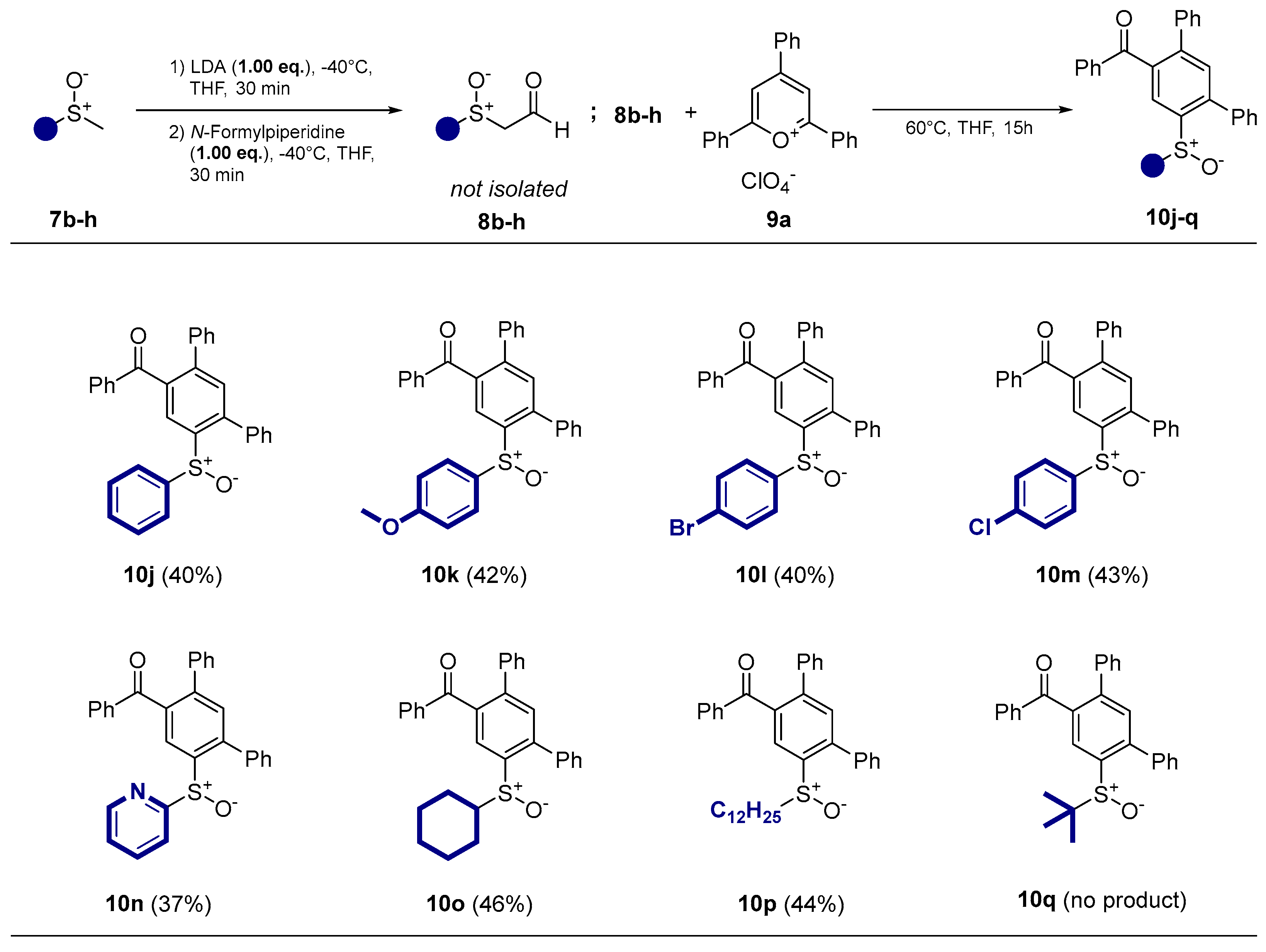
- Phenyl(6’-(phenylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)methanone (10j) Prepared from 309 mg (2.20 mmol) methylphenylsulfoxide. Yield 39% as an off-white solid. mp 140°C. Rf= 0.19 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1663, 1046. 1H NMR (500 MHz, Chloroform-d): δ 8.31 (s, 1H), 7.79 – 7.72 (m, 2H), 7.56 – 7.18 (m, 17H), 7.17 – 7.04 (m, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 144.5q, 143.8q, 142.7q, 142.3q, 139.0q, 138.9q, 137.4q, 137.0q, 133.4, 132.5, 131.2, 130.2, 129.6, 129.1, 129.0, 128.8, 128.8, 128.6, 128.5, 128.1, 125.6, 124.7 ppm. MS (ESI) (m/z): 459.14 [M+H]+. HRMS (ESI) (m/z): Calcd for C31H22O2S [M+H]+ 459.1413; Found 459.1414.
