Submitted:
19 December 2024
Posted:
20 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Zebrafish Strains, Housing, and Husbandry
2.2. Morpholino Injections
2.3. Fin Imaging Measurements and Statistical Analysis
2.4. Materials – Antibodies
| Primaries | Host Organism | Company | Catalog number | RRID | |||||
| Sdc4 | Rabbit | BioVision | Cat# 3644-100 | AB_2183016 | |||||
| Mouse | Santa Cruz Biotechnology | Cat# sc-12766 | AB_628314 | ||||||
| TMEM184a | Rabbit | Thermo Fisher Scientific | Cat# PA5-96834 | AB_2808636 | |||||
| Rat | GenScript | protein G-purified peptide sequence from the N-terminal region of rat TMEM184A (N - PAGPQMDHMGNSSQC) | |||||||
| VE-Cadherin | Goat | Santa Cruz Biotechnology | Cat# sc-6458 | AB_2077955 | |||||
| Rabbit | Cell Signaling Technology | Cat# 2500 | AB_10839118 | ||||||
| Rab4 | Rabbit | Abcam | Cat# ab13252 | AB_2269374 | |||||
| Rab11a | Rabbit | Cell Signaling Technology | Cat# 5589 | AB_10693925 | |||||
| β-Actin | Rabbit | Cell Signaling Technology | Cat# 4970 | AB_2223172 | |||||
| Tubulin | Mouse | Abcam | Cat# 7291 | AB_2241126 | |||||
| Secondaries | Host Species | Company | Catalog number | RRID | |||||
| Alexa 647 anti-mouse | Donkey | Jackson ImmunoResearch Labs | Cat# 715-605-151 | AB_2340863 | |||||
