Submitted:
20 December 2024
Posted:
23 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plasmid Construction
2.2. Protein Expression and Purification
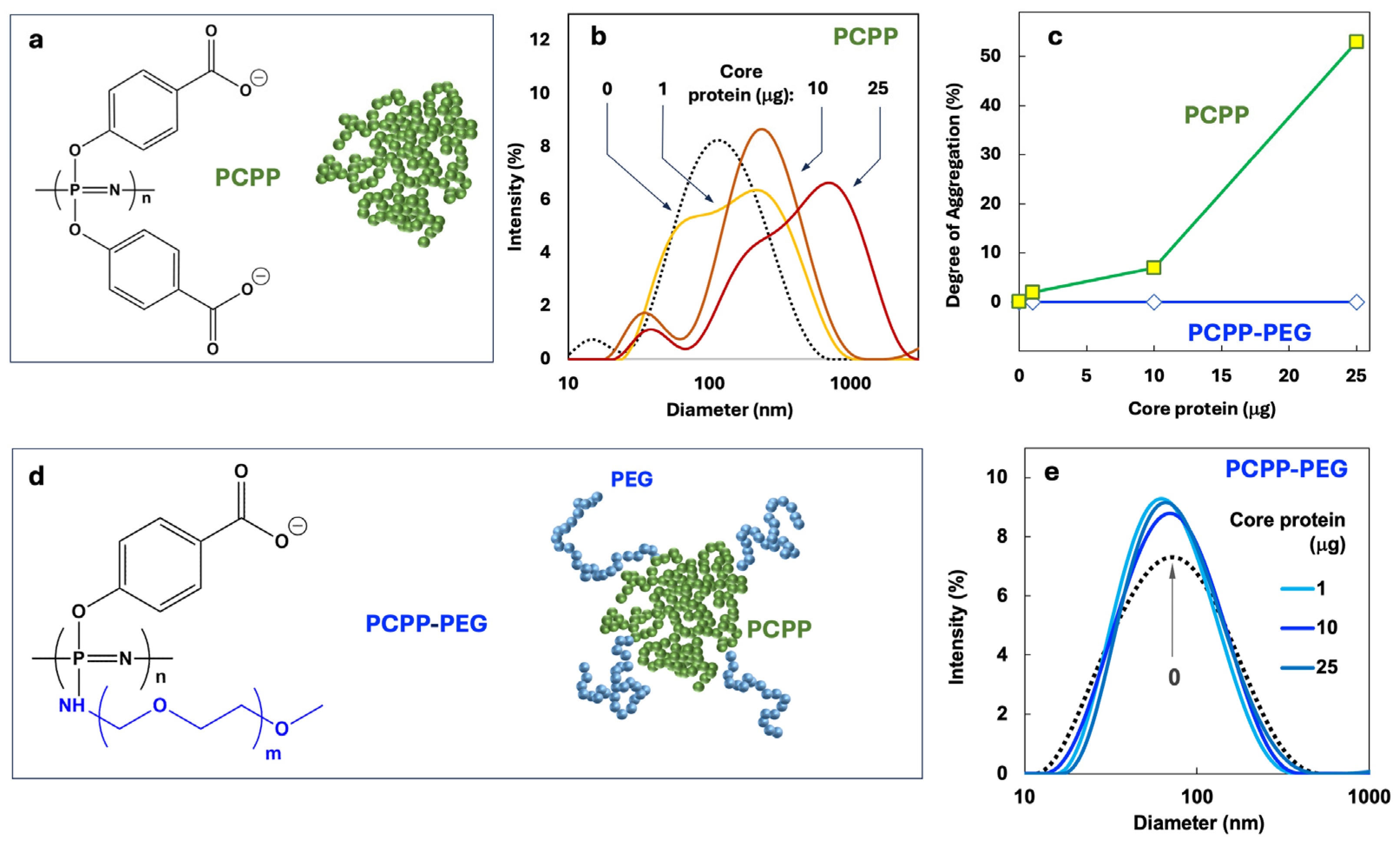
2.3. Synthesis of PCPP-PEG
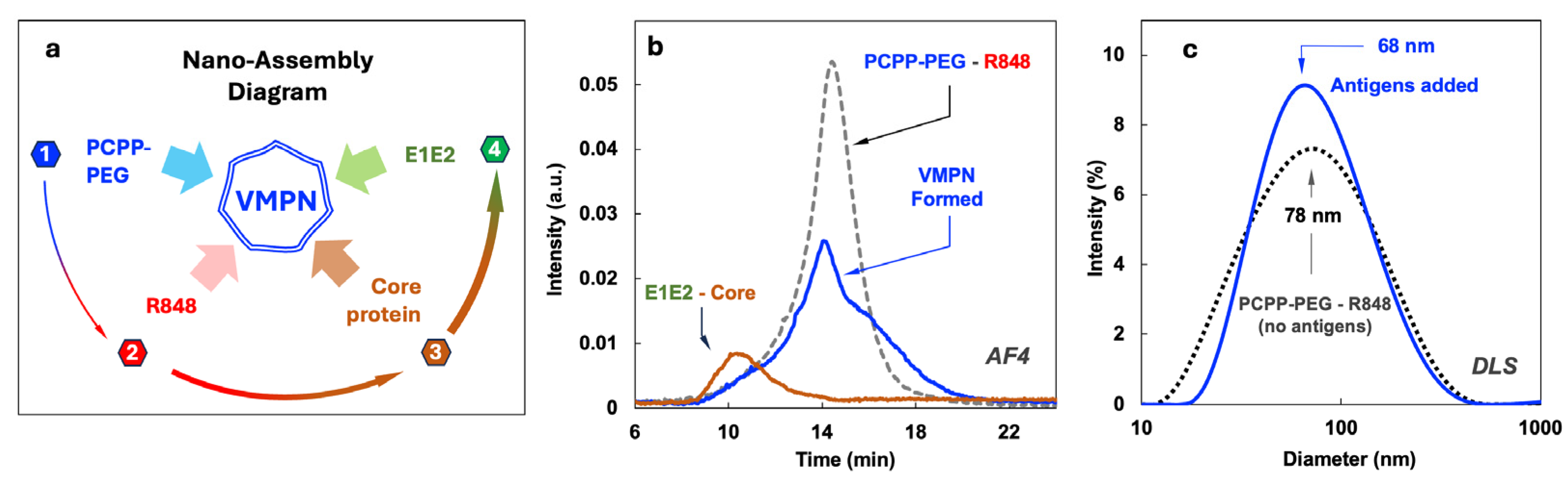
2.4. Preparation of Virus-Mimicking Polymer Nanocomplexes (VMPNs)
