1. Introduction
Yeasts are a heterogeneous group of single cells, eukaryotic microorganisms classified in the fungi kingdom. This large group has some fascinating properties that make it particularly suitable for biological studies, including rapid growth, high production, growth on a variety of feedstocks, ability to compartmentalize reactions in organelles, easy cultivation in small vessels or large-scale bioreactors, facile product separation, resistance to infectious agents, ease of replication and mutant isolation, well-defined genetic system, and most important, a highly versatile DNA transformation system (Sherman, 2002; Barnett & Barnett, 2011). Since the 1950s, the number of described yeast species has increased every year. The importance of yeast analysis, description, and classification is highlighted in its broad-spectrum applications in biotechnology, food, environmental, and pharmaceutical fields, particularly through fermentation, brewing, baking, and as model systems for biological and biomedical research (Barnett & Barnett, 2011).
Although the number of species studied and characterized has increased, several biological, technological, and industrial studies and applications are limited to a small number of species, mostly belonging to Saccharomyces cerevisiae, Pichia pastoris, Candida utilis, and Yarrowia lipolytica. Among these, S. cerevisiae holds a prominent place and a largely exploited microbial platform in the biotechnological field owing to its well-annotated genome, availability, well-known physiological traits, ease of use, and genetic manipulability (Bilal et al., 2022). S. cerevisiae is difficult to surpass by any other yeast in many of its applications. However, despite its numerous advantages, Saccharomyces cerevisiae has several disadvantages that limit its effectiveness in certain industrial applications. One major drawback is its tendency for hypermannosylation, a process in which excessive mannose residues are added to heterologous proteins. This process can affect the stability, solubility, and functionality of these proteins, especially in pharmaceutical and biotechnology applications where precise protein structures are required. Additionally, S. cerevisiae has limitations in biomass production due to its strong Crabtree-positive characteristic, which leads it to favor fermentation over respiration, even in the presence of oxygen. This metabolic trait results in lower yields of biomass and higher ethanol production, which can be counterproductive in processes where high cell density or efficient protein production is desired. (Merico et al., 2004). These disadvantages have led to the study of other types of yeasts, some of which show great potential in industrial applications with fewer limitations. Non-conventional yeasts like Kluyveromyces lactis, Hansenula polymorpha, and Yarrowia lipolytica have also gained importance (reviewed in Castillo-Mendieta et al., 2023).
Among these non-conventional strains, the Kluyveromyces genus has lately been one of the most promising candidates recently due to its versatility and beneficial traits across various industries. Its unique metabolic capabilities make it ideal for applications in the food and beverage sector, where it contributes to fermentation processes, as well as in environmental biotechnology for its role in bioremediation. In addition, in the pharmaceutical industry, bioactive molecules are being explored in the development of pharmaceuticals. Given its extensive utility, Kluyveromyces has captured the attention of researchers and industry professionals alike, who are exploring its potential in the biotechnology sector. (Bilal et al., 2022).
2. Kluyveromyces Genus
The genus Kluyveromyces, with its significant morphological and physiological similarities to Saccharomyces, is an enlarging subject of study. Both are part of the Saccharomyces complex, a subclade of the Saccharomycetes. Unlike S. cerevisiae, the Kluyveromyces genus does not exhibit the limitations mentioned above. When producing proteins, they do not have tendency to undergo hypermannosylation, and most species of Kluyveromyces have a Crabtree-negative effect or possess an aerobic-respiring characteristic (Leonel et al., 2021), which means they do not undergo aerobic alcoholic fermentation, resulting in a much higher, more controlled biomass formation with reduced alcohol contamination (Karim et al., 2020).
Although Kluyveromyces lactis (a species of the Kluyveromyces genus) is considered a non-conventional yeast, it has gained significant popularity in various industries in recent years. Notably, it has been widely used in the dairy industry, particularly in cheese production, due to its ability to produce lactic acid from lactose efficiently and use this lactose as a carbon source (Castillo-Mendieta et al., 2023). It is also widely used in the development of lactose-free dairy products due to its high β-galactosidase activity, which enables it to break down lactose efficiently. K. lactis is also easily amenable to genetic manipulation, and a range of suitable genetic tools are already available for this yeast, thanks to the extensive research focused on this species, including the complete sequencing of its genome (Yamada et al., 2010). The genome structure and ploidy of the genus are not completely resolved, although it has been established that most species are homothallic and can divide into mating groups (Friedrich et al., 2023).
Like S. cerevisiae, K. lactis is haploid and is present in two mating types that can mate to form an unstable diploid that quickly returns to haplophase. However, this trait allows for genetic crosses and is sometimes used to combine mutants and to check the effect of double mutations (Friedrich et al., 2023).
Furthermore, its GRAS (Generally Recognized as Safe) status makes it particularly suitable to produce pharmaceuticals and food-grade proteins, as it can effectively assimilate lactose and utilize this sugar as a carbon source (Lane & Morrissey, 2010).
3. Kluyveromyces marxianus
K. marxianus genome is composed of 8 chromosomes in total including mitochondrial DNA, with 4952 genes identified (reviewed in Lertwattanasakul et al., 2022). Genomic and transcriptomic research has provided valuable insights into Kluyveromyces marxianus, with a growing repository of genome sequences for its various strains (Jeong et al. 2012; Suzuki et al. 2014; Silveira et al. 2014; Inokuma et al. 2015; Lertwattanasakul et al. 2015; Quarella et al. 2016; Schabort et al. 2016; Ortiz-Merino et al. 2018).
K. marxianus strains are primarily heterothallic, with variable ploidy status. Traditionally, this species has been considered haploid; however, recent molecular studies suggest that this is not always the case. For instance, research indicates that the widely used strain K. marxianus CBS 6566 (deposited at the Centraalbureau voor Schimmelcultures) may be diploid (Ribeiro et al., 2007; Ortiz-Merino et al., 2018). Conversely, other molecular approaches have confirmed that certain strains remain haploid. Consequently, it appears that both haploid and diploid forms of K. marxianus are prevalent in both research and industrial contexts (Lane & Morrissey, 2010). This variability offers several advantages for researchers, who can select between haploid and diploid strains based on the specific requirements of their studies. Haploid strains, with only one copy of each chromosome, have more propensity to mutations, making them ideal for genetic studies where induced changes are of primary importance. In contrast, diploid strains, possessing two copies of each chromosome, offer enhanced robustness and genetic stability, enhancing their resistance to environmental challenges. This additional stability in diploid strains provides researchers with greater confidence that their experimental results will be consistent and reliable.
Despite its immense potential across industrial, pharmaceutical, and biological applications, Kluyveromyces marxianus has often been overshadowed by its sister species. However, its unique characteristics and physiology provide it with distinct advantages, positioning it as a potential game-changer in the yeast industry.
One of the most beneficious features of K. marxianus is its ability to be isolated from a wide range of inexpensive habitats, including kefir grains, fermented dairy products, dairy industrial wastes, sugar industry effluents, plants, sisal leaves, soil, sugarcane bagasse, insects, and fruits. In addition to its versatility in being isolated from various sources, these sources are generally inexpensive and easy to obtain (Tulio, 2024).
As previously mentioned, another advantage is that K. marxianus has a Crabtree-negative effect, meaning it possesses aerobic-respiring characteristics and is unlikely to undergo aerobic alcoholic fermentation. This trait allows for the avoidance of ethanol formation as an undesirable byproduct under aerobic conditions. This characteristic is extremely useful for the large-scale biosynthesis of compounds whose products are associated with biomass formation (Bilal et al., 2022; Fonseca et al., 2008).
In addition, K. marxianus is also able to tolerate and efficiently ferment diverse sugars such as monosaccharides of six-carbon sugars (galactose, fructose, and mannose), of five-carbon sugars (xylose, xylitol, and arabinose), disaccharides (sucrose, lactose, and cellobiose), the trisaccharide raffinose, the polysaccharide inulin, and a non-fermentable carbon source of glycerol (reviewed in Lertwattanasakul et al., 2022). K. marxianus converts lignocellulosic biomass into bioethanol or other bioproducts, which helps to reduce the volume of waste that will be converted into agricultural and forestry waste and wastewater. Lignocellulose is a complex biopolymer major component of plant cell walls, made up of cellulose, hemicellulose, and lignin (Saldarriaga-Hernández et al., 2020).