| Alexa 647 anti-Rabbit | Donkey | Jackson ImmunoResearch Labs | Cat# 711-605-152 | AB 2492288 | |||||
| Alexa 647 anti-rabbit FC specific | Donkey | Jackson ImmunoResearch Labs | Cat# 111-605-046 | AB_2338076 | |||||
| Alexa 488 anti-rat | Donkey | Jackson ImmunoResearch Labs | Cat# 712-545-153 | AB_2340684 | |||||
| Alexa 488 anti-rat, F(ab’)2 specific | Donkey | Jackson ImmunoResearch Labs | Cat# 112-545-072 | AB_2338359 | |||||
| Alexa 488 anti-rabbit | Donkey | Jackson ImmunoResearch Labs | Cat# 711-545-152 | AB_2313584 | |||||
| CY3 anti-mouse | Donkey | Jackson ImmunoResearch Labs | Cat# 715-165-150 | AB_2340813 | |||||
| TRITC anti-rabbit | Donkey | Jackson ImmunoResearch Labs | Cat# 711-025-152 | RRID:AB_2340588 | |||||
| TRITC anti-goat | Donkey | Jackson ImmunoResearch Labs | Cat# 705-025-147 | RRID:AB_2340389 | |||||
2.5. Culturing and Transfections
2.6. Immunofluorescent Staining
| Figure | Primary1 | Primary2 | Secondary1 | Secondary2 |
| 1A | Sdc4 rb (1:100) | TMEM NTD rat (1:50) | Alexa 647 α-rb (1:200) | Alexa 488 α -rat (1:500) |
| 1B | Sdc4 mo (1:50) | TMEM CTD rb (1:50) | Alexa 488 α-mo (1:200) | TRITC α-rb (1:200) |
