Submitted:
17 December 2024
Posted:
18 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ichthyophthirius multififiliis Isolation and Fluorescence Labeling
2.2. Pond Selection and Collection of Water Samples
2.3. In-Situ Predation Experiment
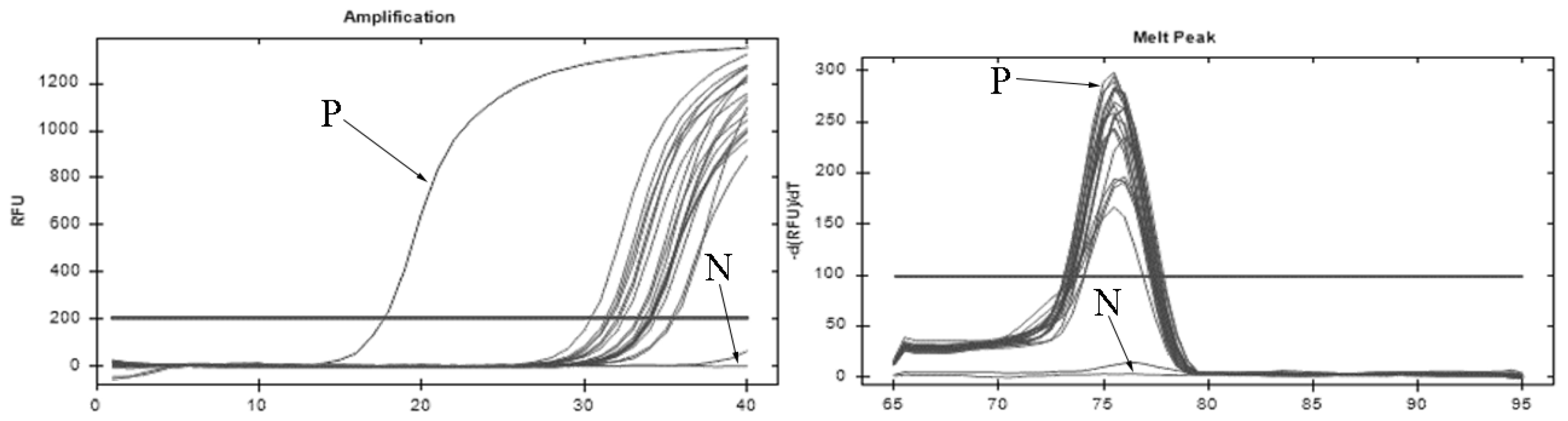
2.4. DNA Extraction and PCR Amplification
