Introduction
A G-quadruplex (G4) is a non-canonical secondary structure formed by a guanine-rich sequence. In a G4, four guanine bases form a plane called a G-tetrad through Hoogsteen hydrogen bonding, and multiple G-tetrads stacking on each other [
1]. G4s play crucial roles in various biological processes within cells, including gene expression regulation, DNA replication, protein translation, maintenance of genome stability, and telomere maintenance [
2,
3,
4]. They are also implicated in cancer and other diseases, highlighting their potential as therapeutic targets [
5]. Direct high-resolution capture of G4 structure formation within an intracellular chromatin context is essential for understanding these mechanisms [
6]. Several methods, combining high-throughput sequencing with G4-specific antibodies, have been proposed to yield genome-wide maps detailing on the positions and frequencies of G4 formation in living cells [
7]. The first protocol for mapping G4s intracellular, known as G4 ChIP–seq, was developed by Hänsel-Hertsch et al., using an engineered antibody called BG4 [
8]. Similarly, Zheng et al. proposed a protocol using an artificial G4 probe protein, G4P, to capture G4s in living cells through a ChIP-Seq-based approach [
9]. Moreover, Lyu et al. combined BG4 with the Tn5-based CUT&Tag procedure, establishing the first G4 CUT&Tag method for genome-wide profiling of native G4s [
10]. Collectively, these methods produce high-throughput sequencing reads across the genome, providing insights into intracellular G4s formed.
Peak calling is a crucial step in analyzing intracellular G4 sequencing data, as it identifies the regions of interest, i.e., intracellular G4 sites, by analyzing stacks of aligned reads across the genome. The peak calling process can be divided into two main steps: first, identifying regions enriched with DNA reads/fragments as candidate peaks across the entire genome, and second, subjecting these candidates to hypothesis testing to determine their statistical significance [
11]. Based on significance thresholds, candidate peaks are then ranked and filtered accordingly. However, peak calling results can be affected by factors such as experimental noise and the accessibility of target regions, which are commonly observed across datasets. To mitigate these challenges, different algorithms employ distinct strategies to optimize the two main steps of peak calling: identifying enriched regions and testing their statistical significance [
12].
Several tools are available for intracellular G4 peak calling. Most published experiments [
8,
9,
10], regardless of the antibody used (BG4 or G4P) or sequencing technique employed (ChIP-Seq or CUT&Tag), have utilized MACS2 [
13] for peak calling. Exceptions include the studies by Hui et al. [
14], which employed SEACR for analyzing G4-CUT&Tag data, and Lago et al. [
15], which applied HOMER to their G4 ChIP-Seq data. The number of peaks generated by these three algorithms is comparable, and the validity of the peaks is supported by the presence of motifs, genomic annotations, or chromatin accessibility. Notably, there are over 40 peak calling algorithms available, but only a limited number have been used in G4-related studies. Although the performance of some of these algorithms have been compared and evaluated [
11,
16,
17], their applicability to intracellular G4 sequencing data have yet to be thoroughly assessed. This is important because G-quadruplex (G4) formation depends on specific sequence motifs and is usually narrower than histone modification or transcription factor binding sites. Therefore, systematic evaluation of peak calling algorithms is essential for accurate identification of G4 structures and better understanding of their biological functions and regulatory roles.
One significant challenge for the comparison of these methods is the absence of a “gold standard” dataset for benchmarking. For intracellular G4 sequencing results, there is a lack of methods that are as credible as the Sanger sequencing is for next-generation sequencing data. Even when studying the same cell type, different antibodies (BG4 or G4P) and sequencing methods (ChIP-Seq or CUT&Tag) yield overlapping yet distinct sets of peaks [
9,
10,
18], highlighting the difficulty and complexity of establishing a standardized test.
In this study, we selected seven peak calling algorithms—MACS2 [
13], HOMER [
19], GEM [
20], SICER [
21], PeakRanger [
22], GoPeaks [
23], and SEACR [
24]—based on criteria including original publications, documentation availability, usability, maintenance status, and underlying design principles, and evaluated the operating characteristics of them on intracellular G4 sequencing data. We prepared benchmark datasets using an integration strategy for benchmarking purposes. The validity of the identified G4 peaks was assessed based on the distributions of putative G4 motifs and associated epigenetic modifications. Additionally, we compared the effects of different candidate peak identification strategies and different hypothesis testing models on G4 data analysis. By synthesizing results across these analyses, we identified the most suitable peak calling algorithms for intracellular G4 sequencing to enhance their performance.
Methods
Selection of G4 Sequencing Datasets
Six G4 sequencing datasets, referred to as 'test datasets' from the Gene Expression Omnibus (GEO) were selected to evaluate the performance of the candidate algorithms. These test datasets included two derived from the K562 cell line and four from the HEK293T cell line. Detailed characteristics of each test dataset are summarized in
Table 1. The selected test datasets encompass two distinct experimental approaches—ChIP-seq and CUT&Tag—utilizing two different antibodies, BG4 and G4P, and incorporating both paired-end and single-end library types.
Selection of Peak Calling Algorithms
Forty-three peak calling algorithms were reviewed (
Supplementary Table S1). Based on criteria including original publications, availability of documentation, usability, maintenance status, and underlying design principles, seven algorithms were selected for detailed evaluation with G4 sequencing data: MACS2, HOMER, GEM, SICER, PeakRanger, GoPeaks, and SEACR. The algorithms differ in their strategies for identifying candidate peaks and performing hypothesis testing. MACS2, HOMER, and PeakRanger utilize a sliding window approach for peak identification, while SICER and GoPeaks divide the genome into fixed intervals. For hypothesis testing, MACS2 applies a dynamic Poisson model for the distribution of sequencing reads/fragments, SICER and HOMER use Poisson models, and GoPeaks and PeakRanger employ binomial models. SEACR, which adjusts thresholds using a global background signal distribution, and GEM, which integrates binding event discovery with motif identification via probabilistic modeling, do not conform to this classification framework.
G4 Sequencing Data Analysis Pipeline
Raw sequencing data were quality-checked and adapter-trimmed using Trim Galore (v0.6.6) [
25]. Reads were aligned to the hg19 reference genome with Bowtie2 (v2.3.5.1) [
26], and alignment results were stored in BAM format using Samtools (v1.9) [
27]. Duplicate reads were removed with Picard (v2.23.4) MarkDuplicates, and genomic blacklist regions were excluded using Bedtools (v2.29.1) intersect [
28].
G4 Peak Identification and Preprocessing
To evaluate algorithm performance, peak callers were applied to six test datasets under the lowest significance thresholds to generate unfiltered candidate peaks. Specific configurations with the lowest thresholds were used for each algorithm: GEM (q=0, fold=1), MACS2 (q=1, -f=BAMPE for paired-end data), HOMER (-F=1, -P=1, -L=1, -LP=1, -C=0, -fdr=1), SICER (fdr=1), PeakRanger (q=1, p=0.999), and SEACR (relaxed mode). For GoPeaks, p-values were adjusted in the code to vary peak counts, as the algorithm does not provide p-values for each interval. Performance differences across test datasets were observed due to algorithm-specific limitations, such as GoPeaks supporting only paired-end data and SEACR requiring paired-end data with an IgG antibody control (
Supplementary Table S2). Candidate peaks identified by each algorithm were ranked by p-value or FDR. The top 10,000 high-confidence intervals were selected as G4 peaks [
18].
