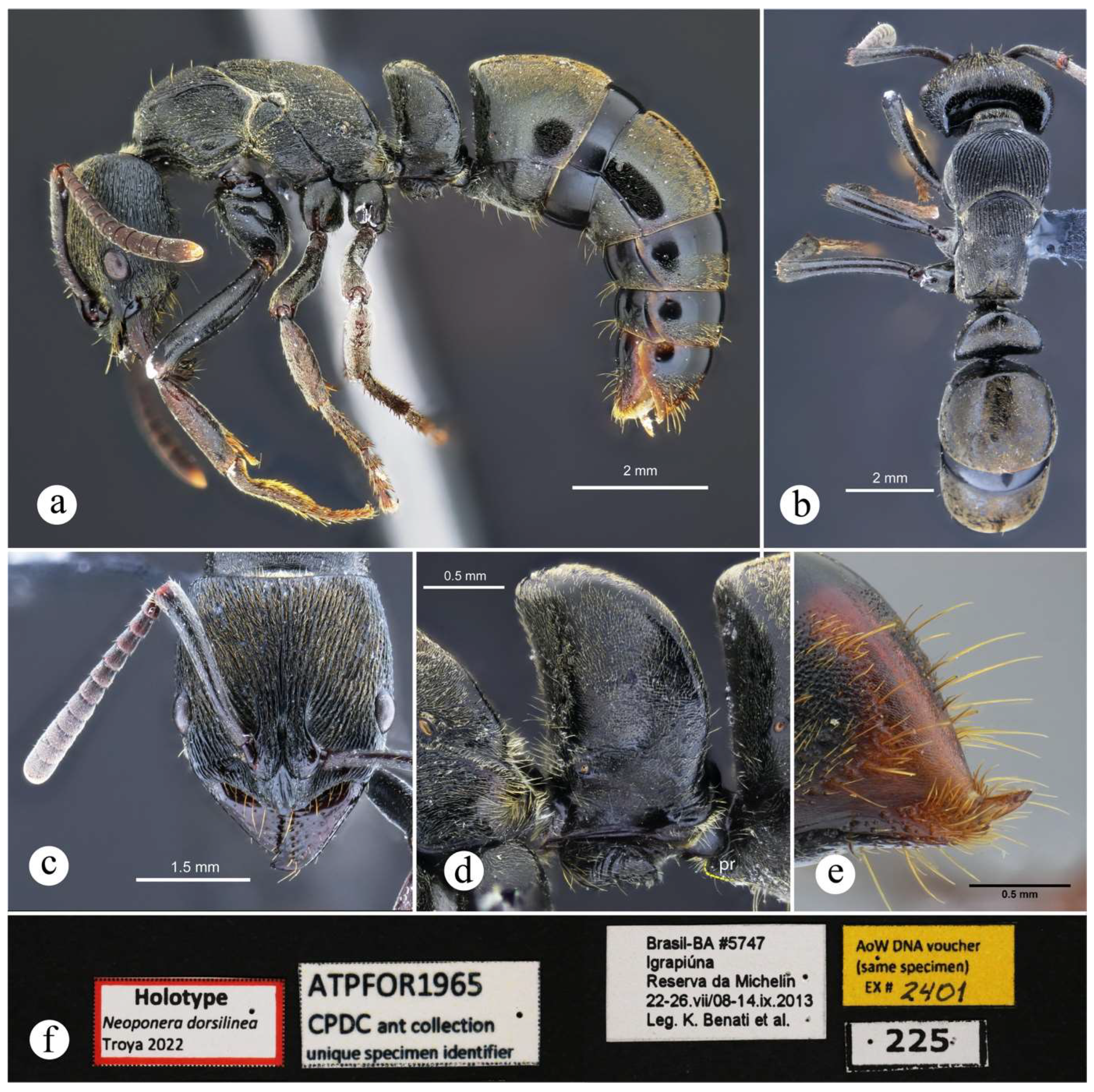
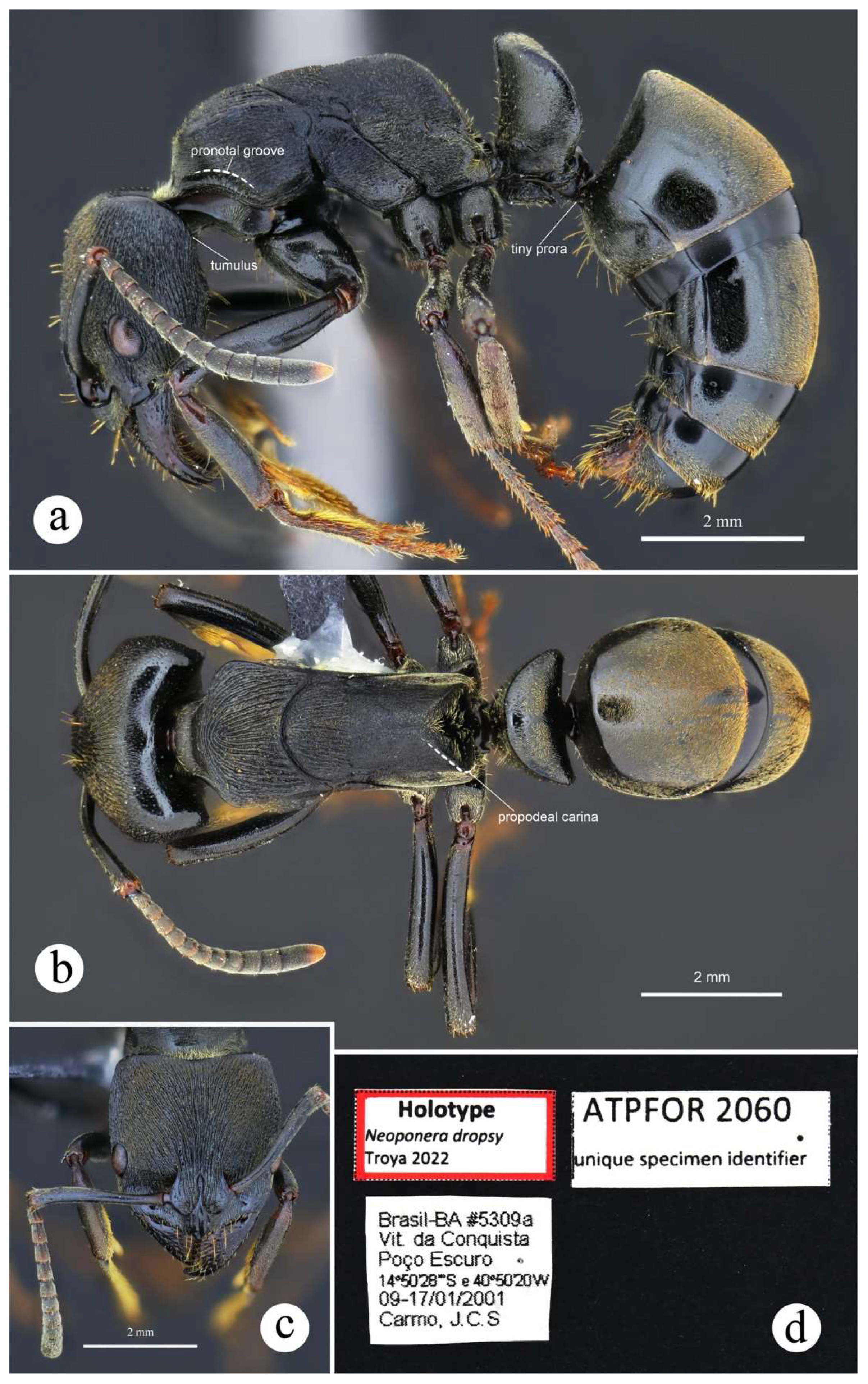
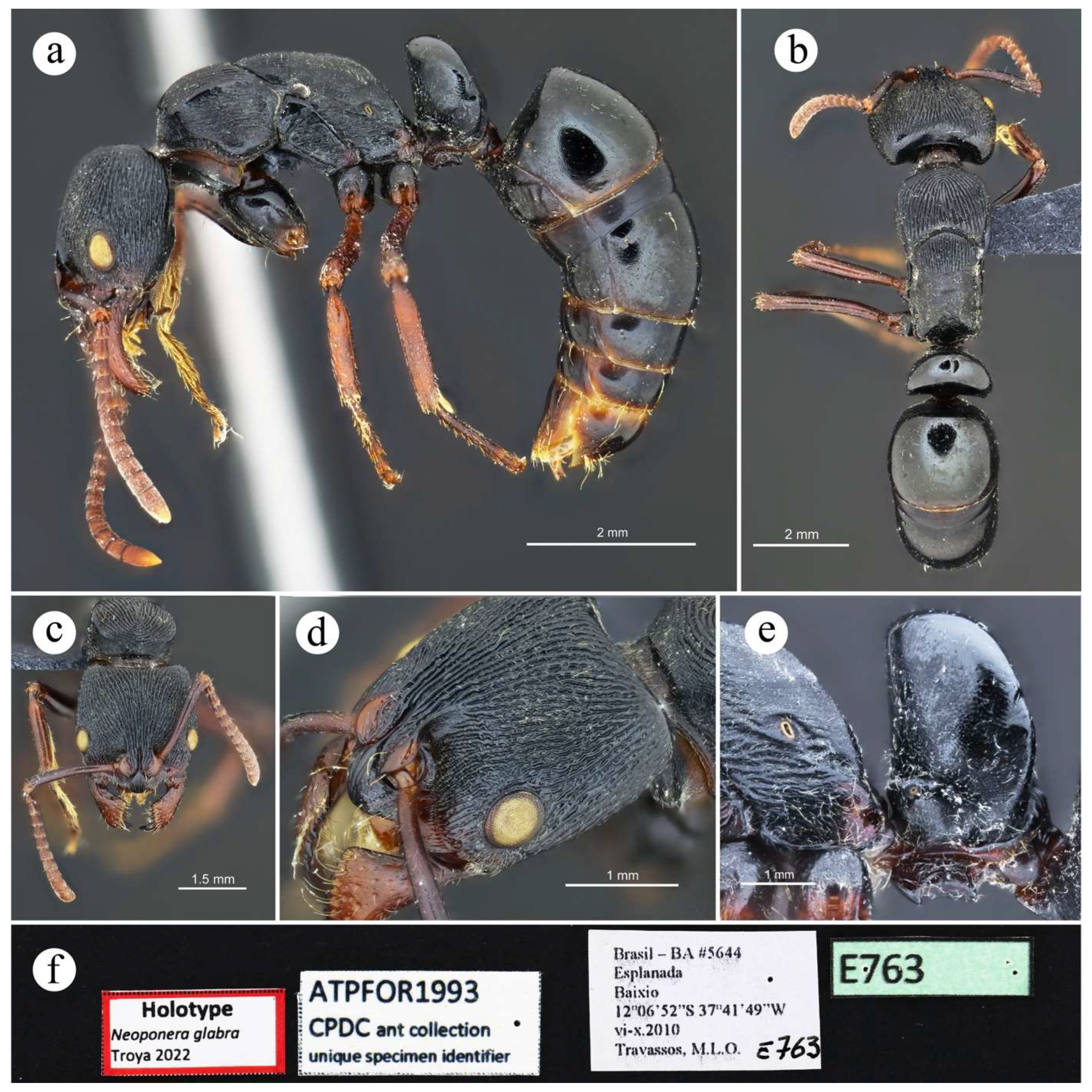
4.2. Species Accounts
4.2.1. Neoponera bugabensis (Forel, 1899)
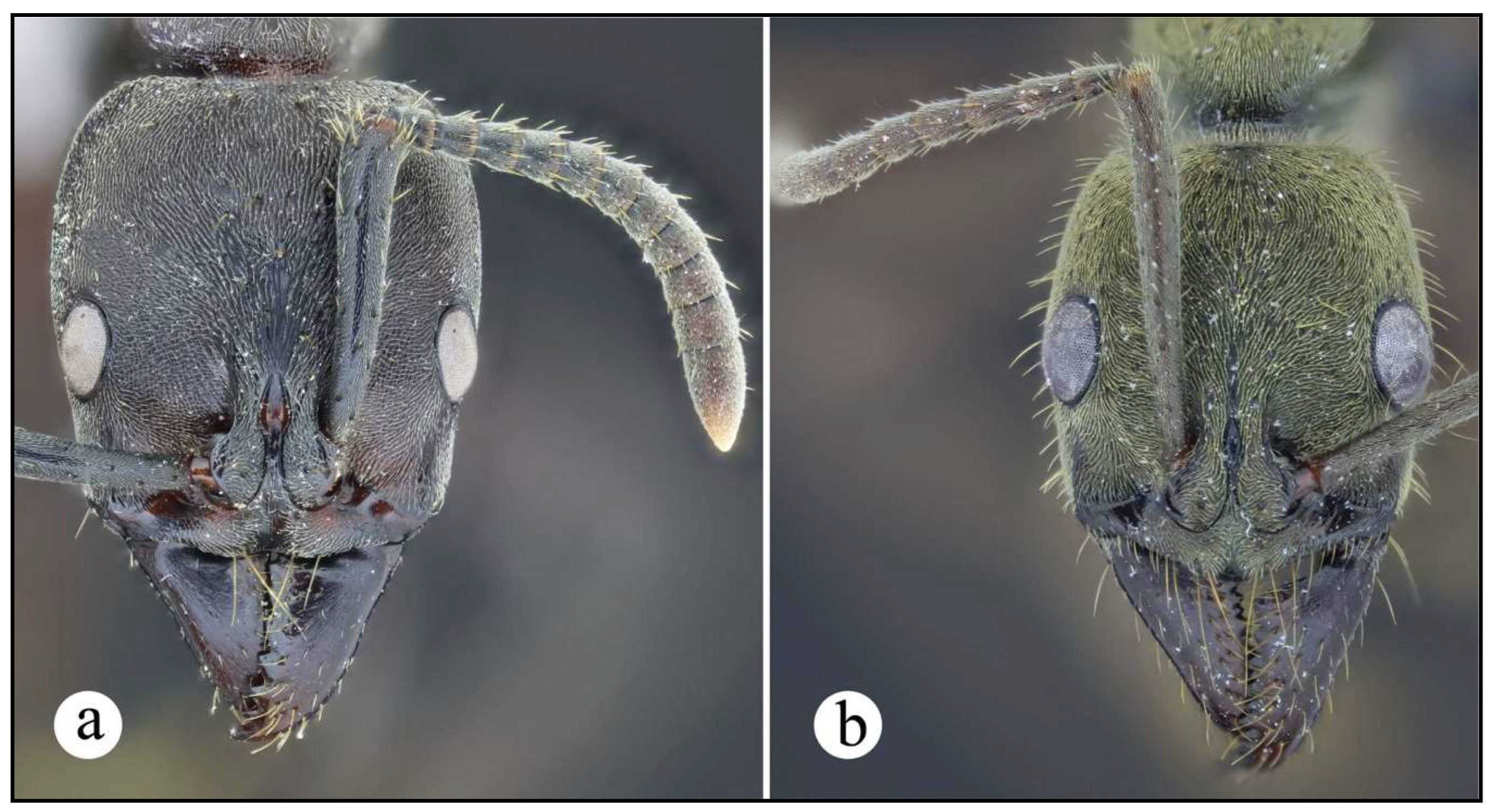
Figures. 1b (head ☿); 13, 15, 16: a – c (☿); 14, 17: a – c (♀︎); 30a (distribution)
Pachycondyla theresiae var. bugabensis Forel, 1899: 14. ☿ lectotype [by designation of Mackay & Mackay, 2010: 224], Panama, Bugaba, Champion [leg.], (MHNG, AntWeb: CASENT0907242) [image examined, link].
Combinations. In Pachycondyla: Brown, 1995: 303; in Neoponera: Emery, 1901: 47; Schmidt and Shattuck, 2014: 151.
Subspecies of Neoponera theresiae: Emery, 1911: 72; Kempf 1972: 162; Bolton 1995: 303; Mackay, 2008: 198.
Status as species: MacKay and Mackay, 2010: 224 (redescription).
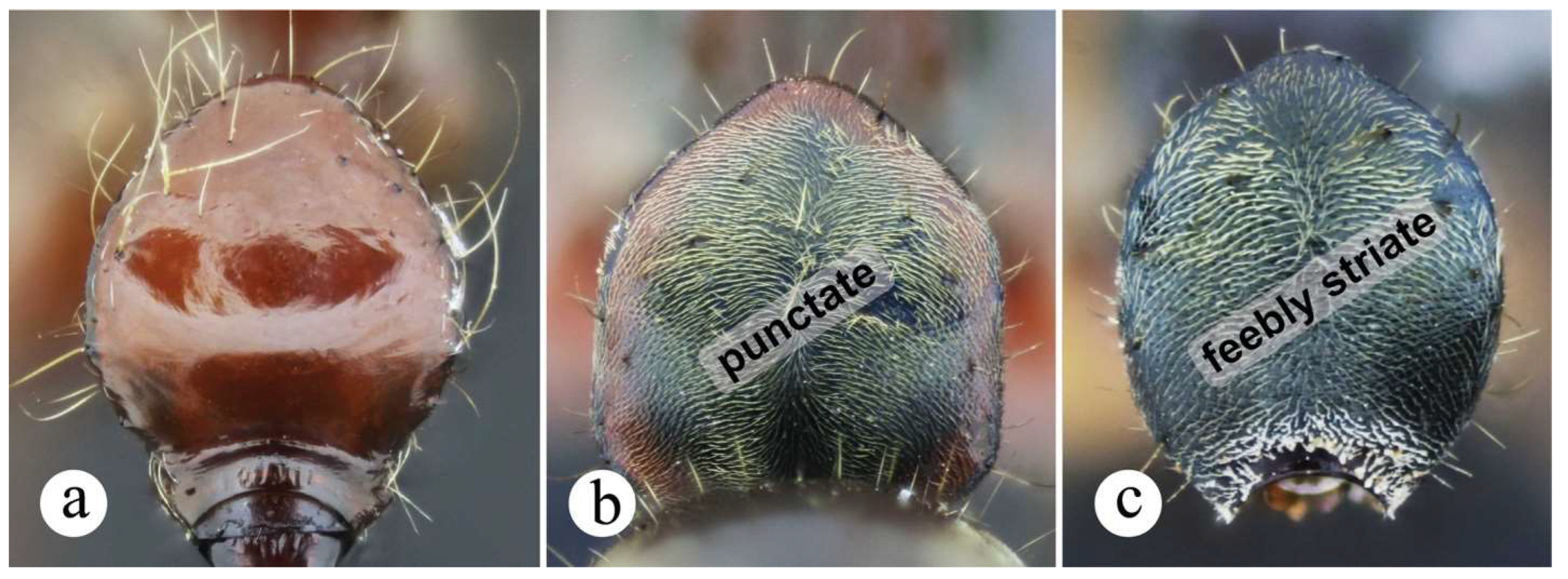
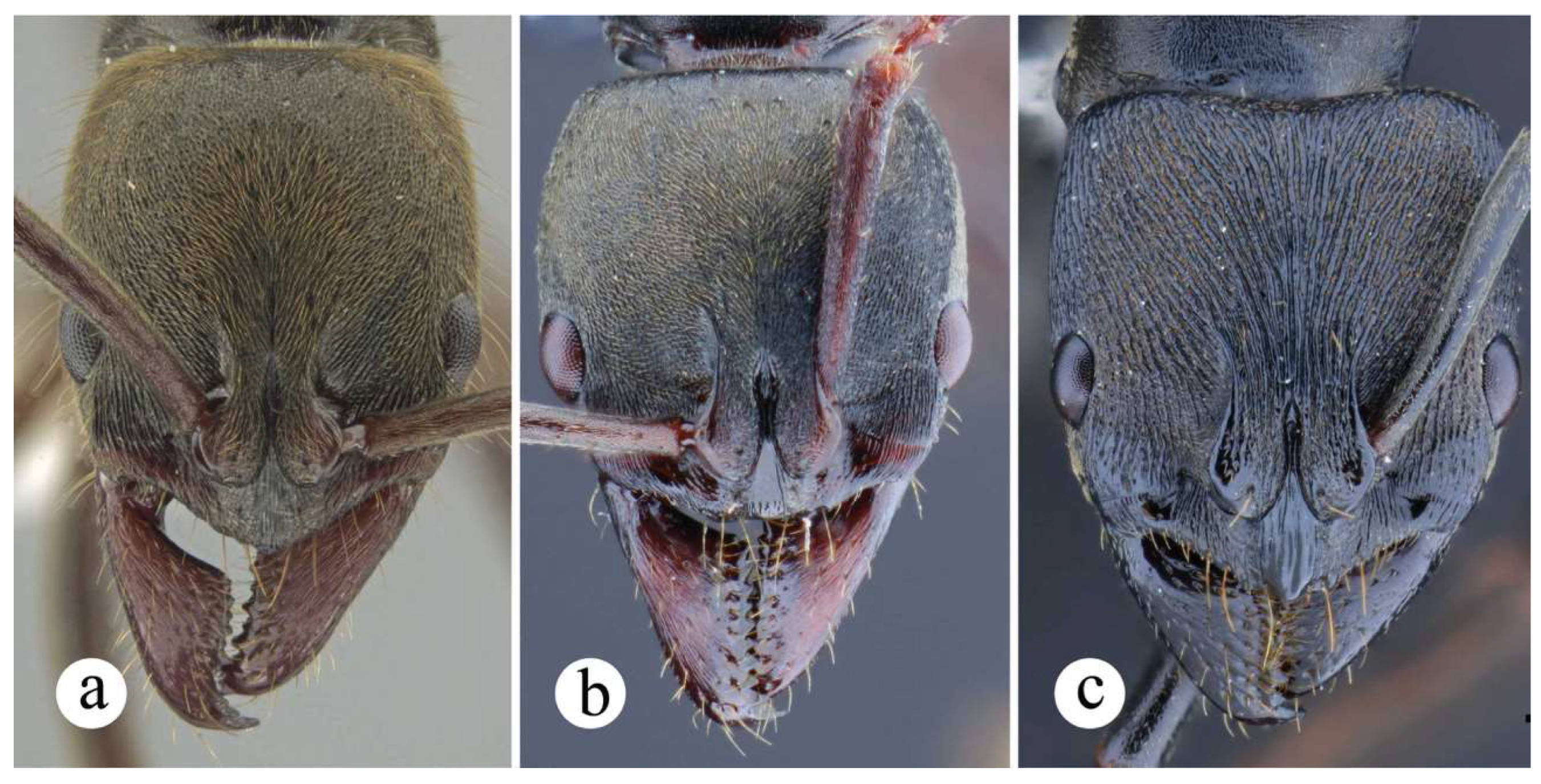
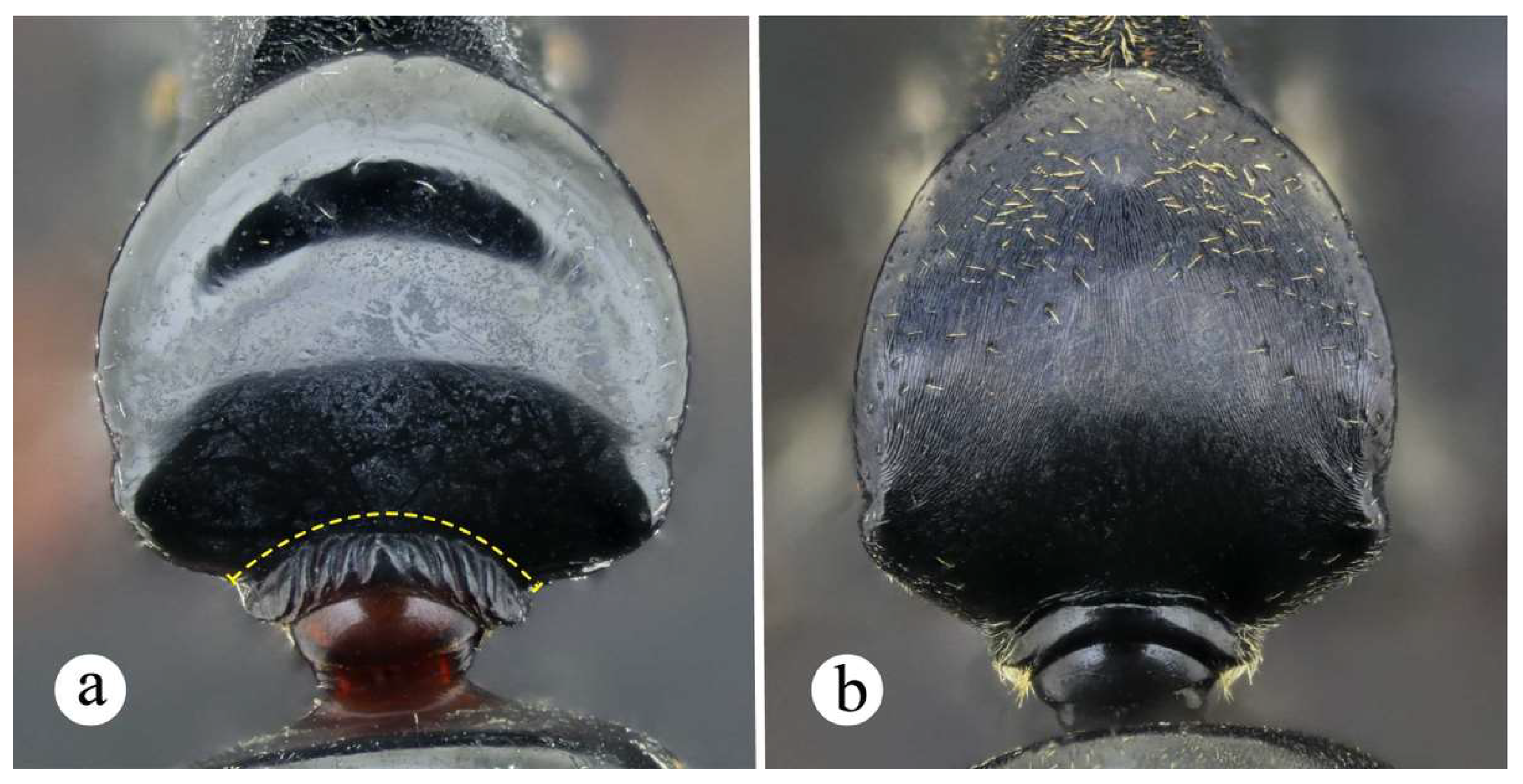
Worker and queen diagnosis. Head subquadrate (Figures 14b, 17b), sometimes slightly longer than broad (Fig. 15b), with longitudinal, weakly to well-impressed, irregular striae (Figs. 16b, 17b); antennal scape, when pulled posterad, exceeding posterior head margin by approximately 1.5 times (Figs. 14b, 16b) to 3 times (Fig. 15b) apical scape width; malar carina well-marked, it usually fades away before reaching anterior eye margin; humeral carina present, slightly salient; posterolateral margin of propodeum weakly carinate (Figs. 15a, 16a) or without carina; anterior margin of petiolar node either slightly inclined posterad (Figs. 8a, 10b) or steeper (Fig. 17a); posterolateral margin of node weakly carinate, carina usually blunt (Figs. 10b, 14a) mostly in Central American populations; anterior margin of node straight (Figs. 10b, 14a, 15a, 16a) or slightly concave, this latter only seen in queens from South America (Fig. 17a); dorsal appressed pilosity varying from pale yellowish-brownish (Fig. 16), to intense, brilliant golden (Figs. 14, 15), erect and suberect hairs on head and mesosomal dorsum either shorter or about as long as maximum eye length.
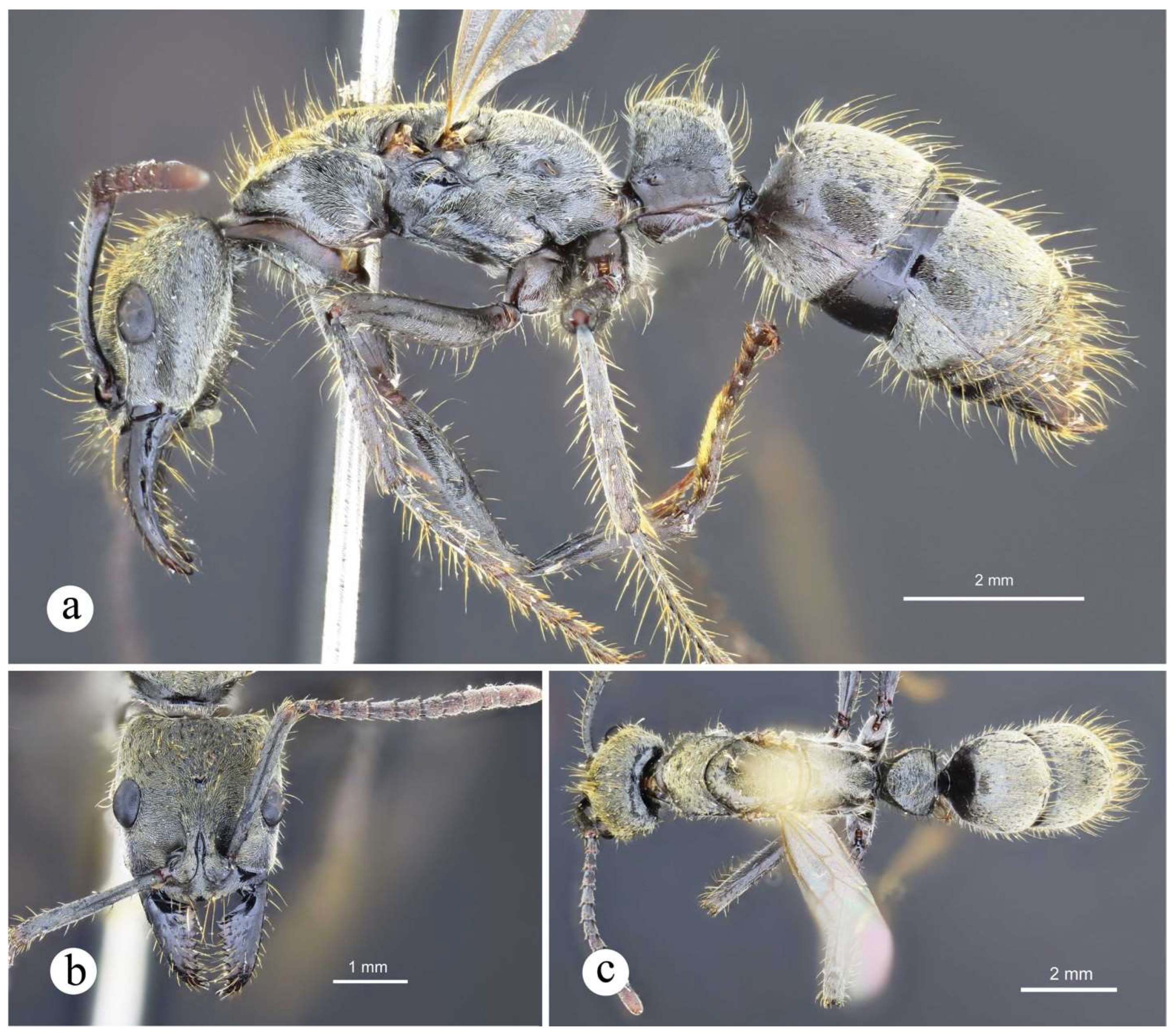
Figure 13.
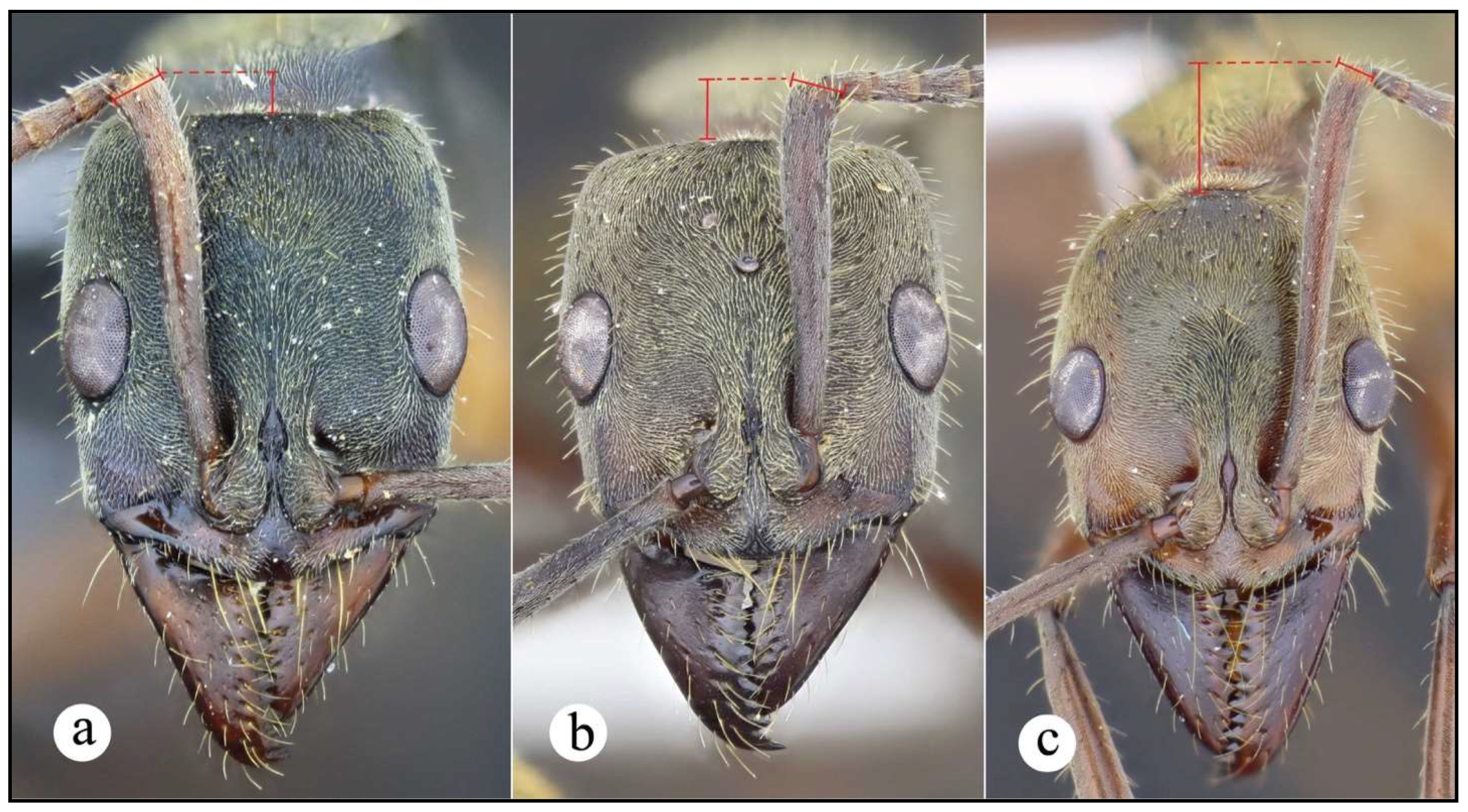
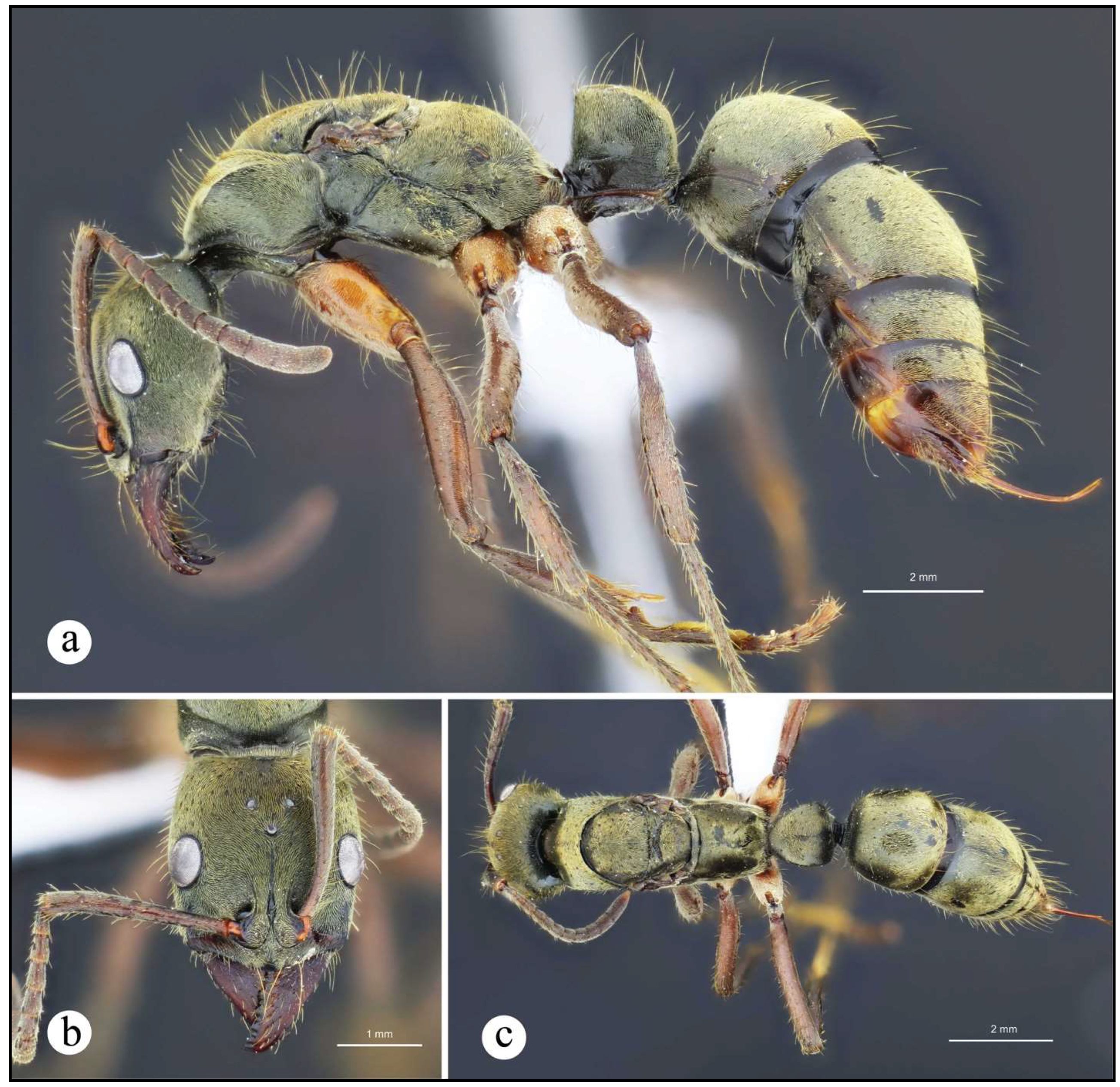
Neoponera bugabensis. ☿ (MUCR: CASENT0637852), Costa Rica: Limón. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 13.
Neoponera bugabensis. ☿ (MUCR: CASENT0637852), Costa Rica: Limón. a. Lateral view; b. Head in frontal view; c. Dorsal view.
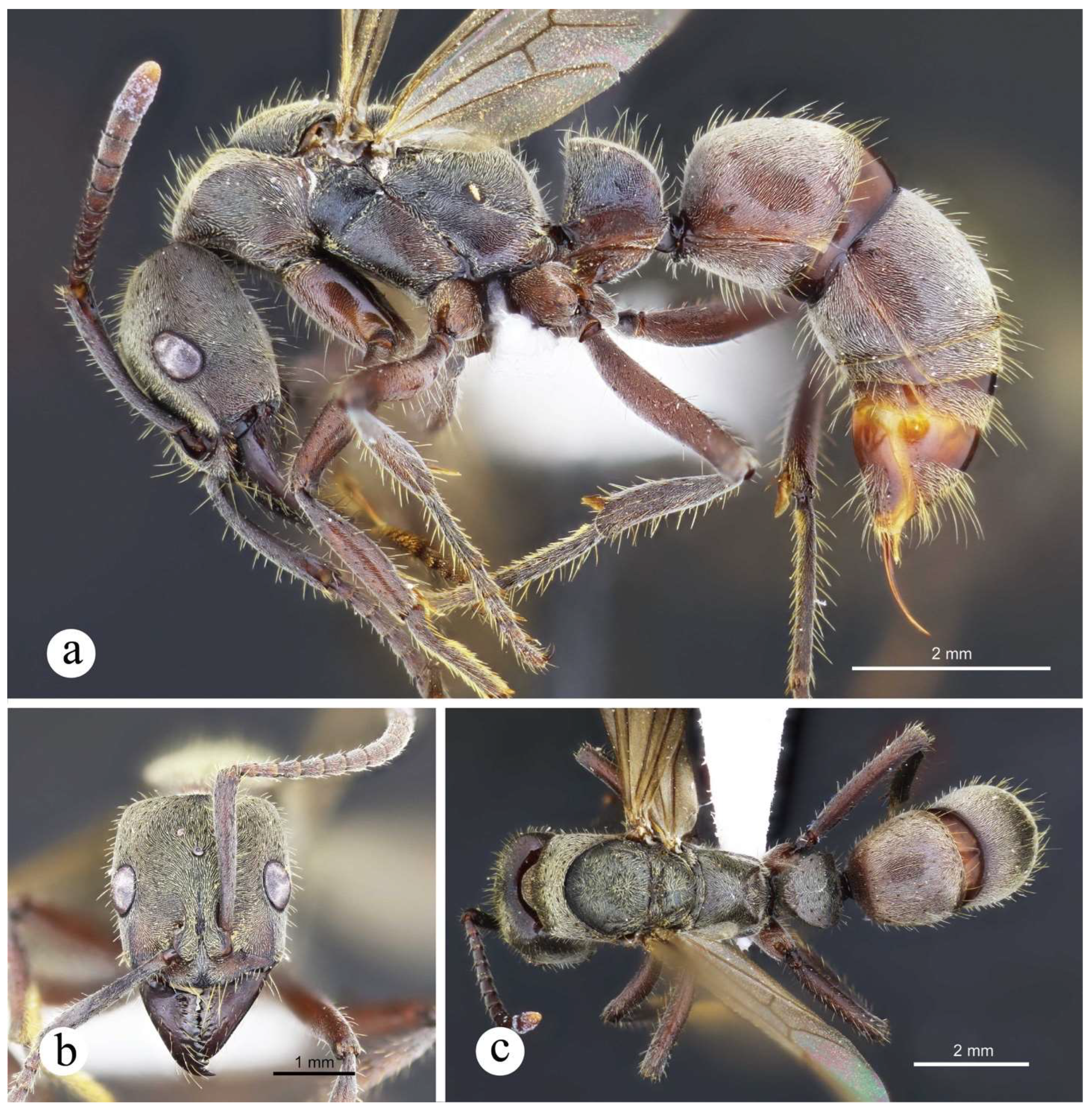
Figure 14.