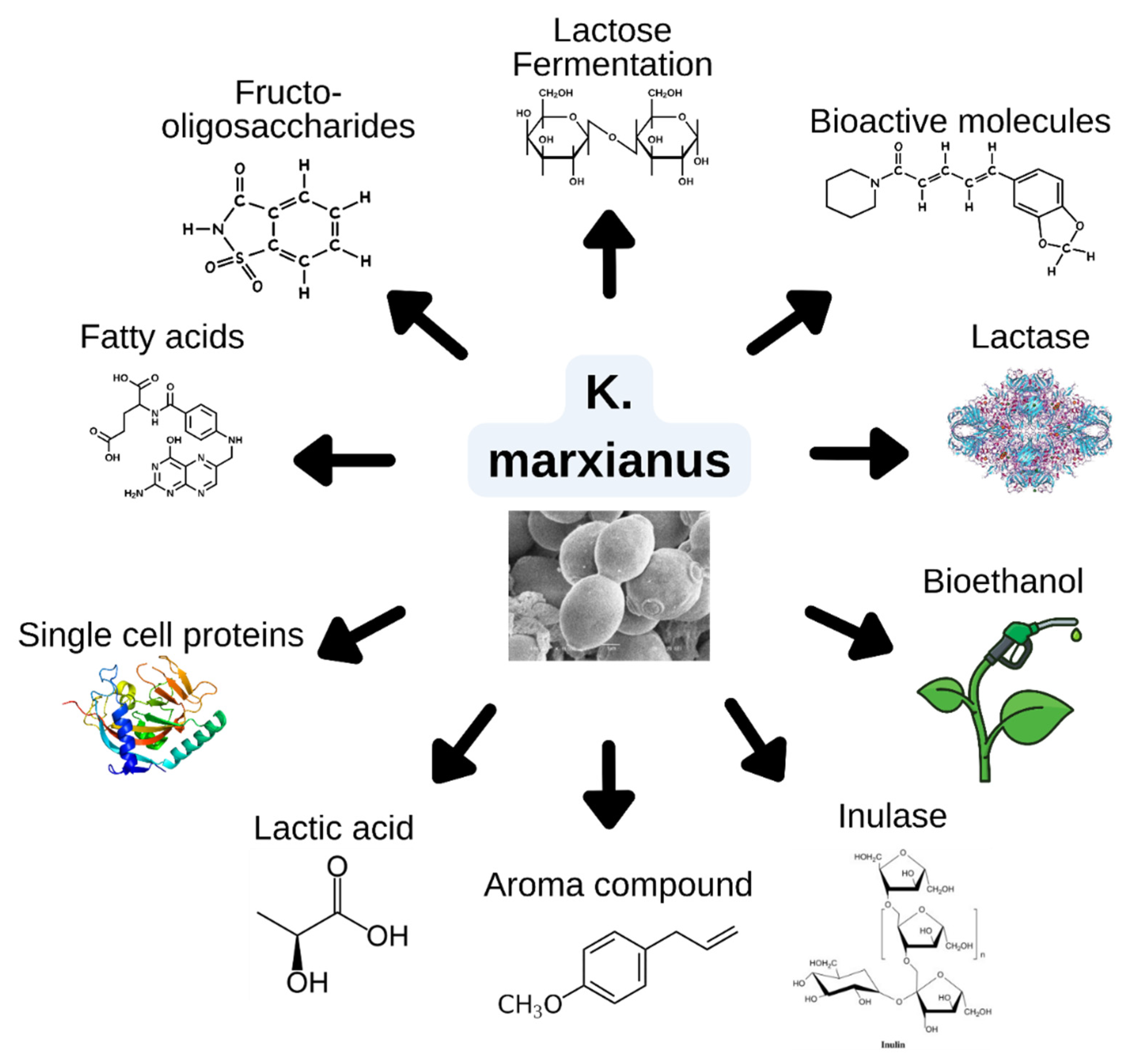
K. marxianus has acquired LAC genes, LAC12 and LAC4, which encode a permease to transport lactose molecules into the cell and a β-galactosidase to breaks down lactose into glucose and galactose. This gives it the capacity to efficiently carry out simultaneous fermentation and respiration, efficiently assimilate lactose, and utilize this sugar as a carbon source, as mentioned before. Furthermore, these polymorphisms enhance its ability to perform fast fermentation processes, which is particularly beneficial for industries that require rapid conversion of substrates, such as in the production of bioethanol or bioproducts from lactose-rich by-products like whey.
Moreover, K. marxianus is a respiratory-fermentative organism, meaning that it can generate energy through two distinct pathways depending on environmental conditions. It can produce energy through the tricarboxylic acid cycle via oxidative phosphorylation when oxygen is available, or it can switch to fermentation, producing ethanol under anaerobic or low-oxygen conditions (Lane & Morrissey, 2010). This metabolic versatility is particularly advantageous in industrial settings, as it allows for adaptation to fluctuating environmental conditions, such as oxygen availability or nutrient limitations. For example, under aerobic conditions, K. marxianus can efficiently produce biomass and byproducts, such as organic acids or enzymes, while under anaerobic conditions, it can switch to ethanol production, which is valuable for the biofuel industry.
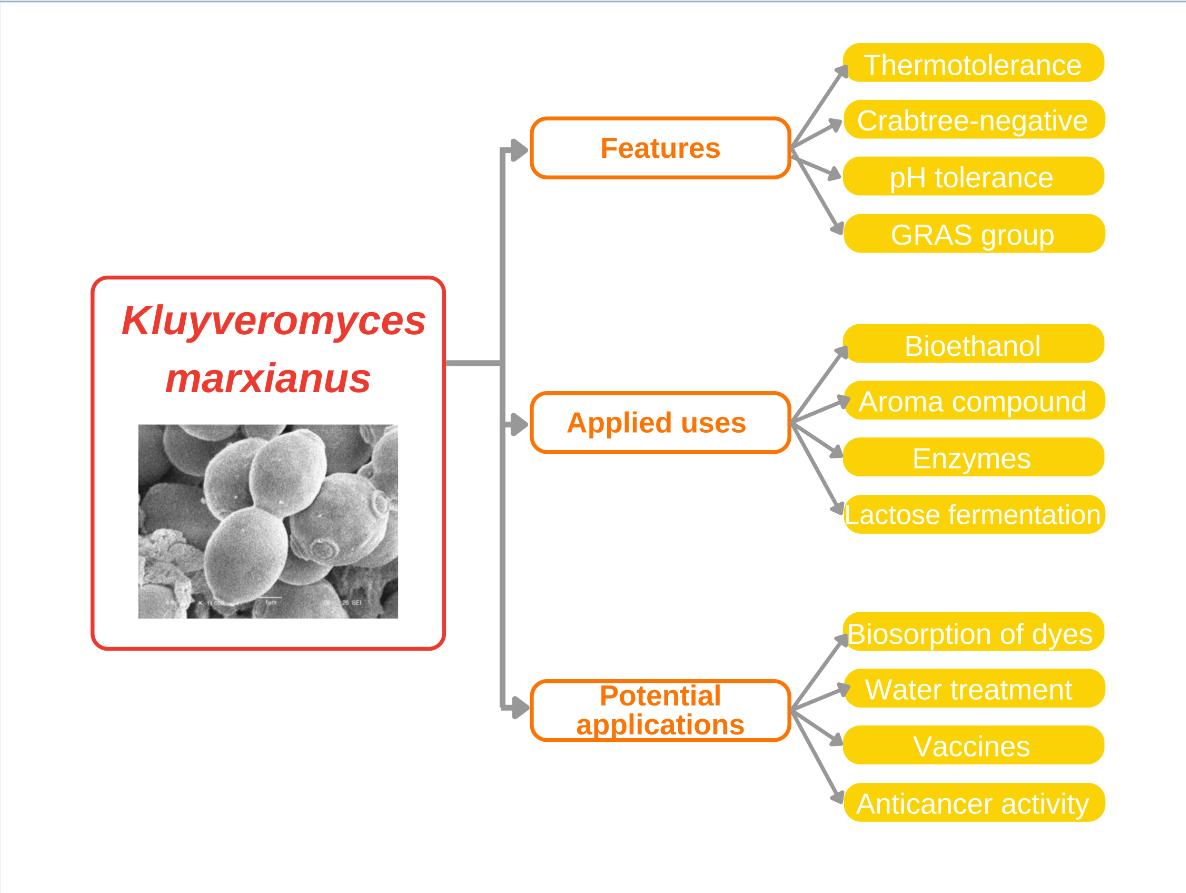
In previous studies, K. marxianus has shown a remarkable ability to grow at elevated temperatures. It withstands conditions from 40 °C to 50 °C growing faster than any eukaryote, up to 0.99/h at 40 °C. In addition, it exhibits strong pH tolerance, thriving in a wide range of pH values. These capabilities not only reduce cooling costs and reduce the risk of contamination, but also make K. marxianus a versatile option for use in various environments and industrial processes (Rajkumar & Morrissey, 2020). The thermal tolerance of K. marxianus is particularly useful for high tropical temperatures, where traditional yeast strains such as Saccharomyces cerevisiae struggle. This trait also opens the possibility for simultaneous saccharification fermentation (SSF). Saccharification is a process in which enzymes break down complex carbohydrates in biomass into simple sugars, such as glucose. In parallel, microorganisms ferment these simple sugars and convert them into desired products, such as ethanol. Both steps occur in the same reactor, preventing the buildup of glucose and other sugars that could inhibit the enzymes. This process is used in bioethanol production, leading to enzymatic hydrolysis at higher temperatures with the possibility of coupling and improving efficiency.