Construction of G4 Benchmark Datasets
The benchmark was generated by identifying the overlap of G4 peaks detected by multiple algorithms within each test dataset (
Supplementary Figure S1). For each algorithm, the peaks were ranked by their p-values or corresponding significance scores, and the top 10,000 peaks were regarded as identified peaks for that algorithm. Peaks identified by more than half of the algorithms were included in to the benchmark. The remaining ones from each algorithm were taken as algorithm-specific peaks.
For the test datasets containing multiple replicate samples, the first replicates from each test dataset were employed for construction of the benchmark, and the other replicates were used for validation of the benchmark.
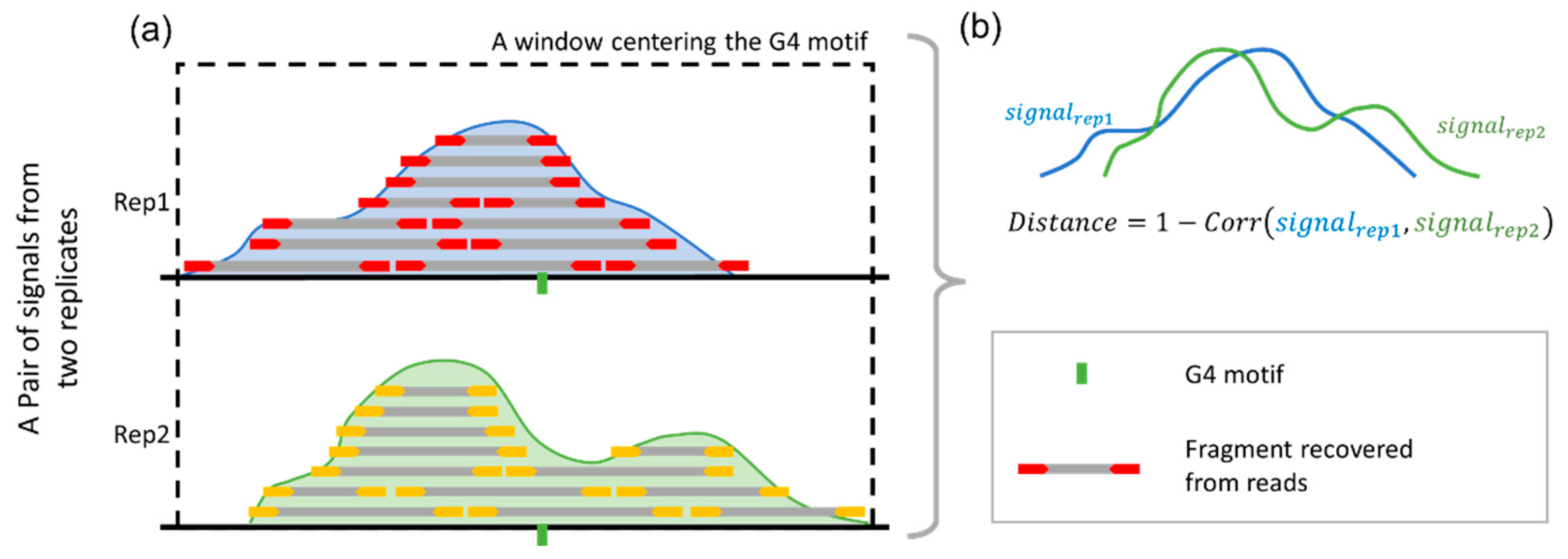
To validate the benchmark, a "distance" value was introduced to evaluate the consistency of G4 signals across replicates (
Figure 1). Here, the peaks from the benchmark were attributed to the G4 motifs nearest to the peak center. The signal values with a 1000-bp window centering these motifs, from both replicates, were saved as vectors for calculating the distance values. The distance is defined as:
where
signalrep1 and
signalrep2 represent the vectors generated within the same window from two technical replicates, and Correlation refers to the Pearson’s correlation coefficient between these two vectors. The distance value ranges from 0 to 2, where 0 indicates perfect correlation and 2 indicates no correlation. Smaller distance values reflect stronger signal consistency across replicates, suggesting that the intracellular G4 is stable and reliably present.
Besides, random groups for each test dataset were generated by utilizing signal vectors from 10,000 randomly selected windows, serving as controls.
Calculating Precision, Recall, and Harmonic Mean for Each Candidate Algorithms
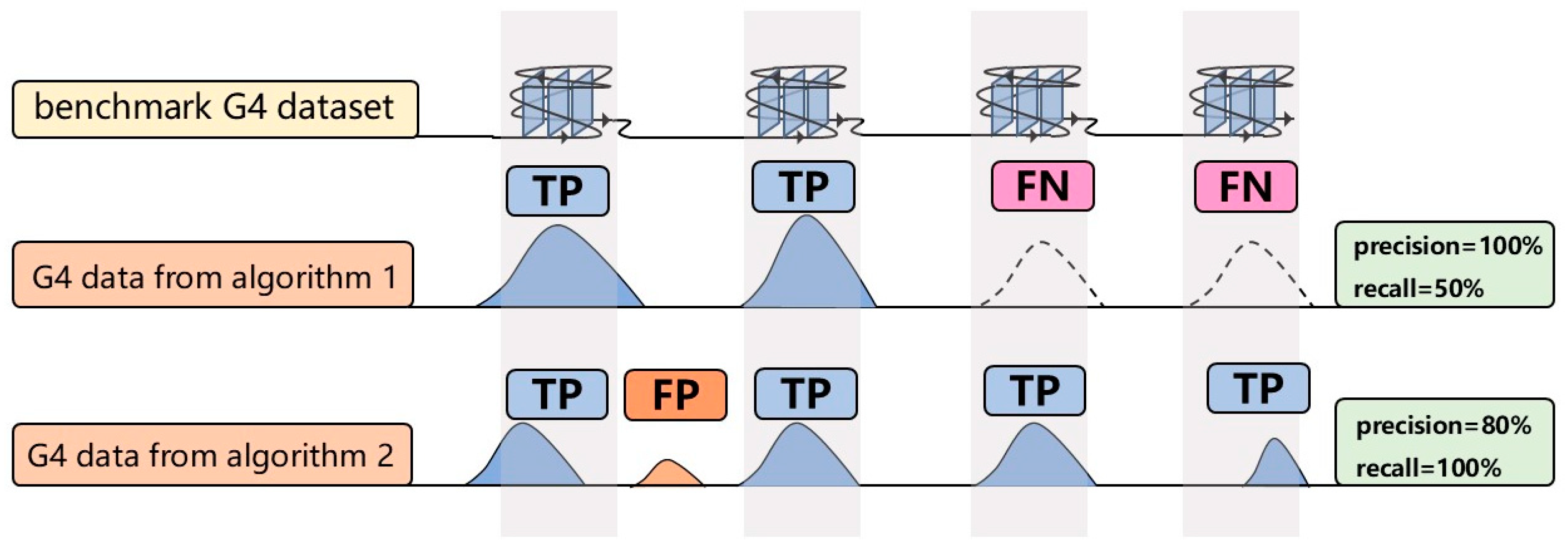
Precision and recall of G4 peaks identified by each algorithm were calculated using the G4 benchmark dataset as the reference standard. The harmonic mean (HM score) of precision and recall was employed as the overall performance metric to emphasize balanced algorithm performance. The HM score, which increases only when both precision and recall are high, provides a balanced measure of algorithm performance. Precision is defined as the proportion of overlapping base pairs between algorithm-identified G4 peaks and the benchmark dataset, while recall represents the proportion of benchmark peaks overlapping with algorithm-identified peaks (
Figure 2). The formulas are:
TP (True Positive) represents algorithm-identified G4 peaks that overlap with the benchmark dataset. FP (False Positive) refers to algorithm-identified peaks that do not overlap with the benchmark dataset, while FN (False Negative) denotes benchmark dataset that do not overlap with algorithm-identified peaks.
