Submitted:
14 December 2024
Posted:
16 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Stable Transfectants
2.2. Antibodies
2.3. Development of Hybridomas
2.4. Flow Cytometric Analysis
2.5. Determination of Dissociation Constant (KD) by Flow Cytometry
2.6. Western Blot Analysis
3. Results
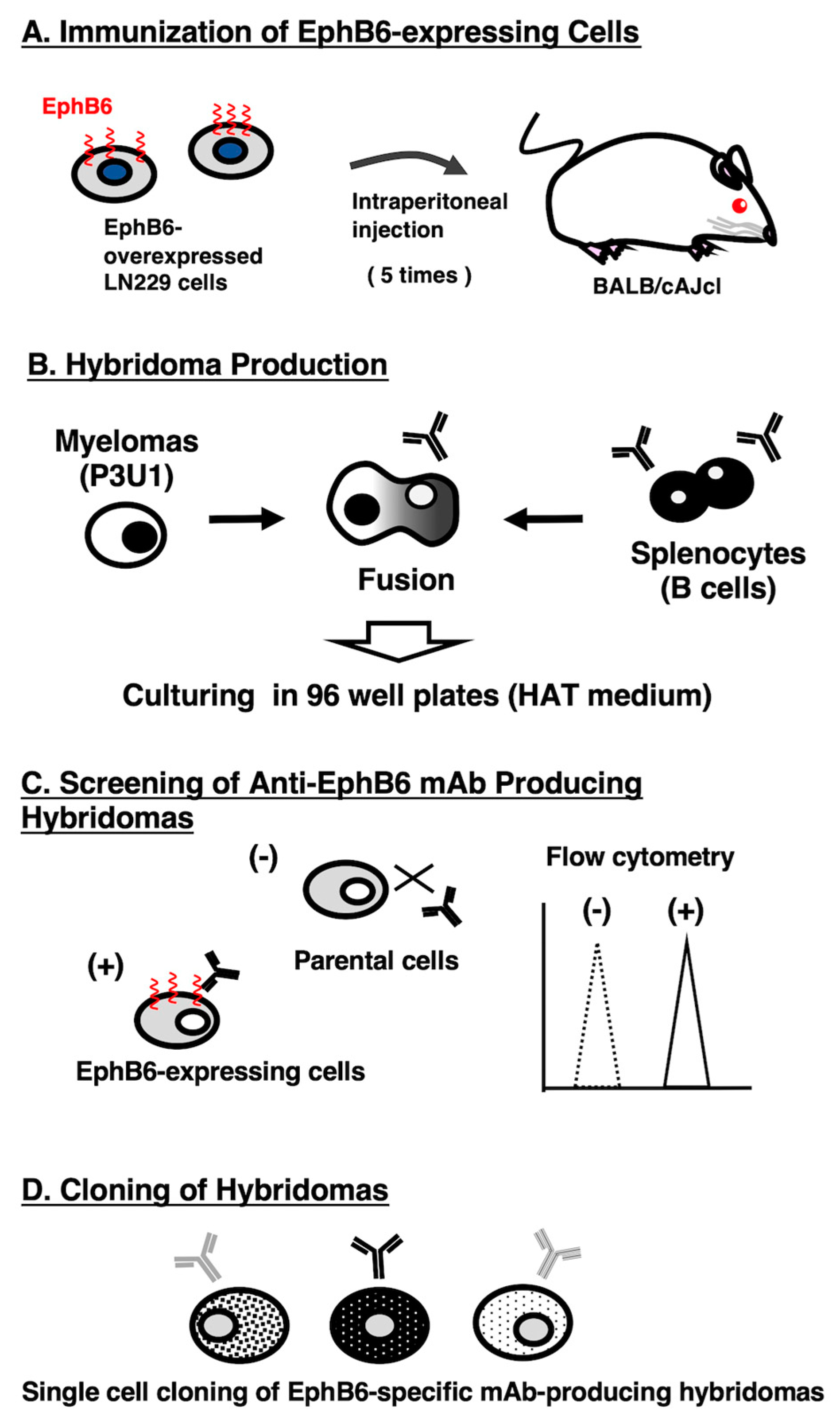
3.1. Development of Anti-EphB6 mAbs Using the CBIS Method
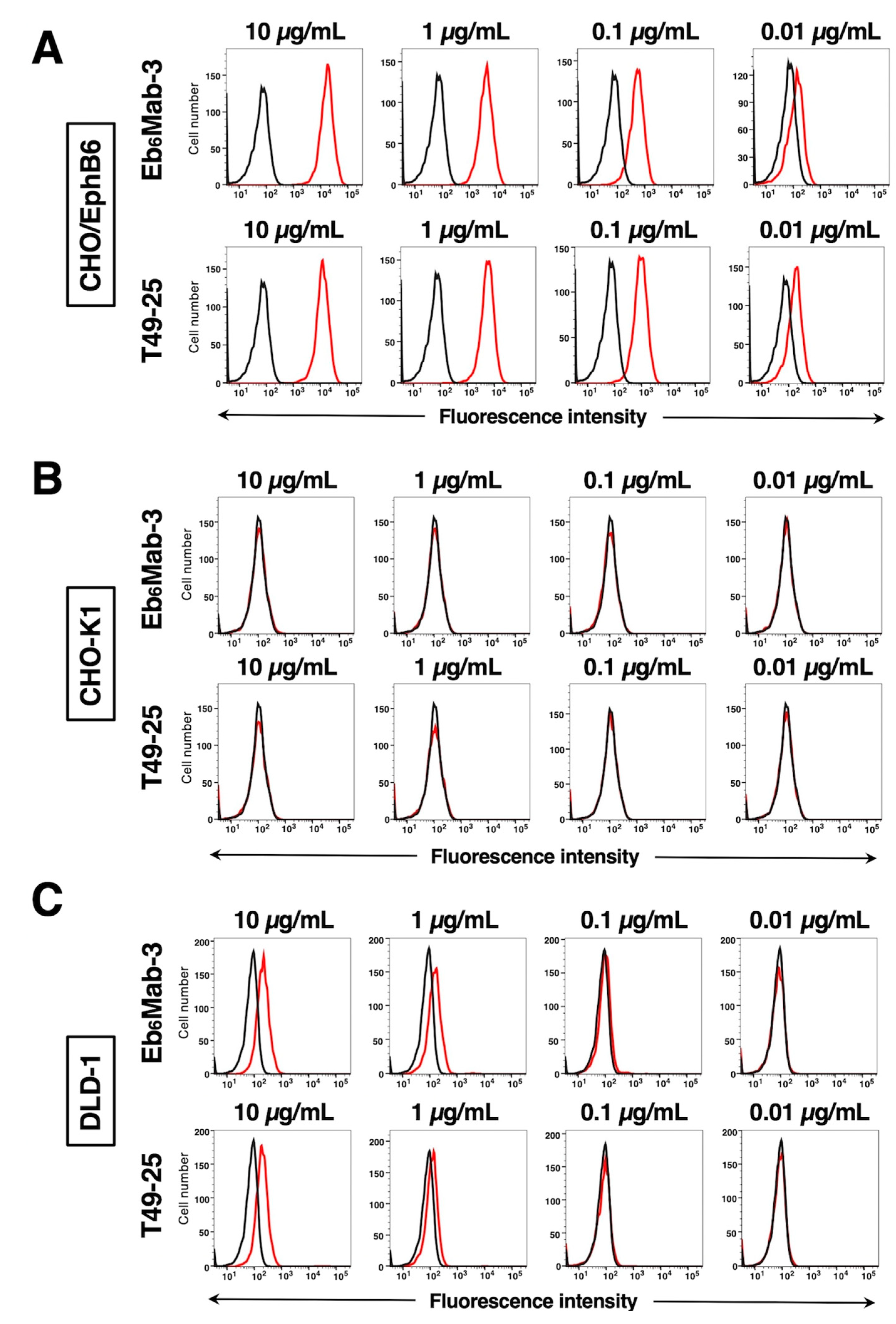