Flexibility extends beyond the biofuel industry to dairy fermentation, enzyme production, and chemical applications because of its viability under industrial conditions with the risk of contamination will occur, especially in large-scale operations.
Even when brought to the attention of researchers recently, K. marxianus has been shown to belong to the GRAS category (Generally Recognized as Safe) and is recognized by the FDA for pharmaceutical and food use (Fonseca et al., 2007). This designation builds on its safety for human consumption, which allows for its integration into food biotechnology, including the manufacture of fermented products such as cheese and yogurt, as well as its use in nutraceuticals and probiotics (Shruthi et al., 2022).
Despite the large number of mentioned features, several useful features remain to be discovered. Therefore, it is essential to identify and understand knowledge gaps related to genes, metabolic pathways, key enzymes, and regulatory mechanisms to gain a comprehensive understanding of how K. marxianus produces relevant metabolites (Karim et al., 2022). Fortunately, thanks to advances in research on K. marxianus, applications that take advantage of its unique features have been implemented in high-impact areas.
4. Implemented Applications of Kluyveromyces marxianus
Due to its useful features,
K. marxianus is currently used in industries for a wide variety of applications (
Figure 1). As mentioned before, the versatile
K. marxianus can be isolated from a wide range of low-cost habitats and, combined with its inherent ability to produce enzymes, rapidly converts this yeast to a native enzyme producer (Bilal et al., 2022). Currently, the most produced enzymes are inulinases, which hydrolyzes a plant fructan called inulin and is not commonly found in other yeasts or fungi, β-galactosidases which lead to lactose degradation, and pectinases, which have been used in the juice and wine manufacturing because of their ability to degrade the cell wall- (Karim, 2020; Lane & Morrissey, 2010; Y. Qiu et al., 2023).
Nowadays, K. marxianus is an aroma compound producer, achieving high product yields. K. marxianus is being exploited in this area due to the need to find new, cheaper ways to synthesize aroma compounds. It can produce significant amounts of 2-phenyl ethanol —the compound responsible for rose aroma; ethyl decanoate, used to provide wines with unique and complex fruit flavors—; benzaldehyde —utilized in dyes, fragrances, and pharmaceuticals—; and 2,3-butanediol —printing inks, perfumes, fumigants—. (Wittmann et al., 2002; Morrissey et al., 2017; Chreptowicz et al., 2018; Fabre et al., 1998).
Another industry K. marxianus application is the production of bioethanol by fermentation of polyfructan substrates, hexoses, and lignocelluloses (Castillo-Mendieta et al., 2023) (cheap, abundant, and renewable) substrates, making it of great interest in second-generation biofuels. Currently, it is synthesized at high temperatures to avoid the costs of freezing and possible contamination and to carry out saccharification and fermentation simultaneously (Karim et al., 2020; Belem et al., 1998). The thermotolerance of K. marxianus can reach these high temperatures. In addition, the ability of K. marxianus to grow in low-cost substrates and the high performance of the product that this yeast leaves makes it a better option to produce bioethanol (Hernández-Mendoza et al., 2024, Park et al., 2015).
5. Potential Biotechnological Applications
The remarkable attributes of
Kluyveromyces marxianus underscore its potential as a versatile and innovative alternative to traditionally used yeasts, extending far beyond its current applications. This section explores the ongoing research efforts that are shaping a future full of possibilities (
Figure 2).
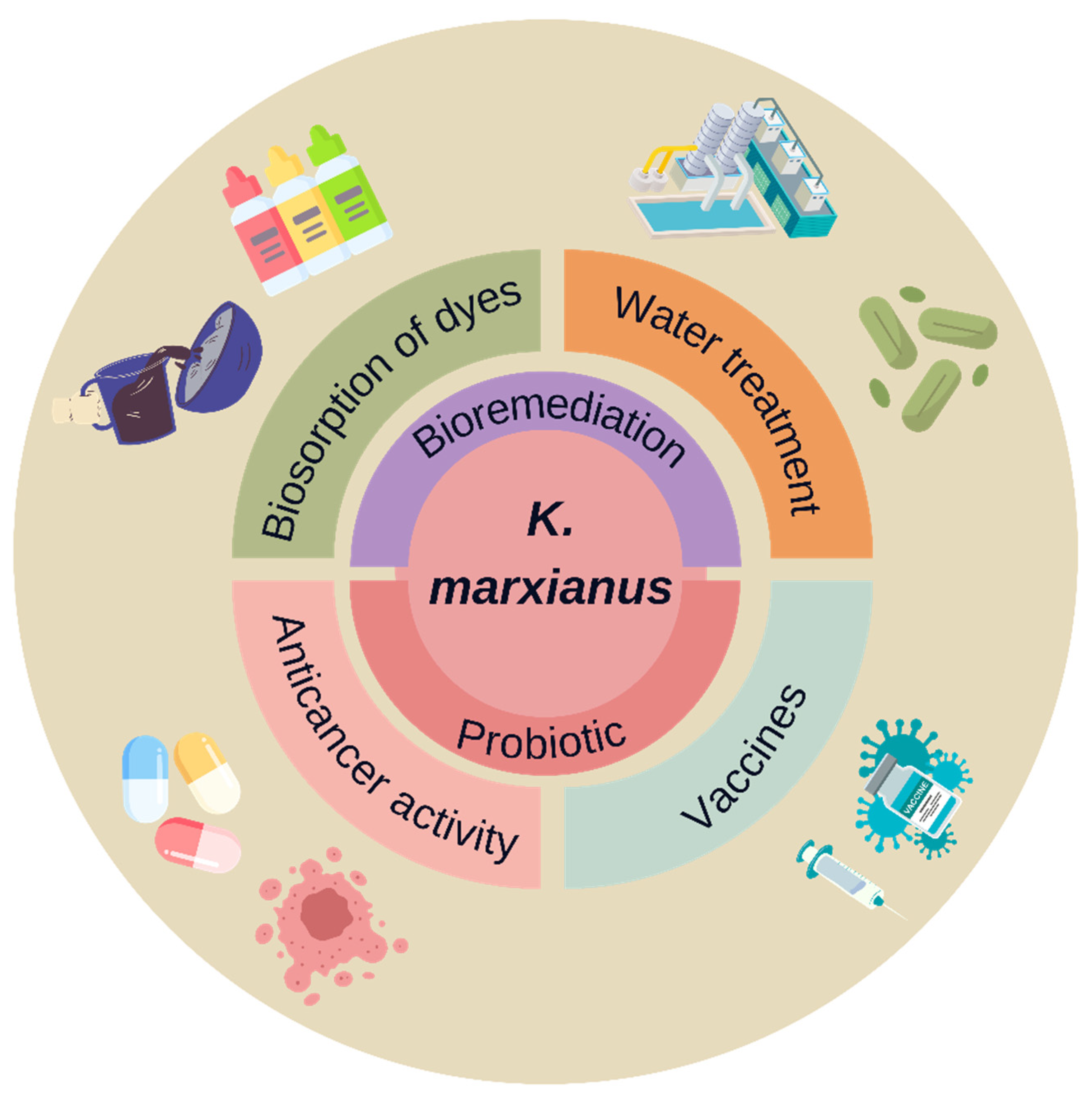
5.1. Anticancer Activity
Cancer is a class of diseases characterized by uncontrolled cell growth and the ability to spread or metastasize to all bodies. In recent years, remarkable progress has been made in understanding the proposed characteristics of cancer development, dreams, and treatment modalities (Baskar et al., 2014). Early treatments of cancer generally rely on the induction of apoptosis -a process that initiates programmed cell death in cancer cells. Conventional approaches that make use of chemotherapy and radiotherapy have been associated with toxicity because they often kill not only the target cancerous cells but also healthy cells (Zhang et al., 2024). This could result in severe side effects that may affect the general well-being of a patient. Because of these facts, one of the active research areas in this field involves studying anticancer agents that would have specific effects on cancerous cells while minimizing the damage to other non-cancerous tissues to finally develop safer and more precise therapies for cancer patients.
Some yeasts have shown promising potential in cancer treatment. For instance, an isolated K. marxianus AS41 strain from traditional dairy products has shown significant apoptotic effects on various epithelial cancer cell lines (Saber et al., 2017). The metabolites secreted by this particular K. marxianus AS41 strain significantly decreased cancer cell viability, with minimal adverse effects on normal epithelial cell lines. An upregulation of genes related to apoptotic pathways, both intrinsic and extrinsic, was observed (Saber et al., 2017). The same characteristics were found in secretions from another K. marxianus YAS secretions (a strain isolated from yogurt) which showed similar anticancer activity on colon cancer cells. Also, they had no significant cytotoxic effects on the epithelial normal cell line.
