Submitted:
23 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Historical
2. Materials and Methods
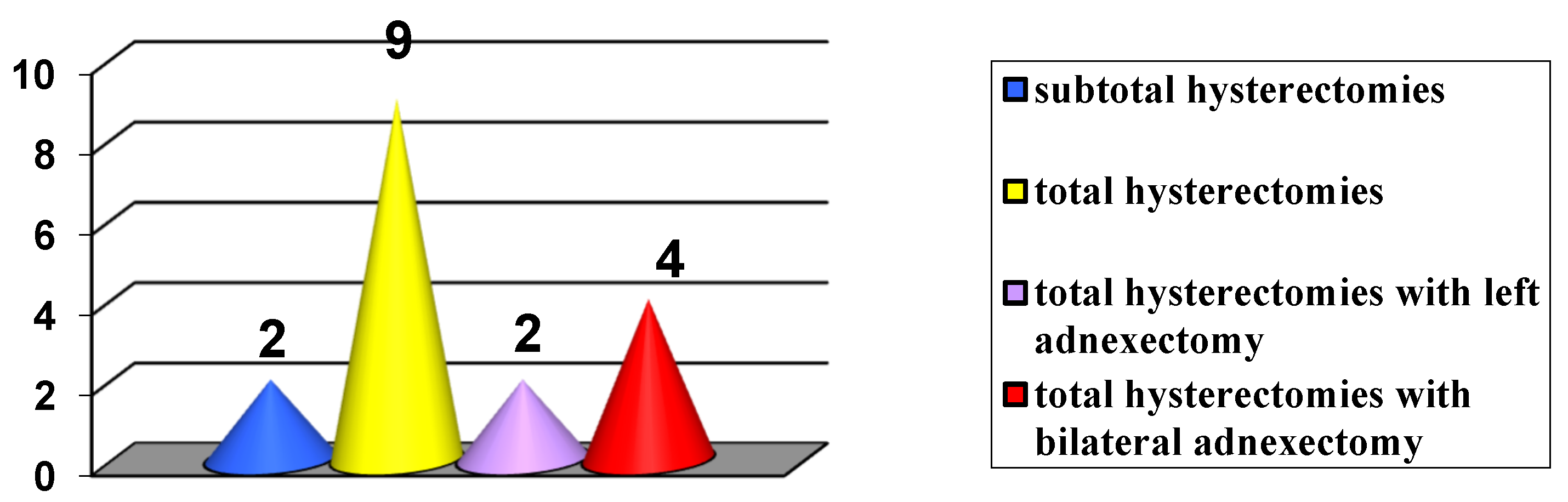
3. Results
4. Discussion
- retroplacental hematoma consists in the rapid formation of a blood collection between the maternal face of the placenta and the uterine wall, before the expulsion of the fetus, after the 20th week of gestation; it is a milder lesion, with reduced symptoms and often discovered only during the inspection of the placenta or during routine ultrasound checks; in this case, the detachment is mild, it may involve one or two placental cotyledons; larger hematomas can also be found, located in the center of the placenta, which can take off up to 50% of the placental surface and can also have an important clinical impact.
- The recurrence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyelese, Y., & Ananth, C. V. (2006). Placental abruption. Obstetrics & Gynecology, 108(4), 1005–1016.
- Ananth, C. V., Smulian, J. C., Srinivas, N., Getahun, D., & Salihu, H. M. (2005). Risk of infant mortality among twins in relation to placental abruption: Contributions of preterm birth and restricted fetal growth. Twin Research and Human Genetics, 8(5), 524–531.
- Berceanu, S., & Georgescu Brăila, M. (1998). Obstetrica, Vol. II, Part II. Craiova: Editura Aius.
- Djakovic, A., Rieger, L., Wirbelauer, J., Kalla, J., & Dietl, J. (2007). Schwere intrauterine Wachstumsretardierung bei Uterus bicornis, Insertio velamentosa und vorzeitiger partieller Plazentalösung in der 26. Schwangerschaftswoche—eine Kasuistik. Z Geburtshilfe Neonatol, 211(4), 169–173.
- Ananth, C. V., Peltier, M. R., Kinzler, W. L., Smulian, J. C., & Vintzileos, A. M. (2007). Chronic hypertension and risk of placental abruption: Is the association modified by ischemic placental disease? American Journal of Obstetrics and Gynecology, 197(3), 273.e1-7.