| S1C | TMEM CTD rb (1:50) | TMEM NTD rat (1:50) | TRITC α-rb (1:200) | Alexa 488 α - rat (1:500) |
| S2A | Sdc4 rb (1:100) | TMEM NTD rat (1:50) | Alexa 647 α-rb (1:200) | Alexa 488 α - rat (1:500) |
| S2B | Sdc4 mo (1:50) | TMEM CTD rb (1:50) | Alexa 488 α-mo (1:200) | TRITC α-rb (1:200) |
| 3/4 | VE cad gt(1:200) | TMEM CTD (1:100) | TRITC α-gt (1:200) | Alexa 647 α-rb (1:200) |
| S3 | VE cad gt (1:200) | TMEM CTD (1:100) | Alexa 488 α-gt (1:200) | Alexa 647 α-rb (1:200) |
2.7. Co-immunoprecipitation and WESTERN BLOTTING
| Figure WB | Primary1 | Primary2 | Secondary1 | Secondary2 |
| 1C/ S2 C | Sdc4 rb (1:100) | TMEM NTD rat (1:200) | Alexa 647 α-rb FC site Specific (1:10,000) | Alexa-488 α-rat F(ab’)2 specific (1:10,000) |
| 3C/4D | VE-cad rb (1:1000) | β-actin rb (1:1000) | Alexa-488 α-rb (1:10,000) | |
| 4C | TMEM CTD rb (1:500) | Tubulin mo (1:10,000) | Alexa-488 α-rb (1:10,000) | Alexa 647 α-mo (1:10,000) |
| S1. A. | TMEM CTD rb (1:500) | Tubulin mo (1:10,000) | Alexa-488 α-rb (1:10,000) | CY3 α- mo (1:10,000) |
| S1. C. | TMEM CTD rb (1:200) | Sdc4 mo (1:200) | Alexa 647 α-rb (1:10,000) | CY3 α- mo (1:10,000) |
| S2. D. | Sdc4 rb (1:1000) | Alexa 647 α-rb FC site Specific (1:10,000) | ||