2.5. Physico-Chemical Characterization
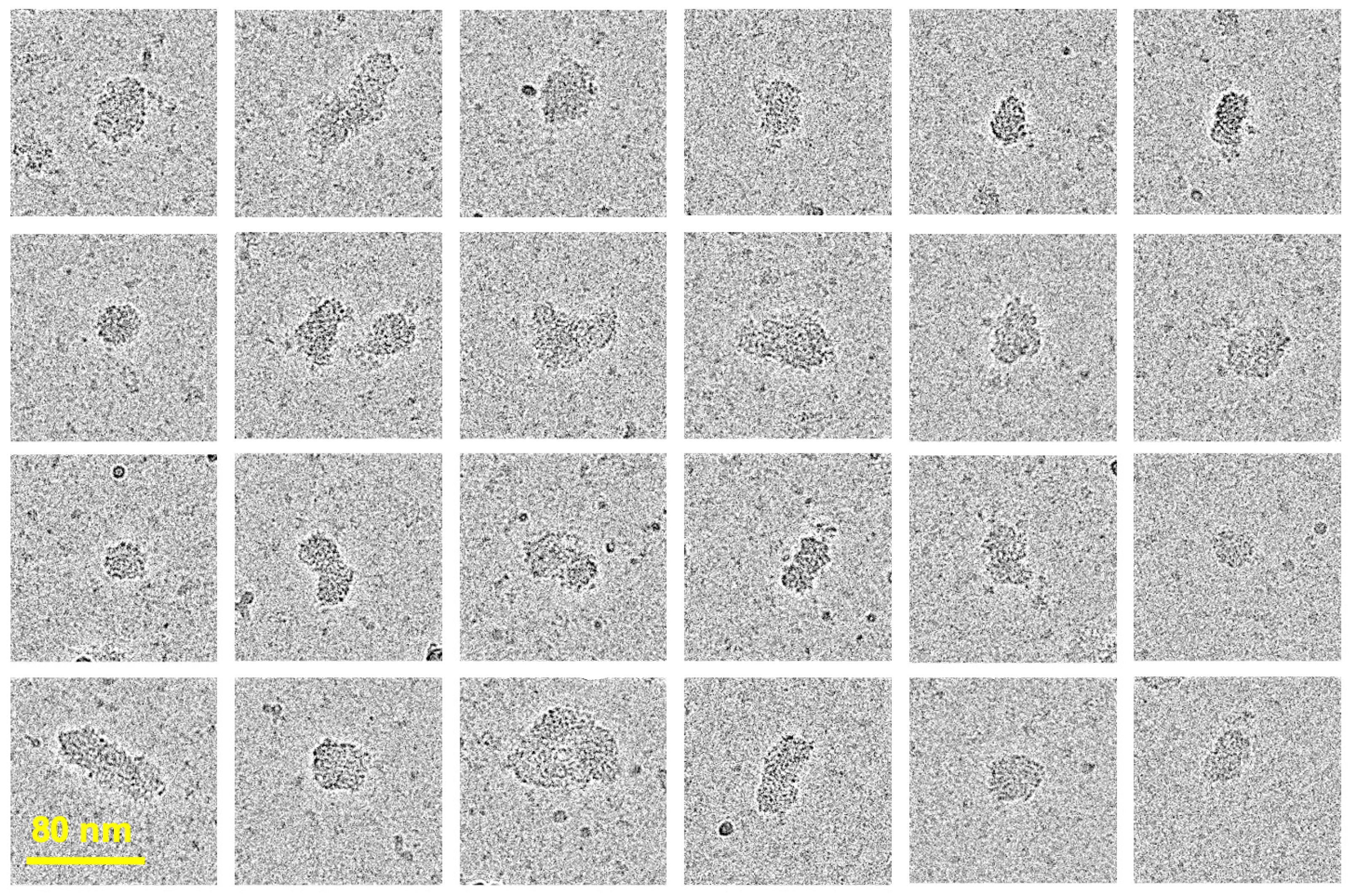
2.6. CryoEM Visualization of Complexes
2.7. Immunization
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. HCV Pseudoparticles (HCVpp) Neutralization Assay
2.10. Intracellular Staining for Cytokines and Low Cytometry Analysis
2.11. Statistical Analysis