- (6’-((4-methoxyphenyl)sulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10k) prepared from 355 mg (2.09 mmol) 4-methoxyphenylmethylsulfoxide. Yield 42% as an off-white solid. mp 94°C. Rf= 0.10 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1664, 1250, 1045, 698. 1H NMR (500 MHz, Chloroform-d): δ 8.35 (s, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.57 – 7.48 (m, 1H), 7.45 – 7.37 (m, 6H), 7.33 – 7.27 (m, 4H), 7.27 – 7.16 (m, 3H), 7.02 (d, J = 8.8 Hz, 2H), 6.75 (d, J = 8.9 Hz, 2H), 3.79 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 161.8q, 143.4q, 142.9q, 142.0q, 138.8q, 138.7q, 137.3q, 136.9q, 135.5q, 133.3, 132.3, 130.1, 129.4, 128.9, 128.6, 128.5, 128.4, 128.4, 127.9, 127.7, 124.3, 114.4, 55.4 ppm. MS (ESI) (m/z): 489.15 [M+H]+, 511.13 [M+Na]+, 527.11 [M+K]+, 977.30 [2M+H]+, 999.28 [2M+Na]+, 1015.25 [2M+K]+. HRMS (ESI) (m/z): Calcd for C32H24O3S [M+H]+ 489.15189; Found 489.15195. Anal. Calc. for C32H24O3S: C 78.66; H 4.95. Found C 78.53; H 5.01.
- (6’-((4-bromophenyl)sulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10l) prepared from 438 mg (2.00 mmol) 4-bromophenylmethylsulfoxid. Yield 43% as a white solid. mp 92°C. Rf= 0.34 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1664, 1048, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.27 (s, 1H), 7.76 – 7.68 (m, 2H), 7.53 – 7.43 (m, 4H), 7.39 (s, 1H), 7.38 – 7.26 (m, 6H), 7.25 – 7.18 (m, 5H), 7.03 – 6.95 (m, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 144.0q, 143.0q, 142.4q, 142.1q, 139.1q, 138.7q, 137.5q, 137.2q, 136.9q, 133.5, 132.6, 130.1, 129.6, 129.3, 129.0, 128.9, 128.6, 128.5, 128.2, 126.9, 124.5 ppm. MS (ESI) (m/z): 537.05 [M+H]+, 559.03 [M+Na]+, 575.00 [M+K]+, 1073.09 [2M+H]+, 1095.07 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C31H21BrO2S [M+H]+ 537.05184; Found 537.05130. Anal. Calc. for C31H21BrO2S: C 75.52; H 4.29. Found C 75.48; H 4.32.
- (6’-((4-chlorophenyl)sulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10m) prepared from 350 mg (2.00 mmol) 4-chlorophenylmethylsulfoxid. Yield 40 % as a white solid. mp 122°C. Rf= 0.34 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1664, 1049, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.53 – 7.43 (m, 4H), 7.41 – 7.30 (m, 7H), 7.28 (m, 2H), 7.25 – 7.19 (m, 3H), 6.92 (d, J = 8.0 Hz, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 144.0q, 143.7q, 142.3q, 142.1q, 139.1q, 138.7q, 137.2q, 136.9q, 133.5, 132.6, 132.3, 130.1, 129.6, 129.0, 128.9, 128.6, 128.5, 128.2, 127.0, 125.8q, 124.5 ppm. MS (ESI) (m/z): 493.10 [M+H]+, 515.08 [M+Na]+, 531.05 [M+K]+, 985.19 [2M+H]+, 1007.17 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C31H21ClO2S [M+H]+ 493.10236; Found 493.10232. Anal. Calc. for C31H21ClO2S: C 69.28; H 3.94. Found C 69.42; H 3.80.
