Submitted:
19 December 2024
Posted:
20 December 2024
You are already at the latest version
Abstract
Epithelial ovarian cancer (EOC) exhibits a unique mode of metastasis, involving spheroid formation in the peritoneum. Our research on EOC spheroid cell biology has provided valuable insights into the signalling plasticity associated with metastasis. We speculate that EOC cells modify their biology between tumor and spheroid states during cancer dormancy, although the specific mechanisms underlying this transition remain unknown. Here, we present novel findings from direct comparisons between cultured EOC spheroids and organoids. Our results indicated that AMP-activated protein kinase (AMPK) activity was significantly upregulated and protein kinase B (Akt) was down-regulated in iOvCa spheroids compared to organoids, suggesting a clear differential phenotype. Through a comprehensive RNA sequencing analysis, we further supported these phenotypic differences and highlighted the significance of cell cycle regulation in organoids. By inhibiting the G2/M checkpoint via kinase inhibitors, we confirmed that this pathway is essential for organoids. Interestingly, our results suggest that specifically targeting aurora kinase A (AURKA) may represent a promising therapeutic strategy since our cells were equally sensitive to Alisertib treatment as both spheroids and or-ganoids. Our findings emphasize the importance of studying cellular adaptations of EOC cells, as there may be different therapeutic targets depending on the step of EOC disease progression.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. 3D Spheroid Culture
2.3. 3D Organoid Culture
2.4. Organoid Growth Analysis
2.5. Antibodies
2.6. Protein Isolation
2.7. Immunoblotting
2.8. Hematoxylin & Eosin Staining
2.9. Immunohistochemistry
2.10. Immunofluorescence
2.11. RNA Isolation
2.12. Transcriptomic Analysis
2.13. Quantitative Reverse Transcription-(qRT)-PCR
2.14. Kinase Inhibitor Treatments
2.15. Statistical Analysis
3. Results
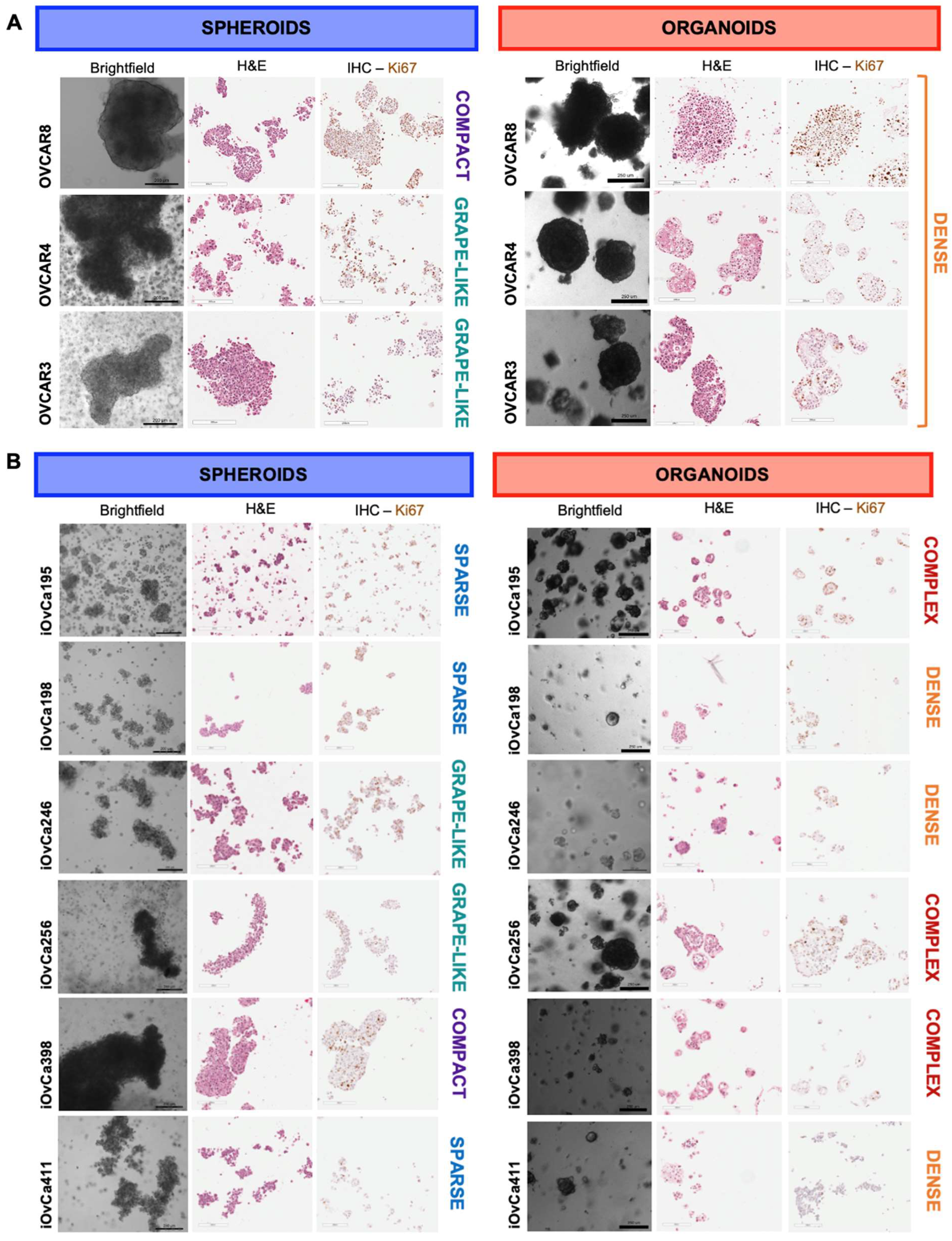
3.1. HGSOC cell lines showed distinct spheroid and organoid morphologies
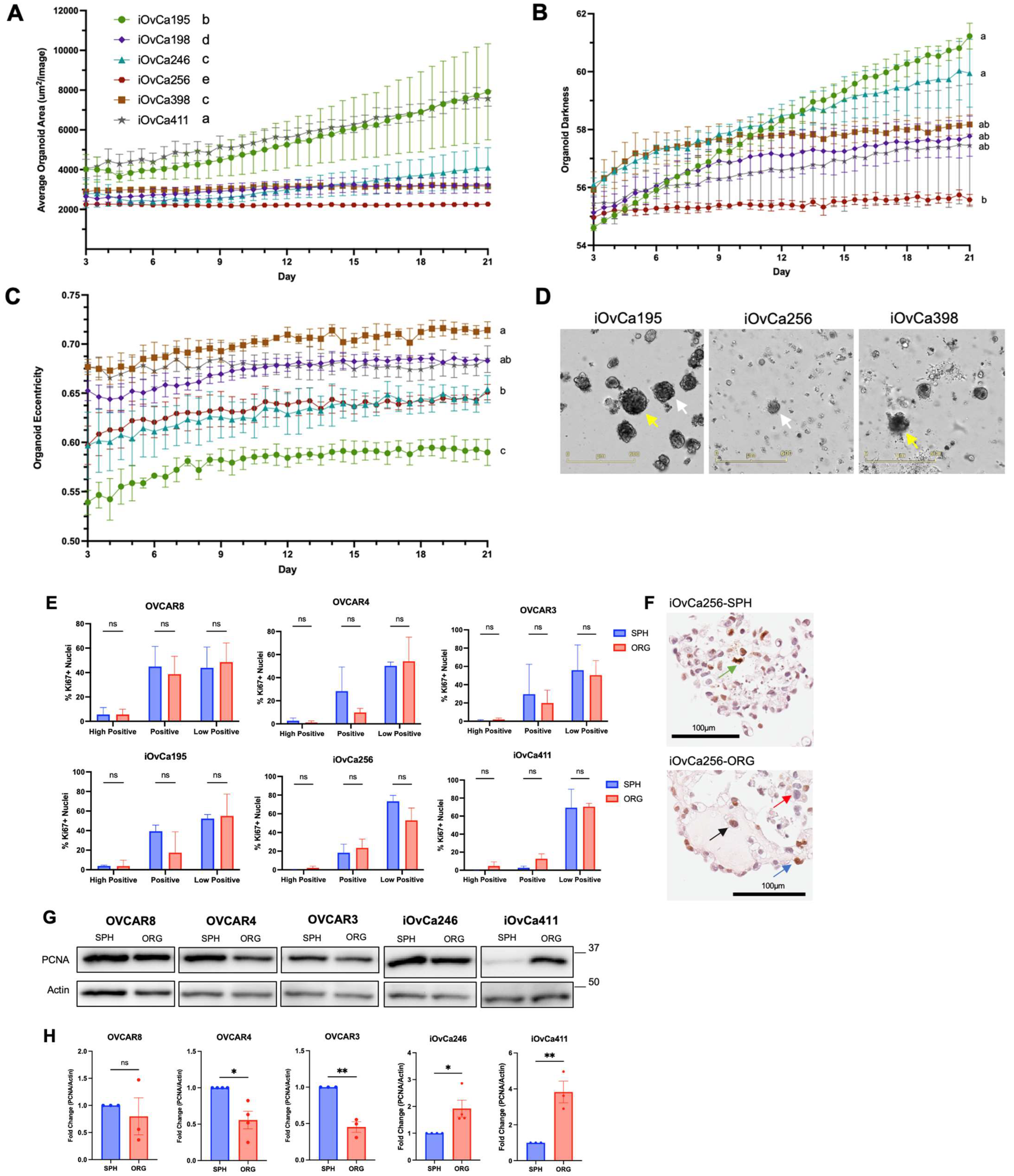
3.2. HGSOC organoid varying growth dynamics relate to morphology
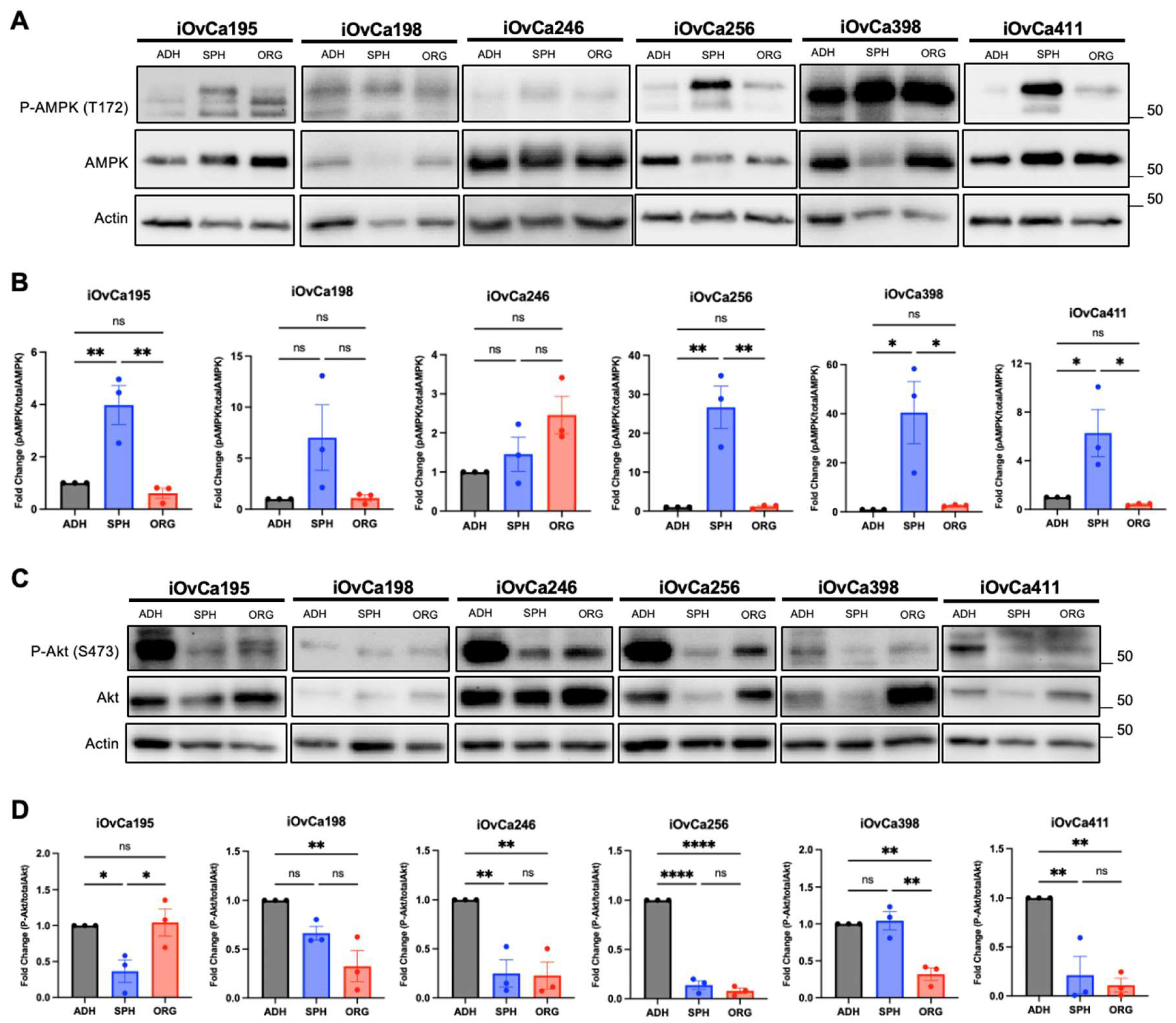
3.3. Altered AMPK and Akt signaling in HGSOC spheroids and organoids indicates biological switching
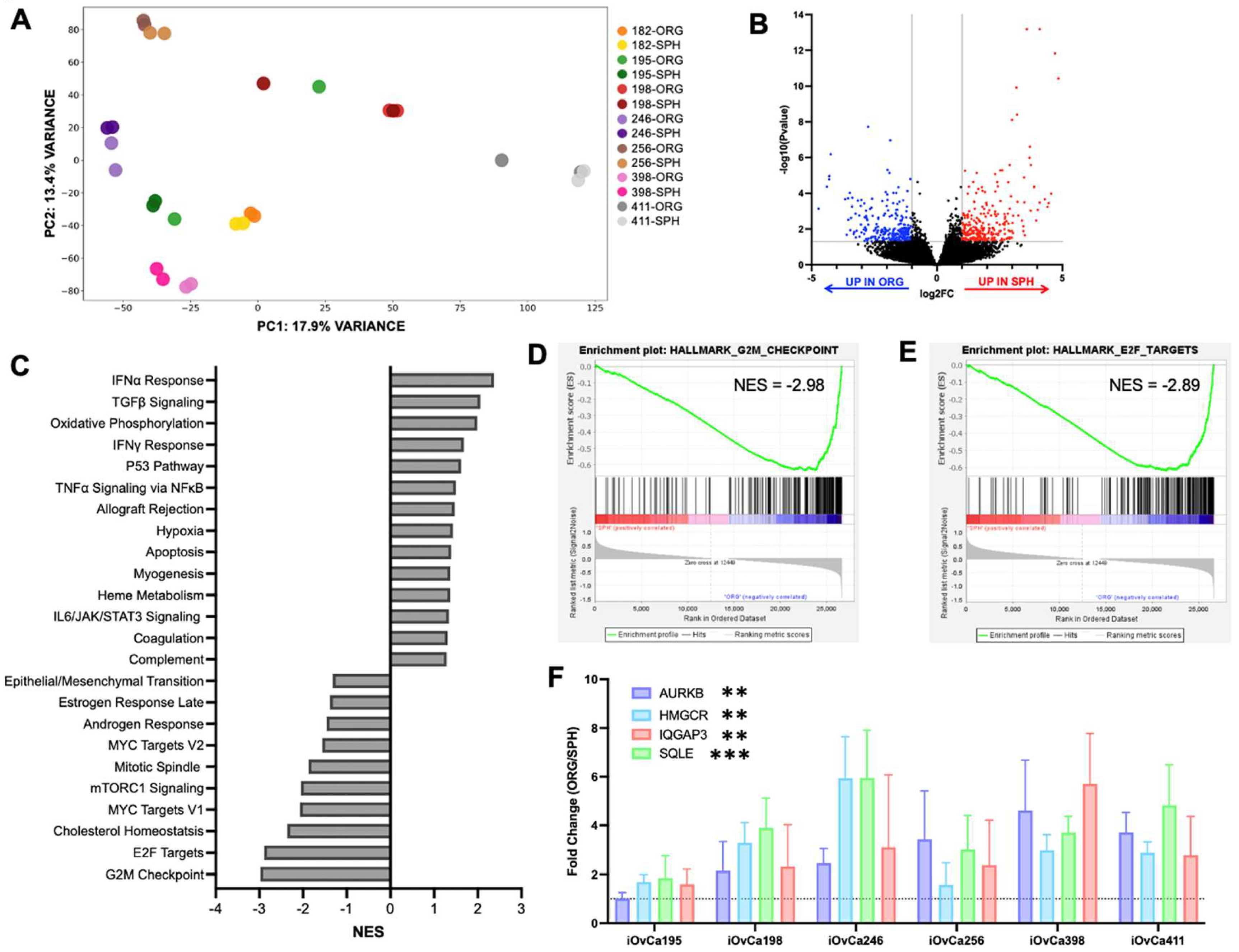
3.4. Differential gene expression between HGSOC spheroids and organoids
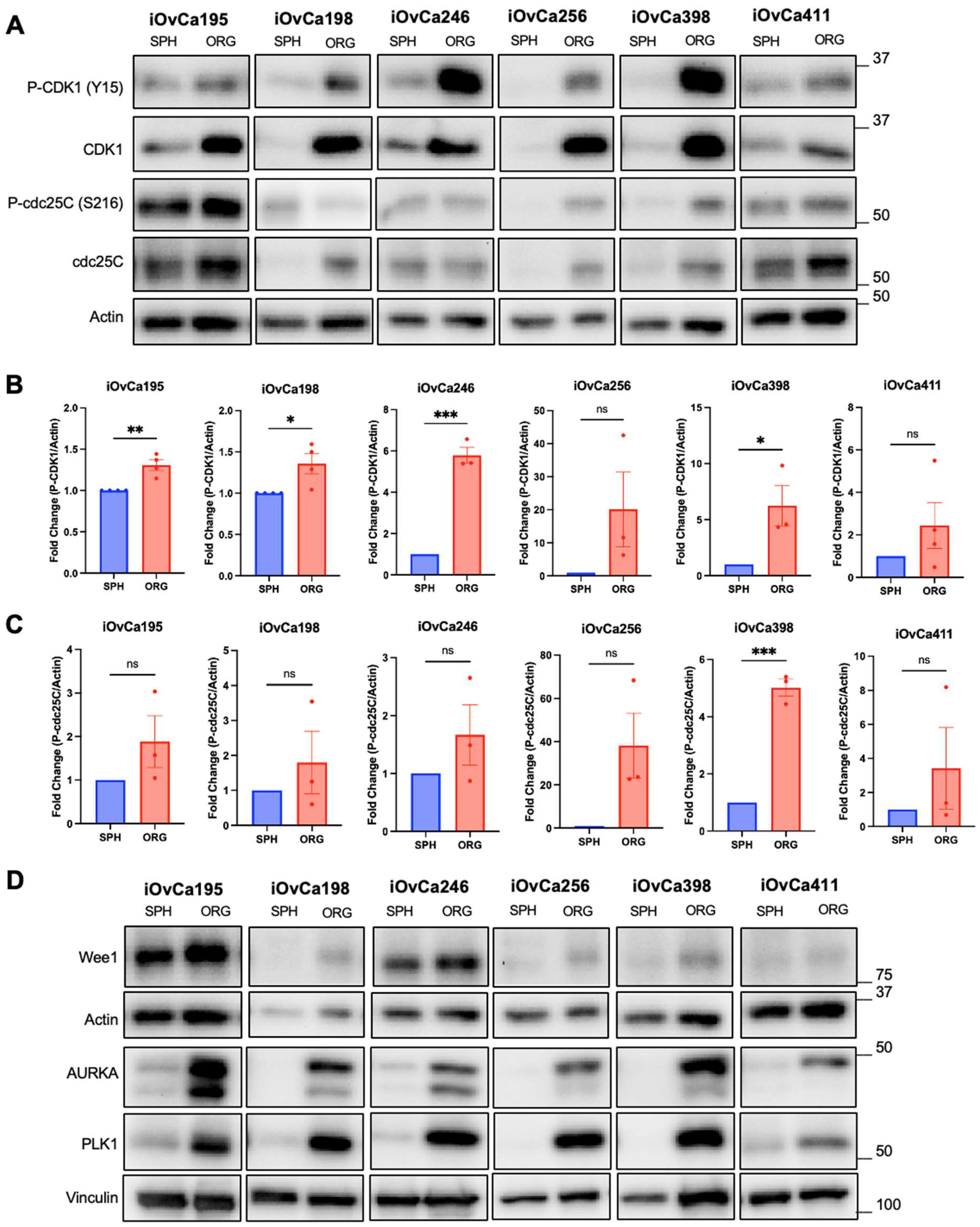
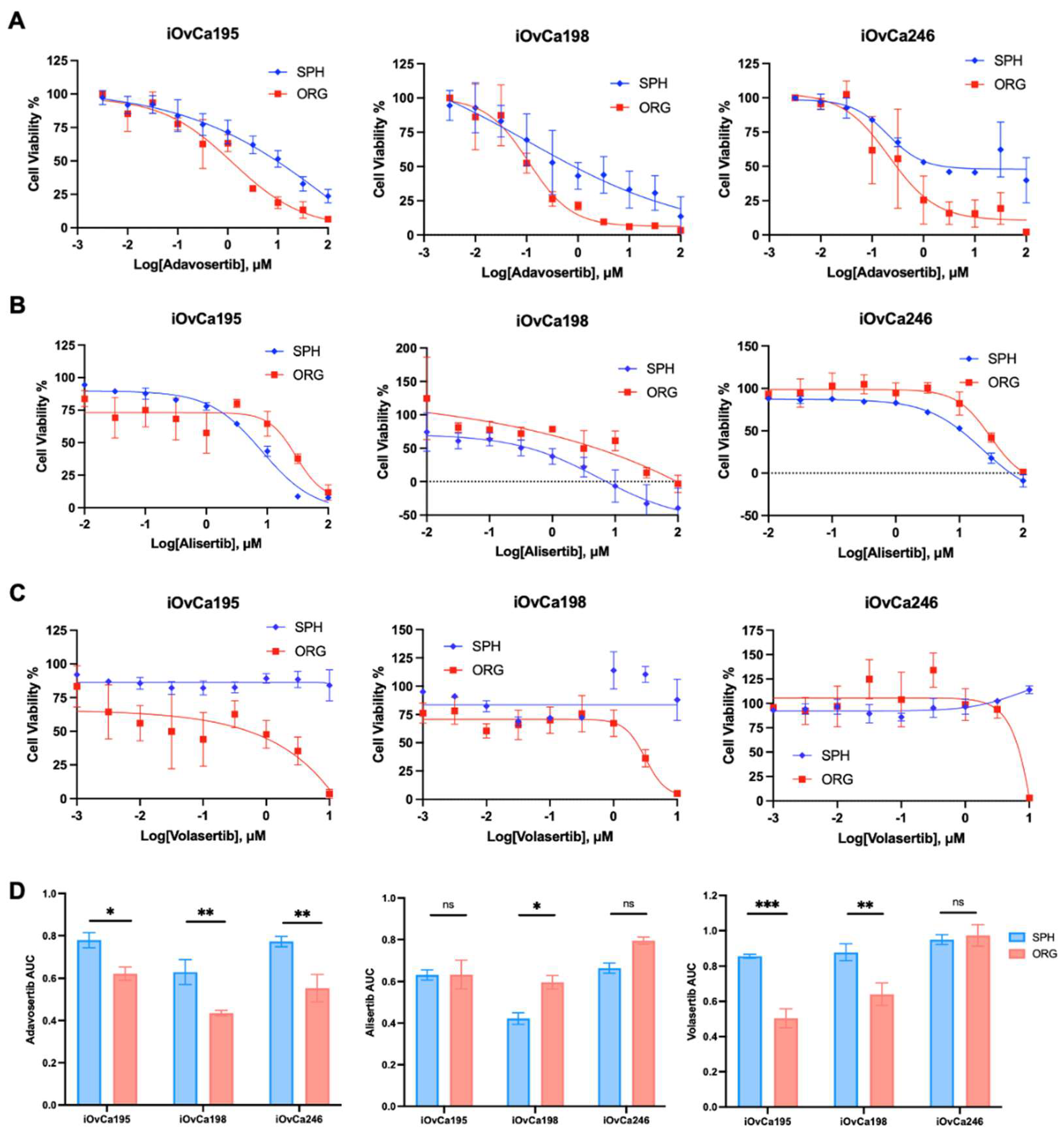
3.5. G2/M checkpoint is a key regulatory pathway in HGSOC organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High Grade Serous Ovarian Carcinomas Originate in the Fallopian Tube. Nat Commun 2017, 8. [Google Scholar] [CrossRef]