- Cunningham, G. F., MacDonald, P. C., Gant, N. F., et al. (1993). Obstetrical hemorrhage. In Williams Obstetrics (19th ed., pp. 819–851). Norwalk, CT: Appleton and Lange.
- Medina, T. M., & Hill, D. A. (2006). Preterm premature rupture of membranes: Diagnosis and management. American Family Physician, 73(4), 659–664.
- Vârtej, P. (1997). Obstetrică fiziologică şi patologică. Bucureşti: Editura All.
- Könegärd, M., & Gennser, G. (1986). Incidence and recurrence rate of abruptio placentae in Sweden. Obstetrics & Gynecology, 67, 523.
- Hladky, K., Yankowitz, J., & Hansen, W. F. (2002). Placental abruption. Obstetrics & Gynecology Survey, 57(5), 299–305.
- Alghamdi, N. G., Khoj, Z. M., et al. (2018). Placental abruption. Indo American Journal of Pharmaceutical Sciences, 5(11), 11900–11904.
- Riihimäki, O., Metsäranta, M., Paavonen, J., Luukkaala, T., Gissler, M., Andersson, S., Nuutila, M., & Tikkanen, M. (2018). Placental abruption and child mortality. Pediatrics, 142(2), e20173915.
- Siddique, S., Afridi, H., Riaz, B., & Afridi, I. U. K. (2021). Fetomaternal outcome in placental abruption. Pakistan Journal of Medical & Health Sciences, 15(8), 1971–1974.
- Downes, K. L., Shenassa, E. D., & Grantz, K. L. (2017). Neonatal outcomes associated with placental abruption. American Journal of Epidemiology, 186(12), 1319–1328.
- Braila, A. D., Gluhovschi, A., Neacsu, A., Lungulescu, C. V., Braila, M., Vircan, E. L., Cotoi, B. V., & Goganau, A. M. (2018). Placental abruption: Etiopathogenic aspects, diagnostic and therapeutic implications. Romanian Journal of Morphology and Embryology, 59(1), 187–195.
- Dafallah, S. E., & Babikir, H. E. (2004). Risk factors predisposing to abruptio placentae. Maternal and fetal outcome. Saudi Medical Journal, 25(9), 1237–1240.
- Thieba, B., Lankoande, J., Akotionga, M., Kyelem, C., Ouedraogo, A., Ouedraogo, C. M., & Koné, B. (2003). Hématome rétroplacentaire: Aspects épidémiocliniques et pronostiques à propos d’une série de 177 cas. Gynecology Obstetrics Fertility, 31(5), 429–433.
- Nayama, M., Tamakloé-Azamesu, D., Garba, M., Idi, N., Djibril, B., Kamayé, M., Marafa, A., Touré, A., Diallo, F. Z., & Houfflin-Debarge, V. (2007). Hématome rétroplacentaire. Prise en charge dans une maternité de référence du Niger. Etude prospective à propos de 118 cas sur un an. Gynecology Obstetrics Fertility, 35(10), 975–981.
- Markhus, V. H., Rasmussen, S., Lie, S. A., & Irgens, L. M. (2011). Placental abruption and premature rupture of membranes. Acta Obstetricia et Gynecologica Scandinavica, 90, 1024–1029.
- Tica, V. I., Şerbănescu, L., & Tica, I. (2006). Etiologic, clinical and prognostic correlations in abruptio placentae. Revista Medico-Chirurgicală a Societății de Medicină și Natură Iași, 110(3), 633–638.
- Vârtej, P. (2000). Apoplexia uteroplacentară. In Munteanu Ioan (Ed.), Tratat de obstetrică (pp. 1234–1245). Bucureşti: Editura Academiei Române.
- Odibo, A. O., Cahill, A. G., Stamilio, D. M., Stevens, E. J., Peipert, J. F., & Macones, G. A. (2007). Predicting placental abruption and previa in women with a previous cesarean delivery. American Journal of Perinatology, 24(5), 299–305.
- Costea, D. O., Șerbănescu, L., Badiu, D., Ardeleanu, V., Branescu, C. M., Zgura, A., & Costea, A. C. (2022). Pain management in the right iliac fossa during the COVID-19 pandemic. Journal of Mind and Medical Sciences, 9(1), 162–167.
- Bordei, P., Surdu, L. S., Șerbănescu, L., & Tudorache, I. S. (2019). Morphometry of placental vessels. Revue de Chimie (Bucharest), 70(11), 4119–4122.