3.2. Evaluation of Antibody Reactivity Using Flow Cytometry
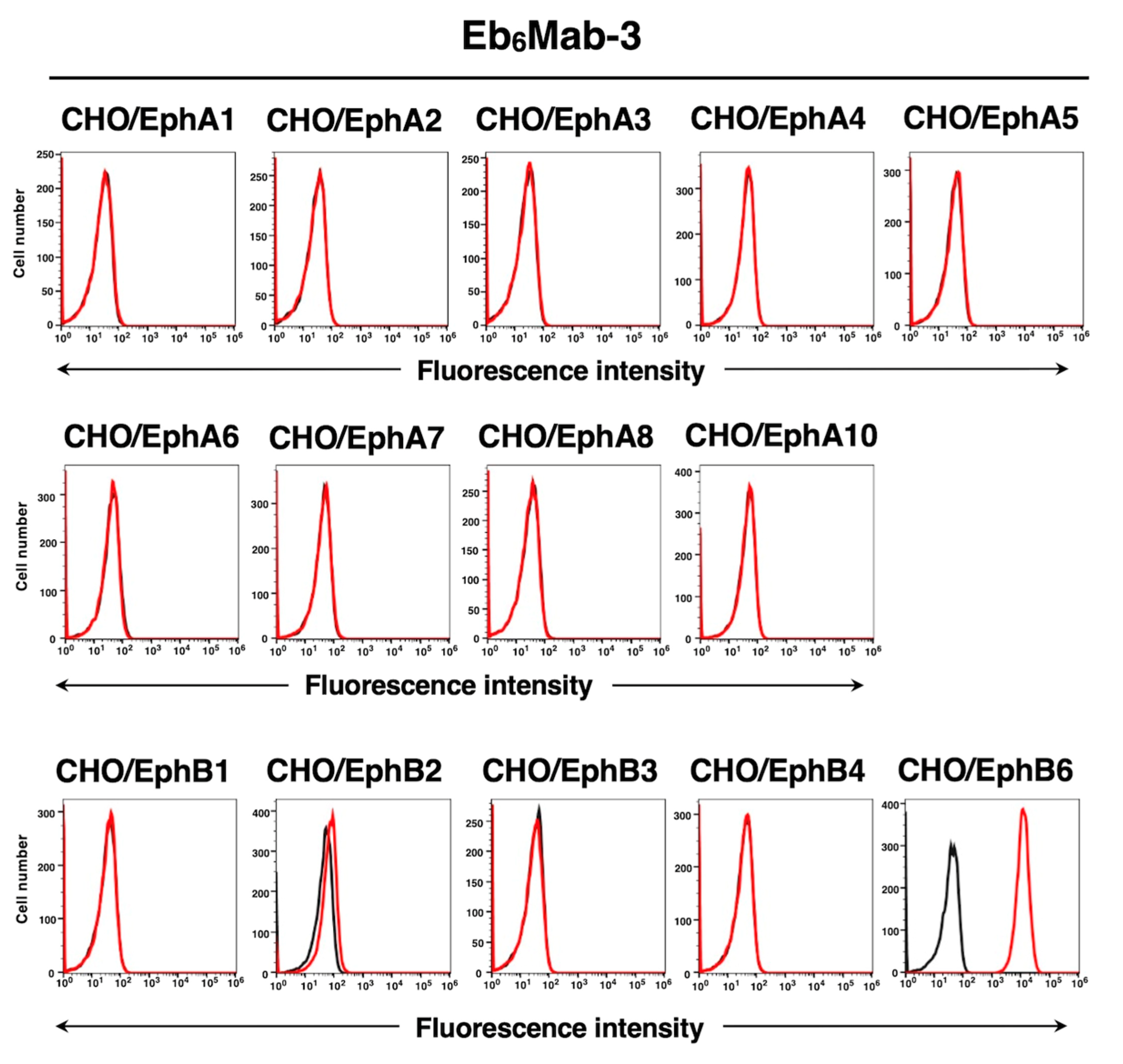
3.3. Specificity of Eb6Mab-3 to Eph Receptor-Overexpressed CHO-K1 Cells
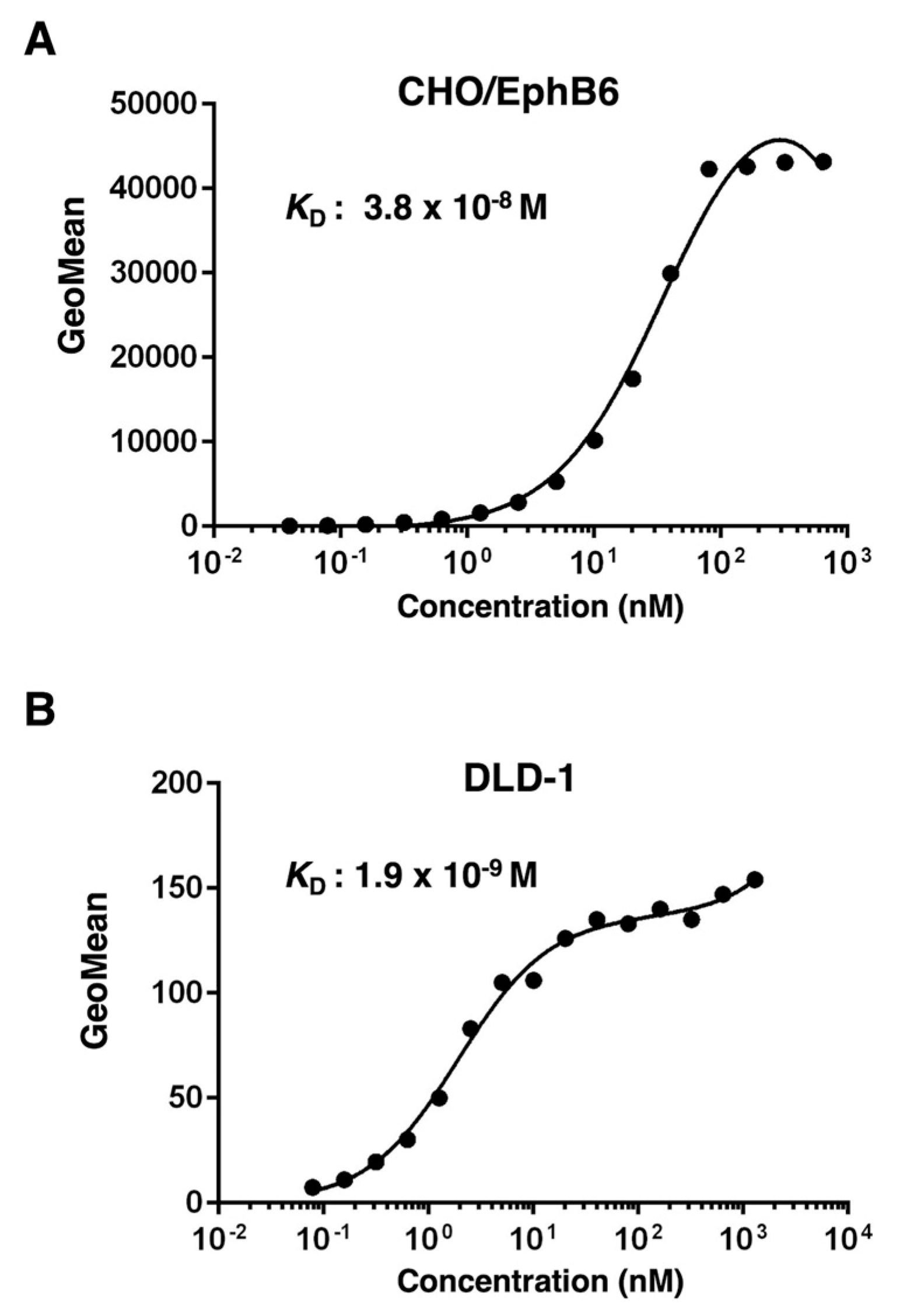