Neoponera bugabensis. ♀︎ (JTLC: CASENT0617486), Honduras: Comayagua. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 14.
Neoponera bugabensis. ♀︎ (JTLC: CASENT0617486), Honduras: Comayagua. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 15.
Neoponera bugabensis_var1. ☿ (MUSENUV: MUSENUV22595), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 15.
Neoponera bugabensis_var1. ☿ (MUSENUV: MUSENUV22595), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 16.
Neoponera bugabensis_var2. ☿ (MUSENUV: HOR0985), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 16.
Neoponera bugabensis_var2. ☿ (MUSENUV: HOR0985), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 17.
Neoponera bugabensis_var2. ♀︎ (MUSENUV: HOR0983), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 17.
Neoponera bugabensis_var2. ♀︎ (MUSENUV: HOR0983), Colombia: Valle del Cauca. a. Lateral view; b. Head in frontal view; c. Dorsal view.
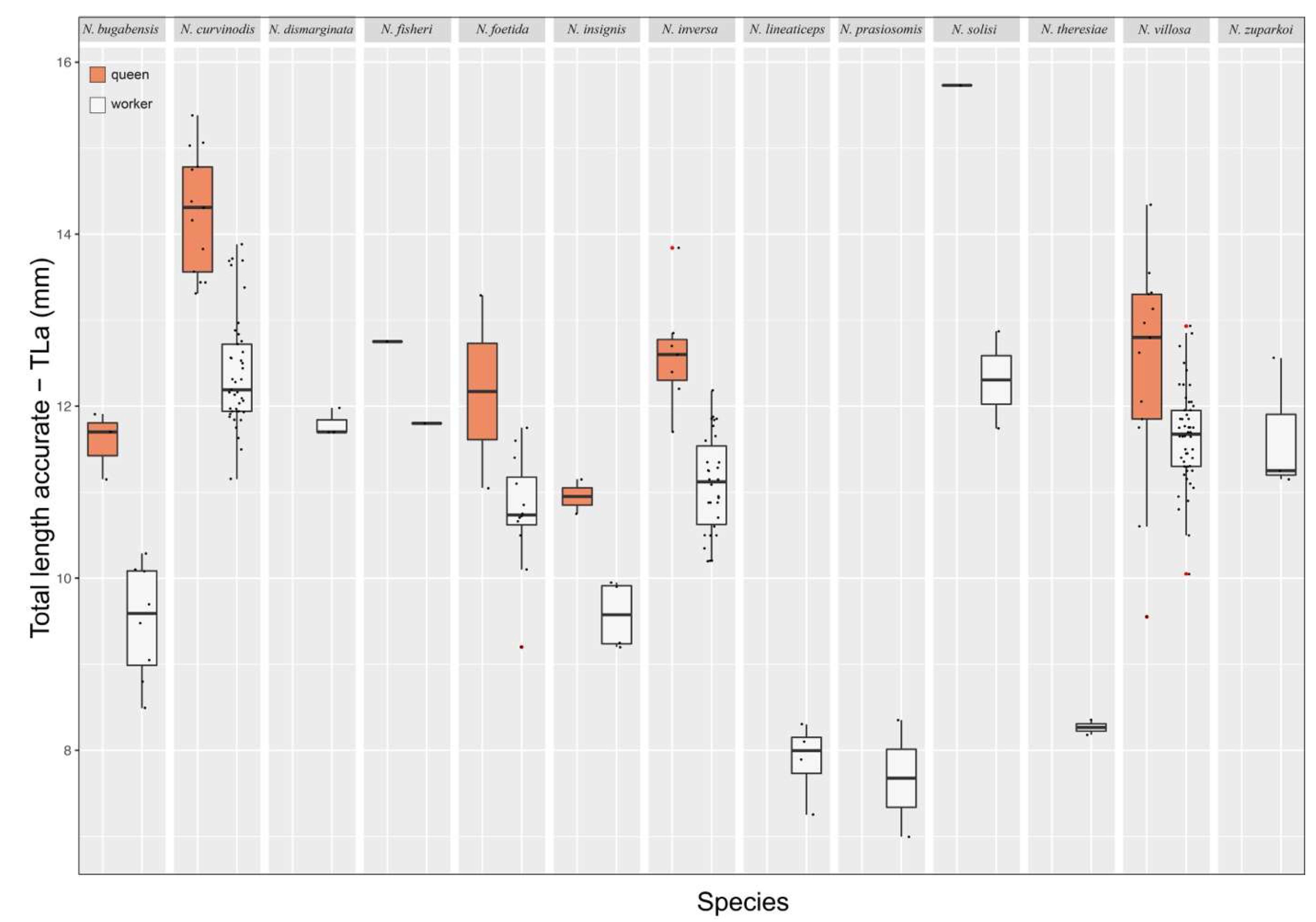
Worker. Measurements (n = 14): HW: 1.73-2.1; HL: 2.02-2.45; EL: 0.5-0.65; SL: 1.72-2.7; WL: 3.15-3.8; PrW: 1.25-1.55; MsW: 0.76-0.95; MsL: 0.63-0.88; PW: 0.9-1.15; PH: 0.6-1.2; PL: 0.9-1.25; GL: 3.29-4.7; A3L: 1.18-1.5; A4L: 1.23-1.7; A3W: 1.4-1.7; A4W: 1.45-1.75; TLa: 8.49-10.7; TLr: 9.61-11.68. Indices. CI: 79.35-88.64; OI: 28.21-34.29; SI: 97.56-131.71; MsI: 105.71-123.33; LPI: 91.67-150.0; DPI: 80.0-111.11.
Queen. Measurements (n = 6): HW: 1.84-2.31; HL: 2.23-2.69; EL: 0.59-0.72; SL: 1.98-2.45; WL: 3.65-4.44; PrW: 1.45-1.81; MsW: 1.33-1.69; MsL: 1.3-2.25; PW: 1.04-1.2; PH: 1.02-1.25; PL: 1.1-1.28; GL: 4.2-5.5; A3L: 1.45-1.8; A4L: 1.6-1.95; A3W: 1.61-2.0; A4W: 1.69-2.12; TLa: 10.14-11.91; TLr: 11.21-13.91. Indices. CI: 82.0-90.2; OI: 29.27-33.68; SI: 95.65-121.28; MsI: 73.91-111.54; LPI: 97.78-109.52; DPI: 92.68-104.35.
Male. Described by MacKay and Mackay (2010).
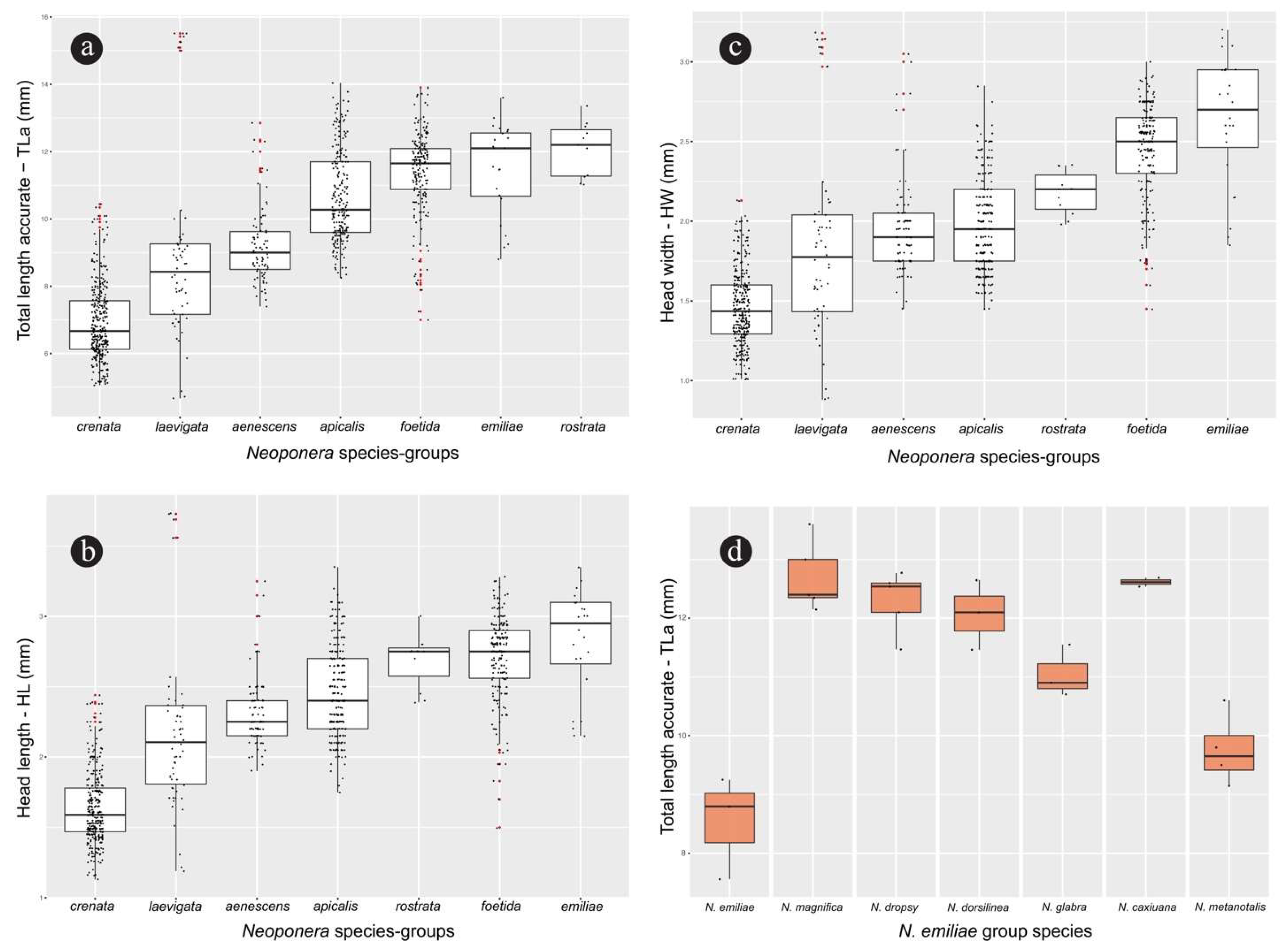
Comments. Neoponera bugabensis is a medium-sized taxon in the N. foetida group (Fig. 12; ☿: TLa = 8.5 – 10.7 mm; ♀︎TLa: 10.1 – 11.9 mm). Populations of Central America have a cuticle with more intense golden pilosity than those from South America, and are amongst the most brilliant in the genus when light is directed to their cuticle. In a similar fashion to N. curvinodis, the examined material of N. bugabensis shows relatively large size variation as judged by the variable TLa in workers (Fig. 12). In addition, some body traits also show variations somewhat correlated with geographic distribution making this species one of the most hard-to-diagnose in the genus together with N. curvinodis.
Based on the below variations, this species can be categorized under three forms: the first matches the traits of the lectotype from Panama, thus this is the “true” N. bugabensis (Figs. 13, 14), and is the most widely distributed form. The other two, which here are called “variant – 1” (Fig. 15), and “variant – 2” (Figs. 16, 17), are more restricted to Costa Rica and to Colombia-Ecuador, respectively. These variants slightly differ from N. bugabensis in the form of the head, petiolar node, pilosity and color. Difference in scape length between N. bugabensis and its variant – 2 was also noticed, but it was not consistent across all individuals. Despite this, they are not described as new in this work since such traits, except for scape length, show some degree of plasticity in other species of the genus, for example, in N. crenata (Roger), N. curvinodis (Forel), N. oberthueri (Emery), N. rugosula Emery. On the other hand, the current specimen-set available for each variant: 5 specimens from Costa Rica belonging to variant – 1, and 5 specimens from Colombia-Ecuador belonging to variant – 2, is not representative enough to detect a putative case of lineage divergence as judged from a pattern of morphological discontinuities departing from the traits characterizing N. bugabensis.
No doubt, this is a “tricky species”, it may just be a highly variable form composed of ecotypes which are in process of adaptive speciation, or it may be a multi-species-in-one case for which morphological analysis alone is insufficiently informative. DNA sequencing may aid, but also increasing the specimen-set representing the whole known distribution is required. In Neoponera, a similar example occurring in this transitional region, i.e., southern Central America and northern South America, is possibly depicted by the gradation of striae on the petiolar node in N. mashpi Troya and Lattke populations: those from Central America show a tenuous nodal sculpture which is more clearly impressed in populations from South America (Troya and Lattke 2022).
The reader may use the following characters to distinguish these two variants from N. bugabensis. In parentheses are given the differential traits for the latter.
Variant – 1: 1) Head longer than broad in frontal view (vs. quadrate to subquadrate). 2) Antennal scape, when pulled posterad, exceeding posterior head margin by 2 – 2.5 times apical scape width (vs. 1.5 – 2 times). In a specimen from Valle del Cauca, Colombia (MUSENUV22595) the scape is even longer, exceeding said head margin by ca. 3 times apical scape width. 3) Petiolar node longer than high in lateral view (vs. relatively more symmetric). 4) Most erect hairs on body dorsum longer than maximum eye length (vs. mostly shorter).
Variant – 2. This form is very similar to variant – 1, including the head shape, antennal scape length, and length of erect hairs. The following characters, however, distinguish it from N. bugabensis: 1) Appressed pilosity pale yellowish, and that of gastral dorsum not entirely covering the integument (vs. intense golden and usually completely covering gastral integument). 2) Carina of dorsolateral margin of petiolar node relatively acute, somewhat similar to that in N. insignis (vs. blunt carina on dorsolateral nodal margin).
Neoponera bugabensis could be confused with the following five species in the N. foetida group: N. insignis (including the variant “villosa_cf2”), N. lineaticeps, N. theresiae, N. prasiosomis sp. nov., N. villosa. Of these, arguably N. insignis is very close phylogenetically to N. bugabensis (Troya et al. unpublished). Neoponera insignis and N. bugabensis occur in sympatry, at least in Costa Rica (Longino 2010), and N. villosa_cf2 is known from a single specimen from Darien, Panama. According to Longino (2010), and Mackay and Mackay (2010), N. insignis is identical to N. bugabensis, and these differ in the striae orientation of the mid clypeus: transverse on the former, and longitudinal in the latter. However, among the material examined, three specimens of N. insignis, a worker and queen from Parque Nacional Katios, Colombia (LACMENT142417), and a worker from Darién, Panama (JTL9077-1), carry longitudinal striae on the mid clypeus. This is another evidence supporting the hypothesis that clypeal striae are a plastic trait in Neoponera and should not be used in species diagnoses. Further evidence is presented under the treatment of N. curvinodis (see below), where more examples of clypeal striae variations are brought together for other species in the genus. Neoponera insignis is indeed nearly indistinguishable from N. bugabensis. The following features are helpful: 1) The head of N. insignis, as measured horizontally from eye to eye, is slightly broader than that of N. bugabensis, and apparently, as inferred from the few specimens examined (n = 4 ☿, 2♀) from Colombia, Costa Rica, and Panama, the eye does not break- but just reaches, or at most feebly exceeds the lateral head margin. In N. bugabensis the eye always exceeds such margin. 2) The antennal scape of N. insignis, when pulled posterad, exceeds the posterior head margin by one- or at most 1.5 times the apical scape width, while in N. bugabensis it always exceeds that margin by 1.5 up to 3 times the apical scape width. 3) The dorsoposterior nodal margin in N. insignis is carinate, with the carina relatively acute. In N. bugabensis that margin is usually blunt, especially in the Central American specimens, but apparently this is not always the case in populations from South America. Two queens from Ecuador, for example, one from Esmeraldas (MEPN38311), a site from the Chocó-Darién, and another from the Amazonian Parque Nacional Yasuní (MEPN34643) show a slightly acute nodal margin, less so however, than in N. insignis.
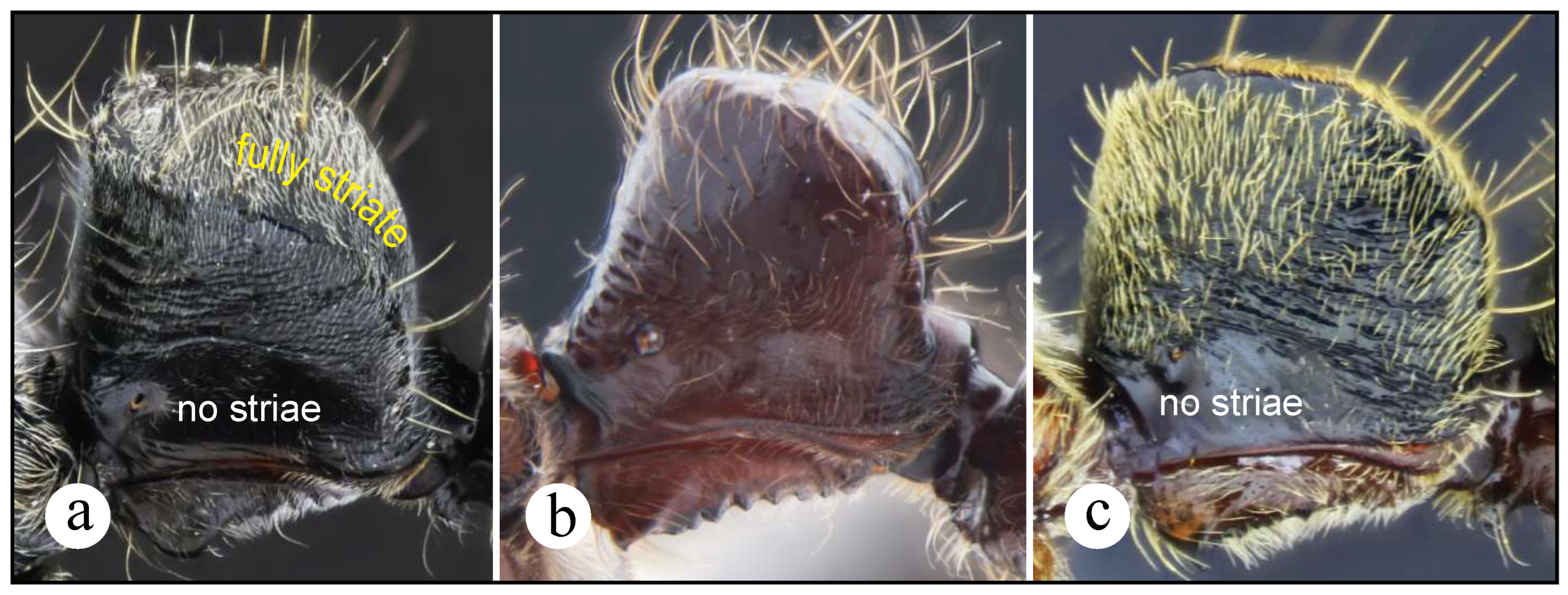
Neoponera lineaticeps is easily distinguishable from N. bugabensis just by examining the dorsum of head and the dorsoposterior nodal face which carry well-impressed longitudinal striae, especially those on head (Fig. 4a) which may also be referred as costae since these are thicker than those on the node. These striae (or costae) are absent in N. bugabensis.
Neoponera theresiae is distinguished from N. bugabensis in: 1) Showing longitudinally divergent striae on the proximal region of the mandibular dorsum (Fig. 6a). Neoponera bugabensis also bears striae on such region but these are significantly more attenuated. 2) Well-impressed striae are also present in N. theresiae laterally on the propodeum and petiolar node, as well as longitudinal, and irregularly shaped on the posterior nodal face. The body dorsum, of N. bugabensis, on the other hand, is virtually striae-less. 3) The head of N. insignis is somewhat circular-shaped rather than subquadrate as in N. bugabensis. This latter trait is perhaps the easiest way to separate both forms.
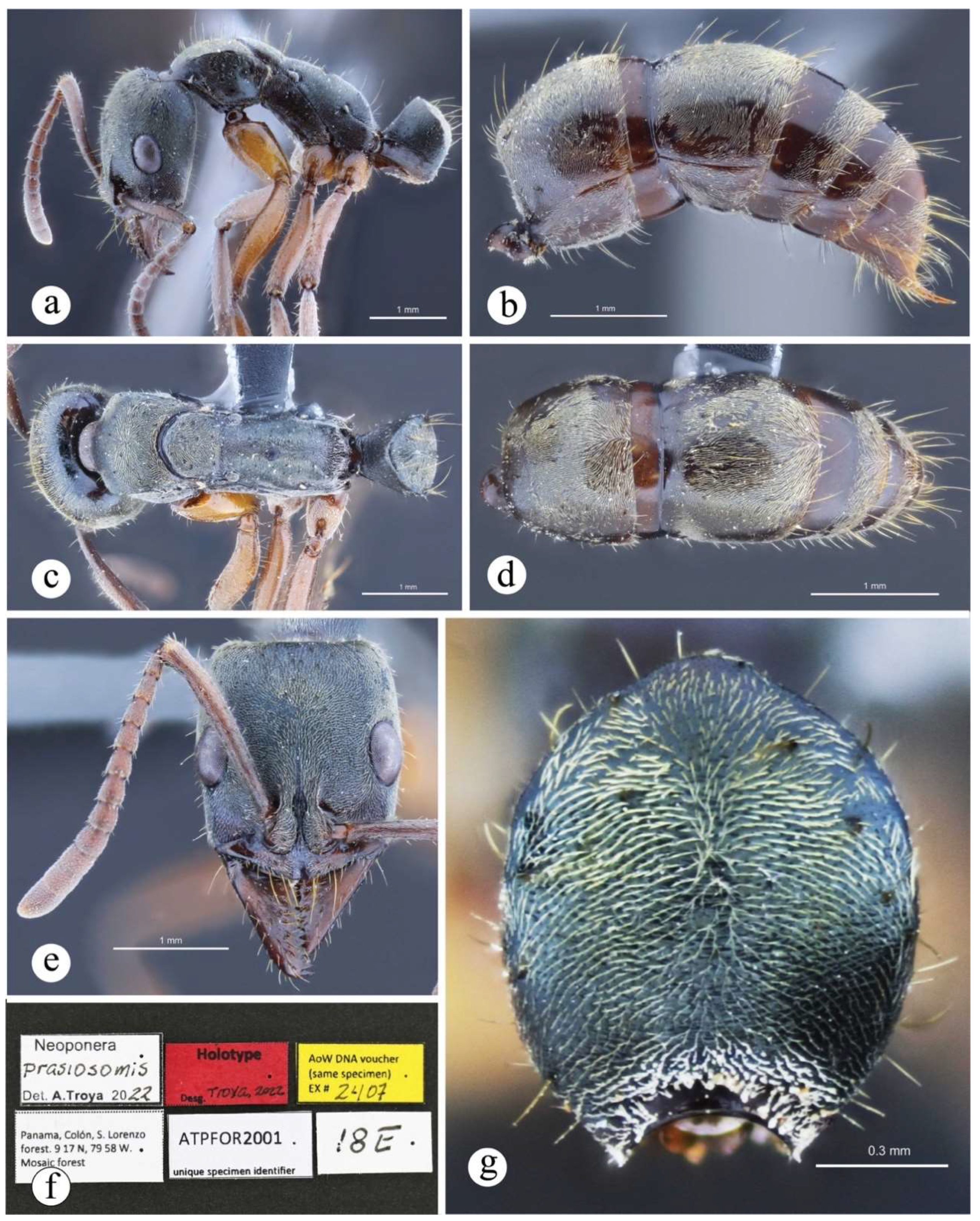
Neoponera prasiosomis sp. nov. has the following differing features as compared to N. bugabensis: 1) The eye is large as compared to head length, it represents at least 29% of that measure, while in N. bugabensis the eye mostly represents 27% or slightly less. 2) The cuticle of N. prasiosomis is greenish (holotype from Panama) or brownish (paratype from Ecuador), while the cuticle of N. bugabensis is usually black, or rarely castaneous in one specimen from Coclé, Panama (CASENT0632922). 3) Appressed hairs in N. prasiosomis are pale yellowish and less abundant, especially on the gastral dorsum, than those in N. bugabensis which are bright golden and abundant, so that the gastral cuticle is not easily discernible. 4) Neoponera prasiosomis is smaller than N. bugabensis (Fig. 12).
Neoponera villosa is larger than N. bugabensis (Fig. 12) and has a tumulus on the posterior petiolar node face. This structure is absent in N. bugabensis.
Neoponera insignis variant N. villosa_cf2 (Fig. 26) differs in the following from N. bugabensis: 1) The petiolar node is higher than long in lateral view, while in N. bugabensis is approximately symmetric. 2) The head and mesosomal cuticle are violaceous-black (see previous comment above about the cuticle of N. bugabensis). 3) The gaster is covered by abundant, relatively opaque, yellow hairs, so that the cuticle is virtually invisible (Fig. 26). Gastral hairs of N. bugabensis are golden bright and the cuticle is slightly discernible. 4) This is a small form (TLa = 8 mm), clearly smaller than N. bugabensis (TLa: 8.5 – 10.3, Fig. 12).
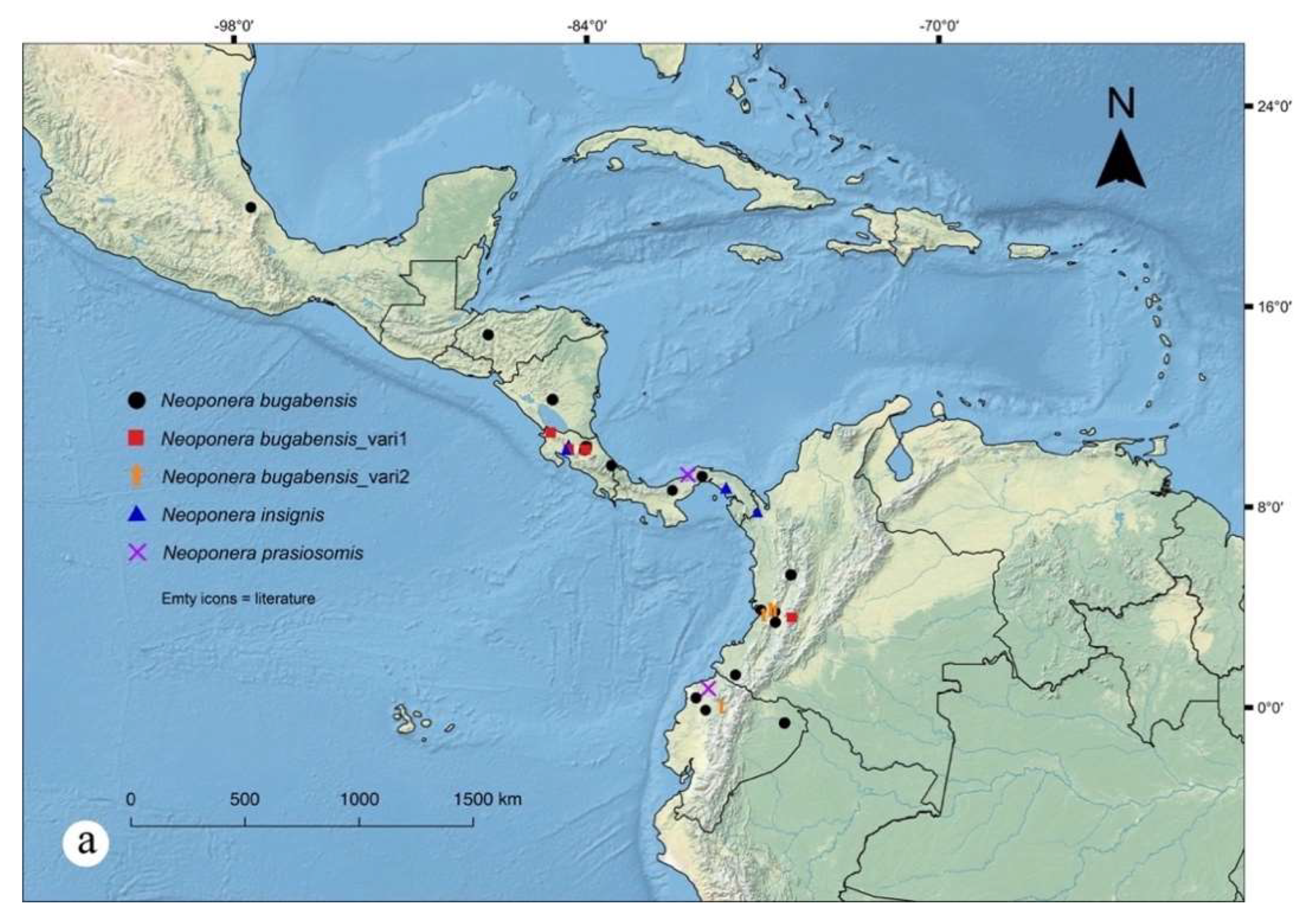
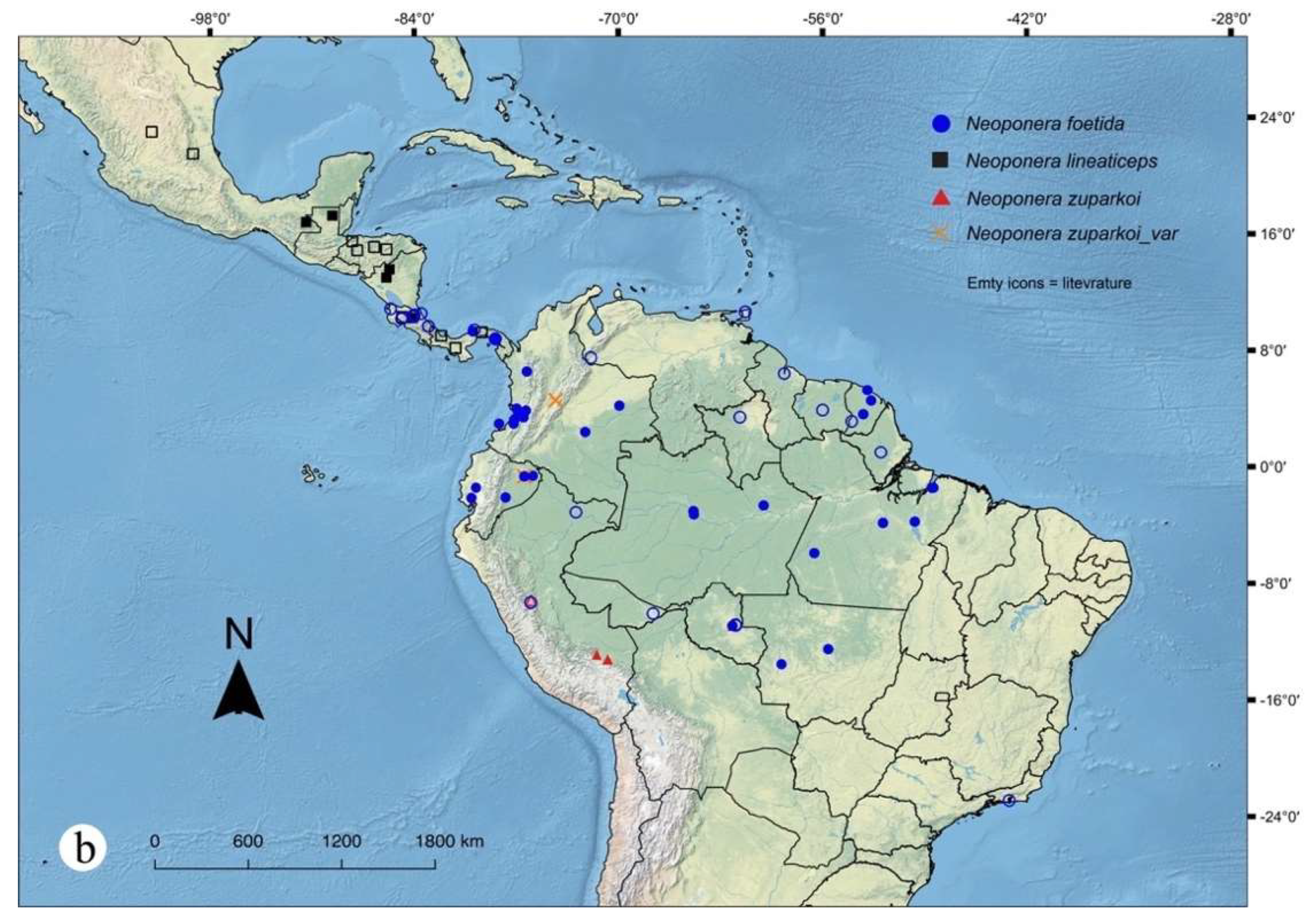
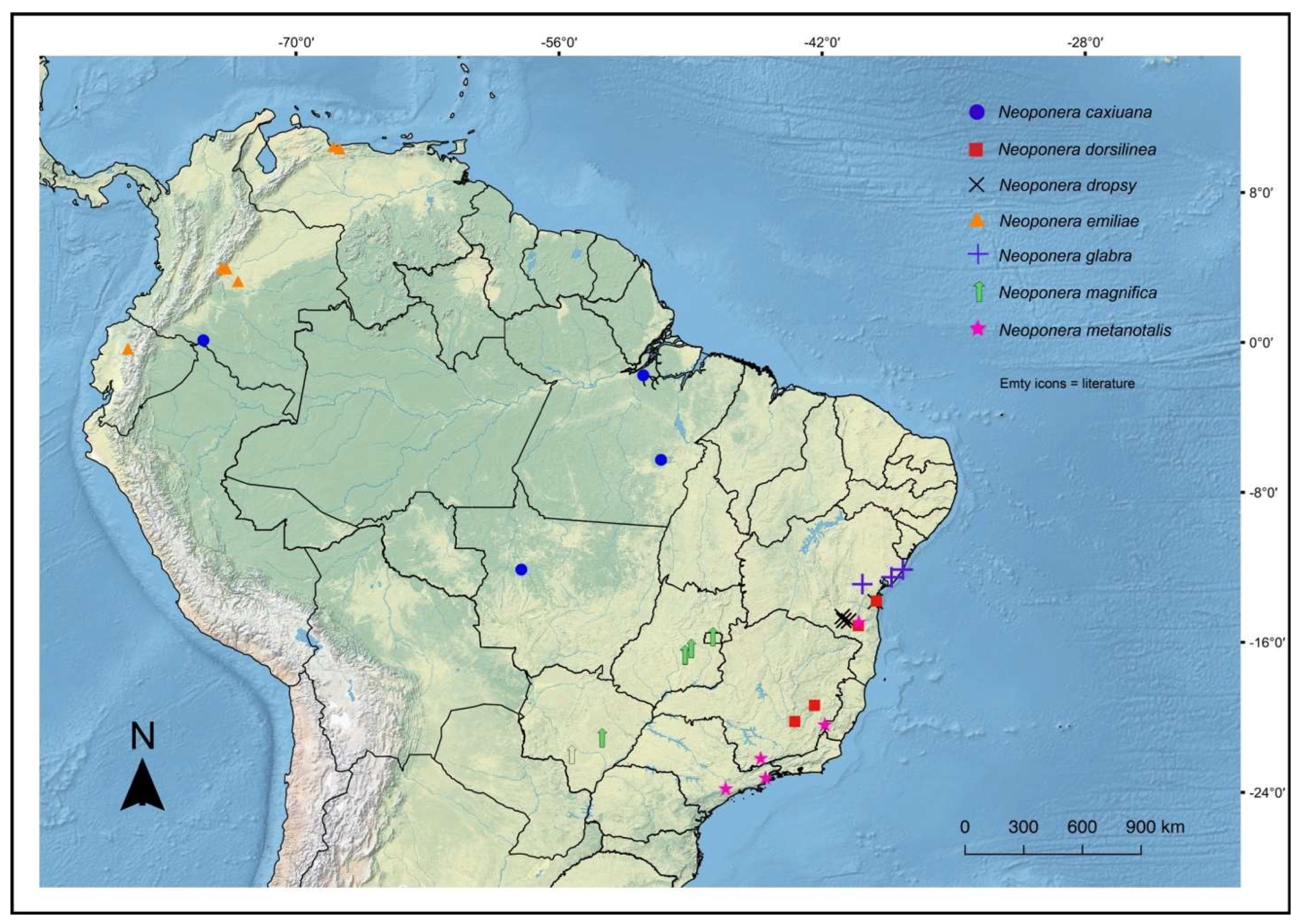
Distribution notes. The elevational range of N. bugabensis spans from nearly the sea level, mostly in lowland rain forests, up to about 2300 m in mountainous Andean humid forests. Examined records belong from a number sites in Central America and northwestern South America. Mackay and Mackay (2010) report also a site near Puerto Maldonado, southeastern Peruvian Amazon. Neoponera bugabensis has been collected in various ecosystem types, from the Oaxacan montane humid forests near the Gulf of Mexico, to the Talamancan montane, Isthmian Atlantic and Pacific forests in Central America, to the Colombian and Ecuadorian Chocó-Darien, and the Napo moist forests (Amazonian) Ecuador.
Natural history notes. See Mackay and Mackay (2010) and Longino (2010). From the present material: this arboreal species has been collected mostly in mature, well-preserved forests, using several techniques like fogging, pitfall, arboreal pitfall, and directly by hand, either in daylight or at night, this latter though through insecticidal canopy knockdown.
Material examined. 22☿️, 3♀. COLOMBIA – Nariño: • 1☿️; Altaquér, Reserva Natural Río Ñambí; 1.3, -78.0833; alt. 1351m; 2010-04-08; Flores, E. leg.; hand collected; (MEPN). – Risaralda: • 6☿️; Puerto de Oro; 5.2995, -75.8869; alt. 1500m; 1991-09-01; Fernández, F. leg.; (ICN). – Valle del Cauca: • 1☿️; Guandal; 3.893, -77.069; alt. 6m; 1998-02-03; Riascos, Y. leg.; (ICN). • 1☿️; Km 18; 3.4, -76.5; (MUSENUV). • 1☿️; Vereda Campo Alegre, R. Bravo; 3.8167, -76.5167; alt. 1413m; 1984-02-05; Cepeda, O. leg.; (ICN). COSTA RICA – Heredia: • 1☿️; 16km SE La Virgen; 10.2682, -84.0842; alt. 500m; 2001-04-11; ALAS leg.; (JTLC). • 1☿️; La Selva Biological Station; 10.4333, -84.0167; alt. 50m; 1991-10-15; Longino, J. leg.; (INBC). – Limón: • 1☿️; Res. Biol. Hitoy Cerere; 9.6531, -83.0229; alt. 650m; 2015-06-11; Longino, J. leg.; (MUCR). ECUADOR – Esmeraldas: • 1♀; Reserva Ecológica Mache Chindul, Laguna de Cube; 0.3875, -79.65; alt. 434m; 2016/11; Troya, A. leg.; fogging; (MEPN). – Orellana: • 1♀; Parque Nacional Yasuní, Tiputini Biodiversity Station, 288 km SE Quito; -0.6319, -76.1442; alt. 230m; 2002-07-21; Erwin, T.; et al. leg.; fogging; (MEPN). • 2☿️; Parque Nacional Yasuní, Tiputini Biodiversity Station, 288 km SE Quito; -0.6167, -76.1333; alt. 240m; 2002-07-21; Erwin, T.; et al. leg.; fogging; (MEPN). – Pichincha: • 1☿️; Puerto Quito; -0.1, -79.2667; 1984/07; Ponce, P. leg.; (QCAZ). HONDURAS – Comayagua: • 1♀; PN Cerro Azul Meámbar; 14.8693, -87.8979; alt. 1170m; 2010-05-22; LLAMA leg.; (JTLC). • 1☿️; Parque Nacional Cerro Azul Meámbar; 14.8698, -87.8987; alt. 1120m; 2010-05-20; LLAMA leg.; (JTLC). MEXICO – Puebla: • 1☿️; 4km WNW Hueytamalco; 19.9567, -97.327; alt. 790m; 2019-06-15; Longino, J. leg.; (JTLC). NICARAGUA – Chontales: • 4km NE Cuapa; 12.2888, -85.3535; alt. 850m; 2011-04-20; Longino, J. leg.; hand collected; (JTLC). • 1☿️; 4km NE Cuapa; 12.2888, -85.3535; alt. 850m; 2011-04-20; Longino, J. leg.; hand collected; (JTLC). PANAMA – Chiriqui: • 1☿️; Bugaba; 8.4833, -82.6167; alt. 350m; Champion leg.; (MHNG). – Coclé: • 1☿️; 6 km NNW El Copé, Parque Nacional Omar Torrijos; 8.6669, -80.5887; alt. 790m; Longino, J. leg.; hand collected; (JTLC). – Panamá: • 1☿️; Cerro Azul, Los Altos; 9.22, -79.41; alt. 838m; 1994-05-24; Smith, N.; Kassabian, R. leg.; (UCDC).
Geographic range. Southern Mexico: Puebla, Honduras, Nicaragua, Costa Rica, Panama, Colombia, Ecuador, Southern Peru*. *: Literature record.
Back_to_table_of_contents
4.2.2. Neoponera curvinodis (Forel 1899)
Figures. 7c (pronotum ☿); 8b, c (petiole ♀︎); 11a (propodeum♀︎); 18: a – e (☿); 30b (distribution).
Pachycondyla villosa curvinodis Forel, 1899:15. ☿ syntype, Guatemala, [Quetzaltenango province], Las Mercedes, Torola, Champion [leg.], MHNG [absent from collection]; ☿ syntype, Panama, [Chiriquí province] Bugaba, Volcan de Chiriqui, Champion, [leg.], (MHNG) [absent from collection].