Another example is the cell wall polysaccharides of K. marxianus CCT7735, a strain from the Tropical Culture Collection (CCT) that showed cell proliferation activity in a macrophage cell line. They were able to activate macrophages and stimulate the inflammatory response against cancer cells. The antiproliferative and anticancerous effect of polysaccharides varies with cell type. This observation may be attributed to the differences in the receptors present on different cell types that interact with different types of polysaccharides (Galinari et al., 2018).
K. marxianus has a high level of (1→3)-β-D-glucan and (1→6)-β-D-glucan in its cell wall, which is associated with important biological activities, such as the inhibition of tumor growth in vivo and antiproliferative effects on cancer cells in vitro, this is the case of ATCC 10022, a K. marxianus strain isolated from plant material (Fortin et al., 2017; Alcázar et al., 2016). (1→3)-β-D-glucan and (1→6)-β-D-glucan have previously been identified as effective in macrophage activation and inhibiting cell proliferation in cancer studies. In addition, it was able to induce the activity of quinone reductase, an enzyme that plays an important role in cellular defense against cancer.
The promising findings described in this section suggest the potential application of K. marxianus strains derived from natural sources as adjuvants in cancer treatment. This underscores the need for further research into the therapeutic use of this non-conventional yeast, developing of novel drugs targeting specific apoptotic genes. Such investigations could unlock exciting new possibilities in the field of cancer therapy.
5.2. Antipathogenic activity
The antipathogenic activity of some microorganisms is particularly useful in the industry making the use of synthetic chemicals unnecessary. There is a need to investigate new and more effective microorganisms with such properties. For instance, K. marxianus AS41 showed significant antimicrobial activity against several pathogens such as Salmonella spp, Candida albicans, Listeria monocytogenes and some Bacilli (Homayouni-Rad et al., 2020). Additionally, research on K. marxianus S-2-05, a strain isolated from a French cheese, inhibited Salmonella typhimurium and induced downregulation of its chromosomal sopD gene, which is involved in epithelial cell invasion and promotes plasma membrane scission (Ceugniez et al., 2017).
K. marxianus may play a role in preventing bacterial infections, which is crucial for intestinal health and food safety. This characteristic may be related to its ability to modulate host immune responses. K. marxianus can influence the production of pro-inflammatory cytokines, which could help strengthen the body’s defenses against pathogens (Shruthi et al., 2022).
On the other hand, Smith and collaborators showed that K. marxianus CBS1553 (another strain kept in the Centraalbureau voor Schimmelcultures) induced a rapid Transepithelial Electrical Resistance (TER) increase of 50 % from baseline levels in response to Salmonella stimulation (Smith et al., 2015). K. marxianus CBS1553 was able to delay the Salmonella-induced TER disruption significantly, suggesting that this yeast can protect human epithelial cells from pathogen invasion.
Moreover, K. marxianus showed a high capacity to secrete lytic enzymes capable of altering the integrity of the cell walls of other yeasts, including protease and amylase. Besides, they can produce biofilms, which play an important role in the biocontrol of phytopathogenic fungi by inhibiting mycelial growth in vitro. Additionally, K. marxianus showed antagonistic activity in vitro against the germination of spores and the growth of the mycelium of the phytopathogenic fungus (Dos Santos et al., 2024).
K. marxianus serves in defense against bacteria that affects a variety of species. An example is K. marxianus XZ1, an industrially isolated strain, which lowered the stimulation of Penicillium expansum—a pathogen that affects plants, animals, and humans who consume the affected fruit—to inhibit the growth of this fungus. In addition, it stimulates the activity of catalase, peroxidase, polyphenol oxidase, chitinase, and phenylalanine ammonia-lyase to participate in the defense of the fruit against P. expansum (Zheng, 2023; Geng et al., 2011; Gao & Daugulis, 2009).
According on new investigations the observed results indicate that the yeast K. marxianus is a potential agent for the biological control of pathogenic fungi with applications in intestinal health and infection prevention postharvest fruit preservation. Making it a promising candidate for use in medicine and the food industry (Homayouni-Rad et al., 2020; Shruthi et al., 2022).
5.3. Probiotic
In general terms, probiotics are defined as live microorganisms that confer a health benefit to the host. In more detail, probiotics are food or pharmaceutical preparations containing non-pathogenic live microorganisms that enhance the beneficial function of the normal gastrointestinal microbiota. (Feldmann, 2012; Tullio, 2024). These include Lactobacillus, Clostridia, Bifidobacterium, Enterococcus, Faecalibacterium, and Propionibacterium (Altieri, 2016). Although scientists have discovered many probiotic yeasts, growing interest in the investigation of new nonconventional yeasts has started to appear (Bilal et al., 2022).
Probiotic microorganisms are expected to be safe and effective, non-pathogenic and non-toxic, susceptible to antibiotics and antifungals, with high antimicrobial activities, and resistant to low pH and bile salts due to their ability to modify adhesion, cellular immunity, and human intestinal microbiota. Some K. marxianus strains meets all these characteristics (Bilal et al., 2022; Homayouni-Rad et al., 2020; Romanin et al., 2015; Qiu et al., 2023; Tullio et al., 2024). For example, K. marxianus AS41, obtained from cheese and yogurt, has shown excellent resistance to acidic pH 2.0, 0.3 % bile salts, and superior antimicrobial activity, showing a stronger activity against a Native strain of E. coli, Enterococcus faecalis PTCC 1394, and Streptococcus mutans PTCC 1683 (Saber et al., 2017).
Another example is K. marxianus VM004, a strain isolated from whey, which has a good antimicrobial capacity against Escherichia coli, Salmonella typhimurium, Serratia sp., and Salmonella sp. This strain also presented resistance in gastrointestinal tract (GIT) conditions and had some beneficial probiotic properties in vitro for animal feed (Díaz-Vergara et al., 2017).
Additionally, the strain, K. marxianus B0399, a kefir isolated strain, reportedly has good GIT adhesion to human enterocyte-like Caco-2 cells and modifies immune function. It controls the inflammatory response by increasing or decreasing proinflammatory cytokines in the presence of inflammatory stimulation with lipopolysaccharide. This strain also maintains the colonic microbiota balance, reducing its cytotoxic potential (Maccaferri et al., 2017).
K. marxianus effects as a mineral bioavailability agent, as a preventative of intestinal diseases, and as an antagonistic agent against pathogens such as Salmonella spp, Candida albicans, Listeria monocytogenes, and some bacilli are also highlighted (Homayouni-Rad et al., 2020). It also helps the immune system by increasing the number of serum IgG and lysozyme levels, induces cell response, and exerts immunomodulatory effects. In another study, K. marxianus supplementation was beneficial for feed efficiency, host immunity, and intestinal structure of broiler chickens. This strain optimized the host immune-related indices (serum IgG and lysozyme levels), and improved intestinal microbial structure, which may contribute to the improved feed efficiency and intestinal structure of broiler chickens (Wang et al., 2017).
K. marxianus also has antioxidant, and hypocholesterolemic characteristics (Karim et al., 2020). For instance, K. marxianus M3 isolated from Tibetan mushrooms can reduce serum triglyceride, total cholesterol, Low-Density Lipoprotein Cholesterol LDL-C levels, and atherogenic index (a biomarker that assesses the risk of cardiovascular disease and atherogenicity) in rats (Xie et al., 2015). In another study, K. marxianus PTCC 5195 was used to produce antioxidant and ACE-inhibitory peptides from the yeast protein hydrolysates (Mirzaei et al., 2017). For another instance, some novel antihypertensive peptides released by K. marxianus from bovine lactoferrin have been identified as an anti-oxidative stress agent (García-Tejedor et al., 2014). To give another example, K. marxianus CIDCA 8154, a strain isolated from kefir, shows anti-inflammatory and anti-oxidative stress properties in in vivo models. It shows that pretreatment of epithelial cells with this strain reduces the levels of intracellular reactive oxygen species (Romanin et al., 2015).
5.4. Vaccines
K. marxianus several advantageous traits, including its rapid growth, the ability to create high biomass yields, and a capacity to perform post-translational modifications make this yeast efficient and economical for the expression of recombinant proteins, allowing efficient production of antigens in a short period. K. marxianus is presented as a highly promising option for vaccine production, mainly the generation of virus-like particles (VLPs). Particles that mimic viruses without containing genetic material (Bilal et al., 2022). Also, K. marxianus is associated with high immunogenicity, meaning that VLPs can induce robust immune responses without the risk of pathogenicity.