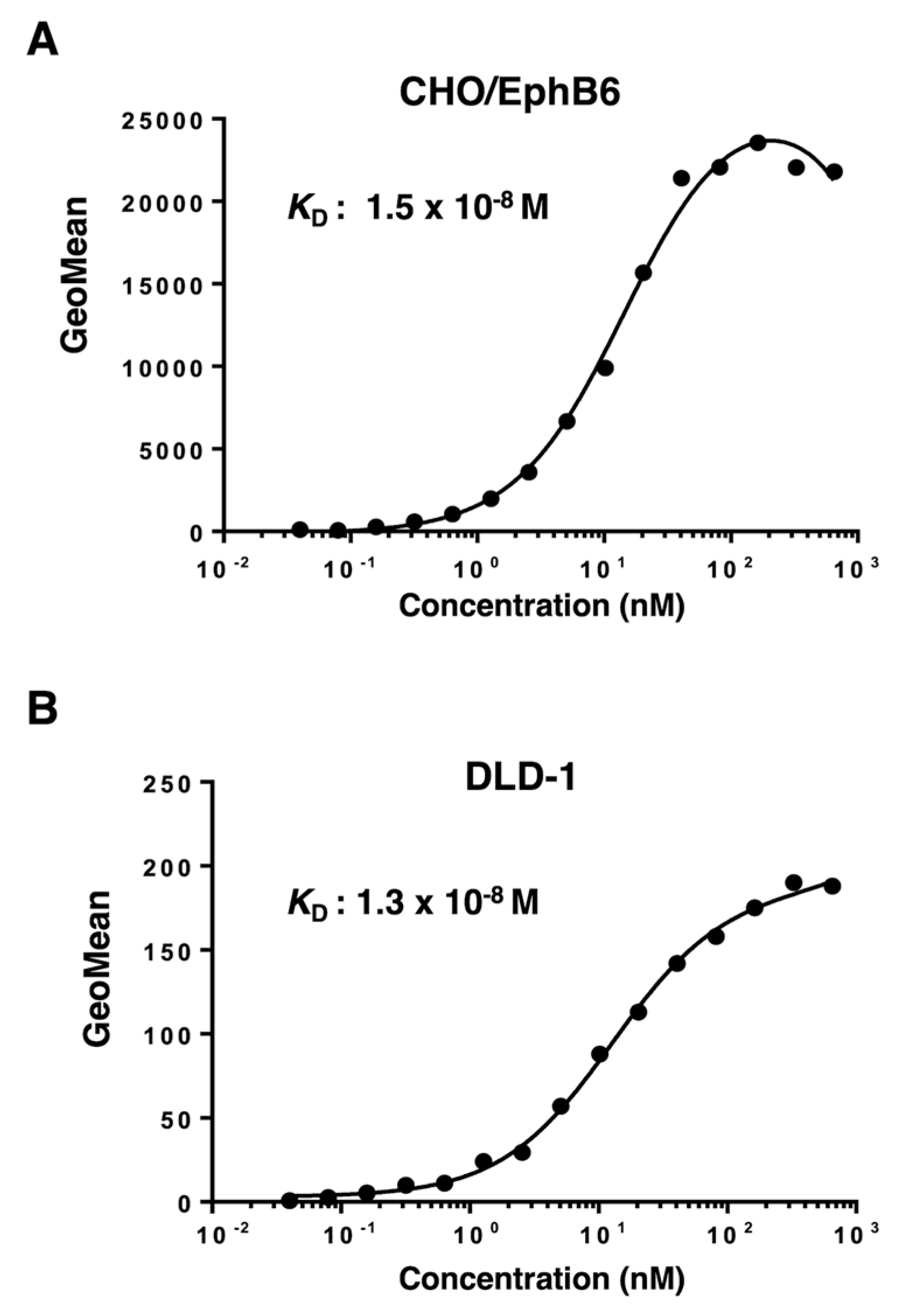
3.4. Calculation of the Binding Affinity of Anti-EphB6 mAbs Using Flow Cytometry
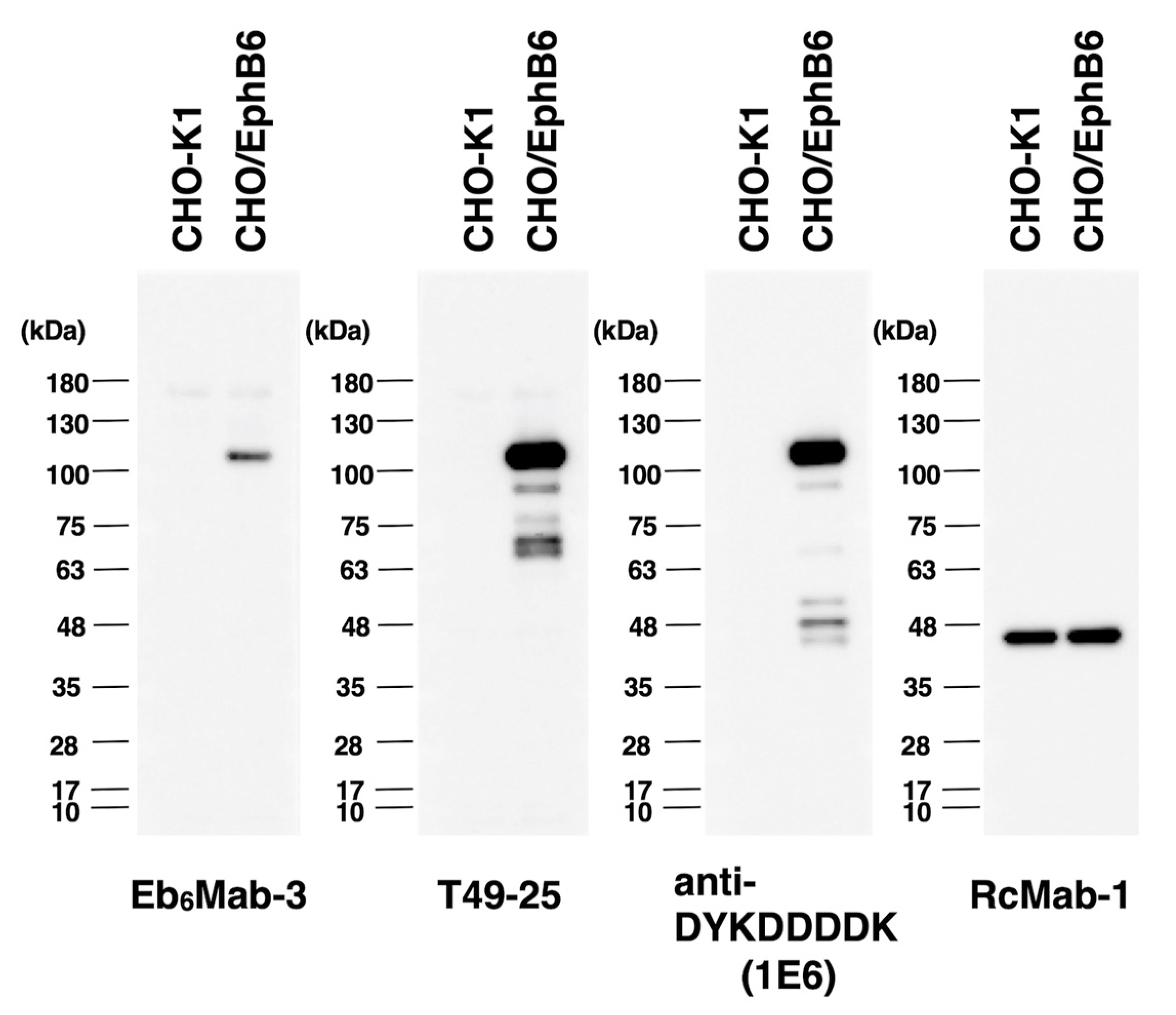
3.5. Western Blot Analyses Using Anti-EphB6 mAbs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008;128(6): 1365-1374. [CrossRef]
- Schneider, M.R.; Werner, S.; Paus, R.; Wolf, E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol 2008;173(1): 14-24.
