Submitted:
24 December 2024
Posted:
24 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
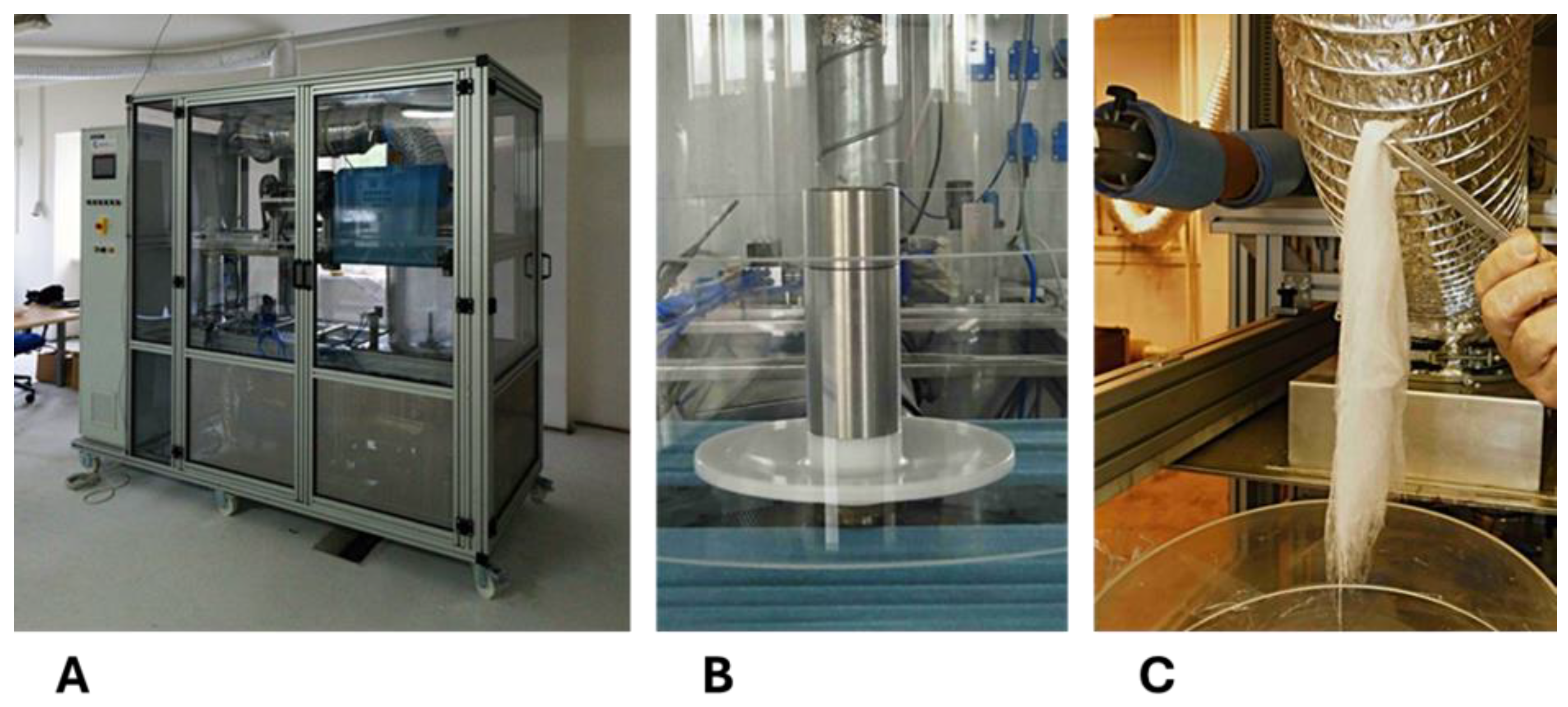
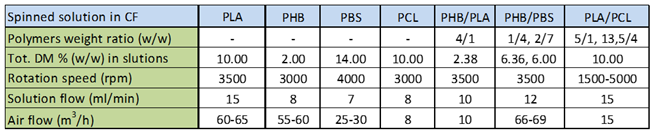
2.1. Preparation of Fibrous Scaffolds
2.2. Characterization of the Scaffold Microstructure by Scanning Electron Microscopy
2.3. Brunauer-Emmett-Teller (BET) Analysis
2.4. Sterilization of the Prepared Fibrous Scaffolds
2.5. Cellular Component, Isolation and Characterization
2.6. Cell Cultivation
2.7. Cell Visualization and Confocal Microscopy
3. Results