- Phenyl(6’-(pyridin-2-ylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)methanone (10n) prepared from 300 mg (2.12 mmol) 2-pyridylmethylsulfoxid. Yield 36% as a white solid. mp 110°C. Rf= 0.04 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 1659, 1048, 689. 1H NMR (500 MHz, Chloroform-d): δ 8.44 (m, 1H), 7.80 (s, 1H), 7.78 – 7.69 (m, 2H), 7.65 – 7.58 (m, 2H), 7.56 – 7.52 (m, 2H), 7.42 (s, 1H), 7.41 – 7.32 (m, 4H), 7.24 – 7.14 (m, 5H), 7.14 – 7.06 (m, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.9q, 165.5q, 150.0, 144.4q, 144.2q, 142.1q, 138.9q (2C), 138.1, 137.6q, 136.9q, 133.3, 132.5, 130.5, 130.1, 129.0, 128.7, 128.5, 128.4, 128.4, 128.2, 127.3, 124.7, 120.2 ppm. MS (ESI) (m/z): 460.13 [M+H]+. HRMS (ESI) (m/z): Calcd for C30H21NO2S [M+H]+ 460.1366; Found 460.1368. Anal. Calc. for C30H21NO2S: C 78.41 ; H 4.61; N 3.05. Found C 78.35 ; H 4.67; N 3.07.
- (6’-(cyclohexylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10o) prepared from 304 mg (2.08 mmol) cyclohexylmethylsulfoxid. Yield 46% as a white solid. mp 160°C. Rf= 0.22 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 2926, 1664, 1046, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.11 (s, 1H), 7.77 – 7.69 (m, 2H), 7.52 – 7.42 (m, 7H), 7.38 – 7.32 (m, 4H), 7.28 – 7.20 (m, 3H), 2.27 (m, 1H), 1.84 – 1.73 (m, 1H), 1.70 – 1.61 (m, 1H), 1.60 – 1.50 (m, 2H), 1.48 – 1.16 (m, 3H), 1.16 – 0.99 (m, 3H), 0.92 – 0.81 (m, 1H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.3q, 143.6q, 142.1q, 139.3q, 138.9q, 138.6q, 137.6q, 137.0q, 133.4, 132.3, 130.1, 129.3, 129.0, 128.9, 128.7, 128.6, 128.4, 128.1, 126.0, 60.7, 27.3, 25.8, 25.3, 25.3, 22.8 ppm. MS (ESI) (m/z): 465.18 [M+H]+, 487.17 [M+Na]+, 503.14 [M+K]+, 951.35 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C31H28O2S 465.18828; Found 465.18810. Anal. Calc. for C31H28O2S: C 80.14; H 6.07. Found C 80.22; H 6.02.
- (6’-(dodecylsulfinyl)-[1,1’:3’,1’’-terphenyl]-4’-yl)(phenyl)methanone (10p) prepared from 465 mg (2.00 mmol) 1-dodecylmethylsulfoxid. Yield 44% as a yellow oil. Rf= 0.40 (20% ethyl acetate in n-pentane). IR (ATR) (cm-1): 2922, 1666, 1047, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.23 (s, 1H), 7.78 (d, J = 8.5 Hz, 2H), 7.58 – 7.45 (m, 6H), 7.45 – 7.33 (m, 4H), 7.28 (dd, J = 14.7, 7.7 Hz, 3H), 2.59 (dd, J = 14.0, 8.0 Hz, 1H), 2.56 – 2.39 (m, 1H), 1.72 – 1.58 (m, 1H), 1.54 – 1.41 (m, 1H), 1.39 – 1.07 (m, 18H), 0.93 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.3q, 143.6q, 141.3q, 141.3q, 139.0q, 138.9q, 137.3q, 137.0q, 133.4, 132.3, 130.1, 129.1, 129.1, 129.0, 128.9, 128.6, 128.5, 128.4, 128.1, 125.0, 54.9, 32.0, 29.7 (2C), 29.6, 29.4, 29.4, 29.0, 28.3, 22.8, 22.2, 14.2 ppm. MS (ESI) (m/z): 551.30 [M+H]+, 573.28 [M+Na]+, 1001.59 [2M+H]+, 1123.57 [2M+Na]+. HRMS (ESI) (m/z): Calcd for C37H43O2S [M+H]+ 551.29783; Found 551.29773. Anal. Calc. for C37H43O2S: C 80.68; H 7.69. Found C 80.38; H 7.75.