Proportion of pG4, oG4, and aG4 in Candidate G4 Peaks
pG4 (putative G-quadruplexes) refers to potential G4 sites predicted from genomic DNA using computational tools. In this study, pG4 sites were predicted using pqsFinder [
29]. oG4 (observed G-quadruplexes) refers to in vitro G4 sites identified through techniques like G4-seq [
30], which stabilize G4s with PDS or K+. The oG4 data were obtained from GSE63874. aG4 (active G-quadruplexes), identified by Zhang et al. [
31], represents G4 sites identified across multiple cancer cell lines, reflecting strong intracellular secondary structure formation. The proportions of G4 peaks identified by each algorithm overlapping with pG4, oG4, and aG4 were calculated using Bedtools intersect [
32].
Chromatin Accessibility Data Processing
Chromatin accessibility and histone modification data for the K562 and HEK293T cell lines were obtained from GEO (K562: GSE215595, GSE176370, GSE127009, GSE127010, GSE213909; HEK293T: GSE178668). Chromatin signals over G4 peaks were calculated using bigWigAverageOverBed (v2) [
33] and log-transformed. Signal differences across G4 peaks were analyzed using the Kruskal-Wallis test [
34].
Construction of the G4 to-Be-Tested Datasets for Hypothesis Testing Evaluation
To evaluate hypothesis testing models, including Binomial, Poisson, and dynamic Poisson distributions, a G4 to-be-tested datasets was constructed with positive samples (known G4 sites) and negative samples (non-G4 sites). Sequencing fragment counts were calculated for each genomic position, and p-values were computed based on fragment density. A threshold was applied to identify G4 peaks, and false positive rates were calculated to assess model performance. The G4 benchmark dataset was used as the positive sample set for each test dataset.
Negative samples were generated by combining all unfiltered G4 peaks identified by candidate algorithms and excluding peaks overlapping the G4 benchmark dataset, those predicted by pqsFinder or G4Hunter [
35], or these annotated by Tan et al. [
9] (GSE133379). The remaining intervals were extracted from hg19 using Bedtools getfasta and filtered to remove sequences matching the pattern
G3-5N1-7G3-5N1-7G3-5N1-7G3-5. A random selection of 10,000 intervals was used as the negative sample set. Positive and negative samples were then combined to construct the G4 to-be-tested datasets.
Reads/Fragment Distribution Models and False Positive Rate Evaluation
Three reads/fragments distribution model commonly used in hypothesis testing of peak calling algorithms—Poisson, Binomial, and dynamic Poisson distribution—were evaluated.
In the Poisson model, the number of sequencing fragments in the treatment replicate at a candidate G4 peak X follows a Poisson distribution:
where the expected value λ is the fragment counts from the control replicate at the same site.
The dynamic Poisson model is similar to the Poisson model but with a dynamic λ, followed the approach described by Zhang et al. [
13]. For the control, genomic intervals of 1kb, 5kb, 10kb, 50kb, 100kb, 500kb, and 1000kb are extended upstream and downstream of the candidate G4 peak. The average number of fragments are counted in each interval, as (
), along with the genome-wide background (
). The expected value of the fragment counts is set as
. The fragment counts in the treatment X follows the Poisson distribution:
In the binomial model, the fragment counts at the candidate G4 peak in the treatment follows a binomial distribution:
where
n is the scaled number of sequencing fragments in the control, and
p is estimated as the average fragment counts in all the candidate peak regions in the control divided by
n.
p-values were computed based on the random variable X, and the corresponding distribution. All calculations for the Poisson and Binomial models were performed using R (v4.1.0).
False positive rates were calculated as the proportion of G4 peaks identified at varying thresholds that did not overlap with known positive G4 peaks:
TP (True Positive) is the number of G4 peaks identified by the hypothesis testing models that overlap with the known positive G4 peaks dataset. FP (False Positive) is the number of identified G4 peaks that do not overlap with the positive G4 peaks dataset.
Results and Discussion
Evaluating the G4 Benchmark Dataset
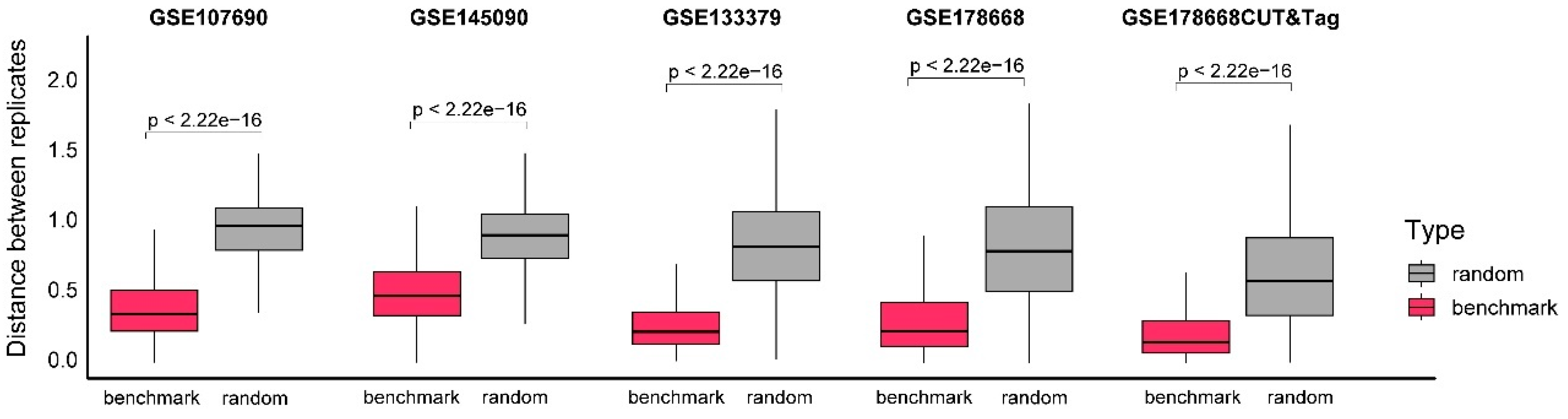
To assess the reliability of the G4 benchmark datasets established by integrating results from multiple algorithms (as described in the Methods section), we calculated the distance of sequencing signals between replicate samples at each benchmark peak. The distance value (Formula 1) measures the similarity of sequencing fragment distribution based on the correlation coefficient of the signal vectors from the two replicates within a 1000-bp window. Smaller distance values indicate greater reproducibility of G4 formations across replicates, reflecting event stability. Five of the six test datasets, containing replicate data, were included in this evaluation.
The analysis indicated that the mean distance values for the five G4 benchmark datasets ranged between 0.2 and 0.5, with two datasets showing mean values below 0.25 (
Figure 3). Compared to randomly selected windows, the benchmark datasets exhibit significantly lower distance values, with p-values less than 2.22e-16, which is the default lower limit printed by the R package. This consistent pattern of low distance values highlights the stability of G4 benchmark datasets and supports their reliability. These findings confirm that the benchmark datasets provide a robust reference for evaluating the performance of candidate algorithms in identifying G4 peaks.