Neoponera bactronica Fernandes et al. 2014: 136. Figs. 1 – 14, 106, 107 (☿, ♂). ☿ holotype, Brazil, Bahia, Ilhéus, CEPEC Genética, PI24 bis Phenotype 2, XI.1998, leg. D. Fresneau, (MZSP) [examined]; 5 ☿, 3 ♂ paratypes: ☿, same data as for the holotype, (INPA: INPA–HYM033260) [examined]; ☿, same data as for the holotype, except: #4905, 17.I.1995, leg. Arouca J., (NHMW) [not examined]; ♂, same data as for the holotype, except: CEPEC#4587, 15.X.1986, leg. P. Terra, (MZSP) [examined]; ♂, same data as for the holotype, except: CEPEC#4587, 23.II.1988, leg. P. Terra, (INPA: INPA–HYM033261) [examined]; ♂, same data as for the holotype, (CPDC) [not examined]; 2 ☿, same data as for the holotype, (CPDC) [not examined]; ☿, same data as for the holotype, except: CEPEC, 6.XI.2007, leg. A.F.R. Carmo & I. C. Nascimento, (MPEG) [not examined]. Syn. nov.
Neoponera billemma Fernandes et al. 2014: 140. Figs. 15 – 29 (☿, ♀︎). ☿ holotype, Brazil, Pará, Benevides, Morelândia, 16.VI.1988, leg. Bittencourt, (MZSP) [examined]; 1 ☿, 2 ♀︎ paratypes: ☿ Brazil, Goiás, 1980, leg. Kent Redford, #124, (INPA: INPA–HYM033262) [examined]; ♀︎, same data as preceding, (NHMW) [not examined]; ♀︎, Brail, São Paulo, Rio Claro, 22.VIII.2000, leg. D. Fresnau, (CPDC) [not examined]. Syn. nov.
Neoponera subversa Lucas et al. 2002: 256. Fig. 1c (☿). (nomen nudum, unavailable). Syn. nov.
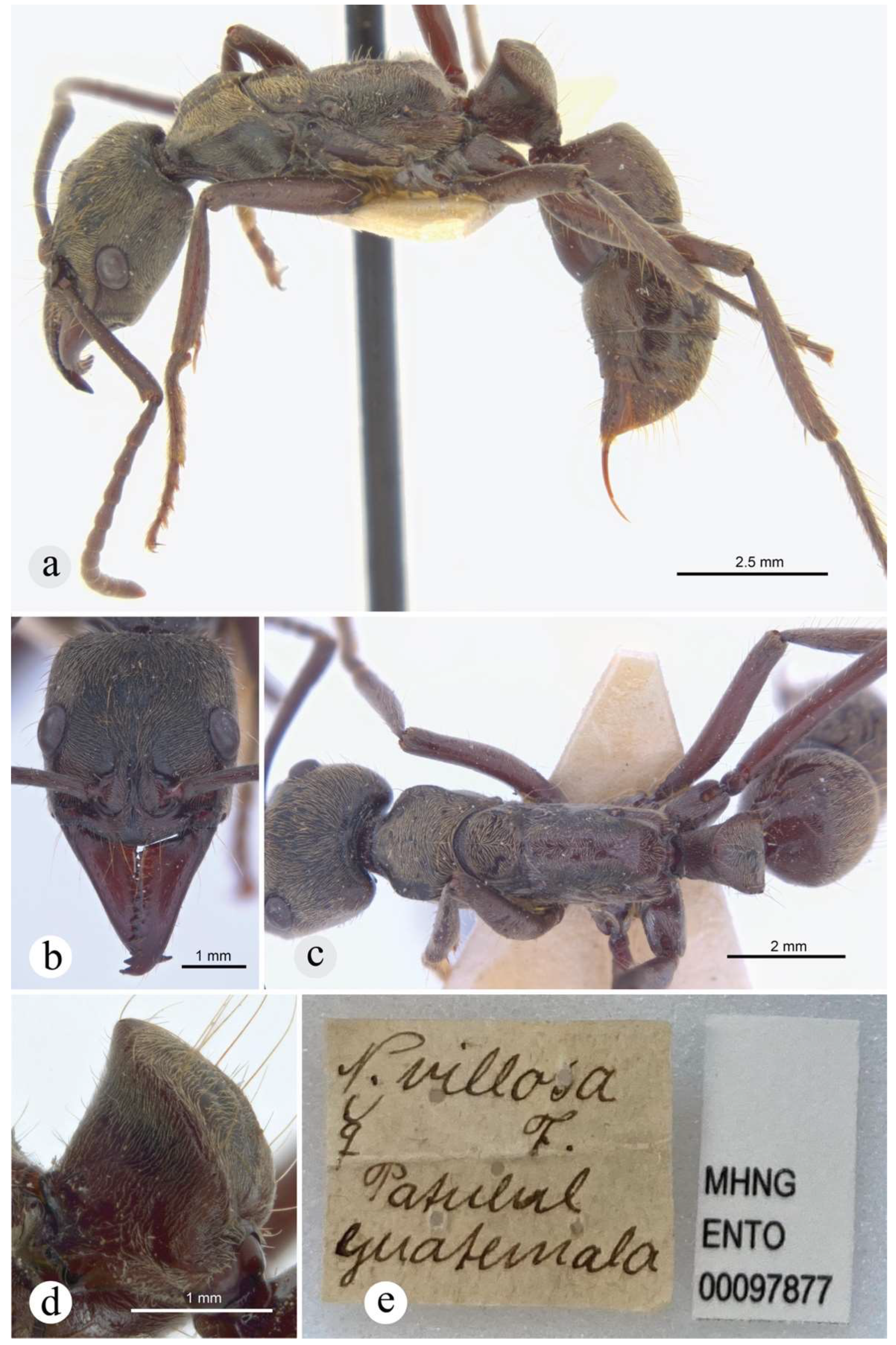
☿ neotype [present designation], Guatemala, Patulul [information on label hand written by A. Forel] (MHNG: MHNGENTO00097877) [image examined]. Note: material from A. Forel collection at MHNG; misidentified by him as N. villosa].
Combinations. In Neoponera: Emery, 1901: 47; in Pachycondyla: MacKay & MacKay, 2010: 297
Subspecies of Neoponera villosa: Forel, 1901: 45.
Status as species. MacKay & MacKay, 2010: 297 [redescription].
Senior synonym of Neoponera inversa: Emery, 1911: 73
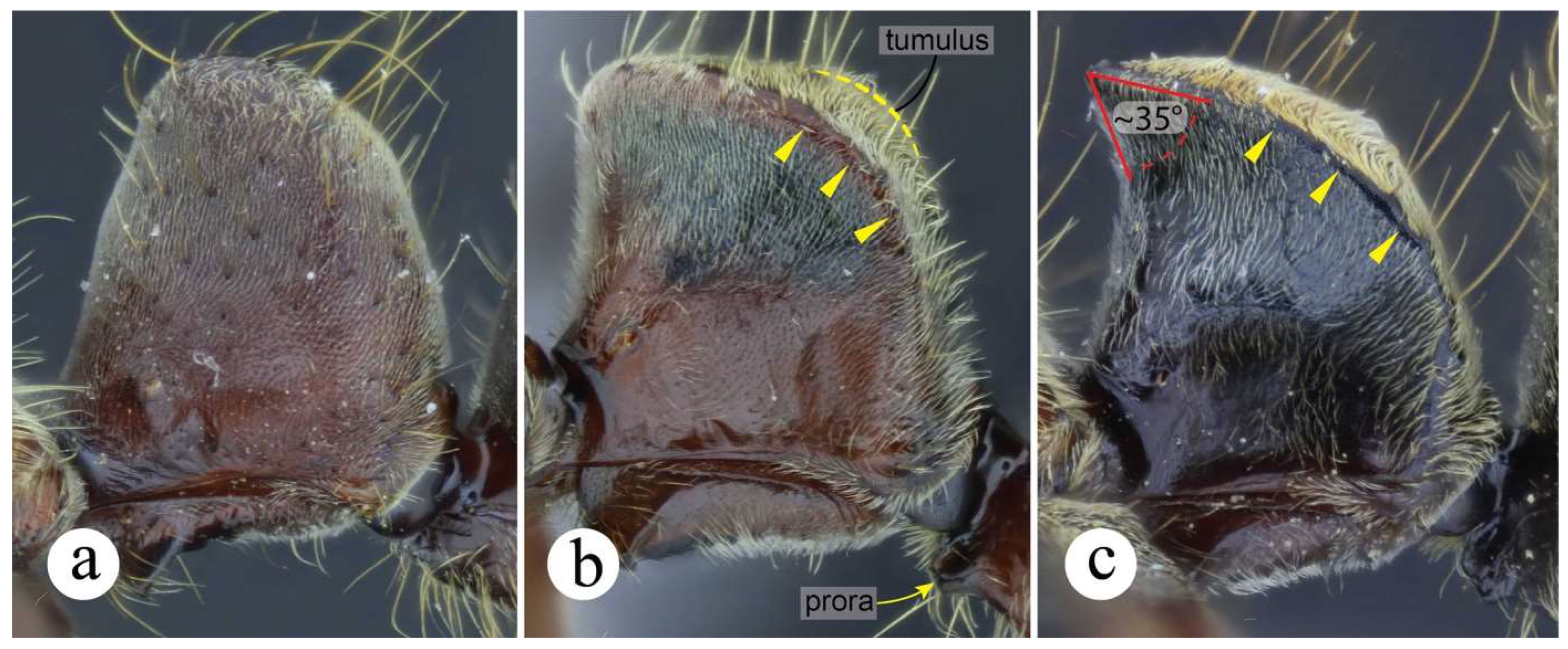
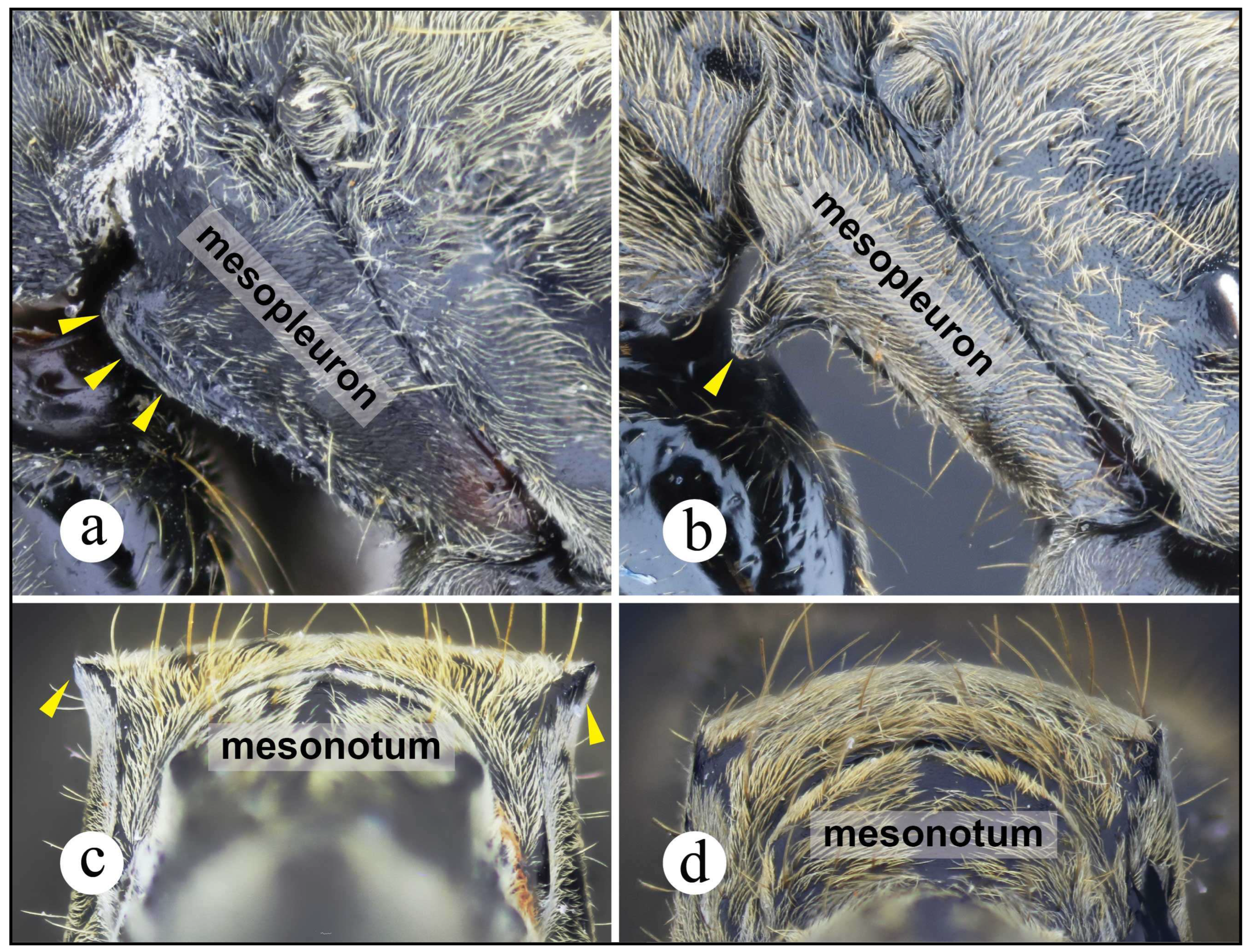
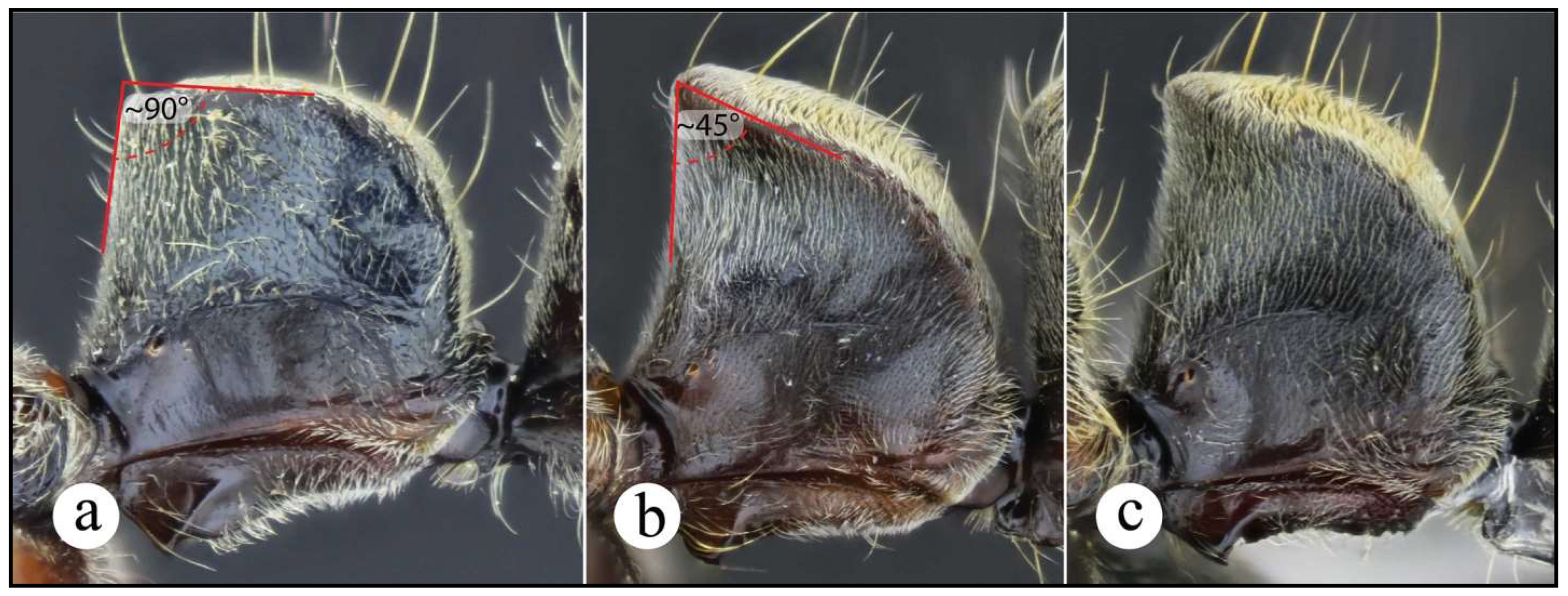
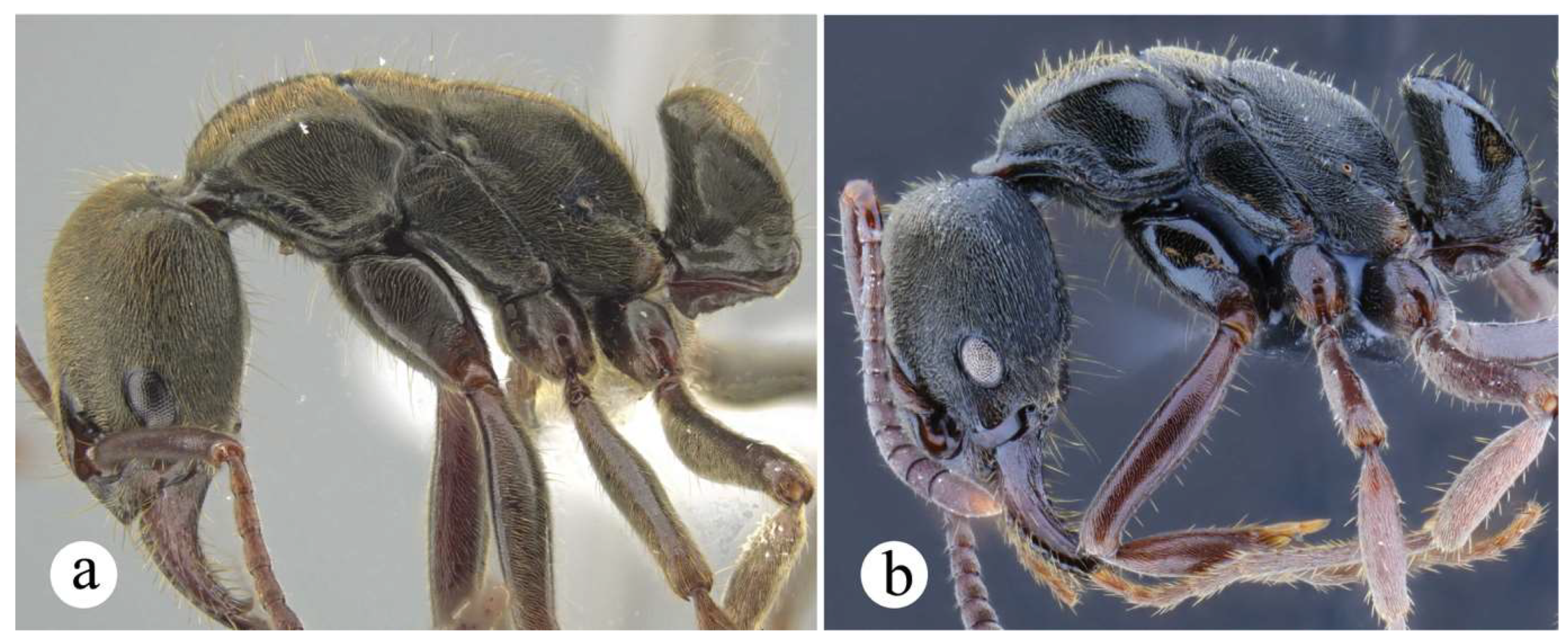
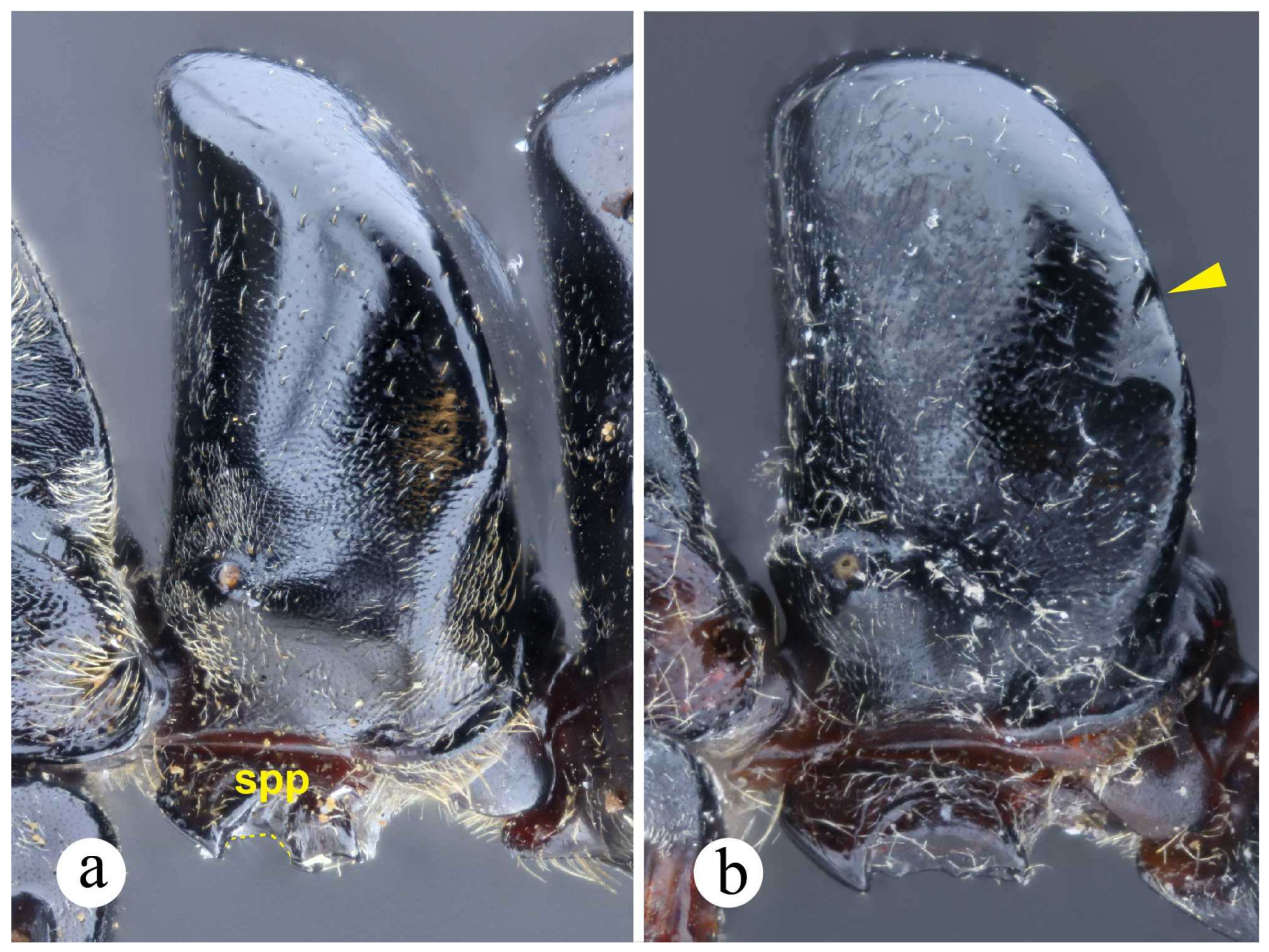
Worker and queen diagnosis. Head rectangular, longer than broad, lateral head margin moderately straight, posterior head margin concave (Fig. 18a); antennal scape, when pulled posterad, exceeding posterior head margin by two times apical scape width, usually slightly more (Fig. 18b); posterolateral propodeal margin with raised carina, feebly crenulate (Fig. 18a, 11a), posterior propodeal face usually slightly concave dorsally, just in between propodeal carinae; in lateral view, anterior margin of petiolar node straight, inclined posterad, distal portion usually slightly curved anterad (Fig. 8b, 18a); in lateral view, posterior nodal margin convex, meeting anterodorsally with anterior margin in acute angle, ca. 45° (Fig. 8b); anterolateral nodal face usually feebly deflate, this is better discernible on the distal nodal portion (Fig. 8b, 18d).
Worker. Measurements (n = 64): HW: 2.12-2.91; HL: 2.38-3.28; EL: 0.56-0.85; SL: 2.44-3.25; WL: 3.94-5.03; PrW: 1.56-2.19; MsW: 1.0-1.44; MsL: 0.75-1.22; PW: 1.12-1.56; PH: 1.25-1.72; PL: 1.08-1.62; GL: 3.69-6.0; A3L: 1.47-2.09; A4L: 1.56-2.16; A3W: 1.81-2.31; A4W: 1.94-2.5; TLa: 10.79-13.91; TLr: 11.5-15.31. Indices. CI: 75.56-118.42; OI: 23.92-32.35; SI: 97.78-129.41; MsI: 108.57-140.74; LPI: 83.7-113.08; DPI: 85.42-115.79.
Queen. Measurements (n = 16): HW: 2.62-3.12; HL: 2.94-3.44; EL: 0.72-0.91; SL: 2.56-3.25; WL: 4.75-5.56; PrW: 2.0-2.56; MsW: 1.81-2.06; MsL: 1.65-2.62; PW: 1.38-1.75; PH: 1.45-1.88; PL: 1.25-1.69; GL: 4.81-6.69; A3L: 2.0-2.38; A4L: 2.0-2.41; A3W: 2.31-2.69; A4W: 2.44-2.84; TLa: 13.31-15.38; TLr: 13.94-17.12. Indices. CI: 88.24-97.87; OI: 24.47-31.82; SI: 93.18-107.14; MsI: 76.54-112.12; LPI: 80.7-98.11; DPI: 83.02-120.0.
Male. Described by Fernandes et al. (2014). The following is added to their diagnosis: notauli not touching posteriorly; shallow to absent anteromedian dorsal groove on propodeum, showing horizontal rugae; propodeum posterolaterally with blunt carina which does not reach dorsum; in lateral view, anterior margin of petiolar node mostly straight (it does not show the typical distally curved projection of workers and queens); lateral nodal margin feebly angulate, but this is hardly discernible since it bears abundant, slightly raised piligerous punctae; abundant yellow subappressed pilosity, most of which is arranged in groups of stacked hairs, like if they were combed; abdominal sternum VII flat to feebly concave, bearing appressed hairs though not abundant.
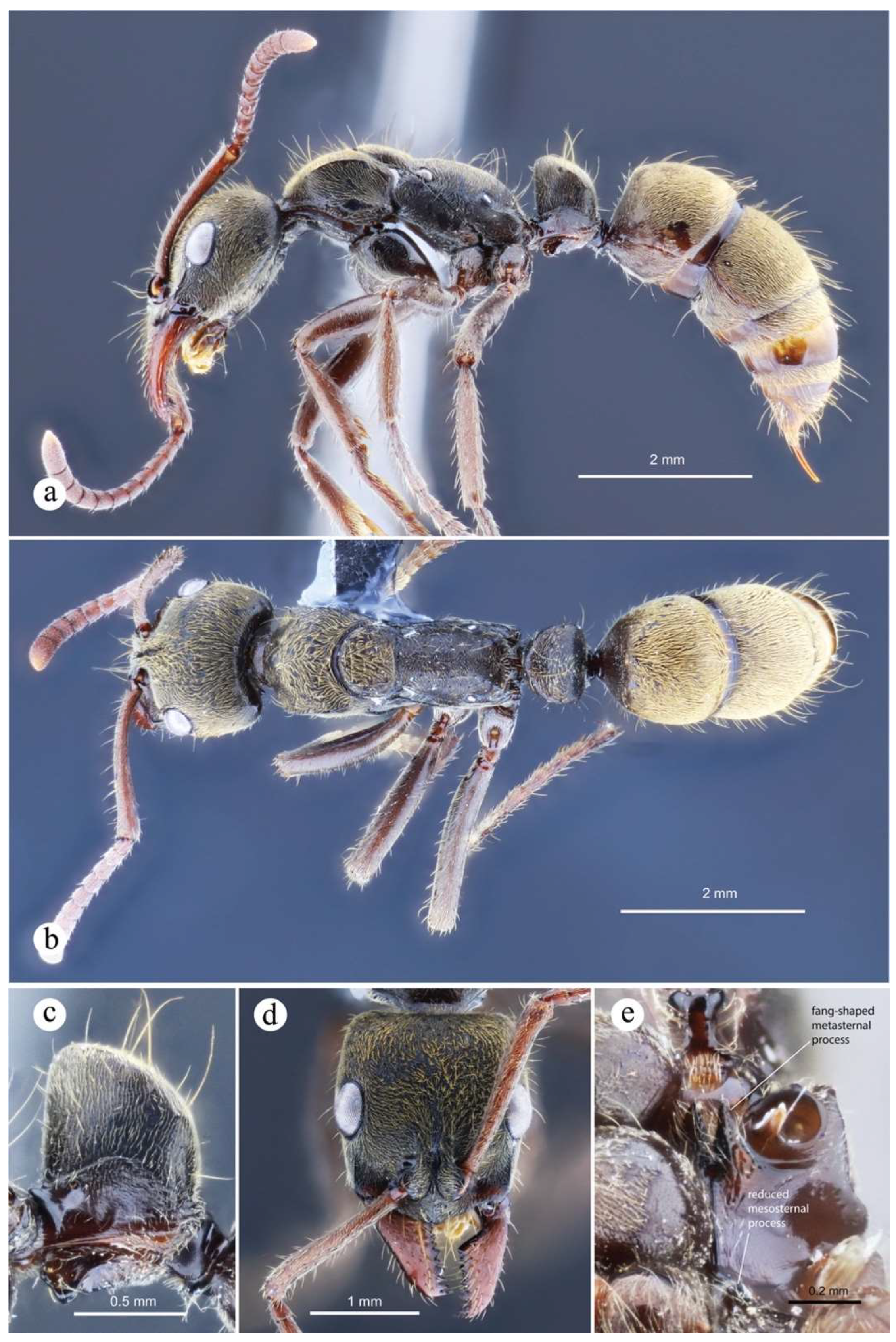
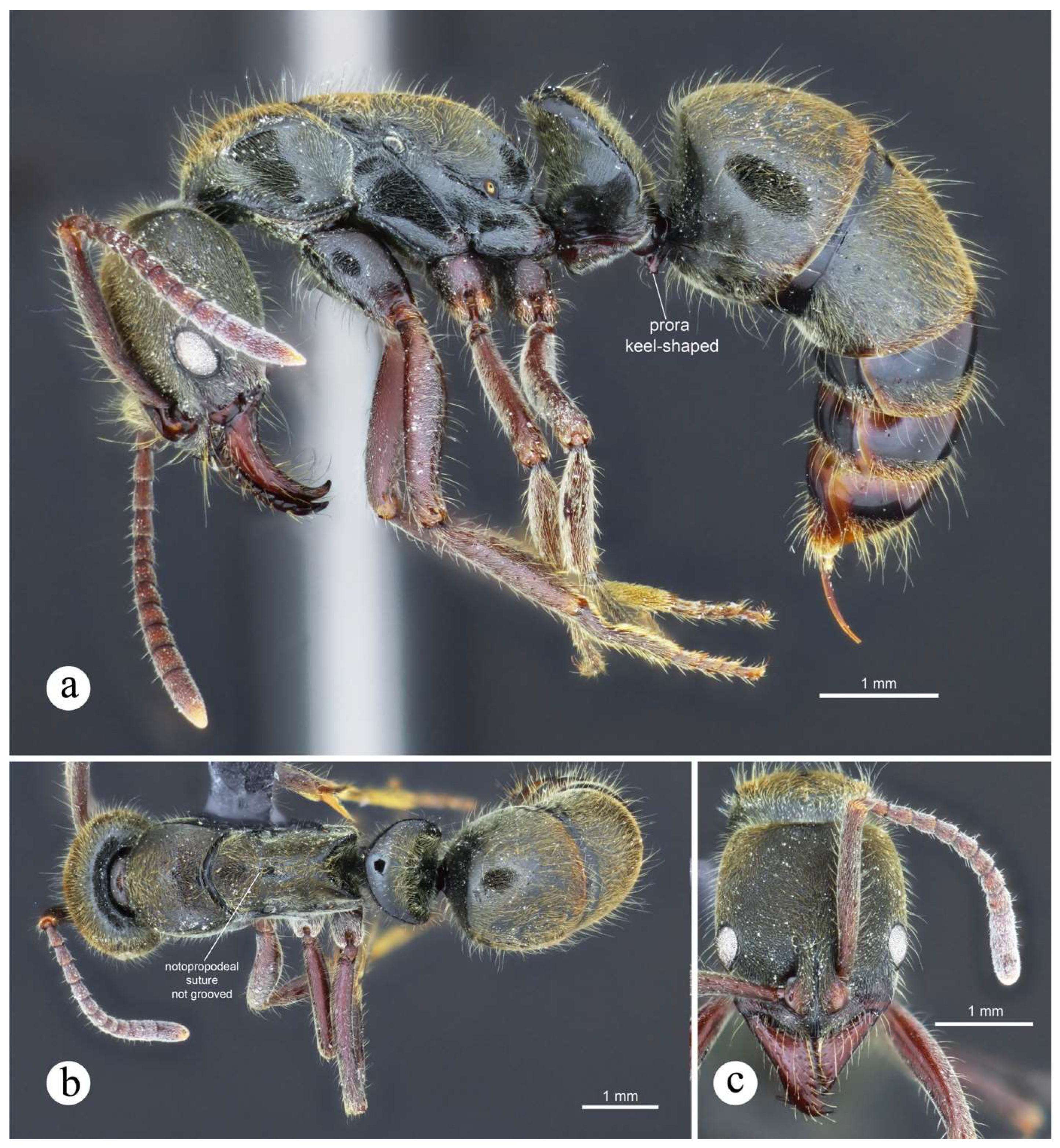
Figure 18.
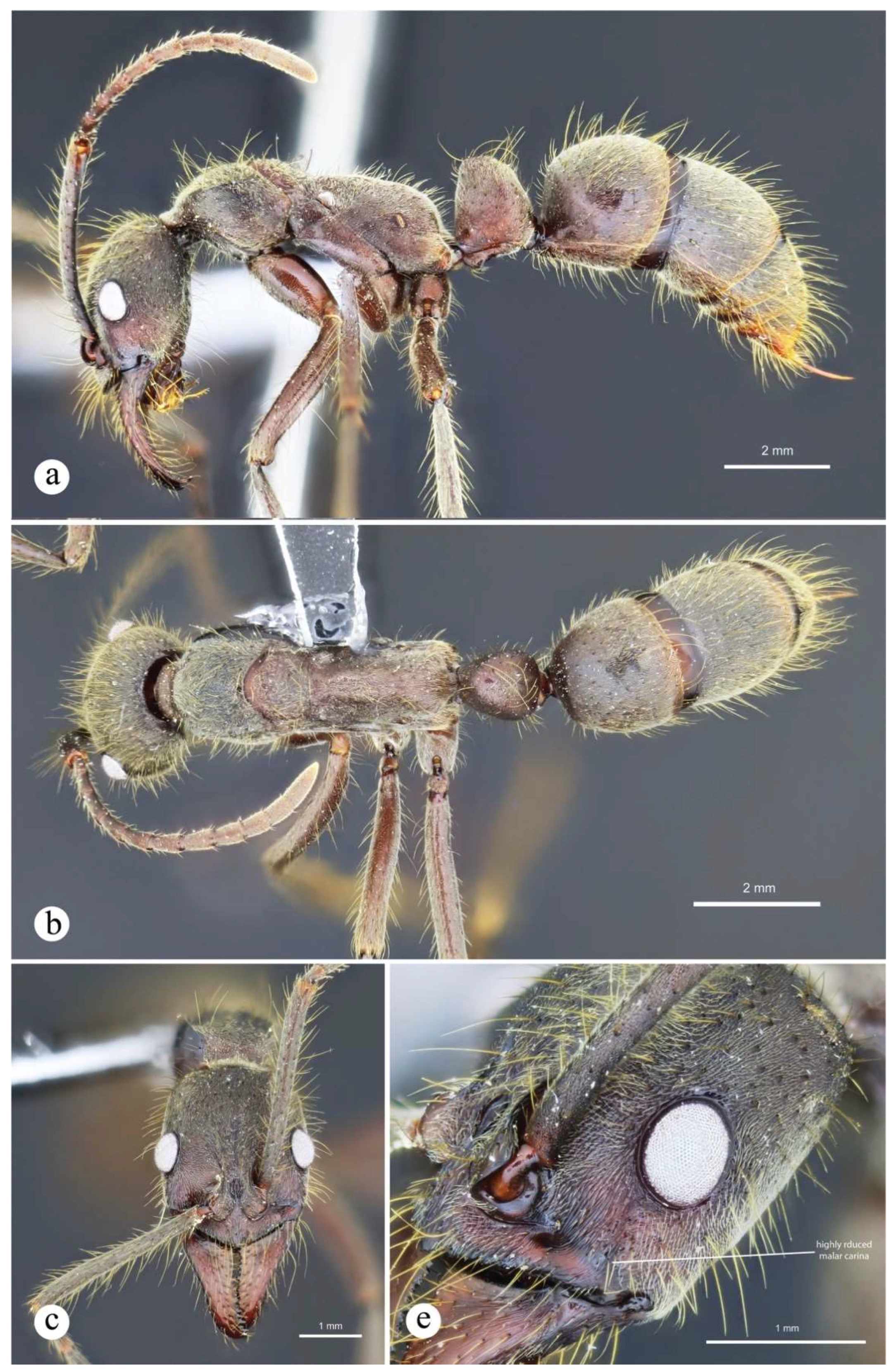
Neoponera curvinodis neotype. ☿ (MHNG: MHNGENTO00097877), Guatemala. a. Lateral view; b. Head in frontal view; c. Dorsal view; d. Petiole in anterolateral view; e. Collection labels. Note: A. Forel misidentified this specimen as N. villosa. Images by Bernard Landry, MHNG.
Figure 18.
Neoponera curvinodis neotype. ☿ (MHNG: MHNGENTO00097877), Guatemala. a. Lateral view; b. Head in frontal view; c. Dorsal view; d. Petiole in anterolateral view; e. Collection labels. Note: A. Forel misidentified this specimen as N. villosa. Images by Bernard Landry, MHNG.
Male. Measurements (n = 4): HW: 1.38-1.72; HL: 1.44-1.69; EL: 0.84-0.89; SL: 0.31-0.38; WL: 4.06-4.5; PrW: 1.62-1.75; MsW: 1.84-2.09; MsL: 2.31-2.5; PW: 0.78-0.88; PH: 0.94-1.09; PL: 1.06-1.19; GL: 5.0-5.62; A3L: 1.44-1.72; A4L: 1.56-1.81; A3W: 1.31-1.59; A4W: 1.81-2.0; TLa: 9.69-10.78; TLr: 12.0-12.88. Indices. CI: 92.59-105.77; OI: 50.91-61.36; SI: 20.0-22.73; MsI: 74.68-90.54; LPI: 103.03-120.0; DPI: 69.44-82.35.
Comments. Neoponera curvinodis is the largest species in the N. foetida group (Fig. 12; ☿ TLa = 10.8 – 13.9 mm; ♀︎TLa = 13.3 – 15.4 mm), and is the second largest in the genus after N. commutata Roger. Size variation in the worker caste is relatively large (SD = 0.69), and it is assumed something similar occurs in the queen, but few specimens were gathered and measured so as to reach a more solid conclusion.