2.5. DNA Metabarcoding Assay of Zooplankton Diversity
2.6. Data Statistics and Analyses
3. Results
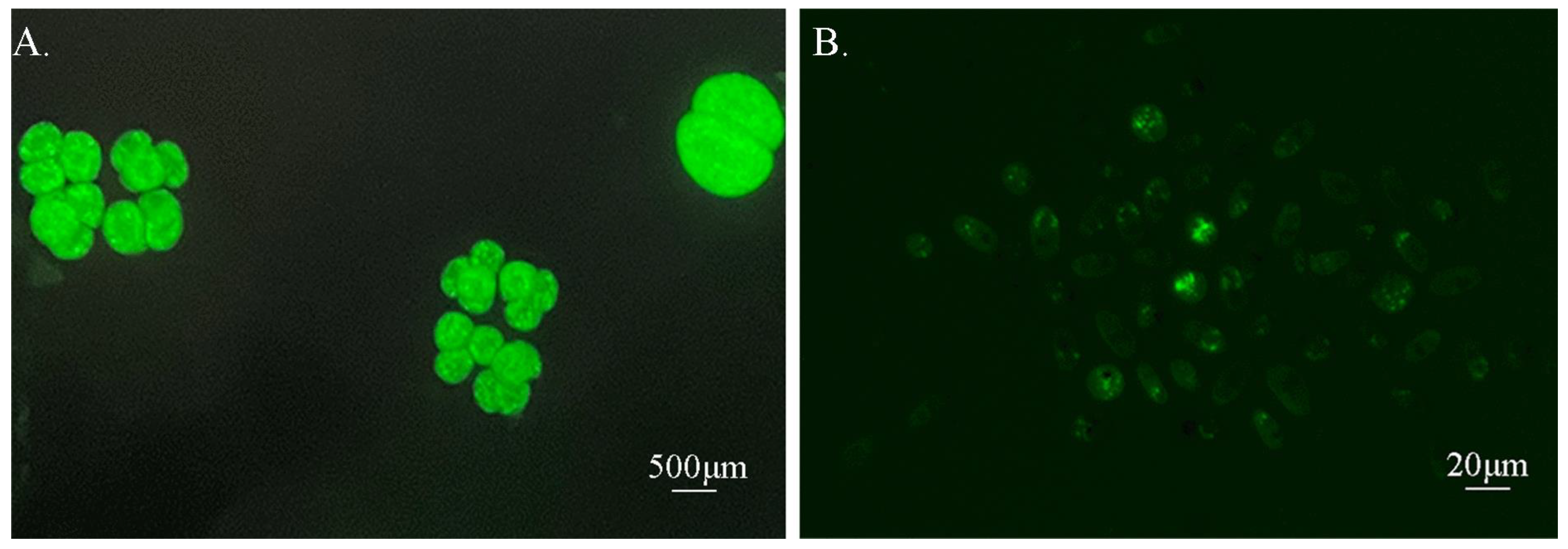
3.1. Feasibility of Theronts Labeled with CFDA-SE
3.2. High Covert Infection with I. multifiliis in Ponds
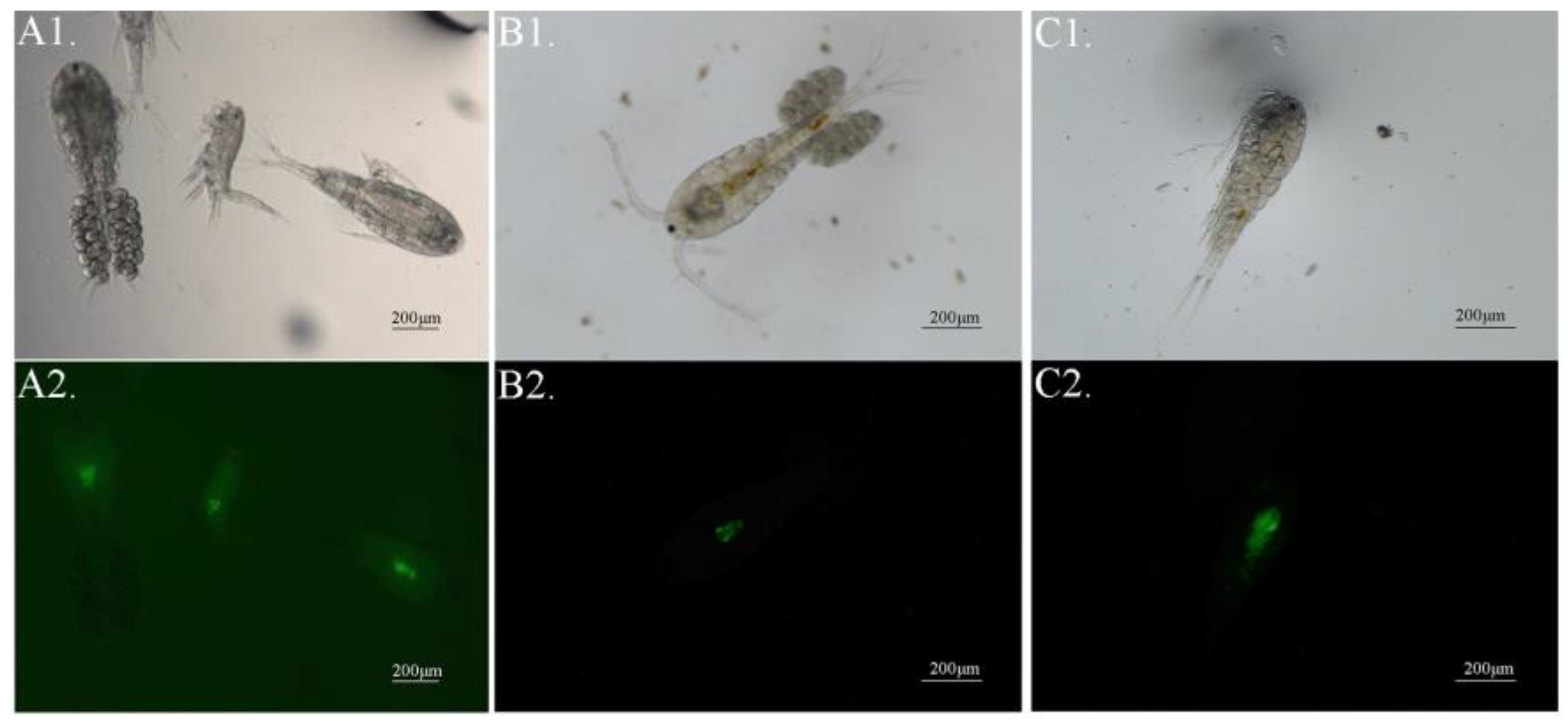
3.3. In-Situ Natural Predators of Theronts in Pond
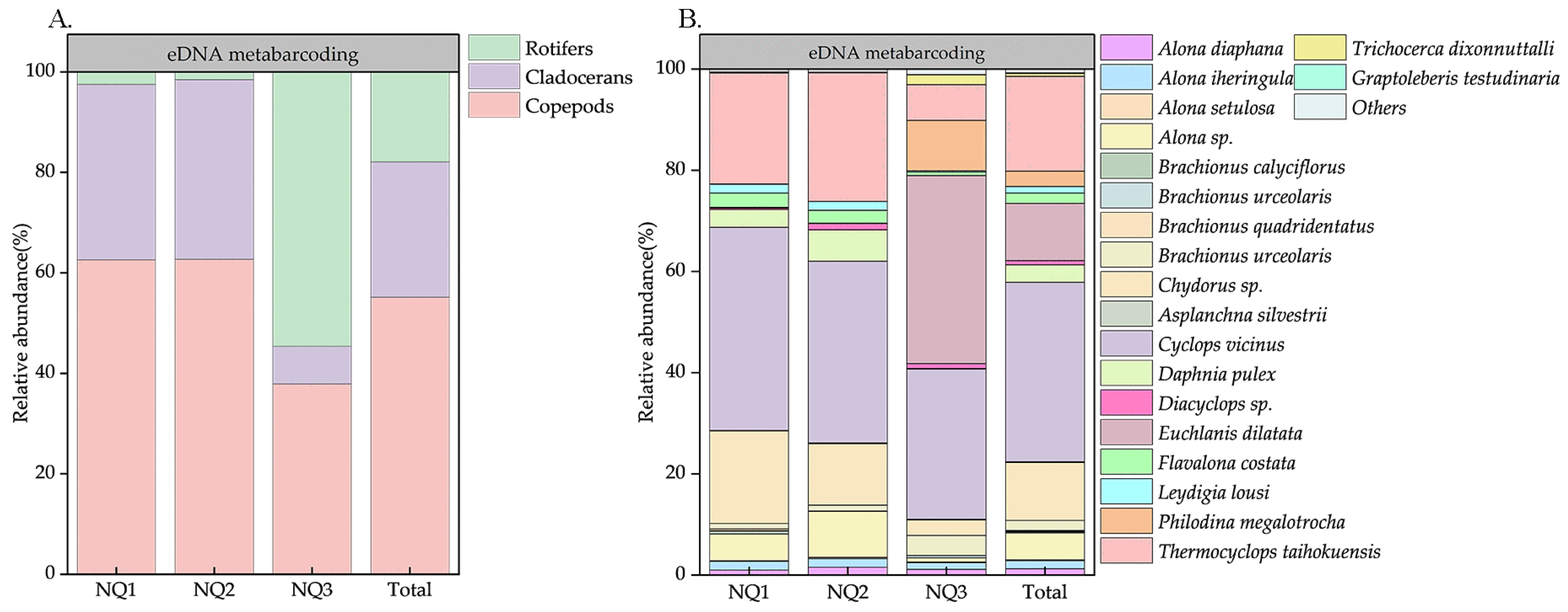
3.4. Zooplankton Composition Based on mt CO1 DNA Metabarcoding Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, R.A. Ichthyophthirius multifiliis Fouquet and ichthyophthiriosis in freshwater teleosts. In Advances in Parasitology; Baker, J.R., Muller, R., Rollinson, D., Eds.; Academic Press, 2005; Vol. 59, pp. 159–241.