Evaluating the Validity of Algorithm-Specific G4 Peaks
Since the sets of G4 peaks identified by different algorithms partially overlap, we define these overlapping regions shared by all the candidates as "common G4 peaks." After removing the common G4 peaks from the results of each algorithm, the remaining ones are referred to as "algorithm-specific G4 peaks." The common G4 peaks are intuitively reliable, as they have been validated by all the candidate algorithm. However, it is still necessary to evaluate the algorithm-specific G4 peaks for each candidate, as their validity specifically reflects the differences in performance of the candidates. Due to the previously mentioned low precision and recall based on the benchmark, as well as the inappropriate peak width and quantity, we excluded SICER and SEACR from further comparisons. The subsequent analyses focused exclusively on MACS2, HOMER, GEM, PeakRanger, and GoPeaks.
Again, pG4 sites, i.e. the G4 motifs, were utilized to validate the reliability of the G4 peaks identified by the candidate algorithms (
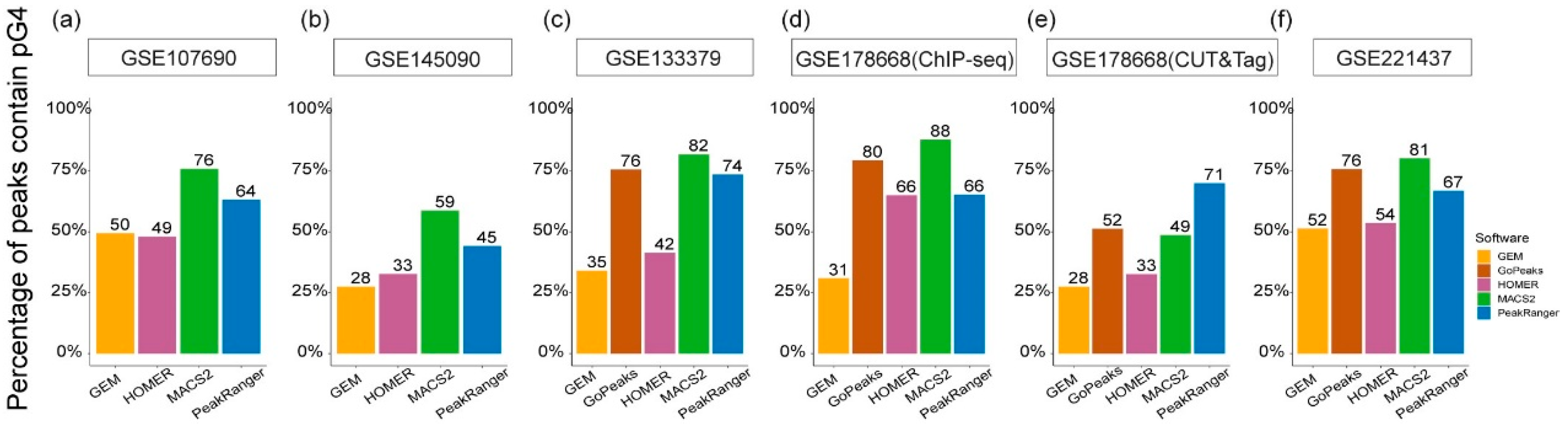
Figure 6). In four out of the six test datasets, over 70% of the MACS2-specific G4 peaks overlap with pG4 sites, and in only one test dataset, the proportion of the pG4-overlapping peaks is less than half (approximately 49%). Similarly, PeakRanger-specific G4 peaks exhibit a trend of over 60% overlap with pG4 sites in five of the six test datasets, and more than 70% of the GoPeaks-specific peaks overlap with pG4s in three of the four datasets applicable to the algorithm. In contrast, the proportions of pG4-overlapping peaks in the HOMER- and GEM-specific results are below 50% in most test datasets.
G4s exhibit a strong tendency to localize in promoters and regions characterized by high chromatin accessibility, as they are closely associated with biological processes such as transcription [
15]. Consequently, epigenetic marks that indicate chromatin state and transcription activity can also be employed as supporting materials for validating the authenticity of these algorithm-specific peaks. We employed ATAC-seq signals to assess chromatin accessibility, alongside four types of histone modifications: H3K4me1, which is usually associated with enhancers; H3K4me2 and H3K4me3, which may regulate RNA polymerase II pausing, and H3K27ac, which is generally considered a marker of active enhancers and promoters [
36].
The strength values of these five types of epigenetic signals were calculated at the algorithm-specific G4 peaks. The MACS2-, PeakRanger-, and GoPeaks-specific peaks exhibit significantly higher chromatin accessibility and histone modification signals compared to those from HOMER and GEM (
Figure 7). This indicates stronger consistency of these algorithm-specific G4 peaks with known biological characteristics of intracellular G4 functions. Consequently, the algorithm-specific G4 peaks identified by MACS2, PeakRanger, and GoPeaks are more likely to be involved in active biological processes, reinforcing their authenticity.
Overall Evaluation of the Candidate Algorithms
According to the performance evaluated using the benchmark datasets, along with validation against known pG4, oG4 and aG4 sites, as well as the validity of the algorithm-specific peaks, we recommend three methods that excel across all the factors as tools for intracellular G4 peak calling: MACS2, PeakRanger, and GoPeaks (
Table 3). In contrast, the other candidates either performed poorly on the benchmark, or had resolution unsuitable for G4 data, or generated peaks not consistent with those known G4 information or epigenetic signals, making them less suitable for the peak calling task for intracellular G4 sequencing data.
Furthermore, we aim to investigate the characteristics that might influence the performance of these algorithms on intracellular G4 sequencing data. The two stages of peak calling—identifying enriched regions as candidate peaks and evaluating their statistical significance against controls through hypothesis testing —were assessed separately. Since SEACR and GEM do not conform to this framework due to their distinct methodologies [
24,
37], our analysis was restricted to MACS2, HOMER, SICER, PeakRanger, and GoPeaks. We compared the strategies employed by these algorithms at each step to determine their impact on G4 peak identification.
In the step of candidate peak identification, there are at least two available strategies. MACS2, HOMER, and PeakRanger employ a sliding window approach to scan the genome for candidate peaks, while SICER and GoPeaks segment the genome into fixed intervals. Across all test datasets, over 96% of intracellular G4 sites were successfully identified by all five algorithms (
Supplementary Figure S1), indicating that both the sliding window and fixed-interval strategies are effective for capturing intracellular G4 sites with no discernible difference in the results between the two strategies.