Yang and his collaborators demonstrated the natural ability of K. marxianus to assemble these VLPs easily and cheaply, as seen in studies where it outperformed other systems such as E. coli and insect cells. Yeast is emerging as a promising host for large-scale production of virus-like particles (VLPs) – safe and practical alternatives to inactivated viruses as a vaccine that can promote a strong protective immune response but without the risk of disease – of porcine parvovirus type 2 (PPV2). In addition to this, spleen lymphocyte proliferation and cytokine appearance were shown, suggesting that PPV VLPs produced by K. marxianus elicited cellular and humoral immune responses in mice. The results showed that VLPs produced in this system not only have a high yield but are also immunogenic (Yang et al., 2021).
Another study evaluated the efficacy of two types of subviral particle-based vaccines (SVPs) to protect chickens against avian bursa disease virus (IBDV), specifically against the novel strain FJ-18. SVPs vaccines of the VP2 protein with a His tag (nvHVP2-SVPs) were found to provide 100 % protection against infection. SVPs were produced using K. marxianus, ensuring a high level of biosecurity, as subunit vaccines do not contain viral genetic material. These findings suggest that the use of nvHVP2-SVPs could be an effective strategy for vaccination against IBDV, contributing to reducing economic losses in the poultry industry (Chen et al., 2018).
Furthermore, K. marxianus’s status as GRAS by the FDA and Qualified Presumption of Safety (QPS) by the EFSA reinforces its suitability for applications in animal health (Yang et al., 2021). The ability of K. marxianus to generate vaccines that are not only safe, highly effective, and cheap due to its accessible substrates represents a significant advance in the fight against infectious diseases in humans and livestock. This makes K. marxianus a key player in the future of vaccine development, where it can not only increase production capacity but also open the door to new immunological strategies, thus contributing to animal and public health, as well as industries and their efficiency.
5.5. Bioremediation and Water Treatment
Dairy companies discharge their wastewater into fresh water, which leads to a severe ecological imbalance. These wastewaters have high chemical oxygen demand (COD) and biochemical oxygen demand (BOD), making them severe pollutants (Karim et al., 2020). Due to this, there is a great need to explore alternative methods of efficient and inexpensive treatment of this water and reduce environmental impact.
Part of these contamination is due to increase of sugars in fresh water, K. marxianus has great potential to help bioremediate wastewater from the dairy industry due to its ability to produce lactase and β-galactosidase enzymes, which are responsible for breaking down lactose into glucose and galactose. These sugars can then be metabolized by the yeast, thereby reducing the contamination of the water. K. marxianus NRRLY-610 (a strain kept in the Northern Regional Research Laboratory (NRRL), has been shown to effectively remove lactic acid and other organic compounds, such as acetic acid, ethanol, and residual sugars (Hang et al., 2003). Lactose in these waters is one of the major causes of elevated COD. Its removal directly reduces the pollutant load in the wastewater. Inefficient management of these waters has led to discharges into water bodies and agricultural fields, generating environmental problems such as eutrophication and soil degradation. In experiments, K. marxianus was able to reduce wastewater COD up to 78.94 %, indicating its potential to improve water quality significantly. The ability of K. marxianus to grow at high temperatures and across large pH ranges extends its suitability for various environmental conditions in wastewater treatment, improving the prospects for large-scale deployments.
Besides, K. marxianus decreases COD and BOD, and the biomass produced can be valorized for further uses, such as bioenergy or animal feed. This production represents a sustainable approach towards the circular economy whereby this waste from the dairy industry can be valorized into value-added byproducts with lessened environmental impact and heightened economic value. Moreover, lactose degradation by yeast, alongside other organic pollutants, prevents eutrophication-a process whereby excess nutrients in water bodies lead to excessive algal bloom growth, an undesired event responsible for oxygen consumption and deterrence of aquatic life. Besides helping to restore the ecological balance, K. marxianus contributes to reducing organic loads in wastewater, thus decreasing costs related to conventional wastewater treatments. Thus, K. marxianus is an economically viable environmental-friendly solution that is offered to industries for their compliance issues with environmental regulations and their footprint reduction.
K. marxianus is used in dairy industry waste management which is an integrated strategy that reduces contaminants and produces valuable biomass at the same time. Thus, it is a means to reduce environmental pollution through waste reduction and achieve zero waste through the recycling of industrial byproducts into useful products. Consequently, these principles favor sustainability and environment-protecting practices. K. marxianus also recently proved successful in converting wastes from the dairy industry to bioethanol, indicating that this microorganism could diversify the field concerning bioremediation and recovery of resources from wasted organic materials
5.6. Biosorption
Biosorption is a technique that uses living or dead biological materials to bind and concentrate pollutants from water, especially those not easily biodegradable, such as metals and dyes (Vijayaraghavan et al., 2014). Recently, this technique has been using bacteria and fungi to absorb the material found in wastewater. The composition of the cell wall of K. marxianus, contains glucan and chitin, which have several functional groups – hydroxyl (-OH), amino (-NH2), and acetylamino (-NHCOCH3) – with a high affinity for metal ions and other contaminants make K. marxianus have biosorption properties. However, although the living cell has these properties, its biosorption ability rate depends on its cell metabolism. For this reason, the ability of residual biomass from K. marxianus to function as a biosorbent has been examined. In addition, the dead cell retains the cell wall and all its characteristics. This feature, coupled with several properties, such as its rapid growth and low cost, makes this yeast a great option for research and applications in biosorption.
Agroindustrial activities and several others, such as mining, pollute water and the ecosystems that rely on it. This polluted water can also be treated thanks to K. marxianus (Lane & Morrissey, 2010), which contains heavy metals such as Cu, Pb, and Cd. This biotechnological method offers a more economical and less energy-intensive solution than traditional physicochemical methods. K. marxianus has shown high efficiency for copper bioaccumulation and the removal of Pb2+ and Cd2+ ions, both as independent contaminants and in the mixture, which is why it represents a viable solution for the decontamination of wastewater and the prevention of eutrophication (Nicula et al., 2023).
Another industry that generates a high level of pollution is the textile industry, which uses inks that will be discharged into large bodies of water to contaminate them later. Their pollution potential stems from the possible toxicity and carcinogenicity posed by such dyes due to components such as benzidine and other aromatic compounds (Meehan et al., 2000). Some articles have shown the potential that this yeast absorbs these dyes from water. For example, K marxianus IMB3 can eliminate between 78 % and 98 % of the Remazol Black-B dye — a water-soluble, synthetic, azo dye commonly used in the textile industry to dye fibers such as cotton, polyester, and others — in different concentrations (25-200 mg/L) under aerobic conditions (Meehan et al., 2000). In other dyes, it has also presented favorable results, especially in Remazol Turquoise Blue, which manages to absorb 98 mg/g of dry biomass, and Remazol Red with 68 mg/g of dry biomass. However, perhaps due to their composition or chemical structure, some dyes, such as Cibacron Orange, only manage to be absorbed at a rate of 8.5 mg/g (Bustard et al., 1998).
The ability of residual biomass from the thermotolerant ethanol-producing yeast strain Kluyveromyces marxianus IMB3 to function as a biosorbent for uranium has been examined. It was found that the biomass had an observed maximum biosorption capacity of 120 mg U/g dry weight of biomass (Bustard et al., 1997). This capacity showed an increase as the temperature increased. Nevertheless, it was also affected by the decrease in pH, causing its biosorption capacity to decrease. Uranium crystals were shown to be concentrated on the outer surface of the cell wall; this agrees with the feature of its cell wall mentioned above, although uranium deposition was also observed in the interior of the cell. This result is consistent with the significant potential for the removal of textile dyes, metals and other contaminants from K. marxianus.
6. Advancing Biotechnological Methods and Synthetic Biological Parts
Kluyveromyces marxianus is one organism that has been very popular in biotechnology, since it is rapidly growing, thermotolerant, and can utilize different carbon sources. However, the utility of this organism in metabolic engineering is currently limited because of the lack of genetic tools, very few auxotrophic markers, and a narrow range of promoters. Because of the arrival of CRISPR-Cas9 and CRISPRi (CRISPR interference) technologies, gene editing in K. marxianus has greatly improved, allowing for precise gene knockouts and targeted gene suppression (Lobs et al., 2017; Lobs et al., 2018; Rajkumar & Morrissey, 2022). The SlugCas9-HF variant, an extremely high-fidelity CRISPR-Cas9 variant, reduces off-target effects and allows the efficient integration of large gene modules (up to 12 kb), as well as simultaneous integration at multiple loci with high efficiency (Zhou et al., 2024).
6.1. Development of Synthetic Biological Parts
The Kit of Kluyveromyces marxianus (KmK), established by Rajkumar and co-workers in The Kit of Kluyveromyces marxianus (KmK), established by Rajkumar and co-workers in 2019, is a set of standardized synthetic biological parts for K. marxianus. Such a toolbox provides a wide variety of promoters and terminators specifically tailored for the optimization and precise activity modulation of gene expression in varied conditions (Rajkumar et al., 2019). This collection includes promoters from K. marxianus CBS6556: PGK1, PDC1, ENO1, and TDH1, and the TDH3 promoter from K. marxianus NBRC1777. Terminators from genes like INU1 and LAC4 are also included. The KmK employed the Yeast Toolkit (YTK) standard utilizing Golden Gate assembly and CRISPR/Cas9 technologies for metabolic engineering and synthetic biology applications.