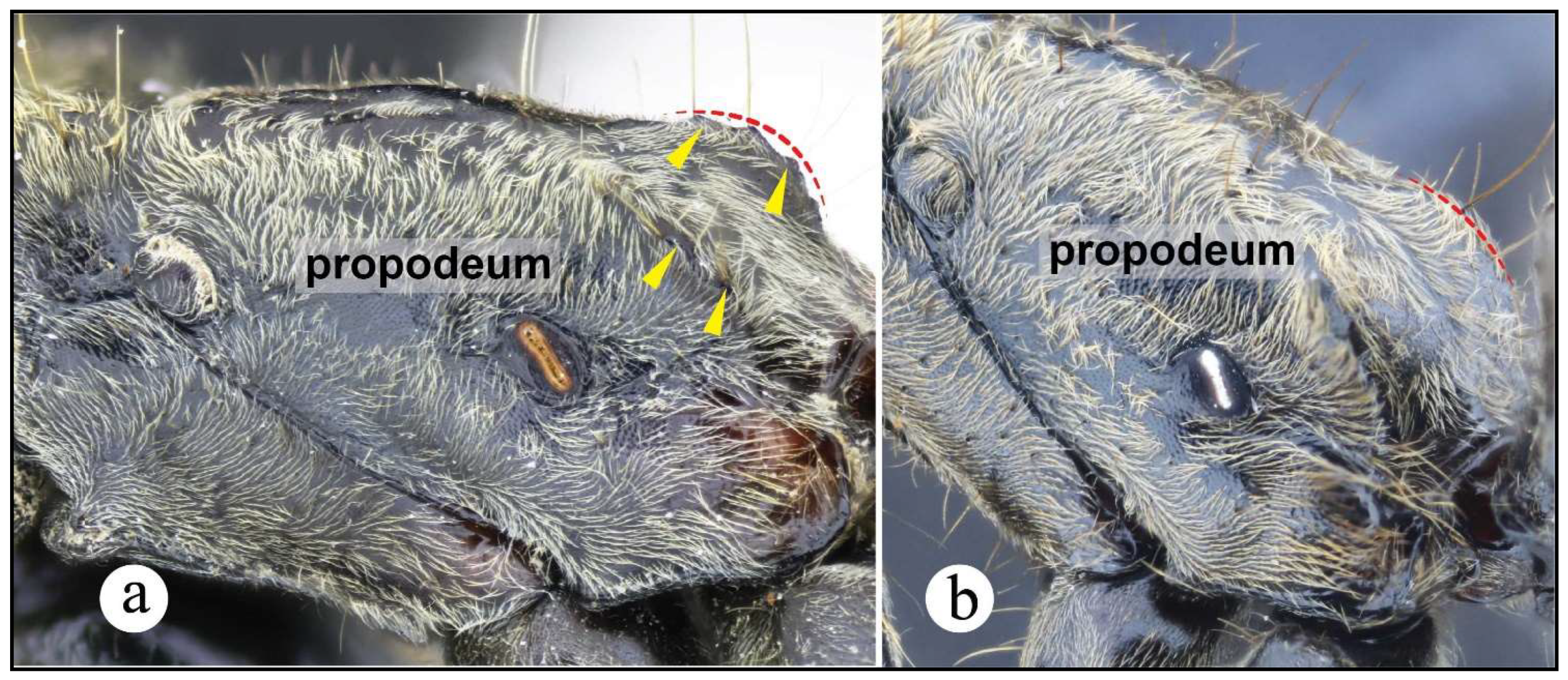
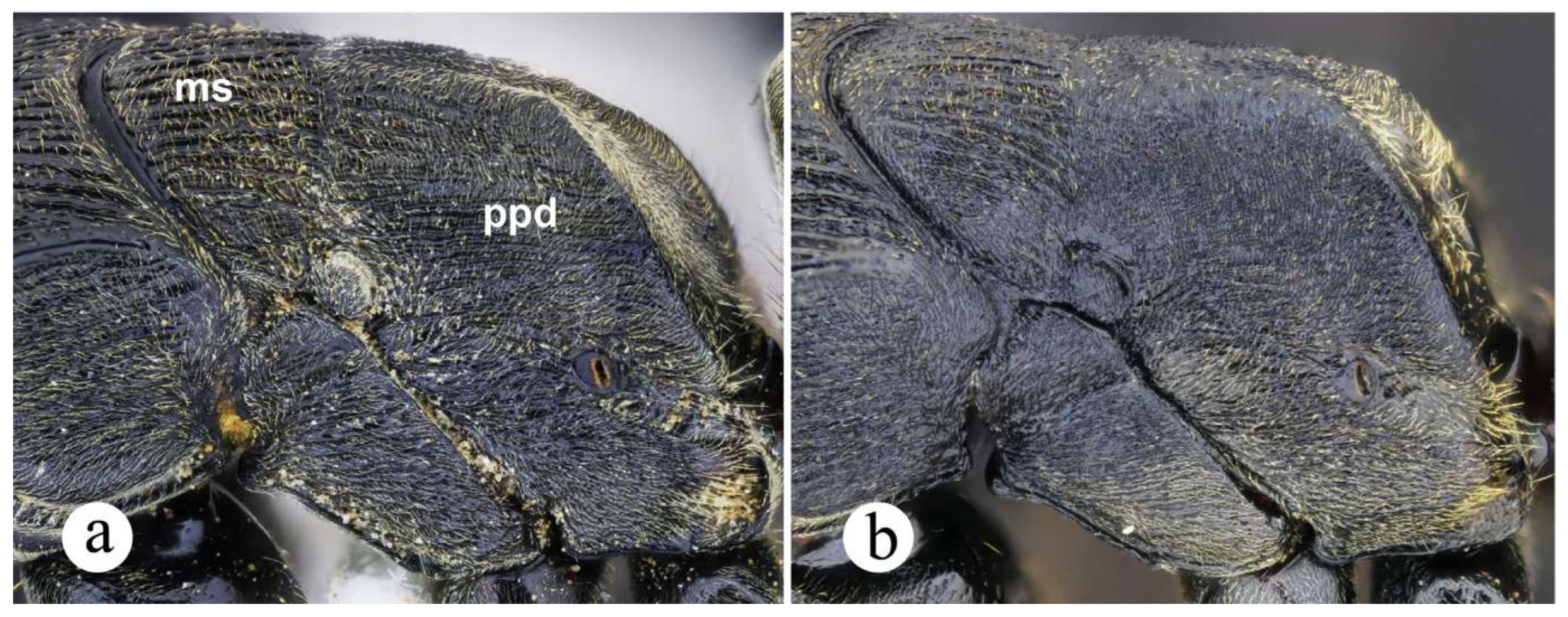
Neoponera zuparkoi, N. villosa, and in particular N. inversa are morphologically most similar to N. curvinodis (see comparison further below). Their distinction can be daunting if diagnostic features are not carefully examined. Plasticity is prevalent in both N. curvinodis and N. inversa. Some variations like the shape and orientation of the mid clypeal striae, or the shape of the humeral carina and of the petiolar node can certainly obscure their taxonomic distinction. For example, the humeral carina can be well-developed and salient (Fig. 7c, 18c), or just feebly emerging from the cuticle.
The following three body regions are considered plastic in both workers and queens in Neoponera curvinodis, none of these variations are geographically correlated: the head lateral margin, the carina at the propodeal posterolateral margin, and the anterior margin of the petiolar node. The first two are less variable than the third. As for the lateral head margin, a moderately straight form (Fig. 18b) is dominant over a somewhat curved form (link). Both variations are usually imperceptible and can be detected only when examining numerous specimens representing a broad geographic range. The propodeal carina, on the other hand, is easier to distinguish, it may be clearly raised with weak crenulae (Fig. 11a, 18a), which is the most common form, or it may be just slightly raised with almost no signs of crenulae (Fig. 11b). The distal region of the anterior nodal margin may be curved anterad (Fig. 8b), or the margin is just slightly concave to straight distally (Fig. 8c). When the first state is present, the anterolateral nodal face is usually deflate. Both forms, concave or straight nodal margin, can be present in specimens obtained from the same collection events, and even from the same nest series (Fig. 8b, c). The degree to which the cuticle of the petiolar node is deflate or contracted is perhaps the clearest example of plasticity in this species, but also in N. inversa. We hypothesize this could be the result of a physiological condition since it is present even among nestmates. We assume it could be due to variations in individual development, maybe linked to factors like diet (Thompson 1999, West-Eberhard 2005). If this hypothesis is further tested and potentially supported, then the question would be ¿Why it is present only in these lineages, i.e., N. curvinodis and N. inversa, and not in, for example, N. villosa and N. zuparkoi which are closely related to them? If this is not supported, and physiology does not play a role here, another potential explanation would come from evolution itself, by considering instead these changes in morphology, especially on the node, an adaptive response over (unknown) selection pressures of the environment. However, since both species occur in sympatry, especially in several South American regions (Fig. 30b), interspecific gene flow may also play a part here. If this is the case, then populational analyses, using for example maternally-inherited genetic material, would be useful for identifying first-generation hybrids, also called F1. A recent study by Weyna et al. (2022), showed that ultraconserved elements can also be used to unveil hybridization history in non-model organisms. They also found that amongst a number of tested organisms, ants showed the highest number of F1 hybrids, which suggests higher rates of recent hybridization.
Whatever the causes involved for explaining this phenomenon in both species, such phenotypic plasticity likely was the source of confusion while identifying N. curvinodis from its very similar congeners, in particular with N. inversa. The following features of workers and queens of N. curvinodis, in combination with the taxonomic key, make easier their distinction: 1) The lateral head margin is moderately straight, so that the head outline is typically rectangular-shaped (slightly convex in N. inversa); 2) The posterolateral propodeal margin usually has a crenulate, raised carina (absent or weakly developed in N. inversa); 3) The petiolar node is relatively broad in lateral view: ☿ PL = 1.1 – 1.63 mm, with the dorsoposterior margin meeting with the anterior margin in 45° angle. In N. inversa the node is narrower: ☿ PL = 1 – 1.25 mm, with its dorsoposterior margin usually meeting with the anterior margin in 30° – 35° angle; 4) The anterolateral distal nodal face is deflate (frequently), or not deflate at all (rarely), while in N. inversa is always deflate, so that the nodal top seems more curved anterodorsad than in N. curvinodis; 5) Neoponera curvinodis is bigger (☿ TLa = 10.8 – 13.9 mm) than N. inversa (☿ TLa = 10.2 – 12.2 mm). Notes: 1. While identifying these two taxa it is recommended making a final decision based on these five characters together; 2. Although the range cited for the variables Petiolar Length (PL) and Total Length accurate (TLa) was obtained from a number of specimens and localities in the Neotropics, the reader may expect these limits to fall out of such ranges.
Both N. curvinodis and N. inversa are distinguishable from N. villosa by examining the shape of the petiolar node: In N. villosa the anterior margin is vertically straight or nearly so, and the convex dorsoposterior face has a tumulus, better discernible in lateral view (Fig. 2b). This tumulus is absent in N. curvinodis and N. inversa. Also, the anterior half of the nodal dorsal margin is almost horizontally straight in N. villosa, it meets in ca. 90° angle with its anterior margin, and the nodal top is convex, in lateral view. In N. curvinodis and N. inversa the nodal dorsal margin is always inclined posterad, and the nodal top is relatively acute.
Neoponera curvinodis is also very similar to N. zuparkoi, but the nodal shape of this latter is not deflate anteriorly, and has a tumulus on the posterodorsal face. The nodal shape of N. zuparkoi is almost identical to that of N. villosa (see prior paragraph). In addition, the humeral carina of N. zuparkoi is not salient, while in N. curvinodis is either strongly or feebly salient. The anterior mesopleural margin of N. zuparkoi has a protruding, anteroventrally directed cuticular lobe (Figs. 7a-b). Such region in N. curvinodis is only carinate as in all other members of the N. foetida group.
Justification for the neotype designation. The primary type material, which should be located in MHNG in Geneve, is absent from collection (Bernard Landry, pers. comm.). Since the worker syntype was collected by Mr. Champion, the other possible repositories for this specimen are the BMNH in London, or the USNM in Washington D.C. Information on this subject was requested to the BMNH and their online collections database was searched. The USNM ant collection was also examined. The type material is not vouchered in those institutions. Brian Fisher’s imaging team could not locate this type in other relevant insect collections either. Designation of a neotype is necessary since this form is barely distinguishable from its highly similar congener, N. inversa, but also from N. zuparkoi and N. villosa, although with relatively less degree of difficulty. The chosen neotype is a worker from Patulul in Guatemala, a site approximately 230 Km west Las Mercedes, the type locality of this species. This specimen was misidentified by Auguste Forel as N. villosa (hand written on original label, Fig. 18e), and its morphology matches that of other specimens collected in Costa Rica, which are part of his collection preserved in MHNG, and identified by him as N. villosa var. curvinodis. The newly designated neotype is well-preserved and potentially suitable for molecular analyses.
Neoponera bactronica and N. billemma as junior synonyms of N. curvinodis. Two sources of evidence support these synonymies:
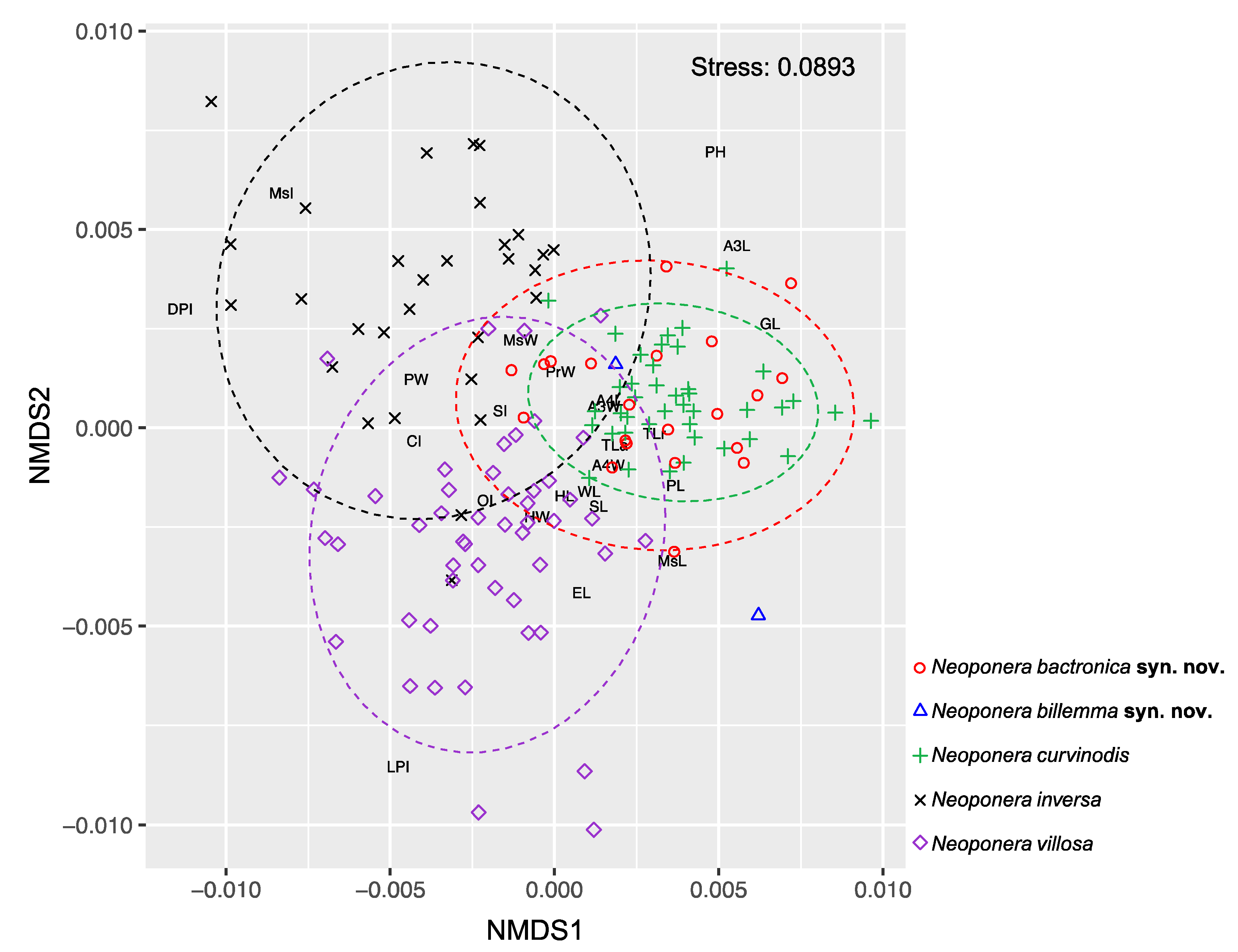
1) The morphology of the examined type material of N. bactronica and N. billemma match the form of the presently designated neotype of N. curvinodis. The above shown variable body regions detected in N. curvinodis are also present in examined populations of the synonymized forms. Furthermore, the NMDS analysis based on 24 morphometric variables shows that specimens previously identified as N. bactronica, N. billemma, and N. curvinodis share almost all of their morphospace represented in the first two dimensions of the ordination with a strong stress value of 0.0893, thus indicating the observations are not randomly grouped (Fig. 19). These results are statistically supported by the pairwise contrast test which suggests these three forms are not different (p adjusted = 1). The global PERMANOVA analysis, on the other hand, rejected the null hypothesis that all observations are equal (p < 0.000999, 33% variance explained). This result is coherent with the taxa that share only some of their morphospace, this is, N. inversa and N. villosa, and does not apply to the other three forms which are grouped under the morphospace of a single species, here considered N. curvinodis.
Figure 19.
Non-metric multidimensional scaling (NMDS) representing the distribution of specimens (icons) in the morphospace of some closely related species of the N. foetida group, two of which are here considered junior synonyms of N. curvinodis. Abbreviated letters represent the 24 morphometric variables involved in the analysis. The closer these are to the observations, the more relevant they are for explaining the observed ordination. Dashed circles represent the 95% confidence intervals.
Figure 19.
Non-metric multidimensional scaling (NMDS) representing the distribution of specimens (icons) in the morphospace of some closely related species of the N. foetida group, two of which are here considered junior synonyms of N. curvinodis. Abbreviated letters represent the 24 morphometric variables involved in the analysis. The closer these are to the observations, the more relevant they are for explaining the observed ordination. Dashed circles represent the 95% confidence intervals.
In addition, the diagnostic characters of both N. bactronica and N. billemma do not support their specific status. As for N. bactronica, the first two characters, namely “head strongly punctate on frontal face; notopropodeal groove strongly marked on dorsum” (Fernandes et al. 2014, pg. 136) are also present in all lineages of the N. foetida group. The dorsal punctae on the head are usually shown in combination with striae of differing depth degree. In Neoponera this trait is highly homoplastic, thus not useful for species diagnoses in most cases, except for few species in the N. aenescens group, like N. carbonaria (Smith) where the head dorsum is typically micropunctate. The second trait, the notopropodeal groove, shows little interspecific variation and is considered apomorphic in the N. foetida group. This trait is absent in almost all species of the N. crenata group, its siter clade, and is regained in the remaining Neoponera groups (Troya et al. unpublished). The third character “petiole without striae and longer than high in lateral view” (Fernandes et al. 2014, p. 136) is partially informative because the ratio of the nodal length vs its height is not intraspecifically constant. Also, most workers identified as N. bactronica have the node slightly higher than long as seen laterally, not longer than high as reported in Fernandes et al. (2014). Similar morphometric measurements were obtained in N. curvinodis and N. inversa. In N. villosa, the opposite pattern is found where the node is slightly longer than high, or rather symmetric. As for N. billemma, a pattern is impossible to discern since only two workers are known. However, the node of the paratype is also higher than long.
Current nodal morphometrics of N. curvinodis, N. inversa, and N. villosa match those of Lucas et al. (2002) for their morphospecies “Pvi2”, “Pvi1”, and “Pvv”, respectively. Lucas et al. (2002) believed Pvi2 belonged to a new species and provisionally called it “Pachycondyla subversa” (currently nomen nudum). Based on Forel’s (1899) illustration of N. curvinodis, specifically on the measurement of the petiolar node (height and length) and on the shape of the anterior margin, i.e., concave, Mackay and Mackay (2010) inferred that Pvi2 closely matches N. curvinodis. This is also supported here since the nodal shape of Pvi2 (Fig. 1c in Lucas et al. 2002) matches that of the neotype of N. curvinodis.
Therefore, if Pvi2 represents N. curvinodis (and not a new, but distinct species from N. inversa according to Mackay and Mackay 2010), then further erection of a new lineage (i.e., N. bactronica) was not required. Since N. curvinodis and N. inversa are so similar, commonly collected, and occur in sympatry in most regions for which we gathered specimens, it is crucial to first examine as much material as possible from their entire range. Examination of the type material is also very important, but also revising historical specimens identified by Forel.
Neoponera billemma, on the other hand, is diagnosed as a species with “Strong transverse striae on the [mid] clypeus; anterior face of petiole lightly striate below and concave” (Fernandes et al. 2014, p. 141). The first trait, the mid clypeal striae, is another plastic feature in Neoponera. Among the material thus far examined for the genus, these striae can vary intraspecifically with no detected geographical pattern. Although these striae are usually longitudinal in Neoponera, sometimes these are also: horizontal e.g., JTLC: INBIOCRI001281564 from Costa Rica (N. insignis); oblique, e.g., CPDC: AT570 from Bahia, Brazil (N. villosa); fingerprint-like, e.g., CPDC: AT922 from Distrito Federal, Brazil (N. curvinodis); mixed: horizontal and longitudinal, e.g., JTLC: CASENT0617097 from Honduras (N. bugabensis); or simply smooth, without striae, e.g., JTLC: JTL7597-S from Nicaragua (N. villosa). Out of the N. foetida group, the horizontally impressed striae also occur in for example, the N. crenata group: MZSP: MZSP123017 from Santa Catarina, Brazil (undescribed species). As for the second trait, “petiolar node lightly striate below”, cited as diagnostic for N. billemma in Fernandes et al. (2014), many specimens from all species in the N. foetida group show these striae in variable depth degree. Finally, the nodal anterolateral concavity is another plastic feature of N. curvinodis, as previously commented.
2) Phylogenetic evidence. The phylogeny of the genus based on ultraconserved elements recovered with high support (100 % bootstrap) N. curvinodis, N. inversa, and N. villosa as distinct but closely related lineages (Troya et al., unpublished). Neoponera zuparkoi and the morphospecies N. ecu2923 (see details further below under that species), emerge as sister to N. inversa, with low support, though. Santos et al. (2018) tested the specific status of the first three species and also included N. bactronica. Based on specimens from two regions of the Brazilian Atlantic Forest they sequenced two mitochondrial genes (CO1, 16S) and studied their karyotypes. They concluded these represent four distinct lineages. We agree with them except for N. bactronica which is grouped with N. curvinodis in the same clade (see their Fig. 2). Nonetheless, they suggested that N. bactronica and N. curvinodis diverged recently, and proposed further research on this issue. Mendoza-Ramírez et al. (2019) sequenced two mitochondrial genes (CO1, Cyt b) and eight nuclear microsatelites of the above species plus N. solisi and N. bugabensis. In their phylogeny N. curvinodis is sister to N. bactronica + N. villosa (Fig. 2 in their work). Neoponera bactronica, however, was represented by specimens from only two regions: a site in Mato Grosso (Brazil), and another from Colombia. Neoponera curvinodis was better represented by four specimens from: Chiapas (Mexico), Guatemala, Honduras, Nicaragua, and two from the same site in Mato Grosso. The authors showed that both N. bactronica and N. curvinodis have considerably high intraspecific genetic distances, up to 5.1% and 9.5 % for the CO1 and Cyt b, respectively, which is depicted in, for example, the long branch separating the Brazilian specimens from those of Colombia. They brought out two potential causes for this result: 1) A marked mitochondrial genetic structure which has been observed in other broadly ranged lineages, like Solenopsis saevissima (Smith), or 2) Each form could represent more than one species. Similarly, in the Neoponera phylogeny one N. curvinodis specimen (INPAHYM033801-2) from Santa Catarina, southern Brazil showed a significantly larger branch than its conspecific (DZUP549372) from Sergipe, northeast Brazil. The observed plasticity of this species probably mirrors its high intraspecific genetic distance, in particular between geographically divergent populations.
In this revision we cannot confirm nor deny a multispecies hypothesis to further understand the systematics of N. curvinodis. Yet, the present data set does not seem to favor a cryptic status since this taxon is now re-diagnosed, although showing some level of intraspecific variation among the proposed characters. This new diagnosis fits well in the phylogeny of Neoponera (Troya et al., unpublished) allowing distinction from its putatively closest species, N. inversa, N. villosa, and N. zuparkoi.
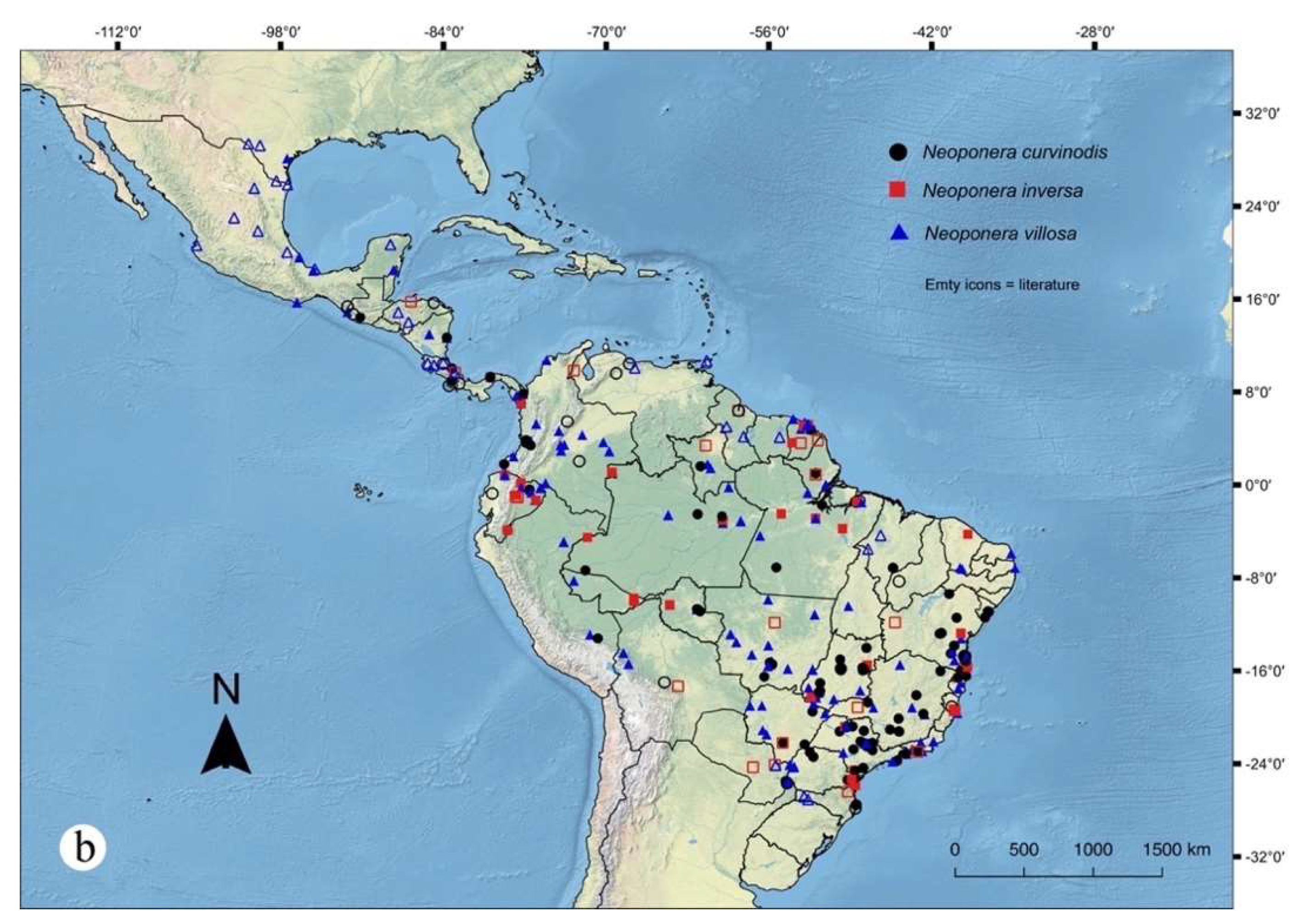
Natural history and distribution notes. See Mackay and Mackay (2010), and Fernandes et al. (2014). From the presently examined material: Neoponera curvinodis has been collected during daylight, in a variety of habitats from primary well-preserved, to second-growth, to relatively disturbed forests, using mostly canopy-aimed techniques like fogging, pitfall and arboreal Malaise, and less intensely through CDC, and mercury light trapping, Malaise, beating, sweeping, pan-trapping, Winkler, and hand collecting. Specimens and/or colonies have been found in Cinnamomum chavarrianum (Lauraceae), Miconia cinerascens, Clidemia hirta (Melastomataceae), Qualea grandiflora (Vochysiaceae), Theobroma cacao (Malvaceae) in agriculture systems, as well as in palms (e.g., Bactris spp.) and bromeliads. The elevational record extends from nearly the sea level up to 2300 m, this latter obtained in Cordillera del Cóndor, Ecuador. Neoponera curvinodis has been abundantly collected in the Atlantic Forest, and a number of records belong also from most Brazilian biomes except for the Pampa. Outside Brazil, records span from Amazonia, to the Venezuelan Cordillera de la Costa and Formación Lara-Falcón, to the Chocó-Darién and the Pacific and Atlantic lowland forests in Central America.
Material examined. 218☿️, 22♀, 15♂. BRAZIL – Acre: • 1☿️; Senador Guiomard; -10.0667, -67.6167; alt. 214m; 2014-05-31; Denicol, M. R.; Santos, A. M. leg.; arboreal pitfall; (DZUP). – Amapá: • 1♀; [no locality given]; 1.0, -52.0; 1978-10-22; Torres, M. leg.; (MPEG). • 1☿️; [no locality given]; 1.0, -52.0; 1978-10-21; Torres, M. leg.; (CPDC). – Amazonas: • 1☿️; Br. 174, Km 44; -2.724, -60.0472; alt. 72m; 1994-11-17; Harada, A. leg.; (INPA). • 1☿️; Carabinaui river; -2.5307, -62.1502; alt. 146m; 1995-04-28; Motta; et al. leg.; light trap; (INPA). • 1♂; Liberdade river, Estirão da preta; -7.35, -71.8167; alt. 175m; 2011-05-11; Rafael, J.; et al. leg.; hand collected; (INPA). • 1☿️; Manaus; -3.1167, -60.0167; alt. 65m; 1989-02-26; Vogh, R. leg.; (INPA). • 1♀; Reserva Florestal Adolpho Ducke, Instituto Nacional de Pesquisas da Amazônia (INPA); -2.9167, -59.9833; alt. 65m; 2010-05-16; Belmont, E. leg.; (INPA). – Bahia: • 2☿️; 1.5 km E of Guaratinga; -16.5833, -39.7667; alt. 151m; 2002-12-06; Santos, J. R. M. leg.; baiting; (CPDC). • 1☿️; 16 km N of Vitoria da Conquista; -15.0333, -49.9; alt. 752m; 2003-07-14; Carmo, J. C. S. leg.; (CPDC). • 1☿️; 20 km E of Anadaraí; -12.7508, -41.1669; alt. 323m; 2001-03-16; Santos, J. R. M. leg.; (CPDC). • 1♂; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 1986-10-15; Terra, P. leg.; (MZSP). • 1♂; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 1988-02-23; Terra, P. leg.; (INPA). • 1♀; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.7659, -39.2159; alt. 53m; 1999-01-28; Delabie, J. H. C. leg.; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 1998-11-01; Fresneau, D. leg.; (MZSP). • 3☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.7659, -39.2159; alt. 53m; 2017-08-01; Carvalho, E.; Queiroz, J. leg.; Malaise; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.7659, -39.2159; alt. 53m; 2012-05-17; Silva, J. A. leg.; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 1998-11-01; Fresnau, D. leg.; (INPA). • 1☿️; Andaraí; -12.8, -41.33; alt. 400m; 1993-01-01; Almeida, C. leg.; (CPDC). • 1☿️; Andaraí; -12.758, -41.177; alt. 340m; 2001-03-16; Santos, J. R. M. leg.; (CPDC). • 1♂; Fazenda Bom Jesus; -7.1397, -45.3467; alt. 214m; 1992-04-11; Cardoso, P. leg.; (INPA). • 3☿️; Fazenda Bom Jesus; -7.1397, -45.3467; alt. 214m; 1992-02-10; Cardoso, P. leg.; (CPDC). • 2☿️; Fazenda Bom Jesus; -7.1397, -45.3467; alt. 214m; 1992-04-11; Cardoso, P. leg.; (INPA). • 3☿️; Fazenda Silêncio ; -13.8585, -40.0838; alt. 214m; 1997-12-02; Argolo, J. S. leg.; (CPDC). • 1☿️; Ilhéus; -14.7972, -39.035; alt. 23m; 1988-09-27; Diniz leg.; hand collected; (DZUP). • 1☿️; Indaiá; -14.52, -40.37; alt. 790m; 1988-02-04; Santos, J. R. M. leg.; (CPDC). • 1☿️; Itabuna; -14.79, -39.28; alt. 60m; 2002-09-01; Santos, J. R. M. leg.; (CPDC). • 1☿️; Itapebi; -15.9687, -39.5321; alt. 195m; 1980-04-11; Forbes; Benton leg.; (CPDC). • 1☿️; Porto Seguro; -16.4455, -39.0658; alt. 3m; 1998-11-07; (CPDC). • 1☿️; Porções; -14.52, -40.36; alt. 770m; 2004-01-25; Mariano, E. leg.; (CPDC). • 1♂; Reserva Biológica do Una; -15.1768, -39.1053; alt. 108m; 2011-11-01; Sena, D. U. leg.; (CPDC). • 1☿️; São Domingos, Praia do Norte ; -14.7466, -39.0627; alt. 5m; 1995-05-07; Delabie, J. H. C. leg.; (CPDC). • 1☿️; São José, Chapada Diamantina; -11.4408, -39.8778; alt. 392m; 2001-03-22; Santos, J. R. M. leg.; Winkler; (CPDC). – Distrito Federal: • 1☿️; APA Gama Cabeça de Veado; -15.8667, -47.8333; alt. 1080m; (CPDC). • 1☿️; Area de Proteção Ambiental Gama Cabeça de Veado; -15.8667, -47.8333; alt. 1080m; 2000-03-02; Mirelle, P. leg.; (DZUP). • 1☿️; Area de Proteção Ambiental Gama Cabeça de Veado; -15.8667, -47.8333; alt. 1080m; 2000-02-01; Mirelle, P. leg.; (CPDC). • 2♀; Brasilia; -15.8261, -47.9207; alt. 1080m; 1976-07-06; Diniz, J. leg.; (DZUP). • 1☿️; Brasilia; -15.8261, -47.9207; alt. 1080m; 1976-07-06; Diniz, J. leg.; (DZUP). • 1☿️; Fazenda Agua Limpa; -15.9488, -47.9342; alt. 1085m; 2007-05-24; Maravalhas, J. leg.; (CPDC). • 1☿️; Parque Nacional de Brasilia; -15.7293, -47.9613; alt. 1120m; 2014-01-18; Sendoya, S. leg.; (DZUP). • 1☿️; RECOR IBGE; -15.9163, -47.8672; alt. 1161m; 2017-11-01; Lasmar, C. leg.; pitfall; (DZUP). – Goiás: • 1☿️; 17 km NE of Jataí, Fazenda Rio Paraíso; -17.7333, -51.6167; alt. 891m; 2011-01-28; Diniz, J. leg.; pan trap; (DZUP). • 1☿️; Açude; -17.8587, -51.727; alt. 788m; 2005-08-20; Paniago, G. leg.; (DZUP). • 1♀; Fazenda Luziana; -17.8715, -51.7309; alt. 831m; 2002-05-08; Diniz.; et al. leg.; (DZUP). • 1☿️; Fazenda Santa Lucia; -17.0635, -51.6155; alt. 537m; 2008-10-10; Diniz, J. leg.; (DZUP). • 1☿️; Goiás; -15.8257, -49.836; 1970-01-01.000001980; Redford, K. leg.; (INPA). • 1☿️; Parque Nacional Chapada dos Veadeiros; -14.0388, -47.623; alt. 1287m; 2003-02-01; Ribas, C.; Madureira, M. leg.; arboreal pitfall; (DZUP). • 1♀; Parque Natural Municipal Mata Do Açude; -17.8605, -51.728; alt. 791m; 2005-11-12; Diniz, J. leg.; (DZUP). • 1☿️; Parque Natural Municipal Mata Do Açude; -17.8605, -51.728; alt. 791m; 2005-12-21; (DZUP). • 1☿️; Pequena Central Hidrelétrica (PCH) de Fazenda velha; -17.9706, -51.7627; alt. 623m; 2009-03-18; pan trap; (DZUP). – Mato Grosso: • 1☿️; Chapada dos Guimarães; -15.45, -55.7333; alt. 726m; 1983-11-27; (DZUP). • 1☿️; Chapada dos Guimarães; -15.45, -55.7333; alt. 726m; 1983-07-08; (DZUP). • 1☿️; Chapada dos Guimarães; -15.3, -55.9333; alt. 257m; 1983-12-04; (DZUP). • 1♀; Colégio Agricultura Buriti, Chapada dos Guimarães; -15.45, -55.7333; alt. 726m; 1982-11-12; Zanuto, M. leg.; (MPEG). • 1☿️; Sesc. Pantanal; -16.5083, -56.4161; alt. 135m; 2017-04-01; Lasmar, C. leg.; pitfall; (DZUP). – Mato Grosso do Sul: • 1♀; 28 km E of Bataiporã; -22.35, -52.9333; alt. 294m; 2012-12-03; Savaris, M.; Lampert, S. leg.; (DZUP). • 1☿️; Dourados Rodovia Itahum Km 2; -22.221, -54.8055; alt. 445m; 2019-07-14; Santos,P.; et al. leg.; (DZUP). – Minas Gerais: • 1☿️; Boa Esperança; -21.0667, -45.6; alt. 845m; 2014-03-19; Queiroz; et al. leg.; pitfall; (DZUP). • 1☿️; Chapada dos Guimarães; -15.3, -55.9333; alt. 308m; 1983-07-04; (DZUP). • 1☿️; Fazenda Recife; -16.0167, -41.2667; 1989-07-21; Paiva, R. leg.; (MZSP). • 1☿️; Itumirim; -21.2333, -44.8167; alt. 925m; 2014-02-19; Queiroz; et al. leg.; arboreal pitfall; (DZUP). • 1☿️; Itumirim; -21.2167, -44.8333; alt. 925m; 2014-02-19; Queiroz; et al. leg.; arboreal pitfall; (DZUP). • 1☿️; Itumirim; -21.2167, -44.8333; alt. 885m; 2014-02-19; Queiroz; et al. leg.; arboreal pitfall; (DZUP). • 1☿️; Parque Estadual do Rio Preto; -18.1167, -43.3333; alt. 845m; 2017-11-01; Reis, A.; et al. leg.; pitfall; (DZUP). • 1☿️; Pq. Floresta do Río Doce; -19.7, -42.7333; alt. 580m; 1989-09-23; Mel, G. A. R. leg.; (DZUP). • 1♀; UFU, Campus Santa Mônica; -19.5167, -52.2667; alt. 870m; 2013-12-23; Bueno, B. leg.; (DZUP). – Paraná: • 1☿️; 7 km NW of Paranavaí, BR376 km 96; -23.0167, -52.5; alt. 486m; 2019-02-20; Azevedo, F.; Freitas, D. S. leg.; pitfall; (DZUP). • 1♀; Antonina; -25.3, -48.7667; alt. 114m; 2014-03-02; Calixto, J.; Feitosa, R. leg.; (DZUP). • 1☿️; Antonina; -25.3, -48.6833; alt. 25m; 2009-09-14; Donatti, A. J.; Souza, J. M. T. leg.; (DZUP). • 1♀; Antonina centro; -25.4307, -48.7127; alt. 40m; 1966-01-19; Azevedo, M. leg.; (DZUP). • 1☿️; Entorno do rio Brumado; -25.3402, -48.885; alt. 397m; 2017-10-18; Pinto, A.; et al. leg.; (DZUP). • 1☿️; Foz do Iguaçu; -25.5, -54.5833; alt. 206m; 2000-08-20; Delabie, J. leg.; hand collected; (CPDC). • 1♀; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1984-12-06; Malaise; (DZUP). • 3☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-12-27; Malaise; (DZUP). • 2☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-05-13; Malaise; (DZUP). • 1☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1984-12-10; Malaise; (DZUP). • 1☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1984-12-03; Malaise; (DZUP). • 1☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-05-17; light trap; (DZUP). • 1☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-01-21; Malaise; (DZUP). • 1☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-02-04; Malaise; (DZUP). • 2☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-01-06; Malaise; (DZUP). • 2☿️; Instituto Agronômico do Paraná; -25.4, -49.25; alt. 963m; 1985-01-28; Malaise; (DZUP). • 1☿️; Morretes; -25.4, -49.25; alt. 963m; 1985-02-25; Malaise; (DZUP). • 1☿️; Ourizona; -23.4167, -52.2; alt. 457m; 2005-07-23; Barbosa, A. C. leg.; (DZUP). • 5☿️; Parque Nacional Iguaçu, 19 Km SE Foz do Iguaçu; -25.65, -54.4333; alt. 225m; 2017-10-01; Troya, A. leg.; hand collected; (MEPN). • 1☿️; Reserva Guaricica, Sede SPVS; -25.3136, -48.6956; alt. 6m; 2019-03-23; (DZUP). • 1☿️; Reserva Natural Guaricica; -25.3, -48.6833; alt. 25m; 2010-01-14; Donatti, A. J.; Souza, J. M. T. leg.; (DZUP). • 1☿️; Reserva Natural Guaricica; -25.3, -48.6833; alt. 25m; 2009-12-09; Donatti, A. J.; Souza, J. M. T. leg.; (DZUP). • 1☿️; Reserva Natural Guaricica; -25.3, -48.6833; alt. 25m; 2010-12-12; Souza, J. M. T. leg.; (DZUP). • 1☿️; Reserva Natural Guaricica; -25.3, -48.6667; alt. 110m; (DZUP). – Pará: • 1☿️; Benevides ; -1.35, -48.25; alt. 31m; 1988-06-16; Bittencourt, N. leg.; (MZSP). • 1☿️; Fazenda Florentino; -7.1167, -55.3833; alt. 967m; 2010-12-12; Krinsk, D. leg.; pitfall; (DZUP). • 1☿️; Floresta Nacional Caxiuanã; -1.7333, -51.45; alt. 21m; 2016-09-29; Silva, R.; et al. leg.; pitfall; (MPEG). – Pernambuco: • 1☿️; Tapera; -9.3898, -40.5146; alt. 381m; 1929-01-26; Pickel, B. leg.; (INPA). – Rio de Janeiro: • 2☿️; Ilha Grande; -23.1518, -44.2289; alt. 991m; 2013-11-18; Leponce, M.; Queiroz, J. leg.; (CPDC). • 1☿️; [locality not given]; -22.9477, -43.212; alt. 280m; 1927-12-22; Conde, O. leg.; (INPA). – Rondônia: • 1♀; Ji-Paraná; -10.88, -61.95; 1984-07-15; Overal, W. leg.; hand collected; (MPEG). • 1☿️; Reserva do INPA; -10.7, -62.2; 1985-03-29; Ramos, F. leg.; hand collected; (MPEG). – Roraima: • 1☿️; Parque Nacional Serra da Mocidade; 1.6, -61.9; alt. 600m; 2016-01-15; Xivier, F.; et al. leg.; (INPA). – Santa Catarina: • 1♀; Unidade de Conservação Ambiental Desterro-UCAD; -27.5167, -48.5; alt. 241m; (CPDC). • 1♀; Unidade de Conservação Ambiental Desterro-UCAD; -27.5167, -48.5; alt. 241m; 2005-08-19; Zillikens, A. leg.; (INPA). • 1☿️; Unidade de Conservação Ambiental Desterro-UCAD; -27.5833, -48.5333; alt. 22m; 2005-08-19; Schmid, V. leg.; (DZUP). • 3☿️; Unidade de Conservação Ambiental Desterro-UCAD; -27.5167, -48.5; alt. 241m; 2005-08-19; Zillikens, A. leg.; (INPA). – Sergipe: • 1☿️; Santa Luzia do Itanhi; -11.4167, -37.4167; alt. 6m; 1993-10-05; Delabie, J. leg.; hand collected; (CPDC). • 1☿️; São Cristovão; -10.9, -37.1833; alt. 21m; 1995-01-01; Feneron , R. leg.; (CPDC). • 1♀; UFS; -10.9167, -37.1; alt. 7m; 2015-06-16; Cruz, N. G. leg.; (DZUP). • 1☿️; Universidade Federal de Sergipe, Campus São Cristóvão; -10.9264, -37.1026; alt. 9m; Feneron, R. leg.; (CPDC). – São Paulo: • 1♀; 33 km SW of José Bonifácio, Rio Tietê; -21.2199, -49.9575; alt. 359m; 1979-09-02; Diniz, J. leg.; (DZUP). • 3☿️; Eng. Schmitt; -20.8669, -49.31; alt. 517m; 1970-11-16; Diniz, J. L. M.; Caballero leg.; (DZUP). • 1☿️; Estação Ecologica de Itirapina; -22.2144, -47.9234; alt. 770m; 2014-03-06; Sendoya, S. leg.; (DZUP). • 1☿️; Fazenda Campininha; -22.2167, -47.1167; alt. 722m; 1977-05-27; (DZUP). • 2☿️; Ilha dos pescadores (Ilha da Vitória); -23.75, -45.0; alt. 33m; 1964-03-24; D. Zoologia leg.; (MZSP). • 2☿️; Instituto de Biociências, Letras e Ciências Exatas (Ibilce), UNESP; -20.7852, -49.3598; alt. 533m; 1996-10-25; Izzo, T. J. leg.; (DZUP). • 1☿️; Iporanga; -24.5859, -48.5945; alt. 96m; 1961-11-01; Lenko, K.; Reichardt leg.; (INPA). • 6♂; Mata de Santa Genebra; -22.8228, -47.1094; alt. 631m; 2009-11-01; Fernandes, I. leg.; (INPA). • 1♀; Mata de Santa Genebra; -22.8228, -47.1094; alt. 631m; 2009-11-01; Fernandes, I. leg.; (INPA). • 61☿️; Mata de Santa Genebra; -22.8228, -47.1094; alt. 631m; 2009-11-01; Fernandes, I. leg.; (INPA). • 1☿️; Pratânia; -22.7667, -48.7333; alt. 657m; 2015-02-01; Veiga, P. A. S. leg.; (DZUP). • 1♂; Ribeirão preto, Campos da USP; -21.166, -47.8499; alt. 605m; 2019-03-02; Glaser, S. leg.; (DZUP). • 1☿️; Sete Barras; -24.3667, -47.9167; alt. 14m; 2004-07-11; Melo, G. leg.; Malaise; (DZUP). • 1☿️; Sete Barras; -24.3667, -47.9167; alt. 14m; 2005-03-24; Melo, G. leg.; Malaise; (DZUP). • 2☿️; Sete Barras; -24.3667, -47.9167; alt. 14m; 2005-01-08; Melo, G. leg.; Malaise; (DZUP). • 1☿️; Severínia; -20.8062, -48.8092; alt. 579m; 1995-09-12; Fowler, H. G. leg.; (CPDC). • 1☿️; Sitio Caranda; -22.0861, -48.1274; alt. 663m; 2016-12-01; Oliveira, J. leg.; CDC; (MPEG). • 1♂; UNESP - Campus; -22.397, -47.5478; alt. 628m; 1999-11-01; Fresneau, D. leg.; (CPDC). • 1☿️; UNESP - Campus; -22.397, -47.5478; alt. 628m; 1999-11-01; Fresneau, D. leg.; (CPDC). COLOMBIA – Chocó: • 3☿️; Estación Silvicultura al Bajo Atrato; 7.0406, -77.3378; alt. 149m; 1992-07-01; Mendoza, L. leg.; (MEPN). • 1☿️; Estación Silvicultura al Bajo Atrato; 7.0406, -77.3378; alt. 149m; 1994-02-01; Ferro, L. leg.; (ICN). • 1☿️; Parque Nacional Natural Los Katios, Centro administrativo Sautatá; 7.85, -77.1335; alt. 96m; 2003-05-20; Cansia, A. leg.; pitfall; (IAvH). – Nariño: • 1☿️; Tumaco; 1.7875, -78.7913; alt. 5m; 2015-09-16; (MEPN). – Valle del Cauca: • 1☿️; Anchicaya ; 3.616, -76.909; alt. 350m; 1970-11-01; (MUSENUV). • 1♀; Bajo Anchicaya ; 3.689, -76.94; alt. 270m; 1983-03-01; Quintero, J. leg.; (MUSENUV). • 2☿️; Bajo Anchicaya ; 3.689, -76.94; alt. 270m; 1983-03-01; Quintero, J. leg.; (MUSENUV). • 1♀; Cali; 3.4, -76.5; 1983-02-01; Manzano, M. leg.; (MUSENUV). • 1☿️; San Cipriano, Buenaventura; 3.84, -76.898; alt. 80m; 2002-02-28; Gutiérrez, C. leg.; (MUSENUV). • 1☿️; San Cipriano, Buenaventura; 3.84, -76.898; alt. 80m; 2002-06-08; Gutiérrez, C. leg.; (MUSENUV). COSTA RICA – Limon: • 2☿️; Zent, 23 Km WNW Puerto Limón; 10.0167, -83.2667; alt. 21m; 1958-11-01; Lara, F. leg.; (MZSP). – Puntarenas: • 1☿️; 14 km E Palmar Norte; 8.95, -83.3333; alt. 120m; 1985-08-01; Ward, P. S. leg.; (PSWC). • 1☿️; Parque Nacional Corcovado, Sirena Station; 8.533, -83.533; 1992-06-03; McDonald, M. J. leg.; (AMNH). ECUADOR – Manabi: • 1☿️; Estación Científica Río Palenque; -0.7333, -79.8; alt. 180m; 1980-12-29; Sandoval, S. leg.; (MZSP). – Napo: • 1☿️; Limoncocha; -0.3998, -76.6001; alt. 280m; 1972-06-30; Kazan, P. leg.; (QCAZ). – Zamora Chinchipe: • 2☿️; Paquisha alto, Hito 2, 15 Km SE Fruta del Norte, Cordillera del Cóndor; -3.9003, -78.4829; alt. 2325m; 2008-03-15; Troya, A. leg.; fogging; (MEPN). FRENCH GUIANA – Cayenne: • 1☿️; Base Vie; 5.1667, -52.65; alt. 10m; (CPDC). • 1☿️; Montagne dês Chevaux; 4.7333, -52.4333; alt. 75m; 2011-11-19; Team, S. E. A. G. leg.; (DZUP). NICARAGUA – Región Autónoma del Atlántico Sur: • 1☿️; RN Kahka Creek; 12.671, -83.7175; alt. 30m; 2011-06-06; LLAMA leg.; Malaise; (JTLC). PANAMA – Colón: • 2♂; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003-10-01; Dejean, A.; et al. leg.; (CPDC). • 1♀; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2004-03-08; Pizon, S. leg.; Malaise; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2522, -79.9894; alt. 96m; 2003-10-27; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2004-10-12; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003-09-01; Schmidl, J. leg.; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003-10-01; Dejean, A.; et al. leg.; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2004-02-17; Springae, N. D; Pizon, S leg.; Malaise; (CPDC). • 2☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003-04-01; (CPDC). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003-10-05; Kitching, R. leg.; Light trap; (CPDC). PERU – Cusco: • 1☿️; Quince Mil, km 8; -13.2167, -70.7167; alt. 633m; 2012-08-20; Cavichioli, R.; et al. leg.; Malaise; (DZUP).