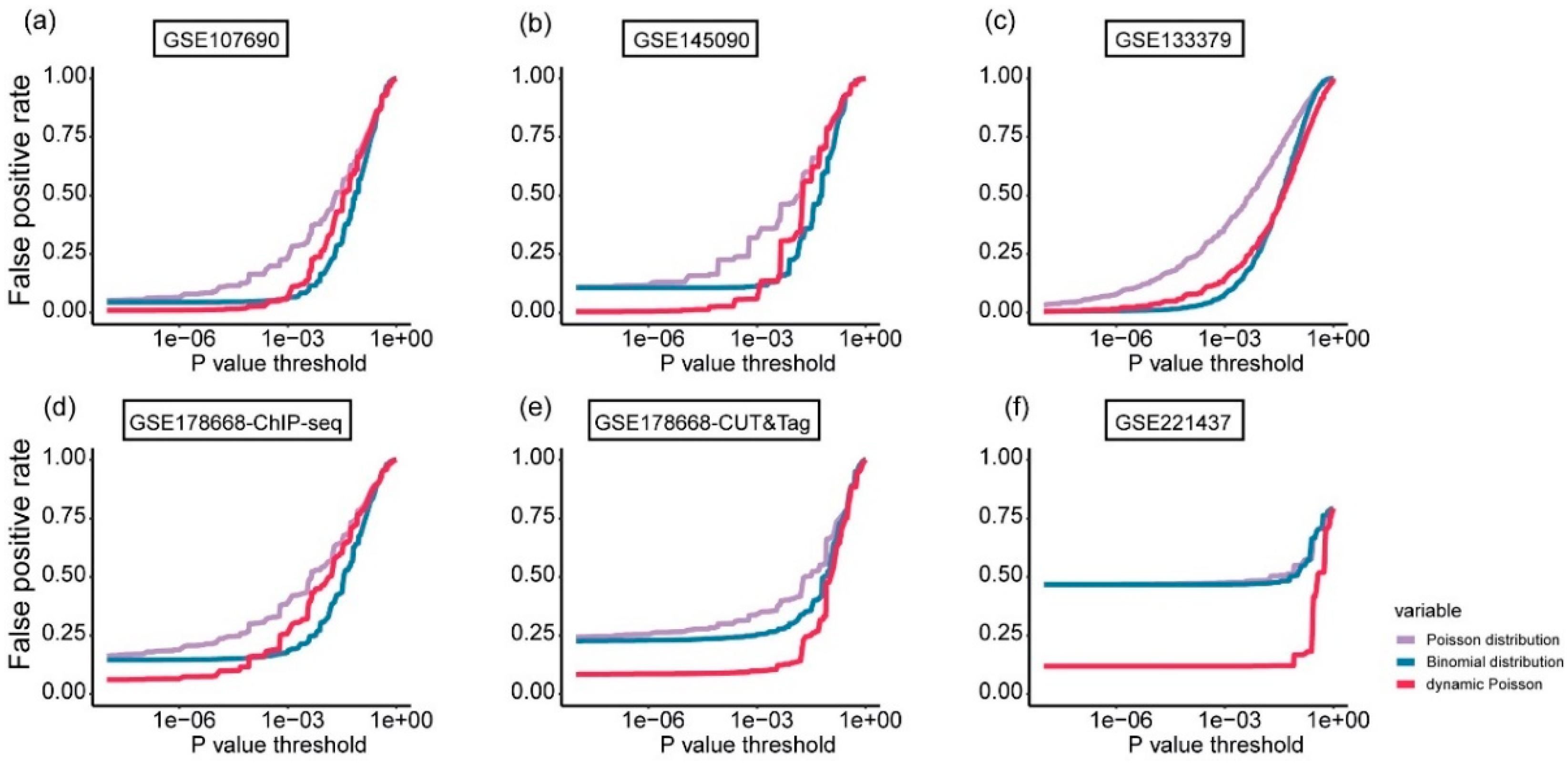
In the second step, i.e. evaluating the significance of the candidate peaks, three distribution models for the sequencing reads/fragments are available: the Poisson model employed by SICER and HOMER, the dynamic Poisson model used by MACS2, and the binomial model utilized by GoPeaks and PeakRanger. We assessed the impact of these distribution models on G4 peak identification by analyzing the false positive rates varying with p values of the hypothesis testing, across the six test datasets (
Figure 8).
The dynamic Poisson model consistently achieves the lowest false positive rates, especially when significance thresholds . This model outperforms both the Binomial and Poisson models and demonstrating its effectiveness in G4 peak identification. For most test datasets, when looser thresholds (p-value ) are applied, the binomial model outperforms the other two models. In certain cases, such as the GSE133379 dataset, it may perform comparably to the dynamic Poisson model at thresholds in certain cases, likely due to higher sequencing depth and a more uniform fragment distribution that favor the binomial assumption.
Furthermore, the Poisson model consistently produces the highest false positive rates across all test datasets and thresholds, highlighting its limitations in accurately identifying G4 peaks. These findings provide a plausible explanation of the superior performance of algorithms such as MACS2 (using the dynamic Poisson model) and GoPeaks and PeakRanger (utilizing the binomial model) compared SICER and HOMER (relying solely on the Poisson model) in G4 identification.
Conclusion
This study systematically evaluated seven peak calling algorithms—MACS2, HOMER, GEM, SICER, PeakRanger, GoPeaks, and SEACR—for intracellular G4 sequencing data. Benchmark datasets established by integrating results from multiple algorithms were generated, and their reliability was assessed based on peak similarity between replicate samples. Additionally, known G4 information, including pG4, oG4 and aG4, was employed to evaluate the results of these candidate algorithms. Furthermore, the performance of these algorithms was also assessed through the validity of the algorithm-specific peaks, by using pG4 sites and known epigenetic signals.
Among the seven candidate algorithms, SICER and SEACR generated high proportions of peaks overlapping with pG4s, oG4s, and aG4s, yet the width of these peaks was not optimal for G4 peak calling tasks. While HOMER and GEM could generate much narrower peaks, indicating higher resolution, they underperformed in other metrics. In contrast, MACS2, PeakRanger, and GoPeaks demonstrated superior performance. These three algorithms not only showed high precision and recall on benchmark datasets, but also produced a moderately high proportion of pG4-, oG4- and aG4-overlapping peaks with moderate widths. Moreover, they effectively identified biologically relevant G4 peaks enriched with known sequence motifs and other epigenetic signals. The successful application of these three algorithms to intracellular G4 data might be partially attributed to the distribution model of sequencing reads/fragments—either dynamic Poisson or binomial—employed during the hypothesis testing step of the peak calling procedures.
Our findings provide practical guidance and rationale for selecting peak caller in intracellular G4 identification, including the preparation of evaluation process as well as the characteristics of the algorithms that could be considered. These results will promote the reliable detection of functional G4 structures across the genome.
Future efforts should focus on developing G4-specific peak calling algorithms that account for the unique sequence and functional characteristics of G4s, including their narrow peak width and sensitivity to chromatin context. Incorporating models tailored to G4 folding dynamics and motif specificity could improve the accuracy of G4 peak identification, thereby facilitating a better understanding of their roles in gene regulation and genomic stability. Such advancements would not only refine G4 mapping but also promote their use as biomarkers and therapeutic targets, thereby expanding the potential applications of G4 identification in molecular biology and clinical settings.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This work was supported by the National Natural Science Foundation of China (No. 62472084 and 62002060), the Leading Technology Program of Jiangsu Province (grant number BK20222008) and the Fundamental Research Funds for the Central Universities of China (No. 2242023K5005).