Kumar and colleagues further extended the genetic toolkit by identifying and cloning strong native promoters and terminators in K. marxianus NBRC1777. Transcriptomic analysis indicated that uncharacterized proteins YDR524C-B and YDR134C promoters drive highly increased protein expression, with the latter exhibiting strain levels two times higher than those driven by the known strong TDH3 promoter (Kumar et al., 2021). They were effective as well across temperature (30 °C and 45 °C) and carbon sources (dextrose and xylose), providing further improvements in versatility for industrial applications. Novelly terminators were also shown to promote the activity of these promoters through stability contribution to mRNA and offer more efficient production of heterologous proteins (Wu et al., 2024). Besides that, promoter-terminator cassettes are also functional in S. cerevisiae, offering broader applications for protein expression and metabolic engineering.
6.2. Applications in Metabolic Engineering
The extended genetic toolkit has enabled the engineering of K. marxianus for improved performance in producing heterologous proteins (Qiu et al., 2023). The identified promoter-terminator cassettes have been used effectively in enhancing β-galactosidase (Bergkamp et al., 1993), a key enzyme in the processing of lactose, in dairy industries. They are also found to work on the Saccharomyces cerevisiae, proving their versatility in transforming different yeast species for protein expression and metabolic engineering (Lee et al., 2013; Li et al., 2022; Yang et al., 2015). Such advancements can make K. marxianus more attractive from synthetic biology and biotechnology applications in the field of industry and research.
Application of highly sophisticated genetic manipulations in K. marxianus has been significantly advanced through the use of state-of-the-art genetic tools and through the identification of very useful biological parts. The increasing variety and availability of biological parts into Kluyveromyces marxianus has augmented its prominence as a distinctive and versatile host for synthetic biology applications and is now well-established for further applications, for biofuels, biochemical production, enzyme production, dairy and pharmaceuticals.
7. Conclusions
Astonishingly varied properties of yeasts have confirmed their relevance in the areas of biotechnology, food, environment, and pharmacy. Among them all, Kluyveromyces marxianus is among the recent non-conventional yeasts whose potential has recently been hailed as a promising all-around candidate for applications. Leaving aside the Crabtree-negative metabolism, this yeast also has thermotolerance, put in the category of GRAS, from the potential interested in the bioethanol production industry through enzyme synthesis to aroma compound generation. These traits have so much attracted interest in exploring the potential from new and fresh areas of application.
Apart from its current industrial applications, K. marxianus is also likely to be useful in anticancer activity, inhibition of pathogens, probiotic activity, vaccine development, and bioremediation. Indeed, these possible applications highlight the organism’s adaptability and relevance to modern biotechnology key challenges such as sustainable development and human health. Continued research along these lines may ultimately lead to revolutionary discoveries further establishing K. marxianus as an important tool toward global challenges.
K. marxianus is thus affected by some of these limitations such as poor characterization of genetic tools, a limited number of auxotrophic markers, and a need for better constitutive and inducible promoters. But recent significant advances, including the incorporation of the CRISPR-Cas9 systems, plasmid-based technologies, and codon optimizations, have just begun to address these challenges. These innovations improve the organism’s capabilities for genetic engineering and make it more efficient as a microbial cell factory.
The research achievement on K. marxianus is becoming utilized in enormous scope into a possible future as an effective, versatile yeast for biotechnological applications. It is also future biotechnology’s pivotal organism because it would adapt to diverse industrial needs while providing sustainable and innovative solutions. Further exploration of its unique properties and improvement of genetic engineering tools will allow massive applications in the future that will have a great impact on quality of life and ultimately become part of global sustainability.
8. Future Directions
Kluyveromyces marxianus is emerging as a frontier in biotechnology, and further development will enable wide application of its benefits in industrial and scientific disciplines. Above all, the potentiality of the yeast would be unlocked further by focused research and development in the following areas:
1. Understanding Basic Mechanisms. Elucidating the underlying mechanisms which contribute to the outstanding growth rate, thermotolerance, and high protein secretion capacity of K. marxianus is extremely necessary. Dissecting genes, regulatory networks, metabolic pathways, and key enzymes will serve to establish a thorough understanding of how this yeast is able to produce valuable metabolites. That knowledge will pave the way for metabolic engineering strategies designed specifically to augment functionality when it comes to certain particular industrial applications.
2. Development of Advanced Genetic Tools. In the last decade genetic tools have been improved making K. marxianus a suitable workhorse organism for genetic applications. Advancement of genome editing technologies-such as CRISPR-Cas combined with the construction of more effective promoters, and development of novel auxotrophic markers would contribute significantly to the flexibility of engineered K. marxianus.
3. Explore Carbon Source Utilization. Extending knowledge about how K. marxianus interacts with a wide variety of carbon sources, including lignocellulosics or unconventional substrates would improve its versatility.
4. Following its metabolic adaptation under extreme conditions, such as high temperatures or low pH, or osmotic processes, will enable this organism to work in industrial environments whose demands are higher. Such a study will not only fortify its descriptor status as a good model organism but also enhance its competitiveness against other microbial cell factories.
Ultimately, Kluyveromyces marxianus harbors a future that is just within reach-to be adapted to and excel across different biotechnological domains. With synergy in the development of genetic tool and systems biology with the added thrust from synthetic biology, this versatile yeast can now be positioned to emerge as a model organism for industrial biotechnology. Clearly, addressing and solving these limitations and also tapping into unexplored potential makes K. marxianus an excellent candidate for breakthrough developments in health, sustainability, and indeed the next frontiers.
References
- Alcázar V, E.M., Arrizon G., J.P., Gschaedler M., A.C., & Lugo C., E.C. (2016). Extraction and quantification of the yeast cell wall polysaccharides (Extracción y cuantificación de los polísacaridos de la pared celular de las levaduras). e-Gnosis, 14, pp. 1-7. Available online: https://www.redalyc.org/articulo.oa?id=73048315002.
- Altieri, C. (2016). Dairy propionibacteria as probiotics: recent evidences. World Journal of Microbiology and Biotechnology, 32(10):172. [CrossRef]
- Barnett, J.A. & Barnett, L. (2011). Yeast Cytology, 1950 to 1990. In Yeast Research pp. 60-75 (eds J.A. Barnett and L. Barnett). [CrossRef]
- Baskar, R., Dai, J., Wenlong, N., Yeo, R., & Yeoh, K.W. (2014). Biological response of cancer cells to radiation treatment. Frontiers in Molecular Biosciences, 1, 24. [CrossRef]
- Belem, M.A.F., & Lee, B.H. (1998). Production of bioingredients from Kluyveromyces marxianus grown on whey: An alternative. Critical Reviews in Food Science and Nutrition, 38(7), 565–598.