| S3. A | VE-cad goat (1:5000) | Tubulin mo (1:10,000) | Alexa-488 α-goat (1:10,000) | Alexa 647 α-mo (1:10,000) |
2.8. WCL Harvest and Cell Fractionation
2.9. RNA Purification and RT-qPCR
| Primer Name | Primer 5’-3’ | Reference |
| GAPDH Forward | ACACCCTCAAGATTGTCAGCAA | [35] |
| GAPDH Reverse | TCATAAGTCCCTCCACGATGC | [35] |
| VE-Cadherin Forward | TCTGCCGGCAAGGTGTTCCG | [36] |
| VE-Cadherin Reverse | CATGGTCTGCCACCGTGGGG | [36] |
| TMEM184a Forward | CTTCTGCAAGCAGCCCAC | |
| TMEM184a Reverse | CCTGAAGTTGCAGGCGTC |
2.10. VEGF Treatment and Vesicle Staining
2.11. Corrected Total Cell Fluorescence (CTCF) and Image J Fiji Quantification
2.12. RStudio Visualization of Immunofluorescent Cell Experiments
2.13. Scratch Wounding and Leading-Edge Analysis
3. Results
3.1. TMEM184A Interacts with Sdc4 in Vascular ECs
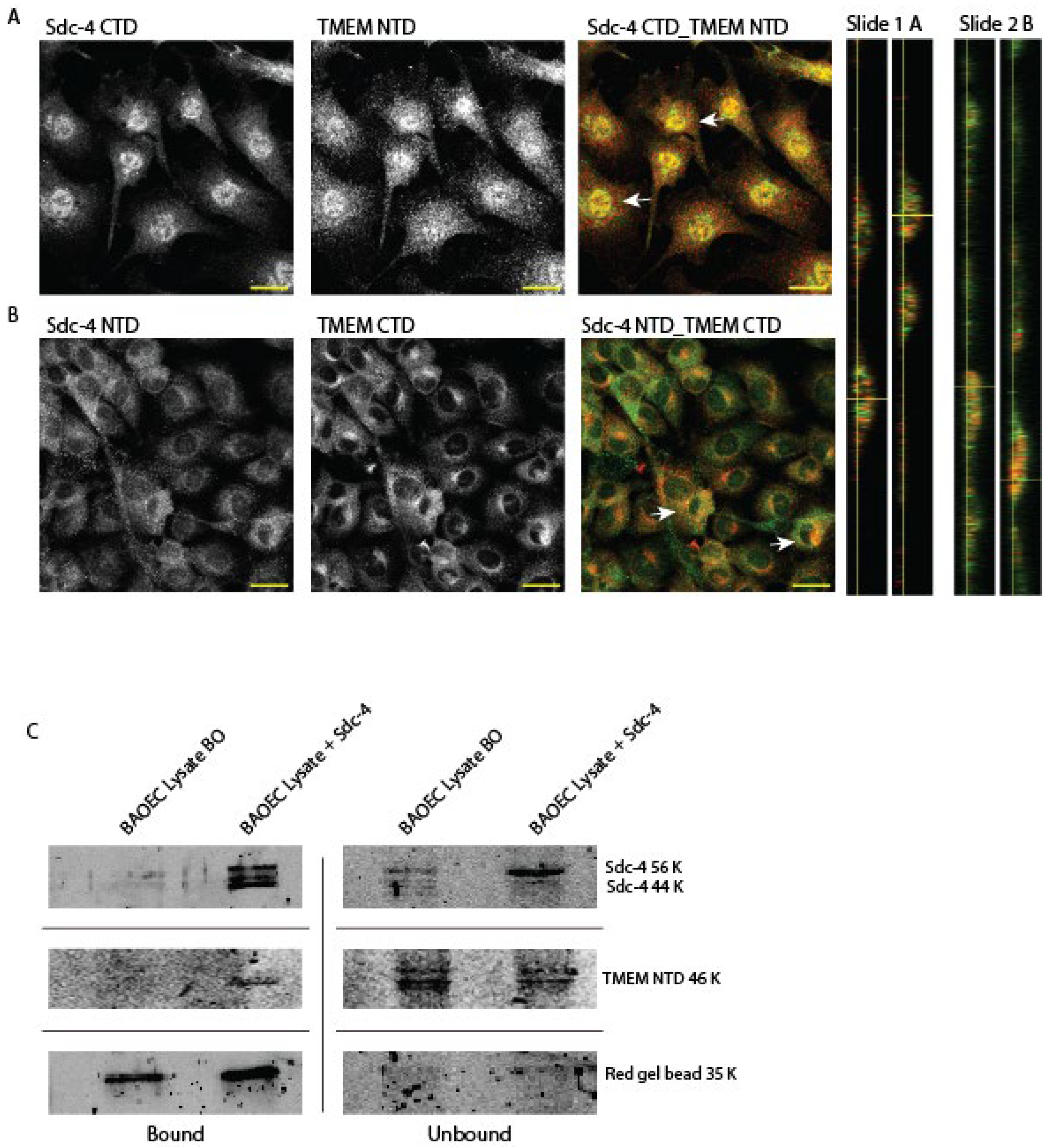
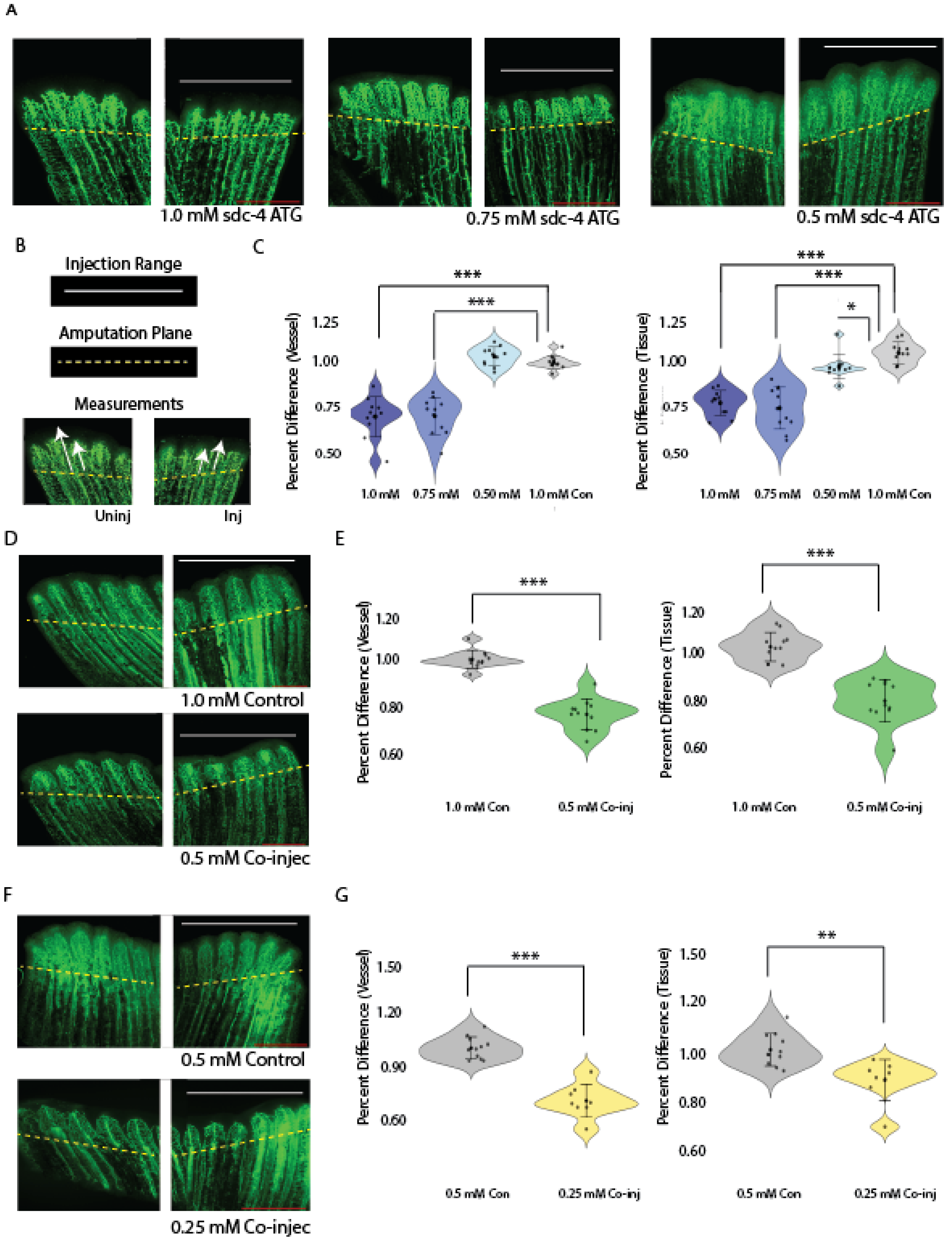
3.2. Sdc4 and Tmem184a Function Cooperatively to Promote Vessel and Tissue Outgrowth
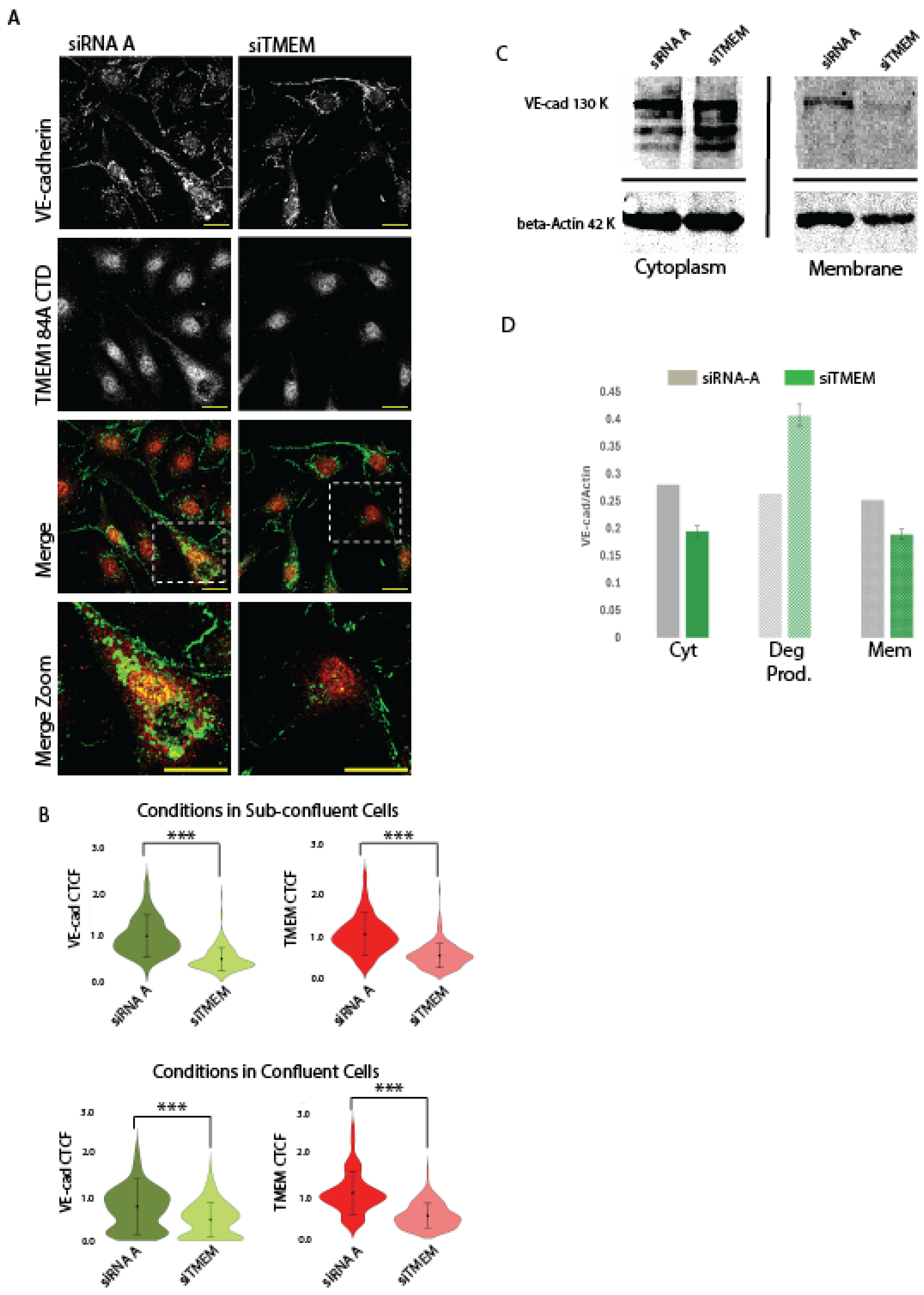
a. TMEM184A is Required to Maintain post-translational VE-cad levels in sub-confluent ECs.
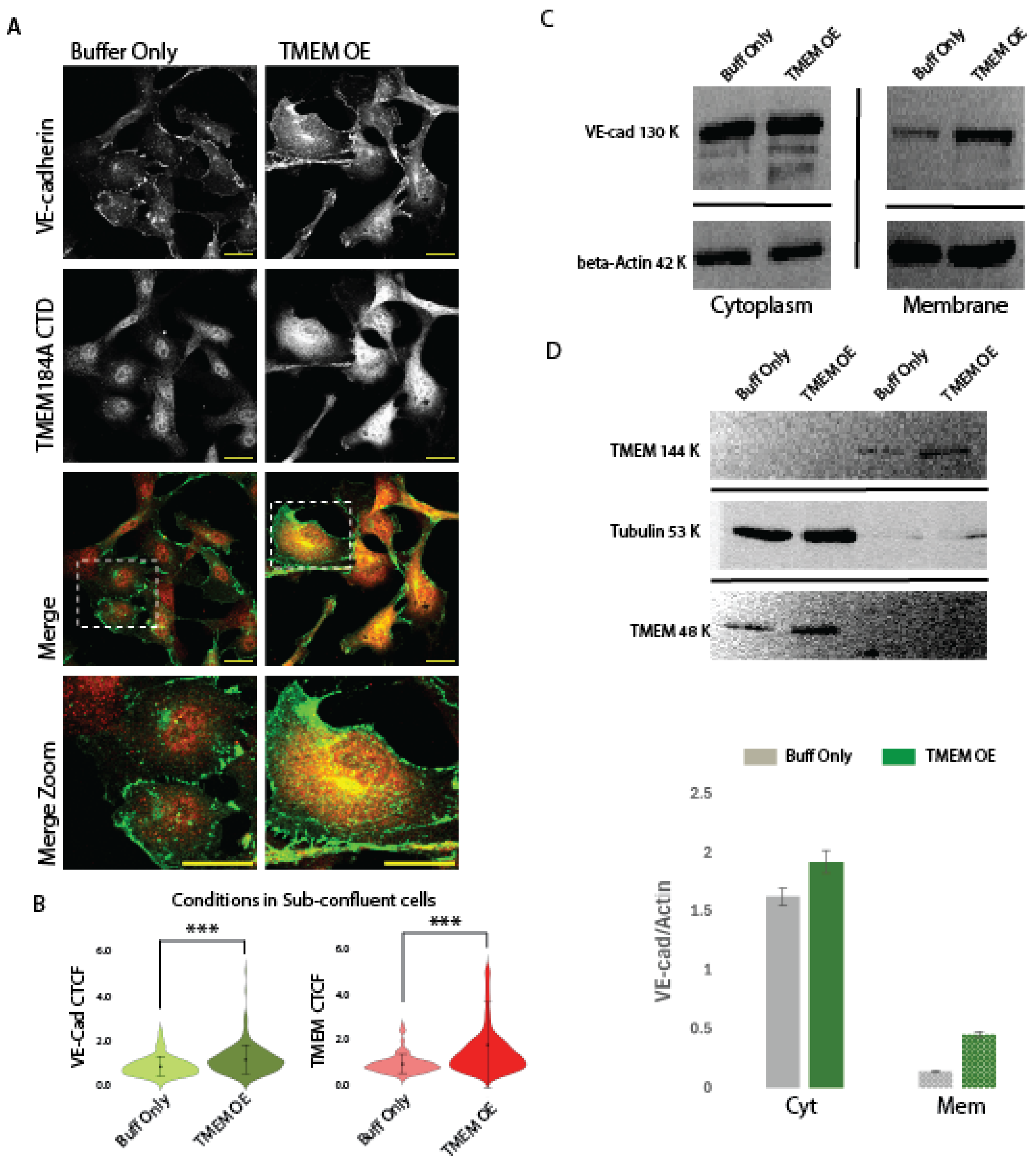
b. TMEM184A-tGFP Expression Colocalizes in VE-cad puncta and Increases total VE-Cad in Sub-Confluent ECs.