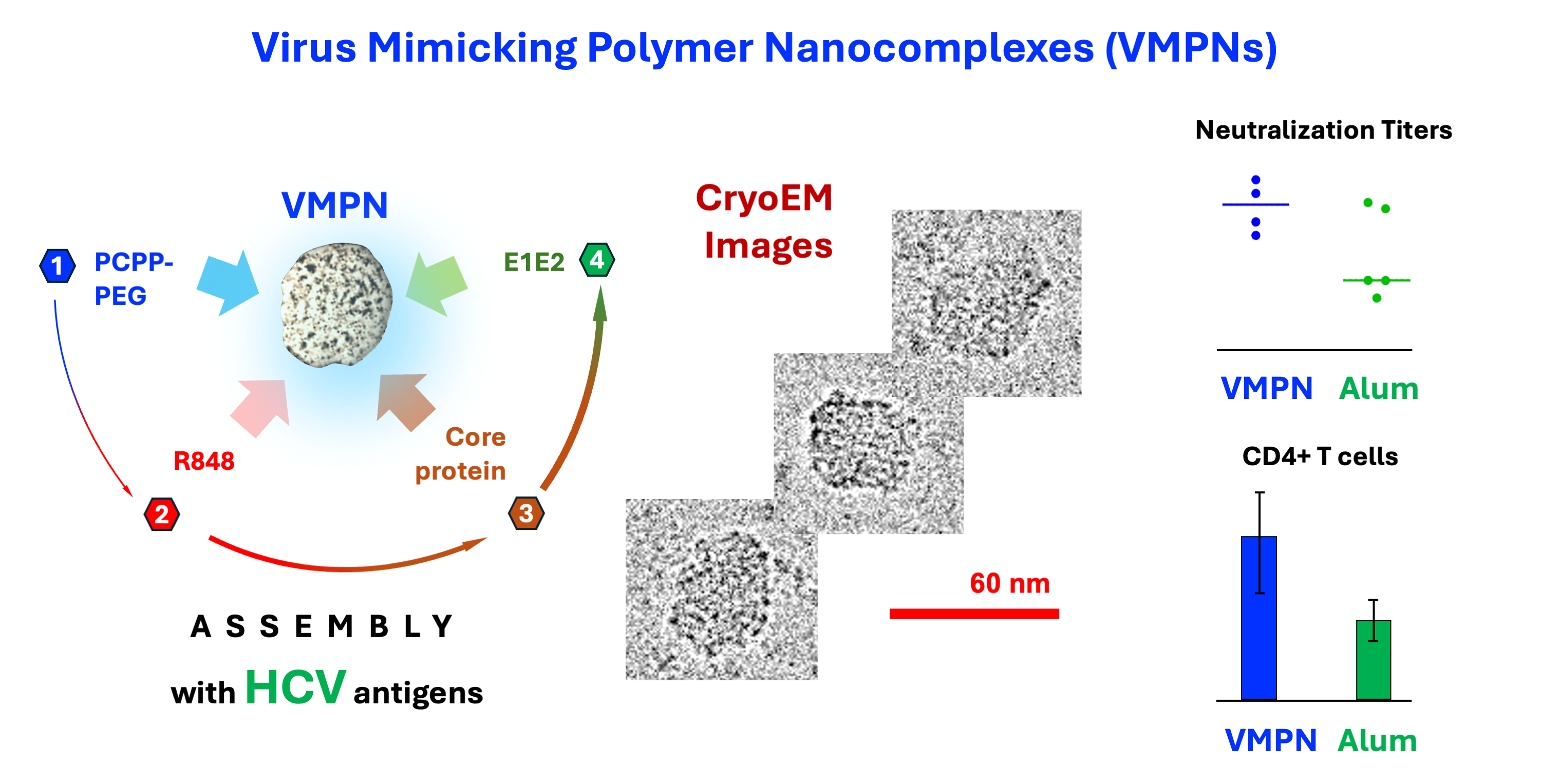
3. Results
3.1. Macromolecular Design of a Polyphosphazene Delivery Vehicle and its Optimization
3.2. Assembly of VMPNs Containing Ternary E1E2 – core protein – TLR7/8 Agonist
3.3. Direct Visualization of VMPNs by cryoEM
3.4. Immunization Studies
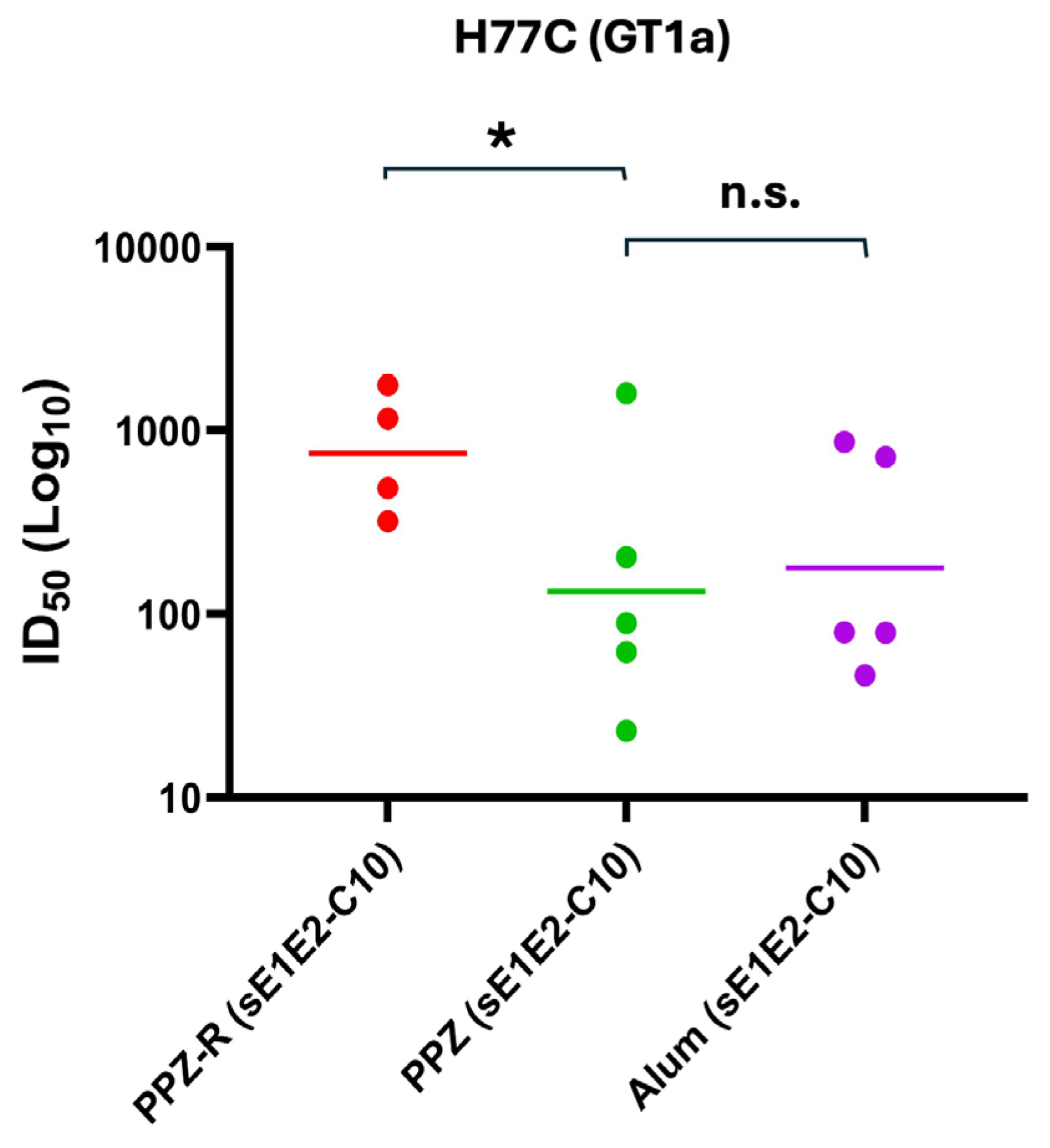