- . [CrossRef]
- Bilal, M., Ji, L., Xu, Y., Xu, S., Lin, Y., Iqbal, H., & Cheng, H. (2022). Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Frontiers in Bioengineering and Biotechnology, 10, 851768. [CrossRef]
- Bustard, M. , Donnellan, N., Rollan, A., & McHale, A.P. (1997). Studies on the biosorption of uranium by a thermotolerant, ethanol-producing strain of Kluyveromyces marxianus. Bioprocess Engineering, 17(1), 45–50. [CrossRef]
- Bustard, M., McMullan, G., & McHale, A.P. (1998). Biosorption of textile dyes by biomass derived from Kluyveromyces marxianus IMB3. Bioprocess Engineering, 19(6), 427–430. [CrossRef]
- Castillo-Mendieta, K., Arias, J., & Gonzales-Zubiate, F.A. (2023). Upgrading non-conventional yeasts into valuable biofactories. In Biotechnology-Biosensors, Biomaterials and Tissue Engineering Annual Volume 2023. IntechOpen. [CrossRef]
- Ceugniez, A., Coucheney, F., Jacques, P., Daube, G., Delcenserie, V., & Drider, D. (2017). Anti-Salmonella activity and probiotic trends of Kluyveromyces marxianus S-2-05 and Kluyveromyces lactis S-3-05 isolated from a French cheese, Tomme d’Orchies. Research in Microbiology, 168(6), 575–582. [CrossRef]
- Chen, P., Zhang, L., Chang, N., Shi, P., Gao, T., Zhang, L., & Huang, J. (2018). Preparation of virus-like particles for porcine circovirus type 2 by YeastFab assembly. Virus Genes, 54(2), 246–255. [CrossRef]
- Chreptowicz, K., Sternicka, M.K., Kowalska, P.D., & Mierzejewska, J. (2018). Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Letters in Applied Microbiology, 66(2), 153–160. [CrossRef]
- Díaz-Vergara, L., Pereyra, C.M., Montenegro, M., Pena, G.A., Aminahuel, C.A., & Cavaglieri, L.R. (2017). Encapsulated whey–native yeast Kluyveromyces marxianus as a feed additive for animal production. Food Additives & Contaminants: Part A, 34(5), 750–759. [CrossRef]
- Dos Santos, A.M., Albuini, F.M., Barros, G.C., Pereira, O.L., da Silveira, W.B., & Fietto, L.G. (2023). Identification of the main proteins secreted by Kluyveromyces marxianus and their possible roles in antagonistic activity against fungi. FEMS Yeast Research, 23, foad007. [CrossRef]
- Fabre, C.E., Blanc, P.J., & Goma, G. (1998). Production of 2-phenylethyl alcohol by Kluyveromyces marxianus. Biotechnology Progress, 14(2), 270–274. [CrossRef]
- Feldmann, H. (2012). Yeast: Molecular and Cell Biology: Second Edition. Yeast: Molecular and Cell Biology: Second Edition. [CrossRef]
- Fonseca, G. G., Gombert, A. K., Heinzle, E., & Wittmann, C. (2007). Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Research, 7(3), 422–435. [CrossRef]
- Fonseca, G.G., Heinzle, E., Wittmann, C., & Gombert, A.K. (2008). The yeast Kluyveromyces marxianus and its biotechnological potential. Applied Microbiology and Biotechnology, 79(3), 339–354. [CrossRef]
- Fortin, O., Aguilar-Uscanga, B., Vu, K.D., Salmieri, S., & Lacroix, M. (2018). Cancer chemopreventive, antiproliferative, and superoxide anion scavenging properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. Boulardii cell wall components. Nutrition and Cancer, 70(1), 83–86. [CrossRef]
- Friedrich, A., Gounot, J.-S., Tsouris, A., Bleykasten, C., Freel, K., Caradec, C., & Schacherer, J. (2023). Contrasting Genomic Evolution Between Domesticated and Wild Kluyveromyces lactis Yeast Populations. Genome Biology and Evolution, 15(2).
- . [CrossRef]
- Galinari, É., Almeida-Lima, J., Macedo, G. R., Mantovani, H. C., & Rocha, H. A. O. (2018). Antioxidant, antiproliferative, and immunostimulatory effects of cell wall α-D-mannan fractions from Kluyveromyces marxianus. International Journal of Biological Macromolecules, 109, 837–846. [CrossRef]
- Gao, F., & Daugulis, A. J. (2009). Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by Kluyveromyces marxianus. Biotechnology and Bioengineering, 104(2), 332–339. [CrossRef]
- García-Tejedor, A., Sánchez-Rivera, L., Castelló-Ruiz, M., Recio, I., Salom, J.B., & Manzanares, P. (2014). Novel antihypertensive lactoferrin-derived peptides produced by Kluyveromyces marxianus: Gastrointestinal stability profile and in vivo angiotensin I-converting enzyme (ACE) inhibition. Journal of Agricultural and Food Chemistry, 62(7), 1609–1616. [CrossRef]
- Geng, P., Chen, S., Hu, M., Rizwan-ul-Haq, M., Lai, K., Qu, F., & Zhang, Y. (2011). Combination of Kluyveromyces marxianus and sodium bicarbonate for controlling green mold of citrus fruit. International Journal of Food Microbiology, 151(2), 190–194. [CrossRef]
- Jeong, H., Lee, D.-H., Kim, S.H., Kim, H.-J., Lee, K., Song, J.Y., Kim, B.K., Sung, B.H., Park, J.C., Sohn, J.H., Koo, H.M., & Kim, J.F. (2012). Genome sequence of the thermotolerant yeast Kluyveromyces marxianus var. marxianus KCTC 17555. Eukaryotic Cell, 11:1584-1585. [CrossRef]
- Hang, Y.D., Woodams, E.E., & Hang, L.E. (2003). Utilization of corn silage juice by Klyuveromyces marxianus. Bioresource technology, 86(3), 305-307. [CrossRef]
- Hernández-Mendoza, A.G., Ruiz, H.A., Ortiz-Ceballos, Á.I., Castro-Luna, A.A., Láinez, M., & Martínez-Hernández, S. (2024). Ethanol production from Agave salmiana leaf juices by consolidated bioprocessing comparing two strains of Kluyveromyces marxianus. Industrial Crops and Products, 208, 2024, 117839. [CrossRef]
- Homayouni-Rad, A., Azizi, A., Oroojzadeh, P., & Pourjafar, H. (2020). Kluyveromyces marxianus as a Probiotic Yeast: A Mini-review. Current Nutrition & Food Science, 16(8), 1163-1169. [CrossRef]
- Inokuma, K., Ishii, J., Hara, K.Y., Mochizuki, M., Hasunuma, T., & Kondo, A. (2015). Complete genome sequence of Kluyveromyces marxianus NBRC1777, a nonconventional thermotolerant yeast. Genome Announcements, 3:10.1128/genomea.00389-15. [CrossRef]
- Karim, A., Gerliani, N., & Aïder, M. (2020). Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. International Journal of Food Microbiology, 333, 108818. [CrossRef]
- Kumar, P., Sahoo, D.K., & Sharma, D. (2021). The identification of novel promoters and terminators for protein expression and metabolic engineering applications in Kluyveromyces marxianus. Metabolic Engineering Communications, 12, 2021, e00160.
- . [CrossRef]
- Lane, M.M., & Morrissey, J.P. (2010). Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biology Reviews, 24(1-2), 17-26. [CrossRef]
- Lee, K.S., Kim, J,S., Heo, P., Yang, T.-J., Sung, Y.-J., Cheon, Y., Koo, H.M., Yu, B.J., Seo, J.-H., Jin, Y.-S., Park, J.C. & Kweon, D.-H. (2013). Characterization of Saccharomyces cerevisiae promoters for heterologous gene expression in Kluyveromyces marxianus. Appl Microbiol Biotechnol 97, 2029-2041. [CrossRef]
- Leonel, L.V., Arruda, P.V., Chandel, A.K., Felipe, M.G.A., & Sene, L. (2021). Kluyveromyces marxianus: a potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Critical Reviews in Biotechnology, 41(8), 1131-1152. [CrossRef]
- Lertwattanasakul, N., Kosaka, T., Hosoyama, A., Suzuki, Y., Rodrussamee, N., Matsutani, M., Murata, M., Fujimoto, N., Suprayogi, T.K., Limtong, S., Fujita, & N., Yamada, M. (2015). Genetic basis of the highly efficient yeast Kluyveromyces marxianus: complete genome sequence and transcriptome analyses. Biotechnol Biofuels 8(1):47. [CrossRef]
- Lertwattanasakul, N., Nurcholis, M., Rodrussamee, N., Kosaka, T., Murata, M., & Yamada, M. (2022). Kluyveromyces marxianus as a Platform in Synthetic Biology for the Production of Useful Materials. In: Darvishi Harzevili, F. (eds) Synthetic Biology of Yeasts. Springer, Cham. [CrossRef]
- Lobs, A.-K., Engel, R., Schwartz, C., Flores, A., & Wheeldon, I. (2017). CRISPR–Cas9-enabled genetic disruptions for understanding ethanol and ethyl acetate biosynthesis in Kluyveromyces marxianus. Biotechnol. Biofuels 10, 164. [CrossRef]
- Lobs, A.-K., Schwartz, C., Thorwall, S., & Wheeldon, I., (2018). Highly multiplexed CRISPRi repression of respiratory functions enhances mitochondrial localized ethyl acetate biosynthesis in Kluyveromyces marxianus. ACS Synth. Biol. 7, 2647-2655. [CrossRef]
- Maccaferri, S., Klinder, A., Brigidi, P., Cavina, P., & Costabile, A. (2012). Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Applied and environmental microbiology, 78(4), 956-964. [CrossRef]