c. TMEM184A Colocalizes with Rab-GTPases in Response to VEGF
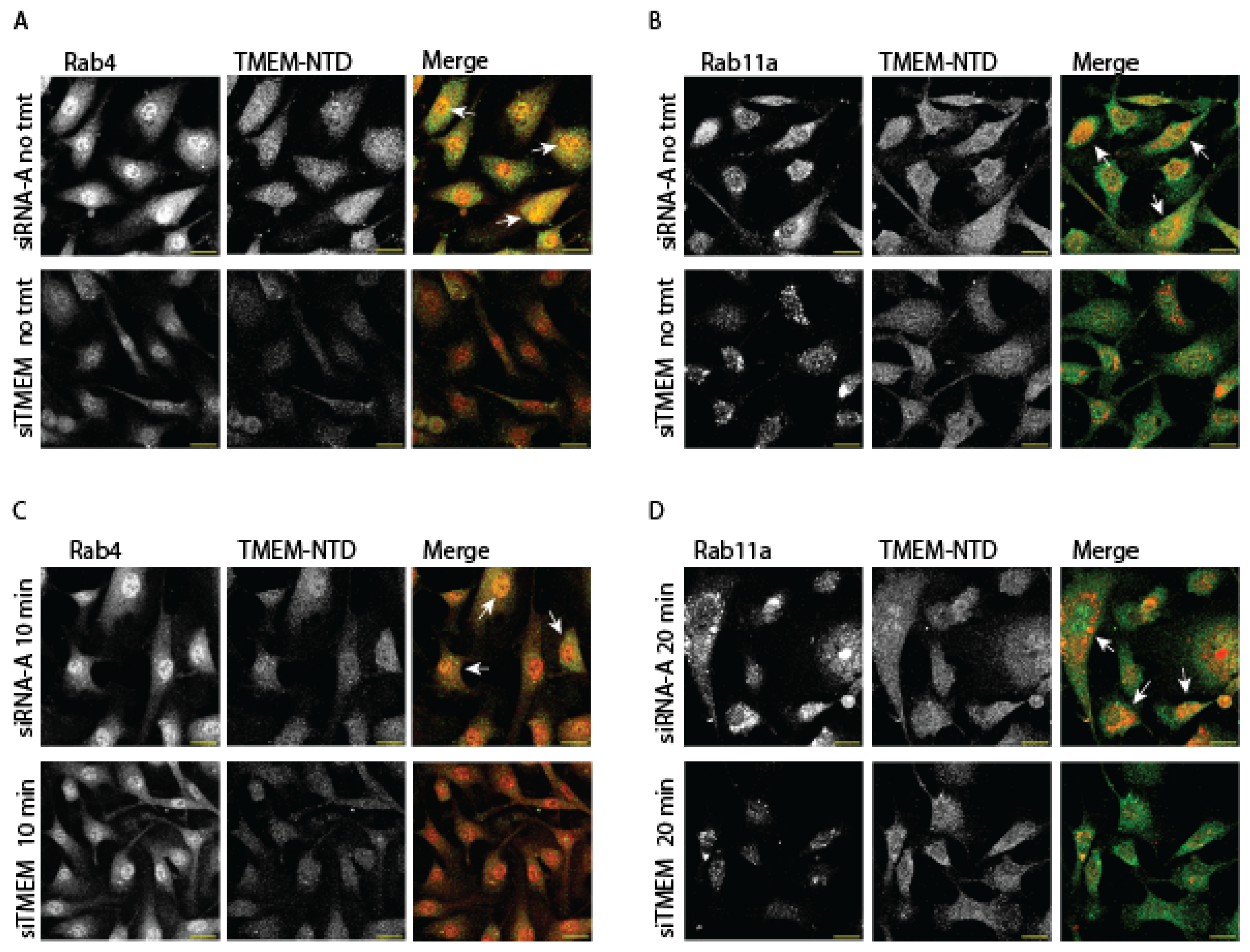
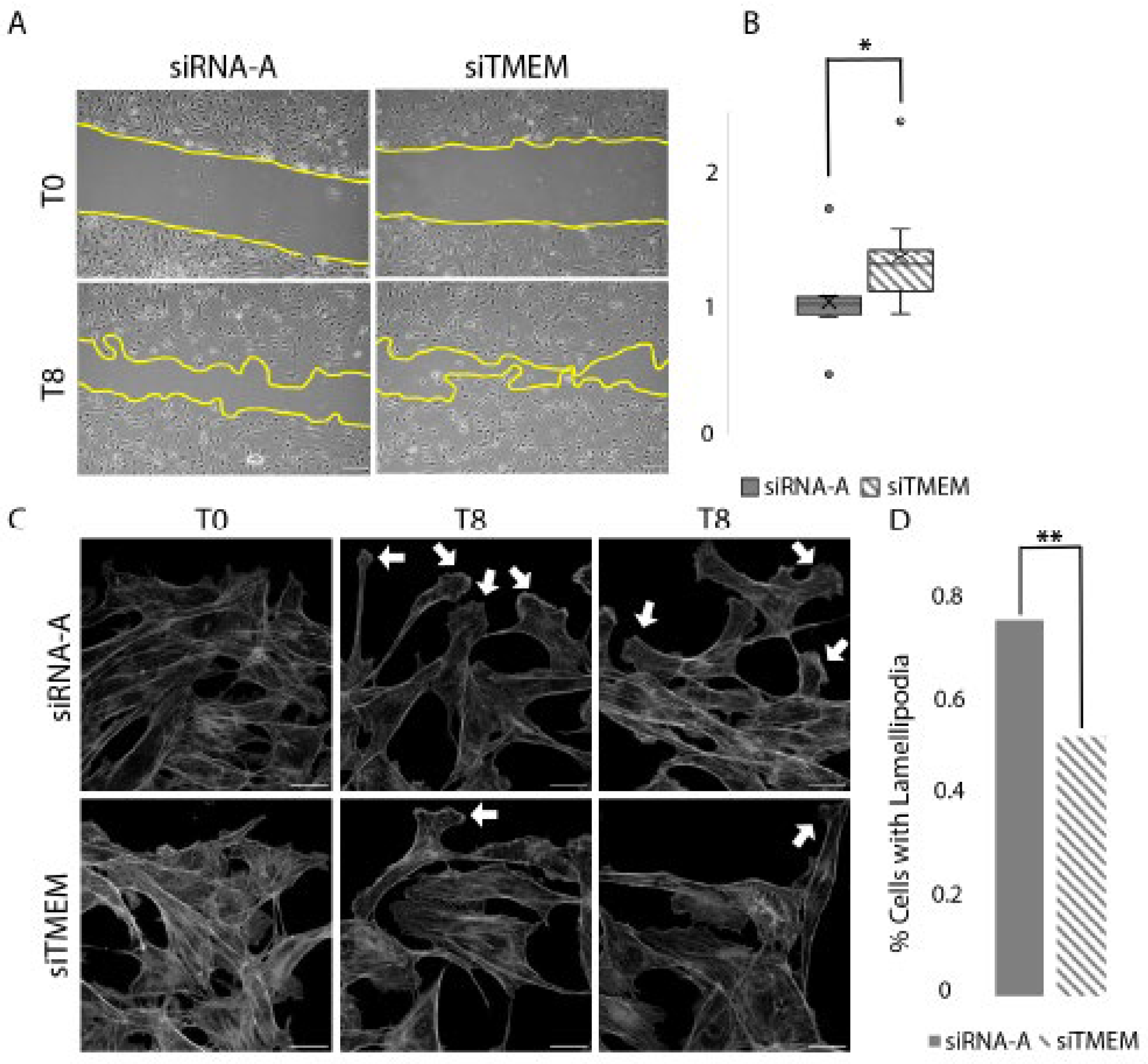
d. TMEM184A KD Cells Migrate Faster Compared to Control siRNA Cells in Wounding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Farwell, S.L.; Kanyi, D.; Hamel, M.; Slee, J.B.; Miller, E.A.; Cipolle, M.D.; Lowe-Krentz, L.J. Heparin Decreases in Tumor Necrosis Factor alpha (TNFalpha)-induced Endothelial Stress Responses Require Transmembrane Protein 184A and Induction of Dual Specificity Phosphatase 1. The Journal of biological chemistry 2016, 291, 5342–5354. [Google Scholar] [CrossRef] [PubMed]
- Gilotti, A.C.; Nimlamool, W.; Pugh, R.; Slee, J.B.; Barthol, T.C.; Miller, E.A.; Lowe-Krentz, L.J. Heparin responses in vascular smooth muscle cells involve cGMP-dependent protein kinase (PKG). Journal of cellular physiology 2014, 229, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Talotta-Altenburg, L.M.; Silimperi, K.A.; Ciabattoni, G.O.; Lowe-Krentz, L.J. Endothelial nitric oxide synthase activation is required for heparin receptor effects on vascular smooth muscle cells. American journal of physiology. Cell physiology 2020, 318, C463–C475. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.J.; Slee, J.B.; Farwell, S.L.; Li, Y.; Barthol, T.; Patton, W.A.; Lowe-Krentz, L.J. Transmembrane Protein 184A Is a Receptor Required for Vascular Smooth Muscle Cell Responses to Heparin. The Journal of biological chemistry 2016, 291, 5326–5341. [Google Scholar] [CrossRef] [PubMed]
- Pukac, L.A.; Carter, J.E.; Ottlinger, M.E.; Karnovsky, M.J. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. Journal of cellular physiology 1997, 172, 69–78. [Google Scholar] [CrossRef]
- Savage, J.M.; Gilotti, A.C.; Granzow, C.A.; Molina, F.; Lowe-Krentz, L.J. Antibodies against a putative heparin receptor slow cell proliferation and decrease MAPK activation in vascular smooth muscle cells. Journal of cellular physiology 2001, 187, 283–293. [Google Scholar] [CrossRef]
- Thourani, V.H.; Brar, S.S.; Kennedy, T.P.; Thornton, L.R.; Watts, J.A.; Ronson, R.S.; Zhao, Z.Q.; Sturrock, A.L.; Hoidal, J.R.; Vinten-Johansen, J. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. American journal of physiology. Heart and circulatory physiology 2000, 278, H2084–2093. [Google Scholar] [CrossRef]
- Barry, A.K.; Wang, N.; Leckband, D.E. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. Journal of cell science 2015, 128, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Dorland, Y.L.; Huveneers, S. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci 2017, 74, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovascular research 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006, 8, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. The Biochemical journal 2011, 437, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mansouri, M.; Rizk, A.; Berger, P. Regulation of VEGFR2 trafficking and signaling by Rab GTPase-activating proteins. Scientific reports 2019, 9, 13342. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Hermanson, S.; Ekker, S.C. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood 2004, 103, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Wang, Y.; Rhodes, J.M.; Atri, D.; Archer-Hartmann, S.; Zhang, J.; Zhuang, Z.W.; Chen, D.; Wang, T.; Wang, Z.; et al. Publisher Correction: N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat Commun 2019, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.T.; Reine, T.M.; Sudworth, A.; Jenssen, T.G.; Kolset, S.O. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J Histochem Cytochem 2015, 63, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Outeiro-Bernstein, M.A.; Juliano, L.; Vardiero, F.; Nader, H.B.; Woods, A.; Legrand, C.; Morandi, V. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. Journal of cellular physiology 2008, 214, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.R.; Hamidi, H.; Bass, M.D.; Warwood, S.; Ballestrem, C.; Humphries, M.J. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev Cell 2013, 24, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Marchant, L.; Carmona-Fontaine, C.; Kuriyama, S.; Larrain, J.; Holt, M.R.; Parsons, M.; Mayor, R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008, 135, 1771–1780. [Google Scholar] [CrossRef]
- Williamson, R.C.; Cowell, C.A.M.; Reville, T.; Roper, J.A.; Rendall, T.C.S.; Bass, M.D. Coronin-1C Protein and Caveolin Protein Provide Constitutive and Inducible Mechanisms of Rac1 Protein Trafficking. The Journal of biological chemistry 2015, 290, 15437–15449. [Google Scholar] [CrossRef]
- De Rossi, G.; Vahatupa, M.; Cristante, E.; Arokiasamy, S.; Liyanage, S.E.; May, U.; Pellinen, L.; Uusitalo-Jarvinen, H.; Bainbridge, J.W.; Jarvinen, T.A.H.; et al. Pathological Angiogenesis Requires Syndecan-4 for Efficient VEGFA-Induced VE-Cadherin Internalization. Arteriosclerosis, thrombosis, and vascular biology 2021, 41, 1374–1389. [Google Scholar] [CrossRef] [PubMed]
- Echtermeyer, F.; Streit, M.; Wilcox-Adelman, S.; Saoncella, S.; Denhez, F.; Detmar, M.; Goetinck, P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. The Journal of clinical investigation 2001, 107, R9–R14. [Google Scholar] [CrossRef]
- Sauteur, L.; Krudewig, A.; Herwig, L.; Ehrenfeuchter, N.; Lenard, A.; Affolter, M.; Belting, H.G. Cdh5/VE-cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep 2014, 9, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Grimsley-Myers, C.M.; Isaacson, R.H.; Cadwell, C.M.; Campos, J.; Hernandes, M.S.; Myers, K.R.; Seo, T.; Giang, W.; Griendling, K.K.; Kowalczyk, A.P. VE-cadherin endocytosis controls vascular integrity and patterning during development. The Journal of cell biology 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Delva, E.; Kowalczyk, A.P. Regulation of cadherin trafficking. Traffic 2009, 10, 259–267. [Google Scholar] [CrossRef]
- Su, W.; Kowalczyk, A.P. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Molecular biology of the cell 2017, 28, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chrifi, I.; Louzao-Martinez, L.; Brandt, M.M.; van Dijk, C.G.M.; Burgisser, P.E.; Zhu, C.; Kros, J.M.; Verhaar, M.C.; Duncker, D.J.; Cheng, C. CMTM4 regulates angiogenesis by promoting cell surface recycling of VE-cadherin to endothelial adherens junctions. Angiogenesis 2019, 22, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Adams, I.R. Sdmg1 is a component of secretory granules in mouse secretory exocrine tissues. Developmental dynamics : an official publication of the American Association of Anatomists 2009, 238, 223–231. [Google Scholar] [CrossRef]