References
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. 2019, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Knipscheer, P. G-quadruplex resolution: From molecular mechanisms to physiological relevance. DNA Repair 2023, 130, 103552. [Google Scholar] [CrossRef] [PubMed]
- Cave, J.W.; Willis, D.E. G-quadruplex regulation of neural gene expression. FEBS J. 2021, 289, 3284–3303. [Google Scholar] [CrossRef]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-quadruplexes: a promising target for cancer therapy. Mol. Cancer 2021, 20, 1–18. [Google Scholar] [CrossRef]
- Hänsel-Hertsch, R.; Beraldi, D.; Lensing, S.V.; Marsico, G.; Zyner, K.; Parry, A.; Di Antonio, M.; Pike, J.; Kimura, H.; Narita, M.; et al. G-quadruplex structures mark human regulatory chromatin. Nat. Genet. 2016, 48, 1267–1272. [Google Scholar] [CrossRef]
- Galli, S.; Flint, G.; Růžičková, L.; Di Antonio, M. Genome-wide mapping of G-quadruplex DNA: a step-by-step guide to select the most effective method. RSC Chem. Biol. 2024, 5, 426–438. [Google Scholar] [CrossRef]
- Hänsel-Hertsch, R.; Spiegel, J.; Marsico, G.; Tannahill, D.; Balasubramanian, S. Genome-wide mapping of endogenous G-quadruplex DNA structures by chromatin immunoprecipitation and high-throughput sequencing. Nat. Protoc. 2018, 13, 551–564. [Google Scholar] [CrossRef]
- Zheng, K.-W.; Zhang, J.-Y.; He, Y.-D.; Gong, J.-Y.; Wen, C.-J.; Chen, J.-N.; Hao, Y.-H.; Zhao, Y.; Tan, Z. Detection of genomic G-quadruplexes in living cells using a small artificial protein. Nucleic Acids Res. 2020, 48, 11706–11720. [Google Scholar] [CrossRef]
- Lyu, J.; Shao, R.; Yung, P.Y.K.; Elsässer, S.J. Genome-wide mapping of G-quadruplex structures with CUT&Tag. Nucleic Acids Res. 2021, 50, e13–e13. [Google Scholar] [CrossRef]
- Thomas, R. Features that define the best ChIP-seq peak calling algorithms. Brief Bioinform 2017, 18, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A. Normalization, bias correction, and peak calling for ChIP-seq. Stat Appl Genet Mol Biol 2012, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Model-based analysis of ChIP-Seq (MACS). Genome Biol 2008, 9, R137. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.W.I.; Simeone, A.; Zyner, K.G.; Tannahill, D.; Balasubramanian, S. Single-cell mapping of DNA G-quadruplex structures in human cancer cells. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Lago, S.; Nadai, M.; Cernilogar, F.M.; Kazerani, M.; Moreno, H.D.; Schotta, G.; Richter, S.N. Promoter G-quadruplexes and transcription factors cooperate to shape the cell type-specific transcriptome. Nat. Commun. 2021, 12, 3885. [Google Scholar] [CrossRef]
- Jeon, H. Comparative analysis of commonly used peak calling programs for ChIP-Seq analysis. Genomics Inform 2020, 18, e42. [Google Scholar] [CrossRef]
- Eder, T.; Grebien, F. Comprehensive assessment of differential ChIP-seq tools guides optimal algorithm selection. Genome Biol 2022, 23, 119. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Yin, Z.; Fang, P.; Xiao, R.; Xiang, Y.; Wang, W.; Li, Q.; Huang, B.; Huang, J.; et al. Ligand-induced native G-quadruplex stabilization impairs transcription initiation. Genome Res. 2021, 31, 1546–1560. [Google Scholar] [CrossRef]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef]
- Guo, Y.; Mahony, S.; Gifford, D.K. High Resolution Genome Wide Binding Event Finding and Motif Discovery Reveals Transcription Factor Spatial Binding Constraints. PLOS Comput. Biol. 2012, 8, e1002638. [Google Scholar] [CrossRef]
- Xu, S. Spatial clustering for identification of ChIP-enriched regions (SICER) to map regions of histone methylation patterns in embryonic stem cells. Methods Mol Biol 2014, 1150, 97–111. [Google Scholar] [PubMed]
- Feng, X.; Grossman, R.; Stein, L. PeakRanger: a cloud-enabled peak caller for ChIP-seq data. BMC Bioinformatics 2011, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Yashar, W.M.; Kong, G.; VanCampen, J.; Curtiss, B.M.; Coleman, D.J.; Carbone, L.; Yardimci, G.G.; Maxson, J.E.; Braun, T.P. GoPeaks: histone modification peak calling for CUT&Tag. Genome Biol. 2022, 23, 1–21. [Google Scholar] [CrossRef]
- Meers, M.P.; Tenenbaum, D.; Henikoff, S. Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Martin, M.J.E.j. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H. , Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Quinlan, A.R. BEDTools: The Swiss-Army Tool for Genome Feature Analysis. Curr. Protoc. Bioinform. 2014, 47, 11.12–1. [Google Scholar] [CrossRef]
- Hon, J.; Martínek, T.; Zendulka, J.; Lexa, M. pqsfinder: an exhaustive and imperfection-tolerant search tool for potential quadruplex-forming sequences in R. Bioinformatics 2017, 33, 3373–3379. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef]
- Zhang, R.; Shu, H.; Wang, Y.; Tao, T.; Tu, J.; Wang, C.; Mergny, J.-L.; Sun, X. G-Quadruplex Structures Are Key Modulators of Somatic Structural Variants in Cancers. Cancer Res. 2023, 83, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Beato, M. bwtool: a tool for bigWig files. Bioinformatics 2014, 30, 1618–1619. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Walmsley, R.P. Learning and Understanding the Kruskal-Wallis One-Way Analysis-of-Variance-by-Ranks Test for Differences Among Three or More Independent Groups. Phys. Ther. 1997, 77, 1755–1761. [Google Scholar] [CrossRef]
- Brázda, V.; Kolomazník, J.; Lýsek, J.; Bartas, M.; Fojta, M.; Šťastný, J.; Mergny, J.-L. G4Hunter web application: a web server for G-quadruplex prediction. Bioinformatics 2019, 35, 3493–3495. [Google Scholar] [CrossRef]
- Calo, E.; Wysocka, J. Modification of Enhancer Chromatin: What, How, and Why? Mol. Cell 2013, 49, 825–837. [Google Scholar] [CrossRef]
- Furey, T.S. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet 2012, 13, 840–852. [Google Scholar] [CrossRef]
Figure 1.
Procedure for validating the reproducibility of benchmark peaks across replicates. Benchmark peak (upper peak) is identified from the first replicate and associated with the nearest G4 motif (green). The second replicate is used for validation, with signal profiles from both replicates (lower peak) extracted within a ±1000 bp window around the G4 motif. The “distance” metric, calculated as 1 minus the Pearson’s correlation between the signal vectors, quantifies the consistency between replicates, with smaller values indicating greater reproducibility.
Figure 1.
Procedure for validating the reproducibility of benchmark peaks across replicates. Benchmark peak (upper peak) is identified from the first replicate and associated with the nearest G4 motif (green). The second replicate is used for validation, with signal profiles from both replicates (lower peak) extracted within a ±1000 bp window around the G4 motif. The “distance” metric, calculated as 1 minus the Pearson’s correlation between the signal vectors, quantifies the consistency between replicates, with smaller values indicating greater reproducibility.
Figure 2.
Precision and recall illustration. The first row shows the G4 benchmark dataset as the reference. The second and third rows depict G4 peaks identified by different algorithms. Blue peaks indicate true positives, orange peaks represent false positives, and dashed peaks denote false negatives.
Figure 2.
Precision and recall illustration. The first row shows the G4 benchmark dataset as the reference. The second and third rows depict G4 peaks identified by different algorithms. Blue peaks indicate true positives, orange peaks represent false positives, and dashed peaks denote false negatives.
Figure 3.