- Yang, H.; Tu, X.; Xiao, J.; Hu, J.; Gu, Z. Investigations on white spot disease reveal high genetic diversity of the fish parasite, Ichthyophthirius multifiliis (Fouquet, 1876) in China. Aquaculture 2023, 562, 738804. [Google Scholar] [CrossRef]
- Al-Jubury, A.; Yin, F.; Abusharkh, T.; Fuad, M.H.; Kania, P.W.; Buchmann, K. Stationary metal sheets (copper, zinc or brass) in fish tanks prevent Ichthyophthirius multifiliis Fouquet, 1876 infection of rainbow trout: in vivo and in vitro effects. Aquaculture 2023, 577, 739945. [Google Scholar] [CrossRef]
- Dickerson, H. Ichthyophthirius multifiliis and Cryptocaryon irritans (Phylum Ciliophora). Fish Diseases and Disorders 2006, 1, 116–153. [Google Scholar] [CrossRef]
- Saleh, M.; Abdel-Baki, A.-A.S.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Silencing of heat shock protein 90 (hsp90): effect on development and infectivity of Ichthyophthirius multifiliis. BMC Veterinary Research 2023, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.; Dong, H.T.; Senapin, S.; Kayansamruaj, P.; Pirarat, N.; Rung-ruangkijkrai, T.; Tiawsirisup, S.; Rodkhum, C. Synergistic infection of Ichthyophthirius multifiliis and Francisella noatunensis subsp. orientalis in hybrid red tilapia (Oreochromis sp.). Microbial Pathogenesis 2020, 147, 104369. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Ahmadifar, E.; Dawood, M.A.O.; Soltani, M. Dietary sodium propionate enhanced the growth performance, immune-related genes expression, and resistance against Ichthyophthirius multifiliis in goldfish (Carassius auratus). Aquaculture 2021, 540, 736720. [Google Scholar] [CrossRef]
- Shinn, A.P.; Picón-Camacho, S.M.; Bron, J.E.; Conway, D.; Yoon, G.H.; Guo, F.C.; Taylor, N.G.H. The anti-protozoal activity of bronopol on the key life-stages of Ichthyophthirius multifiliis Fouquet, 1876 (Ciliophora). Veterinary Parasitology 2012, 186, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bodensteiner, L.R.; Sheehan, R.J.; Wills, P.S.; Brandenburg, A.M.; Lewis, W.M. Flowing water: an effective treatment for ichthyophthiriasis. Journal of Aquatic Animal Health 2000, 12, 209–219. [Google Scholar] [CrossRef]
- Gratzek, J.; Gilbert, J.; Lohr, A.; Shotts, E.; Brown, J. Ultraviolet light control of Ichthyophthirius multifiliis Fouquet in a closed fish culture recirculation system. Journal of Fish Diseases 2006, 6, 145–153. [Google Scholar] [CrossRef]
- Forwood, J.M.; Harris, J.O.; Landos, M.; Deveney, M.R. Life cycle and settlement of an australian isolate of Ichthyophthirius multifiliis Fouquet, 1876 from rainbow trout. Folia Parasitol (Praha) 2015, 62, 2015.013. [Google Scholar] [CrossRef] [PubMed]
- Ewing, M.S.; Ewing, S.A.; Zimmer, M.A. Sublethal copper stress and susceptibility of channel catfish to experimental infections with Ichthyophthirius multifiliis. Bull. Environ. Contam. Toxicol. 1982, 28, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Wang, J.-G.; Liu, Q.-F.; Li, M.; Ye, L.-T.; Gong, X.-N. Prevention of Ichthyophthirius multifiliis infestation in goldfish (Carassius auratus) by potassium ferrate(vi) treatment. Veterinary Parasitology 2010, 168, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-R.; Zhao, W.-S.; Wang, R.-Q.; Zou, H.; Li, W.-X.; Wu, S.-G.; Li, M.; Wang, G.-T. In vitro assessment of copper naphthenate against the free-living stages of Ichthyophthirius multifiliis. Aquaculture Reports 2020, 17, 100404. [Google Scholar] [CrossRef]
- Jørgensen, L. von G. The fish parasite Ichthyophthirius multifiliis – host immunology, vaccines and novel treatments. Fish & Shellfish Immunology 2017, 67, 586–595. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, S.; Huang, K.; Zhao, W.; Zou, H.; Li, W.; Li, M.; Wang, G. Can Chilodonella uncinata induce cross-protection in koi carp (Cyprinus carpio) against Ichthyophthirius multifiliis? Evidence from immune response and challenge experiments. Aquaculture 2024, 579, 740198. [Google Scholar] [CrossRef]