Geographic range. Mexico (Chiapas)*, Guatemala, Honduras*, Nicaragua, Costa Rica, Panama, Colombia, Venezuela*, Guyana*, French Guiana, Ecuador, Peru, Bolivia*, Brazil (Acre, Amapá, Bahia, Distrito Federal, Espirito Santo*, Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pará, Paraná, Piauí*, Rio de Janeiro, Rondônia, Roraima, Santa Catarina, São Paulo, Sergipe), Paraguay*, Argentina (Misiones)*. *: Literature records.
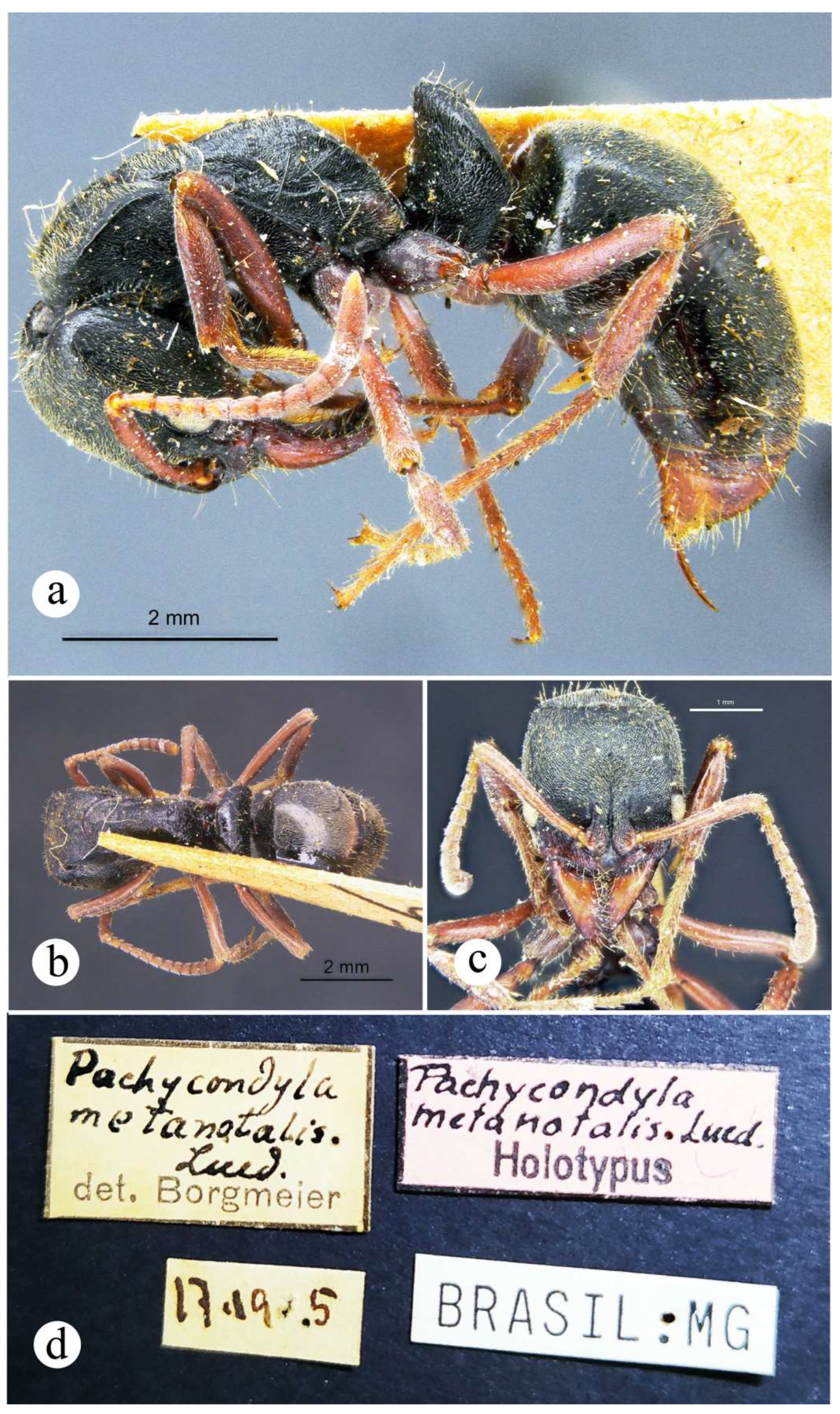
4.2.3. Neoponera dismarginata (Mackay & Mackay 2010)
Figures. 2a (petiole ♀︎); 20: a – e (☿); 31a (distribution).
Pachycondyla dismarginata MacKay & MacKay, 2010: 301, Figs. 111, 256, 257. ☿ holotype, Costa Rica, Heredia Prov., 16 km. N Vol. (Volcán) Barba, 10°17’N, 84 05’W, 950 m., 4-14.vii.1986, J. Longino (leg.) #1380-S, wet forest, workers on vegetation, (MCZC: MCZ-ENT00036273) [image examined, link]. Paratypes: 3 ☿ [same data as for holotype], (INBC, MCZC, WEMC) [not examined]. Note: Collection data transcribed ipsis litteris directly from holotype label.
Combination in Neoponera: Schmidt & Shattuck, 2014: 151.
Status as species: Fernandes et al., 2014: 134.
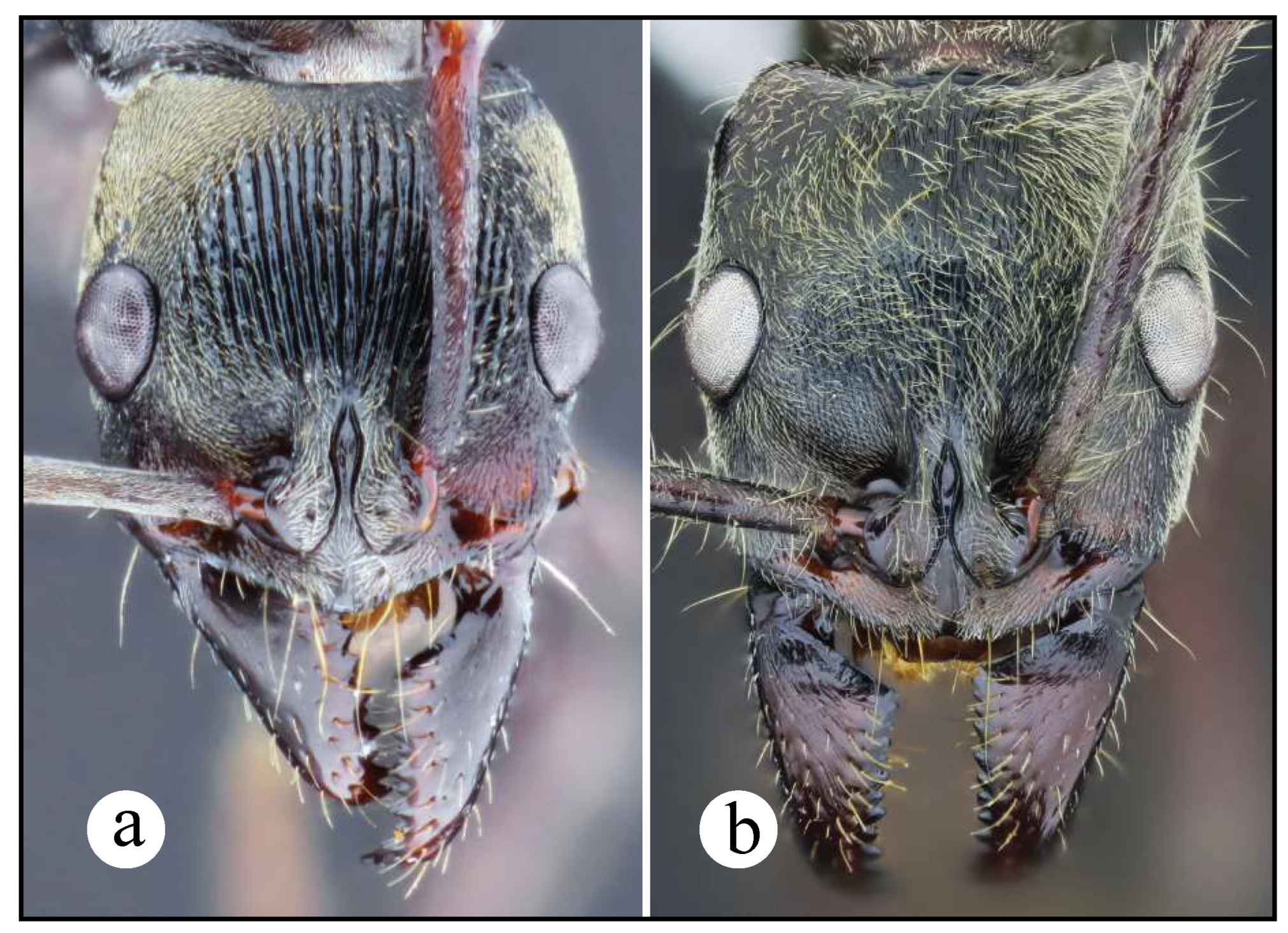
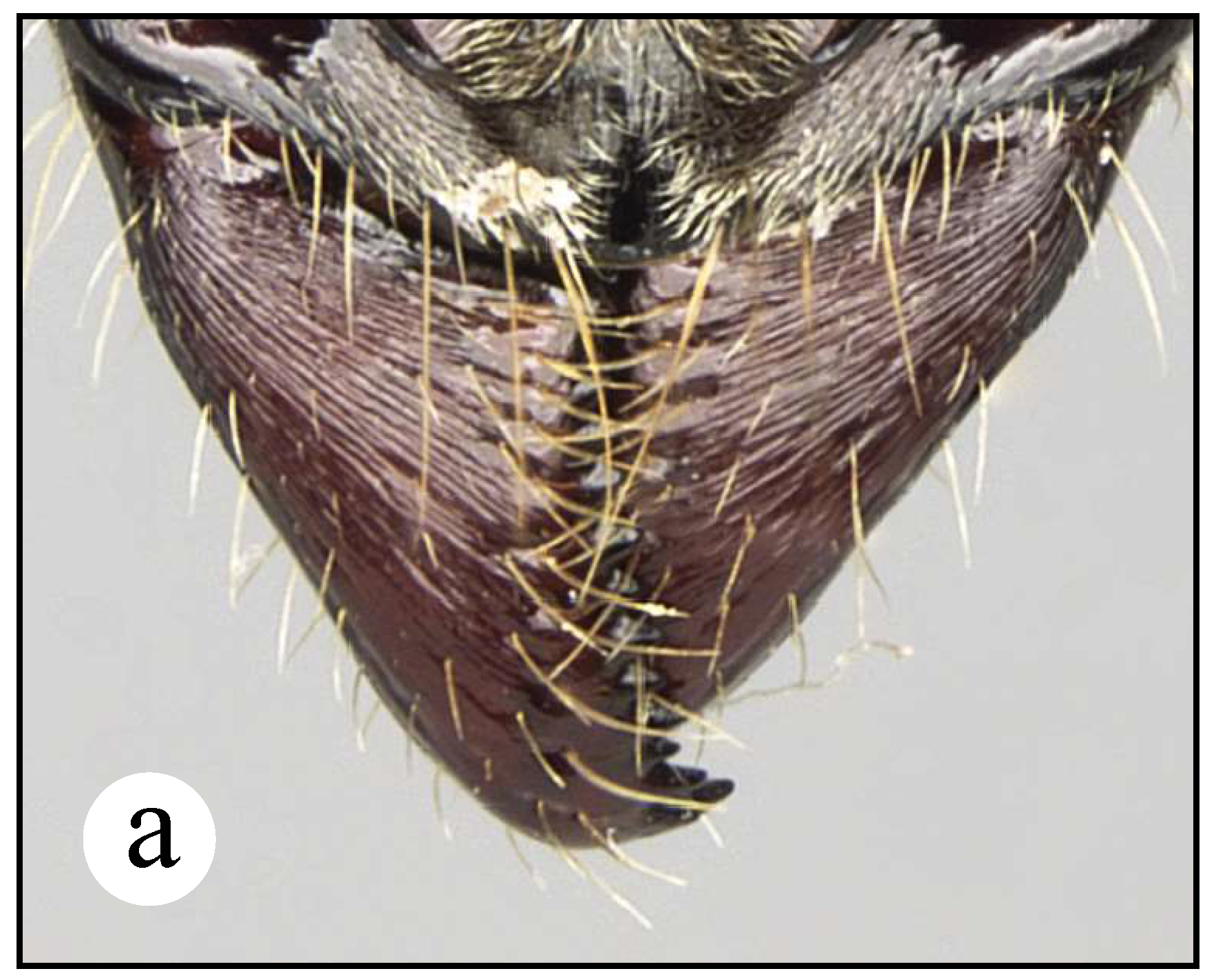
Worker (and probably queen) diagnosis. Head longer than broad in frontal view; malar carina virtually absent, though a tiny longitudinal swelling combined with well-impressed rugae is present anteriorly (Fig. 20e); antennal scape very long, when pulled posterad exceeding posterior head margin by about four times apical scape width (Fig. 20c); eye relatively small (as compared to head length), distance from anterior eye margin to mandibular insertion slightly longer than maximum eye length (Fig. 20e); clypeus bearing well-impressed, irregular longitudinal striae, mid clypeus convex, relatively acute (Fig. 20c); humeral carina present, slightly acute anteriorly, not salient; mesosternal process highly reduced, stump-shaped; metasternal process well-developed as in all congeners of the N. foetida group, dorsally with longitudinal well-impressed striae; posterolateral propodeal margin convex, not carinate (Fig. 20a); petiolar node subtriangular in lateral view, anterior and posterior margins irregularly straight, posterior face convex, nodal top slightly anterior to vertical midline (Fig. 2a, 20a); lateral nodal carina completely absent (Fig. 2a); subpetiolar process with well-developed, acute cusp and relatively flat dome (Fig. 2a); prora very small, slightly discerned laterally, anteriorly blunt (Fig. 2a); appressed pilosity white-brownish, not abundant and composed of tiny hairs (in contrast to most other congeners of the group where these hairs are longer and abundant); cuticle light castaneous.
Worker. Measurements (n = 3): HW: 2.25-2.3; HL: 2.6-2.85; EL: 0.6-0.72; SL: 3.25-3.3; WL: 4.3-4.5; PrW: 1.5-1.75; MsW: 1.0-1.2; MsL: 0.85-0.95; PW: 1.1-1.15; PH: 1.3-1.4; PL: 1.25-1.3; GL: 4.25-4.9; A3L: 1.5-1.75; A4L: 1.75-1.9; A3W: 1.9-2.05; A4W: 2.0-2.05; TLa: 11.7-11.98; TLr: 12.65-13.2. Indices. CI: 80.7-86.54; OI: 26.67-31.09; SI: 141.3-146.67; MsI: 117.65-129.41; LPI: 89.29-100.0; DPI: 88.0-88.46.
Queen and male. Unknown.
Comments. In the N. foetida group, N. dismarginata is the second largest species after N. curvinodis (Fig. 12; ☿ TLa = 11.7 – 12 mm). Since only four specimens were examined, all of them basically equal, no inference about morphological variation is possible for this Costa Rican endemic. Some body features like the long antennal scape, the tiny feebly impressed malar carina, the shape of the node, among others, make this taxon very singular among its congeners in this species-group, but also among species in the genus. Thus, it is unlikely for it to be misidentified with other Neoponera. Only N. apicalis (Latreille) and N. obscuricornis (Emery) show a relatively similar node, but their integument is dull black and the head has large eyes, clearly larger than N. dismarginata. Mackay and Mackay (2010) point out that N. bugabensis and N. insignis show malar carinae which do not reach the anterior eye margin, and because of that they are “placed” with N. dismarginata. Nevertheless, the malar carina of the latter is so poorly developed that it hardly would be closely related to those species based just on this single trait. Longino (2010) mentions that the “cheeks” (= malar region) have distinct carinae which reach the anterior eye margin. This is a typo, however, since the image of a specimen (INBIOCRI002278957) shown just above Longino’s text depicts otherwise.
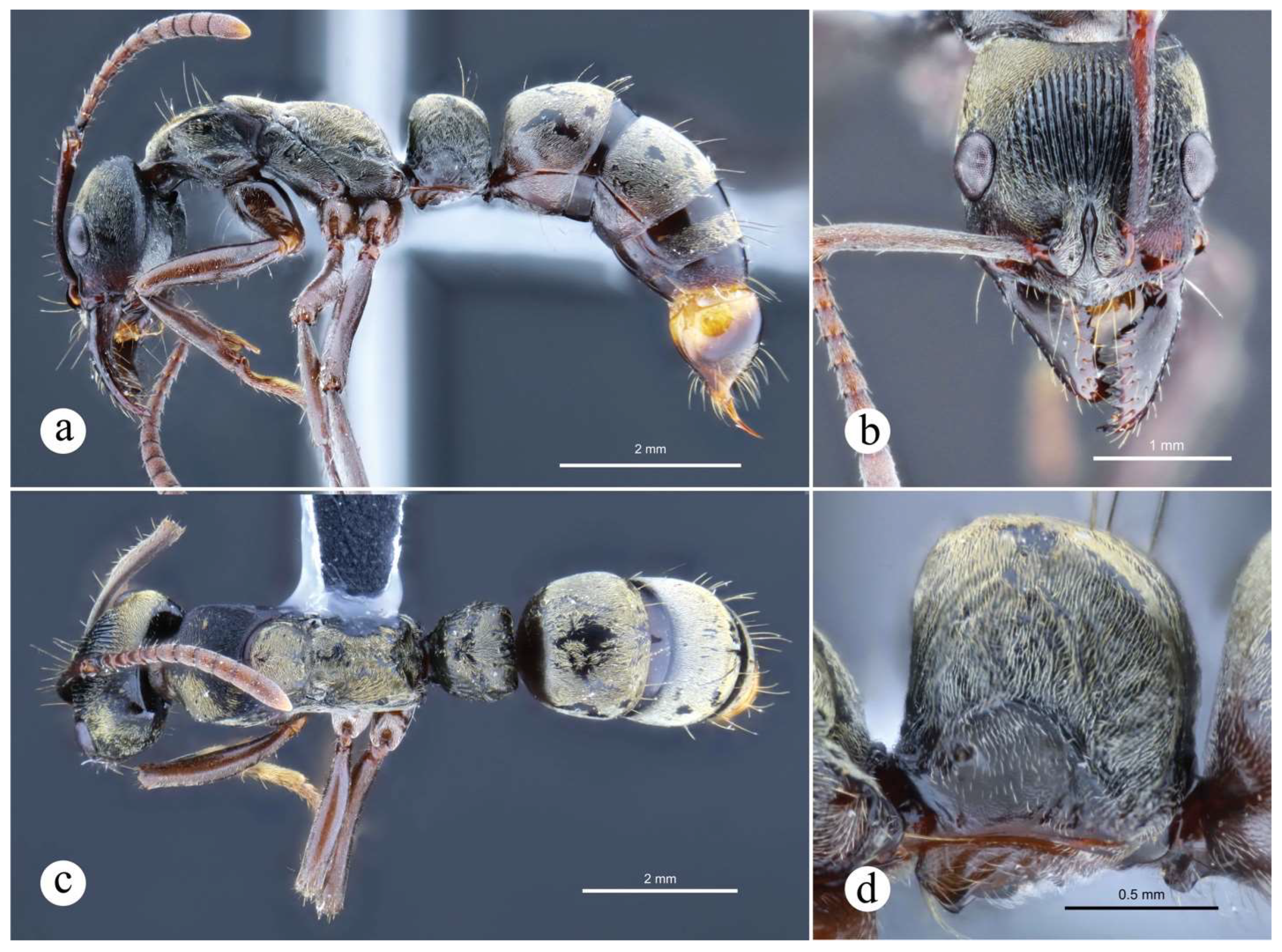
Figure 20.
Neoponera dismarginata. ☿ (JTL: JTL9295). a. Lateral view; b. Dorsal view; c. Head in frontal view; d. Close-up of head in anterolateral view.
Figure 20.
Neoponera dismarginata. ☿ (JTL: JTL9295). a. Lateral view; b. Dorsal view; c. Head in frontal view; d. Close-up of head in anterolateral view.
Neoponera dismarginata is the putative sister lineage of N. solisi (Troya et al. unpublished), but it is quite different to it. The petiolar node of N. solisi is somewhat block-shaped in lateral view with scarce appressed pubescence and the posterior face is glabrous, while the node of N. dismarginata is subtriangular in lateral view and carries relatively abundant appressed pilosity.
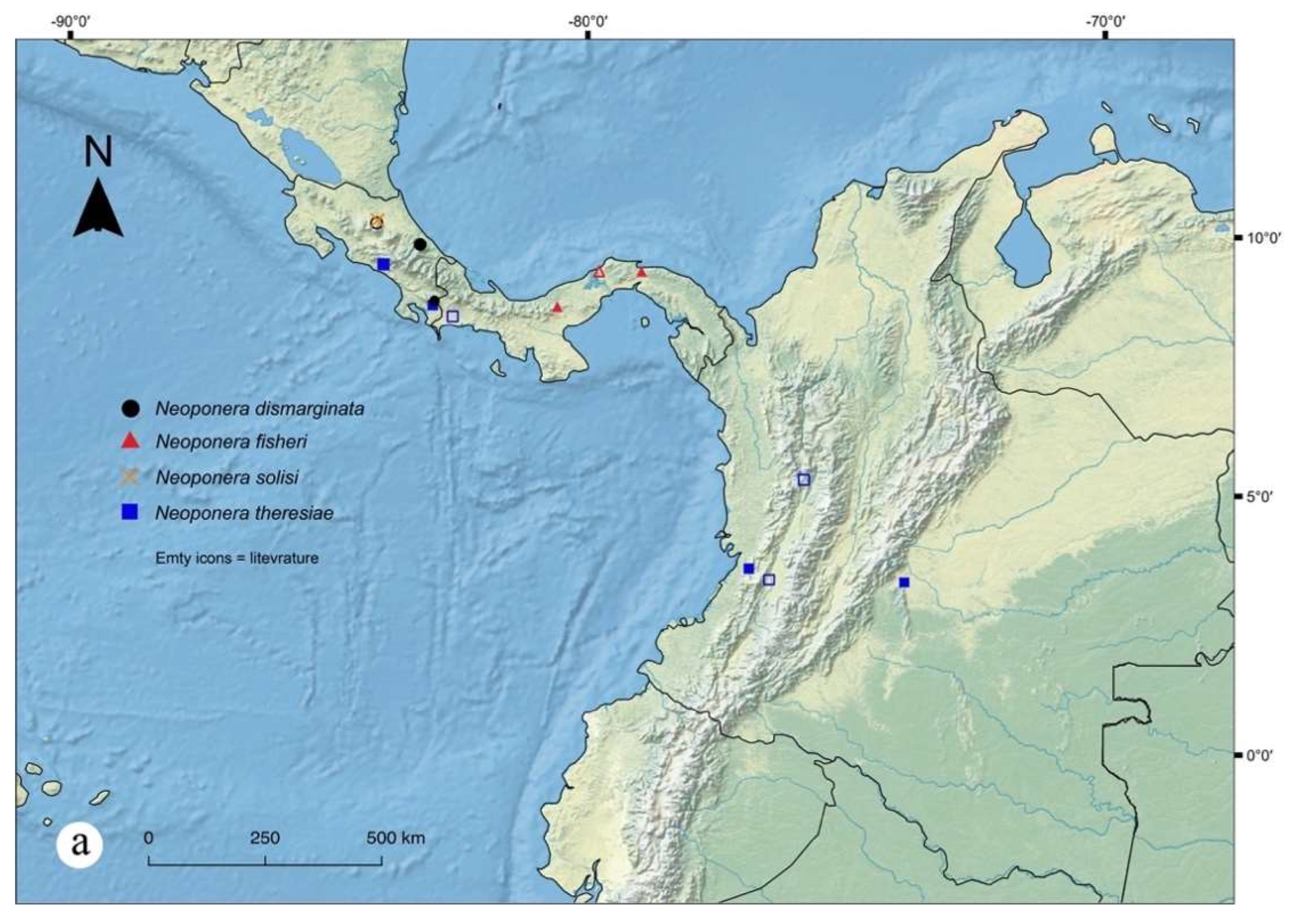
Distribution notes. Populations of N. dismarginata apparently are not rare in Costa Rica from where it has been collected in a number of sites since 1984 up to 2015 according to AntWeb.org. It appears though that this is a true endemic for that region since no other records have been found in several other Central American countries where some sampling campaigns have been carried out (see for example the LLAMA and ADMAC projects led by J. Longino)
Natural history notes. See Longino (2010).
Material examined. 4☿️. COSTA RICA – Heredia: • 1☿️; 16km N Vol. Barba; 10.2833, -84.0833; alt. 950m; 1986-07-09; (MCZC). – Limón: • Cerro Platano, 26km WSW Limón; 9.8682, -83.2408; alt. 1140m; 2015-06-16; Longino, J. leg.; hand collected; (MUCR). • 1☿️; Cerro Platano, 26km WSW Limón; 9.8682, -83.2408; alt. 1140m; 2015-06-16; Longino, J. leg.; hand collected; (MUCR). – Puntarenas: • 2☿️; Wilson Botanical Garden, 4km S San Vito; 8.7833, -82.9667; alt. 1200m; 1990-03-22; Longino, J. leg.; (JTLC).
Geographic range. Costa Rica.
4.2.4. Neoponera fisheri (Mackay & Mackay 2010)
Figures. 1a (head ♀︎); 21: a – c (♀︎); 31a (distribution).
Pachycondyla fisheri MacKay & MacKay, 2010: 328, Figs. 30, 32, 34, 278, 279, 293, 451- 454. ☿ holotype, Panama, Colon Prov., Santa Rita Ridge, 9°21’N, 79°47’W, 250 m., 24.iii.189, B.L. Fisher (leg.), rain forest ex Cecropia hispidissima, (CASC: CASENT092338) [image examined, link]. Paratypes: 9 ☿, 6♀, 2 ♂︎, [same data as for holotype], (CASC, GFMP, IAVH, MCZC, USNM, WEMC) [not examined]. Note: Paratype from IAVH absent from collection.
Combination in Neoponera: Schmidt & Shattuck, 2014: 151.
Worker and queen diagnosis. Head subtrapezoidal, narrowed anteriorly; masticatory margin of mandible with eight to nine teeth, gradually decreasing in size, denticles absent, except for one proximal (Fig. 21b); eye, especially on workers, less globose than in all other congeners in the N. foetida group, and placed slightly anteriorly on head; mid clypeus strongly concave, somewhat like harelip (Fig. 21b); antennal scape, when pulled posterad, fails to reach posterior head margin by about one apical scape width (Fig. 1a, 21b); malar carina present but tiny, feebly protuberant anteriorly (Fig. 1a, 21b); humerus weakly marginate anteriorly, particularly on worker, but a true carina is absent; notopropodeal groove cross ribbed in workers only; mesopleural groove tenuously impressed on workers, and vestigial on queens (Fig. 21a); meso- and metasternal processes well-developed, fang-shaped; in lateral view, petiolar node somewhat block-shaped with broadly curving margin posteriorly in workers (link), and subtriangular in queens, with convex top (Fig. 21a); posterolateral nodal margin lacking carina (Fig. 21a); posterior nodal face glabrous (Fig. 5a); subpetiolar process smooth, lacking horizontal striae, instead bearing one or two longitudinal carinae; pale yellowish appressed pilosity although covering body surface, formed by fine, thin hairs, so that cuticle is clearly discerned.
Figure 21.
Neoponera fisheri. ♀︎ (PSWC: CASENT0843089), Panama, Coclé. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 21.
Neoponera fisheri. ♀︎ (PSWC: CASENT0843089), Panama, Coclé. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Worker. Measurements (n = 1): HW: 2.6; HL: 2.75; EL: 0.65; SL: 1.9; WL: 4.25; PrW: 1.6; MsW: 1.3; MsL: 1.1; PW: 1.35; PH: 1.15; PL: 1.2; GL: 5.09; A3L: 1.65; A4L: 1.95; A3W: 1.95; A4W: 2.25; TLa: 11.8; TLr: 13.29. Indices. CI: 94.55; OI: 25; SI: 73.08; MsI: 118.18; LPI: 104.35; DPI: 112.5.
Queen. Measurements (n = 1): HW: 2.75; HL: 2.85; EL: 0.8; SL: 2.1; WL: 4.55; PrW: 2.2; MsW: 1.2; MsL: 0.8; PW: 1.5; PH: 1.2; PL: 1.2; GL: 5.66; A3L: 2.0; A4L: 2.15; A3W: 2.15; A4W: 2.5; TLa: 12.75; TLr: 14.26. Indices. CI: 96.49; OI: 29.09; SI: 76.36; MsI: 150; LPI: 100; DPI: 125.
Male. Described by Mackay and Mackay (2010).
Comments. Neoponera fisheri is a relatively large species in the N. foetida group (Fig. 12). Few specimens are known, all of them collected in three sites from Panama (AntWeb.org), their body color varies from castaneous to black. Besides this, the overall morphology seems to be quite similar among the scant material available. Nevertheless, a queen from Coclé (CASENT0843089) shows a mid-clypeal triangular-shaped concavity carrying horizontal striae in frontal view, which is different from the equivalent structure in a worker from Nusagandi (MZSP) where this concavity is subrectangular and lacks striae. In addition, this same worker shows an anterior tiny ocellus (about two times smaller than those of the queen) and two posterior ocellar pits (see below a discussion about this).
Neoponera fisheri is another peculiar species in terms of morphology in the N. foetida group. Mackay and Mackay (2010) believed this as a possible link between species in the N. aenescens and the N. foetida groups. They placed N. fisheri in the former although without certainty, and further suggested that it may deserve its own complex. Mackay and Mackay’s view about this species was in some way accurate because it is “almost” a link between two species-groups, but these are the N. crenata and the N. foetida groups. In the phylogeny of the genus, N. fisheri is placed at the base of the latter clade, and sister to all species in it (Troya et al., unpublished). From an evolutionary perspective, this placement makes sense since this lineage shares scant traits with its clade partners, namely the shape of head sculpture, and the fang-shaped metasternal process with broad lobes. Of these, only the latter is considered apomorphic between all species in the N. foetida group. Nevertheless, even with a well-supported phylogeny at hand we would refrain to suggest that the morphology of N. fisheri depicts the ancestral features of the remaining clade members. Whether these unusual features, like the short antennal scapes (unique among species in the genus), the strongly concave mid clypeus, the fused mesopleural plates of the queen, among others, represent ancestral states which have been lost in all other species of the group, or may these traits be independently gained as a function of the environment, for example through adaptive speciation, is a topic which warrants further research.
Out of the N. foetida group, another species with unique morphology features (among its clade partners) is N. luteola which belongs to the N. crenata group. Interestingly, some body regions of both N. fisheri and N. luteola are similar, like the outline of the head bearing somewhat flat eyes (less globose than in most other Neoponera belonging to these two sister clades), the shape and sculpture of the node, and the shape of the mesosoma. Current evidence supports a potential partnership between Neoponera luteola and some Cecropia plants in South America (Gutiérrez-Valencia et al. 2017). No (sufficient) evidence is still available for N. fisheri but it could well represent a similar case since specimens were collected inside Cecropia plants in different sites and years in Panama.
Natural history notes. Almost nothing is known about the biology and ecology of N. fisheri. The labels of the two examined specimens show that the worker and other nestmates (queen and larva) were collected by Phil Ward in three adjacent internodes of a Cecropia insignis (Urticaceae). The other group of individuals, consisting of the type material which was not physically examined here, was collected by Brian Fisher inside a C. hispidissima.
In regards to the ocellus observed in the worker from Nusagandi: ocelli-bearing workers is a relatively rare condition in Neoponera, but it has been seen in for example, members of the N. apicalis (N. apicalis, N. cooki), and N. aenescens groups (N. aenescens, N. carbonaria), being more frequent in N. cooki. Ocelli are usually found in worker ants which show crepuscular and night foraging behavior; these allow them capturing more light in dimly lit conditions (Narendra and Ribi 2017). In contrast to members of the N. apicalis and N. aenescens groups which are predominantly epigeic, where light incidence on the forest floor is reduced as compared to upper forest strata, most species in the N. foetida group, including N. fisheri, are arboreal. Apparently, the ocelli in workers of this group are quite rare, and thus far, has not been seen in workers of the arboreal N. crenata group, except for few specimens of N. goeldii (pers. obs.). Neoponera goeldii is arguably a nocturnal arboreal forager (Orivel et al. 2000). Perhaps, some N. fisheri workers forage at dawn or during the night, more frequently though than others with different schedule in their foraging behavior. Longino ( 2010) observed N. dismarginata workers in Costa Rica foraging at night. However, ocelli are thus far apparently absent from workers of this latter species.
Material examined. 3☿️, 2♀, 1♂. PANAMA – Coclé: • 1♀; 6 km NNW El Copé, Parque Nacional Omar Torrijos; 8.6719, -80.595; alt. 840m; 2015-01-22; Ward, P. S. leg.; hand collected; (PSWC). • 1☿️; 6 km NNW El Copé, Parque Nacional Omar Torrijos; 8.6719, -80.595; alt. 840m; 2015-01-22; Ward, P. S. leg.; hand collected; (DZUP). • 6 km NNW El Copé, Parque Nacional Omar Torrijos; 8.6719, -80.595; alt. 840m; 2015-01-22; Ward, P. S. leg.; hand collected; (DZUP). – Panama: • 1☿️; 42km W of Tupile, Reserva Nusagandi; 9.3489, -78.966; alt. 346m; 1989-10-26; Fisher, B. leg.; hand collected; (MZSP). • 1♂; Puerto Colón, Santa Rita ridge; 9.35, -79.7833; alt. 250m; 1989-03-24; Fisher, B. leg.; hand collected; (UTEP) [image from AntWeb]. • 1♀; Puerto Colón, Santa Rita ridge; 9.35, -79.7833; alt. 250m; 1989-03-24; Fisher, B. leg.; hand collected; (UTEP). • 1☿️; Puerto Colón, Santa Rita ridge; 9.35, -79.7833; alt. 250m; 1989-03-24; Fisher, B. leg.; hand collected; (CAS).
Geographic range. Panama.
4.2.5. Neoponera foetida (Linnaeus, 1758)
Figures. 3a (petiole ☿); 22: a – c (♀︎); 31b (distribution)
Formica foetida Linnaeus, 1758: 582 ♀︎ “America meridionali” [no locality given], Rolander [leg.], (ZMLS) [absent from collection].
Combinations. In Ponera: Smith, 1858: 95; in Pachycondyla: Roger, 1863: 18; Brown, 1995: 305; in Neoponera: Emery, 1901: 47; Schmidt & Shattuck, 2014: 151.