- Field, C.J.; Perez, A.M.; Samet, T.; Ricles, V.; Iovine, M.K.; Lowe-Krentz, L.J. Involvement of transmembrane protein 184a during angiogenesis in zebrafish embryos. Frontiers in physiology 2022, 13, 845407. [Google Scholar] [CrossRef]
- Farwell, S.L.N.; Reylander, K.G.; Iovine, M.K.; Lowe-Krentz, L.J. Novel Heparin Receptor Transmembrane Protein 184a Regulates Angiogenesis in the Adult Zebrafish Caudal Fin. Frontiers in physiology 2017, 8, 671. [Google Scholar] [CrossRef]
- Rawls, J.F.; Frieda, M.R.; McAdow, A.R.; Gross, J.P.; Clayton, C.M.; Heyen, C.K.; Johnson, S.L. Coupled mutagenesis screens and genetic mapping in zebrafish. Genetics 2003, 163, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kathryn Iovine, M. Using Morpholinos to Examine Gene Function During Fin Regeneration. Methods Mol Biol 2017, 1565, 79–85. [Google Scholar] [CrossRef]
- Farwell, S.L.; Slee, J.B.; Li, Y.; Lowe-Krentz, L.J. Using a GFP-tagged TMEM184A Construct for Confirmation of Heparin Receptor Identity. Journal of visualized experiments : JoVE 2017. [Google Scholar] [CrossRef]
- Divari, S.; Berio, E.; Biolatti, B.; Cannizzo, F.T. Reference Gene Selection and Prednisolone Target Gene Expression in Adipose Tissues of Friesian Cattle. J Agric Food Chem 2017, 65, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, M.; Mysiorek, C.; Petrault, O.; Boucau, M.C.; Aijjou, R.; Uzbekov, R.; Berezowski, V. Transient oxygen-glucose deprivation sensitizes brain capillary endothelial cells to rtPA at 4h of reoxygenation. Microvasc Res 2014, 91, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PloS one 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Blaukovitch, C.I.; Pugh, R.; Gilotti, A.C.; Kanyi, D.; Lowe-Krentz, L.J. Heparin treatment of vascular smooth muscle cells results in the synthesis of the dual-specificity phosphatase MKP-1. Journal of cellular biochemistry 2010, 110, 382–391. [Google Scholar] [CrossRef]
- Patton, W.A., 2nd; Granzow, C.A.; Getts, L.A.; Thomas, S.C.; Zotter, L.M.; Gunzel, K.A.; Lowe-Krentz, L.J. Identification of a heparin-binding protein using monoclonal antibodies that block heparin binding to porcine aortic endothelial cells. The Biochemical journal 1995, 311 Pt 2, 461–469. [Google Scholar] [CrossRef]
- Gopal, S.; Multhaupt, H.A.B.; Pocock, R.; Couchman, J.R. Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol 2017, 60-61, 57–69. [Google Scholar] [CrossRef]
- Gulino-Debrac, D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 2013, 1, e24180. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ding, Y.; Richards, M.; Kaakinen, M.; Giese, W.; Baumann, E.; Szymborska, A.; Rosa, A.; Nordling, S.; Schimmel, L.; et al. Tyrosine-protein kinase Yes controls endothelial junctional plasticity and barrier integrity by regulating VE-cadherin phosphorylation and endocytosis. Nat Cardiovasc Res 2022, 1, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Garner, J.; Buckley, K.M.; Vincent, P.A.; Chiasson, C.M.; Dejana, E.; Faundez, V.; Kowalczyk, A.P. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Molecular biology of the cell 2005, 16, 5141–5151. [Google Scholar] [CrossRef] [PubMed]