- Schneider, M.R.; Wolf, E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol 2009;218(3): 460-466.
- Plum, L.; Schubert, M.; Brüning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 2005;16(2): 59-65. [CrossRef]
- Schlessinger, J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol 2014;6(3).
- Bennasroune, A.; Gardin, A.; Aunis, D.; Crémel, G.; Hubert, P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol 2004;50(1): 23-38.
- Templeton, A.J.; Diez-Gonzalez, L.; Ace, O.; et al. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: a meta-analysis. Cancer Treat Rev 2014;40(9): 1048-1055.
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat Rev Cancer 2024;24(1): 5-27. [CrossRef]
- Trinidad, E.M.; Zapata, A.G.; Alonso-Colmenar, L.M. Eph-ephrin bidirectional signaling comes into the context of lymphocyte transendothelial migration. Cell Adh Migr 2010;4(3): 363-367.
- Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008;133(1): 38-52.
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci 2008;5(5): 263-272.
- Miao, H.; Wang, B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol 2009;41(4): 762-770.
- McCarron, J.K.; Stringer, B.W.; Day, B.W.; Boyd, A.W. Ephrin expression and function in cancer. Future Oncol 2010;6(1): 165-176.
- Gurniak, C.B.; Berg, L.J. A new member of the Eph family of receptors that lacks protein tyrosine kinase activity. Oncogene 1996;13(4): 777-786.
- Mendrola, J.M.; Shi, F.; Park, J.H.; Lemmon, M.A. Receptor tyrosine kinases with intracellular pseudokinase domains. Biochem Soc Trans 2013;41(4): 1029-1036. [CrossRef]
- Liang, L.Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph receptor signalling: from catalytic to non-catalytic functions. Oncogene 2019;38(39): 6567-6584.
- Munthe, E.; Rian, E.; Holien, T.; et al. Ephrin-B2 is a candidate ligand for the Eph receptor, EphB6. FEBS Lett 2000;466(1): 169-174.
- Liang, L.Y.; Geoghegan, N.D.; Mlodzianoski, M.; et al. Co-clustering of EphB6 and ephrinB1 in trans restrains cancer cell invasion. Commun Biol 2024;7(1): 461.
- Xu, D.; Yuan, L.; Liu, X.; et al. EphB6 overexpression and Apc mutation together promote colorectal cancer. Oncotarget 2016;7(21): 31111-31121.
- El Zawily, A.; McEwen, E.; Toosi, B.; et al. The EphB6 receptor is overexpressed in pediatric T cell acute lymphoblastic leukemia and increases its sensitivity to doxorubicin treatment. Sci Rep 2017;7(1): 14767. [CrossRef]
- Colucci, M.; Trivieri, N.; Mencarelli, G.; et al. A functional role of Ephrin type-B receptor 6 (EPHB6) in T-cell acute lymphoblastic leukemia. Biomark Res 2023;11(1): 92.
- Dong, Y.; Pan, J.; Ni, Y.; et al. High expression of EphB6 protein in tongue squamous cell carcinoma is associated with a poor outcome. Int J Clin Exp Pathol 2015;8(9): 11428-11433.
- Fox, B.P.; Kandpal, R.P. EphB6 receptor significantly alters invasiveness and other phenotypic characteristics of human breast carcinoma cells. Oncogene 2009;28(14): 1706-1713.
- Jia, X.; Zhang, D.; Zhou, C.; et al. Eph receptor B6 shapes a cold immune microenvironment, inhibiting anti-cancer immunity and immunotherapy response in bladder cancer. Front Oncol 2023;13: 1175183.
- Bulk, E.; Yu, J.; Hascher, A.; et al. Mutations of the EPHB6 receptor tyrosine kinase induce a pro-metastatic phenotype in non-small cell lung cancer. PLoS One 2012;7(12): e44591.
- Freywald, A.; Sharfe, N.; Roifman, C.M. The kinase-null EphB6 receptor undergoes transphosphorylation in a complex with EphB1. J Biol Chem 2002;277(6): 3823-3828.
- Truitt, L.; Freywald, T.; DeCoteau, J.; Sharfe, N.; Freywald, A. The EphB6 receptor cooperates with c-Cbl to regulate the behavior of breast cancer cells. Cancer Res 2010;70(3): 1141-1153.
- Akada, M.; Harada, K.; Negishi, M.; Katoh, H. EphB6 promotes anoikis by modulating EphA2 signaling. Cell Signal 2014;26(12): 2879-2884.
- Giaginis, C.; Alexandrou, P.; Poulaki, E.; et al. Clinical Significance of EphB4 and EphB6 Expression in Human Malignant and Benign Thyroid Lesions. Pathol Oncol Res 2016;22(2): 269-275. [CrossRef]
- Mateo-Lozano, S.; Bazzocco, S.; Rodrigues, P.; et al. Loss of the EPH receptor B6 contributes to colorectal cancer metastasis. Sci Rep 2017;7: 43702.