3.5. Evaluation of Serological Responses and Homologous and Heterologous Neutralization
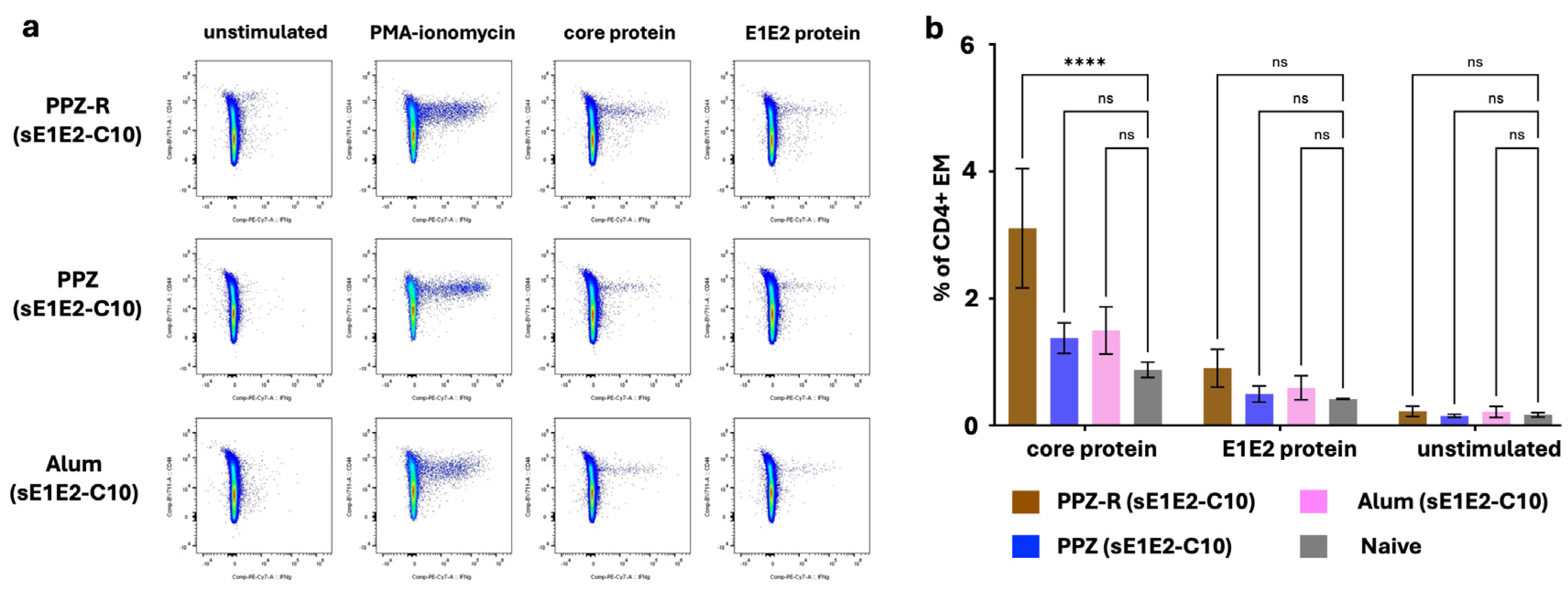
3.6. Evaluation of Memory VMPN T-Cell Responses to sE1E2 and Core Antigens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Hepatitis C Key Facts. https://www/who.int/en/news-room/fact-sheets/detail/hepatitis-c (April 9),.