3.1. Preparation of Fibrous Scaffolds
3.2. Characterization of the Scaffold Microstructure by Scanning Electron Microscopy
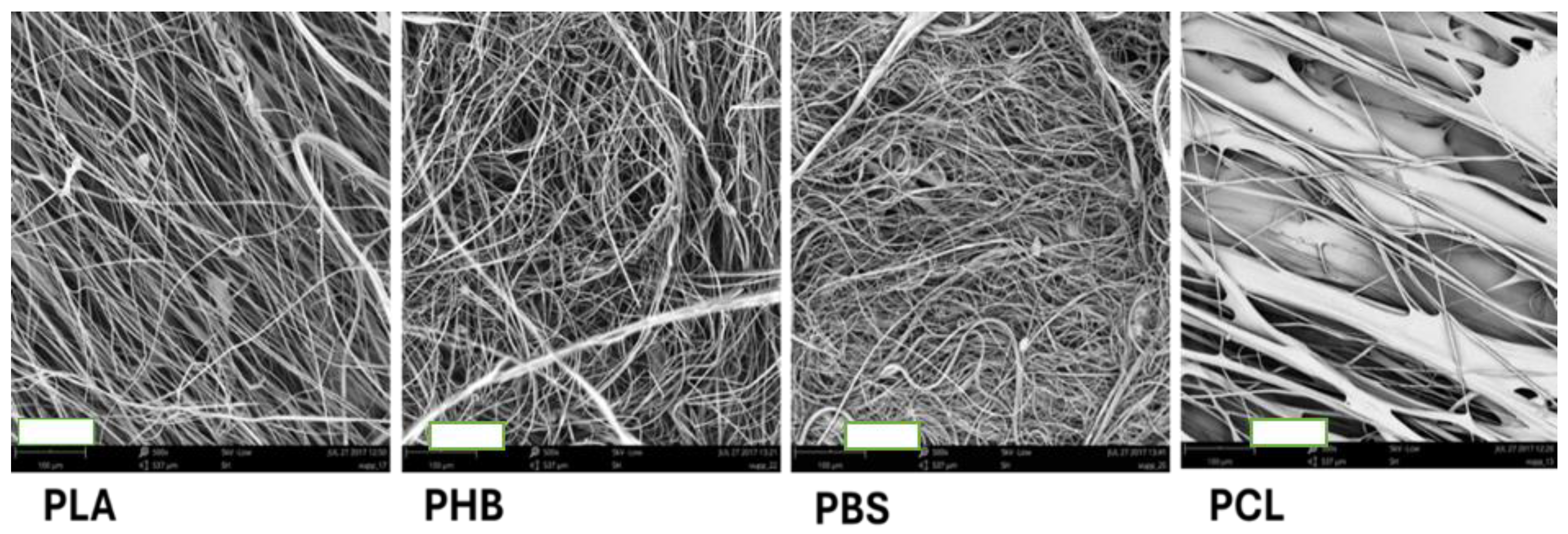
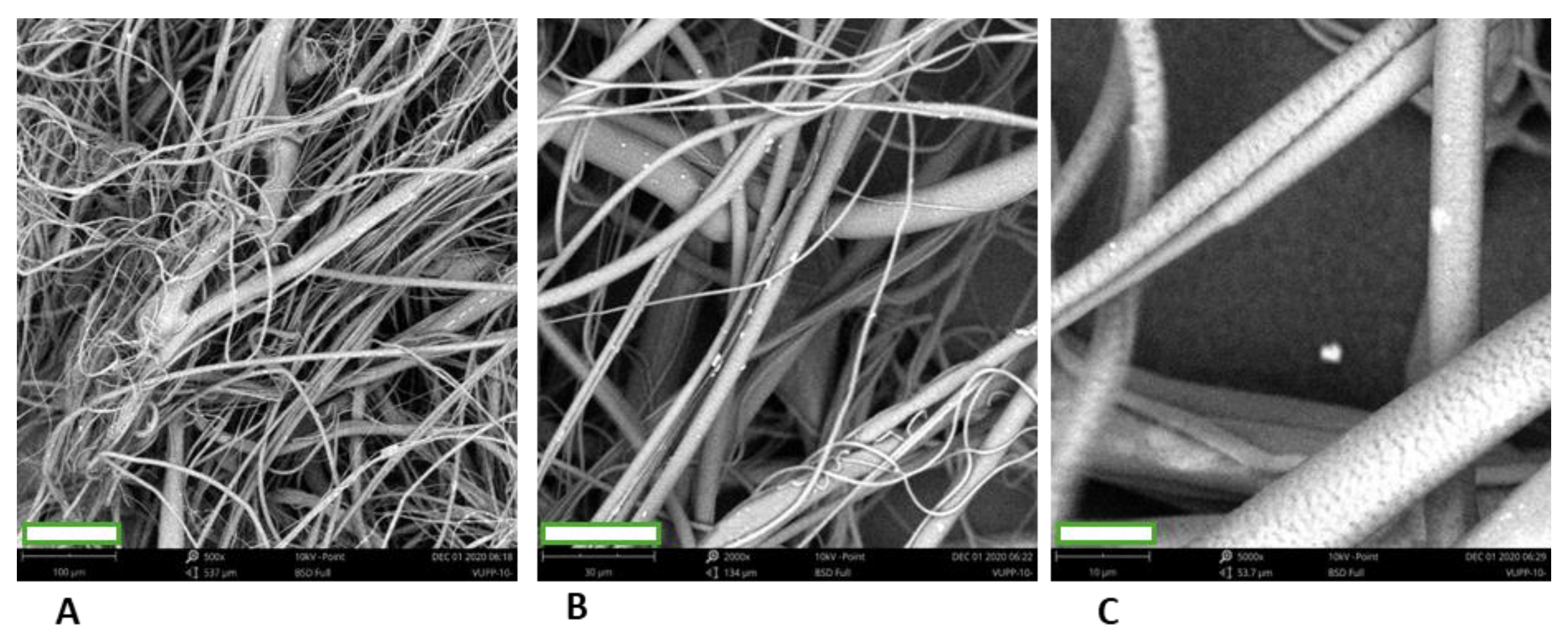
3.2.1. Microstructure of the 3D Fiber „Cotton Wool-like Constructs“ Prepared from the Individual Polymers
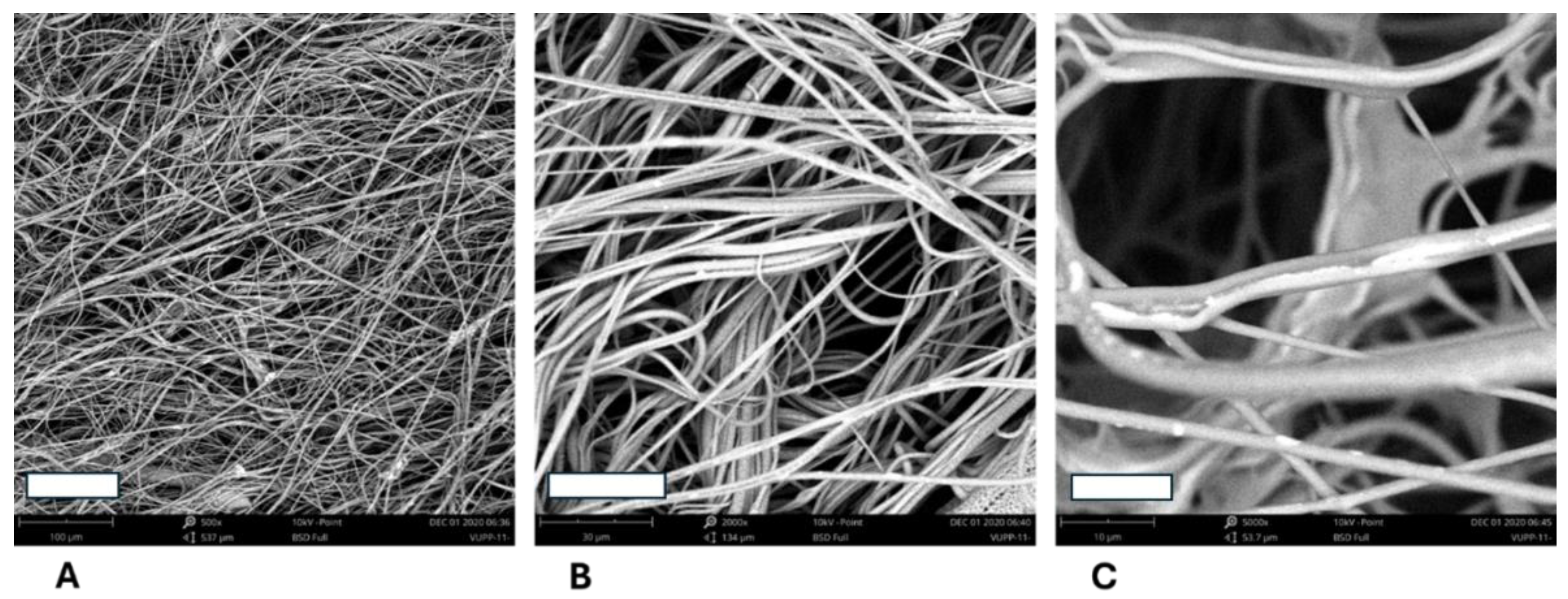
3.2.2. Microstructure of the 3D Fiber „Cotton Wool-like Constructs“ Prepared from the Polymer Blends
3.2.3. Use of PLA and PCL Composite Fibers and Optimal PLA/PCL Ratio in Fibrous Scaffolds for Tissue Engineering Applications