- Karst, A.M.; Levanon, K.; Drapkin, R. Modeling High-Grade Serous Ovarian Carcinogenesis from the Fallopian Tube. Proc Natl Acad Sci U S A 2011, 108, 7547–7552. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhao, L.; Rojas, C.; Bateman, N.W.; Yao, H.; Lara, O.D.; Celestino, J.; Morgan, M.B.; Nguyen, T.V.; Conrads, K.A.; et al. Molecular Analysis of Clinically Defined Subsets of High-Grade Serous Ovarian Cancer. Cell Rep 2020, 31. [Google Scholar] [CrossRef]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.J.; Shah, P.; et al. A Single-Cell Landscape of High-Grade Serous Ovarian Cancer. Nat Med 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Haagsma, J.; Kolendowski, B.; Buensuceso, A.; Valdes, Y.R.; DiMattia, G.E.; Shepherd, T.G. Gain-of-Function P53R175H Blocks Apoptosis in a Precursor Model of Ovarian High-Grade Serous Carcinoma. Sci Rep 2023, 13, 11424. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, A.; Lisio, M.A.; Fu, L.; Srinivasan, R.; Capuccino, J.V.; Gao, Z.H.; Telleria, C. The Capacity of High-Grade Serous Ovarian Cancer Cells to Form Multicellular Structures Spontaneously along Disease Progression Correlates with Their Orthotopic Tumorigenicity in Immunosuppressed Mice. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Das, T.; Meunier, L.; Barbe, L.; Provencher, D.; Guenat, O.; Gervais, T.; Mes-Masson, A.M. Empirical Chemosensitivity Testing in a Spheroid Model of Ovarian Cancer Using a Microfluidics-Based Multiplex Platform. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Ward, M.R.; Rowley, K.R.; Wold, R.M.; Takayama, S.; Buckanovich, R.J.; Mehta, G. Formation of Stable Small Cell Number Three-Dimensional Ovarian Cancer Spheroids Using Hanging Drop Arrays for Preclinical Drug Sensitivity Assays. Gynecol Oncol 2015, 138, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Jin, V.; Bergsten, T.M.; Austin, J.R.; Lantvit, D.D.; Russo, A.; Burdette, J.E. Loss of PTEN in Fallopian Tube Epithelium Results in Multicellular Tumor Spheroid Formation and Metastasis to the Ovary. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Singha, B.; Laski, J.; Ramos Valdes, Y.; Liu, E.; DiMattia, G.E.; Shepherd, T.G. Inhibiting ULK1 Kinase Decreases Autophagy and Cell Viability in High-Grade Serous Ovarian Cancer Spheroids. Am J Cancer Res 2020, 10, 1384–1399. [Google Scholar] [PubMed]
- Fritz, J.L.; Collins, O.; Saxena, P.; Buensuceso, A.; Ramos Valdes, Y.; Francis, K.E.; Brown, K.R.; Larsen, B.; Colwill, K.; Gingras, A.-C.; et al. A Novel Role for NUAK1 in Promoting Ovarian Cancer Metastasis through Regulation of Fibronectin Production in Spheroids. Cancers (Basel) 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Summers, M.A.; McDonald, M.M.; Croucher, P.I. Cancer Cell Dormancy in Metastasis. Cold Spring Harb Perspect Med. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Shepherd, T.G.; Dick, F.A. Principles of Dormancy Evident in High-Grade Serous Ovarian Cancer. Cell Div 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Tomas, E.; Shepherd, T.G. Insights into High-Grade Serous Carcinoma Pathobiology Using Three-Dimensional Culture Model Systems. J Ovarian Res 2023, 16. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact Spheroid Formation by Ovarian Cancer Cells Is Associated with Contractile Behavior and an Invasive Phenotype. Int J Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- MacDonald, J.; Ramos-Valdes, Y.; Perampalam, P.; Litovchick, L.; DiMattia, G.E.; Dick, F.A. A Systematic Analysis of Negative Growth Control Implicates the DREAM Complex in Cancer Cell Dormancy. Molecular Cancer Research 2017, 15, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Buensuceso, A.; Fritz, J.L.; Collins, O.; Valdés, Y.R.; Borrelli, M.J.; DiMattia, G.E.; Shepherd, T.G. Loss of LKB1-NUAK1 Signalling Enhances NF-ΚB Activity in a Spheroid Model of High-Grade Serous Ovarian Cancer. Sci Rep 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.J.M.; Valdes, Y.R.; Peart, T.M.; Fazio, E.N.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. Combination of AKT Inhibition with Autophagy Blockade Effectively Reduces Ascites-Derived Ovarian Cancer Cell Viability. Carcinogenesis 2014, 35, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, S.; Valdes, Y.R.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; Dimattia, G.E.; Shepherd, T.G. TGFβ Signaling Regulates Epithelial-Mesenchymal Plasticity in Ovarian Cancer Ascites-Derived Spheroids. Endocr Relat Cancer 2016, 23, 147–159. [Google Scholar] [CrossRef]
- Peart, T.; Ramos Valdes, Y.; Correa, R.J.M.; Fazio, E.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. Intact LKB1 Activity Is Required for Survival of Dormant Ovarian Cancer Spheroids. Oncotarget 2015, 6, 22424–22438. [Google Scholar] [CrossRef]
- Buensuceso, A.; Ramos Valdes, Y.; DiMattia, G.E.; Shepherd, T.G. AMPK-Independent LKB1 Activity Is Required for Efficient Epithelial Ovarian Cancer Metastasis. Molecular Cancer Research 2020, 18, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Laski, J.; Singha, B.; Wang, X.; Ramos Valdes, Y.; Collins, O.; Shepherd, T.G. Activated CAMKKβ-AMPK Signaling Promotes Autophagy in a Spheroid Model of Ovarian Tumour Metastasis. J Ovarian Res 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Peart, T.M.; Correa, R.J.M.; Valdes, Y.R.; DiMattia, G.E.; Shepherd, T.G. BMP Signalling Controls the Malignant Potential of Ascites-Derived Human Epithelial Ovarian Cancer Spheroids via AKT Kinase Activation. Clin Exp Metastasis 2012, 29, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.J.M.; Peart, T.; Valdes, Y.R.; Dimattia, G.E.; Shepherd, T.G. Modulation of AKT Activity Is Associated with Reversible Dormancy in Ascites-Derived Epithelial Ovarian Cancer Spheroids. Carcinogenesis 2012, 33, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.; Brugge, J.S. Ovarian Cancer Spheroids Use Myosin-Generated Force to Clear the Mesothelium. Cancer Discov 2011, 1, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Puiffe, M.L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.