- Dommisse, J., & Tiltman, J. (1992). Placental bed biopsies in placental abruption. BJOG, 99(8), 651–654.
- Ananth, C. V., Berkowitz, T. S., Savitz, D. A., et al. (1999). Placental abruption and adverse perinatal outcomes. JAMA, 282, 1646–1651.
- Tikkanen, M., Nuutila, M., Hiilesmaa, V., Paavonen, J., & Ylikorkala, O. (2006). Clinical presentation and risk factors of placental abruption. Acta Obstetricia et Gynecologica Scandinavica, 85(6), 700–705.
- Tikkanen, M., Gissler, M., Metsäranta, M., Luukkaala, T., Hiilesmaa, V., Andersson, S., Ylikorkala, O., Paavonen, J., & Nuutila, M. (2009). Maternal deaths in Finland: Focus on placental abruption. Acta Obstetricia et Gynecologica Scandinavica, 88(10), 1124–1127.
- Saeed, M., & Rana, T. (2011). Fetomaternal outcome in pregnancies complicated with placental abruption. Pakistan Journal of Medical & Health Sciences, 5(1), 140–143.
- Usui, R., Matsubara, S., Ohkuchi, A., Kuwata, T., Watanabe, T., Izumi, A., & Suzuki, M. (2008). Fetal heart rate pattern reflecting the severity of placental abruption. Archives of Gynecology and Obstetrics, 277(3), 249–253.