3.2.4. Influence of Fiber and Pore Size in Micro/Nanofibrous Mats on Cell Colonization
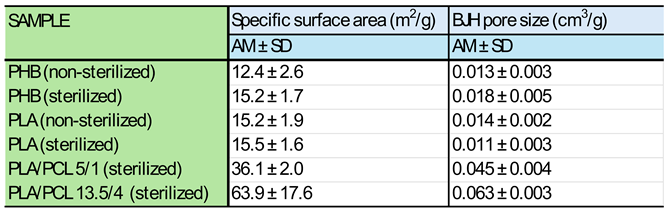
3.3. BET Analysis
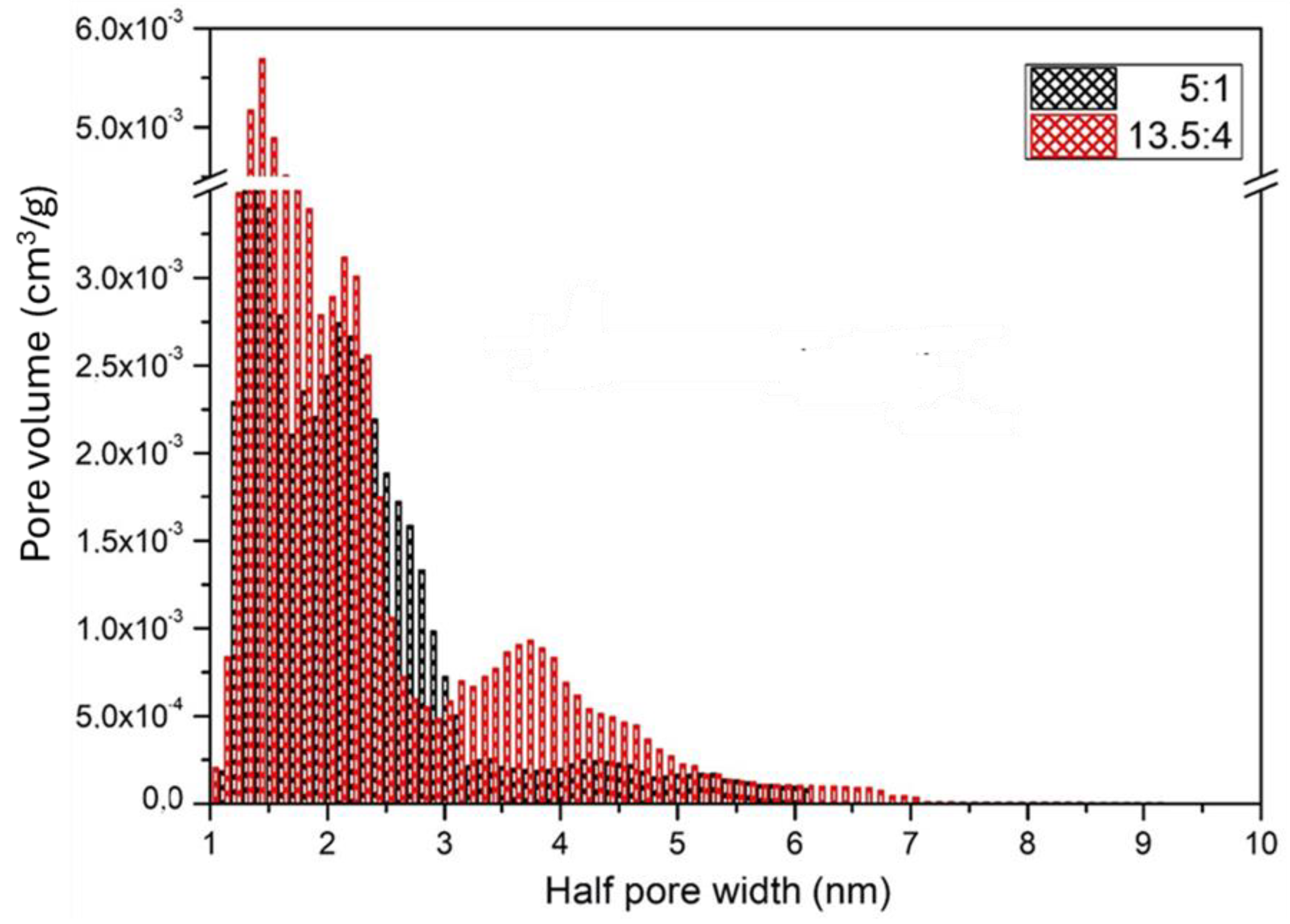
3.3.1. Results of BET Analysis of Micro/Nanofibrous Hybrid Fibrous Scaffolds Prepared from Single Polymers and from 5/1 and 13.5/4 (w/w) PLA/PCL Blends
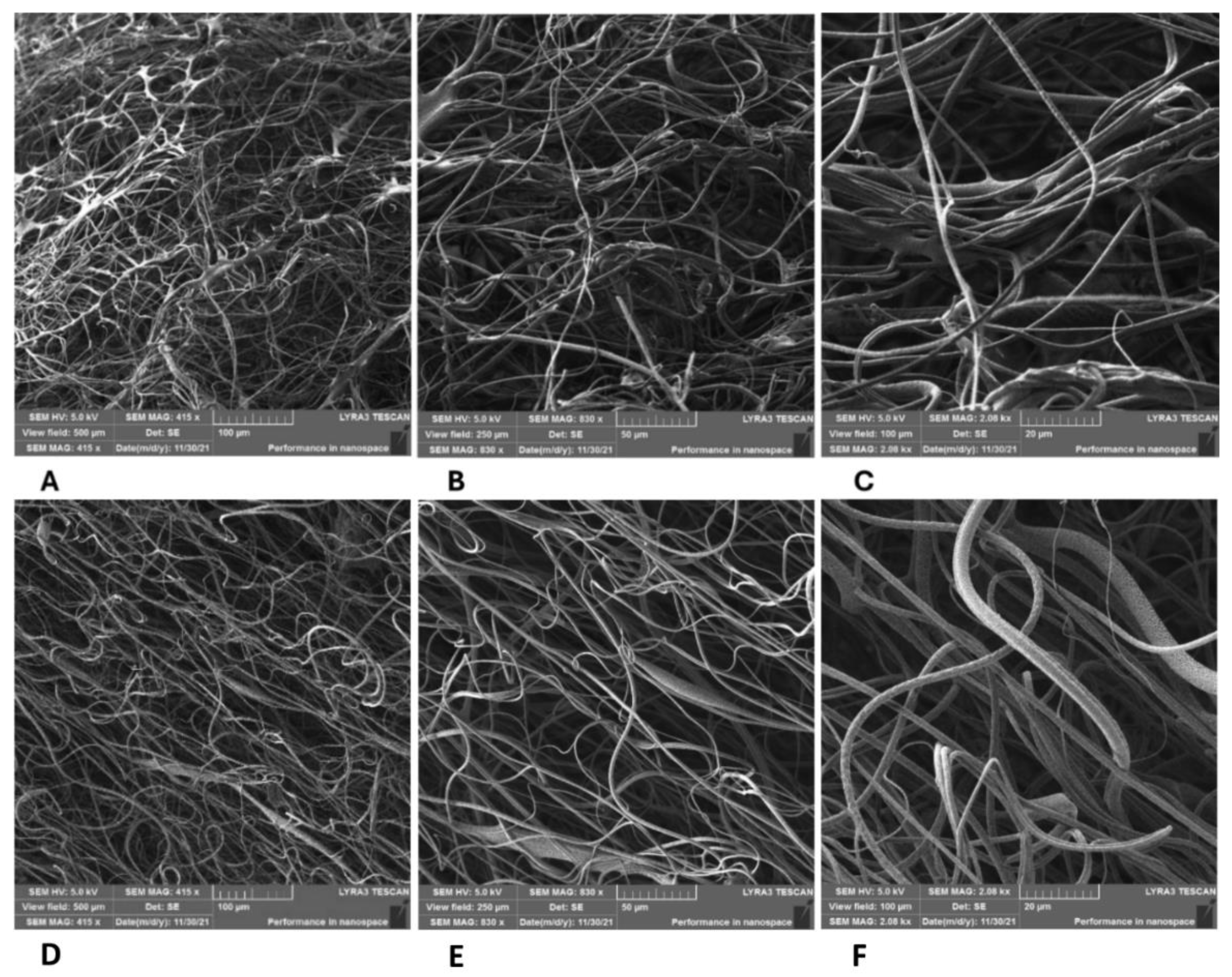
3.3.2. Effect of Micro/Nanofiber Surface Roughness on Cell Colonization
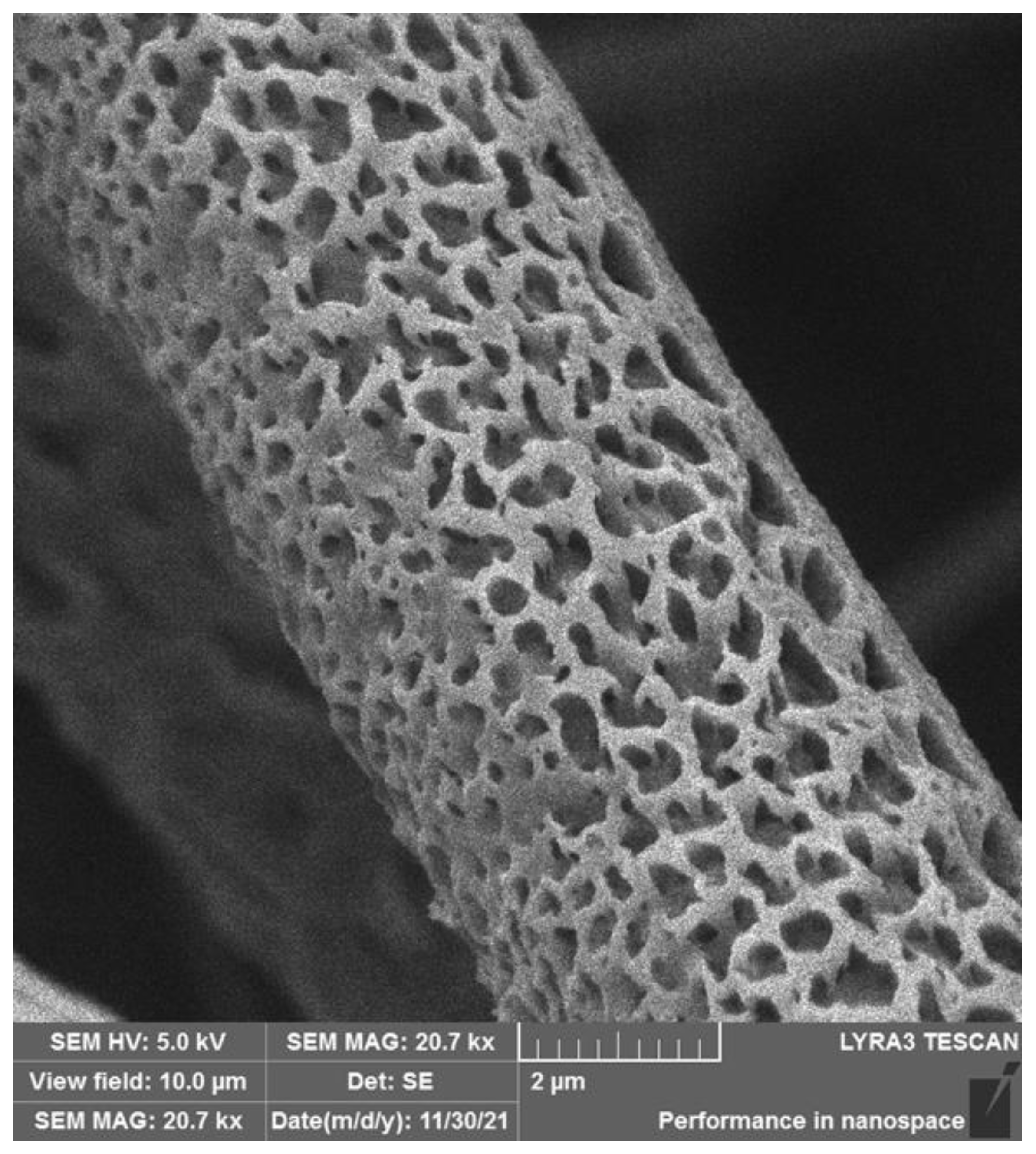