Evaluation of G4 benchmark datasets by signal distance between replicates. The x-axis represents different datasets and groups, and the y-axis represents distances between the signal vectors from two technical replicates within the same windows. Different groups, the benchmark and random, are indicated by different colors. Of the six test datasets utilized in this study, five containing technical replicates were selected for analysis, while GSE221437 was excluded from this evaluation due to the absence of replicate samples. Hypothesis testing between different groups was performed using the Wilcoxon test, and 2.22e-16 is the default lower limit printed by the R package.
Figure 3.
Evaluation of G4 benchmark datasets by signal distance between replicates. The x-axis represents different datasets and groups, and the y-axis represents distances between the signal vectors from two technical replicates within the same windows. Different groups, the benchmark and random, are indicated by different colors. Of the six test datasets utilized in this study, five containing technical replicates were selected for analysis, while GSE221437 was excluded from this evaluation due to the absence of replicate samples. Hypothesis testing between different groups was performed using the Wilcoxon test, and 2.22e-16 is the default lower limit printed by the R package.
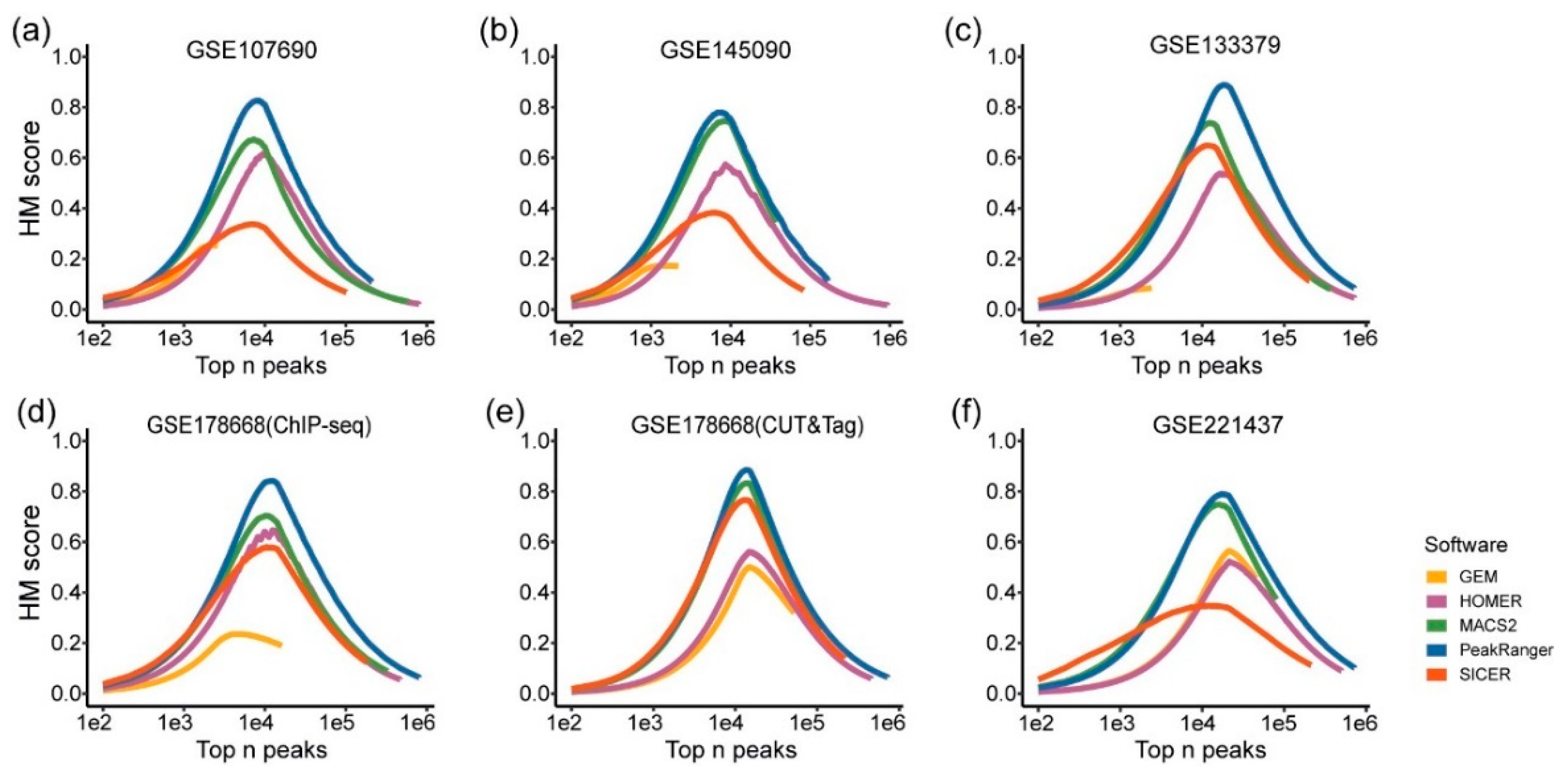
Figure 4.
Comparison of HM scores for G4 peak identified by candidate algorithms. The x-axis represents different numbers of G4 peaks, with higher values indicating a greater number of peaks. The y-axis represents the HM score, ranging from 0 to 1.0. Algorithms are indicated by different colors.
Figure 4.
Comparison of HM scores for G4 peak identified by candidate algorithms. The x-axis represents different numbers of G4 peaks, with higher values indicating a greater number of peaks. The y-axis represents the HM score, ranging from 0 to 1.0. Algorithms are indicated by different colors.
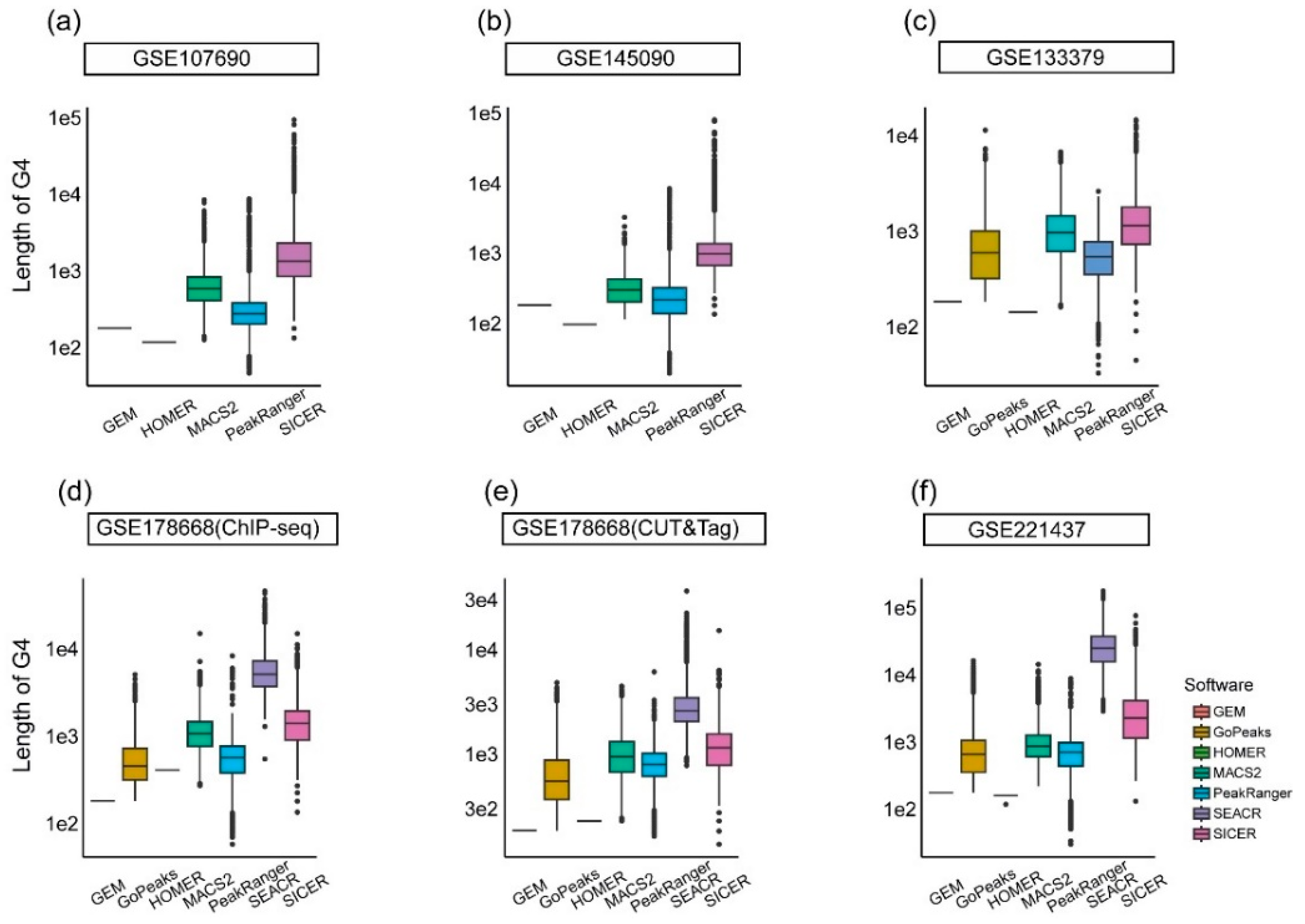
Figure 5.