-M. Characterization of Ovarian Cancer Ascites on Cell Invasion, Proliferation, Spheroid Formation, and Gene Expression in an in Vitro Model of Epithelial Ovarian Cancer. Neoplasia 2007, 9, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Burston, H.E.; Kent, O.A.; Communal, L.; Udaskin, M.L.; Sun, R.X.; Brown, K.R.; Jung, E.; Francis, K.E.; Rose, J. La; Lowitz, J.; et al. Inhibition of Relaxin Autocrine Signaling Confers Therapeutic Vulnerability in Ovarian Cancer. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Willows, R.; Sanders, M.J.; Xiao, B.; Patel, B.R.; Martin, S.R.; Read, J.; Wilson, J.R.; Hubbard, J.; Gamblin, S.J.; Carling, D. Phosphorylation of AMPK by Upstream Kinases Is Required for Activity in Mammalian Cells. Biochemical Journal 2017, 474, 3059–3073. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Owada, S.; Inagaki, Y.; Shida, Y.; Tatemichi, M. Glucose Starvation Induces LKB1-AMPK-Mediated MMP-9 Expression in Cancer Cells. Sci Rep 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Lõhmussaar, K.; Kopper, O.; Korving, J.; Begthel, H.; Vreuls, C.P.H.; van Es, J.H.; Clevers, H. Assessing the Origin of High-Grade Serous Ovarian Cancer Using CRISPR-Modification of Mouse Organoids. Nat Commun 2020, 11. [Google Scholar] [CrossRef]
- de Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lõhmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An Organoid Platform for Ovarian Cancer Captures Intra- and Interpatient Heterogeneity. Nat Med 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Filippou, P.S.; Aleman, J.; Garreta, E.; Wang, Y.; Yang, J.; Huang, S.; Cheng, S.; Jin, Y.; Zhang, N. Application of Ovarian Cancer Organoids in Precision Medicine: Key Challenges and Current Opportunities. Front Cell Dev Biol 2021, 9. [Google Scholar] [CrossRef]
- Nero, C.; Vizzielli, G.; Lorusso, D.; Cesari, E.; Daniele, G.; Loverro, M.; Scambia, G.; Sette, C. Patient-Derived Organoids and High Grade Serous Ovarian Cancer: From Disease Modeling to Personalized Medicine. Journal of Experimental & Clinical Cancer Research 2021 40:1 2021, 40, 1–14. [Google Scholar] [CrossRef]
- Yee, C.; Dickson, K.-A.; Muntasir, M.N.; Ma, Y.; Marsh, D.J. Three-Dimensional Modelling of Ovarian Cancer: From Cell Lines to Organoids for Discovery and Personalized Medicine. Front Bioeng Biotechnol 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Senkowski, W.; Gall-Mas, L.; Marin Falco, M.; Li, Y.; Lavikka, K.; Kriegbaum, M.C.; Oikkonen, J.; Bulanova, D.; Pietras, E.J.; Voßgröne, K.; et al. A Platform for Efficient Establishment, Expansion and Drug Response Profiling of High-Grade Serous Ovarian Cancer Organoids. [CrossRef]