- Meehan, C., Banat, I. M., McMullan, G., Nigam, P., Smyth, F., & Marchant, R. (2000). Decolorization of Remazol Black-B using a thermotolerant yeast, Kluyveromyces marxianus IMB3. Environment international, 26(1-2), 75-79. [CrossRef]
- Merico, A., Capitanio, D., Vigentini, I., Ranzi, B. M., & Compagno, C. (2004). How physiological and cultural conditions influence heterologous protein production in Kluyveromyces lactis. Journal of Biotechnology, 109(1–2), 139–146. [CrossRef]
- Mirzaei, M., Mirdamadi, S., Ehsani, M. R., & Aminlari, M. (2018). Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. Journal of Food and Drug Analysis, 26(2), 696–705. [CrossRef]
- Morrissey, J.P., Varela, J.A., Gethins, L., Stanton, C., & Ross, P. (2017). Applications of Kluyveromyces marxianus in biotechnology. Yeast Diversity in Human Welfare, 439–453. [CrossRef]
- Nicula, N.O., Lungulescu, E.M., Rîmbu, G.A., Marinescu, V., Corbu, V.M., & Csutak, O. (2023). Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency. International Journal of Environmental Research and Public Health, 20(6). [CrossRef]
- Ortiz-Merino, R.A., Varela, J.A., Coughlan, A.Y., Hoshida, H., da Silveira, W.B., Wilde, C., Kuijpers, N.G.A., Geertman, J.-M., Wolfe, K.H., & Morrissey, J.P. (2018). Ploidy variation in Kluyveromyces marxianus separates dairy and non-dairy isolates. Frontiers Genetics 9. [CrossRef]
- Park, J.-B., Kim, J.-S., Jang, S.-W., Hong, E., & Ha, S.-J. (2015). The Application of Thermotolerant Yeast Kluyveromyces marxianus as a Potential Industrial Workhorse for Biofuel Production. KSBB Journal, 30(3), 125–131. [CrossRef]
- Qiu, Y., Lei, P., Wang, R., Sun, L., Luo, Z., Li, S., & Xu, H. (2023). Kluyveromyces as promising yeast cell factories for industrial bioproduction: From bio-functional design to applications. Biotechnology Advances, 64, 108125. [CrossRef]
- Quarella, S., Lovrovich, P., Scalabrin, S., Campedelli, I., Backovic, A., Gatto, V., Cattonaro, F., Turello, A., Torriani, S., Felis, G.E. (2016). Draft genome sequence of the probiotic yeast Kluyveromyces marxianus fragilis B0399. Genome Announcements 4:10.1128/genomea.00923-16. [CrossRef]
- Rajkumar, A.S., Varela, J.A., Juergens, H., Daran, J.-M.G., Morrissey, J.P. (2019). Biological Parts for Kluyveromyces marxianus Synthetic Biology. Frontiers in Bioengineering and Biotechnology, 7, 2019. [CrossRef]
- Rajkumar, A.S., & Morrissey, J.P. (2020). Rational engineering of Kluyveromyces marxianus to create a chassis for the production of aromatic products. Microbial Cell Factories, 19(1), 1-19. [CrossRef]
- Rajkumar, A.S., & Morrissey, J.P. (2022). Protocols for marker-free gene knock-out and knock-down in Kluyveromyces marxianus using CRISPR/Cas9, FEMS Yeast Research, 22, 1, 2022, foab067. [CrossRef]
- Ribeiro, O., Gombert, A.K., Teixeira, J.A., & Domingues, L. (2007). Application of the Cre-loxP system for multiple gene disruption in the yeast Kluyveromyces marxianus. Journal of Biotechnology, 131(1), 20–26. [CrossRef]
- Romanin, D.E., Llopis, S., Genovés, S., Martorell, P., Ramón, V.D., Garrote, G.L., & Rumbo, M. (2016). Probiotic yeast Kluyveromyces marxianus CIDCA 8154 shows anti-inflammatory and anti-oxidative stress properties in in vivo models. Beneficial Microbes, 7(1), 83–93. [CrossRef]
- Saber, A., Alipour, B., Faghfoori, Z., & Yari Khosroushahi, A. (2017). Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. Journal of Functional Foods, 34, 408–421. [CrossRef]
- Saldarriaga-Hernández, S., Velasco-Ayala, C., Flores, P.L.I., de Jesús Rostro-Alanis, M., Parra-Saldivar, R., Iqbal, H.M., & Carrillo-Nieves, D. (2020). Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. International journal of biological macromolecules, 161, 1099-1116. [CrossRef]
- Schabort, D.T.W., Letebele, P.K., Steyn, L., Kilian, S.G., du Preez, J.C. (2016) Differential RNA-seq, multinetwork analysis and metabolic regulation analysis of Kluyveromyces marxianus reveals a compartmentalised response to xylose. PLoS ONE, 11(6):1-31. [CrossRef]
- Sherman, F. (2002). Getting started with yeast, Editor(s): Christine Guthrie, Gerald R. Fink, Methods in Enzymology, Academic Press, 350, 2002, pp. 3-41, ISBN 9780121822538. [CrossRef]
- Shruthi, B., Deepa, N., Somashekaraiah, R., Adithi, G., Divyashree, S., & Sreenivasa, M. Y. (2022). Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnology Reports, 34, 2022, e00716. [CrossRef]
- Silveira, W.B., Diniz, R.H., Cerdán, M.E., González-Siso, M.I., Souza, R.A., Vidigal, P.M., Brustolini, O.J., de Almeida Prata, E.R., Medeiros, A.C., Paiva, L.C., Nascimento, M., Ferreira, E.G., Dos Santos, V.C., Bragança, C.R., Fernandes, T.A., Colombo, L.T., Passos, F.M. (2014). Genomic sequence of the yeast Kluyveromyces marxianus CCT 7735 (UFV-3), a highly lactose-fermenting yeast isolated from the Brazilian dairy industry. Genome Announcements 2:10.1128/genomea.01136-14. [CrossRef]
- Smith, I. M., Baker, A., Arneborg, N., & Jespersen, L. (2015). Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar Typhimurium. Letters in Applied Microbiology, 61(5), 491–497. [CrossRef]
- Suzuki, T., Hoshino, T., & Matsushika, A. (2014). Draft genome sequence of Kluyveromyces marxianus strain DMB1, isolated from sugarcane bagasse hydrolysate. Genome Announcements 2:10.1128/genomea.00733-14. [CrossRef]
- Tullio, V. (2024). Probiotic Yeasts: A Developing Reality? Journal of Fungi (Basel, Switzerland), 10(7), 489. [CrossRef]
- Vijayaraghavan, K., & Yun, Y.S. (2008). Bacterial biosorbents and biosorption. Biotechnology advances, 26(3), 266-291. [CrossRef]
- Wang, W., Li, Z., Lv, Z., Zhang, B., Lv, H., & Guo, Y. (2017). Effects of Kluyveromyces marxianus supplementation on immune responses, intestinal structure and microbiota in broiler chickens. PLoS ONE, 12(7), e0180884. [CrossRef]
- Wittmann, C., Hans, M., & Bluemke, W. (2002). Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast, 19(15), 1351-1363. [CrossRef]
- Wu, P., Mo, W., Tian, T., Song, K., Lyu, Y., Ren, H., Zhou, J., Yu, Y., & Lu, H. (2024). Transfer of disulfide bond formation modules via yeast artificial chromosomes promotes the expression of heterologous proteins in Kluyveromyces marxianus. mLife. 2024; 3: 129-142. [CrossRef]
- Xie, Y., Zhang, H., Liu, H., Xiong, L., Gao, X., Jia, H., Lian, Z., Tong, N., & Han, T. (2015). Hypocholesterolemic effects of Kluyveromyces marxianus M3 isolated from Tibetan mushrooms on diet-induced hypercholesterolemia in rat. Brazilian Journal of Microbiology: Publication of the Brazilian Society for Microbiology, 46(2), 389–395. [CrossRef]
- Yamada, R., Tanaka, T., Ogino, C., & Kondo, A. (2010). Gene copy number and polyploidy on products formation in yeast. Applied Microbiology and Biotechnology, 88(4), 849–857. [CrossRef]
- Yang, D., Chen, L., Duan, J., Yu, Y., Zhou, J., & Lu, H. (2021). Investigation of Kluyveromyces marxianus as a novel host for large-scale production of porcine parvovirus virus-like particles. Microbial Cell Factories, 20(1). [CrossRef]
- Yang, D., Zhang, L., Duan, J., Huang, Q., Yu, Y., Zhou, J., Lu, H., Yang, D., & Catelli, E. (2021). A Single Vaccination of IBDV Subviral Particles Generated by Kluyveromyces marxianus Efficiently Protects Chickens against Novel Variant and Classical IBDV Strains. Vaccines 2021, 9, pp. 1443, 9(12), 1443. [CrossRef]
- Zhang, Q.Y., Wang, F.X., Jia, K.K., & Kong, L.D. (2018). Natural product interventions for chemotherapy and radiotherapy-induced side effects. Frontiers in Pharmacology, 9, 415590. [CrossRef]
- Zhou, H., Tian, T., Liu, J., Lu, H., Yu, Y., & Wang, Y. (2024). Efficient and markerless gene integration with SlugCas9-HF in Kluyveromyces marxianus. Communications Biology, 7(1), 797. [CrossRef]
- Zheng, X., Zheng, L., Xia, F., Li, J., Zhou, W., Yuan, L., Rao, S., & Yang, Z. (2023). Biological control of blue mold rot in apple by Kluyveromyces marxianus XZ1 and the possible mechanisms of action. Postharvest Biology and Technology, 196, 112179. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).