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, J.-K. Natural Terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Fucole, polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Kouno, I.; Hashimoto, A.; Kawano, N.; Yang, C.-S. New Sesqui-neolignan from the Pericarps of Illicium macranthum. Chem. Pharm. Bull. 1989, 37, 1291–1292. [Google Scholar] [CrossRef]
- Taro, N.; Hideki, K.; Kazuhiro, T.; Toshio, F. Structure of Mulberrofuran R, a Novel 2-Arylbenzofuran Derivative from the Cultivated Mulberry Tree (Morus lhou Koidz.). Heterocycles 1987, 26, 759. [Google Scholar] [CrossRef]
- Wu, C.-A.; Chou, H.-H.; Shih, C.-H.; Wu, F.-I.; Cheng, C.-H.; Huang, H.-L.; Chao, T.-C.; Tseng, M.-R. Synthesis and physical properties of meta-terphenyloxadiazole derivatives and their application as electron transporting materials for blue phosphorescent and fluorescent devices. J. Mater. Chem. 2012, 22, 17792–17799. [Google Scholar] [CrossRef]
- Kamath, L.; Manjunatha, K.B.; Shettigar, S.; Umesh, G.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. Investigation of third-order nonlinear and optical power limiting properties of terphenyl derivatives. Opt. Laser Technol. 2014, 56, 425–429. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, B.; Gao, Y.; Chen, H.; Dong, B.; Xu, Y.; Li, J.; Wang, H.; Li, W. Triplet collection for highly efficient single-emitting-layer pure fluorescent WOLED based thermally activated delayed fluorescent host of acridine/sulfone derivative. Opt. Mater. 2020, 110, 110510. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, J.Y. A hole transport material with ortho- linked terphenyl core structure for high power efficiency in blue phosphorescent organic light-emitting diodes. Org. Electron. 2014, 15, 399–404. [Google Scholar] [CrossRef]
- Liao, H.-R.; Lin, Y.-J.; Chou, Y.-M.; Luo, F.-T.; Wang, B.-C. Theoretical study of optical and electronic properties of p-terphenyls containing cyano substituents as promising light-emitting materials. J. Lumin. 2008, 128, 1373–1378. [Google Scholar] [CrossRef]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m-Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Su, S.-J.; Cai, C.; Kido, J. RGB Phosphorescent Organic Light-Emitting Diodes by Using Host Materials with Heterocyclic Cores: Effect of Nitrogen Atom Orientations. Chem. Mater. 2011, 23, 274–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B.; Tan, J.; Mu, G.; Yi, W.; Lv, X.; Zhuang, S.; Liu, W.; Wang, L. Optimized electron-transport material based on m-terphenyl-diphenylphosphine oxide with the harmonious compatibility of high ET and electron mobility for highly efficient OLEDs. J. Mater. Chem. C. 2017, 5, 8516–8526. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Novel star-shaped host materials for highly efficient solution-processed phosphorescent organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6131–6137. [Google Scholar] [CrossRef]
- Sasabe, H.; Pu, Y.-J.; Nakayama, K.-i.; Kido, J. m-Terphenyl-modified carbazole host material for highly efficient blue and green PHOLEDS. Chem. Commun. 2009, 6655–6657. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhao, Y.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Tuning the Photophysical Properties and Energy Levels by Linking Spacer and Topology between the Benzimidazole and Carbazole Units: Bipolar Host for Highly Efficient Phosphorescent OLEDs. J. Phys. Chem. C 2010, 114, 5193–5198. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Al-Jammal, W.K.; El-Khateeb, M.Y.; McDonald, R. Synthesis of Diiodinated Biphenyls and Iodinated meta-Terphenyls by Regioselective Suzuki–Miyaura Cross-Coupling Reactions of 5-Substituted 1,2,3-Triiodobenzenes. Eur. J. Org. Chem. 2015, 2015, 3374–3384. [Google Scholar] [CrossRef]