3.3.3. Effect of Internal Porosity of Individual Micro/Nanofibers on Cell Colonization
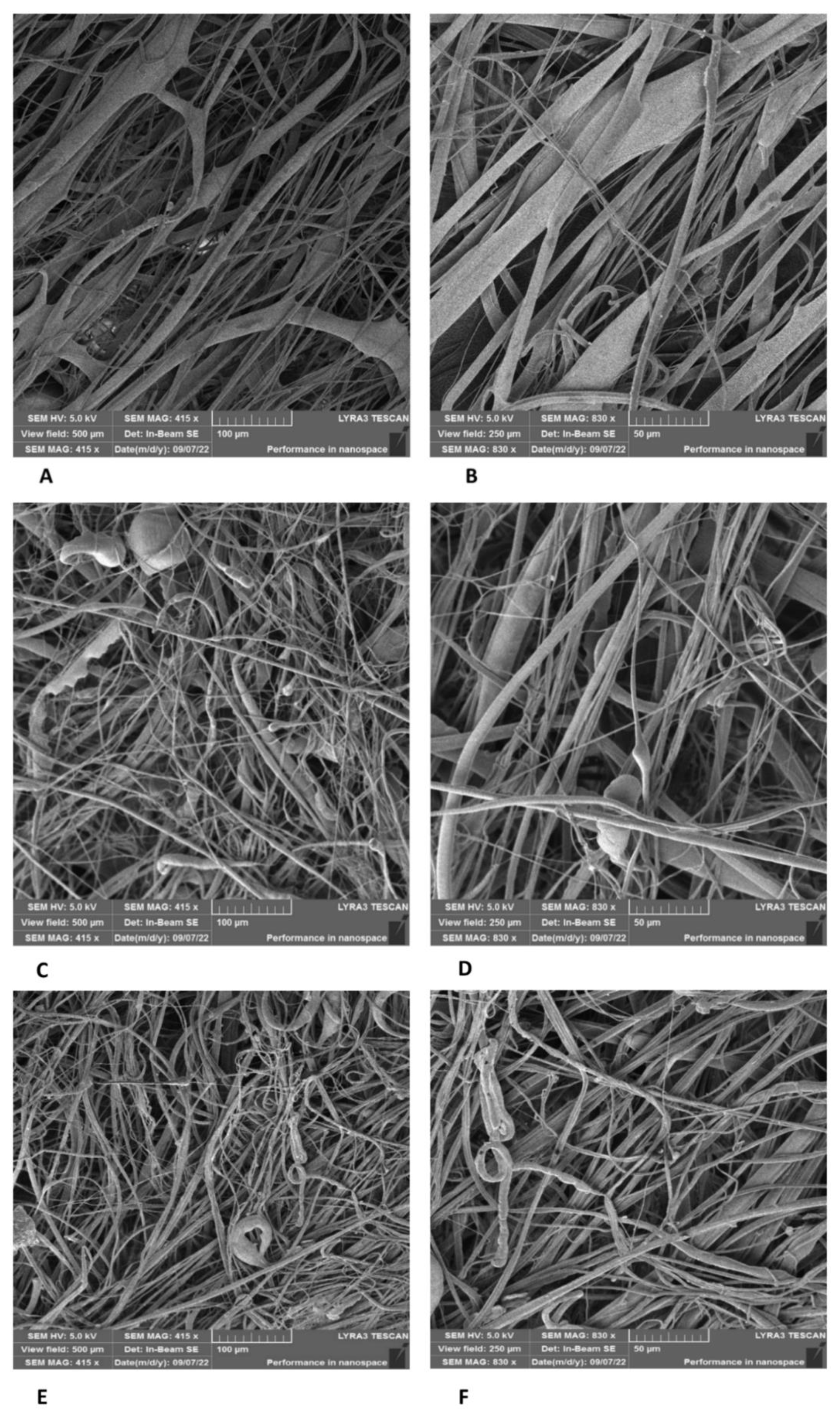
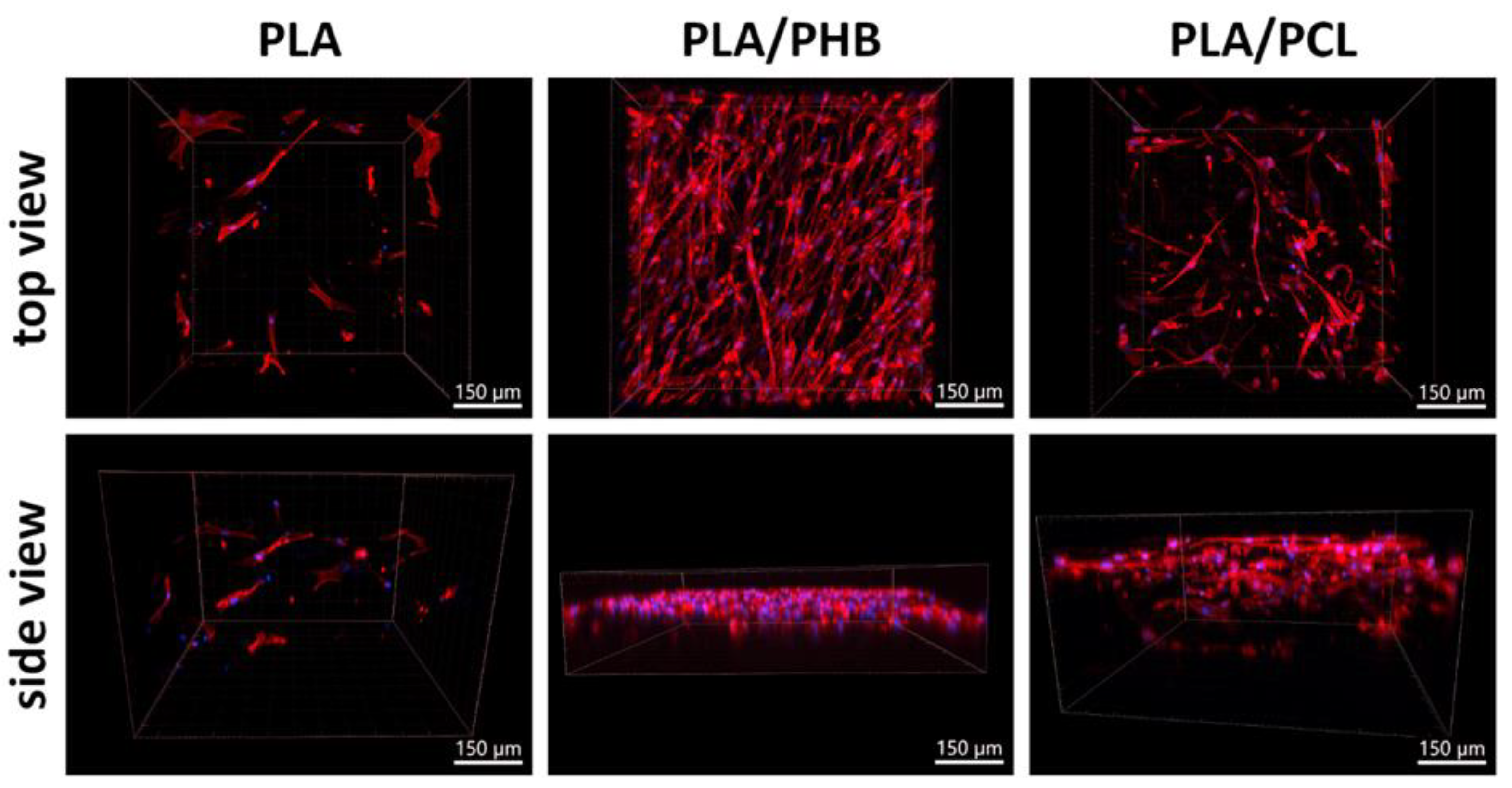
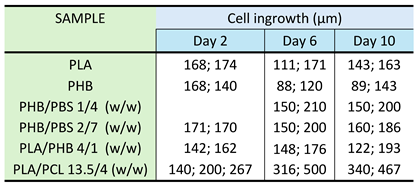
3.4. Biocompatibility and Cell Colonization of the Prepared Scaffolds
3.4.1. Comparison of Cell Colonization of Selected Scaffolds
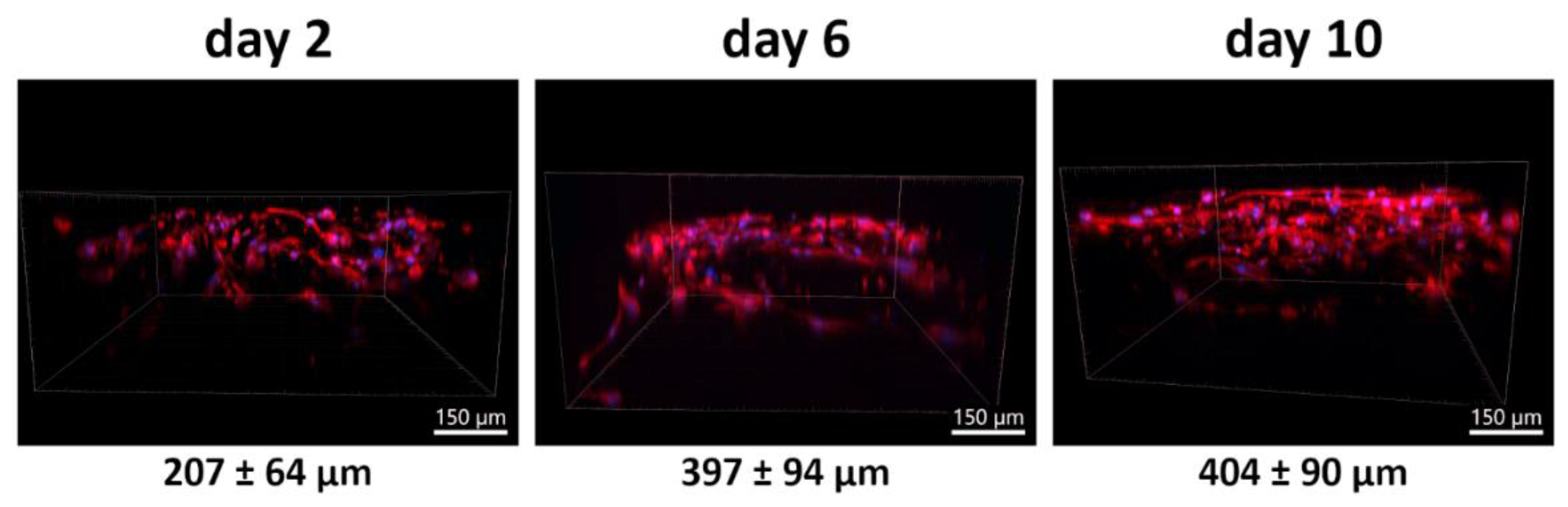
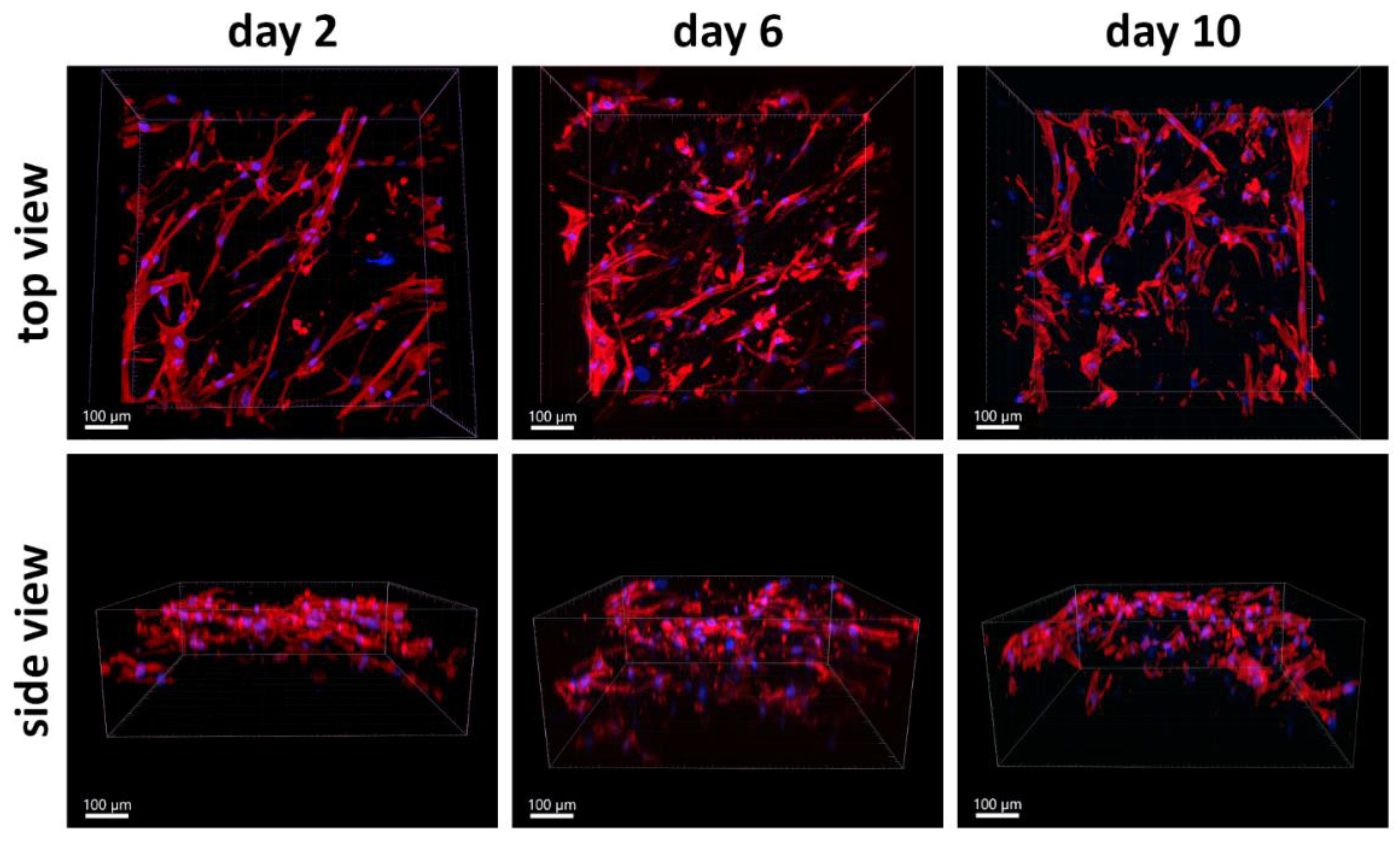
3.4.2. Time Course of PLA/PCL Scaffold Colonization
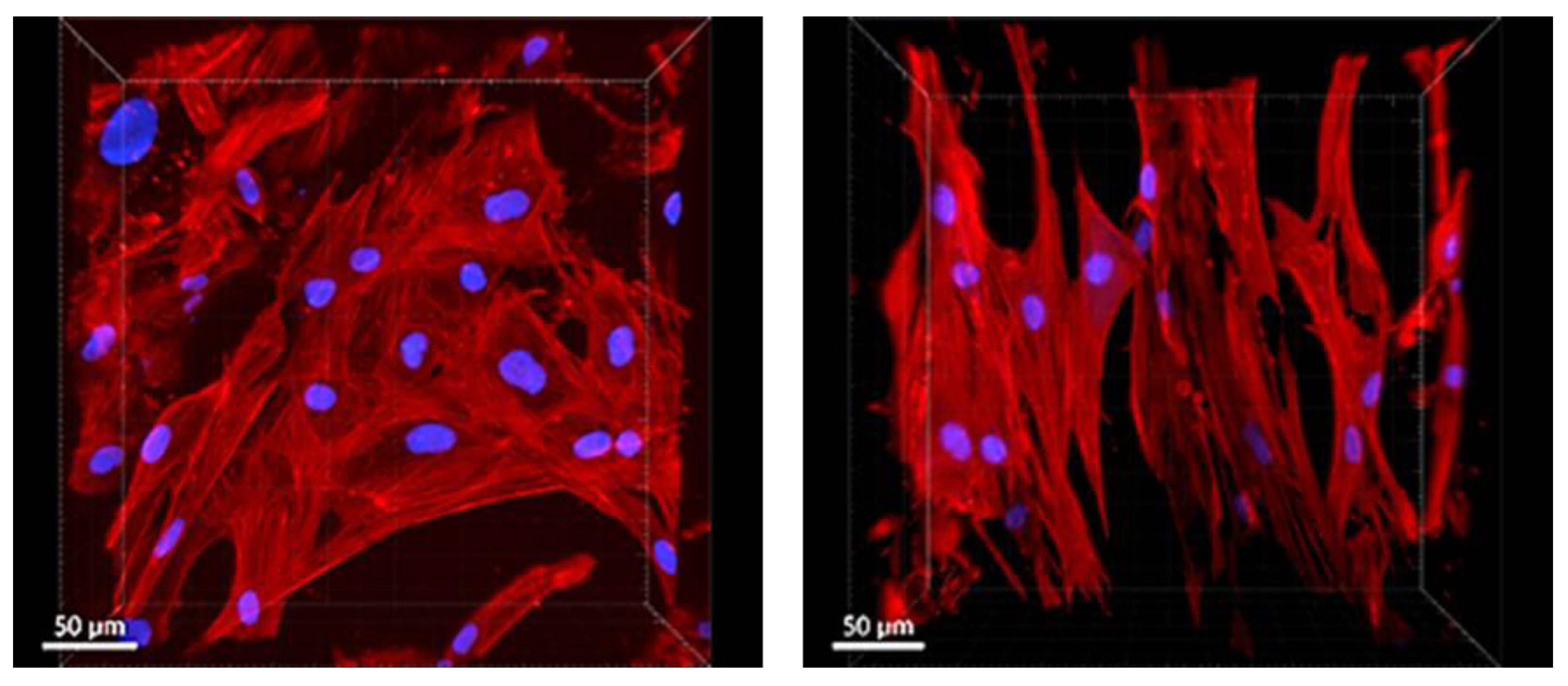
3.4.3. Cell Morphology on PLA/PCL Scaffolds
3.4.4. Suitability of the Scaffolds from a Biological Point of View
4. Conclusions and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacakova, L.; Bacakova, M.; Pajorova, J.; Kudlackova, R.; Stankova, L.; Filova E.; Musilkova, J.; Potocky, S.; Kromka, A. Nanofibrous scaffolds as promising cell carriers for tissue engineering. In Nanofiber research- reaching new heights; Rahman, M.M., Asiri, A.M., Eds.; Intechopen Limited: United Kingdom; 2016, pp. 29-54.
- Malik, S.; Sundarrajan, S.; Hussain, T.; Nazir, A.; Ayyoob, M.; Berto, F.; Ramakrishna, S. Sustainable nanofibers in tissue engineering and biomedical applications. Mat Design Process Comm. 2021, 3(6), e202. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int J Nanomedicine 2006, 1(1), 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Naqvi, S.; Gopinath, P. Chapter 7 - Applications of Nanofibers in Tissue Engineering. In Micro and Nano Technologies, Applications of Nanomaterials, Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S. Eds.; Woodhead Publishing: United Kingdom; 2018, pp. 179-203.