- Cao, Z.-Y.; Xi, B.-W.; Zhou, Q.-J.; Chen, K.; Xie, J. Predation of cyclopoid copepods on the theronts of Ichthyophthirius multifiliis: shedding light on biocontrol of white spot disease. Pathogens 2023, 12, 860. [Google Scholar] [CrossRef]
- Kosiba, J.; Krztoń, W. Insight into the role of cyanobacterial bloom in the trophic link between ciliates and predatory copepods. Hydrobiologia 2022, 849, 1195–1206. [Google Scholar] [CrossRef]
- Li, J.; Yang, K.; Chen, F.; Lu, W.; Fang, T.; Zhao, X.; Li, H.; Cui, K. The impacts of crustacean zooplankton on a natural ciliate community: a short-term incubation experiment. Acta Protozoologica 2017, 56, 289–301. [Google Scholar] [CrossRef]
- Tang, H.-J.; Sun, S. Predation of several dominant copepods on microzooplankton in Changjiang river estuary. OLS 2015, 46. [Google Scholar]
- Fu, Y.-W.; Guo, S.-Q.; Luo, J.-J.; Sang, C.-G.; Lin, D.-J.; Liu, Y.-M.; Zhang, Q.-Z. Effectiveness assessment of plant mixtures against Ichthyophthirius multifiliis in grass carp Ctenopharyngodon idella. Aquaculture 2021, 530, 735742. [Google Scholar] [CrossRef]
- Shen, C.J. Fauna Sinica: Crustacea Freshwater Copepoda; Science Press: Beijing, China, 1979; Volume 10, ISBN 978-7-03-030797-2. [Google Scholar]
- Cao, Z.-Y.; Zhou, Q.-J.; Chen, K.; Xi, B.-W.; Xie, J.; Pan, L.-K.; Mao, Y. Establishment and application of PCR and SYBR Green real-time PCR assays for detection of Ichthyophthirius multifiliis. scxb 2024, 48, 109415–109418. [Google Scholar] [CrossRef]
- Prosser, S.; Martínez-Arce, A.; Elías-Gutiérrez, M. A new set of primers for COI amplification from freshwater microcrustaceans. Molecular Ecology Resources 2013, 13, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Swindell, S.R.; Plasterer, T.N. SEQMAN. In Sequence Data Analysis Guidebook; Swindell, S.R., Ed.; Springer New York: Totowa, NJ, 1997; pp. 75–89. ISBN 978-1-59259-556-3. [Google Scholar]
- Wangensteen, O.S.; Palacín, C.; Guardiola, M.; Turon, X. DNA metabarcoding of littoral hard-bottom communities: high diversity and database gaps revealed by two molecular markers. PeerJ 2018, 6, e4705. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Ji, F.; Han, D.; Yan, L.; Yan, S.; Zha, J.; Shen, J. Assessment of benthic invertebrate diversity and river ecological status along an urbanized gradient using environmental DNA metabarcoding and a traditional survey method. Science of The Total Environment 2022, 806, 150587. [Google Scholar] [CrossRef]
- Hansen, P.J.; Bjornsen, P.K.; Hansen, B.W. Zooplankton grazing and growth: scaling within the 2-2,000-μm body size range. Limnol. Oceanogr. 1997, 42, 687–704. [Google Scholar] [CrossRef]
- Kosiba, J.; Wilk-Wozniak, E.; Krzton, W.; Strzesak, M.; Pociecha, A.; Walusiak, E.; Pudas, K.; Szarek-Gwiazda, E. What underpins the trophic networks of the plankton in shallow oxbow lakes? Microb. Ecol. 2017, 73, 17–28. [Google Scholar] [CrossRef]
- Ni, D.; Li, L. Studies on the morphology and life cycle of Ichthyophthirius nultifliis and its control, with a description of a new species. ssswxb 1960, 0, 197–215. [Google Scholar] [CrossRef]
- Coyne, R.S.; Hannick, L.; Shanmugam, D.; Hostetler, J.B.; Brami, D.; Joardar, V.S.; Johnson, J.; Radune, D.; Singh, I.; Badger, J.H.; et al. Comparative genomics of the pathogenic ciliate Ichthyophthirius multifiliis, its free-living relatives and a host species provide insights into adoption of a parasitic lifestyle and prospects for disease control. Genome Biology 2011, 12, R100. [Google Scholar] [CrossRef]
- Mehlhorn, H. Ichthyophthirius multifiliis. In Encyclopedic Reference of Parasitology: Biology, Structure, Function; Springer: Berlin, Heidelberg, 2001; pp. 303–303. ISBN 978-3-540-29834-2. [Google Scholar]