Status as species. Linnaeus, 1767: 965; Roger, 1860: 312 [redescription]; MacKay & MacKay, 2010: 332 [redescription].
Neoponera pedunculata Smith, 1858: 96. Syn. nov.
Senior synonym of Neoponera lobata: Retzius 1783: 75.
Worker and queen diagnosis. Head subrectangular; antennal scapes, when pulled posterad, exceeding posterior head margin by about 1.5 times apical scape width; humeral carina salient, shelf-like (Figs. 7c, 22a); petiolar node block-shaped, with dorsal margin horizontally straight up to about the mid region and immediately broadly curving posterad, anterior margin mostly straight (Figs. 3a, 22a); node covered by usually gross horizontal striae, except by mid ventral region on lateral face (Fig. 3a); prora keel-shaped, tip usually acute, directed ventrad.
Worker. Measurements (n = 12): HW: 2.0-2.45; HL: 2.2-2.75; EL: 0.6-0.75; SL: 1.9-2.55; WL: 3.45-4.45; PrW: 1.5-1.9; MsW: 0.8-1.1; MsL: 0.75-0.97; PW: 1.1-1.5; PH: 0.9-1.46; PL: 0.95-1.19; GL: 3.7-4.44; A3L: 1.25-1.75; A4L: 1.35-1.85; A3W: 1.35-2.15; A4W: 1.5-2.2; TLa: 9.2-11.75; TLr: 10.3-12.46. Indices. CI: 86.96-98.0; OI: 25.53-35.0; SI: 95.0-107.5; MsI: 94.12-122.22; LPI: 79.4-127.78; DPI: 100.0-136.36.
Figure 22.
Neoponera foetida. ♀︎ (MPEG: ATPFOR1848), Brazil, Amazonas. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 22.
Neoponera foetida. ♀︎ (MPEG: ATPFOR1848), Brazil, Amazonas. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Queen. Measurements (n = 2): HW: 2.2-2.75; HL: 2.45-2.8; EL: 0.6-0.7; SL: 2.25-2.75; WL: 4.25-5.09; PrW: 1.75-2.2; MsW: 1.0-1.65; MsL: 0.7-1.75; PW: 1.4-1.55; PH: 1.15-1.2; PL: 1.25-1.25; GL: 4.3-5.66; A3L: 1.5-1.95; A4L: 1.6-2.2; A3W: 2.1-2.3; A4W: 2.2-2.45; TLa: 11.05-13.29; TLr: 12.25-14.8. Indices. CI: 89.8-98.21; OI: 25.45-27.27; SI: 100.0-102.27; MsI: 94.29-142.86; LPI: 104.17-108.7; DPI: 112.0-124.0.
Male. Unknown.
Comments. Neoponera foetida is a medium-sized taxon in the N. foetida group (Fig 12; (☿ TLa = 9.2 – 11.75 mm; ♀︎TLa = 11 – 13.29 mm). Because of the coarse striae covering most of the petiolar node, this species is fairly easy to distinguish from all others in the group. Another potentially apomorphic populational trait is the salient humeral carina, which is slightly less protruding in specimens from Central America than those from South America. Other than that, the overall morphology of N. foetida is typical of this group, and it shows closer resemblance to the morphology of N. villosa, N. curvinodis, N. inversa, and N. zuparkoi, with which it forms a clade (Troya et al., unpublished).
This is the only species in the N. foetida group for which, thus far there are no clues about the location of the primary type material for any caste, except for the male which is still unknown. The ZMLS collection in Sweden could be a probable candidate, however, while writing this manuscript the museum staff confirmed to us it is not there either (ZMLS staff, pers. comm.). Roger (1860) redescribed this species based on a queen but this is also apparently lost since it is not vouchered at the MNHU collection. Despite the nodal striae being conspicuous in N. foetida, these are almost identically displayed on the node of at least two other Neoponera: N. striatinodis (Emery) and an unidentified Neoponera from Orellana, Ecuador, which belongs to the N. crenata group (collection codes: MEPNINV3375, MEPNINV28815). Neoponera foetida is today relatively easy to identify, but probably it was not in the past due to the scarcity of reference specimens in collections. It is thanks to Roger’s (1860) redescription and to De Geer's (1773) illustration of the nodal striae of N. lobata (currently senior synonym of N. foetida) that this species is now identifiable, and most importantly, diagnosable. For this reason, no attempt is made here to designate a neotype. The reader can use the present diagnosis and taxonomic key to recognize this species. Fernandes et al. (2014), Mackay and Mackay (2010), and AntWeb.org images (link) are also useful resources.
Natural history notes. See Longino (2010), and Mackay and Mackay (2010). Although some of the examined records suggest this species could be adapting to relatively anthropized environments, for example, grasslands, near pasture zones, and even in major cities, the frequency of these captures is still relatively low. Well-preserved, mature, and second-growth forests seem to host more populations of N. foetida than those impacted by humans. This is more apparent in southern Central America, and in northern and central South America. Thus far, the last trace for this species in South America is central Mato Grosso (Fig. 31b). Surprisingly, while writing this manuscript, we noted that the holotype of N. pedunculata Smith, 1858, which here is designated as junior synonym of N. foetida, is a very old specimen from Rio de Janeiro, Brazil, presumably collected before the 20th Century; this specimen is vouchered at the BMNH collection. According to AntCat.org (2022) there is another, also very old specimen preserved in OXUM, in London, which matches the traits of N. pedunculata. However, no collection data is provided on the label of that specimen, only the word “pedunculata”. Current specimen records of N. foetida represented in those directly examined here, which were also validated from AntWeb, and Antmaps, and from other confident literature sources that were used to represent the distribution of this species (see under references and map), suggest that populations of N. foetida are no longer encountered in nature in the Atlantic Forest, where they hypothetically used to inhabit in the past. We consulted a number of researchers in ant taxonomy and ecology, most of them Brazilians who have spent years collecting in various regions of the Atlantic Forest. They confirmed being unaware of no recent, nor past collections or sightings of this species in such biome. From any species in Neoponera, this could be the first confirmed case of a local extinction in the continent. Future field campaigns in this major Brazilian ecoregion may further inform about this concern.
Material examined. 40☿️, 6♀. BRAZIL – Amazonas: • 1☿️; 5 Km SW Alvarães; -3.2695, -64.823; alt. 70m; 1993-09-23; Gorayeb, I.; Silveira, O. leg.; Malaise; (MPEG). • 1☿️; Fazenda Universidade Federal do Amazonas - UFAM; -2.6458, -60.0408; alt. 91m; 2011-08-02; Fernandes, I. leg.; (INPA). • 1♀; Mamirauá; -3.0333, -64.85; alt. 51m; 1993-09-25; Gorayeb, I.; Silveira, O. leg.; arboreal Malaise; (MPEG). • 1☿️; Mamirauá; -3.0333, -64.85; alt. 51m; 1994-06-14; Gorayeb, I.; Silveira, O. leg.; arboreal Malaise; (MPEG). – Mato Grosso: • 1☿️; 65 Km S Sinop; -12.5167, -55.6167; alt. 430m; 1974/10; Alvarenga, M.; Roppa leg.; (MPEG). • 1☿️; Sapezal, Fazenda Tupanci; -13.5479, -58.8161; alt. 560m; 2017-12-11; Ferreira, J. leg.; pitfall; (DZUP). – Pará: • 1♀; Belém; -1.45, -48.4833; alt. 26m; 1992-01-08; Torres, M. leg.; (MPEG). • 1☿️; Cuiú-Cuiú; -5.9167, -56.5667; alt. 160m; 2018-06-18; Siqueira, E. leg.; arboreal pitfall; (MPEG). • 1☿️; Mocambo Reserve; -1.4333, -48.4; alt. 29m; 1977-09-15; Torres, M. leg.; (MPEG). • 1☿️; Tucuruí; -3.7655, -49.6702; alt. 35m; 1929-03-02; Overal, W. leg.; (MPEG). • 1☿️; Xingu river, right shore; -3.8519, -51.8523; alt. 173m; 2001-03-03; Santos, R.; Dias, J. leg.; (MPEG). • 1☿️; Xingu river, right shore; -3.8519, -51.8523; alt. 173m; 2001-03-11; Maciel, C..; Dias, J. leg.; arboreal Malaise; (MPEG). – Rio de Janeiro: • 1☿️; Constancia; -22.9, -43.2; (BMNH). – Rondônia: • 1♀; St. Elena River, left shore; -10.9023, -62.168; alt. 230m; 1985-03-20; Torres, M. leg.; (MPEG). COLOMBIA • 1♀; Leticia; 4.2, -69.93; alt. 80m; (ICN). – Antioquia: • 1☿️; Parque Nacional Natural Las Orquídeas; 6.549, -76.283; alt. 985m; 1976-10-21; Amat, G. leg.; (IAvH). – Caqueta: • 1☿️; Araracuara; 2.399, -72.282; alt. 140m; 1988-11-15; Fernández, F. leg.; (IAvH). – Cauca: • 1☿️; Parque Nacional Natural Gorgona; 2.966, -78.183; alt. 30m; 25/5/2001; Duque, R. leg.; (IAvH). • 1☿️; Parque Nacional Natural Gorgona, Alto El Mirador; 2.9667, -77.1833; alt. 180m; 2000-03-01; Sharkey, M. leg.; Malaise; (IAvH). – Valle del Cauca: • 1☿️; Bajo Calima; 3.998, -76.975; alt. 30m; 1996-05-17; hand collected; (MUSENUV). • 1☿️; Cali, km 18; 3.4, -76.5; 2003-02-08; Mazo, A. leg.; (MUSENUV). • 1☿️; Córdoba, San Cipriano; 3.84, -76.898; alt. 80m; Chacón, P. leg.; (MUSENUV). • 1☿️; Guandal, R. Yurumangui; 3.265, -77.12; alt. 6m; 1998-01-28; Riascos, F. leg.; (IAvH). • 1♀; Laguna de Sonso; 3.861, -76.349; alt. 930m; 2000-10-07; Mogollon, J. leg.; (MUSENUV). ECUADOR – Guayas: • 1☿️; Bosque Protector Cerro Blanco, Quebrada Condor; -2.1333, -80.0833; alt. 223m; 2016/11; Troya, A. leg.; pitfall; (MEPN). • 1☿️; Bosque Protector Cerro Blanco, Quebrada Condor; -2.1333, -80.0833; alt. 223m; 2016/11; Troya, A. leg.; fogging; (MEPN). – Los Rios: • 1☿️; C. R. R. Palenque; -1.432, -79.752; alt. 27m; 1977-03-02; De Vries, T. leg.; (QCAZ). – Morona Santiago: • 1☿️; Reserva Wisui, Cordillera del Cutucú; -2.1, -77.7333; alt. 657m; 2008-02-28; Simbaña, M. leg.; Malaise; (MEPN). – Orellana: • 1☿️; Estación Chiruisla; -0.6139, -75.8761; alt. 200m; 2005-09-15; Donoso, D. leg.; fogging; (QCAZ). • 1☿️; Parque Nacional Yasuní, 32 Km SSE Limoncocha, Onkonegare Km 39 Pompeya Sur; -0.658, -76.452; alt. 216m; 1995-07-03; Erwin, T.; et al. leg.; fogging; (MEPN). • 1☿️; Parque Nacional Yasuní, 32 Km SSE Limoncocha, Onkonegare Km 39 Pompeya Sur; -0.658, -76.452; alt. 216m; 1994-06-21; Erwin, T.; et al. leg.; fogging; (MEPN). FRENCH GUIANA – Cayenne: • 2☿️; 12km East from the village of Sinnamary. Paracou Station; 5.2667, -52.9167; alt. 50m; 1996/11; Corbara, B.; et al. leg.; (CPDC). • 1☿️; Mont Kaw, Relais de Patawa; 4.55, -52.6667; alt. 300m; 2005/11; Cerda, J. A. leg.; Malaise; (CPDC). – Saint-Laurent-du-Maroni: • 1♀; Belvedere de Saul; 3.6167, -53.2; alt. 326m; 2011-05-20; Team, S. E. A. G. leg.; (DZUP). • 1☿️; Belvedere de Saul; 3.6167, -53.2; alt. 326m; 2011-10-23; Team, S. E. A. G. leg.; (DZUP). • 1☿️; Itoupe, P6; 3.1222, -53.9932; alt. 600m; 2014-11-15; Orivel, J.; Fichaux, M. leg.; Pitfall72h; (EcoFoG). PANAMA – Colón: • 1☿️; Bosque Protector San Lorenzo; 9.3167, -80.0; alt. 20m; 2005-09-05; (DZUP). • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; (CPDC). • 3☿️; Bosque Protector San Lorenzo; 9.283, -79.966; alt. 16m; 2003-10-01; Dejean, A.; et al. leg.; (CPDC). • 2☿️; Bosque Protector San Lorenzo; 9.283, -79.966; alt. 16m; 2004-02-28; Springae, N.; Pinzon, S. leg.; Malaise; (CPDC). – Darién: • 1☿️; 7 km SW Platanilla; 8.7769, -78.4341; alt. 440m; 2015-01-20; Longino, J. leg.; hand collected; (JTLC). PERU – Loreto: • 1☿️; ACEER Lab; -3.1167, -72.9167; alt. 150m; 1994-08-05; Olson, D. M. leg.; (PSWC).
Geographic range. Costa Rica, Panama, Colombia, Trinidad and Tobago*, Venezuela, Guyana*, Suriname*, French Guiana, Ecuador, Peru*, Brazil: Acre*, Amapá*, Amazonas, Rondônia, Mato Grosso, Pará. *: Literature records.
4.2.6. Neoponera insignis (Mackay & Mackay 2010).
Figures. 10a (petiole ☿); 23: a – d (☿); 24: a – c (♀︎); 25: a – e (♂︎); 30a (distribution).
Figure 23.
Neoponera insignis. ☿ (MCZC: MCZ-ENT00036274), Costa Rica, Alajuela. a. Lateral view; b. Dorsal view; c. Head in frontal view; d. Collection labels. Images credit: Museum of Comparative Zoology, Harvard University, ©President and Fellows of Harvard College.
Figure 23.
Neoponera insignis. ☿ (MCZC: MCZ-ENT00036274), Costa Rica, Alajuela. a. Lateral view; b. Dorsal view; c. Head in frontal view; d. Collection labels. Images credit: Museum of Comparative Zoology, Harvard University, ©President and Fellows of Harvard College.
Pachycondyla insignis MacKay & MacKay, 2010: 405. Figs. 112, 269, 530-532. ☿ holotype, Costa Rica, Alajuela, Monteverde Cloud Forest Reserve, Esperanza, 800 m., 10°19’N, 84°43’W, 29.ix.1989, wet forest, ex Cecropia insignis, B.L. Fisher (leg.) # 108, (MCZC: MCZ-ENT00036274) [image examined]; Paratypes: ☿, ♀︎, [same data as for holotype], (MCZC, WEMC) [not examined].
Combination in Neoponera: Schmidt & Shattuck: 151
Status as species: Fernandes et al., 2014: 135.
Worker and queen diagnosis. Head subquadrate, sometimes slightly broadened at mid length in frontal view; eye slightly exceeding lateral head margin, sometimes just reaching it (Fig. 23c); antennal scape, when pulled posterad, exceeding posterior head margin by about one apical scape width (Figs. 9b, 23c); humeral carina weakly salient; posterolateral margin of petiolar node broadly convex and carinate, dorsal margin meeting with anterior margin in 80° angle approximately (Figs. 10a, 23a, 24a); subpetiolar process with acute cusp and inclined dome, the latter sometimes with acute longitudinal carina and feebly impressed longitudinal striae laterally (Figs. 10a, 24a), instead of the usually horizontal; anterior margin straight to slightly concave (Figs. 10a, 23a, 24a).
Worker. Measurements (n = 3): HW: 2.0-2.05; HL: 2.2-2.35; EL: 0.55-0.6; SL: 1.75-2.05; WL: 3.5-3.65; PrW: 1.4-1.5; MsW: 0.9-1.0; MsL: 0.75-0.85; PW: 1.1-1.15; PH: 0.7-1.0; PL: 0.9-0.95; GL: 4.2-4.55; A3L: 1.25-1.5; A4L: 1.4-1.6; A3W: 1.55-1.65; A4W: 1.65-1.75; TLa: 9.25-9.95; TLr: 10.8-11.5. Indices. CI: 85.11-90.91; OI: 27.5-29.27; SI: 87.5-100.0; MsI: 105.88-133.33; LPI: 94.74-135.71; DPI: 115.79-122.22.
Queen. Measurements (n = 2): HW: 2.2-2.2; HL: 2.35-2.4; EL: 0.55-0.6; SL: 1.95-1.95; WL: 3.95-3.95; PrW: 1.6-1.75; MsW: 1.3-1.65; MsL: 0.75-1.35; PW: 1.25-1.25; PH: 1.0-1.0; PL: 1.0-1.0; GL: 4.05-4.75; A3L: 1.7-1.85; A4L: 1.75-1.95; A3W: 1.5-1.95; A4W: 1.8-2.0; TLa: 10.75-11.15; TLr: 11.4-12.05. Indices. CI: 91.67-93.62; OI: 25.0-27.27; SI: 88.64-88.64; MsI: 122.22-173.33; LPI: 100.0-100.0; DPI: 125.0-125.0.
Figure 24.
Neoponera insignis. ♀︎ (JTLC: LACM-ENT142417), Colombia, Chocó. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 24.
Neoponera insignis. ♀︎ (JTLC: LACM-ENT142417), Colombia, Chocó. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 25.
Neoponera insignis. ♂︎ (JTLC: INBIOCRI002278907), Costa Rica, Alajuela. a. Lateral view; b. Dorsal view; Ocelli in dorsal view; d. Head in frontal view; e. Fore and hind wings.
Figure 25.
Neoponera insignis. ♂︎ (JTLC: INBIOCRI002278907), Costa Rica, Alajuela. a. Lateral view; b. Dorsal view; Ocelli in dorsal view; d. Head in frontal view; e. Fore and hind wings.
Male diagnosis. Notauli well-impressed, not touching posteriorly (Fig. 25b); mesopleural sulcus well-impressed, cross ribbed; metanotum without tumulus or carina; in lateral view, petiolar node slightly longer than high, lacking posterolateral carina (Fig. 25a); hind wing bearing 10-12 notauli.
Male (first description). Measurements (n = 2): HW: 1.25-1.25; HL: 1.3-1.35; EL: 0.7-0.75; SL: 0.2-0.25; WL: 3.15-3.5; PrW: 1.35-1.4; MsW: 0.85-0.9; MsL: 0.65-0.65; PW: 0.7-0.7; PH: 0.5-0.65; PL: 0.8-0.85; GL: 3.75-3.8; A3L: 1.1-1.15; A4L: 1.25-1.3; A3W: 1.15-1.25; A4W: 1.15-1.5; TLa: 7.75-8.0; TLr: 9.05-9.45. Indices. CI: 92.59-96.15; OI: 56.0-60.0; SI: 16.0-20.0; MsI: 130.77-138.46; LPI: 130.77-160.0; DPI: 82.35-87.5.
Head. Frontal view: subrhomboid. Mandible spatulate, longer than wide, blunt apically. About two mandalus (maximum length) fit on mandibular dorsum, mandibular apex, when mandible is closed, barely touches lateral margin of labrum. Prementum with well-developed dome placed proximally. Palp formula: 6,4. Stipes mostly smooth, internal longitudinal groove present. Labrum dorsum smooth. Clypeus anteromedially straight to slightly concave, somewhat globular-shaped in lateral view, covering anteclypeus. Area between posterior margin of clypeus and supraclypeal area weakly concave. Posterior supraclypeal area round. Supraclypeal area slightly protruding from cuticule. Distance between internal margins of antennal sockets approximately one socket diameter. Torular lobe strongly reduced. Eye suboval, slightly notched in the dorsal third, maximum length slightly less than half head length, convex, globose, placed at cephalic mid-length. Ocellar area not protruding from cuticle. Ocellar area placed slightly posterad to vertex. Anterior ocellus maximum length subequal to that of posterior ocelli. Ocelli not equidistant to each other, distance between anterior ocellus to posterior ocelli slightly shorter than interposterior ocellar distance. Distance from posterior ocellar margin to posterior margin of head roughly one apical scape width. Distance between anterior and posterior ocelli about one anterior ocellus maximum length. Occipital carina present, well-developed. Scape subquadrate, about twice as long as pedicel. Length of first flagellomere longer than second flagellomere. Mesosoma. Lateral view: Dorsal margin convex. Pronotum lacking humeral carina, completely rounded. Anterior subalar area approximately as broad as tegular maximum width. Anapleural sulcus well-developed, running laterally only on mesopleuron, not reaching ventral mesopleural region. Metanotal trough deeply impressed. Metapleuropropodeal suture relatively shallow. Posterolateral margin of propodeal declivity with feeble, blunt carina, without crenulae. Anterolateral propodeal corner rounded. Propodeal spiracle slit-shaped. Posteroventral cuticular flap at metapleural gland opening reduced, weakly projecting dorsad, gland orifice completely discernible laterally. Dorsal view: mesoscutum about as long as broad, slightly more than two times length of mesoscutellum. Mesoscutum anterior triangular impression present, strongly impressed. Parapsidal lines evident, slightly divergent. Notauli present, strongly impressed, weakly cross ribbed, not meeting posteriorly, but close to do so, not extending to transscutal line. Transscutal sulcus well-developed. Scutoscutellar sulcus present, straight, strongly impressed, cross ribbed, not completely separating mesoscutum from mesoscutellum. Mesoscutellum subtrapezoid, domed. Metascutellum well-developed, clearly discernible dorsally, surface with slightly salient longitudinal carina. Anterodorsal median propodeal sulcus present or absent, when present weakly impressed. Posterior face of propodeal declivity feebly convex. Propodeal lateral margins subparallel, not converging dorsomedially. Ventral view: probasisternum triangular-shaped, strongly grooved. Probasisternal posterior projection acute. Mesosternal process well-developed, tooth-shaped, internal margins of lobes convergent, relatively flattened anteroposteriorly, space between lobes approximately equal to width of each. Metasternal process well-developed, triangular-shaped, flattened anteroposteriorly, apex shorter than half height of metacoxal internal margin, space between lobes narrower than width of each, internal margins running parallel. Wings. Hyaline. Weakly infuscated. Forewing venation Ogata type Ia: submarginal cells 1, 2 and discoidal present, vein 2M present, reduced, 2r-rs offset from Rs+m, Rs+M ca. three times longer than 2M, setose layer evenly covering surface. Hindwing venation Cantone and Von Zuben Type I: basal and subbasal cells and vein 2M present, 1R well-developed, 1Rs poorly developed. Jugal lobe present. Hindwing bearing 10–12 hamuli. Legs. Mesofemur slightly thicker than metafemur, in dorsal or ventral view. Ventral surface of meso- and metafemora flattened roughly on distad half, surface even without groove. Tibial spur formula 2(1s, 1p), 2(1s, 1p). Anterior mid tibial spur half-length of posterior spur. Anterior hind tibial spur half-length of posterior spur. Pretarsal claw arched, with well-developed median tooth. Arolium present, roughly 0.5 times length of pretarsal claw. Petiole. Lateral view: Petiolar node anterior margin straight, inclined ca. 30° posterad; posterior margin straight, inclined ca. 30° anterad, anterior and posterior margins reaching approximately same level dorsally, middorsal margin convex, highest point at nodal longitudinal midline, posterior face slightly convex. Subpetiolar process subtriangular, with blunt, keel-shaped, grooved anterior cusp, followed posteriorly by relatively flat region bordered by longitudinal carinae, mostly smooth, with two weakly raised longitudinal carinae medially. Lateral projection of anteroventral nodal carina well-developed, with blunt tip feebly projecting dorsad. Lateral nodal carina vestigial. Anteroventral nodal carina incomplete, strongly concave medially. Gaster. Lateral view: Prora well-developed, with blunt tip projecting anteroventrad; posterior region slightly flattened and broadened. Anterior margin of tergum of A3 convex. Cinctus moderate. Dorsal view: Tergum of A3 equal in length to A4. Abdominal sternum 7 with concave surface, bearing scattered erect and suberect hairs. Spine at posterior margin of tergum of A8 well-developed, tip not surpassing posterior magin of sternum of A9, acute, slightly flattened laterally. Sternum of A9 longer than broad, posterior margin convex. Pygostyles present, about three times longer than broad. Color. Mandibles and legs yellowish brownish or dark brown, antennae ferruginous brown. Pilosity. Body with moderately abundant, flexuous, golden to dark golden, erect and suberect hairs. Appressed pubescence abundant. Meso- and metatarsi bearing scant spine-like setate and abundant finer setae. Sculpture. Integument polished, with abundant, shallowly impressed piligerous punctures. Striae on head absent, mesosoma lacking striae, except for axilar areas and few feebly impressed anterolaterally on propodeum. Striae on petiolar node absent.
Comments. Neoponera insignis is a relatively small to medium-sized species in the N. foetida group (Fig. 12, ☿ TLa = 9.20 – 9.95 mm; ♀︎: 10.75 – 11.15 mm). Since only few workers and queens were here examined (n = 6), no profound inferences are possible in regards to morphology variations across its range. Nevertheless, specimens from Alajuela, Costa Rica (northernmost range) differ little from those of Chocó, Colombia (southernmost range) in the form of the petiolar node: the anterior margin is slightly concave in a queen from the latter site, while in those from Costa Rica the margin is straight.
Mackay and Mackay (2010) and Longino (2010) noted that N. insignis is essentially identical to N. bugabensis. They claimed that transverse clypeal striae of N. insignis differentiate it from N. bugabensis where this sculpture is longitudinal or absent. However, the clypeal striae are a plastic feature, sometimes transverse or horizontal, and sometimes longitudinal in this species (see also comments on Neoponera clypeal striae under N. curvinodis). Nonetheless, we certainly agree with Longino and Mackay and Mackay in considering both species morphologically identical. These two forms live in sympatry in Central America, use similar resources for housing their nests on the canopy (although Longino 2010 note that N. bugabensis is a general forager and opportunistic nester), and are closely related according to recent phylogenomic evidence (Troya et al., unpublished). It would be no surprise one lineage diverged from the other very recently.
Figure 26.
Neoponera insignis (variant, referred in text as N. villosa_cf2). ☿ (PSWC: CASENT0249167), Panama, Darién. a. Lateral view; b. Dorsal view; c. Petiole in lateral view; d. Head in frontal view; e. Mesosternum in anteroventral view, showing the fang-shaped metasternal process, and the right lobe of the mesosternal process.
Figure 26.
Neoponera insignis (variant, referred in text as N. villosa_cf2). ☿ (PSWC: CASENT0249167), Panama, Darién. a. Lateral view; b. Dorsal view; c. Petiole in lateral view; d. Head in frontal view; e. Mesosternum in anteroventral view, showing the fang-shaped metasternal process, and the right lobe of the mesosternal process.
As hypothesized by Longino (2010), who observed the behavior of N. insignis in Costa Rica, it could have evolved through sympatric speciation from N. bugabensis, N. villosa or some ancestral version of them. Note that Longino also considered N. villosa highly similar to N. insignis, but these two species are easily distinguished from each other since N. villosa is larger and bears a tumulus on the posterior nodal face, which is absent in N. insignis. The reader may use the traits commented previously under the treatment of N. bugabensis to separate both species.
Neoponera insignis is also similar to N. lineaticeps, N. theresiae, N. prasiosomis sp. nov., and the morphospecies N. villosa_cf2 (see notes below about this latter). Some differing traits between these species and N. bugabensis were previously referred. These traits also apply to N. insignis since this is virtually identical to N. bugabensis.
Distribution notes. Previously considered a Costa Rican endemic (Longino 2010, Mackay and Mackay 2010), now it is known that N. insignis reaches the northern lowland rain forests of the Colombian Chocó-Darién, and since many Cecropia ant-plants reach further southern regions in the continent as for example, Ecuador, Peru, Brazil, it is likely this species be found there in future sampling events. Due to its high similarity with N. bugabensis, which has been found as far south to Peru, is also plausible that a proportion of specimens cited in the literature are misidentifications.
Neoponera villosa_cf2 as variant of N. insignis. The only known specimen of N. villosa_cf2, a worker, is sister to N. insignis and these two forms are closely related to N. bugabensis and N. prasiosomis sp. nov. (Troya et al., unpublished). Neoponera villosa_cf2 is very similar to N. insignis except for: 1) The petiolar node is shorter (PL = 0.75 mm vs. 0.9 – 1 mm), and the posterolateral nodal margin is less convex than in N. insignis, but relatively straight and more inclined, it meets the anterior margin in 50° – 60° angle vs. 70° – 80° angle in N. insignis; 2) The gastral dorsum carries more appressed hairs than in N. insignis so that the cuticle is poorly discernible, while in N insignis is always discernible; 3) It is smaller (TLa = 8 mm vs. 9.2 – 9 mm). At first sight, N. villosa_cf2 is easily distinguished from N. insignis just by looking at its yellowish, pilose gaster. This latter feature, together with the nodal shape and comparatively small body size would suffice to consider two hypothetical different lineages. Nonetheless, this case is similar to that between N. lineaticeps and its variant from Guatemala which, despite showing clear variations on the nodal sculpture, it seems that variant belongs to a N. lineaticeps population under ongoing differentiation since it shows a relatively short branch in the genus phylogeny (Troya et al., unpublished; see further comments under that species). We are here inclined to support the same hypothesis for N. insignis and N. villosa_cf2 since a similar picture is depicted in the phylogeny, both being sister and showing short branches. Neoponera villosa_cf2 apparently would still belong to an N. insignis population which is already showing signs of morphological differentiation. This specimen was collected by D. M. Olson in the southern side of the Panamanian Darién National Park; this collection site is ca. 50 km west from another collection site in the Colombian Katios National Park, where M. Aide found a worker and queen of N. insignis (JTLC: LACMENT142417).
Overall body size is subject to variation across all lineages in Neoponera, so the differences cited between both forms may not count as determinant traits for proposing N. villosa_cf2 as a new lineage. However, the nodal shape is an important variation, since it clearly differs from the general pattern observed in both N. insignis and in N. bugabensis (including its two variants) which share a very similar nodal shape. Still, we do not consider this single nodal deviation, as well as the more abundant gastral appressed pilosity, sufficiently informative so as to confidently erect a new species. Therefore, N. villosa_cf2 is here considered a variant of N. insignis until further material become available.
Natural history notes. See Longino (2010) who provides an extensive, fine-grain account of observations on Costa Rican N. insignis, based on which William Brown was planning a manuscript (comment from Longino 2010).
Material examined. 4☿️, 2♀, 2♂. COLOMBIA – Chocó: • 1♀; Parque Nacional Natural Los Katios; 7.8167, -77.2; alt. 236m; 1990-06-14; Aide, M. leg.; (JTLC). • 1☿️; Parque Nacional Natural Los Katios; 7.8167, -77.2; alt. 236m; 1990-06-14; Aide, M. leg.; (JTLC). COSTA RICA – Alajuela: • 1♂; north side Laguna Arenal; 10.5, -84.7167; alt. 500m; 1987-06-12; Longino, J. leg.; (JTLC). • 1☿️; north side Laguna Arenal; 10.5, -84.7167; alt. 500m; 1987-06-12; Longino, J. leg.; (JTLC). – Puntarenas: • 1♀; Cerro Plano de Monteverde; 10.3, -84.8167; alt. 1300m; 1989-06-11; Longino, J. leg.; (JTLC). • 1☿️; Cerro Plano de Monteverde; 10.3, -84.8167; alt. 1300m; 1989-06-11; Longino, J. leg.; (JTLC). PANAMA – Darién: • 1♂; 9 km WSW Platanilla; 8.7759, -78.4539; alt. 750m; 2015-01-20; Longino, J. leg.; hand collected; (JTLC). • 9 km WSW Platanilla; 8.7759, -78.4539; alt. 750m; 2015-01-20; Longino, J. leg.; hand collected; (JTLC). • 1☿️; 9 km WSW Platanilla; 8.7759, -78.4539; alt. 750m; 2015-01-20; Longino, J. leg.; hand collected; (JTLC).
Geographic range. Costa Rica, Panama, Colombia.
4.2.7. Neoponera inversa (Smith, 1858)
Figures. 2c (petiole ☿); 30b (distribution).
Ponera inversa Smith, 1858: 96. ☿ lectotype [by designation of Fernandes et al. 2014: 149], Ecuador [“South America”], Napo, “51/70”, (BMNH: BMNH1015556, AntWeb: CASENT0902512) [image examined, link].
Combinations: In Pachycondyla: Mayr, 1886: 358; Emery, 1901: 45: Brown, 1995:306. In Neoponera: Emery, 1904: 597; Schmidt & Shattuck, 2014: 151.
Subspecies of Neoponera villosa: Emery, 1904: 597.
Status as species. Mayr, 1863: 448; MacKay & MacKay, 2010: 409 [redescription]; Fernandes et al., 2014: 149 [redescription].
Worker and queen diagnosis. Same diagnostic features as for N. curvinodis, except: Head subquadrate, lateral margin posterior to eye usually convex (link); posterolateral propodeal margin carina-less or with blunt, not raised carina (Fig. 11b); in lateral view, anterior margin of petiolar node moderately to strongly concave, distal portion curved anterad (Fig. 2c); anterolateral nodal face deflate (Fig. 2c); in lateral view, dorsoposterior nodal margin usually meeting with anterior margin in 30° – 35° angle (Fig. 2c).
Worker. Measurements (n = 26): HW: 2.15-2.6; HL: 2.4-2.95; EL: 0.55-0.77; SL: 2.25-2.65; WL: 3.6-4.45; PrW: 1.55-1.9; MsW: 0.95-1.4; MsL: 0.7-1.0; PW: 1.2-1.5; PH: 1.0-1.5; PL: 0.95-1.31; GL: 2.8-4.95; A3L: 1.1-2.0; A4L: 1.3-2.0; A3W: 1.3-2.14; A4W: 1.55-2.15; TLa: 10.05-12.23; TLr: 10.35-13.48. Indices. CI: 81.13-96.15; OI: 23.4-29.57; SI: 93.88-113.95; MsI: 115.79-200.0; LPI: 71.43-115.0; DPI: 104.17-142.86.
Queen. Measurements (n = 6): HW: 2.55-2.85; HL: 2.4-3.0; EL: 0.6-0.75; SL: 2.3-2.7; WL: 4.35-4.75; PrW: 2.0-2.1; MsW: 1.5-1.8; MsL: 1.3-1.5; PW: 1.5-1.75; PH: 1.25-1.75; PL: 1.05-1.3; GL: 4.15-5.6; A3L: 1.7-2.0; A4L: 1.8-2.1; A3W: 2.15-2.45; A4W: 2.25-2.45; TLa: 11.7-12.85; TLr: 12.8-14.45. Indices. CI: 91.38-118.75; OI: 21.05-27.45; SI: 80.7-101.89; MsI: 103.45-126.92; LPI: 68.57-96.15; DPI: 128.0-152.38.
Male. Described by Fernandes et al. (2014). The following is added to their diagnosis: ocelli comparatively small, maximum length of each shorter than diameter of antennal socket acetabulum; notauli well-impressed, touching posteriorly; mesopleural sulcus well-marked but not sculpted; metanotum with feebly raised median carina.
Comments. Neoponera inversa is a medium-sized species in the N. foetida group (Fig. 12; ☿ TLa = 10.2 – 12.18 mm; ♀︎TLa: 10.2 – 12.18 mm). Its external morphology is virtually identical to that of N. curvinodis which is closely related to it (Troya et al. unpublished). The reader can find a detailed discussion on how to distinguish these two lineages apart under the treatment of N. curvinodis. Besides N. curvinodis, only two species in the N. foetida group are morphologically most similar to N. inversa: N. villosa, and N. zuparkoi. The first has a nearly vertically straight anterior nodal margin, and a tumulus on the posterior nodal face, while in N. inversa the anterior nodal margin is concave and lacks a tumulus on the posterior nodal face. Neoponera zuparkoi differs from N. inversa in showing a reduced, not salient humeral carina, and a lobate anterior projection on the mesopleuron. In examined workers and queens of N. inversa the humeral carina is always slightly to moderately salient, and the mesopleuron lacks a lobate anterior projection. Fernandes et al. 2014 considered N. inversa smaller than N. curvinodis, but my measurements of scape length (SL) and scape index (SI) differ from those reported by them (N. curvinodis ☿ SL: 2.63 – 2.70 mm, SI: 104.24 – 105.2 mm; N. inversa ☿ SL: 2.3 – 2.38 mm; SI: 94.65 – 95.2 mm) as follows: N. inversa ☿ SL: 2.25 to 2.65 mm, SI: 94 to 114 mm (n = 30), and N. curvinodis ☿ SL: 2.5 to 3.25 mm, SI: 102.3 to 115 mm (n = 64).
As in N. curvinodis, N. inversa shows some variation in the following body regions which are not geographically correlated: the humeral carina can be feebly- to moderately salient; the propodeal posterolateral margin in most cases is carina-less, but sometimes can carry a weak, blunt carina, somewhat similar to the state present in some N. curvinodis; and the anterolateral nodal face which may vary from weakly- to moderately deflate. This latter is the most common state and it gives the node its characteristic concavity. As in N. curvinodis, these variations are continuous, and they are not associated with body size. In contrast to Fernandes et al. (2014), we did not detect variations in color among specimens belonging to regions closer to the equator, as compared to others obtained from regions distantly separated from that zone.
Distribution notes. Most records of N. inversa belong to lowland forests up to ca. 1000 m of elevation, with an extreme of 2300 m at Cordillera del Cóndor, a mountainous region in southern Ecuador. Populations of N. inversa apparently prefer well-preserved forests as most specimens were collected in national parks and/or poorly degraded regions. This species is broadly distributed in South America and part of Central America; it inhabits in all Brazilian biomes (except for Pantanal), in the Chaco Húmedo of Paraguay, north to Amazonia of Bolivia, Peru, Ecuador, and Colombia. On the northwestern side of the continent records reach the Chocó-Darién and the Caribbean region of Colombia and Venezuela. In regards to Central America, some authors like Kempf (1972), Mackay and Mackay (2010), Branstetter and Sáenz (2012), among others (see AntCat.org) include Mexico and Guatemala as the northernmost range for N. inversa. These authors based their records for this species on Forel (1899) who did not mention those countries as part of the distribution of N. inversa. Forel recorded Las Mercedes in Guatemala as one of the type localities for N. curvinodis which is extremely similar to N. inversa. Thus far, the present literature search and the material examined support only northern Honduras (at the Atlantic) as the northernmost range for this species.
Natural history notes. See Mackay and Mackay (2010), and Fernandes et al. (2014). From this study: Neoponera inversa seems to prefer foraging and nesting on the canopy strata, most specimen-records for which the collection method is known, have been obtained using fogging, pitfall and arboreal Malaise, and beating.