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng B Rev. 2011, 17(5), 349–64. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Electrospun Nanofibers for Wound Dressing and Tissue Engineering Applications. HJBC 2020, 48, 459–481. [Google Scholar] [CrossRef]
- Kharaghani, D.; Kaffashsaei, E.; Md, K.H.; Kim, I.S. The effect of polymeric nanofibers used for 3D-printed scaffolds on cellular activity in tissue engineering: A review. Int. J. Mol. Sci. 2023, 24(11), 9464. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Laurencin, C.T. Nanofiber Technology for Regenerative Engineering. ACS Nano 2020, 14(8), 9347–9363. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D. K.; Alam, M.; Rajinikanth, P. S.; Ao, Q. Electrospun biomimetic nanofibrous scaffolds: A promising prospect for bone tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2022, 23(16), 9206. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.; Shin, Y.M. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Udomluck, N.; Koh, W.G.; Lim, D.J.; Park, H. Recent developments in nanofiber fabrication and modification for bone tissue engineering. Int. J. Mol. Sci. 2020, 21(1), 99. [Google Scholar] [CrossRef]
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, J.; Pang, L.; Chen, J.; Qi, M.; You, S.; Ren, N. An anisotropic three-dimensional electrospun micro/nanofibrous hybrid PLA/PCL scaffold. RSC Adv. 2019, 9(17), 9838–9844. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.E.; Edmondson, D.; Chang, F.C.; Wood, D.; Gong, A.; Levengood, S.L.; Zhang, M. High-throughput and high-yield fabrication of uniaxially-aligned chitosan-based nanofibers by centrifugal electrospinning. Carbohydr Polym. 2015, 134, 467–474. [Google Scholar] [CrossRef]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibers via Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8(4), 1187–1191. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54(4), 677–701. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Duan, Y.-S.; Xu, Q.; Zhang, B. A review on nanofiber fabrication with the effect of high-speed centrifugal force field. J. Eng. Fiber Fabr. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Zannini Luz, H.; dos Santos, L.A.L. Centrifugal spinning for biomedical use: a review. Rev. Solid State Mater. Sci. 2023, 48(4), 519–534. [Google Scholar] [CrossRef]
- Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers 2023, 15, 1253. [Google Scholar] [CrossRef]
- Gholipour-Kanani, A.; Daneshi, P. A Review on Centrifugal and Electro-Centrifugal Spinning as New Methods of Nanofibers Fabrication. JTP 2022, 10(1), 41–55. [Google Scholar]
- Mahalingam, S.; Edirisinghe, M. Forming of Polymer Nanofibers by a Pressurised Gyration Process. Macromol Rapid Commun. 2013, 34(14), 1134–1139. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Chaudhry, H.; Livingston Arinzeh, T. Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng A 2011, A 17, 831–840. [Google Scholar] [CrossRef]
- Beran, M.; Drahorád, J.; Hušek, Z.; Toman, F. A device for the production of nanofibres or microfibres from solutions, emulsions, liquid suspensions or melts containing a spinning substance. Czech patent 30609, 2017.
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 2010, 5, 1294–1311. [Google Scholar] [CrossRef] [PubMed]
- Travnickova, M.; Kasalkova, N.S.; Sedlar, A.; Molitor, M.; Musilkova, J.; Slepicka, P.; Svorcik, V.; Bacakova, L. Differentiation of adipose tissue-derived stem cells towards vascular smooth muscle cells on modified poly(L-lactide) foils. Biomed Mater. 2021, 16(2), 025016. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; Molitor, M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018, 36(4), 1111–1126. [Google Scholar] [CrossRef]
- Ren, L.; Pandit, V.; Elkin, J.; Denman, T.; Cooper, J.A.; Kotha, S.P. Large-scale and highly efficient synthesis of micro- and nano-fibers with controlled fiber morphology by centrifugal jet spinning for tissue regeneration. Nanoscale 2013, 5(6), 2337–2345. [Google Scholar] [CrossRef]
- Loordhuswamy, A.M.; Krishnaswamy, V.R.; Korrapati, P.S.; Thinakaran, S.; Rengaswami, G.D. Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater Sci Eng C Mater Biol Appl. 2014, 42, 799–807. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Yang, B. Centrifugally spun starch-based fibers from amylopectin rich starches. Carbohydr Polym. 2016, 137, 459–465. [Google Scholar] [CrossRef]
- Ren, L.; Kotha, S.P. Centrifugal jet spinning for highly efficient and large-scale fabrication of barium titanate nanofibers. Mater Lett. 2014, 117, 153–157. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26(27), 5474–5491. [Google Scholar] [CrossRef]
- Liao, G.-Y.; Zhou, X.-P.; Chen, L.; Zeng, X.-Y.; Xie, X.-L.; Mai, Y-W. Electrospun aligned PLLA/PCL/functionalized multiwalled carbon nanotube composite fibrous membranes and their bio/mechanical properties. Compos. Sci. Technol. 2012, 72(2), 248-255.
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun polycaprolactone 3d nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv. Healthc. Mater. 2015, 4(15), 2238–2246. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.H.; Edris, H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2013, 24, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hairaldin, S.Z.; Wan Yunus, W.M.Z.; Ibrahim, N.A. Effect Addition of Octadecylamine Modified Clay (ODA-MMT) to Polylactide/Polycaprolactone (PLA/PCL) Blend. AMR 2011, 364, 317–321. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Aminatun Huriah, R.; Hikmawati, D.; Hadi, S.; Amrillah, T.; Abdullah, C.A.C. Nanofiber Scaffold Based on Polylactic Acid-Polycaprolactone for Anterior Cruciate Ligament Injury. Polymers 2022, 14(15), 2983. [Google Scholar] [CrossRef]
- Xu, T.; Yao, Q.; Miszuk, J.M.; Sanyour, H.J.; Hong, Z.; Sun, H.; Fong, H. Tailoring weight ratio of PCL/PLA in electrospun three-dimensional nanofibrous scaffolds and the effect on osteogenic differentiation of stem cells. Colloids Surf B Biointerfaces 2018, 171, 31–39. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Sherbiny, I.M.; Lotfy, A.; El-Badawy, A.; El-Badri, N. Mesenchymal stem cells growth and proliferation enhancement using PLA vs PCL based nanofibrous scaffolds. Int J Biol Macromol. A, 2016, 93, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Zhou, L.; Bi, Z.; Shi, M.; Wang, D.; Li, Q. Fabrication of a novel Three-Dimensional porous PCL/PLA tissue engineering scaffold with high connectivity for endothelial cell migration. Eur. Polym. J. 2021, 161, 110834. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Tambrchi, P.; Mahdavi, A.H.; DaliriJoupari, M.; Soltani, L. Polycaprolactone-co-polylactic acid nanofiber scaffold in combination with 5-azacytidine and transforming growth factor-β to induce cardiomyocyte differentiation of adipose-derived mesenchymal stem cells. Cell Biochem Funct. 2022, 40(7), 668–682. [Google Scholar] [CrossRef]
- Beran, M.; Drahorád, J.; Vltavský, O. Centrifugal Nozzleless Spinning - Alternative Technology to Produce Nanofiber Constructs. Advanced Materials Vid. Proc. Adv. Mater. 2021, 2, 210160. [Google Scholar]
- Guneta, V.; Loh Q.L.; Choong C. Cell-secreted extracellular matrix formation and differentiation of adipose-derived stem cells in 3D alginate scaffolds with tunable properties. J. Biomed. Mater. Res. A, 2016, 104(5), 1090-1101.
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Biopolymer based three-dimensional biomimetic micro/nanofibers scaffolds with porous structures via tailored charge repulsions for skin tissue regeneration. Polym. Adv. Technol. 2021, 32(9), 3535–3548. [Google Scholar]
- Kim, B.S.; Park, K.E.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H.; Kim, Y.J. Effect of nanofiber content on bone regeneration of silk fibroin/poly(ε-caprolactone) nano/microfibrous composite scaffolds. Int J Nanomedicine 2015, 10(1), 485–502. [Google Scholar] [PubMed]
- Hsia, H.C.; Nair, M.R.; Mintz, R.C.; Corbett, S.A. The fiber diameter of synthetic bioresorbable extracellular matrix influences human fibroblast morphology and fibronectin matrix assembly. Plast Reconstr Surg. 2011, 127(6), 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Samie, M.; Khan, A.F.; Hardy, J.G.; Yameen, M. A. Electrospun antibacterial composites for cartilage tissue engineering. Macromol. Biosci. 2022, 22(9).