- Liu, J.; Xu, B.; Xu, G.; et al. Reduced EphB6 protein in gastric carcinoma and associated lymph nodes suggests EphB6 as a gastric tumor and metastasis inhibitor. Cancer Biomark 2017;19(3): 241-248.
- Yu, H.; Qin, X.K.; Yin, K.W.; et al. EphB6 deficiency in intestinal neurons promotes tumor growth in colorectal cancer by neurotransmitter GABA signaling. Carcinogenesis 2023;44(8-9): 682-694. [CrossRef]
- Yu, J.; Bulk, E.; Ji, P.; et al. The EPHB6 receptor tyrosine kinase is a metastasis suppressor that is frequently silenced by promoter DNA hypermethylation in non-small cell lung cancer. Clin Cancer Res 2010;16(8): 2275-2283.
- Bailey, C.M.; Kulesa, P.M. Dynamic interactions between cancer cells and the embryonic microenvironment regulate cell invasion and reveal EphB6 as a metastasis suppressor. Mol Cancer Res 2014;12(9): 1303-1313.
- Gu, Y.; Li, F.; Qian, N.; et al. Expression of EphB6 in ovarian serous carcinoma is associated with grade, TNM stage and survival. J Clin Pathol 2016;69(5): 448-453.
- Tang, X.X.; Evans, A.E.; Zhao, H.; et al. High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res 1999;5(6): 1491-1496.
- Luo, H.; Yu, G.; Tremblay, J.; Wu, J. EphB6-null mutation results in compromised T cell function. J Clin Invest 2004;114(12): 1762-1773.
- Luo, H.; Yu, G.; Wu, Y.; Wu, J. EphB6 crosslinking results in costimulation of T cells. J Clin Invest 2002;110(8): 1141-1150.
- Yoon, S.; Choi, J.H.; Kim, S.J.; et al. EPHB6 mutation induces cell adhesion-mediated paclitaxel resistance via EPHA2 and CDH11 expression. Exp Mol Med 2019;51(6): 1-12.
- Satofuka H, S.H., Tanaka T, Li G, Kaneko MK, Kato Y. An Anti-Human EphA2 Monoclonal Antibody Ea2Mab-7 Shows High Sensitivity for Flow Cytometry, Western Blot, and Immunohistochemical Analyses. Preprint 2024.
- Ubukata R, S.H., Hirose M, Satofuka H, Tanaka T, Kaneko MK, Kato Y. Establishment of a Highly-sensitive Anti-EphB2 Monoclonal Antibody Eb2Mab-3 for Flow Cytometry. Preprint 2024.
- Nanamiya, R.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of an Anti-EphB4 Monoclonal Antibody for Multiple Applications Against Breast Cancers. Monoclon Antib Immunodiagn Immunother 2023;42(5): 166-177.
- Tanaka, T.; Yamamoto, H.; Kaneko, Y.; et al. Ea8Mab-9: A Novel Monoclonal Antibody Against Erythropoietin-Producing Hepatocellular Receptor A8 for Flow Cytometry. Preprints 2024. [CrossRef]
- Ikota, H.; Nobusawa, S.; Arai, H.; et al. Evaluation of IDH1 status in diffusely infiltrating gliomas by immunohistochemistry using anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol 2015;32(4): 237-244.
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002;298(5600): 1912-1934.
- Boudeau, J.; Miranda-Saavedra, D.; Barton, G.J.; Alessi, D.R. Emerging roles of pseudokinases. Trends Cell Biol 2006;16(9): 443-452.
- Strozen, T.G.; Sharpe, J.C.; Harris, E.D.; Uppalapati, M.; Toosi, B.M. The EphB6 Receptor: Kinase-Dead but Very Much Alive. Int J Mol Sci 2021;22(15).
- Ogawa, K.; Wada, H.; Okada, N.; et al. EphB2 and ephrin-B1 expressed in the adult kidney regulate the cytoarchitecture of medullary tubule cells through Rho family GTPases. J Cell Sci 2006;119(Pt 3): 559-570.
- Luo, H.; Wu, Z.; Tremblay, J.; et al. Receptor tyrosine kinase Ephb6 regulates vascular smooth muscle contractility and modulates blood pressure in concert with sex hormones. J Biol Chem 2012;287(9): 6819-6829.
- Freywald, A.; Sharfe, N.; Rashotte, C.; Grunberger, T.; Roifman, C.M. The EphB6 receptor inhibits JNK activation in T lymphocytes and modulates T cell receptor-mediated responses. J Biol Chem 2003;278(12): 10150-10156.
- Matsuoka, H.; Obama, H.; Kelly, M.L.; Matsui, T.; Nakamoto, M. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem 2005;280(32): 29355-29363.