- Fiehn, F.; Beisel, C.; Binder, M. Hepatitis C virus and hepatocellular carcinoma: carcinogenesis in the era of direct-acting antivirals. Curr Opin Virol 2024, 67, 101423. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.L.; Sautto, G.A. Exploring T-Cell Immunity to Hepatitis C Virus: Insights from Different Vaccine and Antigen Presentation Strategies. Vaccines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Tamori, A.; Iio, E.; Ogawa, S.; Yoshida, K.; Uchida-Kobayashi, S.; Enomoto, M.; Tanaka, Y.; Kawada, N. Hepatitis C virus recurrence in two patients who achieved sustained viral response with interferon-free direct-acting antiviral therapy: reinfection or relapse? Clinical journal of gastroenterology 2019, 12, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Gil-Martin, A.; Jarrin, I.; Montes, M.L.; Dominguez, L.; Aldamiz-Echevarria, T.; Tellez, M.J.; Santos, I.; Troya, J.; Losa, J.E.; et al. Reinfection by hepatitis C virus following effective all-oral direct-acting antiviral drug therapy in HIV/hepatitis C virus coinfected individuals. AIDS 2019, 33, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Butt, Z.A.; Wong, S.; Buxton, J.A.; Islam, N.; Yu, A.; Darvishian, M.; Gilbert, M.; Wong, J.; Chapinal, N.; et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. Journal of hepatology 2018, 69, 1007–1014. [Google Scholar] [CrossRef]
- Who, Global Hepatitis Report 2024. World Health Organization: 2024.
- Bartenschlager, R.; Ahlborn-Laake, L.; Yasargil, K.; Mous, J.; Jacobsen, H. Substrate determinants for cleavage in cis and in trans by the hepatitis C virus NS3 proteinase. J Virol 1995, 69, 198–205. [Google Scholar] [CrossRef]
- Grakoui, A.; Wychowski, C.; Lin, C.; Feinstone, S.M.; Rice, C.M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol 1993, 67, 1385–95. [Google Scholar] [CrossRef]
- Cox, A.L. Challenges and Promise of a Hepatitis C Virus Vaccine. Cold Spring Harb Perspect Med 2020, 10, (2).
- Guest, J.D.; Wang, R.; Elkholy, K.H.; Chagas, A.; Chao, K.L.; Cleveland, T.E. t.; Kim, Y.C.; Keck, Z.Y.; Marin, A.; Yunus, A.S.; et al. Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Wang, R.; Suzuki, S.; Guest, J.D.; Heller, B.; Almeda, M.; Andrianov, A.K.; Marin, A.; Mariuzza, R.A.; Keck, Z.Y.; Foung, S.K.H.; et al. Induction of broadly neutralizing antibodies using a secreted form of the hepatitis C virus E1E2 heterodimer as a vaccine candidate. Proc Natl Acad Sci U S A 2022, 119, e2112008119. [Google Scholar] [CrossRef]
- Toth, E.A.; Chagas, A.; Pierce, B.G.; Fuerst, T.R. Structural and Biophysical Characterization of the HCV E1E2 Heterodimer for Vaccine Development. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a novel hepatitis C virus genotype from Punjab, India: Expanding classification of hepatitis C virus into 8 genotypes. Journal of Infectious Diseases 2018, 218, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Thio, C.L.; Wojcik, G.L.; Goedert, J.J.; Mangia, A.; Latanich, R.; Kim, A.Y.; Lauer, G.M.; Chung, R.T.; Peters, M.G.; et al. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Annals of internal medicine 2013, 158, 235–45. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.M.; Zeisel, M.B.; Blaser, E.; Schurmann, P.; Bartosch, B.; Cosset, F.L.; Patel, A.H.; Meisel, H.; Baumert, J.; Viazov, S.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 2007, 104, 6025–30. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–51. [Google Scholar] [CrossRef] [PubMed]
- Logvinoff, C.; Major, M.E.; Oldach, D.; Heyward, S.; Talal, A.; Balfe, P.; Feinstone, S.M.; Alter, H.; Rice, C.M.; McKeating, J.A. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 2004, 101, 10149–54. [Google Scholar] [CrossRef]
- Huang, X.-j.; Lü, X.; Lei, Y.-f.; Yang, J.; Yao, M.; Lan, H.-y.; Zhang, J.-m.; Jia, Z.-s.; Yin, W.; Xu, Z.-k. Cellular immunogenicity of a multi-epitope peptide vaccine candidate based on hepatitis C virus NS5A, NS4B and core proteins in HHD-2 mice. J. Virol. Methods 2013, 189, 47–52. [Google Scholar] [CrossRef]
- Martínez-Donato, G.; Piniella, B.; Aguilar, D.; Olivera, S.; Pérez, A.; Castañedo, Y.; Alvarez-Lajonchere, L.; Dueñas-Carrera, S.; Lee, J.W.; Burr, N.; et al. Protective T Cell and Antibody Immune Responses against Hepatitis C Virus Achieved Using a Biopolyester-Bead-Based Vaccine Delivery System. Clinical and Vaccine Immunology 2016, 23, 370–378. [Google Scholar] [CrossRef]
- Roohvand, F.; Aghasadeghi, M.-R.; Sadat, S.M.; Budkowska, A.; Khabiri, A.-R. HCV core protein immunization with Montanide/CpG elicits strong Th1/Th2 and long-lived CTL responses. Biochem. Biophys. Res. Commun. 2007, 354, 641–649. [Google Scholar] [CrossRef]
- Guest, J.D.; Pierce, B.G. Structure-Based and Rational Design of a Hepatitis C Virus Vaccine. Viruses 2021, 13, 837. [Google Scholar] [CrossRef]