- Lembach, A.; Tan, H.; Roisman, I. V.; Gambaryan-Roisman, T.; Zhang, Y.; Tropea, C.; Yarin, A. L. Drop impact, spreading, splashing, and penetration into electrospun nanofiber mats. Langmuir 2010, 26(12), 9516–9523. [Google Scholar] [CrossRef]
- Wulkersdorfer, B.; Kao, K. K.; Agopian, V. G.; Ahn, A.; Dunn, J. C.; Wu, B. M.; Stelzner, M. Bimodal porous scaffolds by sequential electrospinning of poly(glycolic acid) with sucrose particles. Int. J. Polym. Sci. 2010, 1–9. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Gil, M.; Song, S.; Kim, S. W.; Lee, K. J. Preparation of poly-1-butene nanofiber mat and its application as shutdown layer of next generation lithium ion battery. Polymers 2020, 12(10), 2267. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng 2007, 13, 2249–2257. [Google Scholar] [CrossRef]
- Simonet, M.; Schneider, O.; Neuenschwander, P.; Stark, W. Ultraporous 3d polymer meshes by low-temperature electrospinning: use of ice crystals as a removable void template. Polym Eng Sci 2007, 47(12), 2020–2026. [Google Scholar] [CrossRef]
- Baker, B.; Gee, A.; Metter, R.; Nathan, A.; Marklein, R.; Burdick, J.; Mauck, R. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomat. 2008, 29(15), 2348–2358. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomat. 2011, 32(6), 1583–1590. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7(10), 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Mahjour, S.B.; Sefat, F.; Polunin, Y.; Wang, L.; Wang, H. Improved cell infiltration of electrospun nanofiber mats for layered tissue constructs. J. Biomed. Mater. Res. A 2016, 104(6), 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Jeong, S.; Bae, M.S.; Yang, D.H.; Heo, D.N.; Kim, C.H.; Alsberg, E.; Kwon, I. K. Highly porous electrospun nanofibers enhanced by ultrasonication for improved cellular infiltration. Tissue Eng. A 2011, 17(21-22), 2695-2702.
- Zhang, X.; Meng, S.; Huang, Y.; Xu, M.; He, Y.; Lin, H.; Han, J.; Chai, Y.; Wei, Y.; Deng, X. Electrospun gelatin/β-tcp composite nanofibers enhance osteogenic differentiation of bmscs andin vivobone formation by activating Ca2+-sensing receptor signaling. Stem Cells Int. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, S.J.; Soscia, D.A.; Oztan, B.; Mosier, A.P.; Jean-Gilles, R.; Gadre, A.; Cady, N.C.; Yener, B.; Castracane, J.; Larsen, M. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous plga scaffolds. Biomaterials 2012, 33(11), 3175–3186. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Zhao, C.; Yang, D.; Cui, T.; Liu, Y.; Min, Y. Regulated Surface Morphology of Polyaniline/Polylactic Acid Composite Nanofibers via Various Inorganic Acids Doping for Enhancing Biocompatibility in Tissue Engineering. Nanoscale Res Lett. 2021, 16(1).
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B. D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32(13), 3395–3403. [Google Scholar] [CrossRef]
- Díaz-Gómez, L.; Ballarin, F.M.; Abraham, G.A.; Concheiro, A. Random and aligned PLLA : PRGF electrospun scaffolds for regenerative medicine. J. Appl. Polym. Sci. 2014, 132(5), 41372. [Google Scholar] [CrossRef]
- Oktay, B.; Kayaman-Apohan, N.; Erdem-Kuruca, S.; Süleymanoğlu, M. Fabrication of collagen immobilized electrospun poly (vinyl alcohol) scaffolds. Polym. Adv. Technol. 2015, 26(8), 978–987. [Google Scholar] [CrossRef]
- Dubey, P.; Bhushan, B.; Sachdev, A.; Matai, I.; Kumar, S.; Gopinath, P. Silver-nanoparticle-incorporated composite nanofibers for potential wound-dressing applications. J. Appl. Polym. Sci. 2015, 132(35).
- Gugulothu, D.; Barhoum, A.; Afzal, S.M.; Venkateshwarlu, B.; Uludag, H. Structural Multifunctional Nanofibers and Their Emerging Applications. In Handbook of Nanofibers. Barhoum, A., Bechelany, M., Makhlouf, A. Eds.; Springer; 2019, pp. 693–732.
- Salalha, W.; Dror, Y.; Khalfin, R.; Cohen, Y.; Yarin, A. L.; Zussman, E. Single-walled carbon nanotubes embedded in oriented polymeric nanofibers by electrospinning. Langmuir 2004, 20(22), 9852–9855. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Lu, W.; Guo, Y. Antibacterial porous coaxial drug-carrying nanofibers for sustained drug-releasing applications. Nanomater. 2021, 11(5), 1316. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Naebe, M.; Amiri, M.; Shirvanimoghaddam, K.; Anwar, S.; Michels, J.J.; Asadi, K. Hierarchically structured porous piezoelectric polymer nanofibers for energy harvesting. Adv. Sci 2020, 7(13), 2000517. [Google Scholar] [CrossRef]
- Gulfam, M.; Lee, J.M.; Kim, J.; Lim, D.W.; Lee, E.K.; Chung, B.G. Highly porous core-shell polymeric fiber network. Langmuir 2011, 27(17), 10993–10999. [Google Scholar] [CrossRef] [PubMed]
- Serag, E.; El-Aziz, A.M.A.; El-Maghraby, A.; Nahla, T. Electrospun non-wovens potential wound dressing material based on polyacrylonitrile/chicken feathers keratin nanofiber. Sci Rep 2022, 12, 15460. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Sheng, W.; Huang. K.; Hou, C.; Yue, H.; Hu, B.; Wang, M.; Wei, D.; Li Q.; Zhao, L.; Dong, W.; Zhao, Z.; Li, Y. Novel cigarlike TiO2 nanofibers: fabrication, improved mechanical, and electrochemical performances. ACS Appl Mater Interfaces 2013, 5(6), 2278-2282.
- Nathani, A.; Sharma, C.S. Electrospun mesoporous poly(styrene-block-methyl- methacrylate) nanofibers as biosensing platform: effect of fibers porosity on sensitivity. Electroanalysis 2019, 31(11), 2138–2144. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Kirac-Aydin, A.; Sumer Bolu, B; Sanyal, R.; Sanyal, A. Diels-Alder "Clickable" Biodegradable Nanofibers: Benign Tailoring of Scaffolds for Biomolecular Immobilization and Cell Growth. Bioconjug Chem. 2017, 28(9), 2420-2428.
- Linh, N.T.B.; Min, Y.K.; Song, H.; Lee, B. Fabrication of polyvinyl alcohol/gelatin nanofiber composites and evaluation of their material properties. J Biomed Mater Res B Appl Biomater 2010, 95B(1), 184–191. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Materials Today 2013, 16(6), 229–241. [Google Scholar] [CrossRef]
- Oliveira, C.; Costa-Pinto, A.R.; Reis, R.L.; Martins, A.; Neves, N.M. Biofunctional nanofibrous substrate comprising immobilized antibodies and selective binding of autologous growth factors. Biomacromolecules 2014, 15(6), 2196–2205. [Google Scholar] [CrossRef]
- Priyanto, A.; Hapidin, D.A.; Suciati, T.; Khairurrijal, K. Current Developments on Rotary Forcespun Nanofibers and Prospects for Edible Applications. Food Eng Rev 2022, 14, 435–461. [Google Scholar] [CrossRef]
- Depan, D.; Misra, R. Processing–structure–functional property relationship in organic–inorganic nanostructured scaffolds for bone-tissue engineering: the response of preosteoblasts. J. Biomed. Mater. Res. A 2012, 100A(11), 3080–3091. [Google Scholar] [CrossRef]
- Ghalei, S.; Li, J.; Douglass, M.; Garren, M.; Handa, H. Synergistic approach to develop antibacterial electrospun scaffolds using honey and s-nitroso-n-acetyl penicillamine. ACS Biomater Sci Eng. 2021, 7(2), 517–526. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Li, J.; Zhang, N.; Xu, B.; Li, Y.; Ding, N.; Ge, B. Self-assembly and cross-linking preparation of tilapia-skin-derived collagen/alginate hydrogels for efficient wound repairing. Polym. Eng. Sci. 2024, 64(5), 2146–2156. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.; Zheng, R.; Zhang, Y.; Cui,W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomaterialia 2017, 49, 66–77. [CrossRef]
- Bacakova, L.; Novotna, K.; Hadraba, D.; Musilkova, J.; Slepicka, P.; Beran, M. Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers 2022, 14, 602. [Google Scholar] [CrossRef]

 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