- Hafner, C.; Schmitz, G.; Meyer, S.; et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem 2004;50(3): 490-499.
- Mujoo, K.; Choi, B.K.; Huang, Z.; Zhang, N.; An, Z. Regulation of ERBB3/HER3 signaling in cancer. Oncotarget 2014;5(21): 10222-10236.
- Colomba, A.; Claus, J.; Gao, F.; et al. Targeting the HER3 pseudokinase domain with small molecule inhibitors. Methods Enzymol 2022;667: 455-505.
- Pawar, A.B.; Sengupta, D. Resolving the conformational dynamics of ErbB growth factor receptor dimers. J Struct Biol 2019;207(2): 225-233.
- Xie, T.; Lim, S.M.; Westover, K.D.; et al. Pharmacological targeting of the pseudokinase Her3. Nat Chem Biol 2014;10(12): 1006-1012.
- Arter, Z.L.; Nagasaka, M. Spotlight on Patritumab Deruxtecan (HER3-DXd) from HERTHENA Lung01. Is a Median PFS of 5.5 Months Enough in Light of FLAURA-2 and MARIPOSA? Lung Cancer (Auckl) 2024;15: 115-121.
- Yu, J.; Bulk, E.; Ji, P.; et al. The kinase defective EPHB6 receptor tyrosine kinase activates MAP kinase signaling in lung adenocarcinoma. Int J Oncol 2009;35(1): 175-179.
- Wang, J.; Zhang, Y.; Ma, J.; et al. Determining the effects of Ephrin Type B Receptor 6 and Type A Receptor 3 on facilitating colorectal epithelial cell malignant transformation. Neoplasma 2021;68(5): 955-964.
- Toosi, B.M.; El Zawily, A.; Truitt, L.; et al. EPHB6 augments both development and drug sensitivity of triple-negative breast cancer tumours. Oncogene 2018;37(30): 4073-4093.
- Hanover, G.; Vizeacoumar, F.S.; Banerjee, S.L.; et al. Integration of cancer-related genetic landscape of Eph receptors and ephrins with proteomics identifies a crosstalk between EPHB6 and EGFR. Cell Rep 2023;42(7): 112670.
- Kennedy, S.P.; Han, J.Z.R.; Portman, N.; et al. Targeting promiscuous heterodimerization overcomes innate resistance to ERBB2 dimerization inhibitors in breast cancer. Breast Cancer Res 2019;21(1): 43.
- Zhou, Y.; Yamada, N.; Tanaka, T.; et al. Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2. Nat Commun 2015;6: 7679.
- Shi, X.; Lingerak, R.; Herting, C.J.; et al. Time-resolved live-cell spectroscopy reveals EphA2 multimeric assembly. Science 2023;382(6674): 1042-1050.
- Zhou, Y.; Sakurai, H. Emerging and Diverse Functions of the EphA2 Noncanonical Pathway in Cancer Progression. Biol Pharm Bull 2017;40(10): 1616-1624.
- Hafner, C.; Bataille, F.; Meyer, S.; et al. Loss of EphB6 expression in metastatic melanoma. Int J Oncol 2003;23(6): 1553-1559.
- Pitulescu, M.E.; Adams, R.H. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev 2010;24(22): 2480-2492.
- Shitara, K.; Satoh, T.; Iwasa, S.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: a first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J Immunother Cancer 2019;7(1): 219.
- Gan, H.K.; Parakh, S.; Lee, F.T.; et al. A phase 1 safety and bioimaging trial of antibody DS-8895a against EphA2 in patients with advanced or metastatic EphA2 positive cancers. Invest New Drugs 2022;40(4): 747-755.
- Swords, R.T.; Greenberg, P.L.; Wei, A.H.; et al. KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3, in patients with advanced hematologic malignancies: Results from a phase 1 study. Leuk Res 2016;50: 123-131.
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021;40(4): 162-167.
- Okada, Y.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Epitope Mapping of an Anti-Mouse CD39 Monoclonal Antibody Using PA Scanning and RIEDL Scanning. Monoclon Antib Immunodiagn Immunother 2024;43(2): 44-52.
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts. Int J Mol Sci 2023;25(1).
- Tanaka, T.; Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Anti-Podoplanin Monoclonal Antibody, PMab-117-mG(2a) Exerts Antitumor Activities in Human Tumor Xenograft Models. Cells 2024;13(22).
- Tanaka, T.; Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. Antitumor activities against breast cancers by an afucosylated anti-HER2 monoclonal antibody H(2) Mab-77-mG(2a) -f. Cancer Sci 2024;115(1): 298-309.
- Arimori, T.; Mihara, E.; Suzuki, H.; et al. Locally misfolded HER2 expressed on cancer cells is a promising target for development of cancer-specific antibodies. Structure 2024;32(5): 536-549.e535.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