Material examined. 57☿️, 9♀. BOLIVIA – Santa Cruz: • 1♀; 7.5 K NNE Villa Yapacaní, near Parque Nacional Amboró; -17.3436, -63.8372; alt. 290m; Kozue leg.; ([no museum voucher]). BRAZIL – Acre: • 1☿️; Fazenda Experimental Catuaba; -10.0667, -67.6167; alt. 226m; 2014-03-11; Denicol, M. R.; Santos, A. M. leg.; pitfall; (DZUP). • 1☿️; Humaita; -9.75, -67.6333; alt. 168m; 1992-06-15; Gorayeb, I.; Hernriques, E. leg.; (MPEG). – Amazonas: • 1☿️; AM 170, Km 10; -3.0476, -59.9784; alt. 40m; 1982-08-07; Harada, A. leg.; hand collected; (INPA). • 1☿️; Estirão do Equador; -4.5167, -71.6167; alt. 91m; 1979/10; Alvarenga, M. leg.; (MPEG). – Bahia: • 1☿️; 3 km s of Guaratinga; -16.6167, -39.7833; alt. 220m; 2007-07-26; Santos, J. R. M. leg.; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2014-05-02; Feitosa, R. M. leg.; (DZUP). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2012-12-19; Silva, J. A. leg.; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2005-02-02; Watkins, K.; M. Cobb. leg.; (CPDC). • 1☿️; 4k NW of Una; -15.2667, -39.0333; alt. 34m; 2005-10-27; Santos, J. R. M. leg.; (CPDC). • 1♀; Buerarema; -14.9843, -39.2583; alt. 132m; 2006-05-03; Maia, J. R.; et al. leg.; (CPDC). • 2☿️; Buerarema; -14.9843, -39.2583; alt. 132m; 2006-05-03; Maia, J. R.; et al. leg.; (CPDC). • 1☿️; Canavieiras; -15.6777, -38.9417; alt. 7m; 1988-04-21; do Carmo, J. leg.; (CPDC). • 1♀; Ilhéus; -14.7833, -39.05; alt. 36m; 2014-05-02; Feitosa, R. M. leg.; (DZUP). • 1☿️; Ilhéus; -14.7833, -39.05; alt. 36m; 2014-05-02; Feitosa, R. M. leg.; (DZUP). • 1♀; Reserva Biológica do Una; -15.1768, -39.1053; alt. 108m; 2011/11; Sena, D. U. leg.; (CPDC). • 1☿️; Reserva Biológica do Una; -15.1768, -39.1053; alt. 108m; 2011-11-01; Uzel-Sena, D. leg.; (CPDC). • 2☿️; Serra da Jibólia; -12.7717, -39.522; alt. 219m; 2004-06-04; Rodrigues, R. M. leg.; (CPDC). – Ceará: • 1☿️; 2km S of Pacoti; -4.25, -38.9167; alt. 700m; 2001; Hites, N. leg.; (CPDC). – Distrito Federal: • Aguas emendadas; -15.5, -47.55; alt. 992m; 2014-06-13; Guaraldo, A. leg.; (DZUP). – Espírito Santo: • 1☿️; Floresta Nacional Goytacazes; -19.4353, -40.072; alt. 26m; 2013-02-01; Simon, S. leg.; pitfall; (UFVLABECOL). – Goiás: • 1☿️; Chapadão do Céu, Parque Nacional das Esmas; -18.2889, -52.3672; alt. 619m; 2014-01-07; Pinto, A.; et al. leg.; (DZUP). – Mato Grosso do Sul: • 1☿️; Dourados; -22.2186, -54.8325; alt. 442m; 2009-01-01; Silvestre R. leg.; hand collected; (MuBio-UFGD). – Paraná: • 1☿️; Entorno do rio Brumado; -25.3402, -48.885; alt. 397m; 2017-10-18; Pinto, A.; et al. leg.; (DZUP). • 1☿️; P. E. Pico do Marumbi; -25.4833, -48.9833; alt. 968m; 2014-07-15; Feitosa, R. M.; et al. leg.; (DZUP). • 1☿️; Reserva Bicudinho do Brejo; -25.8833, -48.5667; alt. 7m; 2015-06-26; Oliveira, D. A. leg.; (DZUP). • 1☿️; Reserva Natural Guaricica, Guaricica trail; -25.3667, -48.7667; alt. 9m; 2019-03-22; Feitosa, R.; et al. leg.; Malaise; (DZUP). • 1☿️; Reserva Natural Guaricica, dos Fornos trail; -25.3, -48.65; alt. 36m; 2018-11-06; Troya, A. leg.; beating; (MEPN). – Pará: • 1☿️; Alter do Chão; -2.5041, -54.9548; alt. 23m; 2002-04-30; Vilhena, J. leg.; (INPA). • 1♀; Bairro Marco, Dr. Freitas; -1.4167, -48.45; alt. 22m; 2018/07; Prado, L. leg.; hand collected; (MPEG). • 1♀; Tucuruí; -3.7658, -49.6777; alt. 48m; 1980-08-04; Nunes de Mello leg.; (INPA). • 1☿️; Tucuruí; -3.7655, -49.6702; alt. 35m; 1979/02; Alvarenga, M. leg.; (MPEG). • 1☿️; Utinga State Park; -1.4258, -48.4442; alt. 15m; 1979-01-27; Tadeu, P. leg.; (MPEG). • 1☿️; Xingu river, left shore; -2.8817, -52.0095; alt. 4m; 2000-11-20; Maciel, C.; Dias, J. leg.; Malaise; (MPEG). – Rio de Janeiro: • 1☿️; Paineras; -11.8425, -45.1739; 1964-03-29; Ross, C. E.; Ross, E. S. leg.; (CAS). – Rondônia: • 1☿️; Parque Estadual do Guajara-Mirim, 18 km SW of Nova Dimensão; -10.3167, -64.55; alt. 152m; 1998-03-02; Santos, J. R. M. leg.; Malaise; (CPDC). – São Paulo: • 1♀; Mirassol; -20.8161, -49.5042; alt. 572m; 1977-12-12; Diniz, J. leg.; (DZUP). • 1☿️; Parque Estadual Turistico do Alto Ribeira, Gruta Alambari de Baixo; -24.5514, -48.6803; alt. 181m; 2012-10-02; (MPEG). COLOMBIA – Chocó: • 1☿️; Estación Silvicultura al Bajo Atrato; 7.0406, -77.3378; alt. 149m; 1992/08; Mendoza, L. leg.; (ICN). – Vaupes: • 1☿️; Estación Biológica Caparú; 1.0756, -69.5136; alt. 97m; 2003-04-26; Benavides, L. leg.; (ICN). COSTA RICA – Limón: • 1♀; Res. Biol. Hitoy-Cerere; 9.6716, -83.0243; alt. 160m; 2015-06-12; Krissy Dominguez leg.; beating; (MUCR). ECUADOR – Esmeraldas: • 1☿️; Reserva Ecológica Cotacachi Cayapas, La Tabla, 7 km EES Playa de Oro; 0.845, -78.744; alt. 120m; 2001-04-01; Araujo, P.; et al. leg.; fogging; (MEPN). – Napo: • 1☿️; 3km NNE Archidona; -0.8833, -77.8; alt. 650m; 2003-12-09; Wild, A.; Donoso, D. leg.; (UTIC). • 1☿️; Jatun Sacha 7km ESE Pto. Misahualli; -1.0667, -77.6167; alt. 400m; 1991-08-04; Ward, P. S. leg.; (PSWC). • 1☿️; [no locality given]; -0.99, -77.81; (BMNH). – Orellana: • 1☿️; Parque Nacional Yasuní, Guacamayo saladero; -1.294, -76.06; alt. 215m; 2014-02-02; Troya, A.; Duque, P. leg.; fogging; (MEPN). – Sucumbíos: • 1☿️; Reserva Ecológica Cofán Bermejo; 0.1667, -77.35; alt. 920m; 2005-12-27; Troya, A. leg.; fogging; (MEPN). – Zamora Chinchipe: • 1☿️; Paquisha alto, Hito 2, 15 Km SE Fruta del Norte, Cordillera del Cóndor; -3.9003, -78.4829; alt. 2325m; 2008-03-15; Troya, A. leg.; fogging; (MEPN). FRENCH GUIANA – Cayenne: • 1☿️; Base Vie; 5.1667, -52.65; alt. 10m; 2000-07-06; Durou, S.; et al. leg.; (DZUP). • 3☿️; Base Vie; 5.1667, -52.65; alt. 7m; 2000-12-06; Durou, S. leg.; (CPDC). • 1☿️; Base Vie; 5.1667, -52.65; alt. 7m; 2000-07-06; Durou, S.; et al. leg.; (CPDC). • 1☿️; Montagne dês Chevaux; 4.7333, -52.4333; alt. 75m; 2011-08-07; Team, S. E. A. G. leg.; (DZUP). • 3☿️; Petit Saut; 5.0667, -53.0333; alt. 67m; 2013-10-30; Lenoir, A. leg.; (CPDC). • 2☿️; Petit Saut; 5.0667, -53.0333; alt. 67m; 2003-04-03; Orivel, J. leg.; (CPDC). • 1☿️; Petit Saut; 5.0667, -53.0333; alt. 67m; 1996-07-08; Dejean, A. leg.; (CPDC). • 1☿️; Petit Saut; 5.0667, -53.0333; alt. 67m; 1997/05; Orivel, J.; Dejean, A. leg.; (CPDC). – Saint-Laurent-du-Maroni: • 1☿️; Galbao, P500-3; 3.6008, -53.2668; alt. 465m; 2018-10-13; Fichaux, M.; Jackie, O.; Touchard, A. leg.; Winkler48h; (EcoFoG). • 1☿️; Galbao, P700-1; 3.6036, -53.2807; alt. 688m; 2018-10-18; Fichaux, M.; Jackie, O.; Touchard, A. leg.; Pitfall48h; (EcoFoG). • 1☿️; Maripasoula; 3.6333, -54.0333; alt. 97m; 1999-07-01; Durou, S.; et al. leg.; (CPDC). PANAMA – Colón: • 1♀; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2004-03-09; Springatte; Pizon, S leg.; Malaise; (CPDC).
Distribution. Honduras*, Costa Rica, Panama, Venezuela*, Colombia, Guyana*, French Guiana, Ecuador, Peru, Bolivia, Brazil (Acre, Amazonas, Bahia, Ceará, Distrito Federal, Espírito Santo*, Goiás, Mato Grosso*, Mato Grosso do Sul, Pará, Paraná, Rondônia, Rio de Janeiro*, Santa Catarina*, São Paulo), Paraguay*. *: Literature records.
4.2.8. Neoponera lineaticeps (Mayr, 1866)
Figures. 4a (head ☿); 27: a – d (☿ variant); 31b (distribution).
Pachycondyla lineaticeps Mayr, 1866: 502. ☿ lectotype [by designation of MacKay & MacKay, 2010: 437], Mexico [no further data], (NHMW, AntWeb: CASENT0915667, link) [image examined]. Paralectotypes: 2 ☿, 1♀︎ [same data as for lectotype], (NHMW).
Combinations. In Neoponera: Emery, 1901: 47; Schmidt & Shattuck, 2014: 151. In Pachycondyla: Brown, 1995: 306.
Status as species. Emery, 1890: 73; MacKay & MacKay, 2010: 437 [redescription].
Worker and queen diagnosis. Head subquadrate, posterior margin concave in frontal view (Fig.27b) ; antennal scape, when pulled posterad, exceeding posterior head margin by about 1.5 times apical scape width (link); head vertex, including region near internal eye margin, costate (Fig. 27b); humeral carina salient; in lateral view, anterior margin of petiolar node straight, weakly inclined posterad, meeting dorsal margin in 85° to 90° angle, nodal top blunt (link); anterior and lateral nodal face with gross, irregularly shaped striae (link), sometimes feebly impressed (link); posterior face with patch of gross, longitudinal striae.
Worker. Measurements (n = 4): HW: 1.7-1.95; HL: 1.5-2.05; EL: 0.5-0.5; SL: 1.55-1.95; WL: 2.75-3.05; PrW: 1.25-1.3; MsW: 0.75-0.8; MsL: 0.6-0.7; PW: 0.85-1.05; PH: 0.8-1.02; PL: 0.75-0.9; GL: 3.6-4.1; A3L: 1.0-1.25; A4L: 1.1-1.25; A3W: 1.4-1.55; A4W: 1.5-1.65; TLa: 7.25-8.3; TLr: 8.75-9.73. Indices. CI: 94.87-113.33; OI: 25.64-29.41; SI: 91.18-105.41; MsI: 107.14-133.33; LPI: 88.67-100.0; DPI: 94.44-126.67.
Queen and male. Described by Mackay and Mackay (2010).
Comments. Neoponera lineaticeps is amongst the smallest species in the N. foetida group (Fig. 12; ☿ TLa = 7.25 – 8.3 mm). It shows a homogeneous external morphology across its range except for variations on the sculpture of the petiolar node which may either carry feebly impressed striae laterally (CASENT0249153, link, Puntarenas, Costa Rica) or gross, irregular striae (CASENT0217563, link, Chiapas, Mexico). Despite being irregular, the striae on the lateral nodal face are always horizontally oriented. In this respect, a variant of N. lineaticeps from El Petén, Guatemala (Fig. 27), hereafter referred as N. JTL021, differs from N. lineaticeps in bearing more abundant and brighter appressed pilosity; the striae on the lateral nodal face is not horizontally arranged but rather vertically curved (Fig. 27d); and the nodal top is convex, not blunt as usual in N. lineaticeps (Fig. 27d). This unique specimen was subjected to DNA extraction, and according to the UCE phylogeny (Troya et al. unpublished) it is sister to N. lineaticeps, but the branches of both specimens are relatively short, thus suggesting a recent divergence.
The variation of said characters between these two forms are evident, but as observed previously in other species in the N. foetida group, for example, N. curvinodis, N. inversa, several body regions are subject to plasticity. Either these variants in Central America are the product of hybridization between several sympatric forms, or N. JTL021 is perhaps a distinct lineage belonging to poorly known, possibly small populations. Since the evidence, both molecular and morphological, is currently insufficient to make further judgments, we leave N. JTL021 as a variant of N. lineaticeps until more material is available.
Figure 27.
Neoponera lineaticeps (variant, referred in text as N. JTL021). ☿ (JTLC: CASENT0610994), Guatemala, Petén. a. Lateral view; b. Head in frontal view; c. Dorsal view; d. Petiole in lateral view.
Figure 27.
Neoponera lineaticeps (variant, referred in text as N. JTL021). ☿ (JTLC: CASENT0610994), Guatemala, Petén. a. Lateral view; b. Head in frontal view; c. Dorsal view; d. Petiole in lateral view.
Because of its size, N. lineaticeps can only be confused with N. bugabensis, N. insignis, and N. theresiae, which have a very similar petiolar node. However, these species lack costae on the head dorsum. The node of N. theresiae is laterally striate but in no way similar to the usually gross striae of N. lineaticeps.
Distribution notes. Neoponera lineaticeps inhabits in central – to southeastern Mexico and throughout Central America. Examined material originates from Central American moist and dry forests, as well as from Isthmian-Pacific moist forests, ranging from ca. 300 m up to ca. 1500 m elevation. Fernandez and Palacio (1995) and Fernández et al. (1996) report this species for the Colombian departments of Cauca and Tolima, respectively. Later, however, Fernández in (Fernández and Guerrero 2019) report this species with the status “probably present in Colombia” p. 532. Further sampling in northern Colombia may bring support to the hypothetical incursion of this taxon in South America.
Natural history notes. See Mackay and Mackay (2010), and Longino (2010).
Material examined. 8☿️. COSTA RICA – Alajuela: • 1☿️; Monteverde Cloud Forest Reserve; 10.2912, -84.8158; alt. 1220m; 2018-03-28; J. Longino leg.; beating; (JTLC). – Heredia: • 10km NE Vara Blanca; 10.2362, -84.1177; alt. 1500m; 2005-03-09; Longino, J. leg.; hand collected; (JTLC). • 1☿️; 10km NE Vara Blanca; 10.2362, -84.1177; alt. 1500m; 2005-03-09; Longino, J. leg.; hand collected; (JTLC). GUATEMALA – Petén: • 1☿️; Parque Nacional Tikal; 17.2436, -89.6217; alt. 270m; 2009-05-23; Longino, J. leg.; hand collected; (JTLC). MEXICO – Chiapas: • 1☿️; Ocasinga, Laguna Ocotal Grande; 16.8, -91.4; alt. 971m; 1954/07; Dressler, R. L. leg.; (MZSP). • 1☿️; [no locality given]; 23.0, -102.0; 1986-12-01; Sichel leg.; (NHMW). NICARAGUA – Jinotega: • 2☿️; RN Cerro Kilambé; 13.5652, -85.6982; alt. 1290m; 2011-05-23; Prebus, M. leg.; hand collected; (MEPN). – Matagalpa: • 1☿️; Hotel Selva Negra; 12.9989, -85.9092; alt. 1285m; 2003-07-07; Mackay, W.; Mackay, E. leg.; hand collected; (ICN).
Geographic range. Mexico (Chiapas, Querétaro*, Veracruz*), Guatemala, Honduras*, Nicaragua, Costa Rica, Panama. * Literature records.
4.2.9. Neoponera prasiosomis sp. nov.
Figures. 5c (petiolar node ☿); 28: a – g (☿); 30a (distribution).
Type material. Holotype. 1 ☿; PANAMA: Colón: S. Lorenzo forest, 9°17’ N, 79°58’ W, [October 2003], mosaic forest, [Dejean, A.; et al. leg.], (DZUP: ATPFOR2001).
Etymology. The specific epithet is derived from the Greek words πράσινος (prásinos), meaning green, and σῶμαsôma (sôma), meaning body, alluding to the tenuous greenish cuticle, especially discernible on the head and mesosoma of the holotype. The name is a noun in apposition, thus invariable.
Worker (and probably queen) diagnosis. Head rectangular, antennal scape, when pulled posterad, exceeding posterior head margin by 0.5 to 1 X apical scape width (Fig. 28e); anterior mid clypeal margin convex (Fig. 28e); humeral carina slightly salient; in lateral view, anterior margin of petiolar node straight, meeting with posterior margin in ca. 80° angle, top of node relatively acute (Fig. 28a); posterior face of node either feebly striate (Figs. 5c, 28g) or punctate; integument of head, mesosoma and petiole slightly metallic greenish, gaster castaneous to dark brown.
Figure 28.
Neoponera prasiosomis sp. nov. Holotype ☿ (ICN: ATPFOR2001). Panama, Colón. a. Head, mesosoma and petiole in lateral view; b. Gaster in lateral view; c. Head, mesosoma and petiole in dorsal view; d. Gaster in dorsal view; e. Head in frontal view; f. Collection labels; g. Posterior face of petiolar node showing weakly impressed horizontal striae.
Figure 28.
Neoponera prasiosomis sp. nov. Holotype ☿ (ICN: ATPFOR2001). Panama, Colón. a. Head, mesosoma and petiole in lateral view; b. Gaster in lateral view; c. Head, mesosoma and petiole in dorsal view; d. Gaster in dorsal view; e. Head in frontal view; f. Collection labels; g. Posterior face of petiolar node showing weakly impressed horizontal striae.
Worker description. Measurements (n = 2; holotype in parenthesis): HW: 1.45-1.6 (1.45); HL: 1.7-1.95 (1.7); EL: 0.5-0.55 (0.5); SL: 1.5-1.7 (1.5); WL: 2.6-3.0 (2.6); PrW: 1.0-1.15 (1.0); MsW: 0.75-0.9 (0.75); MsL: 0.6-0.65 (0.6); PW: 0.7-0.9 (0.7); PH: 0.6-0.9 (0.6); PL: 0.7-0.75 (0.7); GL: 2.45-3.25 (2.45); A3L: 1.0-1.35 (1.0); A4L: 1.0-1.3 (1.0); A3W: 1.0-1.05 (1.0); A4W: 1.2-1.25 (1.25); TLa: 7.0-8.35 (7.0); TLr: 7.45-8.95 (7.45). Indices. CI: 82.05-85.29 (85.29); OI: 34.38-34.48 (34.48); SI: 103.45-106.25 (103.45); MsI: 125.0-138.46 (125.0); LPI: 83.33-116.67 (116.67); DPI: 100.0-120.0 (100.0).
Head. Frontal view: subrectangular. Mandible triangular, longer than wide, distal region slightly bent ventrad, dorsolateral margin mostly straight. Masticatory margin of mandible with eight to nine similarly sized teeth distally, and three smaller teeh proximally. Prementum with reduced transverse dome. Palp formula: 4,4. Stipes mostly smooth, internal longitudinal groove present. Labrum dorsum smooth. Clypeus anteromedially convex, with subtriangular-shaped, relatively acute extension of the cuticle projecting anterad. Posterior margin of frontoclypeal sulcus not reaching ocular mid-length. Frontal carina not reaching ocular mid-length. Torular lobe subtriangular, covering approximately 60% of acetabulum of antennal socket, posterolateral margin straight. Malar carina well-developed, not reaching anterointernal eye margin. Eye oval, maximum length ca. one-fourth head length, convex, slightly globose, reaching- to silghtly surpassing lateral margin of head, placed at cephalic mid-length. Posterior margin of head straight to slightly concave. Occipital carina vestigial. Scape, when pulled posterad, surpassing posterior margin of head by about one apical scape width. Mesosoma. Lateral view: Dorsal margin straight to slightly convex. Pronotum carinate, with carina slightly projecting from cuticle. Anapleural sulcus feebly developed, usually merged with surrounding striae. Posterolateral margin of propodeal declivity slightly marginated. Propodeal spiracle slit-shaped. Ventrolateral propodeal declivity without deep groove. Posteroventral cuticular flap at metapleural gland opening well-developed, slightly bent dorsad, gland orifice clearly visible in posterolateral view. Pheromone venting canal at metapleural gland opening present, well-developed. Notopropodeal suture present, forming shallow groove, mesonotum and propodeum separated. Posterior face of propodeal declivity flat. Propodeal lateral margins subparallel, not converging dorsomedially. Ventral view: probasisternum triangular-shaped, strongly grooved. Probasisternal posterior projection acute. Mesosternal process well-developed, tooth-shaped, internal margins of lobes convergent, relatively flattened anteroposteriorly, inclined posterad ca. 20–30 degrees in lateral view, space between lobes broader than width of each. Metasternal process well-developed, inclined posterad, 20°-30° in lateral view, triangular-shaped, flattened anteroposteriorly, apex higher than half height of metacoxal internal margin, space between lobes narrower than width of each, internal margins running parallel. Metasternal process external margins convergent. Legs. Mesofemur slightly thicker than metafemur, in dorsal or ventral view. Ventral surface of meso- and metafemora flattened roughly on distad half, surface even without groove. Tibial spur formula 2(1s, 1p), 2(1s, 1p). Anterior mid tibial spur about half-length of posterior spur. Anterior hind tibial spur about half-length of posterior spur. Pretarsal claw arched, unarmed (without accessory teeth on surface). Arolium present, roughly three times shorter than pretarsal claw. Petiole. Lateral view: Petiolar node with straight anterior margin and convex posterior margin, middorsal margin mostly flat, anterodorsal margin angulate, posterior face slightly convex. Subpetiolar process subtriangular, with acute, keel-shaped, slightly grooved anterior cusp, followed posteriorly by straight, slightly flat dome, dome devoid of- or with scant, feebly impressed striae. Lateral projection of anteroventral nodal carina well-developed, with acute to slightly blunt tip moderately bent dorsad. Lateral nodal carina weakly impressed. Anteroventral nodal carina incomplete, feebly concave medially. Gaster. Lateral view: Prora poorly developed, similar to cuticular lip, slightly projected ventrad. Anterior margin of tergum of A3 straight. Dorsalmost limit of anterior tergal margin of A3 nearly as high as maximum height of petiolar node margin. Anterodorsal margin of tergum of A3 convex. Cinctus moderate. Dorsal view: Tergum of A3 slightly shorter than A4. Posterior dorsum of epipygium completely smooth. Color. Appendages mandibles lighter than body integument, antennae dark brown to ferruginous brown, legs light brown. Pilosity. Body with flexuous, tenuously golden, erect and suberect hairs. Appressed pubescence moderately abundant, especially on gastral dorsum, though integument always discernible. Most erect hairs on body dorsum approximately equal to maximum eye length. Scape pilosity mostly composed of erect and suberect hairs and pruinose pubescence, erect hairs shorter than apical scape width. Meso- and metatarsi bearing moderately abundant spine-like setae interspersed with abundant suberect fine setae. Posterior ventrolateral margin of hypopygium (just next to the base of sting) bearing scattered flexuous hairs, shorter than eye maximum length. Posterior dorsum of epipygium surrounded by 20–30 long flexuous hairs, mostly longer than eye maximum length. Sculpture. Integument polished, with abundant, densely-packed piligerous punctures, most of which touch each other and form irregular, feebly impressed striae, especially on head dorsum where these are more raised. Mandibles without striae dorsally. Striae on petiolar node mostly absent, except for few feebly impressed on anterior nodal face.
Queen and male. Unknown.
Comments. Neoponera prasiosomis is amongst the smallest taxa in the N. foetida group (Fig. 12, ☿ TLa = 7 – 8.35 mm), and the specimen from Esmeraldas, Ecuador (MEPN: MEPN4862) is the smallest specimen of this species-group. No strong inference is possible in regards to morphological variation since only two specimens are known. However, the specimen from Ecuador differs from the holotype in the following: the posterolateral margin of the petiolar node is almost horizontally straight, while that of the holotype is slightly inclined, meeting with the anterior margin in ca. 80° angle; the posterior nodal face is punctate, while that of the holotype has feebly impressed horizontal striae; the body integument is mostly castaneous (rather than greenish on the holotype) although a tenuous greenish hue is discernible.
Neoponera prasiosomis could be confused with N. insignis, N. bugabensis, N. theresiae, and N. villosa_cf2 (variant of N. insignis) in the N. foetida group. In particular, the first two species are very similar to it, the reader can use the following characters to separate them: N. prasiosomis has a rectangular head, clearly longer than broad, while the head of the other two species is mostly subquadrate; the antennal scape of N. prasiosomis exceeds the posterior head margin by mostly one apical scape width, while that of the other species is longer, surpassing the posterior head margin by one up to 2.5 times apical scape width; N. prasiosomis shows a greenish integument while that of the other species is mostly black, to brownish; in lateral view, the petiolar node of N. prasiosomis is shorter (☿ PL = 0.70 – 0.75 mm) than those of N. insignis (☿ PL = 0.9 – 1 mm), and N. bugabensis (☿ PL = 0.9 – 1.25 mm).
Neoponera theresiae, on the other hand, is more easily distinguished from N. prasiosomis since it has convex postocular head margins so that the overall head shaped in frontal view seems suboval, while in N. prasiosomis the postocular head margins are straighter. Also, N. theresiae has striae on the proximal mandibular region and laterally on the nodal face. Neoponera prasiosomis is striae-less on such regions.
Finally, N. villosa_cf2 (here considered variant of N. insignis) differs from N. prasiosomis in having a mostly quadrate head in frontal view; the posterolateral nodal margin is significantly more inclined than in N. prasiosomis; and the gaster is abundantly covered with yellow appressed pilosity so that the integument is barely visible, while in N. prasiosomis the gaster is clearly less pilose allowing visibility of the integument.
Outside the N. foetida group, perhaps only N. rugosula and N. unidentata which belong to the N. crenata group, are similar. The easiest form to distinguish them is by examining the mesosomal dorsum where the notopropodeal suture is absent to vestigial, while in all species of the N. foetida group it is always present.
Distribution notes. The elevational range of currently known specimens of N. prasiosomis spans from nearly the sea level up to 150 m. The Panamanian holotype was collected in the Isthmian-Atlantic moist forests, while the specimen from Ecuador belongs from the western Ecuadorian moist forests, which is part of the southern Chocó-Darién.
Natural history notes. Nothing is known about this species except that it is probably arboreal, the specimen from Ecuador was collected using insecticidal knockdown, in a well-preserved, remote primary forest, about 20 years ago. Presently, the forests of this region are highly threatened by human activities, mainly wood extraction and mining (Rebolledo Monsalve et al. 2022).
Other material examined. 1☿️. ECUADOR – Esmeraldas: • 1☿️; Reserva Ecológica Cotacachi Cayapas, Pajonal, 4 km S Gualpi; 0.7584, -79.154; alt. 150m; 2001-04-01; Araujo, P.; et al. leg.; fogging; (MEPN).
Geographic range. Panama, northwestern Ecuador.
4.2.10. Neoponera solisi (Mackay & Mackay 2010)
Figures. 3b, 5a (petiole ☿); 4b (head ☿); 29: a – c (♀︎); 31a (distribution).
Pachycondyla solisi MacKay & MacKay, 2010: 514, Figs. 113, 253, 635-638. ☿ holotype, Costa Rica, Heredia, Est. El Ceibo, P.N. Braulio Carrillo, 400-600 m., ii.1990, C. Chaves [leg,], (INBC, AntWeb: INBIOCRI000312327, link) [image examined]. Paratypes: 1 ☿, 1♀︎, [same data as for lectotype], (INBC, WEMC) [not examined].
Combination in Neoponera: Schmidt & Shattuck, 2014: 151
Status as species: Fernandes et al., 2014: 135.
Worker and queen diagnosis. Head subtrapezoid, posterior margin concave, with posterior corner slightly protruding in frontal view (Fig. 29a); anterior mid clypeal margin concave, with well-impressed longitudinal groove extending through supraclypeal area (Fig. 29a); malar carina present, weakly impressed; antennal scape, when pulled posterad, exceeding posterior head margin by about two times apical scape width; posterior head dorsum with gross longitudinal striae (Fig. 29a); humeral carina well-developed, salient anteriorly; posterolateral propodeal margin carinate; in lateral view, top of petiolar node convex, posterolateral margin carinate, ventral half of anterior face grossly striate (Fig. 3b); posterior nodal face mostly glabrous, except for some marginal long erect hairs (Fig. 5a); prora highly reduced to a feeble swelling (Fig. 3b); integument dark castaneous.
Worker. Measurements (n = 2): HW: 2.59-2.9; HL: 2.94-3.25; EL: 0.73-0.88; SL: 2.9-2.96; WL: 4.2-4.5; PrW: 1.8-2.3; MsW: 1.0-1.28; MsL: 0.9-1.15; PW: 1.1-1.29; PH: 1.28-1.48; PL: 1.28-1.39; GL: 4.5-5.1; A3L: 1.6-1.93; A4L: 1.72-1.8; A3W: 1.9-2.3; A4W: 2.3-2.3; TLa: 11.74-12.87; TLr: 12.92-14.24. Indices. CI: 88.1-89.23; OI: 28.19-30.34; SI: 102.07-111.97; MsI: 111.11-111.3; LPI: 93.92-100.0; DPI: 85.94-92.81.
Queen. Measurements (n = 1): HW: 3.5; HL: 3.7; EL: 1.0; SL: 3.2; WL: 5.5; PrW: 2.5; MsW: 2.0; MsL: 2.65; PW: 1.49; PH: 1.6; PL: 1.68; GL: 7.2; A3L: 2.45; A4L: 2.4; A3W: 2.65; A4W: 2.8; TLa: 15.73; TLr: 18.08. Indices. CI: 94.59; OI: 28.57-; SI: 91.43; MsI: 75.47; LPI: 105.0; DPI: 88.69.
Male. Unknown.
Comments. Neoponera solisi is a medium-sized to large species in the N. foetida group (Fig. 12; ☿ TLa = 11.7 – 12.9 mm; ♀︎TLa: 15.7 mm). Only three specimens were physically examined out of the scant collected through the ALAS project team in Parque Nacional Braulio Carrillo, in Heredia, Costa Rica, the only site in the Neotropics where this taxon is known from.
The morphology of this species, in particular the mid anterior clypeal region and the petiolar node, are very distinctive characters which make easier its distinction from any other species in this group, as well from any other in the genus. Mackay and Mackay (2010) point out it could be confused with N. chyzeri (which belongs to the N. aenescens group), and with N. lineaticeps. This is unlikely though, since both species have very different nodal shape. Also, N. lineaticeps has a black integument and is much smaller, while N. chyzeri is also black, lacks malar carinae, and the body bears abundant, golden appressed hairs. Neoponera solisi inhabits in the Isthmian-Atlantic moist forests at ca. 500 m elevation.
Natural history notes. Nothing is known about this species. However, the morphology of its head, i.e., subtrapezoidal, is reminiscent of arboreal species like N. fisheri or N. luteola (Roger) which have been collected inside Cecropia spp. (Urticaceae) plants, in particular N. luteola which holds a mutualistic association with C. membranacea Trécul. in Peru (Gutiérrez-Valencia et al. 2017).
Figure 29.
Neoponera solisi. ♀︎ (INBC: INB0003662417), Costa Rica, Heredia. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Figure 29.
Neoponera solisi. ♀︎ (INBC: INB0003662417), Costa Rica, Heredia. a. Lateral view; b. Head in frontal view; c. Dorsal view.
Material examined. 4☿️, 1♀. COSTA RICA – Heredia: • 1☿️; Estación El Ceibo; 10.328, -84.0804; alt. 400m; 1990-02-01; Chaves, C. leg.; (INBC). • 1☿️; Estación El Ceibo; 10.328, -84.0804; alt. 400m; 1990-02-01; Chaves, C. leg.; (UTEP). • 1♀; Parque Nacional Braulio Carrillo, 11 km SE La Virgen; 10.3333, -84.0667; alt. 500m; 2003-02-12; ALAS leg.; Malaise; (INBC). • 2☿️; Parque Nacional Braulio Carrillo, 11 km SE La Virgen; 10.3333, -84.0667; alt. 500m; 2003-02-12; ALAS leg.; Malaise; (INBC).
Geographic range. Northeastern Costa Rica.
4.2.11. Neoponera theresiae (Forel, 1899)
Figures. 3c (petiole ☿); 6a (mandible ☿); 31a (distribution).
Pachycondyla theresiae Forel, 1899: 13, Plate 1, Fig. 11. ☿ lectotype [by designation of MacKay & MacKay, 2010: 549], Panama, Bugaba, Volcán de Chiriqui, Champion [leg.], (MHNG, AntWeb: CASENT0907241, link) [image examined].
Combinations. In Pachycondyla: Brown, 1995: 310; in Neoponera: Emery, 1901: 47; Schmidt & Shattuck, 2014: 152.
Status as species. Emery, 1911: 72; MacKay & MacKay, 2010: 549 [redescription].
Worker (and probably queen) diagnosis. Head subquadrate, lateral margin posterior to eye relatively round, posterior margin feebly concave to straight (link); anterior mid clypeal margin convex; proximal region of mandibular dorsum with well-impressed, divergent striae, distal region usually smooth (Fig. 6a); humeral carina present, slightly salient; posterolateral propodeal margin blunt; in lateral view, anterior margin of petiolar node straight, weakly inclined posterad, nodal top blunt to convex, posterolateral margin convex, carinate, meeting anterior margin in ca. 80° angle (link); posterior propodeal face, and anterolateral nodal face with well-marked horizontal striae (Fig. 3c).
Worker. Measurements (n = 2): HW: 1.9-2.08; HL: 2.05-2.09; EL: 0.45-0.5; SL: 2.0-2.0; WL: 2.8-3.0; PrW: 1.15-1.19; MsW: 0.74-0.75; MsL: 0.65-0.68; PW: 0.9-0.9; PH: 0.78-0.8; PL: 0.88-0.9; GL: 3.0-3.85; A3L: 1.15-1.2; A4L: 1.21-1.25; A3W: 1.4-1.45; A4W: 1.49-1.5; TLa: 8.18-8.35; TLr: 8.95-9.62. Indices. CI: 92.68-99.52; OI: 23.68-24.04; SI: 96.15-105.26; MsI: 108.82-115.38; LPI: 112.5-112.82; DPI: 100.0-102.27.
Queen and male. Unknown.
Comments. Neoponera theresiae is amongst the smallest species in the N. foetida group (Fig. 12; ☿ TLa = 8.18 – 8.35 mm). Although relatively few specimens were examined from Costa Rica, Panama and Colombia, no major deviations from the diagnostic limits of this species were detected. The nodal lateral striae of a Costa Rican specimen from San José (CASENT0843316) are slightly less impressed than those from two Colombian specimens from Valle del Cauca (MUSENUV16069). Besides this, the overall morphology of examined N. theresiae is quite homogeneous.
This species is somewhat hard to distinguish mainly due to the shape of the petiolar node which is very similar to that of N. bugabensis, N. insignis, N. villosa, N. foetida, and N. lineaticeps. In the first three, however, the node is striae-less, while the other two carry well-marked striae, more impressed than in N. theresiae. In N. foetida these striae cover most of the nodal surface, and in N. lineaticeps just a portion, but the striae are gross, clearly raised from cuticle. If doubts persist, the reader can look at the lateral head margins posterior to eyes which in N. theresiae are broadly convex, giving it the appearance of an ovoid shape, rather than subquadrate or rectangular. Another distinctive feature of N. theresiae are the divergent, well-marked striae on the mandibular dorsum. The other species all show striae on their mandibles but these are tenuously (e.g., N. lineaticeps) to moderately impressed (e.g., N. villosa), never strongly marked as in N. theresiae. An exception perhaps is N. foetida, where these striae are virtually absent, still some traces are distinguished.
Distribution notes. Neoponera theresiae inhabits the Isthmian-Pacific moist forests in Central America, south to the Chocó-Darién, Cauca Valley montane forests, and Apure-Villavicencio dry forests in Colombia, spanning an elevation range from 300 m to ca. 1000 m. Wheeler (1923) cites “Cucuruzinho, Rio Autaz”, p. 2. Rio Autaz is a river on the eastern side of the state of Amazonas, Brazil, while Cucuruzinho is not a site but a common name of an Amazonian frog. Emery (1911) cites Peru, without specific site though. Antmaps.org refers Gomes et al. (2010) to a supposed record in Alagoas; this is a probable misidentification, however, maybe with N. foetida which also carries striae in the lateral side of the node. We believe an Amazonian distribution for current N. theresiae populations is unlikely since not a single record for that Biome has been reported in past years.
Natural history notes. See Mackay and Mackay (2010), and Longino (2010).
Material examined. 8☿️. COLOMBIA – Meta: • 1☿️; San Juan de Arama; 3.34, -73.889; alt. 430m; 1995-06-08; Fernández, F. leg.; (MUSENUV). – Valle del Cauca: • 1☿️; Central Hidroeléctrica Anchicayá; 3.61, -76.891; 1980-05-09; Reyes-Blanco, R. leg.; (MUSENUV). COSTA RICA – Puntarenas: • 1☿️; 6 Km S San Vito; 8.7, -83.0; alt. 500m; 1967-03-13; OTS course leg.; (QCAZ). – San José: • 1☿️; Ranchos Tinamú; 9.4857, -83.9572; alt. 870m; 2015-07-13; Mata, I. leg.; beating; (MUCR). • 1☿️; Ranchos Tinamú; 9.4857, -83.9612; alt. 950m; 2015-07-04; Ward, P. S. leg.; beating; (PSWC). • 1☿️; Ranchos Tinamú; 9.4858, -83.9544; alt. 760m; 2015-07-09; Ward, P. S. leg.; beating; (PSWC). • 1☿️; Ranchos Tinamú; 9.4865, -83.9525; alt. 730m; 2015-07-11; ADMAC leg.; miniWinkler; (JTLC). PANAMA – Chiriquí: • 1☿️; Bugaba; 8.4833, -82.6167; alt. 350m; Champion leg.; (MHNG).
Geographic range. Costa Rica, Panama, Colombia.
4.2.12. Neoponera villosa (Fabricius, 1804)
Figures. 2a (petiole); 30b (distribution).
Formica villosa Fabricius, 1804: 409. ♀︎ lectotype [by designation of Fernandes et al. 2014: 155], Suriname, Essequibo, (ZMUC) [image from Fernandes et al. 2014, examined].
Combinations. In Ponera: Lepeletier de Saint-Fargeau, 1835: 192. In Pachycondyla: Mayr, 1862: 720; Brown, 1995: 311. In Neoponera: Emery, 1901: 43; Schmidt & Shattuck, 2014: 152.
Status as species. Lepeletier de Saint-Fargeau, 1835: 192; Roger, 1861: 1 [redescription]; Wheeler, 1908: 403 [redescription]; Gallardo, 1918: 56 [redescription]; MacKay & MacKay, 2010: 571 [redescription]; Fernandes et al., 2014: 154 [redescription].
Neoponera pilosa Smith, 1858: 95. [Junior synonym of Pachycondyla crassinoda. Syn. nov.]
Senior synonym of Neoponera bicolor: Roger, 1861: 1.
Worker and queen diagnosis. The following is added to the diagnosis of Fernandes et al. (2014): Head subquadrate, slightly longer than broad; humeral carina present, sometimes feebly salient; posterior propodeal margin carinate, carina feebly raised; posterolateral margin of petiolar node carinate; posterior face of node with tumulus; prora well developed, truncate anteriorly.
Worker. Measurements (n = 50): HW: 2.15-3.0; HL: 2.5-3.15; EL: 0.65-0.85; SL: 2.2-3.1; WL: 3.6-5.28; PrW: 1.45-2.0; MsW: 0.9-1.4; MsL: 0.75-1.15; PW: 1.15-1.6; PH: 1.0-1.5; PL: 1.0-1.5; GL: 3.5-5.28; A3L: 1.15-1.8; A4L: 1.45-1.95; A3W: 1.45-2.25; A4W: 1.65-2.35; TLa: 10.05-12.93; TLr: 11.1-14.69. Indices. CI: 84.75-98.36; OI: 23.64-30.23; SI: 81.82-109.8; MsI: 86.96-156.25; LPI: 83.33-125.0; DPI: 96.0-131.82.
Queen. Measurements (n = 13): HW: 2.1-3.1; HL: 2.2-3.25; EL: 0.6-0.9; SL: 2.25-3.35; WL: 1.85-5.35; PrW: 1.5-2.25; MsW: 1.15-2.15; MsL: 1.0-2.15; PW: 1.2-1.75; PH: 1.0-1.55; PL: 1.0-1.7; GL: 3.8-5.91; A3L: 1.45-2.1; A4L: 1.65-2.25; A3W: 2.05-2.55; A4W: 2.2-2.65; TLa: 9.55-14.34; TLr: 10.65-15.95. Indices. CI: 87.69-96.88; OI: 25.86-29.82; SI: 94.83-111.67; MsI: 76.74-143.33; LPI: 90.32-121.43; DPI: 102.94-136.0.
Male. Described by Mackay and Mackay (2010) and diagnosed by Fernandes et al. (2014). The following is added to their diagnoses: notauli sometimes touching posteriorly, thus forming a Y shaped impression; mesopleural sulcus sculpted, slightly reaching ventral pleural surface; metanotum medially carinate; posterior propodeal margin sometimes with blunt carina, though mostly carina-less; posterior face of petiolar node with longitudinally oriented tumulus; node about as long as high in lateral view.
Male Measurements (n = 12): HW: 1.2-1.9; HL: 1.05-1.8; EL: 0.75-0.95; SL: 0.25-0.4; WL: 3.95-4.78; PrW: 1.25-1.8; MsW: 1.25-2.15; MsL: 0.75-1.8; PW: 0.75-1.25; PH: 0.95-1.1; PL: 0.75-1.2; GL: 4.5-6.1; A3L: 1.05-1.7; A4L: 0.65-1.9; A3W: 1.5-1.95; A4W: 1.8-2.15; TLa: 9.0-11.0; TLr: 10.85-13.22. Indices. CI: 82.76-147.62; OI: 47.37-75.0; SI: 13.16-33.33; MsI: 86.21-246.67; LPI: 75.0-121.05; DPI: 62.5-126.67.
Comments. Neoponera villosa is a medium-sized to relatively large species in the N. foetida group (Fig. 12; ☿ TLa = 10.05 – 12.93 mm; ♀︎TLa = 9.55 – 14.34 mm). Neoponera bugabensis, N. curvinodis, N. insignis, N. inversa, and N. zuparkoi are most similar to it. They can be distinguished from N. villosa by examining the following (differential traits indicated in parentheses): N. bugabensis and N. insignis have a convex (vs. concave) mid clypeus; N. inversa has a strongly to slightly concave (vs. straight) anterior nodal margin; and in N. curvinodis the anterior nodal margin may also be slightly concave to straight, but the nodal top is slightly curved anterad (vs. straight). The posterior nodal face of N. curvinodis is just convex, while in N. villosa besides being convex it has a tumulus (Fig. 2b). This latter feature is decisive for separating N. villosa from all cited species, except N. zuparkoi which also has a tumulus on the same surface, but this species shows an anteriorly projected mesopleural margin, forming lobate or lamellate cuticular extension (Fig. 7a – b). In N. villosa the mesopleural anterior margin is only carinate or slightly lobate anteriorly (Fig 11a).