- Nakano, S.; Manage, P.M.; Nishibe, Y.; Kawabata, Z. Trophic linkage among heterotrophic nanoflagellates, ciliates and metazoan zooplankton in a hypereutrophic pond. Aquat. Microb. Ecol. 2001, 25, 259–270. [Google Scholar] [CrossRef]
- Declerck, S.A.J.; Domis, L.N. de S. Contribution of freshwater metazooplankton to aquatic ecosystem services: an overview. Hydrobiologia 2023, 850, 2795–2810. [Google Scholar] [CrossRef]
- Lu, X.; Weisse, T. Top-down control of planktonic ciliates by microcrustacean predators is stronger in lakes than in the ocean. Sci Rep 2022, 12, 10501. [Google Scholar] [CrossRef] [PubMed]
- Kim Hue, N.T.; Deruyck, B.; Decaestecker, E.; Vandamme, D.; Muylaert, K. Biological control of ciliate contamination in Chlamydomonas culture using the predatory copepod Acanthocyclops robustus. Algal Research 2019, 37, 269–276. [Google Scholar] [CrossRef]
- Wang, S.; Xie, P.; Wu, S.; Wu, A. Crustacean zooplankton distribution patterns and their biomass as related to trophic indicators of 29 shallow subtropical lakes. Limnologica 2007, 37, 242–249. [Google Scholar] [CrossRef]
- Kobari, T.; Ban, S. Life cycles of two limnetic cyclopoid copepods, Cyclops vicinus and Thermocyclops crassus, in two different habitats. J. Plankton Res. 1998, 20, 1073–1086. [Google Scholar] [CrossRef]
- Maier, G. Variable life-cycles in the fresh-water copepod Cyclops vicinus (Uljanin 1875) - support for the predator avoidance hypothesis. Arch. Hydrobiol. 1989, 115, 203–219. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Jeong, K.-S.; Joo, G.-J. Zooplankton community distribution in shallow reservoirs during winter: influence of environmental factors on Cyclops vicinus (copepoda: cyclopoida). Journal of Ecology and Environment 2014, 37, 99–104. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Jing, Y.; Wang, C.; Zhang, Y. Response of copepod community characteristics to environmental factors in the backshore wetland of expo garden, Shanghai. Huanjing Kexue 2012, 33, 3941–3948. [Google Scholar]
- McEwan, G.F.; Groner, M.L.; Cohen, A.A.B.; Imsland, A.K.D.; Revie, C.W. Modelling sea lice control by lumpfish on atlantic salmon farms: interactions with mate limitation, temperature and treatment rules. Dis. Aquat. Org. 2019, 133, 69–82. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Mo, Z.; Li, A.; Dan, X. Cryptocaryon irritans (Brown, 1951) is a serious threat to aquaculture of marine fish. Rev. Aquac. 2022, 14, 218–236. [Google Scholar] [CrossRef]
| Source | Species | Numbers | Genbank Similar Species | |
| Species (Acc. No.) | Sequence Similarity | |||
| NQ1 | C. vicinus | 45 | C. vicinus (LC604938) | 99.05% ~ 100% |
| T. taihokuensis | 3 | T. taihokuensis (LC215456) | 99.08% ~ 99.09% | |
| Cyclops sp. | 4 | C. vicinus (LC604938) | 96.36% | |
| Thermocyclops sp. | 2 | T. taihokuensis (LC215458) | 98.02% ~ 98.12% | |
| NQ2 | C. vicinus | 47 | C. vicinus (LC604938) | 99.05% ~ 100% |
| T. taihokuensis | 5 | T. taihokuensis (LC215456) | 99.09% ~ 99.85% | |
| Mesocyclops sp. | 1 | Mesocyclops sp. (KJ020568) | 96.94% | |
| Cyclops sp. | 6 | Cyclops sp. (LC215454) | 84.43% ~ 98.49% | |
| Eucyclops sp. | 2 | Eucyclops sp. (KJ020567) | 98.94% ~ 100% | |
| NQ3 | C. vicinus | 42 | C. vicinus (LC604938) | 99.05% ~ 100% |
| Eucyclops sp. | 4 | Eucyclops sp. (KJ020567) | 98.94% ~ 100% | |
| Cyclops sp. | 1 | Cyclops sp. (LC215454) | 84.83% | |
| Phylum | Order | Family | Genus | species | |||||||||||
| NQ1 | NQ2 | NQ3 | NQ1 | NQ2 | NQ3 | NQ1 | NQ2 | NQ3 | NQ1 | NQ2 | NQ3 | ||||
| Arthropoda | 3 | 3 | 3 | 7 | 8 | 10 | 18 | 21 | 21 | 32 | 35 | 34 | |||
| Rotifera | 3 | 3 | 3 | 9 | 7 | 11 | 8 | 4 | 15 | 15 | 14 | 34 | |||
| Total | 6 | 22 | 39 | 78 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