- Fuenmayor, J.; Godia, F.; Cervera, L. Production of virus-like particles for vaccines. N Biotechnol 2017, (Pt B), 174–180. [Google Scholar] [CrossRef]
- Baumert, T.F.; Ito, S.; Wong, D.T.; Liang, T.J. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol 1998, 72, 3827–36. [Google Scholar] [CrossRef] [PubMed]
- Masavuli, M.G.; Wijesundara, D.K.; Torresi, J.; Gowans, E.J.; Grubor-Bauk, B. Preclinical Development and Production of Virus-Like Particles As Vaccine Candidates for Hepatitis C. Frontiers in microbiology 2017, 8, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Rostami, S.; Meshkat, Z. Progress in the development of vaccines for hepatitis C virus infection. World journal of gastroenterology 2015, 21, 11984–2002. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, E.; Roingeard, P.; Beaumont, E. Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies. Viruses, 2023; 15. [Google Scholar]
- Christiansen, D.; Earnest-Silveira, L.; Grubor-Bauk, B.; Wijesundara, D.K.; Boo, I.; Ramsland, P.A.; Vincan, E.; Drummer, H.E.; Gowans, E.J.; Torresi, J. Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery. Sci Rep 2019, 9, 9251. [Google Scholar] [CrossRef]
- Lechmann, M.; Murata, K.; Satoi, J.; Vergalla, J.; Baumert, T.F.; Liang, T.J. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology 2001, 34, 417–23. [Google Scholar] [CrossRef]
- Jeong, S.H.; Qiao, M.; Nascimbeni, M.; Hu, Z.; Rehermann, B.; Murthy, K.; Liang, T.J. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol 2004, 78, 6995–7003. [Google Scholar] [CrossRef]
- Earnest-Silveira, L.; Christiansen, D.; Herrmann, S.; Ralph, S.A.; Das, S.; Gowans, E.J.; Torresi, J. Large scale production of a mammalian cell derived quadrivalent hepatitis C virus like particle vaccine. J Virol Methods 2016, 236, 87–92. [Google Scholar] [CrossRef]
- Earnest-Silveira, L.; Chua, B.; Chin, R.; Christiansen, D.; Johnson, D.; Herrmann, S.; Ralph, S.A.; Vercauteren, K.; Mesalam, A.; Meuleman, P.; et al. Characterization of a hepatitis C virus-like particle vaccine produced in a human hepatocyte-derived cell line. J Gen Virol 2016, 97, 1865–76. [Google Scholar] [CrossRef]
- Polakos, N.K.; Drane, D.; Cox, J.; Ng, P.; Selby, M.J.; Chien, D.; O'Hagan, D.T.; Houghton, M.; Paliard, X. Characterization of hepatitis C virus core-specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J. Immunol. 2001, 166, 3589–98. [Google Scholar] [CrossRef]
- Drane, D.; Maraskovsky, E.; Gibson, R.; Mitchell, S.; Barnden, M.; Moskwa, A.; Shaw, D.; Gervase, B.; Coates, S.; Houghton, M.; et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX™ vaccine: A phase I study in healthy volunteers. Hum. Vaccines 2009, 5, 151–157. [Google Scholar] [CrossRef]
- Eshaghi, B.; Fofana, J.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Virus-Mimicking Polymer Nanoparticles Targeting CD169+ Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals. ACS Appl. Mater. Interfaces 2022, 14, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Li, X.; Wang, Z.-G.; Liu, S.-L. Virus-mimicking nanosystems: from design to biomedical applications. Chem. Soc. Rev. 2023, 52, 8481–8499. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Kuroda, S. i. Development of a virus-mimicking nanocarrier for drug delivery systems: The bio-nanocapsule. Adv. Drug Delivery Rev. 2015, 95, 77–89. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Yin, P.; Chen, K. Enhanced immune responses by virus-mimetic polymeric nanostructures against infectious diseases. Front. Immunol. 2022, 12, 804416. [Google Scholar] [CrossRef]
- Lou, B.; De Beuckelaer, A.; Boonstra, E.; Li, D.; De Geest, B.G.; De Koker, S.; Mastrobattista, E.; Hennink, W.E. Modular core-shell polymeric nanoparticles mimicking viral structures for vaccination. J. Controlled Release 2019, 293, 48–62. [Google Scholar] [CrossRef]
- Somiya, M.; Liu, Q.; Kuroda, S. i. Current progress of virus-mimicking nanocarriers for drug delivery. Nanotheranostics 2017, 1, 415. [Google Scholar] [CrossRef]
- van Rijn, P.; Schirhagl, R. Viruses, Artificial Viruses and Virus-Based Structures for Biomedical Applications. Adv. Healthcare Mater. 2016, 5, 1386–1400. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Sun, Y.; Zhang, X.; Cheng, H.; Mao, S. Design of Virus-Mimicking Polyelectrolyte Complexes for Enhanced Oral Insulin Delivery. J. Pharm. Sci. 2019, 108, 3408–3415. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, J.; Lee, T.; Zhang, W.; Xu, L.; Choi, J.E.; Park, J.H.; Song, J.K.; Jang, S.; Eom, C.-Y.; et al. Virus-mimetic polymer nanoparticles displaying hemagglutinin as an adjuvant-free influenza vaccine. Biomaterials 2018, 183, 234–242. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Langer, R. Polyphosphazene immunoadjuvants: Historical perspective and recent advances. J. Controlled Release 2021, 329, 299–315. [Google Scholar] [CrossRef]