- Gorski, J.W.; Zhang, Z.; McCorkle, J.R.; DeJohn, J.M.; Wang, C.; Miller, R.W.; Gallion, H.H.; Dietrich, C.S.; Ueland, F.R.; Kolesar, J.M. Utilizing Patient-Derived Epithelial Ovarian Cancer Tumor Organoids to Predict Carboplatin Resistance. Biomedicines 2021, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Wu, X. The Role of Patient-Derived Ovarian Cancer Organoids in the Study of PARP Inhibitors Sensitivity and Resistance: From Genomic Analysis to Functional Testing. Journal of Experimental and Clinical Cancer Research 2021, 40. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Sun, F.; Wang, J.; Wang, Y.; Zhu, H.; Chen, M.; Liu, L.; Liu, L.; Lin, H.; Wu, X. Developing Patient-Derived Organoids to Predict PARP Inhibitor Response and Explore Resistance Overcoming Strategies in Ovarian Cancer. Pharmacol Res 2022, 179. [Google Scholar] [CrossRef] [PubMed]
- Nanki, Y.; Chiyoda, T.; Hirasawa, A.; Ookubo, A.; Itoh, M.; Ueno, M.; Akahane, T.; Kameyama, K.; Yamagami, W.; Kataoka, F.; et al. Patient-Derived Ovarian Cancer Organoids Capture the Genomic Profiles of Primary Tumours Applicable for Drug Sensitivity and Resistance Testing. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.G.; Thériault, B.L.; Campbell, E.J.; Nachtigal, M.W. Primary Culture of Ovarian Surface Epithelial Cells and Ascites-Derived Ovarian Cancer Cells from Patients. Nat Protoc 2007, 1, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Reports 2020, 14, 717–729. [Google Scholar] [CrossRef]
- Burleson, K.M.; Casey, R.C.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Skubitz, A.P.N. Ovarian Carcinoma Ascites Spheroids Adhere to Extracellular Matrix Components and Mesothelial Cell Monolayers. Gynecol Oncol 2004, 93, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Xie, J.; Gurler, H.; Muralidhar, G.G.; Sacks, J.D.; Burdette, J.E.; Barbolina, M.V. Versican Regulates Metastasis of Epithelial Ovarian Carcinoma Cells and Spheroids. J Ovarian Res 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Frederick, M.I.; Nassef, M.Z.; Borrelli, M.J.; Kuang, S.; Buensuceso, A.; More, T.; Cordes, T.; O’Donoghue, P.; Shepherd, T.G.; Hiller, K.; et al. Metabolic Adaptation in Epithelial Ovarian Cancer Metastasis. BBA - Molecular Basis of Disease 2024, 1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gupta Vallur, P.; Jones, V.M.; Worley, B.L.; Shimko, S.; Shin, D.H.; Crawford, L.T.C.; Chen, C.W.; Aird, K.M.; Abraham, T.; et al. Context-Dependent Activation of SIRT3 Is Necessary for Anchorage-Independent Survival and Metastasis of Ovarian Cancer Cells. Oncogene 2020, 39, 1619–1633. [Google Scholar] [CrossRef]