- Pelinescu-Onciul, D., & Bari, M. (2005). Ecografia în obstetrică şi ginecologie: Semiologie ecografică normală. Bucureşti: Editura Didactică şi Pedagogică.
- Glantz, C., & Purnell, L. (2002). Clinical utility of sonography in the diagnosis and treatment of placental abruption. Journal of Ultrasound in Medicine, 21(8), 837–840.
- Sohn, C., Voigt, H. J., & Vetter, K. (2004). Vascular supply of the uteroplacental unit and techniques for examination of individual vessels. In Doppler Ultrasound in Gynecology and Obstetrics (pp. 123–145). Stuttgart: Thieme.
- Urban, G., Vergani, P., Ghidini, A., Tortoli, P., Ricci, S., Patrizio, P., & Paidas, M. J. (2007). State of the art: Non-invasive ultrasound assessment of the uteroplacental circulation. Seminars in Perinatology, 31(4), 232–239.
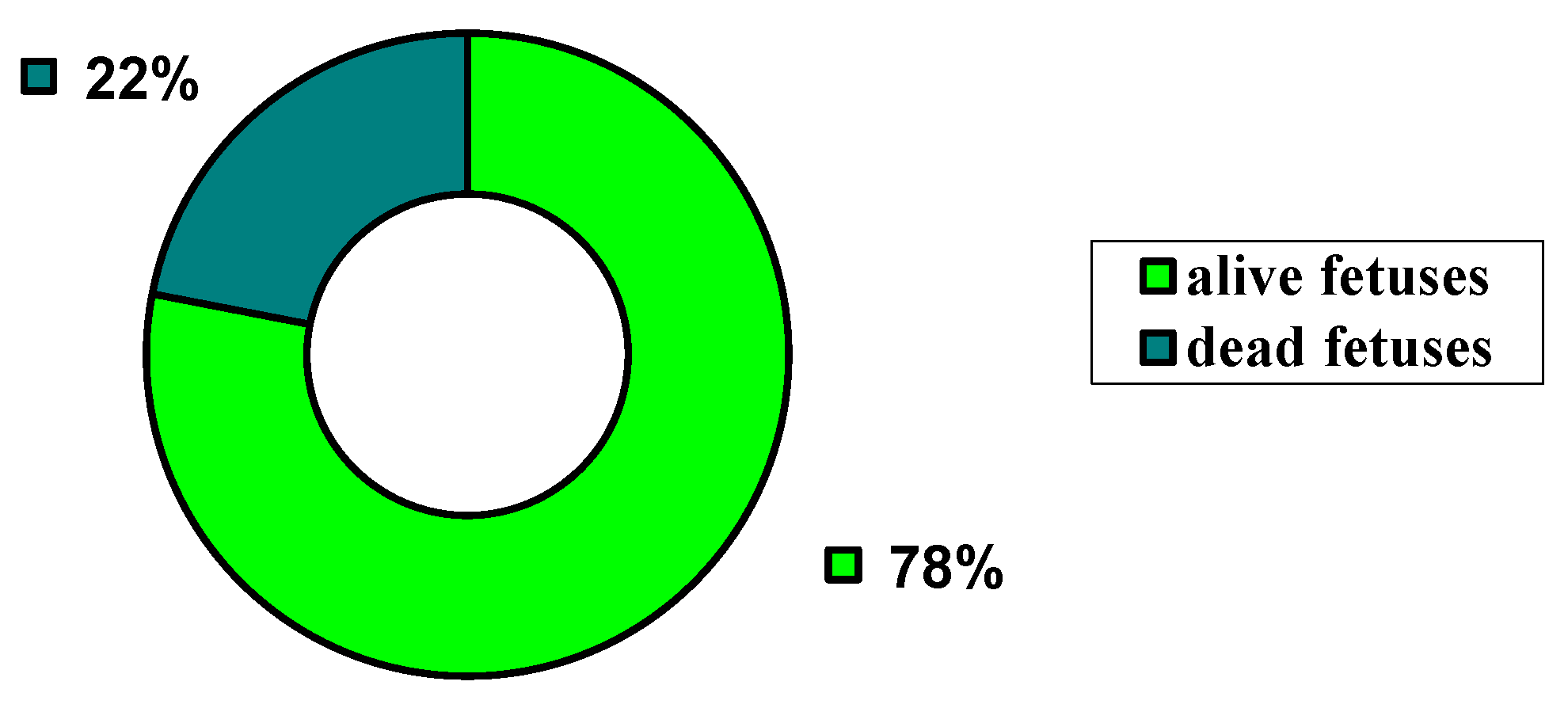
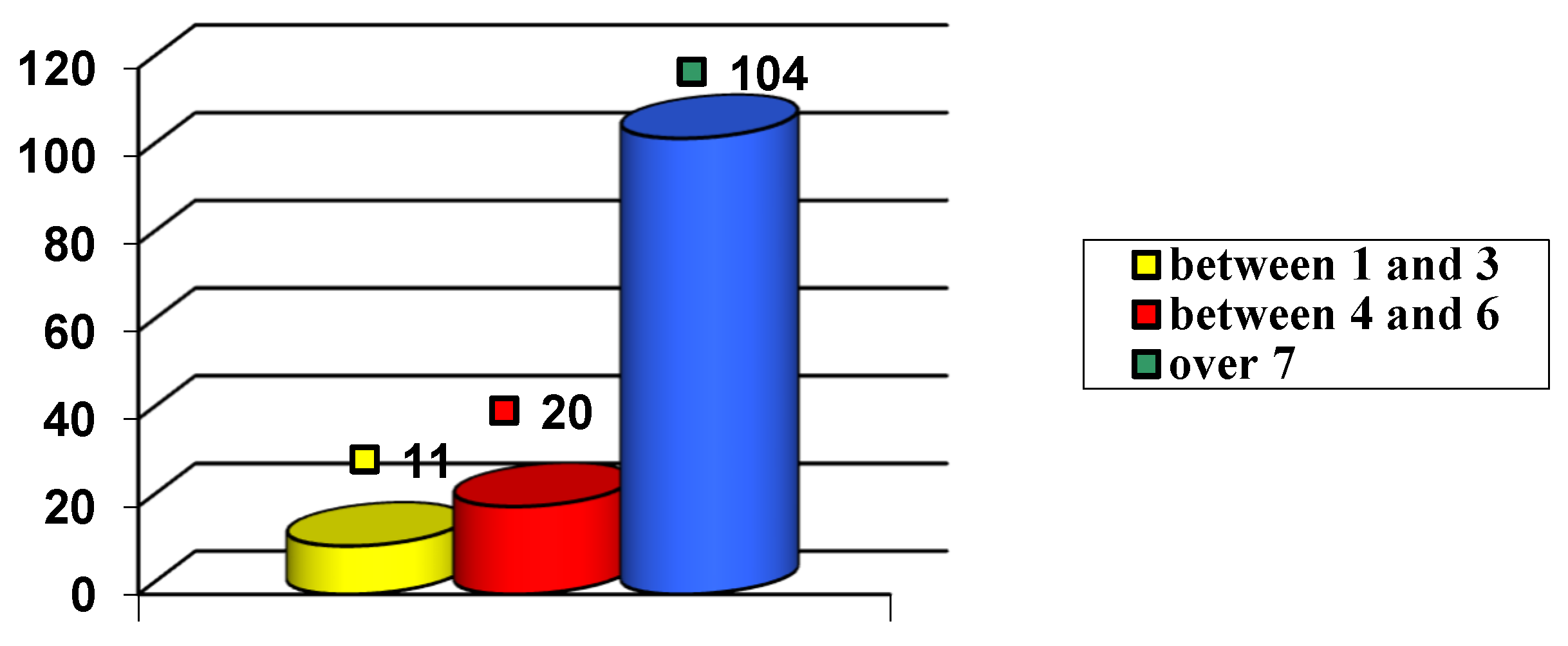
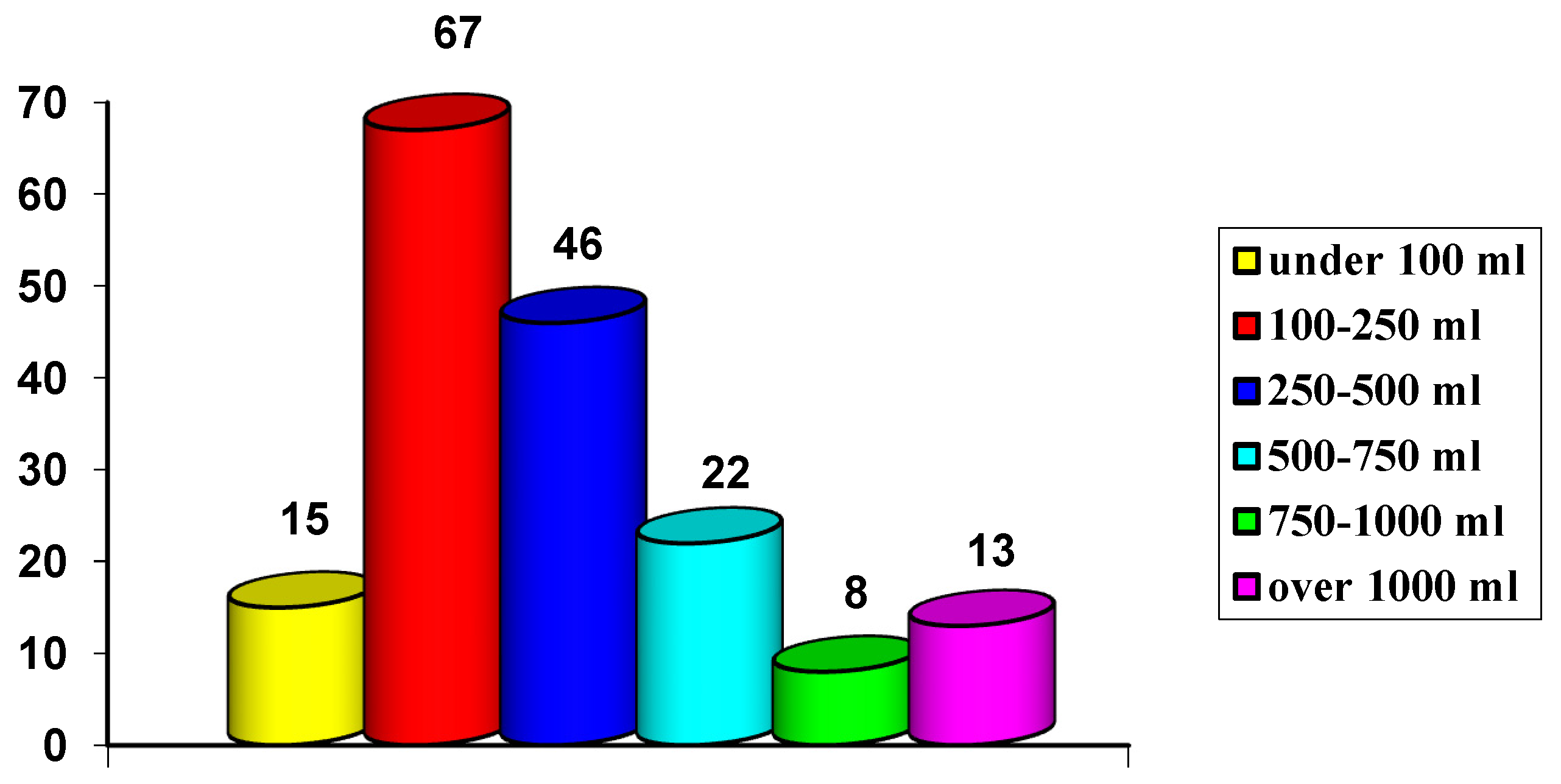
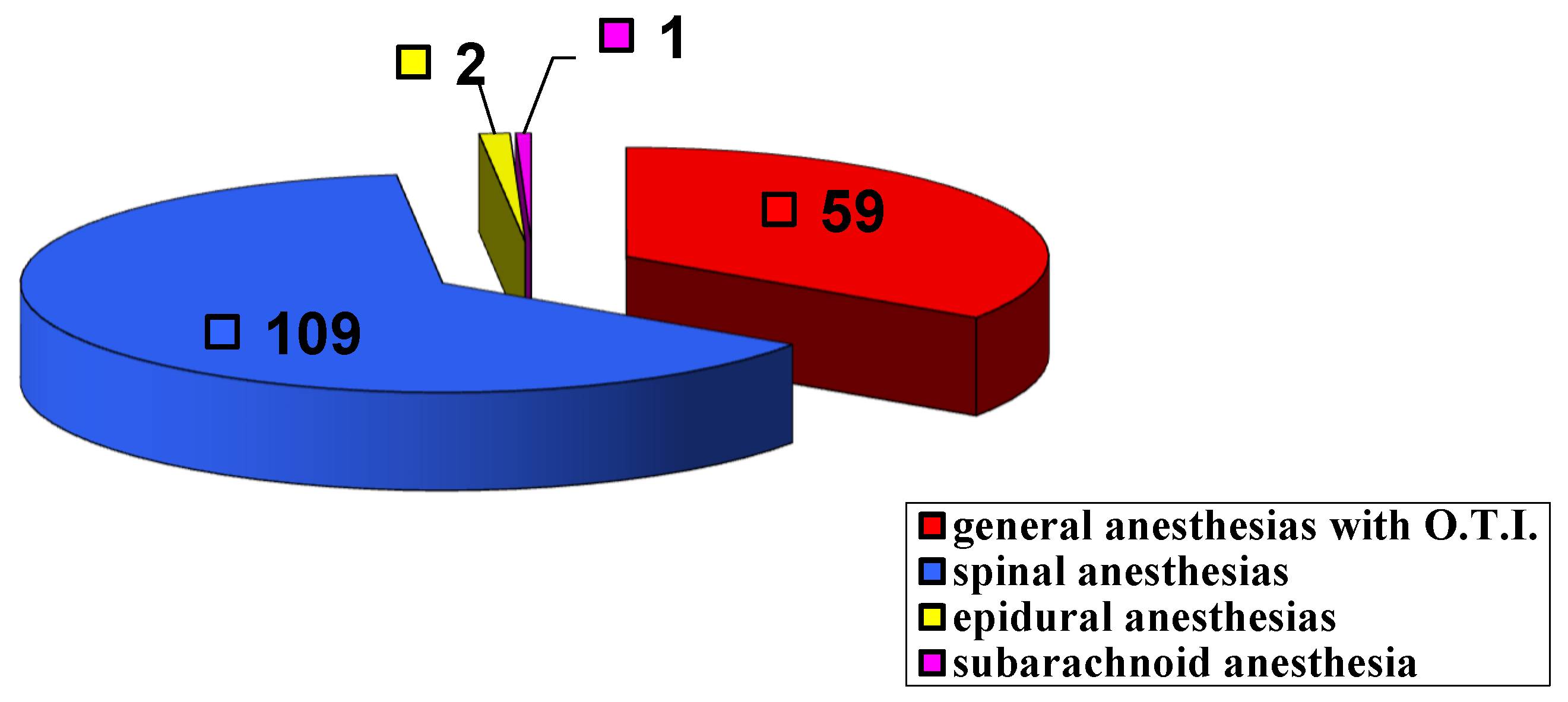
| I G | II G | III G | IV G | V G | VI G | VII G | VIII G | IX G |
| 74 | 35 | 23 | 11 | 14 | 3 | 4 | 4 | 3 |
| I P | II P | III P | IV P | V P | VI P | VII P | VIII P |
| 114 | 35 | 12 | 3 | 4 | 0 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