- Antelo Miguez, J.M.; Adrio, L.A.; Sousa-Pedrares, A.; Vila, J.M.; Hii, K.K. A Practical and General Synthesis of Unsymmetrical Terphenyls. J. Org. Chem. 2007, 72, 7771–7774. [Google Scholar] [CrossRef]
- Camacho, D.H.; Salo, E.V.; Guan, Z. Synthesis and Structure of m-Terphenyl-Based Cyclophanes with Nitrogen Intra-annular Functional Groups. Org. Lett. 2004, 6, 865–868. [Google Scholar] [CrossRef]
- Adrio, L.A.; Míguez, J.M.A.; Hii, K.K. Synthesis of Terphenyls. Org. Prep. Proced. Int. 2009, 41, 331–358. [Google Scholar] [CrossRef]
- Poudel, T.N.; Tamargo, R.J.I.; Cai, H.; Lee, Y.R. Recent Progress in Transition-Metal-Free, Base-Mediated Benzannulation Reactions for the Synthesis of a Diverse Range of Aromatic and Heteroaromatic Compounds. Asian J. Org. Chem. 2018, 7, 985–1005. [Google Scholar] [CrossRef]
- Zimmermann, T. Ringtransformationen heterocyclischer Verbindungen. VII. 2,4,5-Triaryl-benzophenone aus 2,4,6-Triaryl-pyryliumsalzen und Arylacetaldehyden: Erste Pyryliumringtransformationen mit Aldehyden als Kohlenstoffnucleophile. J. Prakt. Chem./Chem.-Ztg. 1994, 336, 303–306. [Google Scholar] [CrossRef]
- Andersen, K.K. Synthesis of (+)-ethyl p-tolyl sulfoxide from (−)-menthyl (−)-p-toluenrsulfinate. Tetrahedron Lett. 1962, 3, 93–95. [Google Scholar] [CrossRef]
- Solladié, G. Asymmetric synthesis using nucleophilic reagents containing a chiral sulfoxide group. Synthesis 1981, 185–196. [Google Scholar] [CrossRef]
- Solladié, G.; Hutt, J.; Girardin, A. Improved Preparation of Optically Active Methyl p-Tolyl Sulfoxide. Synthesis 1987, 173–173. [Google Scholar] [CrossRef]
- Pflieger, P.; Mioskowski, C.; Salaun, J.P.; Weissbart, D.; Durst, F. Synthesis of optically active α-sulfinylacetaldehyde. Tetrahedron Lett. 1988, 29, 6775–6778. [Google Scholar] [CrossRef]
- Vidal, M.; Rezende, M.C.; Pastene, C.; Aliaga, C.; Domínguez, M. Solvatochromism of conjugated 4-N,N-dimethylaminophenyl-pyridinium donor–acceptor pairs. New J. Chem. 2018, 42, 4223–4231. [Google Scholar] [CrossRef]
- Banfi, L.; Colombo, L.; Gennari, C.; Annunziata, R.; Cozzi, F. Stereospecific synthesis of chiral.alpha.-sulfinylhydrazones. Synthesis 1982, 829–831. [Google Scholar] [CrossRef]
- Balaban, A.T.; Nenitzescu, C.D. 699. Reaction of pyrylium salts with alkali cyanides. J. Chem. Soc. 1961, 3566–3572. [Google Scholar] [CrossRef]
- Kuthan, J. Pyrans, Thiopyrans, and Selenopyrans. In Adv. Heterocycl. Chem.; Katritzky, A.R., Ed.; Academic Press, 1983; Volume 34, pp. 145–303. [Google Scholar]
- Williams, A. Hydrolysis of pyrylium salts. Kinetic evidence for hemiacetal intermediates. J. Am. Chem. Soc. 1971, 93, 2733–2737. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Sakai, N.; Shimada, R.; Ogiwara, Y. Indium-Catalyzed Deoxygenation of Sulfoxides with Hydrosilanes. Asian J. Org. Chem. 2021, 10, 845–850. [Google Scholar] [CrossRef]
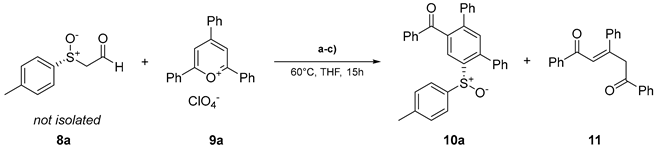 | |||||
|---|---|---|---|---|---|
| Entry | eq. 8a | eq. 9a | Base | Yield 10a / % | Yield 11 / % |
| 1 | 1.0 | 1.0 | NaOAc | 32 | 26 |
| 2b | 1.0 | 1.0 | NaOAc | 35 | 16 |
| 3 | 2.0 | 1.0 | NaOAc | 9 | 27 |
| 4 | 1.0 | 1.0 | - | 38 | <10 |
| 5 | 1.0 | 1.8 | - | 49 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