- Chuang, T.C.; Shao, W.S.; Hsu, S.C.; Lee, S.L.; Kao, M.C.; Wang, V. Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Zhang, Y.; Wang, W.; Song, E.; Fan, Y.; Wei, B. E2F1: A Promising Regulator in Ovarian Carcinoma. Tumor Biology 2016, 37, 2823–2831. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, F.; He, Z.; Zuo, M.Z. E2F2/5/8 Serve as Potential Prognostic Biomarkers and Targets for Human Ovarian Cancer. Front Oncol 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.N.; Liu, J.F.; Kesten, N.; Chow, K.H.; Shetty, A.; He, R.; Afreen, M.F.; Yuan, L.; Matulonis, U.A.; Growdon, W.B.; et al. Enhanced Efficacy of Aurora Kinase Inhibitors in G2/m Checkpoint Deficient Tp53 Mutant Uterine Carcinomas Is Linked to the Summation of Lkb1–Akt–P53 Interactions. Cancers (Basel) 2021, 13, 2195. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Xu, M.; Yang, S.; Yu, T.; Zheng, C.; Huang, X.; Pan, Y.; Chen, Y.; Long, J.; et al. ODF2L Acts as a Synthetic Lethal Partner with WEE1 Inhibition in Epithelial Ovarian Cancer Models. Journal of Clinical Investigation 2023, 133. [Google Scholar] [CrossRef]
- Roering, P.; Siddiqui, A.; Heuser, V.D.; Potdar, S.; Mikkonen, P.; Oikkonen, J.; Li, Y.; Pikkusaari, S.; Wennerberg, K.; Hynninen, J.; et al. Effects of Wee1 Inhibitor Adavosertib on Patient-Derived High-Grade Serous Ovarian Cancer Cells Are Multiple and Independent of Homologous Recombination Status. Front Oncol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Zhou, Z.W.; Ha, C.F.; Zhang, X.Y.; Pan, S.T.; He, Z.X.; Edelman, J.L.; Wang, D.; Yang, Y.X.; Zhang, X.; et al. Alisertib, an Aurora Kinase A Inhibitor, Induces Apoptosis and Autophagy but Inhibits Epithelial to Mesenchymal Transition in Human Epithelial Ovarian Cancer Cells. Drug Des Devel Ther 2015, 9, 425–464. [Google Scholar] [CrossRef] [PubMed]
- Gasimli, K.; Raab, M.; Tahmasbi Rad, M.; Kurunci-Csacsko, E.; Becker, S.; Strebhardt, K.; Sanhaji, M. Sequential Targeting of PLK1 and PARP1 Reverses the Resistance to PARP Inhibitors and Enhances Platin-Based Chemotherapy in BRCA-Deficient High-Grade Serous Ovarian Cancer with KRAS Amplification. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Borah, N.A.; Reddy, M.M. Aurora Kinase B Inhibition: A Potential Therapeutic Strategy for Cancer. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.L.; Sundararajan, V.; Tan, D.S.P. Exploiting Replicative Stress in Gynecological Cancers as a Therapeutic Strategy. International Journal of Gynecologic Cancer 2020, 30, 1224–1238. [Google Scholar] [CrossRef]
- Kroeger, P.T.; Drapkin, R. Pathogenesis and Heterogeneity of Ovarian Cancer. Curr Opin Obstet Gynecol 2017, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, K.; Sproul, D.; Grun, B.; Notaridou, M.; Benjamin, E.; Jacobs, I.J.; Dafou, D.; Sims, A.H.; Gayther, S.A. Modelling Genetic and Clinical Heterogeneity in Epithelial Ovarian Cancers. Carcinogenesis 2011, 32, 1540–1549. [Google Scholar] [CrossRef]