- Chand, D.J.; Magiri, R.B.; Wilson, H.L.; Mutwiri, G.K. Polyphosphazenes as Adjuvants for Animal Vaccines and Other Medical Applications. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Hlushko, R.; Pozharski, E.; Prabhu, V.M.; Andrianov, A.K. Directly visualizing individual polyorganophosphazenes and their single-chain complexes with proteins. Commun. Mater. 2024, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Kethanapalli, S.H.; Andrianov, A.K. Immunopotentiating Polyphosphazene Delivery Systems: Supramolecular Self-Assembly and Stability in the Presence of Plasma Proteins. Mol. Pharm. 2024, 21, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Grado, V.H.; Marin, A.; Hlushko, R.; Yunus, A.S.; Promeneur, D.; Luckay, A.; Lazaro, G.G.; Hamm, S.; Dimitrov, A.S.; Broder, C.C.; et al. Nano-Assembled Polyphosphazene Delivery System Enables Effective Intranasal Immunization with Nipah Virus Subunit Vaccine. ACS Applied Bio Materials 2024, 7, 4133–4141. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Molecular-Level Interactions of Polyphosphazene Immunoadjuvants and Their Potential Role in Antigen Presentation and Cell Stimulation. Biomacromolecules 2016, 17, 3732–3742. [Google Scholar] [CrossRef]
- Vasilakos, J.P.; Tomai, M.A. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines 2013, 12, 809–819. [Google Scholar] [CrossRef]
- Tomai, M.A.; Vasilakos, J.P. TLR-7 and -8 agonists as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 405–407. [Google Scholar] [CrossRef]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Delivery Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. ImmunoHorizons 2018, 2, 185–197. [Google Scholar] [CrossRef]
- Midgard, H.; Bjøro, B.; Mæland, A.; Konopski, Z.; Kileng, H.; Damås, J.K.; Paulsen, J.; Heggelund, L.; Sandvei, P.K.; Ringstad, J.O.; et al. Hepatitis C reinfection after sustained virological response. J. Hepatol. 2016, 64, 1020–1026. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Wang, R.; Karauzum, H.; Chowdhury, A.; Agnihotri, P.; Yunus, *!!! REPLACE !!!*; Abdul, *!!! REPLACE !!!*; Mariuzza, R.A.; Fuerst, T.R. Supramolecular assembly of Toll-like receptor 7/8 agonist into multimeric water-soluble constructs enables superior immune stimulation in vitro and in vivo. ACS Appl. Bio Mater. 2020, 3, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Messaud, F.A.; Sanderson, R.D.; Runyon, J.R.; Otte, T.; Pasch, H.; Williams, S.K.R. An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog. Polym. Sci. 2009, 34, 351–368. [Google Scholar] [CrossRef]
- Pitkänen, L.; Striegel, A.M. AF4/MALS/QELS/DRI characterization of regular star polymers and their “span analogs”. Analyst 2014, 139, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Wang, R.; Chowdhury, A.; Agnihotri, P.; Yunus, A.S.; Pierce, B.G.; Mariuzza, R.A.; Fuerst, T.R. In Vivo and In Vitro Potency of Polyphosphazene Immunoadjuvants with Hepatitis C Virus Antigen and the Role of Their Supramolecular Assembly. Mol. Pharm. 2021, 18, 726–734. [Google Scholar] [CrossRef]
- Weissenberger, G.; Henderikx, R.J.M.; Peters, P.J. Understanding the invisible hands of sample preparation for cryo-EM. Nat. Methods 2021, 18, 463–471. [Google Scholar] [CrossRef]
- Yip, K.M.; Fischer, N.; Paknia, E.; Chari, A.; Stark, H. Atomic-resolution protein structure determination by cryo-EM. Nature 2020, 587, 157–161. [Google Scholar] [CrossRef]
- Cheng, Y. Single-particle cryo-EM - How did it get here and where will it go. Science 2018, 361, 876–880. [Google Scholar] [CrossRef]
- Gopal, A.; Zhou, Z.H.; Knobler, C.M.; Gelbart, W.M. Visualizing large RNA molecules in solution. RNA 2012, 18, 284–99. [Google Scholar] [CrossRef]
- Lyu, Z.; Yao, L.; Chen, W.; Kalutantirige, F.C.; Chen, Q. Electron Microscopy Studies of Soft Nanomaterials. Chem. Rev. 2023, 123, 4051–4145. [Google Scholar] [CrossRef]
- Wang, F.; Gnewou, O.; Solemanifar, A.; Conticello, V.P.; Egelman, E.H. Cryo-EM of Helical Polymers. Chem. Rev. 2022, 122, 14055–14065. [Google Scholar] [CrossRef]
- Mai, D.J.; Schroeder, C.M. 100th Anniversary of Macromolecular Science Viewpoint: Single-Molecule Studies of Synthetic Polymers. ACS Macro Lett. 2020, 9, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.L.; Bomans, P.H.H.; Holder, S.J.; Sommerdijk, N.A.J.M.; Biagini, S.C.G. Cryo Electron Tomography Reveals Confined Complex Morphologies of Tripeptide-Containing Amphiphilic Double-Comb Diblock Copolymers. Angew. Chem. Int. Ed. 2008, 47, 8859–8862. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ting, J.M.; Tirrell, M.V. Mechanism of Dissociation Kinetics in Polyelectrolyte Complex Micelles. Macromolecules 2020, 53, 102–111. [Google Scholar] [CrossRef]
- Lueckheide, M.; Vieregg, J.R.; Bologna, A.J.; Leon, L.; Tirrell, M.V. Structure–Property Relationships of Oligonucleotide Polyelectrolyte Complex Micelles. Nano Letters 2018, 18, 7111–7117. [Google Scholar] [CrossRef] [PubMed]