- Juettner, V.V.; Kruse, K.; Dan, A.; Vu, V.H.; Khan, Y.; Le, J.; Leckband, D.; Komarova, Y.; Malik, A.B. VE-PTP stabilizes VE-cadherin junctions and the endothelial barrier via a phosphatase-independent mechanism. The Journal of cell biology 2019, 218, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Sahlender, D.A.; Walther, N.; Peden, A.A.; Adams, I.R. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development 2008, 135, 1415–1425. [Google Scholar] [CrossRef]
- Bennett, M.; Cantini, M.; Reboud, J.; Cooper, J.M.; Roca-Cusachs, P.; Salmeron-Sanchez, M. Molecular clutch drives cell response to surface viscosity. Proceedings of the National Academy of Sciences of the United States of America 2018, 115, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Ciobanasu, C.; Wang, H.; Henriot, V.; Mathieu, C.; Fente, A.; Csillag, S.; Vigouroux, C.; Faivre, B.; Le Clainche, C. Integrin-bound talin head inhibits actin filament barbed-end elongation. The Journal of biological chemistry 2018, 293, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, A.; Thorpe, S.D.; Cortes, E.; Lachowski, D.; Rice, A.J.; Mykuliak, V.V.; Rog, T.; Lee, D.A.; Hytonen, V.P.; Del Rio Hernandez, A.E. Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat Mater 2020, 19, 669–678. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, A.; Badu-Nkansah, K.; Whittaker, C.A.; Crowley, D.; Bronson, R.T.; Lacy-Hulbert, A.; Hynes, R.O. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development 2010, 137, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Meerschaert, K.; Reekmans, G.; Leenaerts, I.; Small, J.V.; Vandekerckhove, J.; David, G.; Gettemans, J. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol Cell 2002, 9, 1215–1225. [Google Scholar] [CrossRef]
- Troyanovsky, R.B.; Sergeeva, A.P.; Indra, I.; Chen, C.S.; Kato, R.; Shapiro, L.; Honig, B.; Troyanovsky, S.M. Sorting of cadherin-catenin-associated proteins into individual clusters. Proceedings of the National Academy of Sciences of the United States of America 2021, 118. [Google Scholar] [CrossRef]
- Bosseboeuf, E.; Chikh, A.; Chaker, A.B.; Mitchell, T.P.; Vignaraja, D.; Rajendrakumar, R.; Khambata, R.S.; Nightingale, T.D.; Mason, J.C.; Randi, A.M.; et al. Neuropilin-1 interacts with VE-cadherin and TGFBR2 to stabilize adherens junctions and prevent activation of endothelium under flow. Science signaling 2023, 16, eabo4863. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Knoerzer, M.; Khoshmanesh, K.; Mitchell, A.; McIntyre, P. Shear Stress Regulates TRPV4 Channel Clustering and Translocation from Adherens Junctions to the Basal Membrane. Scientific reports 2017, 7, 15942. [Google Scholar] [CrossRef]
- Vellino, S.; Oddou, C.; Rivier, P.; Boyault, C.; Hiriart-Bryant, E.; Kraut, A.; Martin, R.; Coute, Y.; Knolker, H.J.; Valverde, M.A.; et al. Cross-talk between the calcium channel TRPV4 and reactive oxygen species interlocks adhesive and degradative functions of invadosomes. The Journal of cell biology 2021, 220. [Google Scholar] [CrossRef]
- Cappelli, H.C.; Kanugula, A.K.; Adapala, R.K.; Amin, V.; Sharma, P.; Midha, P.; Paruchuri, S.; Thodeti, C.K. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer letters 2019, 442, 15–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