Width distribution of G4 peaks identified by the candidate algorithms. The x-axis represents different algorithms, and the y-axis indicates the width of G4 peaks identified by each algorithm. (a) and (b) use test datasets with single-end sequencing data, applicable to only five of the candidate algorithms. (c) uses a test dataset compatible with six algorithms, while (d) to (f) utilize test datasets that accommodate all seven algorithms.
Figure 5.
Width distribution of G4 peaks identified by the candidate algorithms. The x-axis represents different algorithms, and the y-axis indicates the width of G4 peaks identified by each algorithm. (a) and (b) use test datasets with single-end sequencing data, applicable to only five of the candidate algorithms. (c) uses a test dataset compatible with six algorithms, while (d) to (f) utilize test datasets that accommodate all seven algorithms.
Figure 6.
Proportion of pG4 sites in algorithm-specific G4 peaks from different test datasets. The x-axis represents different algorithms, and the y-axis indicates the percentage of overlapping pG4 sites. (a) and (b) use single-end test datasets, applicable to four algorithms to be run, while (c) - (f) employ test datasets that accommodate all five algorithms.
Figure 6.
Proportion of pG4 sites in algorithm-specific G4 peaks from different test datasets. The x-axis represents different algorithms, and the y-axis indicates the percentage of overlapping pG4 sites. (a) and (b) use single-end test datasets, applicable to four algorithms to be run, while (c) - (f) employ test datasets that accommodate all five algorithms.
Figure 7.
Comparison of epigenetic signals at algorithm-specific G4 peaks across different test datasets. The x-axis represents different algorithms, and the y-axis represents the log-transformed signal values of chromatin accessibility (ATAC) or histone modifications (H3K27ac, H3K4me1, H3K4me2, H3K4me3). (a) and (b) are derived from single-end test datasets applicable to four algorithms, (c) - (f) are based on test datasets applicable to all five algorithms. The P-values displayed were calculated using the Kruskal-Wallis test to assess the statistical significance of differences among the groups identified by different algorithms.
Figure 7.
Comparison of epigenetic signals at algorithm-specific G4 peaks across different test datasets. The x-axis represents different algorithms, and the y-axis represents the log-transformed signal values of chromatin accessibility (ATAC) or histone modifications (H3K27ac, H3K4me1, H3K4me2, H3K4me3). (a) and (b) are derived from single-end test datasets applicable to four algorithms, (c) - (f) are based on test datasets applicable to all five algorithms. The P-values displayed were calculated using the Kruskal-Wallis test to assess the statistical significance of differences among the groups identified by different algorithms.
Figure 8.
a)-(f): False positive rate comparison of the hypothesis testing using the three distribution models—Poisson, binomial, and dynamic Poisson—across six test datasets. The x-axis represents the p-value, while the y-axis shows the corresponding false positive rate.
Figure 8.
a)-(f): False positive rate comparison of the hypothesis testing using the three distribution models—Poisson, binomial, and dynamic Poisson—across six test datasets. The x-axis represents the p-value, while the y-axis shows the corresponding false positive rate.
Table 1.
Summary of data characteristics for six test datasets.
Table 1.
Summary of data characteristics for six test datasets.
| GEO Accession Number |
Cell Line |
Antibody Type |
Sequencing Technique |
Library Type |
| GSE107690 |
K562 |
BG4 |
G4-ChIP-seq |
Single-end |
| GSE145090 |
K562 |
BG4 |
G4-ChIP-seq |
Single-end |
| GSE133379 |
HEK293T |
G4P |
G4P-ChIP-seq |
Paired-end |
| GSE178668 |
HEK293T |
BG4 |
G4-ChIP-seq |
Paired-end |
| GSE178668 |
HEK293T |
BG4 |
G4 CUT&Tag |
Paired-end |
| GSE221437 |
HEK293T |
BG4 |
G4 CUT&Tag |
Paired-end |
Table 2.
Proportion of pG4-, oG4-, and aG4-overlapping peaks generated by the candidate peak calling algorithms.
Table 2.
Proportion of pG4-, oG4-, and aG4-overlapping peaks generated by the candidate peak calling algorithms.
| Candidate Algorithm |
Proportion of pG4-overlapping peaks |
Proportion of oG4-overlapping peaks |
Proportion of aG4-overlapping peaks |
| SEACR |
99%-100% |
79%-100% |
83%-93% |
| SICER |
76%-98% |
53%-85% |
44%-83% |
| MACS2 |
76%-95% |
52%-78% |
54%-83% |
| PeakRanger |
66%-91% |
50%-71% |
60%-82% |
| GoPeaks |
73%-91% |
49%-72% |
58%-88% |
| HOMER |
50%-82% |
35%-61% |
54%-78% |
| GEM |
42%-69% |
29%-52% |
34%-76% |
Table 3.
Summary of performance of peak calling algorithms.
Table 3.
Summary of performance of peak calling algorithms.
| Peak calling algorithm |
Performance on benchmark |
Performance based on known G4 information |
Validity of Algorithm-specific Peaks |
| Peak width |
Proportion of overlapping peaks |
Percentage of pG4-overlaping peaks |
Consistency with epigenetic signals |
| MACS2 |
High HM score |
Moderate |
Moderately high |
High |
High |
| PeakRanger |
Highest HM score |
Moderate |
Moderately high |
High |
High |
| GoPeaks |
- |
Moderate |
Moderately high |
High |
High |
| HOMER |
Moderate HM score |
Narrow |
Low |
Low |
Low |
| GEM |
Much fewer peaks |
Narrow |
Low |
Low |
Low |
| SICER |
Moderate HM score |
Excessively high |
High |
- |
- |
| SEACR |
- |
Excessively high |
High |
- |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).