- Marras, A.E.; Vieregg, J.R.; Ting, J.M.; Rubien, J.D.; Tirrell, M.V. Polyelectrolyte Complexation of Oligonucleotides by Charged Hydrophobic—Neutral Hydrophilic Block Copolymers. Polymers 2019, 11, 83. [Google Scholar] [CrossRef]
- Yu, X.; Qiao, M.; Atanasov, I.; Hu, Z.; Kato, T.; Liang, T.J.; Zhou, Z.H. Cryo-electron microscopy and three-dimensional reconstructions of hepatitis C virus particles. Virology 2007, 367, 126–134. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. Immunology 2008, 8, 247–58. [Google Scholar] [CrossRef]
- Bouveret Le Cam, N.N.; Ronco, J.; Francon, A.; Blondeau, C.; Fanget, B. Adjuvants for influenza vaccine. Res. Immunol. 1998, 149, 19–23. [Google Scholar] [CrossRef]
- Ison, M.G.; Mills, J.; Openshaw, P.; Zambon, M.; Osterhaus, A.; Hayden, F. Current research on respiratory viral infections: Fourth International Symposium. Antiviral Res. 2002, 55, 227–278. [Google Scholar] [CrossRef]
- O'Connell, R.J.; Excler, J.-L.; Polonis, V.R.; Ratto-Kim, S.; Cox, J.; Jagodzinski, L.L.; Liu, M.; Wieczorek, L.; McNeil, J.G.; El-Habib, R. Safety and Immunogenicity of a randomized Phase I prime-boost trial with ALVAC-HIV (vCP205) and Oligomeric gp160 MN/LAI-2 Adjuvanted in Alum or Polyphosphazene. J. Infect. Dis. 2016, 213, 1946–1954. [Google Scholar] [CrossRef]
- O'Connell, R.; Polonis, V.; Ratto-Kim, S.; Cox, J.; Jagodzinski, L.; Malia, J.; Michael, N.; Excler, J.; Robb, M.; Kim, J. Safety and immunogenicity of a randomized phase I prime-boost trial with ALVAC-HIV (vCP205) and gp160 MN/LAI-2 adjuvanted in alum or polyphosphazene. Retrovirology 2012, 2 (Suppl 2)), O50. [Google Scholar] [CrossRef]
- Laera, D.; HogenEsch, H.; O’Hagan, D.T. Aluminum Adjuvants—‘Back to the Future’. Pharmaceutics 2023, 15, 1884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, P.; Guo, D.; Chen, K.; Hu, Z.; Zou, Y. Research Progress of Aluminum Phosphate Adjuvants and Their Action Mechanisms. Pharmaceutics 2023, 15, 1756. [Google Scholar] [CrossRef] [PubMed]
- Jully, V.; Mathot, F.; Moniotte, N.; Préat, V.; Lemoine, D. Mechanisms of Antigen Adsorption Onto an Aluminum-Hydroxide Adjuvant Evaluated by High-Throughput Screening. J. Pharm. Sci. 2016, 105, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, G. Nano alum: A new solution to the new challenge. Hum. Vaccines Immunother. 2022, 18, 2060667. [Google Scholar] [CrossRef]
- Kurzątkowski, W.; Kartoğlu, Ü.; Górska, P.; Główka, M.; Woźnica, K.; Zasada, A.A.; Szczepańska, G.; Trykowski, G.; Gniadek, M.; Donten, M. Physical and chemical changes in Alhydrogel™ damaged by freezing. Vaccine 2018, 36, 6902–6910. [Google Scholar] [CrossRef]
- Mardliyati, E.; Hawa Syaifie, P.; El Muttaqien, S.; Ria Setyawati, D. Nanoscale alum-based adjuvants: Current status and future prospects. Materials Today: Proceedings.
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat Med 2013, 19, 1597–608. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. TLR agonists as vaccine adjuvants in the prevention of viral infections: an overview. Frontiers in microbiology 2023, 14, 1249718. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Wang, R.; Karauzum, H.; Chowdhury, A.; Agnihotri, P.; Yunus, A.S.; Mariuzza, R.A.; Fuerst, T.R. Supramolecular Assembly of Toll-like Receptor 7/8 Agonist into Multimeric Water-Soluble Constructs Enables Superior Immune Stimulation In Vitro and In Vivo. ACS Applied Bio Materials 2020, 3, 3187–3195. [Google Scholar] [CrossRef]
- Kannanganat, S.; Ibegbu, C.; Chennareddi, L.; Robinson, H.L.; Amara, R.R. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007, 81, 8468–76. [Google Scholar] [CrossRef]
- Pierce, B.G.; Felbinger, N.; Metcalf, M.; Toth, E.A.; Ofek, G.; Fuerst, T.R. Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development. Viruses 2024, 16. [Google Scholar] [CrossRef]
- Vijayamahantesh, V.; Patra, T.; Meyer, K.; Alameh, M.G.; Reagan, E.K.; Weissman, D.; Ray, R. Modified E2 Glycoprotein of Hepatitis C Virus Enhances Proinflammatory Cytokines and Protective Immune Response. J Virol 2022, 96, e0052322. [Google Scholar] [CrossRef]

| Group | sE1E2-H445P (µg) |
Core (µg) |
Vehicle | Reference | ||
|---|---|---|---|---|---|---|
| PCPP-PEG (µg) |
R848 (µg) |
Alum (µg) |
||||
| 1 | 25 | - | 100 | 20 | - | PPZ-R (sE1E2) |
| 2 | 25 | 1 | 100 | 20 | - | PPZ-R (sE1E2-C1) |
| 3 | 25 | 10 | 100 | 20 | - | PPZ-R (sE1E2-C10) |
| 4 | 25 | 25 | 100 | 20 | - | PPZ-R (sE1E2-C25) |
| 5 | 25 | 10 | 100 | 20 | - | PPZ (sE1E2-C10) |
| 6 | 25 | 10 | - | - | 35 | Alum (sE1E2-C10) |
| 7 | 25 | 10 | - | - | - | (sE1E2-C10) |
| HCVpp * | PPZ-R (sE1E2-C10) | PPZ (sE1E2-C10) | Alum (sE1E2-C10) |
|---|---|---|---|
| GT1a | 587.9 | 173.3 | 184.4 |
| GT1b | 36.7 | 65.6 | 72.4 |
| GT2a | 125.9 | 113.7 | 102.0 |
| GT2b | 73.4 | 109.9 | 63.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