- Geistlinger, L.; Oh, S.; Ramos, M.; Schiffer, L.; LaRue, R.S.; Henzler, C.M.; Munro, S.A.; Daughters, C.; Nelson, A.C.; Winterhoff, B.J.; et al. Multiomic Analysis of Subtype Evolution and Heterogeneity in High-Grade Serous Ovarian Carcinoma. Cancer Res 2021, 80, 4335–4345. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, D.; Lu, N.; Luo, L. Role of the LKB1/AMPK Pathway in Tumor Invasion and Metastasis of Cancer Cells (Review). Oncol Rep 2015, 34, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissell, M.J. Organoids: A Historical Perspective of Thinking in Three Dimensions. Journal of Cell Biology 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, M.N.; Simeone, K.; Leclerc-Deslauniers, K.; Fleury, H.; Carmona, E.; Provencher, D.M.; Mes-Masson, A.M. Carboplatin Response in Preclinical Models for Ovarian Cancer: Comparison of 2D Monolayers, Spheroids, Ex Vivo Tumors and in Vivo Models. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Khashaba, M.; Fawzy, M.; Abdel-Aziz, A.; Eladawei, G.; Nagib, R. Subtyping of High Grade Serous Ovarian Carcinoma: Histopathological and Immunohistochemical Approach. J Egypt Natl Canc Inst 2022, 34. [Google Scholar] [CrossRef] [PubMed]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the Value of PCNA and Ki-67 as Markers of Cell Proliferation in Ameloblastic Tumors. Med Oral Patol Oral Cir Bucal 2013, 18. [Google Scholar] [CrossRef]
- Fernando, M.; Duijf, P.H.G.; Proctor, M.; Stevenson, A.J.; Ehmann, A.; Vora, S.; Skalamera, D.; Adams, M.; Gabrielli, B. Dysregulated G2 Phase Checkpoint Recovery Pathway Reduces DNA Repair Efficiency and Increases Chromosomal Instability in a Wide Range of Tumours. Oncogenesis 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, S.; Liu, Y.; Li, X.; Wu, J.; Sun, Y.; Liu, G. DNA Damage Response and PD-1/PD-L1 Pathway in Ovarian Cancer. DNA Repair (Amst) 2021, 102. [Google Scholar] [CrossRef] [PubMed]
- Barnaba, N.; LaRocque, J.R. Targeting Cell Cycle Regulation via the G2-M Checkpoint for Synthetic Lethality in Melanoma. Cell Cycle 2021, 20, 1041–1051. [Google Scholar] [CrossRef]
- Chen, C.P.; Yeh, C.N.; Pan, Y.R.; Huang, W.K.; Hsiao, Y.T.; Lo, C.H.; Wu, C.E. Wee1 Inhibition by MK1775 Potentiates Gemcitabine through Accumulated Replication Stress Leading to Apoptosis in Biliary Tract Cancer. Biomedicine and Pharmacotherapy 2023, 166. [Google Scholar] [CrossRef] [PubMed]
- Affatato, R.; Carrassa, L.; Chilà, R.; Lupi, M.; Restelli, V.; Damia, G. Identification of PLK1 as a New Therapeutic Target in Mucinous Ovarian Carcinoma. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Pérez-Fidalgo, J.A.; Gambardella, V.; Pineda, B.; Burgues, O.; Piñero, O.; Cervantes, A. Aurora Kinases in Ovarian Cancer. ESMO Open 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, R.N.; Norris, E.J.; Sutker, A.P.; Klotz, K.E.; Ganapathi, M.K. Targeting Aurora A Kinase (AAK) in Platinum-Resistant High Grade Serous Ovarian Cancer. Front Oncol 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.G.; Ecsedy, J.A.; Chakravarty, A.; Silverman, L.; Zhang, M.; Hoar, K.M.; Stroud, S.G.; Chen, W.; Shinde, V.; Huck, J.J.; et al. Characterization of Alisertib (MLN8237), an Investigational Small-Molecule Inhibitor of Aurora A Kinase Using Novel in Vivo Pharmacodynamic Assays. Clinical Cancer Research 2011, 17, 7614–7624. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Acevedo, A.N.; Orengo-Orengo, J.A.; Cruz-Robles, M.E.; Saavedra, H.I. Mitotic Kinases Are Emerging Therapeutic Targets against Metastatic Breast Cancer. Cell Division 2024, 19. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, K.J.; Crane, J.E.; Sak, M.; Jorgensen, C.J.; O’Donnell, J.P.; Zumbar, C.T.; Wozniak, J.A.; Salazar, C.R.; Parwani, A.V.; Lehman, N.L. Aurora-A Kinase Is Differentially Expressed in the Nucleus and Cytoplasm in Normal Müllerian Epithelium and Benign, Borderline and Malignant Serous Ovarian Neoplasms. Diagn Pathol 2021, 16. [Google Scholar] [CrossRef]
- Kim, S.; Choung, S.; Sun, R.X.; Ung, N.; Hashemi, N.; Fong, E.J.; Lau, R.; Spiller, E.; Gasho, J.; Foo, J.; et al. Comparison of Cell and Organoid-Level Analysis of Patient-Derived 3D Organoids to Evaluate Tumor Cell Growth Dynamics and Drug Response. SLAS Discovery 2020, 25, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Cody, N. AL; Zietarska, M.; Filali-Mouhim, A.; Provencher, D.M.; Mes-Masson, A.-M.; Tonin, P.N. Influence of Monolayer, Spheroid, and Tumor Growth Conditions on Chromosome 3 Gene Expression in Tumorigenic Epithelial Ovarian Cancer Cell Lines. BMC Med Genomics 2008, 1. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Histotype | FIGO Staging | CNV Status | Mutations † | p53 Immunoblot Signal | Chemotherapy Prior to Derivation |
|---|---|---|---|---|---|---|
| OVCAR8 | HGSC | TP53 (SA) # KRAS P121H ERBB2 G776V CTNNB1 E26R |
Low | N/A | ||
| OVCAR4 | HGSC | TP53 L130V # | Low | N/A | ||
| OVCAR3 | HGSC | TP53 R248Q # PIK3R1 c.1746-2A>T (SA) |
High | N/A | ||
| iOvCa182 | HGSC | IIIB | 84.74% | TP53 K132R | High | 6 cycles of carboplatin & paclitaxel; trebananib, angiopoietin inhibitor + weekly paclitaxel for 6 months |
| iOvCa195 | Mixed HGSC and Endometrioid | IV | 77.89% | TP53 E171fs*61 BRCA1 Y978* |
NS | NONE |
| iOvCa198 | HGSC | IIIB | 80.37% | TP53 Q192* PIK3CA I391M KDR Q472H |
NS | 6 cycles of carboplatin & paclitaxel |
| iOvCa246 | HGSC | IIIC | 81.09% | TP53 c.560-2A>T (SA) | NS | NONE |
| iOvCa256 | HGSC Endometrial | IIIA | 55.63% | TP53 Y220C KIT M541L |
Low | 4 cycles of carboplatin and weekly paclitaxel |
| iOvCa398 | HGSC | IC | 70.88% | n.d. | High | NONE |
| iOvCa411 | HGSC | IIIC | 67.84% | TP53 Y234H | High | 6 cycles of carboplatin & paclitaxel; 5 cycles doxorubicin; weekly taxol for 8 months; topotecan; etoposide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