Neoponera villosa has a remarkably stable morphology across its distribution range, in comparison to other closely related congeners like N. curvinodis and N. inversa which show plasticity in some body regions, like the petiolar node (see comments under those species). The cuticular hue is the only detected variable trait in workers and queens of N. villosa, it can sometimes be dark brown combined with castaneous, particularly on the petiolar node (link), or simply dark brown to ferruginous brown (link). The morpho-stability in N. villosa is the only evident case known in the genus for a broadly-ranged lineage. Current observations converge with those of Mendoza-Ramírez et al. (2019) who demonstrated the existence of genetic (mitochondrial) cohesiveness between its populations, which in turn is reflected on their minimally variable external morphology. Arguably, this could be the result of a relatively young divergence from its most recent ancestor which, according to said authors, occurred during the late Pliocene to middle Pleistocene (3.03–1.26 mya), or even more recently in the late Pleistocene (0.75–0.3 mya).
Fernandes et al. (2014) noted that specimens from near the equator have darker mesosoma, gaster and legs, while Mendoza-Ramírez et al. (2019) detected that certain specimens from Peru and Colombia, which show slightly higher genetic distances than the rest in their specimen pool, have a more pilose mesosoma and gaster. In Neoponera, color and pilosity are usually variable traits not useful as species-level diagnostics, unless these clearly differ from those of other related lineages. In this treatment, no major external character variations were observed among the material examined. Thus, it appears that present genetic cohesiveness in N. villosa would be correlated with its morphological stability. Yet, this is a rare case in the genus and additional specimens from further South American regions and biomes need to be included in Mendoza-Ramírez et al.’s (2019) data set so as to confirm that hypothesis.
Within the N. foetida clade, N. foetida and N. villosa are older than N. curvinodis, N. inversa, and N. zuparkoi, and the morphospecies N. ecu2923 (Troya et al. unpublished), the latter considered here a variation of N. zuparkoi (see comments under that species). All being relatively young as judged by their respective comparatively short branches. Of these, populations of N. inversa and N. curvinodis show evident plasticity. As for the other two no inference is possible since scant specimens were examined. Is plasticity in those lineages a signature of ongoing speciation? Are these populations still in course of stablishing differentiated phenotypes? in contrast to what is seen in N. villosa and N. foetida whose morphology is significantly less variable. To tackle these questions more material and approaches are required rather than just morphology-driven inferences. Since N. inversa and N. curvinodis are so similar, and thus far, no evidence seems to indicate they occupy different ecological niches, they could even represent a single but highly variable species.
Distribution notes. Neoponera villosa has the broadest latitudinal range of all species in the genus, it spans from central-eastern Texas to northern Argentina. This is also amongst the most commonly collected taxon in Neoponera, and very frequently collected among all ponerines. Only N. apicalis, and N. verenae Forel have a similar broad distribution. According to Antmaps.org, only three Brazilian states, Piauí, Alagoas and Sergipe lack records of this taxon. Neoponera villosa also inhabits a wide range of biomes and ecosystems, including: the Central and Southern mixed grasslands in central-southern Texas, the drylands of the Tamaulipan Mezquital in southern Texas and northeastern Mexico, the Mexican matorrales (xeric schrublands) of the Meseta Central, the Central American Isthmian Atlantic and Pacific moist forests, the Chocó-Darién, the Venezuelan xeric shrublands of the Cordillera de la Costa, numerous sites in Amazonia as well in all Brazilian biomes, the Bolivian Yungas, the dry and humid Chaco of Argentina and Paraguay, among others. This species mostly likes humid, lowland forests from near the sea level up to about 1400 m elevation, with a record extreme of 2500 m in the city of Bogotá, Colombia. This latter though may just be an interception and not a specimen from a truly established population.
Natural history notes. Neoponera villosa is a heavily studied species in several research lines, see for example, comments on biology and ecology in Perez et al. (1985), Dejean (1990), Longino (2010), Mackay and Mackay (2010), Fernandes et al. (2014), Pérez-Lachaud et al. (2021). Along with N. apicalis these are perhaps the most well-known lineages in the genus with respect to several fields of knowledge, but probably mostly in behavioral biology.
Material examined. 188☿️, 14♀, 18♂. ARGENTINA • 1☿️; Puerto Iguzú, Parque Nacional Iguazú; -25.6831, -54.4546; alt. 180m; 1905-07-08; Peres I. leg.; (DZUP). BOLIVIA – La Paz: • 1☿️; 30 km N Apolo; -14.48, -68.52; alt. 860m; 1993-09-08; Pease, G. leg.; (PSWC). – La paz: • 2☿️; Sarampiuni; -15.4, -68.0833; alt. 754m; 2005-02-13; Rodriguez, J. leg.; Malaise; (DZUP). BRAZIL – Acre: • 1☿️; 6 km E of Porto Walter; -8.25, -72.8; alt. 239m; 1997-02-05; Caldwell, J. P. leg.; (CPDC). – Amapá: • 1☿️; Rod. Duque de Caixas, Km 9; 0.0361, -51.1322; alt. 21m; 1997-10-19; Vilhena, J. leg.; (INPA). – Amazonas: • 1♂; Amana lake; -2.6003, -64.6795; alt. 52m; 1979-09-28; Best, R. leg.; light trap; (INPA). • 1♂; Amana lake; -2.6003, -64.6795; alt. 52m; 1979-09-08; Best, R. leg.; light trap; (INPA). • 1☿️; Balbina; -0.1918, -59.469; alt. 27m; 1992-07-04; Keiroz, M. leg.; (CPDC). • 1☿️; Br. 174, Km 44; -2.724, -60.0472; alt. 72m; 1994-06-24; Harada, A. leg.; (INPA). • 1☿️; Marchantaria island, Camaleão lake; -3.25, -59.9667; alt. 7m; 1992-09-02; Adis, J.; et al. leg.; fogging; (INPA). • 1☿️; Marchantaria island, Camaleão lake; -3.25, -59.9667; alt. 7m; 2008-05-29; (INPA). • 1☿️; Reserva Florestal Adolpho Ducke, Instituto Nacional de Pesquisas da Amazônia (INPA); -2.9333, -59.9667; alt. 106m; 1991-08-21; Adis, J.; et al. leg.; (INPA). • 3♂; São Francisco; -3.1227, -58.433; alt. 17m; 2009-10-02; Fernandes, I. leg.; (INPA). • 1♀; São Francisco; -3.1227, -58.433; alt. 17m; 2009-10-02; Fernandes, I. leg.; (INPA). • 23☿️; São Francisco; -3.1227, -58.433; alt. 17m; 2009-10-02; Fernandes, I. leg.; (INPA). – Bahia: • 3♂; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 1997/09; Santos, J. R. M. leg.; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2012-12-19; Silva, J. A. leg.; (CPDC). • 2☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2004-05-04; pitfall; (CPDC). • 2☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2014-05-04; baiting; (CPDC). • 1☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.75, -39.2167; alt. 61m; 2007-05-02; Santos, P. P. leg.; (CPDC). • 13☿️; 3.3 Km N main entrance CEPLAC-CEPEC, Ilhéus-Itabuna road; -14.7659, -39.2159; alt. 53m; 2017/08; Carvalho, E.; Queiroz, J. leg.; Malaise; (CPDC). • 1☿️; 6 km NW of Boa Nova; -14.3167, -40.25; alt. 844m; 2014-07-05; Silva, A. P. leg.; pitfall; (CPDC). • 2☿️; Buerarema; -14.9844, -39.2583; alt. 130m; 2006-05-03; Maia, J. R.; et al. leg.; (CPDC). • 1☿️; Ilhéus, CEPLAC; -14.7979, -39.0375; alt. 8m; 1988-09-27; Diniz.; et al. leg.; (DZUP). • 1☿️; Itamaraju; -17.0391, -39.5293; alt. 103m; 1994-01-21; Cardoso, I. leg.; (CPDC). • 2☿️; Itororó; -15.1158, -40.0646; alt. 600m; 2007-06-02; Santos, J. R. M. leg.; (CPDC). • 1☿️; Mutuípe; -13.228, -39.504; alt. 220m; 1988-06-21; Crispim, J. leg.; (CPDC). • 1♂; Teixeira de Freitas; -17.5403, -39.7431; alt. 119m; 2004-09-10; Santos, J. R. M. leg.; (CPDC). • 1☿️; Teixeira de Freitas; -17.5403, -39.7431; alt. 119m; 2004-09-10; Santos, J. R. M. leg.; (CPDC). – Ceará: • 1☿️; Chapada do Aroripe; -7.134, -39.594; alt. 770m; 1997-12-28; Almeida, A. leg.; (CPDC). • 1☿️; Crato ; -7.23, -39.4123; alt. 430m; 2007-07-30; Tardim, T. leg.; (CPDC). – Distrito Federal: • 3☿️; Area de Proteção Ambiental Gama Cabeça de Veado; -15.8667, -47.8333; alt. 1080m; 2000-03-02; Mirelle, P. leg.; (DZUP). • 1☿️; Area de Proteção Ambiental Gama Cabeça de Veado; -15.8667, -47.8333; alt. 1080m; 2000-03-02; Mirelle, P. leg.; (CPDC). – Espírito Santo: • 2♂; 13 km SW of Linhares, Bonita; -19.4667, -39.9167; alt. 17m; 2007-07-14; Santos, J. R. M. leg.; (CPDC). • 1☿️; 13 km SW of Linhares, Bonita; -19.4667, -39.9167; alt. 17m; 2007-07-14; Santos, J. R. M. leg.; (CPDC). • 1☿️; Linhares; -19.3947, -40.0653; alt. 31m; 1966-10-11; Niella leg.; (CPDC). • 1☿️; Linhares; -19.3947, -40.0653; alt. 31m; 1991-08-14; Araujo, C. leg.; (CPDC). • 1☿️; Regência; -19.646, -39.826; alt. 6m; 1994-01-23; Delabie, J. leg.; (CPDC). – Goiás: • 1☿️; 17 km NE of Jataí, Fazenda Rio Paraíso; -17.7333, -51.6333; alt. 869m; 2011-01-28; Diniz, J. leg.; pan trap; (DZUP). • 1☿️; 24 km N of Serranópoli, fazenda São Cristóvão; -18.0833, -52.0333; alt. 817m; 2009-01-10; Santos, G. G. leg.; (DZUP). • 1☿️; Fazenda Lageado; -17.8791, -51.7283; alt. 849m; 1998-09-09; Diniz leg.; (DZUP). • 1☿️; Fazenda Lageado; -17.8791, -51.7283; alt. 849m; 2004-09-18; Gilmar leg.; (DZUP). • 1☿️; Fazenda Mato; -17.8791, -51.7283; alt. 849m; 2001-08-31; Diniz, Baiano. leg.; (DZUP). • 1☿️; Ipameri; -17.719, -48.159; alt. 760m; 2011-07-01; Rodrigues, C. leg.; (CPDC). • 1☿️; Jataí; -17.8833, -51.7333; alt. 771m; 2008-04-23; Fachi, M. leg.; (DZUP). • 1☿️; Mineiros, Fazenda Flores.; -17.458, -52.5988; alt. 868m; 2008-07-18; Rezende, F. leg.; (DZUP). • 1☿️; Pequena Central Hidrelétrica (PCH) Retiro Velho; -18.8222, -52.174; alt. 565m; 2011-04-14; Gilmar; James leg.; (DZUP). • 1☿️; Pousada dos Guardiões; -18.306, -51.958; alt. 746m; 2004-05-29; Souza, K. leg.; (DZUP). • 1☿️; Quirinopolis; -18.4476, -50.4552; alt. 510m; 2012-08-05; Daiane leg.; pitfall; (DZUP). – Mato Grosso: • 1♂; 16 km N Chapada dos Guimarães; -15.1667, -55.7833; alt. 734m; 2013-01-17; Savaris, M.; Lampert, S. leg.; (DZUP). • 2☿️; Barra da Garça; -15.8914, -52.2619; alt. 313m; Negrett leg.; (DZUP). • 1☿️; Chapada dos Guimarães; -15.45, -55.9; alt. 726m; 1983-12-01; (DZUP). • 1☿️; Cuiabá; -15.6008, -56.0968; alt. 172m; 1985-02-19; Zanuto, M. leg.; Malaise; (MPEG). • 1☿️; Fazenda Burití; -13.8333, -56.0667; alt. 459m; 1996-05-05; Ribeiro, G. C.; Mendes, H. F. leg.; (DZUP). • 2☿️; Nhambiquara; -12.85, -59.3; alt. 464m; 1960-11-01; Alvarenga, M. leg.; (DZUP). • 1☿️; Poxoréu, Ponte do Santo; -15.826, -54.4016; alt. 375m; 2005-07-23; Diniz.; et al. leg.; (DZUP). • 1☿️; Sapezal, Fazenda Tupanci; -13.5479, -58.8161; alt. 560m; 2017-12-10; Ferreira, J. leg.; pitfall; (DZUP). • 2☿️; Serra Roncador; -11.1667, -52.0833; alt. 303m; 1968-07-13; Laroca & Azevedo. leg.; (DZUP). • 1☿️; Tangará da Serra; -14.6182, -57.4877; alt. 390m; 2008-05-30; Maria, P. R. leg.; (CPDC). • 1☿️; UNEMAT- campus; -9.8833, -56.0833; alt. 287m; 2015-06-27; dos Santos, S. R. leg.; (DZUP). – Mato Grosso do Sul: • 1☿️; 6km W of Jardim; -21.4833, -56.2167; alt. 316m; 2012-12-03; Savaris, M.; Lampert, S. leg.; (DZUP). • 1♂; 7 km NW of Bonito; -21.1, -56.55; alt. 362m; 2011-12-12; Savaris, M.; Lampert, S. leg.; Light trap; (DZUP). • 1☿️; Corumbá; -19.0, -57.65; alt. 124m; 2016-01-13; Filho, R., et al. leg.; hand collected; (DZUP). • 1☿️; Fazenda Nhumirim; -18.988, -56.619; alt. 106m; 2016-07-18; Filho, R., et al. leg.; pitfall; (DZUP). • 2☿️; Fazenda olho d'agua; -19.6742, -51.1903; alt. 385m; 1972-07-12; Diniz, J. leg.; (DZUP). – Minas Gerais: • 1☿️; Pandeiros; -15.4833, -44.75; alt. 510m; 2014-06-16; Santiago, et al. leg.; pitfall; (DZUP). • 2☿️; Parque Estadual do Río Doce; -19.75, -42.6167; alt. 307m; 2015-10-14; Leponce, M, et al. leg.; (DZUP). • 1☿️; Patrocínio; -19.1833, -47.0333; alt. 896m; 2011-06-06; Frizzo, T. leg.; (DZUP). • 1☿️; Santana do Riacho; -19.1685, -43.7148; alt. 764m; 2001-02-19; Soares, S. M. leg.; (CPDC). – Paraná: • 1☿️; 4 km SE Santa Terezinha de Itaipu; -25.4667, -54.35; alt. 296m; 2017-07-25; Filho, R., et al. leg.; pitfall; (DZUP). • 1☿️; Foz do Iguaçu; -25.5, -54.5833; alt. 206m; 1966-12-03; (DZUP). • 1♀; Guairá; -24.0833, -54.2333; alt. 260m; 1982-09-01; Cordeirol, A. M. leg.; (DZUP). • 1♀; Guairá; -24.0833, -54.2333; alt. 260m; 1982-08-01; Cordeirol, A. M. leg.; (DZUP). • 1☿️; Guairá; -24.0833, -54.2333; alt. 260m; 1982-09-01; Cordeirol, A. M. leg.; (DZUP). • 1♂; Reserva Natural Guaricica, Guaricica trail; -25.3, -48.65; alt. 36m; 2008/04; Feola, G. leg.; Malaise; (MEPN). • 2☿️; São Camilo; -24.2667, -53.8333; alt. 335m; 2013-02-01; Gonçalves, R.; Artmann, N. leg.; Malaise; (DZUP). • 1☿️; São Camilo; -24.3, -53.9167; alt. 330m; 2013-02-01; Gonçalves, R.; Artmann, N. leg.; Malaise; (DZUP). – Paraíba: • 1☿️; Universidade Federal do Paraíba; -7.143, -34.85; alt. 47m; 1995-04-02; Santos, M. leg.; (CPDC). – Pará: • 1♀; Bajurú; -1.51, -48.04; alt. 13m; 1978-01-12; França, W. leg.; (MPEG). • 1☿️; Monte Dourado- área 75; -0.7, -52.7; alt. 50m; 2010-11-10; Marsh, C. J. leg.; (DZUP). • 1☿️; Parque Nacional Amazonia; -4.3799, -56.781; alt. 99m; 1978-11-13; Overal, W. leg.; (MPEG). • 1☿️; Xingu river, right shore; -2.8817, -52.0095; alt. 4m; 2000-12-12; Maciel, C.; Dias, J. leg.; Malaise; (MPEG). – Rio Grande do Norte: • 1☿️; Parque do Jiqui; -5.9071, -35.1984; alt. 26m; 2008-11-04; Pinheiro, M. P. G. leg.; (CPDC). – Rio de Janeiro: • 1☿️; Conceição de Malabu; -22.0839, -41.8723; alt. 51m; 1978/09; Alvarenga, M. leg.; (MPEG). • 1☿️; Ilha da Gipóia; -23.0333, -44.35; alt. 113m; 1981-01-22; Rosado leg.; (DZUP). • 1☿️; Maricá; -22.9194, -42.8187; alt. 32m; 2009-06-18; (DZUP). • 1☿️; Universidade Federal Rural do Rio de Janeiro; -22.7713, -43.6863; alt. 23m; 2004-02-03; Delabie, J. H. C. leg.; (CPDC). • 1☿️; Vila do Abraão; -23.1413, -44.1685; alt. 5m; 2012-08-29; Queiroz, J.; et al. leg.; (CPDC). – Rondônia: • 1☿️; CEPLAC; -10.7167, -62.2333; alt. 359m; 2016-12-14; Silva, E. leg.; arboreal pitfall; (MPEG). • 1☿️; Parque Estadual do Guajara-Mirim, 18 km SW of Nova Dimensão; -10.3167, -64.55; alt. 152m; 1998-02-12; Santos, J. R. M. leg.; hand collected; (CPDC). – Roraima: • 1☿️; Estação Ecológica Caracaraí; 1.7764, -61.3049; alt. 71m; 1976-04-30; Nilu leg.; (INPA). • 1♀; Parque Nacional do Viruá; 1.4412, -61.0437; alt. 406m; 2017-12-06; Lattke, J.; et al. leg.; (DZUP). – São Paulo: • 1♂; Corumbataí; -22.222, -47.623; alt. 580m; 1963-09-01; (DZUP). • 1☿️; Fazenda Boa Vista; -15.8947, -52.2624; alt. 308m; 2003-11-26; Diniz leg.; (DZUP). • 1☿️; Fazenda Boa Vista; -15.8947, -52.2624; alt. 308m; 2003-11-26; Diniz.; et al. leg.; (DZUP). • 1☿️; Ipaussu; -23.0552, -49.6237; alt. 575m; 1928-09-20; Pessoa, S. B. leg.; (DZUP). • 1☿️; Itirapina; -22.2526, -47.8156; alt. 804m; 1990-04-10; Paiva, R. leg.; (DZUP). • 1☿️; Itirapina-Cerrado; -22.25, -47.8167; alt. 762m; 2015-11-09; Martins, A. L.; Moericke, P. S. leg.; (DZUP). • 4♀; São José do Rio Preto; -22.1784, -42.966; alt. 601m; 1973-09-02; Taddei leg.; (DZUP). • 2♀; São José do Rio Preto; -22.1784, -42.966; alt. 601m; 1979-08-20; Latorre, J. R. leg.; (DZUP). • 1♀; São José do Rio Preto; -20.7851, -49.3599; alt. 533m; 1978-11-22; (DZUP). • 1♀; São Sebastião ; -23.8064, -45.4018; alt. 2m; 1978-09-04; Diniz, J. leg.; (DZUP). – Tocantins: • 1☿️; Pium; -10.4433, -49.1794; alt. 275m; 2009; Leite, G. leg.; (CPDC). COLOMBIA • 1☿️; Parque Nacional Natural Amacayacu, Matamata; 3.6833, -70.25; alt. 150m; 2001-02-02; Chota, D. leg.; Malaise; (IAvH). • 1☿️; Parque Nacional Natural Serranía de La Macarena; 2.9692, -73.9006; alt. 1253m; 1986/10; (MUSENUV). • 1☿️; Vía Tarapacá km 11; 2.9, -69.744; alt. 85m; 2002-05-27; (ICN). – Chocó: • 1☿️; Estación Silvicultura al Bajo Atrato; 7.0406, -77.3378; alt. 149m; 1992-07-01; Mendoza, L. leg.; hand collected; (ICN). • 1☿️; Parque Nacional Natural Los Katios, Centro administrativo Sautatá; 7.85, -77.1335; alt. 96m; 2001-05-01; Ramírez, D. leg.; Malaise; (IAvH). – Cundinamarca: • 1☿️; Bogotá; 4.64, -74.086; alt. 2500m; 1985-05-24; Parada, C. leg.; (ICN). – Magdalena: • 1☿️; Caribio; 10.76, -75.13; alt. 26m; 1982/10; Norberto, H. leg.; (MUSENUV). – Meta: • 1☿️; La Virginia; 3.463, -73.622; alt. 300m; 1983-03-20; (ICN). • 1☿️; Parque Nacional Natural Serranía de La Macarena; 2.9692, -73.9006; alt. 1253m; 1986/10; (MUSENUV). • 1☿️; Parque Nacional Natural Serranía de La Macarena, Vereda Caño Curia; 3.4067, -73.9537; alt. 520m; 1992-07-18; Villalba, W. leg.; (ICN). • 1☿️; Parque Nacional Natural Serranía de La Macarena, Vereda Caño Curia; 3.4067, -73.9537; alt. 100m; 2004-01-16; Villalba, W. leg.; Malaise; (IAvH). • 1☿️; Puerto Gaitán; 4.312, -72.082; alt. 150m; 1975-12-22; Mackay, W.; Mackay, E. leg.; (ICN). – Nariño: • 1☿️; Iscuande; 2.45, -77.979; alt. 6m; 1976/04; (MUSENUV). – Putumayo: • 1☿️; Parque Nacional Natural La Paya; 0.1551, -75.2231; alt. 219m; 2008-02-20; Jiménez, L. leg.; (ICN). • 1☿️; Parque Nacional Natural La Paya; 0.1551, -75.2231; alt. 219m; 2008-02-20; Jiménez, L. leg.; Malaise; (ICN). – Risaralda: • 1☿️; Santa Cecilia; 5.238, -76.036; alt. 1300m; 1992-02-01; Fernández, F. leg.; (ICN). – Valle del Cauca: • 1☿️; Vereda Campo Alegre; 3.8167, -76.5167; alt. 1413m; 1984-02-05; Cepeda, O. leg.; (ICN). ECUADOR – Esmeraldas: • 1☿️; Reserva Ecológica Cotacachi Cayapas, La Tabla, 7 km EES Playa de Oro; 0.845, -78.744; alt. 120m; 2001-04-01; Araujo, P.; et al. leg.; fogging; (MEPN). – Napo: • 1☿️; Limoncocha; -0.3998, -76.6001; alt. 280m; 1970-07-02; Kazan, P. leg.; (QCAZ). – Orellana: • 2☿️; Parque Nacional Yasuní, 32 Km SSE Limoncocha, Onkonegare Km 39 Pompeya Sur; -0.658, -76.452; alt. 216m; 1995-02-08; Erwin, T.; et al. leg.; fogging; (MEPN). – Pastaza: • 1☿️; Parque Nacional Yasuní, Bameno, sendero Curaray 2; -1.301, -76.143; alt. 240m; 2014-01-13; Troya, A.; Duque, P. leg.; fogging; (MEPN). – Sucumbíos: • 1☿️; Nuevo Sucumbíos; -0.229, -77.326; alt. 750m; 2005-12-27; Troya, A. leg.; fogging; (MEPN). • 1☿️; Reserva de Producción Faunística Cuyabeno, Trocha Zábalo-Güepi; -0.26, -75.69; alt. 270m; 2000-08-08; Araujo, P. leg.; fogging; (MEPN). FRENCH GUIANA – Cayenne: • 1☿️; Campus, P2; 5.1728, -52.6552; alt. 2m; 2018-10-31; Petitclerc, F.; Jackie, O.; Fichaux, M.; Lepage, P. leg.; Winkler48h; (EcoFoG). • 1☿️; Paracou Station; 5.2833, -52.9; alt. 72m; 2009/04; Groc, S. leg.; Winkler; (CPDC). • 1☿️; Sinnamary; 5.3746, -52.9547; alt. 7m; 1999-08-15; Durou, S. leg.; (CPDC). – Saint-Laurent-du-Maroni: • 2☿️; Awala-Yalimapo; 5.691, -53.9328; alt. 5m; 2008-05-17; Groc, S. leg.; (CPDC). • 1☿️; Cayenne; 4.8228, -53.2764; alt. 126m; 2002/04; Orivel, J. leg.; (CPDC). MEXICO – Chiapas: • 1☿️; Tapachula; 14.9, -92.26; alt. 173m; 1992-05-10; Fresnau, D. leg.; hand collected; (CPDC). – Nuevo León: • 1☿️; Parque Natural La Estanzuela Monterrey; 25.5488, -100.2708; alt. 700m; Velasco, J. leg.; ([no museum voucher]). – Oaxaca: • 1☿️; 2km E, La Grua, Chacahua; 15.7028, -96.6031; alt. 17m; 1985-11-30; Quiroz, L. leg.; (CPDC). – Puebla: • 1☿️; Cascada Las Hamacas, 8km ENE Cuetzalán; 20.0343, -97.4512; alt. 280m; 2016-07-03; Longino, J. leg.; search; (JTLC). – Quintana Roo: • 2☿️; Chetumal; 18.5009, -88.2961; alt. 6m; 2011/09; Poteauj, Ch. leg.; (CPDC). – San Luís de Potosí: • 1☿️; Area Natural Protegida Parque Estatal Palma Larga; 21.8712, -99.9598; alt. 990m; 2021-06-25; Stevens, L. leg.; ([no museum voucher]). – Veracruz: • 3♂; La Mancha; 19.595, -96.3955; alt. 9m; 1998-05-06; Fresneau, D. leg.; (CPDC). • 1☿️; La Mancha; 19.595, -96.3955; alt. 9m; 1997-06-10; Quiroz, L. leg.; (CPDC). • 1☿️; Los Tuxtlas, 10km NNW Sontecomapan; 18.5833, -95.0833; alt. 200m; 1985-03-24; P.S. Ward leg.; (PSWC). • 3☿️; Reserva de la Biosfera Los Tuxtlas; 18.4476, -95.2044; alt. 345m; (CPDC). – Yucatán: • 1☿️; Chichén Itzá; 20.6833, -88.5667; alt. 33m; 2004-08-10; Zappi, I. leg.; hand collected; (MSNG). • 1☿️; [no locality given]; 23.0, -102.0; (ZSBS). NICARAGUA – Matagalpa: • 3☿️; RN Cerro Musún; 12.9549, -85.2303; alt. 620m; 2011-04-30; Prebus, M. leg.; hand collected; (MEPN). – Región Autónoma del Atlántico Sur: • 1♀; RN Kahka Creek; 12.6674, -83.7224; alt. 40m; 2011-06-06; Longino, J. leg.; hand collected; (JTLC). • 1☿️; RN Kahka Creek; 12.6674, -83.7224; alt. 40m; 2011-06-06; Longino, J. leg.; hand collected; (JTLC). PANAMA – Colón: • 1☿️; Bosque Protector San Lorenzo; 9.2833, -79.9667; alt. 13m; 2003/10; Dejean, A.; et al. leg.; (CPDC). – Darién: • 1☿️; Cana; 7.7167, -77.7; alt. 500m; 1987-08-23; Olson, D. M. leg.; (PSWC). PARAGUAY – Canindey√∫: • 1☿️; Reserva Natural del Bosque Mbaracayú, Lagunita; -24.1333, -55.4333; alt. 200m; 1996-09-20; A.L. Wild leg.; (ALWC). PERU – Cusco: • 1☿️; Estación Biológica Villa Carmen, 1.5 Km N Pillcopata; -12.8833, -71.4; alt. 521m; 2013-08-05; Ant course 2013 leg.; (DZUP). – Loreto: • 2☿️; Jenaro Herrera; -4.9082, -73.6668; alt. 108m; 1989-10-22; Couturier, G. leg.; (CPDC). UNITED STATES – Texas: • 1☿️; 9 km NE Sinton; 28.1026, -97.4561; alt. 17m; 1956/04; (MEPN). VENEZUELA – Aragua: • 1☿️; La Toma. Rancho Grande; 10.0669, -67.5485; alt. 1100m; 1992-04-07; García, J. L.; Chacón, A. leg.; T. Intercep; (MIZA).
Geographic range. United States (central* and southern Texas), Mexico (Campeche*, Chiapas, Guerrero*, Hidalgo*, Jalisco*, Distrito Federal*, Michoacán*, Nayarit*, Nuevo León, San Luís Potosí, Oaxaca, Puebla*, Querétaro*, Quintana Roo, Sonora*, Sinaloa*, Tabasco*, Tamaulipas*, Veracruz, Yucatán), Belize*, Guatemala*, El Salvador*, Honduras*, Nicaragua, Costa Rica*, Panama, Puerto Rico*, Trinidad and Tobago*, Venezuela*, Colombia, Guyana*, Suriname*, French Guiana, Ecuador, Peru, Bolivia, Brazil (Acre, Amapá, Amazonas, Bahía, Ceará, DF, Espírito Santo, Maranhão*, Mato Grosso, Distrito Federal, Goiás, Minas Gerais, Espírito Santo, Mato Grosso, Mato Grosso Do Sul, Pará, Paraíba, Paraná, Rio de Janeiro, Rio Grande do Norte, Rondônia, Santa Catarina*, São Paulo, Tocantins), Paraguay*, Argentina (Chaco*, Formosa*, Jujuy*, Misiones, Salta*, Tucumán*). * Literature records.
4.2.13. Neoponera zuparkoi (Mackay & Mackay 2010)
Fig. 31b (distribution).
Pachycondyla zuparkoi MacKay & MacKay, 2010: 580, Figs. 114, 267, 685-687. ☿ holotype, Peru, Huánuco, Monson Valley, Tingo María, 15.x.1954, E.I. Schlinger & E.S. Ross (leg.), (CASC) [not examined].
Combination in Neoponera: Schmidt & Shattuck, 2014: 152.
Status as species. Fernandes et al., 2014: 134; Bezděčková et al., 2015: 124.
Worker (and probably queen) diagnosis. Antennal scape, when pulled posterad, exceeding posterior head margin by 1.5 to two times apical scape width; anterior mid clypeal margin straight to convex (link); humeral carina present, not salient (Fig. 7d); anterior mesopleural margin with anteroventrally directed lobate (Fig. 7b) or lamellate projection (Fig. 7a); posterolateral propodeal margin carinate, carina not raised, without crenulae (Fig. 11b); anterior margin of petiolar node straight to feebly concave, posterolateral margin of node carinate, posterior nodal face with tumulus (Fig. 2b); prora well-developed with round tip (link).
Worker. Measurements (n = 5): HW: 2.5-2.75; HL: 2.4-3.06; EL: 0.65-0.75; SL: 2.4-2.62; WL: 3.95-4.53; PrW: 1.6-1.94; MsW: 1.15-1.38; MsL: 0.8-1.0; PW: 1.35-1.44; PH: 0.95-1.44; PL: 1.0-1.34; GL: 3.95-5.0; A3L: 1.3-1.75; A4L: 1.55-1.94; A3W: 1.95-2.19; A4W: 2.05-2.25; TLa: 10.75-12.56; TLr: 11.95-13.69. Indices. CI: 87.72-114.58; OI: 25.45-28.57; SI: 87.27-102.0; MsI: 129.03-150.0; LPI: 80.0-126.32; DPI: 106.98-140.0.
Queen and male. Unknown.
Comments. Neoponera zuparkoi is a medium-sized taxon in the N. foetida group (Fig. 12, ☿ TLa = 10.8 – 12.56 mm), and is very similar to N. villosa, N. inversa, N. curvinodis, and especially to the morphospecies N. ecu2923 (link) which we consider a variant of N. zuparkoi (see below), and hereafter refer to it as N. zuparkoi_var. These four lineages are closely related according to the phylogeny of the genus (Troya et al. unpublished). The lobate mesopleural projection of N. zuparkoi is the easiest form to separate it from those species. In N. zuparkoi_var the mesopleural lobe is expanded in the form of a lamella-shaped projection (Fig. 7a) which is significantly different from the common mesopleural carina present in all species of the N. foetida group, but also in all species of the genus. The humeral carina of N. zuparkoi is not absent, as stated by Mackay and Mackay (2010) and Fernandes et al. (2014), it is present but does not protrude laterally (salient) from the cuticle. Thus, this carina is somewhat poorly developed as compared to all other species in the N. foetida group. However, as previously observed in N. curvinodis and N. inversa, the humeral carina is subject to plasticity, and since just three specimens of this species were examined: two from southern Peru, and one from Rondônia, Brazil, no strong reliance on this character is possible, at least for this species. Likewise, the anterior nodal margin is variable in N. zuparkoi (concave to straight) as in said species. This leaves the putative autapomorphic lobate and/or lamella-shaped mesopleural projection as the only distinguishing feature, potentially useful to effectively separate it from all other species in the N. foetida group. Yet, this trait is apparently another body region which shows certain degree of plasticity in these related group of species, except for N. villosa. This margin is mostly carinate, not protruding, for example in N. curvinodis and N. inversa, but sometimes this carina is also slightly expanded in those species, never anteriorly protruding as in N. zuparkoi, however. Despite this, an untrained eye may not find strong differences between a carinate, lobate, or lamella-shaped mesopleural margin. Differences become more obvious only after examining numerous specimens. The type locality of N. zuparkoi is the central Peruvian Amazonian locality of Tingo María; perhaps collecting more material in this biologically poorly known and vast region would shed further light on the morphology of this species which seems to represent a morphological link between N. inversa and N. curvinodis + N. villosa.
Neoponera zuparkoi_var is ancestral to N. zuparkoi in the Neoponera tree, but this relationship is poorly supported (Troya et al. unpublished). Since their morphology is highly similar with no strong, i.e., non-plastic morphological features justifying a potential taxonomic split, and given that scant specimens from both forms were examined (Mackay and Mackay 2010 erected N. zuparkoi based on a single specimen), we leave N. zuparkoi_var as a variant of N. zuparkoi until additional evidence become available.
Distribution notes. Neoponera zuparkoi inhabits in well-preserved lowland forests in South America. The only four known records belong from the Peruvian Ucayali moist forests, the southern Peruvian Yungas, and the western Amazonian Madeira-Tapajós moist forests in Brazil. Whereas, N. zuparkoi_var is known from the Napo moist forests in Amazonian Ecuador, and from the Magdalena Valley montane forests in the Colombian Andean biome.
Natural history notes. Besides being collected using fogging, Malaise, and Winkler, nothing is known about this species.
Material examined. 5☿️. BRAZIL – Rondônia: • 1☿️; 18 Km SSE, St. Elena River station; -10.9023, -62.168; alt. 230m; 1985-03-23; Torres, M. leg.; (MPEG). COLOMBIA – Cauca: • 1☿️; Parque Nacional Natural Serranía de los Churumbelos Auka-Wasi; 4.5709, -74.2973; alt. 2512m; 1998-07-02; Bustos, J. leg.; Winkler; (MUSENUV). ECUADOR – Orellana: • 1☿️; Parque Nacional Yasuní, 32 Km SSE Limoncocha, Onkonegare Km 39 Pompeya Sur; -0.658, -76.452; alt. 216m; 1995-02-09; Erwin, T.; et al. leg.; fogging; (MEPN). PERU – Cusco: • 1☿️; Quince Mil, km 8; -13.2167, -70.7167; alt. 633m; 2012-08-20; Cavichioli, R.; et al. leg.; Malaise; (DZUP). – Madre de Dios: • 1☿️; Villa Carmen Biological Station; -12.8786, -71.4809; alt. 605m; 2013-08-08; Ward, P. S. leg.; (PSWC).
Geographic range. Peru, Brazil (Rondônia).
Figure 30.
Distribution of species in the Neoponera foetida group. a. Neoponera bugabensis (and its variants), N. insignis, N. prasiosomis sp. nov.; b. Neoponera curvinodis, N. inversa, N. villosa. Empty icons belong to the same species as those with filled icons in each figure, but these represent records obtained from the literature (see references below).
Figure 30.
Distribution of species in the Neoponera foetida group. a. Neoponera bugabensis (and its variants), N. insignis, N. prasiosomis sp. nov.; b. Neoponera curvinodis, N. inversa, N. villosa. Empty icons belong to the same species as those with filled icons in each figure, but these represent records obtained from the literature (see references below).
Figure 31.
Distribution of species in the Neoponera foetida group. a. Neoponera dismarginata, N. fisheri, N. solisi, N. theresiae; b. Neoponera foetida, N. lineaticeps, N. zuparkoi (and its variant). Empty icons belong to the same species as those with filled icons in each figure, but these represent records obtained from the literature (see references below).
Figure 31.
Distribution of species in the Neoponera foetida group. a. Neoponera dismarginata, N. fisheri, N. solisi, N. theresiae; b. Neoponera foetida, N. lineaticeps, N. zuparkoi (and its variant). Empty icons belong to the same species as those with filled icons in each figure, but these represent records obtained from the literature (see references below).
4.2.14. The N. emiliae Species-Group
Worker-Based Key to Species of the
N. emiliae Species-Group
| 1 |
Dorsum of head and pronotum with mixture of punctae and striae (Fig. 1a, b); N. emiliae subclade ………………………………………………………….. 2 |
| |
Dorsum of head and pronotum costate (Fig. 1c); N. magnifica subclade …... 3 |
Figure 1.
Head sculpture variation. a, b. Punctate-striate: a. ☿ N. emiliae, b. ☿ N. metanotalis; c. Costate (☿ N. dropsy).
Figure 1.
Head sculpture variation. a, b. Punctate-striate: a. ☿ N. emiliae, b. ☿ N. metanotalis; c. Costate (☿ N. dropsy).
| 2 (1) |
Anterior mid clypeal margin convex, relatively acute (Fig. 1a); body dorsum with abundant appressed copperish hairs (Fig. 1a, 2a); northwestern South America ……………………………………………………….….. N. emiliae
|
| |
Anterior mid clypeal margin horizontally straight or weakly concave (Fig. 1b); body dorsum with pale golden appressed pilosity, significantly less abundant than in prior lead (Fig. 1b, 2b); eastern Brazil………………... N. metanotalis
|
Figure 2.
Appressed pilosity. a. Abundant, copperish (☿ N. emiliae); b. Less abundant, pale golden (☿ N. metanotalis).
Figure 2.
Appressed pilosity. a. Abundant, copperish (☿ N. emiliae); b. Less abundant, pale golden (☿ N. metanotalis).
| 3 (1) |
Subpetiolar process notched (or concave) medially (Fig. 3a); head dorsum with two patches of appressed golden hairs (Fig. 13c); central Brazil ……..………………………………………………………………….. N. magnifica
|
| |
Subpetiolar process convex or straight medially (Fig. 3b, 12d); head dorsum with scant appressed golden pilosity (Fig. 1c), otherwise pilosity more abundant (Fig. 8c, e) but not as described in prior lead ……………...…... 4
|
| 4 (3) |
Posterior face of petiolar node glabrous (Fig. 4a); head dorsum with irregular, not continuous costae (Fig. 12d) better discernible on lateral side of head; eastern Brazil……………………………………….…………….... N. glabra
|
| |
Posterior face of petiolar node striate, punctate or with mixture of both (Fig. 4b); costae on head dorsum less irregular than above, and continuous, without breaks (Fig. 8e) …………………………………………………………..... 5
|
Figure 3.
Petiole in lateral view. a. Subpetiolar process notched medially (☿ N. magnifica); b. Subpetiolar process horizontally straight or feebly convex medially (N. glabra). spp: subpetiolar process. Arrowhead on b. shows an incision.
Figure 3.
Petiole in lateral view. a. Subpetiolar process notched medially (☿ N. magnifica); b. Subpetiolar process horizontally straight or feebly convex medially (N. glabra). spp: subpetiolar process. Arrowhead on b. shows an incision.
Figure 4.
Posterior face of petiolar node. a. Glabrous face, posteroventral nodal margin (in yellow dashed) showing scrobiculae (☿ N. glabra); b. Striate-punctate face, posteroventral nodal margin smooth (☿ N. magnifica).
Figure 4.
Posterior face of petiolar node. a. Glabrous face, posteroventral nodal margin (in yellow dashed) showing scrobiculae (☿ N. glabra); b. Striate-punctate face, posteroventral nodal margin smooth (☿ N. magnifica).
| 5 (4) |
Posteroventral margin of petiolar node scrobiculate (Fig. 4a); mid-lateral margin of node with incision (Fig. 3b); meso and metapleuron mostly micropunctate (Fig. 8a); western and central Amazonia………... N. caxiuana
|
| |
Posteroventral margin of petiolar node smooth (Fig. 4b); mid-lateral margin of node without incision (Fig. 3a); meso and metapleuron costate (Fig. 12a) ……………………………………………………………………………….. 6 |
| 6 (5) |
Propodeum costate, except posterior face which has scattered piligerous punctae, costae on mesonotum are somewhat continued with those of propodeum (Fig. 5a, link); prora lip-shaped, comparatively small but well-discernible in lateral view (Fig. 9d); eastern Brazil……..….….... N. dorsilinea
|
| |
Propodeum mostly striate, dorsally with micro striae, and posteriorly with scattered piligerous punctate, costae of mesonotum reach only its posterior margin (Fig. 5b); prora tiny or vestigial, not discernible in lateral view (Fig. 10a); eastern Brazil ……………………………………………….... N. dropsy
|
Figure 5.
Mesosoma in lateral view. a. Propodeum costate (☿ N. dorsilinea); b. Propodeum striate (☿ N. dropsy). ms: mesonotum; ppd: propodeum.
Figure 5.
Mesosoma in lateral view. a. Propodeum costate (☿ N. dorsilinea); b. Propodeum striate (☿ N. dropsy). ms: mesonotum; ppd: propodeum.